Abstract
Aging leads to substantial structural changes in the skin. Elastic fibers maintain skin structure, but their degeneration and loss of function with age result in wrinkle formation and loss of skin elasticity. Oxytalan fiber, a type of elastic fiber, extends close to the dermal–epidermal junction (DEJ) from the back of the dermis. Oxytalan fibers are abundant in the papillary layer and contribute to skin elasticity and texture. However, to accurately understand the mechanisms of skin elasticity, the interaction between elastic fibers and DEJ should be elucidated. Here, we investigated elastic fibers and DEJ and their structural alterations with aging. Several basement membrane proteins [collagen (COL) IV, COLVII, and laminin 332], fibrous tropoelastin, and fibrillin-1 in excised human skin tissue were observed using three-dimensional imaging. Age-related alterations in COLVII, elastic fibers, and fibrillin-1 were evaluated. We found that COLVII forms long hanging structures and is co-localized with fibrous tropoelastin in young skin but not aged skin. Fibrillin-1-rich regions were observed at the tips of elastin fibers in young skin tissue, but rarely in aged skin. This co-localization of elastic fiber and COLVII may maintain skin structure, thereby preventing wrinkling and sagging. COLVII is a potential therapeutic target for skin wrinkling:
Keywords: 3D structure, aging, computational analysis, dermal–epidermal junction, elasticity, imaging, skin
Introduction
Elastic fibers are associated with elasticity and structural integrity of the skin.1,2 Age-related degeneration of elastic fibers has been shown to cause wrinkling and loss of elasticity of the skin. 3 A study using skin sections from elderly and young individuals has shown a correlation between the degradation of dermal elastic fibers and the presence of wrinkles. 4 Elastic fibers are divided into three types, namely, elastic, elaunin, and oxytalan fibers. 5 In the reticular dermis, elastic fibers extend parallel to the skin surface and interact with elaunin fibers. Elaunin fibers are slightly thicker than oxytalan fibers and extend to the foot of the papillary layer from the reticular dermis. Oxytalan fibers extend from elaunin fibers to the dermal–epidermal junction (DEJ), and their terminals contain a large proportion of fibrillin. In particular, the papillary dermis is rich in oxytalan fibers, displaying candelabra-like structures.6,7 These fibers are associated with skin resilience and surface texture and help resist skin aging–related changes. 8 Elastic fibers of the papillary dermis disappear in areas constantly exposed to sunlight (photo-aged skin), and a large number of amorphous fibers are deposited in the lower layers; furthermore, elastic fibers naturally decrease with aging in areas not exposed to sunlight. 9 Elastic fiber turnover is a long process, and the regeneration of elastic fibers becomes difficult when the fibers are damaged.10–12 Previously, using three-dimensional (3D) observation and analysis, we found that elastic fibers incurve, shorten, and thicken with age. 13 However, to decipher the mechanism of skin elasticity more accurately, information about elastic fiber structure must be supplemented with knowledge on the connections and interactions of fibers with the epidermal basement membrane.
The DEJ of the skin enables structural adhesion between the epidermis and the dermis. It has important functions, including the regulation of epithelial–mesenchymal interactions, acting as a permeability barrier, participating in signal transduction, maintaining the architectural integrity of the epidermis, and providing protection against shearing forces. The four major subregions of the DEJ can be differentiated using transmission electron microscopy. 14 The structure of the basement membrane at the epidermis–dermis junction consists of multiple proteins provided by fibroblasts and/or keratinocytes. From the epidermal side, mainly integrin, laminin 332, COLXVII, COLIV, and COLVII are arranged by specific interactions between the structural domains of the individual components. 15 The DEJ exhibits stiffness and shows undulating structure; it connects the dermal and epidermal layers, enhances crosstalk, and contributes to the maintenance and regeneration of the dermis and epidermis. However, due to intrinsic and extrinsic factors, numerous structural alterations occur with aging. These alterations weaken the substructure of the DEJ and its function, contributing to the decline in the overall skin physiology. 16 Moreover, COLVII, which is located in the lowermost layer of the basement membrane and forms anchoring fibrils, serves as a junction with dermal collagen fibers. Congenital or acquired deficiencies and abnormalities of these proteins weaken the bond with collagen on the dermal side, inducing skin fragility and blistering.17,18
It is speculated that elastic fibers are also bound to the epidermal basement membrane to contribute to the maintenance of the structure and elasticity of the skin. However, the details of the bonding structure between the basement membrane and the elastic fibers are not clear. We previously observed that there is a gap ranging from several micrometers to several tens of micrometers between the tip of the candelabra structure of oxytalan fibers and the basement membrane. In this study, we investigated the structure that fills the gap between and binds the elastic fiber and basement membrane, as well as alterations in the structure that occur with aging.
Materials and Methods
Human Skin Tissue Samples
Fixed human abdominal skin tissues from 33 Caucasian women (aged 23–78 years, with a mean age of 51.1 ± 11.2 years) were purchased from KAC Co., Ltd. (Kyoto, Japan) and used to perform 3D imaging.
Immunofluorescence Staining and Decolorization
Each fixed skin tissue sample was sliced to 1-mm-thick sections in the epidermis–dermis orientation. The skin sections were washed with 0.05% Tween 20 in phosphate-buffered saline and blocked with StartingBlock blocking buffer (Thermo Fisher Scientific; Waltham, MA). Consequently, they were labeled with anti-tropoelastin mouse monoclonal antibody clone 10B8 (Santa Cruz Biotechnology; Dallas, TX) and anti-collagen-4 (COLIV) rabbit polyclonal antibody (pAb) (Abcam; Cambridge, UK), anti-COLVII rabbit pAb (Abcam), anti-laminin 332 rabbit pAb (Abcam), and anti-fibrillin-1 rabbit pAb (Elastin Products Company; Owensville, MO), diluted in StartingBlock blocking buffer to 1:500. After washing, the sections were stained with Goat Anti-Mouse IgG H&L Alexa Fluor 488 and Goat Anti-Rabbit IgG H&L Alexa Fluor 647 (Thermo Fisher Scientific) at a dilution of 1:1000. Furthermore, nuclear staining was performed using 4’,6-diamidino-2-phenylindole (DAPI) (DOJINDO Lab; Kumamoto, Japan) at 1:5000 dilution. After washing, these sections were subsequently decolorized using RapiClear 1.49 (SunJin Lab Co.; Hsinchu, Taiwan).
3D Imaging and Structural Analysis
After decolorization, nuclear, tropoelastin, and basement membrane proteins (COLIV, COLVII, and laminin 332) in each skin section were observed along the epidermis–dermis orientation, using a confocal laser scanning microscope (FV-1000; Olympus, Tokyo, Japan, or A1R; Nikon, Tokyo, Japan) or a super-resolution microscope (SpinSR10; Olympus). Using the tailing function associated with the microscope, cross-sectional images (X:Y = 450:450 µm) were continuously obtained along the epidermis–dermis orientation at 0.25–0.8 µm intervals up to a depth of 80–150 µm. ImageJ (NIH; Bethesda, MD) was used for co-localization analysis of the elastic fibers and basement membrane proteins. The overlapped regions of each basement membrane protein and tropoelastin were extracted using the “and operation” of the processing method in the Image J software, and their areas were calculated. Moreover, the images of COLVII were processed through binarization, and the volumes were measured using the 3D structural analysis software Simpleware (JSOL; Tokyo, Japan).
Statistical Analysis
A correlation analysis was performed using the Pearson product–moment correlation test. All statistical analyses were conducted using Bell Curve for Excel version 7.0 (Social Survey Research Information Co., Ltd.; Tokyo, Japan). Results were considered significant at p<0.05 (*p<0.05, **p<0.01, ***p<0.001).
Ethical Statement
There were no study participants included, as the skin samples were purchased. Accordingly, ethical approval and informed consent were not required.
Results
COLVII Extends to the Dermis and Elastic Fibers
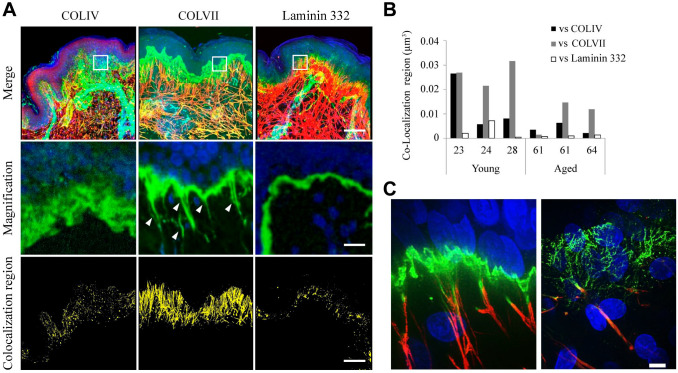
To investigate the association between basement membrane proteins (COLIV, COLVII, and laminin 332) and elastic fibers, we assessed the localization of each protein and elastic fiber in excised human skin tissue. The co-stained tropoelastin and basement membrane proteins show that COLIV, COLVII, and laminin 332 were localized to the basement membrane (Fig. 1A). Furthermore, COLVII, but neither laminin 332 nor COLIV, extended to the dermis from the basement membrane in the fibrous form. This extended region of COLVII was co-localized with elastic fiber, indicating that COLVII is co-localized with elastic fibers over a wider range than that of laminin 332 and COLIV. Quantitative co-localization analysis verified that COLVII most often co-localizes with fibrous tropoelastin (Fig. 1B). Super-resolution imaging at 120-nm resolution with SpinSR10 (Olympus) showed that COLVII and elastic fibers were bound to each other in young skin tissue samples. However, it was observed that the COLVII structure was denatured in aged skin samples, suggesting that the binding property may be dampened (Fig. 1C).
Figure 1.
Collagen (COL) VII extends from the basement membrane to the dermis and binds to elastic fibers. Immunofluorescent three-dimensional images of elastic fibers and basement membrane proteins: (A) merged images (top), magnified images of each white square frame in the merged images (center), and extracted co-localized regions of elastic fibers and each basement membrane protein (below). COLIV, COLVII, and laminin 332 are localized in the basement membrane, and only COLVII extends from the basement membrane toward the dermis (white arrow). The structure of COLVII extending to the dermis is widely co-localized with elastic fibers; however, COLIV and laminin 332 showed almost no co-localization with elastic fibers. Top: blue: nuclear; green: COLIV, COLVII, or laminin 332; red: tropoelastin. Scale bar = 80 µm. Center: blue: nuclear; green: COLIV, COLVII, or laminin 332. Scale bar = 10 µm. Bottom: yellow: co-localization region. Scale bar = 80 µm. (B) The graph represents a quantitative comparison of co-localized regions of elastic fiber and basement membrane proteins in skin tissues excised from women in their 20s and 60s. The co-localization region of elastic fibers and COLVII is wider than that of COLIV and laminin 332 in both generations. The number on the horizontal axis indicates age. (C) The co-localization region of tropoelastin and COLVII is wider than that of COLIV and laminin 332 in both generations. Super-resolution image of COLVII and elastic fiber in young (left) and aged (right) skin tissue samples. Blue: nuclear; green: COLVII; red: tropoelastin. Scale bar = 5 µm.
Fibrillin-1-rich Regions at Oxytalan Fiber Tips Disappear in Aged Skin
The co-staining images of elastin and fibrillin-1 demonstrated that elastin and fibrillin-1 co-localized mostly to oxytalan fibers. In young skin, oxytalan fibers with candelabra-like structures were observed just below an undulating papillary layer, and a fibrillin-1-rich region was confirmed at the fiber tips. However, in aged skin, the papillary layer was flattened, the elastic fibers were short and curved, the fiber tip structure was unclear, and no fibrillin-1-rich region was observed (Fig. 2).
Figure 2.
Fibrillin-1 has a rich region at the tip of the oxytalan fiber and co-localizes with elastic fiber in young skin. Fluorescence immunostaining images of fibrillin-1 and elastic fibers in the skin tissue of subjects aged 23, 24, and 27 years (starting from the left of the upper row) and subjects aged 61, 61, and 64 years (starting from the left of the lower row). Blue: nucleus; green: tropoelastin; red: fibrillin-1. Scale bar = 30 µm.
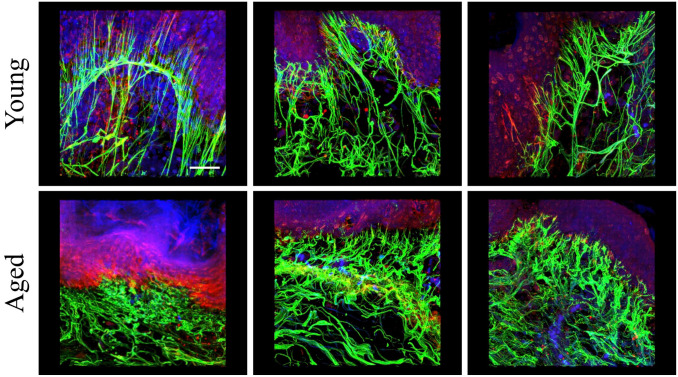
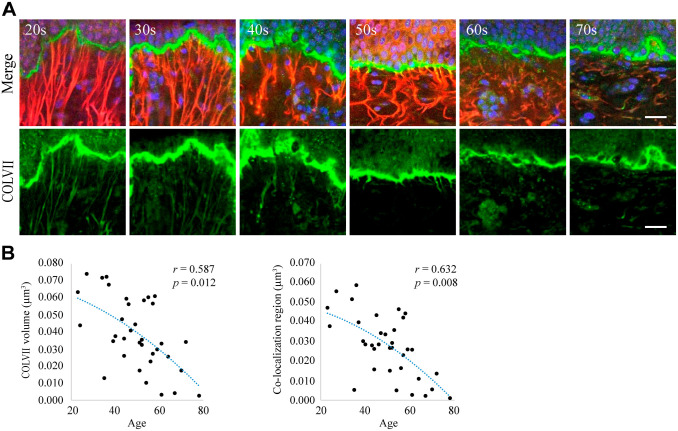
Long Hanging Structure of COLVII Shrinks With Age
To investigate the relationship between the hanging structure of COLVII in the basement membrane and aging, 3D observation of the skin tissue of 33 females in their 20s to 70s was performed using immunofluorescence staining for COLVII and elastin. The hanging and straight structures of COLVII were observed in samples from women of a younger age, but COLVII appeared atrophied and curved in samples from older individuals and disappeared in aged skin. Figure 3A shows typical images from each generation. These observations were verified using a numerical analysis of the images. The hanging region of COLVII decreased with age. The co-localization region between COLVII and elastic fibers also decreased with age (Fig. 3B).
Figure 3.
The hanging structure of COLVII and its co-localization with elastic fiber decrease with age. (A) Immunofluorescent three-dimensional images of COLVII and elastic fibers in the skin tissue from women in their 20s to 70s. Each picture depicts the merged images (above) and COLVII (below). The results indicate that the elongation of COLVII to the dermis decreases with age. Blue: nuclear; green: COLVII; red: tropoelastin. Scale bar = 10 µm. (B) The graphs represent the correlation analysis for age, elastin structure, co-localization region, and elastic fiber length. Each graph reveals the relationship between the extended structure of COLVII and age (left) and the co-localization region and age (right). Both elongation of COLVII to the dermis and co-localization with fibrous tropoelastin were found to decrease with aging.
Discussion
It is pertinent for elastic fibers to preserve their interconnection with the surrounding structures, especially the epidermis, to prevent age-related fragility of skin structures. However, the molecular mechanism that connects the tip of the oxytalan fiber to the basement membrane has not yet been deciphered. In a previous study, we observed that there was a gap of approximately 100 µm between the tip of the oxytalan fiber and the epidermal basement membrane. In this study, we detected the presence of proteins that bind to this gap.
First, we examined elastic fibers with the three major proteins configuring the basement membrane: COLIV, laminin 332, and COLVII. We found that COLVII was elongated from the basement membrane to the dermis and co-localized with elastic fibers in human skin tissue but not COLIV or laminin 332. COLVII exists on the dermal surface among all basement membrane proteins and is further known to bind to COLI, which is important for epidermal–dermal interactions. 16 The length of the anchoring fibrils has been reported to be approximately 200–750 nm, as determined using an electron microscope. 19 However, in this study, we determined that COLVII hangs down to the dermis and that the length of the hanging structure is a few micrometers to 100 µm in the direction of the dermis, which is substantially long. In addition, super-resolution microscopy images showed that the tips of oxytalan fibers and COLVII are co-localized, indicating that the hanging structure of COLVII and elastic fiber exists in the adjacent space within about 200 nm. The association between COLVII and elastic fiber remains unclear. We found that this hanging structure of COLVII potentially has a relationship with elastic fiber, which has an important role in supporting the skin structure, especially in maintaining the candelabra structures and papillary layer. To clarify the structural relationship between COLVII and elastic fibers, additional studies are needed. Although elastic fibers were stained with elastin antibody in this study, it is also necessary to investigate the relationship between COLVII and other elastic fiber component proteins, such as fibrillin-1. Elastin and fibrillin-1 co-localize in the majority of oxytalan fibers, but fibrillin-1 is rich in the tips of oxytalan fibrils. 20 Our observations also confirmed similar fibrillin-1-rich regions in young skin. This suggests that fibrillin-1 would co-localize with COLVII as with elastin, and more extensively at the tip. However, in aged skin, all fibers were atrophied and sloughed, and no fibrillin-1-rich regions were observed at the tips. Further research is needed to determine whether the loss of this fibrillin-1-rich region contributes to the flattening of the papillary layer and the degeneration of entire elastic fibers.
Next, to investigate whether this structure alters with age, the structures of COLVII and elastic fibers in the skin tissue of 33 females were observed. As a result, it was confirmed that the hanging structure of COLVII shortened with aging and even disappeared in some cases. Furthermore, oxytalan fibers shorten with aging; this also accords with previous reports of degeneration and disappearance of fibers with aging.4,13 In addition, a quantitative analysis of 33 young and aged skin samples confirmed that both COLVII and the co-localization region with elastin decreased with age. In this study, we examined abdominal skin tissues, which are non-exposed areas. Accordingly, it can be inferred that the observed age-related alterations are most probably ascribed to natural aging rather than photo-aging. The relationship with elastic fibers subsides with the disappearance of the hanging structure of COLVII. Accordingly, we speculated that the relationship between the epidermis and the dermis is weakened, reducing the tension of the elastic fibers and consequently reducing the elasticity of the dermis. Therefore, the relationship between COLVII and elastic fibers is a potential target for the improvement of age-related alterations in the skin. Moreover, COLVII upregulation may contribute to the enhancement of the relationships between COLVII and elastic fibers.
Although the binding between the basement membrane and the dermis is crucial in maintaining the skin structure, the underlying mechanisms remain obscure. Further research should be undertaken to investigate the mechanism by which COLVII extends into the dermis and oxytalan fibers and the connection between COLVII and oxytalan fiber–constituting proteins. This will provide us with pertinent knowledge on the maintenance of skin structure, particularly involving the relationship between COLVII and fibrillin-1. Elucidating the significance of these relationships can lead to the development of treatments that prevent structural collapse of the skin.
In summary, this study has shown that COLVII forms a long hanging structure and co-localizes with oxytalan fibers. However, this structure disappears with age. Conversely, this structure and co-localization were not observed in COLIV and laminin, or even in the proteins constituting the basement membrane. Taken together, these results suggest that COLVII plays an important role in the connectivity of the dermis and epidermis and the structural mechanism that maintains the skin structure, especially the papillary layer, and is involved in age-related changes in skin structure.
Acknowledgments
We thank Olympus Corporation for permitting the use of super-resolution microscopes. We would like to thank Editage (www.editage.com) for English language editing and journal submission support. The authors have authorized the submission of this manuscript through Editage.
Footnotes
Author Contributions: TT conceived, coordinated, and managed the execution of the study; collected human skin tissue samples; carried out imaging experiments of the skin samples; and drafted the manuscript. SN carried out imaging experiments of the skin samples. SK collected human skin tissue samples and was involved in the design and coordination of the study, planning the methods, and drafting the manuscript. SI supported the research methods, planning the study, and drafting of manuscripts and managed the research team. TS raised the funds needed for the research and managed the organization. All authors have read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by FANCL Corporation (Yokohama, Japan).
Data Availability Statement: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
ORCID iDs: Takeshi Tohgasaki  https://orcid.org/0000-0001-8357-1973
https://orcid.org/0000-0001-8357-1973
Shino Nishizawa  https://orcid.org/0000-0002-2260-7706
https://orcid.org/0000-0002-2260-7706
Shinya Kondo  https://orcid.org/0000-0002-4616-1374
https://orcid.org/0000-0002-4616-1374
Shioji Ishiwatari  https://orcid.org/0000-0002-8710-4608
https://orcid.org/0000-0002-8710-4608
Tetsuhito Sakurai  https://orcid.org/0000-0003-3106-7534
https://orcid.org/0000-0003-3106-7534
Contributor Information
Takeshi Tohgasaki, FANCL Research Institute, FANCL Corporation, Yokohama, Japan.
Shino Nishizawa, FANCL Research Institute, FANCL Corporation, Yokohama, Japan.
Shinya Kondo, FANCL Research Institute, FANCL Corporation, Yokohama, Japan.
Shioji Ishiwatari, FANCL Research Institute, FANCL Corporation, Yokohama, Japan.
Tetsuhito Sakurai, FANCL Research Institute, FANCL Corporation, Yokohama, Japan.
Literature Cited
- 1. Doubal S, Klemera P. Visco-elastic response of human skin and aging. J Am Aging Assoc. 2002;25:115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249–56. [DOI] [PubMed] [Google Scholar]
- 3. Weihermann AC, Lorencini M, Brohem CA, de Carvalho CM. Elastin structure and its involvement in skin photoageing. Int J Cosmet Sci. 2017;39:241–7. [DOI] [PubMed] [Google Scholar]
- 4. Lee JY, Kim YK, Seo JY, Choi CW, Hwang JS, Lee BG, Chang IS, Chung JH. Loss of elastic fibers causes skin wrinkles in sun-damaged human skin. J Dermatol Sci. 2008;50:99–107. [DOI] [PubMed] [Google Scholar]
- 5. Böck P, Stockinger L. Light and electron microscopic identification of elastic, elaunin and oxytalan fibers in human tracheal and bronchial mucosa. Anat Embryol. 1984;170:145–53. [DOI] [PubMed] [Google Scholar]
- 6. Cotta-Pereira G, Guerra Rodrigo F, Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. J Invest Dermatol. 1976;66:143–8. [DOI] [PubMed] [Google Scholar]
- 7. Braverman IM, Fonferko E. Studies in cutaneous aging: I. The elastic fiber network. J Invest Dermatol. 1982;78:434–43. [DOI] [PubMed] [Google Scholar]
- 8. Sherratt MJ. Tissue elasticity and the ageing elastic fiber. Age. 2009;31:305–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. [DOI] [PubMed] [Google Scholar]
- 10. Compton CC, Gill JM, Bradford DA, Regauer S, Gallico GG, O’Connor NE. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989;60:600–12. [PubMed] [Google Scholar]
- 11. Compton CC. Current concepts in pediatric burn care: the biology of cultured epithelial autografts: an eight-year study in pediatric burn patients. Eur J Pediatr Surg. 1992;2:216–22. [DOI] [PubMed] [Google Scholar]
- 12. Raghunath M, Bächi T, Meuli M, Altermatt S, Gobet R, Bruckner-Tuderman L, Steinmann B. Fibrillin and elastin expression in skin regenerating from cultured keratinocyte autografts: morphogenesis of microfibrils begins at the dermo-epidermal junction and precedes elastic fiber formation. J Invest Dermatol. 1996;106:1090–5. [DOI] [PubMed] [Google Scholar]
- 13. Tohgasaki T, Kondo S, Nishizawa S, Ishiwatari S, Sakurai T, Ishikawa S, Takeda A. Evaluation of elastin fibers in young and aged eyelids and abdominal skin using computational 3D structural analysis. Skin Health Dis. 2021;1:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briggaman RA, Wheeler CE. The epidermal-dermal junction. J Invest Dermatol. 1975;65:71–84. [DOI] [PubMed] [Google Scholar]
- 15. Aumailley M. Laminins and interaction partners in the architecture of the basement membrane at the dermal-epidermal junction. Exp Dermatol. 2021;30:17–24. [DOI] [PubMed] [Google Scholar]
- 16. Roig-Rosello E, Rousselle P. The human epidermal basement membrane: a shaped and cell instructive platform that aging slowly alters. Biomolecules. 2020;10:1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, McGrath JA, Mellerio JE, Murrell DF, Shimizu H, Uitto J, Vahlquist A, Woodley D, Zambruno G. The classification of inherited epidermolysis bullosa (EB): report of the third international consensus meeting on diagnosis and classification of EB. J Am Acad Dermatol. 2008;58:931–50. [DOI] [PubMed] [Google Scholar]
- 18. Fine JD, Bruckner-Tuderman L, Eady RA, Bauer EA, Bauer JW, Has C, Heagerty A, Hintner H, Hovnanian A, Jonkman MF, Leigh I, Marinkovich MP, Martinez AE, McGrath JA, Mellerio JE, Moss C, Murrell DF, Shimizu H, Uitto J, Woodley D, Zambruno G. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103–26. [DOI] [PubMed] [Google Scholar]
- 19. Ghadially FN. Extracellular matrix (extracellular components). In: Ghadially FN, editor. Ultrastructural pathology of the cell and matrix. 3rd ed. Oxford: Butterworth-Heinemann; 1988. p. 1215–303. [Google Scholar]
- 20. Langton AK, Graham HK, Griffiths CEM, Watson REB. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp Dermatol. 2019;28:981–84. [DOI] [PMC free article] [PubMed] [Google Scholar]