Abstract
Cognitive impairment (CI) is a major health concern in aging populations. It impairs patients’ independent life and may progress to dementia. Vascular cognitive impairment (VCI) encompasses all cerebrovascular pathologies that contribute to cognitive impairment (CI). Moreover, the majority of CI subtypes involve various aspects of vascular dysfunction. Recent research highlights the critical role of reduced cerebral blood flow (CBF) in the progress of VCI, and the detection of altered CBF may help to detect or even predict the onset of VCI. Arterial spin labeling (ASL) is a non-invasive, non-ionizing perfusion MRI technique for assessing CBF qualitatively and quantitatively. Recent methodological advances enabling improved signal-to-noise ratio (SNR) and data acquisition have led to an increase in the use of ASL to assess CBF in VCI patients. Combined with other imaging modalities and biomarkers, ASL has great potential for identifying early VCI and guiding prediction and prevention strategies. This review focuses on recent advances in ASL-based perfusion MRI for identifying patients at high risk of VCI.
Keywords: Vascular cognitive impairment, vascular dementia, perfusion MRI, arterial spin labeling, neuroimaging, cerebral blood flow, neurovascular unit
Introduction
Cognitive impairment (CI) is a clinical syndrome defined as cognitive decline that is more pronounced than expected for the patient’s age or education level. In adults older than 65 years, the prevalence of mild cognitive impairment (MCI) ranges from 3% to 19%, 1 and increases to 22.2% in patients at the age of 71 years or higher. The prevalence of dementia in the elderly (>65 years) is 6.4%, while that of Alzheimer’s disease (AD) and vascular dementia (VaD) is 4.4% and 1.6% respectively.2,3 Individuals suffering from MCI have a significantly increased risk to develop dementia. 4 CI is becoming a major concern in aging populations due to its heavy medical and socioeconomic burden for patients, families, and the society. Indeed, the CI-associated burden on the medical care system has surpassed that of cancer and heart diseases in the United States. 5
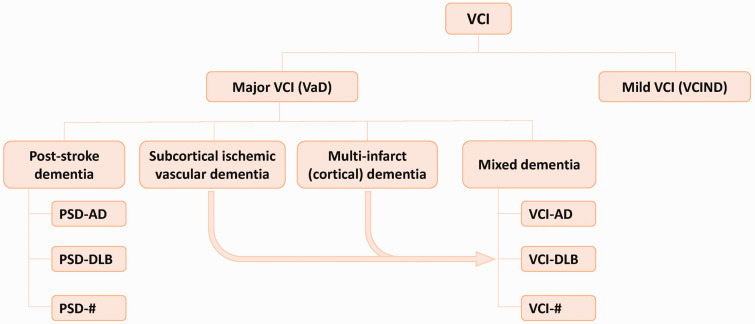
The term vascular cognitive impairment (VCI) was proposed to describe the contribution of cerebrovascular pathologies to any severity of CI.6,7 According to the Vascular Impairment of Cognition Classification Consensus Study (VICCCS), VCI contains mild VCI (VCI-no dementia, VCIND) and major VCI (i.e. vascular dementia, VaD) (Figure 1). The latter is further classified into 4 subtypes: post-stroke dementia (PSD), subcortical ischemic VaD (SIVD), multi-infarct (cortical) dementia, and mixed dementia with additional neurodegenerative pathologies (e.g. VCI-AD, VCI-DLB).4,8
Figure 1.
Classification of VCI according to the Vascular Impairment of Cognition Classification Consensus Study (VICCCS) guideline. VCI is divided into mild VCI (VCI-no dementia, VCIND) and major VCI (vascular dementia, VaD). VaD can be further classified into 4 subtypes: post-stroke dementia (PSD), subcortical ischemic VaD, multi-infarct (cortical) dementia, and mixed dementia with nonvascular pathologies.
In addition to hypertension, diabetes, hypercholesterinemia and other factors compromising cerebrovascular function, aging is recognized as a major risk factor of cerebrovascular pathology. 9 Indeed, the risk of developing VaD doubles with every 5.3 years after the age of 65. Early VCI identification and prediction will be crucial in preventing or delaying full VaD onset since early treatment of cerebral vascular dysfunction is directly associated with lower incidence of VaD. 10
Recent studies suggest that reduction in cerebral blood flow (CBF) occurs prior to the clinical onset of VCI.11–13 Consequently, CBF measurement may aid in distinguishing cognitively normal adults from those at risk for or exhibiting VCI. 14 In addition, CBF reduction is a sensitive predictor of cognitive decline and its progression with age.15,16 This suggests that early detection of CBF changes may be an appropriate method for identifying individuals at risk for VCI. Moreover, the degree of cognitive deficit in patients with subcortical VCI is correlated with reduced regional CBF (rCBF). 17
Hypoperfusion is also prevalent in AD patients and can precede the onset of clinical AD by several years. 18 The classical amyloid cascade hypothesis attributes the decrease in the CBF to neuronal hypometabolism. However, according to the vascular hypothesis, AD pathology begins with perfusion changes, resulting in dysfunction of neurons and surrounding cells, 19 and alterations of both large and small cerebral vessels, prominently seen in, but not limited to the penetrating vasculature of white matter (WM), are considered key drivers in AD. 20 Thus, CBF measurements may also be used to identify individuals in the presymptomatic stages of AD. Moreover, the most predominant type of cognitive impairment is mixed VCI-AD. Since the role of cerebral hypoperfusion in AD is not yet entirely clear and subject to ongoing research, the primary focus of this study will be VCI.
This review discusses recent applications and advances in the use of arterial spin labeling (ASL) in VCI patients and individuals at high risk for developing VCI.
The emerging association between VCI and CBF changes
Due to the strong correlation between CBF and neuronal function and metabolism, CBF is recognized as a clinically relevant marker of brain function. 21 Intact CBF regulation as well as normal cerebral metabolism are essential for the maintenance of cognitive function. 22 In addition, impaired CBF is associated with an increased risk of developing all types of dementia. 23 In VCI, changes in CBF can occur before clinical symptoms.10,11,13 Evidence shows that in patients with subcortical VCI, pathological alterations including CBF reduction and distribution change are closely related to the degree of CI. 17 Thus, a thorough understanding of CBF in the pathophysiologic cascade of VCI is crucial to preserve the possibility of timely intervention.
Cerebral microinfarcts and other ischemic brain tissue injury, especially in the WM, are major pathological hallmarks of VCI.24,25 Chronic cerebral metabolic dysfunction as well as deterioration of pre-existing systemic vascular risk factors such as hypertension, hyperlipidemia, diabetes mellitus, or atrial fibrillation, are important contributors to developing CI.21,26,27 Resulting structural changes in cerebral blood vessels such as hypertensive remodeling or atherosclerosis in high-risk patient groups often lead to vascular occlusion, abnormal cerebral perfusion, and impaired autoregulation, culminating into CBF reduction and CI.
Dysfunction of the neurovascular unit leads to impaired CBF regulation
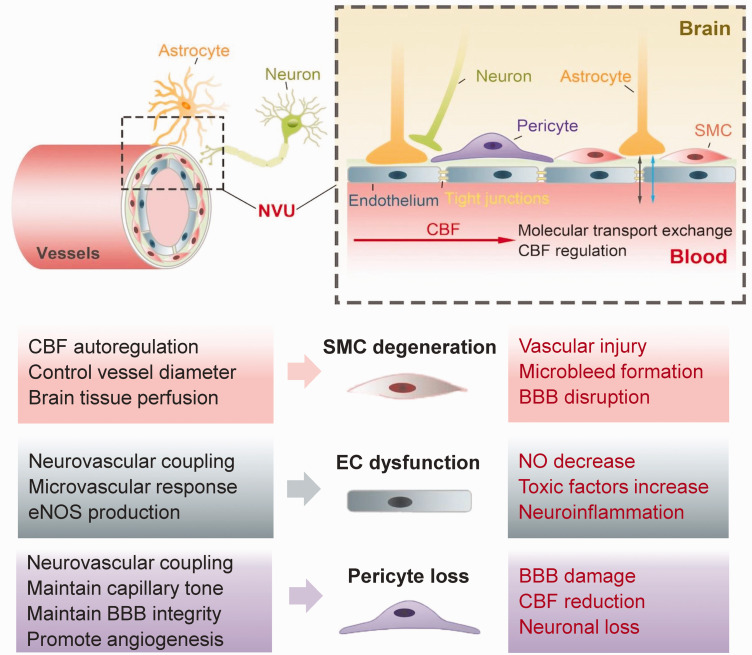
Proper CBF regulation assures both constant and stable brain tissue perfusion which is crucial to meet the brain’s metabolic demands and maintain normal neuronal activity.28,29 The neurovascular unit (NVU), which is comprised of neurons, astrocytes, vascular smooth muscle cells (SMCs), endothelial cells (ECs) and pericytes, plays a crucial role in coupling vascular perfusion and thus regional CBF to neuronal activity, attracting increasing attention in VCI pathophysiology.30,31 Disruption of any component of the NVU in cerebrovascular pathologies has significant impact on CBF modulation and neuronal function (Figure 2). 32 The precise neurovascular coupling is therefore of vital importance, even mild impairments can affect brain function and cause cognitive decline. 33
Figure 2.
Schematic diagram and detailed functions of the cellular components of the neurovascular unit (NVU). The NVU is the functional unit helping CBF regulation. Detailed functions (in black) of normal pericytes, vascular smooth muscle cells (SMCs) and endothelial cells, three components of the NVU, are depicted. Dysfunction of any cellular component of the NVU (in red) can contribute to vascular cognitive impairment (VCI). SMCs can directly control the vessel diameter and have autoregulatory effects on brain tissue perfusion. SMCs degeneration disturbs steady cerebral perfusion and is associated with vascular injury, microbleed development and blood-brain barrier (BBB) disruption. Endothelial cells mediate neurovascular coupling, microvascular responses, endothelial nitric oxide synthase (eNOS) production. Dysfunction of endothelium causes increased toxic factors and reduced production of NO, which can promote the development of VCI. Pericytes are crucial in CBF controlling, BBB permeability maintaining, and angiogenesis promotion. The loss of pericytes in VCI leads to BBB damage, CBF reduction, and neuronal loss.
SMCs in the NVU can directly control vessel diameter and thus regional CBF.32,34 Proper myogenic responses of SMCs are important for CBF regulation and steady capillary perfusion, protecting the brain against potentially negative effects of any rapid blood pressure change. 35 In addition, myogenic responses of SMCs are coupled to neuronal activity as nearby neurons and astrocytes release prostaglandins, nitric oxide, K+ and Ca2+ to SMCs,28,36 which precisely regulates brain tissue blood perfusion through SMC contraction or dilation. 37 In chronic hypertension and aging, SMCs undergo degeneration and the autoregulatory responses is often impaired, causing vascular injury, microbleeds and increased blood-brain barrier (BBB) permeability. 33
ECs are another critical NVU component responsible for mediating dynamic microvascular responses and neurovascular coupling. ECs respond to changing rCBF demands through multiple factors and mechanisms including endothelial nitric oxide synthase (eNOS), neurotransmitters, and metabolic reactions.38,39 In VCI, cerebral ECs undergo pathological changes and produce pro-inflammatory mediators and toxic factors, magnifying neuroinflammation and BBB disruption. 40 Moreover, pathologically altered ECs promote NVU uncoupling through dysregulated VEGF/angiogenesis and ROS/NO axes. Decreased NO bioavailability can further lead to ineffective CBF regulation and cerebral hypoperfusion, which ultimately causes neuronal death and CI.41,42
Pericytes enwrap brain microvasculature, maintain basal capillary tone, and contribute to neurovascular coupling. 43 They are also crucial for BBB integrity, angiogenesis and clearance of toxic cellular metabolites.44–46 Pericyte coverage significantly decreases during aging, and the loss of pericytes is associated with BBB dysfunction, CBF alteration, neuronal loss, WM damage, and cognitive decline.47–49 Brain ischemia can induce capillary constriction by pericytes followed by regional pericyte death due to the loss of energy supply and excitotoxicity, which may irreversibly decrease capillary blood flow and damage the BBB even after reperfusion.50,51 VCI risk factors such as hypertension can also lead to impaired pericyte function. 52
Dysfunction of the abovementioned NVU components all contribute to impaired CBF regulation and BBB disruption in cerebrovascular pathologies. CBF alteration is further related to WM injury, lacunes, cerebral microbleeds, brain atrophy and cognitive deficits in several VCI subtypes both in animals and humans.14,17,24,53–57 A comprehensive understanding of the impacts of CBF patterns on disease onset and progression, the relationship between global CBF and rCBF changes, and standardized criteria for CBF determination is of great importance to validate CBF as a biomarker in assessing VCI.
Application of ASL for detecting CBF alterations in VCI and related conditions
ASL for the measurement of cerebral blood flow
Neuroimaging is essential for precise VCI assessment, including T2-weighted MRI for the detection of lacunar infarcts, susceptibility weighted imaging (SWI) for microbleeds, and fluid-attenuated inversion recovery (FLAIR) sequences for white matter hyperintensities (WMH). Recently, it is recommended that arterial spin labelling (ASL) can quantitatively measure subtle perfusion changes which are untraceable with structural MRI, and add specificity to VCI diagnosis. 6 The technology of ASL was first proposed by Williams et al. in 1992. 58 It has been validated for qualitative and quantitative CBF analysis in different brain disorders, such as CI, AD, acute stroke, and migraine.59–64 ASL labels blood water to act as an endogenous tracer for CBF mapping by changing the magnetization of water proton spins in the arterial blood at the neck region.65,66 Two brain images are acquired; the first (control) image is subtracted from the second (labeled) image in order to remove the static brain tissue signal and to obtain the trajectory of blood flow. 67 If necessary, a series of these image pairs can be acquired for the detection of dynamic changes over the course of the examination.11,68
Although dynamic susceptibility contrast-enhanced perfusion weighted-imaging (DSC-PWI) and [15O]-water positron emission tomography (PET) are well-established standards for CBF measurements, ASL has multiple advantages over these methods, including its non-invasiveness, avoidance of radiation or contrast agent use, high reproducibility, as well as more widespread availability, thus can be a valuable noninvasive alternative to assess brain perfusion.69–71 ASL has been assessed in several clinical studies and is sensitive for detecting CBF changes in VCI and AD patients.14,17,72,73
Moreover, multimodal imaging with the combination of ASL and other MRI sequences provides more comprehensive assessment for VCI pathologies. 74 Recently, combined ASL and blood oxygenation level-dependent (BOLD) functional MRI has been used to study the change of neurovascular coupling in VCI and chronic stroke as the relationship between regional CBF and neuronal activity can be analyzed.75,76 In addition, using ASL with fluorine 18 fluorodeoxyglucose (FDG) PET can reveal the coupling of perfusion and metabolism in different brain regions. 77 Therefore, we can further obtain the correlation between CBF at different locations and neuronal activity or metabolic state by combining ASL technique with other imaging modalities.
ASL in major forms of VCI
Subcortical ischemic VaD, post-stroke dementia, and mixed VCI-AD dementia are the 3 most commonly studied types of CI with vascular contribution, and ASL can be used to evaluate CBF changes in respective patients (Table 1). ASL showed reduced CBF in the frontal and parietal cortices and corresponding subcortical WM lesions in 8 patients with subcortical ischemic VaD compared to 18 elderly subjects with normal cognitive function. 53 Another study compared 53 subcortical VaD patients with significant CI to 23 matched subcortical ischemic vascular disease patients without CI, found diffusely decreased CBF in the temporal and frontal lobes, and in deeper structures such as the hippocampus, thalamus and insula in subcortical VaD patients. 17
Table 1.
ASL studies in VCI and high-risk individuals.
| Study | Patient cohort | N in each group | ASL methods | Main findings |
|---|---|---|---|---|
| Schuff et al. 53 | SIVD | 8 SIVD + 18 HC | pASL | Reduced CBF in the frontal cortex and WM lesions. |
| Sun et al. 17 | SVCI | 53 subcortical VaD + 23 subcortical ischemia without CI | pcASL | Diffusely decreased CBF in temporal and frontal lobes, also in hippocampus, thalamus and insula. |
| Liu et al. 75 | SVCI | 28 SVCI + 26 subcortical ischemia without CI + 24 HC | pcASL | Decreased global ReHo-CBF coupling and decreased ReHo-CBF ratio mainly in cognition-related regions. |
| Firbank et al. 82 | PSD | 8 PSD + 31 PSND + 29 HC | pASL | GM CBF/WM CBF ratio was reduced in PSD group and predicted dementia in PSND group. |
| Hays et al. 90 | SCD | 35 SCD + 35 HC | pcASL | Negative associations between verbal memory and rCBF in SCD patients. |
| Ferro et al. 24 | cSVD risk factors | 74 dementia+ 78 CIND + 29 NCI | pcASL | Cerebral cortical microinfarcts were associated with reduction in global CBF. |
| Promjunyakul et al. 74 | cSVD risk factors | 82 with cSVD risk factors | pASL | CBF penumbra was more extensive than structural penumbras of WMH. |
| Promjunyakul et al. 81 | NCI elderly | 61 NCI | pASL | CBF surrounding WMH could predict future WMH expansion. |
| Jann et al. 14 | cSVD risk factors | 45 with cSVD risk factors | pcASL | Relative CBF in MCA territories is positively correlated with executive functions and MoCA scores. |
| Bangen et al. 91 | Vascular risk burden | 16 high vascular risk + 55 low vascular risk | pASL | The correlation among increasing age, reduced cortical CBF, and CI was only significant in patients with high vascular risk burden. |
| Bangen et al. 92 | T2DM | 11 T2DM, 38 without diabetes | pcASL | Decreased rCBF in diabetes was associated with decline in several cognition domains. |
| Xekardaki et al. 94 | Cognitive decline | 73 cognitive decline + 75 HC | pASL | Reduced CBF in the posterior cingulate cortex could indicate early neuropsychological decline. |
SIVD: subcortical ischemic VaD; HC: health control (here refers to cognitively normal elderly subjects); SVCI: subcortical VCI; ReHo: regional homogeneity; PSD: post-stroke dementia; PSND: post-stroke no-dementia; SCD: subjective cognitive decline; cSVD: cerebral small vessel disease; CIND: cognitive impairment–no dementia; NCI: no cognitive impairment; WMH: white matter hypertensities; MoCA: Montreal Cognitive Assessment; T2DM: Type 2 diabetes mellitus.
Subcortical ischemic VaD also belongs to the umbrella of cerebral small vessel disease (cSVD). cSVD causes diffuse brain injury and is strongly associated with VCI. 78 Key neuroimaging findings in cSVD include small subcortical infarcts, WMH, lacunes, cerebral microbleeds, enlarged perivascular spaces. 79 Cortical microinfarcts have also been described. 80 Cortical microinfarcts and confluent WMH have been shown to be associated with significant reduction in global CBF.24,54 In addition, CBF surrounding WMH can predict future WMH expansion.74,81
In one study of post-stroke dementia, researchers selected 39 elderly patients six years after stroke, of which eight developed dementia. In these patients, the ratio of CBF in the gray matter (GM CBF) to CBF in the white matter (WM CBF) was reduced. Moreover, this ratio predicted the occurrence of dementia in post-stroke patients without dementia. 82 However, normalized CBF values were calculated by dividing them by the mean WM CBF in the respective study, which is considered as a simple calibration method with lower sensitivity and reproductibility. Thus, the analysis of CBF changes in post-stroke dementia warrants further investigation.
Mixed VCI-AD dementia is probably the most common form of CI.83,84 Patients with AD symptoms or prodromal AD exhibit global and regional hypoperfusion in the parietal and medial temporal lobes, as well as in the precuneus, posterior cingulate cortex, and hippocampus.85,86 Many patients clinically diagnosed with AD have considerable vascular pathology, and may be assumed to be indeed mixed-type VCI-AD patients. The CBF patterns in VCI-AD need to be much better elucidated in future studies for better understanding of their implications in mixed VCI-AD. 87
ASL in mild VCI and high-risk individuals
Early identification of mild VCI is critical for timely interventions aiming to avoid or delay progression to major VCI. Cerebral hypoperfusion measured by ASL is an early indicator of VCI in patients presenting with very mild symptoms. In patients with mild VCI (VCIND), the rCBF reduction in specific regions can be related to cognitive deficits. For example, a study comparing VCIND with different domain impairment found that the group of 16 non-amnestic VCIND patients with single domain impairment showed CBF reduction in the left temporal lobe, left lenticular nucleus, and bilateral periventricular WM. 88 Moreover, a study combined ASL and BOLD-fMRI to compare 26 subcortical ischemic vascular disease patients without CI and 28 patients with mild CI, used the regional homogeneity (ReHo)-CBF coupling and ReHo-CBF ratio to represent neurovascular coupling. In patients without CI, the ReHo/CBF ratio in the left precentral gyrus was positively correlated to Mini-mental State Examination (MMSE) scores. The mild CI group showed further decreased global ReHo-CBF coupling and decreased ReHo-CBF ratio mainly in the left insula, left precentral gyrus, right middle temporal gyrus, and right precuneus, indicating the role of impaired neurovascular coupling at the early stage of VCI and during disease progression. 75
Subjective cognitive decline (SCD) is distinct from objective cognitive decline (mild VCI and VaD), but is associated with increased risk of future cognitive decline compared to individuals without any symptoms. 89 Evidence from 35 SCD patients compared to elderly subjects with normal cognition suggested that SCD patients have negative associations between verbal memory and rCBF measured by ASL, which may reflect neurovascular dysfunction at an early stage of SCD. 90
ASL can predict cognitive decline in high-risk patients. In patients with vascular risk factors of cSVD such as hypertension, diabetes, or hypercholesterolemia, a cohort showed that relative CBF (vs. global mean CBF) in leptomeningeal middle cerebral artery (MCA) territories is positively correlated with executive functions and Montreal Cognitive Assessment (MoCA) scores. 14 Another study of 71 subjects also showed that in non-demented older adults with multiple vascular risk factors, advancing age was correlated with reduced cortical CBF, which was in turn associated with CI, whereas no such relationship was observed in patients with low vascular risk factor burden. 91 Specifically, decreased rCBF in type 2 diabetes is associated with decline in several cognitive domains, including memory, learning, attention, and execution.26,92 Hypertension can further exacerbate CBF decrease in patients with diabetes. 93 Even in healthy elderly individuals, ASL may help to predict CI as reduced CBF in the posterior cingulate cortex can indicate early neuropsychological decline as shown in a prospective study of 148 elderly individuals. 94 Thus, CBF measured by ASL is a potential functional biomarker of VCI, 95 which may have a high value in early detection of CBF alteration in high risk populations (Table 1).
Technical advances of ASL enable CBF imaging
Recent technical advances expanding the clinical use of ASL as a CBF measurement tool
The practical advantages of ASL lie in its avoidance of invasive needling, radioactive tracers, potentially nephrotoxic contrast agents, long preparations, and extensive scan times. 96 Multiple studies have demonstrated good feasibility, applicability and reproducibility of ASL in geriatric populations.71,97 The clinical application of ‘classical’ ASL imaging is still limited though. In contrast to DSC-PWI, which requires contrast agent application but provides hemodynamic parameters including CBF, cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP), ASL is relatively limited to CBF analysis. Due to the use of subtracted images in order to determine ASL signals, there is an unavoidable lower signal-to-noise ratio (SNR) compared to direct contrast agent measurements. 98 Moreover, the significant diversity of ASL parameters used by different investigators limits its application in multicenter research comparisons that need prior determination of consistent protocols.
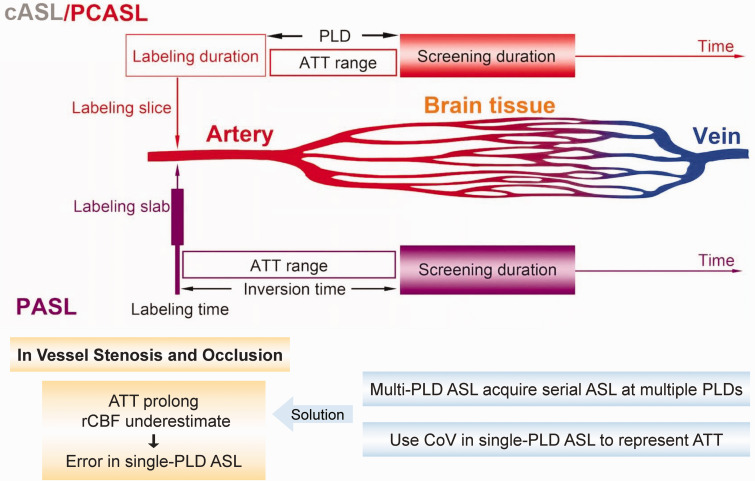
However, recent technical advances have expanded the clinical use of ASL as a CBF measurement tool. According to different labeling schemes, ASL is classified into pulsed ASL (pASL), continuous ASL (cASL), and pseudo-continuous ASL (pcASL) (Figure 3).99,100 pASL refers to a single short pulse about 10 ms to label inflowing arterial blood, the inverted blood flows from neck to brain and gradually loses labeling. cASL is a continuous pulse at a thin slice through the neck over a period of time. However, the need for continuous radio frequency apparatus and low labeling efficiency severely restricts its clinical application. 101 pcASL uses more than 1000 short pulses (1–2 seconds) with high frequency instead of a long continuous pulse, which can be considered as an “upgraded” pASL with higher SNR. 66 Both pcASL and pASL are commonly used in clinical settings with pcASL becoming the more preferred choice. Many studies have shown both high scan-rescan repeatability and excellent inter-site reproducibility of pcASL in cerebrovascular diseases.14,102–104
Figure 3.
Schematic diagram displaying the main differences between pulsed arterial spin labeling (pASL), continuous ASL (cASL), and pseudo-continuous ASL (pcASL), in labeling zone and duration. pASL refers to a single short pulse to label inflowing arterial blood, while cASL/pcASL involves a continuous pulse or over 1000 shaped pulses with high frequency at a thin slice, through the neck over a period of time. Arterial transit time (ATT) refers to the time between labeling and screening, which can lead to cerebral blood flow (CBF) underestimation in ASL due to relaxation of labeled spins. The post-labeling delay (PLD, or inversion time in pASL) is artificially preset to be longer than the longest ATT to delay the screening time and minimize the inaccuracy caused by ATT. However, single-PLD ASL can cause errors due to the mismatch between the single PLD and ATT, especially in vessel stenosis and occlusion, which is common in VCI patients. This challenge can be overcomed by multi-PLD ASL or the use of spatial coefficient of variation (CoV).
Minimized arterial transit time (ATT) for improved CBF imaging
One of the important technical issue of ASL is the consideration of inflow time or arterial transit time (ATT), which is the time delay between labeling in the neck region and the arrival of labeled blood in the brain. Prolonged ATT is thought to cause CBF underestimation in ASL, as relaxation of inverted or saturated spins (‘de-labeling’) during the blood passage from labeling location to screening location can occur.105,106 The ASL accuracy can be improved by minimizing ATT, which means inverting spins as close as possible to the screening areas. 107 The calculation of final CBF using ATT also prevents underestimation. However, ATT can vary significantly, based on differences in cerebral regions, patients’ age, blood flow velocity in diverse arteries, and a longer travel distance caused by the probable presence of collateral pathways.66,67
To avoid imprecise measurement due to heterogeneous ATT in clinical settings, the artificially preset post-labeling delay (PLD) (or inversion time in pASL) can be refined by delaying the screening time to roughly imitate ATT in the brain.92,108 PLD is optimized to be longer than the longest ATT to ensure that the labeled blood has reached the tissue at the time of screening (Figure 3). 109 However, single PLD-ASL which applies a single PLD time that is set between 1.5 to 2 s for CBF estimation, may cause errors due to the mismatch between the single PLD and ATT.110,111 Especially in the case of proximal vessel occlusion, the delayed inflow and perfusion can be falsely recognized by single-PLD ASL as reduced CBF in the corresponding vascular territories, producing arterial transit artifacts. 77 This disadvantage can be overcomed by multi-PLD ASL, a recent ASL technology acquiring serial ASL images at multiple PLDs which improves the accuracy of CBF measurement and provide more hemodynamic parameters including ATT, but requires relatively long scanning time.112,113 Another recent solution is the use of spatial coefficient of variation (CoV) of CBF images from single-PLD ASL as an alternative for ATT measurement, which can detect subtle CBF change without long-time scanning.59,114,115 As the impact of vessel stenosis and occlusion in poorly perfused areas on ASL accuracy is common in VCI, these strategies help to overcome the challenge and improve the application value of advanced ASL in VCI assessment.
Other strategies to improve the efficiency and accuracy of CBF measurement
New ASL strategies are under development to improve the efficiency of CBF measurements. For example, time-encoded pcASL, measuring dynamic perfusion, and methods that show combined 4 D-angiography with perfusion information are gradually being applied for CBF measurements. 116 Optimized acquisition and analysis frameworks as well as capable MRI scanners will enhance the clinical use of ASL to quantitatively measure brain perfusion.108,117
ASL implementation varies, among others, in hardware considerations, pulsing approaches, time delay setting, readout approaches, and postprocessing methods. In 2014, the International Society for Magnetic Resonance in Medicine (ISMRM) and the European consortium ASL in Dementia (AID) reached a consensus concerning an optimal clinical implementation for CBF measurements. 109 The consensus recommends pcASL, background suppression, segmented 3 D readouts, calculation and presentation of both label/control difference images, and CBF reported in absolute units. 118 To obtain ATT data and avoid abnormally long ATT, multiple-PLD ASL is suggested, while single-PLD ASL is recommended for rapid CBF measurements. 109
Concluding remarks and perspectives
The early recognition and identification of VCI are attracting increasing attention. Cerebral hypoperfusion might play a crucial role in developing and accelerating VCI. A considerable body of evidence supports that decreases in CBF detected by ASL are an early indicator of VCI, and play a major role in both mixed type VCI-AD and AD. This may further widen the application of ASL as an imaging tool for the prediction of cognitive decline. However, there is still a great diversity in ASL parameters applied by different research groups, and the harmonization of imaging modalities is crucial for increasing reproducibility of imaging findings. Future studies exploring the possibility of screening VCI in different patient populations are highly warranted.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P.L. is supported by the National Natural Science Foundation of China (NSFC, 91957111, 81971096, 81722017, 82061130224), New Frontier Technology Joint Research sponsored by Shanghai Shenkang Hospital Development Center (SHDC12019102), and Shanghai Municipal Education Commission-Gaofeng Clinical Medical Grant Support (20181805), “Shuguang Program” (20SG17) supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission and the “Outstanding Academic Leaders Plan” (20XD1422400) supported by Shanghai Municipal Science and Technology Committee of Shanghai. PL and JB are supported by a Newton Advanced Fellowship grant provided by the UK Academy of Medical Sciences (NAF\R11\1010). PL is supported by Innovative research team of high-level local universities in Shanghai (SHSMU-ZLCX20211602).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: DH, XG, LP and PL drafted the manuscript. ZZ drafted the figures. DH and YG prepared the references. YG, ZC, FY, RMD, MD, JB and PL revised the manuscript. All authors agreed on the final draft.
ORCID iDs: Rick M Dijkhuizen https://orcid.org/0000-0002-4623-4078
Marco Duering https://orcid.org/0000-0003-2302-3136
Johannes Boltze https://orcid.org/0000-0003-3956-4164
Peiying Li https://orcid.org/0000-0002-5721-9914
References
- 1.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet (London, England) 2006; 367: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 2005; 76 Suppl 5: v2–v7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Duering M, Hachinski V, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol 2019; 73: 3326–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med 2013; 368: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Primers 2018; 4: 18003. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skrobot OA, Black SE, Chen C, et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement 2018; 14: 280–292. [DOI] [PubMed] [Google Scholar]
- 9.Yang T, Sun Y, Lu Z, et al. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev 2017; 34: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017; 120: 573–591. [DOI] [PubMed] [Google Scholar]
- 11.Malojcic B, Giannakopoulos P, Sorond FA, et al. Ultrasound and dynamic functional imaging in vascular cognitive impairment and Alzheimer's disease. BMC Med 2017; 15: 27–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee G, Wilson D, Jäger HR, et al. Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochim Biophys Acta 2016; 1862: 926–938. [DOI] [PubMed] [Google Scholar]
- 13.Ye Q, Bai F. Contribution of diffusion, perfusion and functional MRI to the disconnection hypothesis in subcortical vascular cognitive impairment. Stroke Vasc Neurol 2018; 3: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jann K, Shao X, Ma SJ, et al. Evaluation of cerebral blood flow measured by 3D PCASL as biomarker of vascular cognitive impairment and dementia (VCID) in a cohort of elderly Latinx subjects at risk of small vessel disease. Front Neurosci 2021; 15: 627627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beason-Held LL, Goh JO, An Y, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 2013; 33: 18008–18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez DL, Thomas KR, Edmonds EC, et al. Regional hypoperfusion predicts decline in everyday functioning at three-year follow-up in older adults without dementia. J Alzheimers Dis 2020; 77: 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Cao W, Ding W, et al. Cerebral blood flow alterations as assessed by 3D ASL in cognitive impairment in patients with subcortical vascular cognitive impairment: a marker for disease severity. Front Aging Neurosci 2016; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays CC, Zlatar ZZ, Wierenga CE. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer's disease. Cell Mol Neurobiol 2016; 36: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solis E, Jr, Hascup KN, Hascup ER. Alzheimer's disease: the link between amyloid-β and neurovascular dysfunction. J Alzheimers Dis 2020; 76: 1179–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney MD, Kisler K, Montagne A, et al. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci 2018; 21: 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci 2017; 67: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parthasarathy AB, Gannon KP, Baker WB, et al. Dynamic autoregulation of cerebral blood flow measured non-invasively with fast diffuse correlation spectroscopy. J Cereb Blood Flow Metab 2018; 38: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation 2017; 136: 719–728. [DOI] [PubMed] [Google Scholar]
- 24.Ferro DA, Mutsaerts HJ, Hilal S, et al. Cortical microinfarcts in memory clinic patients are associated with reduced cerebral perfusion. J Cereb Blood Flow Metab 2020; 40: 1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jokinen H, Koikkalainen J, Laakso HM, et al. Global burden of small vessel disease-related brain changes on MRI predicts cognitive and functional decline. Stroke 2020; 51: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Sun L, He G, et al. Cerebral perfusion alterations in type 2 diabetes mellitus – a systematic review. Front Neuroendocrinol 2021; 62: 100916. [DOI] [PubMed] [Google Scholar]
- 27.Dai W, Lopez OL, Carmichael OT, et al. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 2008; 39: 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab 2018; 38: 2192–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadlbauer A, Kinfe TM, Zimmermann M, et al. Association between tissue hypoxia, perfusion restrictions, and microvascular architecture alterations with lesion-induced impairment of neurovascular coupling. J Cereb Blood Flow Metab 2022: 42: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Wang Y, Yan XL, et al. Pathological changes in neurovascular units: lessons from cases of vascular dementia. CNS Neurosci Ther 2021; 27: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lok J, Gupta P, Guo S, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res 2007; 32: 2032–2045. [DOI] [PubMed] [Google Scholar]
- 32.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toth P, Tarantini S, Csiszar A, et al. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017; 312: H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shvedova M, Litvak MM, Roberts JD, Jr, et al. cGMP-dependent protein kinase I in vascular smooth muscle cells improves ischemic stroke outcome in mice. J Cereb Blood Flow Metab 2019; 39: 2379–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 2006; 9: 1397–1403. [DOI] [PubMed] [Google Scholar]
- 37.Toth P, Tucsek Z, Tarantini S, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 2014; 34: 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Song W, Li L, et al. Endothelial nitric oxide synthase: a potential therapeutic target for cerebrovascular diseases. Mol Brain 2016; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards DN, Salmeron K, Lukins DE, et al. Integrin α5β1 inhibition by ATN-161 reduces neuroinflammation and is neuroprotective in ischemic stroke. J Cereb Blood Flow Metab 2020; 40: 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousef H, Czupalla CJ, Lee D, et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat Med 2019; 25: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graves SI, Baker DJ. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin Pharmacol Toxicol 2020; 127: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hariharan A, Jing Y, Collie ND, et al. Altered neurovascular coupling and brain arginine metabolism in endothelial nitric oxide synthase deficient mice. Nitric Oxide 2019; 87: 60–72. [DOI] [PubMed] [Google Scholar]
- 43.Hartmann DA, Berthiaume AA, Grant RI, et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci 2021; 24: 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Z, Chopp M, Chen J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab 2020; 40: 1381–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erdő F, Denes L, de Lange E. Age-associated physiological and pathological changes at the blood-brain barrier: a review. J Cereb Blood Flow Metab 2017; 37: 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse Central nervous system. Nat Med 2018; 24: 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Tuo QZ, Zou JJ, Lei P. Rodent models of vascular cognitive impairment. J Mol Neurosci 2021; 71: 1–12. [DOI] [PubMed] [Google Scholar]
- 50.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng J, Korte N, Nortley R, et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol 2018; 136: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toth P, Tucsek Z, Sosnowska D, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 2013; 33: 1732–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuff N, Matsumoto S, Kmiecik J, et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009; 5: 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bastos-Leite AJ, Kuijer JP, Rombouts SA, et al. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol 2008; 29: 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hattori Y, Enmi J, Kitamura A, et al. A novel mouse model of subcortical infarcts with dementia. J Neurosci 2015; 35: 3915–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hattori Y, Enmi J, Iguchi S, et al. Gradual carotid artery stenosis in mice closely replicates hypoperfusive vascular dementia in humans. JAHA 2016; 5: e002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuloaga KL, Johnson LA, Roese NE, et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab 2016; 36: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 1992; 89: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibaraki M, Nakamura K, Toyoshima H, et al. Spatial coefficient of variation in pseudo-continuous arterial spin labeling cerebral blood flow images as a hemodynamic measure for cerebrovascular steno-occlusive disease: a comparative (15)O positron emission tomography study. J Cereb Blood Flow Metab 2019; 39: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lacalle-Aurioles M, Mateos-Pérez JM, Guzmán-De-Villoria JA, et al. Cerebral blood flow is an earlier indicator of perfusion abnormalities than cerebral blood volume in Alzheimer's disease. J Cereb Blood Flow Metab 2014; 34: 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas KR, Osuna JR, Weigand AJ, et al. Regional hyperperfusion in older adults with objectively-defined subtle cognitive decline. J Cereb Blood Flow Metab 2021; 41: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harston GW, Okell TW, Sheerin F, et al. Quantification of serial cerebral blood flow in acute stroke using arterial spin labeling. Stroke 2017; 48: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Younis S, Christensen CE, Vestergaard MB, et al. Glutamate levels and perfusion in pons during migraine attacks: a 3T MRI study using proton spectroscopy and arterial spin labeling. J Cereb Blood Flow Metab 2021; 41: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thamm T, Guo J, Rosenberg J, et al. Contralateral hemispheric cerebral blood flow measured with arterial spin labeling can predict outcome in acute stroke. Stroke 2019; 50: 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grade M, Hernandez Tamames JA, Pizzini FB, et al. A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology 2015; 57: 1181–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hernandez-Garcia L, Lahiri A, Schollenberger J. Recent progress in ASL. NeuroImage 2019; 187: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston ME, Lu K, Maldjian JA, et al. Multi-TI arterial spin labeling MRI with variable TR and bolus duration for cerebral blood flow and arterial transit time mapping. IEEE Trans Med Imaging 2015; 34: 1392–1402. [DOI] [PubMed] [Google Scholar]
- 68.Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed 2010; 23: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wintermark M, Sesay M, Barbier E, et al. Comparative overview of brain perfusion imaging techniques. Stroke 2005; 36: e83–99. [DOI] [PubMed] [Google Scholar]
- 70.Fan AP, Jahanian H, Holdsworth SJ, et al. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: a systematic review. J Cereb Blood Flow Metab 2016; 36: 842–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sigurdsson S, Forsberg L, Aspelund T, et al. Feasibility of using pseudo-continuous arterial spin labeling perfusion in a geriatric population at 1.5 tesla. PloS One 2015; 10: e0144743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Binnewijzend MA, Kuijer JP, Benedictus MR, et al. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology 2013; 267: 221–230. [DOI] [PubMed] [Google Scholar]
- 73.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Promjunyakul NO, Lahna DL, Kaye JA, et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: a multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab 2016; 36: 1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Cheng R, Chen L, et al. Altered neurovascular coupling in subcortical ischemic vascular disease. Front Aging Neurosci 2021; 13: 598365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blicher JU, Stagg CJ, O'Shea J, et al. Visualization of altered neurovascular coupling in chronic stroke patients using multimodal functional MRI. J Cereb Blood Flow Metab 2012; 32: 2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haller S, Zaharchuk G, Thomas DL, et al. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology 2016; 281: 337–356. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab 2016; 36: 6–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ter Telgte A, Wiegertjes K, Gesierich B, et al. Temporal dynamics of cortical microinfarcts in cerebral small vessel disease. JAMA Neurol 2020; 77: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Promjunyakul N, Lahna D, Kaye JA, et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. NeuroImage Clin 2015; 8: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Firbank MJ, He J, Blamire AM, et al. Cerebral blood flow by arterial spin labeling in poststroke dementia. Neurology 2011; 76: 1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider JA, Arvanitakis Z, Bang W, et al. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 84.Román GC. Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci 2002; 203–204: 7–10. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Wang Q, He C, et al. Altered regional cerebral blood flow and brain function across the Alzheimer's disease spectrum: a potential biomarker. Front Aging Neurosci 2021; 13: 630382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer's disease. J Alzheimers Dis 2011; 26 Suppl 3: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chui HC, Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimers Res Ther 2015; 7: 21–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao X, Guo Q, Zhao Q, et al. The neuropsychological characteristics and regional cerebral blood flow of vascular cognitive impairment-no dementia. Int J Geriatr Psychiatry 2010; 25: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 89.Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol 2020; 19: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hays CC, Zlatar ZZ, Campbell L, et al. Subjective cognitive decline modifies the relationship between cerebral blood flow and memory function in cognitively normal older adults. J Int Neuropsychol Soc 2018; 24: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bangen KJ, Nation DA, Clark LR, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front Aging Neurosci 2014; 6: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bangen KJ, Werhane ML, Weigand AJ, et al. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes. Front Aging Neurosci 2018; 10: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia W, Rao H, Spaeth AM, et al. Blood pressure is associated with cerebral blood flow alterations in patients with T2DM as revealed by perfusion functional MRI. Medicine (Baltimore) 2015; 94: e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xekardaki A, Rodriguez C, Montandon ML, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015; 274: 490–499. [DOI] [PubMed] [Google Scholar]
- 95.Smith EE, Biessels GJ, De Guio F, et al. Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration. Alzheimers Dement (Amst) 2019; 11: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang K, Herzog H, Mauler J, et al. Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. J Cereb Blood Flow Metab 2014; 34: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin T, Qu J, Zuo Z, et al. Test-retest reliability and reproducibility of long-label pseudo-continuous arterial spin labeling. Magn Reson Imaging 2020; 73: 111–117. [DOI] [PubMed] [Google Scholar]
- 98.Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. MAGMA 2012; 25: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jezzard P, Chappell MA, Okell TW. Arterial spin labeling for the measurement of cerebral perfusion and angiography. J Cereb Blood Flow Metab 2018; 38: 603–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollock JM, Tan H, Kraft RA, et al. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am 2009; 17: 315–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho ML. Arterial spin labeling: clinical applications. J Neuroradiol 2018; 45: 276–289. [DOI] [PubMed] [Google Scholar]
- 102.Larkin JR, Simard MA, Khrapitchev AA, et al. Quantitative blood flow measurement in rat brain with multiphase arterial spin labelling magnetic resonance imaging. J Cereb Blood Flow Metab 2019; 39: 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woods JG, Chappell MA, Okell TW. Designing and comparing optimized pseudo-continuous arterial spin labeling protocols for measurement of cerebral blood flow. NeuroImage 2020; 223: 117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Binnie LR, Pauls MMH, Benjamin P, et al. Test-retest reliability of arterial spin labelling for cerebral blood flow in older adults with small vessel disease. Transl Stroke Res 2022; 13: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishida S, Kimura H, Isozaki M, et al. Robust arterial transit time and cerebral blood flow estimation using combined acquisition of Hadamard-encoded multi-delay and long-labeled long-delay pseudo-continuous arterial spin labeling: a simulation and in vivo study. NMR Biomed 2020; 33: e4319. [DOI] [PubMed] [Google Scholar]
- 106.Zhang LX, Woods JG, Okell TW, et al. Examination of optimized protocols for pCASL: sensitivity to macrovascular contamination, flow dispersion, and prolonged arterial transit time. Magn Reson Med 2021; 86: 2208–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai W, Robson PM, Shankaranarayanan A, et al. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012; 67: 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woods JG, Chappell MA, Okell TW. A general framework for optimizing arterial spin labeling MRI experiments. Magn Reson Med 2019; 81: 2474–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European Consortium for ASL in Dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang R, Yu S, Alger JR, et al. Multi-delay arterial spin labeling perfusion MRI in moyamoya disease – comparison with CT perfusion imaging. Eur Radiol 2014; 24: 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaneta T, Katsuse O, Hirano T, et al. Voxel-wise correlations between cognition and cerebral blood flow using arterial spin-labeled perfusion MRI in patients with Alzheimer's disease: a cross-sectional study. BMC Neurol 2017; 17: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mezue M, Segerdahl AR, Okell TW, et al. Optimization and reliability of multiple postlabeling delay pseudo-continuous arterial spin labeling during rest and stimulus-induced functional task activation. J Cereb Blood Flow Metab 2014; 34: 1919–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shirzadi Z, Stefanovic B, Chappell MA, et al. Enhancement of automated blood flow estimates (ENABLE) from arterial spin-labeled MRI. J Magn Reson Imaging 2018; 47: 647–655. [DOI] [PubMed] [Google Scholar]
- 114.Mutsaerts HJ, Petr J, Václavů L, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab 2017; 37: 3184–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morgan CA, Melzer TR, Roberts RP, et al. Spatial variation of perfusion MRI reflects cognitive decline in mild cognitive impairment and early dementia. Sci Rep 2021; 11: 23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Osch MJ, Teeuwisse WM, Chen Z, et al. Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow. J Cereb Blood Flow Metab 2018; 38: 1461–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lahiri A, Fessler JA, Hernandez-Garcia L. Optimizing MRF-ASL scan design for precise quantification of brain hemodynamics using neural network regression. Magn Reson Med 2020; 83: 1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dolui S, Vidorreta M, Wang Z, et al. Comparison of PASL, PCASL, and background-suppressed 3D PCASL in mild cognitive impairment. Hum Brain Mapp 2017; 38: 5260–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]