Abstract
Asymptomatic low-grade carotid artery stenosis (LGCS) is a common finding in patients with manifest arterial disease, however its relationship with brain MRI changes and cognitive decline is unclear. We included 902 patients (58 ± 10 years; 81% male) enrolled in the Second Manifestations of Arterial Disease – Magnetic Resonance (SMART-MR) study without a history of cerebrovascular disease. LGCS was defined as 1–49% stenosis on baseline carotid ultrasound, whereas no LGCS (reference category) was defined as absence of carotid plaque. Brain and white matter hyperintensity (WMH) volumes and cognitive function were measured at baseline and after 4 (n = 480) and 12 years (n = 222) of follow-up. Using linear mixed-effects models, we investigated associations of LGCS with progression of brain atrophy, WMH, and cognitive decline. LGCS was associated with greater progression of global brain atrophy (estimate −0.03; 95%CI, −0.06 to −0.01; p = 0.002), and a greater decline in executive functioning (estimate −0.02; 95%CI, −0.031 to −0.01; p < 0.001) and memory (estimate −0.012; 95%CI, −0.02 to −0.001; p = 0.032), independent of demographics, cardiovascular risk factors, and incident brain infarcts on MRI. No association was observed between LGCS and progression of WMH. Our results indicate that LGCS may represent an early marker of greater future brain atrophy and cognitive decline.
Keywords: Brain atrophy, cognitive decline, cohort studies, low-grade carotid artery stenosis, white matter hyperintensity
Introduction
Carotid artery stenosis refers to the buildup of atherosclerotic plaque along the lining of the carotid arteries and represents a well-recognized cause of atheroembolic stroke. 1 At the highest levels of stenosis, carotid atheroma may also lead to hemodynamic stroke through flow restriction and cerebral ischemic injury. 2
Mild carotid atheroma resulting in low-grade (1–49%) carotid artery stenosis (LGCS) is associated with a relatively low risk of atheroembolic stroke compared to moderate or severe stenosis,3,4 but is a common finding in older individuals and patients with atherosclerotic disease.5–8 In clinical practice, LGCS is frequently identified incidentally in asymptomatic patients on imaging studies. 9 Results from recent cross-sectional studies suggest that asymptomatic LGCS may be of clinical importance as a risk factor for smaller brain volumes and worse cognitive performance. 10 The cross-sectional design of these studies, however, precludes establishing a cause-effect relationship. To the best of our knowledge, no previous studies examined the longitudinal relationship of LGCS with brain MRI changes and cognitive decline in patients without a history of cerebrovascular disease.
Here, we tested the hypothesis that asymptomatic LGCS may represent a risk factor for greater progression of brain atrophy, WMH and cognitive decline. Using data from the Second Manifestations of ARTerial disease-Magnetic Resonance (SMART-MR) study, we compared trajectories of brain volumes, WMH volumes, and cognitive domains between patients with asymptomatic LGCS and patients without LGCS over 12 years of follow-up, adjusting for demographics and cardiovascular risk factors.
Methods
Study population
We used data from the SMART-MR study, a prospective cohort study at the University Medical Center Utrecht to investigate risk factors and consequences of brain changes on MRI in patients with manifest arterial disease.11,12 A total of 1,309 adult patients newly referred to the University Medical Center Utrecht for treatment of atherosclerotic disease (manifest coronary artery disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm) between 2001 and 2005 were included for baseline measurements, including a 1.5 T brain MRI.11,12 During a 1-day visit to the University Medical Center Utrecht, a physical examination, ultrasonography of the carotid arteries, blood and urine samplings, and a 1.5 T brain MRI scan were performed.11,12 Neuropsychological assessment was added to the research protocol from 2003 onwards. We used questionnaires to assess demographics, risk factors, medical history, medication use, and cognitive and physical functioning.11,12 Of the 1,309 patients included, 754 patients had follow-up measurements four years later between January 2006 and May 2009. Between November 2013 and October 2017, all patients alive were invited for a second follow-up, including a 1.5 T brain MRI. Second follow-up measurements were obtained from 329 patients.
The SMART-MR study was approved by the medical ethics committee of the University Medical Center Utrecht according to the guidelines of the Declaration of Helsinki of 1975. Written informed consent was obtained from all patients participating in the SMART-MR study.
Study sample
Of the 1,309 patients included in the SMART-MR study, carotid ultrasound data were irretrievable or incomplete in 56 patients and 181 patients were categorized as having moderate (50-69%) or severe (>70%) carotid stenosis. These patients were excluded from the present analyses. In addition, we excluded 170 patients with a history of cerebrovascular disease (defined as transient ischemic attack, stroke, cerebral ischemia, amaurosis fugax, or retinal infarction) 13 as these may include patients with a symptomatic LGCS, resulting in a study sample of 902 patients (LGCS n = 713; no LGCS n = 189). Flow diagrams of patients with available neuroimaging and cognition data at each visit are shown in Figure 1 and Figure 2 of the Supplemental Material, respectively.
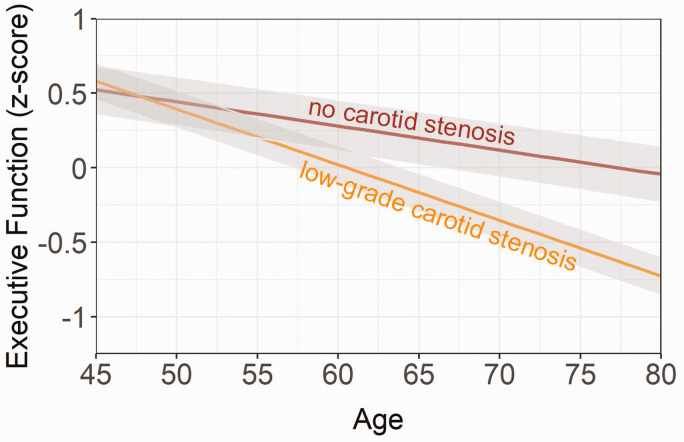
Figure 1.
Longitudinal relationship between executive functioning (z-score), low-grade carotid stenosis, and no stenosis. Age of patients at each visit was chosen as the time variable. The shaded grey area represents the 95% confidence interval. Results adjusted for sex, education level, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years, alcohol use and practice effect.
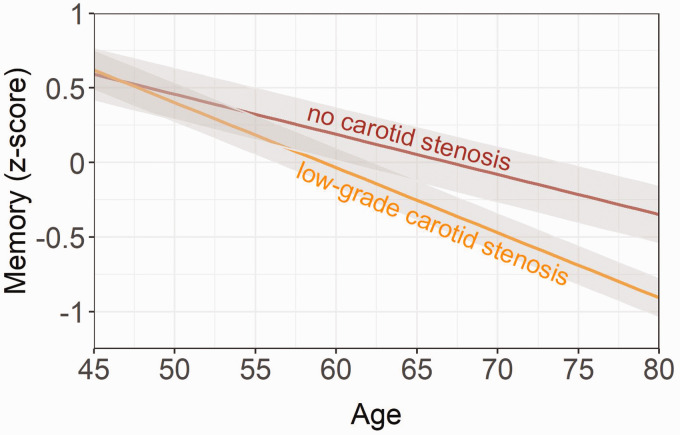
Figure 2.
Longitudinal relationship between memory (z-score), low-grade carotid stenosis, and no stenosis. Age of patients at each visit was chosen as the time variable. The shaded grey area represents the 95% confidence interval. Results adjusted for sex, education level, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years, alcohol use and practice effect.
Carotid stenosis
At baseline, ultrasonography consisting of color Doppler-assisted duplex scanning was performed with a 10 MHz linear-array transducer (ATL Ultramark 9) by experienced ultrasound technicians to determine the presence and degree of carotid stenosis. The severity of carotid stenosis was evaluated based on blood flow velocity patterns and presence of plaque, and was recorded on a categorical scale.14,15 The greatest stenosis observed on the right or the left side of the common or internal carotid artery was taken to determine the severity of carotid artery disease. Carotid stenosis 1–29% and 30–49% were defined as presence of plaque with a peak systolic velocity (PSV) ≤ 100 cm/s and >100 to ≤150 cm/s, respectively. In the present study, we defined LGCS as 1–49% stenosis. No LGCS (reference category) was defined as absence of carotid plaque.
MRI protocol
MR imaging of the brain was performed on a 1.5 T whole-body system (Gyroscan ACS-NT, Philips Medical Systems, Best, the Netherlands) using a standardized scan protocol. 11 Transversal T1-weighted [repetition time (TR) = 235 ms; echo time (TE) = 2 ms], T2-weighted [TR = 2200 ms; TE = 11 ms], fluid-attenuated inversion recovery (FLAIR) [TR = 6000 m; TE = 100 ms; inversion time (TI) = 2000 ms] and T1-weighted inversion recovery images [TR = 2900 ms; TE = 22 ms; TI = 410 ms] were acquired with a voxel size of 1.0 × 1.0 × 4.0 mm3 and contiguous slices. 12
Brain infarcts
Although we excluded patients with a history of symptomatic cerebrovascular disease, patients in the study sample may show clinically non-manifest brain infarcts on MRI (i.e., silent cerebrovascular disease), which may confound the relationship between LGCS and change in neuroimaging and cognitive outcomes. We therefore accounted for brain infarcts on MRI in our analyses. Brain infarcts were visually rated by a neuroradiologist blinded to patient characteristics on the T1-weighted, T2-weighted, and FLAIR images of the MRI scans. Lacunes were defined as focal lesions between 3 to 15 mm according to the STRIVE criteria, 16 whereas non-lacunar lesions were categorized in large infarcts (i.e., cortical infarcts and subcortical infarcts not involving the cerebral cortex) and infarcts located in the cerebellum or brain stem.
Brain volume measurements
White matter hyperintensity (WMH) volumes and brain volumes were obtained using the k-nearest neighbor (kNN) automated segmentation program on the T1-weighted, FLAIR, and T1-weighted inversion recovery sequences of the MRI scans. 17 The kNN segmentation method has been shown to be suitable for detecting longitudinal brain volume changes.11,18 All WMH segmentations were visually checked by an investigator (RG) blinded to patient characteristics using an image processing framework (MeVisLab 2.7.1., MeVis Medical Solutions AG, Bremen, Germany). Incorrectly segmented voxels were added to the correct segmentation volumes using the image processing framework.
Total brain volume (including the volume of the cerebellum) was calculated by summing the volumes of gray matter, white matter, WMH and, if present, the volumes of brain infarcts. Total intracranial volume (ICV) was calculated by summing the total brain volume and the volume of cerebrospinal fluid. Total brain volume, sulcal cerebrospinal fluid volume and ventricular volume were normalized for ICV and expressed as brain parenchymal fraction (BPF), sulcal cerebrospinal fluid fraction (CSFF) and ventricular fraction (VF), and were used as indicators of global, cortical, and subcortical atrophy, respectively. Similarly, WMH volume was normalized for ICV. Natural log transformation was performed on WMH volumes due to a non-normal distribution.
Cognitive functioning
Cognitive functioning was measured at baseline, and first and second follow-up visits with a set of standard neuropsychological tests covering the domains of memory and executive functioning. Memory was assessed with the 15 Word Learning test (immediate recall based on five trials and delayed recall) and with the delayed recall of the Rey-Osterrieth Complex Figure test.19,20 Executive functioning was assessed by the Visual Elevator test (10 trials), the Brixton Spatial Anticipation test, and the Verbal Fluency test (letter A with a time span of 60 seconds).21–23 Visual Elevator test scores were natural log-transformed due to a non-normal distribution and multiplied by minus one so that higher scores represented better performance. Similarly, Brixton test scores were multiplied by minus one so that higher scores represented better performance.
To assess change in cognitive functioning, we converted test scores from each visit to z-scores based on the baseline population mean and standard deviation (SD). These z-scores were averaged to create domain-specific z-scores for memory and executive functioning, which were subsequently standardized to the baseline domain-specific z-score mean and SD for all patients.
Covariates
At baseline, age, sex, smoking habits, alcohol intake and highest level of education were assessed using questionnaires. Height and weight were measured, and the body mass index (BMI) was calculated (kg/m2). Systolic blood pressure (SBP) (mmHg) and diastolic blood pressure (DBP) (mmHg) were measured three times with a sphygmomanometer, and the average of these measures was calculated. Hypertension was defined as a mean SBP of >160 mmHg, a mean DBP of >95 mmHg, or self-reported use of antihypertensive drugs. Threshold values of SBP and DBP for hypertension were determined according to criteria established in 2001. An overnight fasting venous blood sample was taken to determine glucose and lipids. Diabetes mellitus was defined as fasting serum glucose levels of ≥7.0 mmol/l, and/or use of glucose-lowering medication, and/or known history of diabetes.
Education level was categorized into three categories based on the Dutch education system and ranged from no education/primary school to university education. Low level education included no education or primary school only (comparable to up to six years of education), whereas high level education included higher professional education and university education (comparable to ≥15 years of education). All other educational levels were defined as intermediate (comparable to around 7–14 years of education).
Statistical analysis
Baseline characteristics of the total study sample, and stratified by presence and absence of LGCS were reported with descriptive statistics. We compared baseline characteristics of patients with LGCS versus those without using an independent samples t-test and Chi square test for continuous and dichotomous variables, respectively.
Linear mixed-effects models
We used linear mixed-effects models with random effects to assess changes in neuroimaging outcomes and cognitive functioning over time. 24 The age of patients at each visit was chosen as the time variable, which was centered at 58 years (the mean value at the first visit) and hereinafter referred to as ‘time’. LGCS was represented by a dichotomous variable with absence of LGCS as the reference category.
Models were run in two steps. In the first model, time, LGCS, and an interaction term between LGCS and time (our primary coefficient of interest) were entered, together with sex, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years and alcohol use at baseline as covariates. Models that estimated cognitive change in addition included education level and a practice effect, which was modeled using an indicator fixed at the square root of the number of prior visits. 25
Incident brain infarcts and lacunes may act as a confounder on the relationship between LGCS and change in neuroimaging and cognitive outcomes. Therefore, in a second model, the covariates indicating large infarcts and lacunes on baseline MRI were replaced with time-varying covariates indicating the presence of large infarcts and lacunes at both baseline and follow-up MRI.
To determine whether brain atrophy was associated with cognitive functioning at baseline and follow-up, we also added BPF as a time-varying predictor to the models that estimated cognitive change.
Adequacy of the linear mixed-effects models was determined by examining the residuals for approximate normality and homoscedasticity. We concluded that model assumptions were adequately met.
Missing covariates
To reduce the risk of bias due to complete case analysis, we performed chained equations imputation on missing baseline covariates to generate 10 imputed datasets using SPSS 25.0 (Chicago, IL, USA). The linear mixed-effects models were performed on the imputed datasets and the pooled results were presented. Statistical significance was set at p ≤ 0.05.
Sensitivity analysis
The substantial attrition during follow-up in the present study may lead to informative dropout. To determine whether this was the case in the study sample, we used joint models that allow for controlling the results of the linear mixed models for dropout (including due to death) using correlated survival data. 26 Joint models consist of a longitudinal and a survival submodel. 26 The longitudinal submodel consisted of the linear mixed-effects models used in the primary analyses with adjustment for demographics, cardiovascular risk factors and brain infarcts on MRI at baseline. The survival submodel consisted of a Cox proportional hazards regression model with baseline age, sex and LGCS (with absence of LGCS as the reference category) as predictors. Follow-up data for the survival submodel were obtained from questionnaires that patients received biannually and are described in detail in previous work. 12 We defined dropout (i.e., the “event” in the survival submodel) as having a missing outcome for the second follow-up measurement, either due to death or any other reason.
We compared joint models using different baseline hazard functions and we selected the baseline hazard function that yielded the lowest Akaike information criterion. The Weibull baseline hazard function was chosen for models that estimated change in brain volumes, whereas the piecewise baseline hazard function was chosen for models that estimated change in cognitive functioning. The JM package for R version 4.0.5 (R Core Team, 2021) was used for the joint model analysis. 26
Results
Baseline characteristics of the study sample (n = 902; mean age 58 ± 10 years; 81% male) are shown in Table 1. LGCS was present in 713 patients (79%) at baseline, whereas 189 patients (21%) did not show any carotid stenosis on ultrasound.
Table 1.
Baseline characteristics of patients with low-grade carotid stenosis, patients without stenosis and the total study sample.
| Low-grade carotid stenosis (n = 713) | No carotid stenosis (n = 189) | All patients (n = 902) | p-valuea | |
|---|---|---|---|---|
| Age (years) | 59 ± 9 | 51 ± 10 | 58 ± 10 | <0.001 |
| Sex, % men | 81.5 | 78.3 | 80.8 | 0.09 |
| BMI (kg/m2) | 27 ± 4 | 26 ± 4 | 27 ± 4 | 0.08 |
| Smoking, pack yearsb | 20 (0, 52) | 14 (0, 42) | 19 (0, 49) | <0.001c |
| Alcohol use, % | ||||
| Current | 75 | 74 | 75 | 0.52 |
| Former | 10 | 9 | 10 | 0.36 |
| Abstinent | 15 | 17 | 15 | 0.12 |
| Hypertension, % | 48.8 | 37.6 | 46.5 | <0.001 |
| Diabetes mellitus, % | 20.9 | 10.1 | 18.6 | <0.001 |
| Education level, % | ||||
| Low | 13.0 | 8.4 | 11.9 | 0.005 |
| Intermediate | 67.5 | 63.5 | 66.6 | 0.08 |
| High | 19.5 | 28.1 | 21.5 | <0.001 |
| Infarcts on MRI, % | ||||
| Large | 3.5 | 1.1 | 3.0 | 0.002 |
| Cerebellar | 2.7 | 2.7 | 2.5 | 0.47 |
| Brainstem | 1.4 | 0.5 | 1.2 | 0.09 |
| Lacunes on MRI, % | 10.9 | 3.2 | 9.3 | <0.001 |
| pCBF, ml/min per 100 ml brain volume | 52.2 ± 10.3 | 53.2 ± 9.0 | 52.4 ± 10.1 | 0.03 |
| BPF, % ICV | 79.0 ± 2.8 | 80.7 ± 2.5 | 79.3 ± 2.8 | <0.001 |
| CSFF, % ICV | 18.9 ± 2.3 | 17.5 ± 2.0 | 18.6 ± 2.3 | <0.001 |
| VF, % ICV | 2.1 ± 1.1 | 1.8 ± 1.0 | 2.0 ± 1.0 | <0.001 |
| WMH volume on MRI, mlb | 0.9 (0.2, 5.6) | 0.5 (0.1, 2.2) | 0.8 (0.2, 4.9) | <0.001c |
| Executive functioning, z-score | −0.02 ± 0.96 | 0.23 ± 0.98 | 0.05 ± 0.97 | <0.001 |
| Memory, z-score | −0.10 ± 0.97 | 0.27 ± 0.96 | 0.00 ± 0.98 | <0.001 |
Characteristics are presented as mean ± SD or %.
aP-value for independent samples t-test or Chi square test (if proportions) for comparison between patients with low-grade carotid stenosis and patients without carotid stenosis.
bMedian (10th percentile, 90th percentile).
cNatural log-transformed due to a non-normal distribution in the statistical analysis.
BMI: body mass index; SD: standard deviation; WMH: white matter hyperintensity; pCBF: parenchymal cerebral blood flow; BPF: brain parenchymal fraction; ICV: total intracranial volume; CSFF: sulcal cerebrospinal fluid fraction; VF: ventricular fraction.
Patients with LGCS were older, had a less favorable cardiovascular profile, more often had a low education level, and showed smaller brain volumes and lower executive functioning and memory z-scores compared to the reference group (Table 1).
Mean time between baseline and first follow-up measurements was 3.9 ± 0.4 years (range 2.9–5.8 years), whereas there were 12.0 ± 0.4 years (range 11.1–13.5 years) between baseline and the second follow-up measurements.
Associations between LGCS and brain MRI changes
Mean decrease in BPF per year for the study sample was 0.25% ICV (95% CI, –0.28 to –0.22), whereas CSFF and VF were estimated to increase at 0.19% ICV (95% CI, 0.16 to 0.22) and 0.06% ICV (95% CI, 0.05 to 0.06) per year, respectively (Table 2). Mean increase in WMH per year was 0.08 natural log-transformed ml (95% CI, 0.07 to 0.10).
Table 2.
Output of the linear mixed-effects models with age of patients at each visit as the time variable, neuroimaging outcomes as dependent variables and low-grade carotid stenosis as independent variable.
| BPF |
CSFF |
VF |
WMHb |
|||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Intercept | ||||||||
| Model 1 | 79.6 (78.5 to 80.8) | <0.001 | 18.4 (17.5 to 19.5) | <0.001 | 1.99 (1.54 to 2.43) | <0.001 | –3.34 (–3.94 to –2.74) | <0.001 |
| Model 2 | 79.5 (78.3 to 80.6) | <0.001 | 18.6 (17.5 to 19.6) | <0.001 | 2.08 (1.64 to 2.52) | <0.001 | –3.21 (–3.81 to –2.61) | <0.001 |
| Time | ||||||||
| Model 1 | –0.25 (–0.28 to –0.22) | <0.001 | 0.19 (0.16 to 0.22) | <0.001 | 0.06 (0.05 to 0.07) | <0.001 | 0.08 (0.07 to 0.10) | <0.001 |
| Model 2 | –0.24 (–0.27 to –0.21) | <0.001 | 0.19 (0.16 to 0.21) | <0.001 | 0.06 (0.05 to 0.07) | <0.001 | 0.08 (0.07 to 0.10) | <0.001 |
| LGCSa | ||||||||
| Model 1 | 0.07 (–0.30 to 0.40) | 0.717 | 0.07 (–0.23 to 0.37) | 0.661 | –0.10 (–0.24 to 0.03) | 0.134 | 0.00 (–0.18 to 0.17) | 0.987 |
| Model 2 | 0.04 (–031 to 0.37) | 0.858 | 0.09 (–0.22 to 0.38) | 0.591 | –0.09 (–0.22 to 0.05) | 0.200 | 0.03 (–0.15 to 0.20) | 0.763 |
| LGCS x Time | ||||||||
| Model 1 | –0.03 (–0.06 to –0.01) | 0.002 | 0.03 (0.01 to 0.05) | 0.011 | 0.01 (0.002 to 0.02) | 0.019 | 0.01 (–0.03 to 0.02) | 0.162 |
| Model 2 | –0.03 (–0.05 to –0.01) | 0.004 | 0.02 (0.01 to 0.04) | 0.014 | 0.01 (0.001 to 0.02) | 0.026 | 0.01 (–0.03 to 0.02) | 0.156 |
Model 1: adjusted for sex, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years and alcohol use at baseline.
Model 2: model 1 with time-varying covariates for large infarcts and lacunes on MRI.
aNo LGCS as the reference category.
bNatural log-transformed and standardized for total intracranial volume.
CI: confidence interval; LGCS: low-grade carotid stenosis; BPF: brain parenchymal fraction; CSFF: sulcal cerebrospinal fluid fraction; VF: ventricular fraction; WMH: white matter hyperintensity volume.
At age 58 (i.e., intercept), no main effects were observed of LGCS on BPF (estimate 0.07; 95% CI, –0.30 to 0.40; p = 0.717), CSFF (estimate 0.07; 95% CI, –0.23 to 0.37; p = 0.661), VF (estimate –0.10; 95% CI, –0.24 to 0.03; p = 0.134), or WMH volume (estimate 0.00; 95% CI, –0.18 to 0.17; p = 0.978). Significant main effects of sex, lacunes on MRI, diabetes mellitus, and smoking pack years were observed on BPF, CSFF, VF and WMH volume (Supplementary Table 1).
LGCS, compared with no LGCS, was associated with greater change in BPF (estimate –0.03; 95% CI, –0.06 to –0.01; p = 0.002), CSFF (estimate 0.03; 95% CI, 0.01 to 0.05; p = 0.011) and VF (estimate 0.01; 95% CI, 0.002 to 0.02; p = 0.019), and these results did not substantially change after adjusting for incident large brain infarcts or lacunes (Table 2). LGCS was not associated with greater change in WMH volume over time (estimate 0.01; 95% CI, –0.03 to 0.02; p = 0.162).
In the joint model analysis, parameter estimates for the time effect were slightly smaller for BPF, CSFF, VF and WMH compared with the primary analysis (Supplementary Table 3). Controlling for death/dropout, LGCS versus no LGCS remained significantly associated with a greater decline in BPF (estimate –0.036; 95% CI, –0.06 to –0.01; p = 0.001), and a greater increase in CSFF (estimate 0.03; 95% CI, 0.01 to 0.05; p = 0.006) and VF (estimate 0.01; 95% CI, 0.002 to 0.017; p = 0.013). Consistent with the primary analysis, LGCS was not related to a greater change in WMH volume (estimate 0.007; 95% CI, –0.003 to 0.017; p = 0.179). Estimates of association parameters were significant for CSFF (estimate 0.0485; p = 0.036) and VF (estimate 0.143; p = 0.002), thereby indicating that death/dropout impacted average change in CSFF and VF over time, whereas this was not the case for BPF (estimate –0.037; p = 0.063) and WMH volume (estimate –0.012; p = 0.722) (Supplementary Table 3).
Associations between LGCS and cognitive domain changes
Executive functioning was estimated to decrease by 0.06 z-score units (95% CI, –0.08 to –0.05; p < 0.001) on average per year. For memory, mean decrease was estimated at 0.06 z-score units (95% CI, –0.04 to –0.08; p < 0.001) per year for the study sample (Table 3).
Table 3.
Output of the linear mixed-effects models with age of patients at each visit as the time variable, cognition domain-specific z-scores as dependent variables and low-grade carotid stenosis as independent variable.
| Executive functioning |
Memory |
|||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI | p-value | |
| Intercept | ||||
| Model 1 | –0.84 (–1.37 to –0.32) | 0.002 | –0.29 (–0.84 to 0.27) | 0.311 |
| Model 2 | –0.78 (–1.31 to –0.25) | 0.004 | –0.30 (–0.86 to 0.26) | 0.293 |
| Time | ||||
| Model 1 | –0.06 (–0.08 to –0.05) | <0.001 | –0.06 (–0.04 to –0.08) | <0.001 |
| Model 2 | –0.06 (–0.07 to –0.04) | <0.001 | –0.06 (–0.04 to –0.07) | <0.001 |
| LGCSa | ||||
| Model 1 | –0.06 (–0.20 to 0.09) | 0.403 | 0.05 (–0.10 to 0.20) | 0.496 |
| Model 2 | –0.04 (–0.18 to 0.10) | 0.577 | 0.04 (–0.11 to 0.19) | 0.586 |
| LGCS x Time | ||||
| Model 1 | –0.020 (–0.031 to –0.01) | <0.001 | –0.012 (–0.02 to –0.001) | 0.032 |
| Model 2 | –0.017 (–0.028 to –0.006) | 0.003 | –0.010 (–0.02 to 0.001) | 0.082 |
Model 1: adjusted for sex, education level, practice effect, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years and alcohol use at baseline.
Model 2: model 1 with time-varying covariates for large infarcts and lacunes on MRI.
aNo LGCS as the reference category.
CI: confidence interval; LGCS: low-grade carotid stenosis.
At age 58 (i.e., intercept), LGCS versus no LGCS was not associated with a lower z-score in executive functioning (estimate –0.06; 95% CI, –0.20 to 0.09; p = 0.403) or memory (estimate 0.05; 95% CI, –0.10 to 0.20; p = 0.496), and these estimates did not substantially change after accounting for incident large brain infarcts or lacunes (Table 3). Significant main effects of sex, education level, and alcohol use were observed on executive functioning and memory (Supplementary Table 2).
LGCS, compared with no LGCS, was associated with a greater decline in executive functioning by 0.02 z-score units (95% CI, –0.031 to –0.01; p < 0.001; Figure 1) per year. The association between LGCS and change in executive functioning persisted after controlling for incident large brain infarcts and lacunes (Table 3). For memory, LGCS versus no LGCS was associated with a greater decline by 0.012 z-score units (95% CI, –0.02 to –0.001; p = 0.032; Figure 2) per year. The association between LGCS and change in memory slightly attenuated after controlling for incident large brain infarcts and lacunes (Table 3).
When adding BPF as a time-varying predictor, we observed that lower BPF was associated with a lower z-score in executive functioning at baseline and follow-up (estimate –0.05; 95% CI, –0.07 to –0.02; p = 0.001) in a model that controlled for sex, large infarcts on MRI, lacunes on MRI, hypertension, diabetes mellitus, body mass index, smoking pack years and alcohol use at baseline. In this model, LGCS (compared with no LGCS) remained associated with a greater decline in executive functioning by 0.01 z-score units (95% CI, –0.02 to –0.01; p = 0.007) per year. Lower BPF was associated with a lower z-score in memory at baseline and follow-up, however the association was not significant (estimate –0.02; 95% CI, –0.05 to 0.01; p = 0.161). In this model, we observed that LGCS (compared with no LGCS) was associated with a greater decline in memory by 0.01 z-score units per year, however the association was not significant (95% CI, –0.02 to 0.00; p = 0.103).
In the joint model analysis, parameter estimates for the time effect were comparable with the primary analyses (Supplementary Table 3). Controlling for death/dropout, LGCS remained associated with a greater decline in executive functioning (estimate –0.017; 95% CI, –0.026 to –0.01; p < 0.001) and memory (estimate –0.011; 95% CI, –0.017 to –0.004; p = 0.002). Estimates of association parameters were significant for both executive functioning (estimate –1.34; p < 0.001) and memory (estimate –0.546; p < 0.001), indicating that death/dropout impacted average change in executive functioning and memory over time.
Discussion
In this cohort of patients with manifest arterial disease, we observed that asymptomatic low-grade carotid artery stenosis (LGCS) was associated with greater progression of global, cortical and subcortical brain atrophy, but not with white matter hyperintensities (WMH) compared with absence of stenosis. LGCS was also associated with a greater decline in executive functioning and memory throughout the follow-up period of 12 years. These relationships were independent of demographics, cardiovascular risk factors and brain infarcts on MRI.
In clinical practice, emphasis is on the detection of carotid stenosis due to the associated risk of atheroembolic stroke. The risk of atheroembolic stroke is relatively low in LGCS but increases substantially in moderate and severe stenosis.3,4 The findings of this long-term follow-up study, however, suggest that asymptomatic LGCS may be of clinical importance as a marker of greater future brain atrophy and cognitive decline. Our results are consistent with a recent cross-sectional study in which mild carotid atheroma was related to cortical thinning and worse fluid intelligence. 10
The exact mechanisms underlying the association of LGCS with greater brain atrophy and cognitive decline remain to be determined. One such mechanism may be cerebral hypoperfusion secondary to LGCS, however this explanation is less likely because carotid artery stenosis <50% is usually considered hemodynamically insignificant. Symptomatic or silent brain infarcts and lacunes are also less likely to explain the observed relationships because we included only asymptomatic patients with LGCS and we adjusted the analyses for prevalent and incident silent brain infarcts on MRI. We also observed that cardiovascular risk factors such as diabetes mellitus or hypertension did not explain the association of LGCS with greater brain atrophy or cognitive decline, even though patients with LGCS did show a less favorable cardiovascular profile at baseline. In this context, it is possible that LGCS represents a proxy marker for more profound atherosclerotic vascular changes within the cerebrum or physiological changes associated with more severe generalized atherosclerosis such as low-grade systemic inflammation.10,27 These processes, which may be difficult to measure in patients, may negatively impact brain health over time leading to greater brain atrophy and cognitive decline.
To the best of our knowledge, no previous studies compared trajectories of brain MRI changes and cognitive functioning between asymptomatic patients with LGCS and those without. Studies examining the impact of carotid plaque (irrespective of degree of stenosis) on cognitive functioning have reported conflicting findings. In the Northern Manhattan Study, carotid plaque was not related to greater cognitive decline throughout the follow-up period of 5 years. 28 In the Tromsø Study, however, presence of carotid plaque at baseline was associated with lower cognitive test scores measured 7 years later. 29 With respect to WMH, our findings are in line with a prospective analysis of the Rotterdam Scan Study in which increasing carotid plaque severity was not associated with progression of WMH over 3 years of follow-up. 30
Limitations of this study include, first, the substantial attrition during follow-up. However, we addressed this issue by performing sensitivity analyses using joint models and we observed that the relations between LGCS, progression of brain atrophy and cognitive decline held after controlling for death/dropout. Second, cognitive testing in this analysis was limited to only two cognitive domains. Third, as follow-up measurements of vascular risk factors were available only in a limited number of patients, our analyses did not account for changes in vascular risk factors during follow-up. Fourth, the volumetric MRI technique used in our study did not allow us to measure region-specific brain volume changes. Results from a recent cross-sectional analysis in the Lothian Birth Cohort 1936 indicate that carotid atheroma was predominantly associated with smaller volumes in specific anterior and posterior cortical regions, whereas regions of the primary motor and sensory cortex were relatively spared. 10 Lastly, volumetry in this study was performed on MRI sequences with a slice thickness of 4 mm instead of 1 mm, which is likely more sensitive in detecting brain volume changes.
Strengths of this study are the large number of patients included, the long follow-up period and the multiple brain MRI and cognitive functioning measurements recorded over time. In addition, we accounted for silent cerebrovascular disease on baseline MRI in the analyses. Also, we used prospective MRI data to adjust the analyses for incident brain infarcts and lacunes during follow-up. Lastly, we also accounted for a potential practice effect in the cognitive analyses due to the relatively short interval between the baseline and first follow-up measurement of 4 years.
Overall, our findings demonstrate that asymptomatic LGCS is associated with greater cognitive decline and greater progression of global, cortical, and subcortical brain atrophy over 12 years of follow-up, independent of demographics, cardiovascular risk factors, or brain infarcts on MRI. These results indicate that LGCS, a common finding in older individuals and patients with manifest arterial disease, may be a clinical marker of greater future brain atrophy and cognitive decline.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221133859 for Low-grade carotid artery stenosis is associated with progression of brain atrophy and cognitive decline. The SMART-MR study by Rashid Ghaznawi, Jet MJ Vonk, Maarten HT Zwartbol, Jeroen de Bresser, Ina Rissanen, Jeroen Hendrikse and Mirjam I Geerlings in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We gratefully acknowledge the contribution of the research nurses; R. van Petersen (data-manager); B. van Dinther (study manager) and the members of the Utrecht Cardiovascular Cohort-Second Manifestations of ARTerial disease-study group (UCC-SMART-study group): F.W. Asselbergs and H.M. Nathoe, Department of Cardiology; G.J. de Borst, Department of Vascular Surgery; M.L. Bots and M.I. Geerlings, Julius Center for Health Sciences and Primary Care; M.H. Emmelot, Department of Geriatrics; P.A. de Jong and T. Leiner, Department of Radiology; A.T. Lely, Department of Obstetrics/Gynaecology; N.P. van der Kaaij, Department of Cardiothoracic Surgery; L.J. Kappelle and Y. Ruigrok, Department of Neurology; M.C. Verhaar, Department of Nephrology, F.L.J. Visseren (chair) and J. Westerink, Department of Vascular Medicine, University Medical Center Utrecht and Utrecht University.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this paper was received as part of a grant from the Netherlands Organization for Scientific Research-Medical Sciences (NWO-MW: project No. 904-65-095). This funding source had no role in the design, data collection, data analyses and data interpretation of the study or writing of the report. We also gratefully acknowledge the funding from the European Research Council under the European Union’s Horizon 2020 Programme (H2020)/ERC grant agreement n°637024 and n°66681 (SVDs@target).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: 1) conception and design of the study: RG, JV, JH, MIG
2) acquisition and analysis of data: RG, JV, MHTZ, JB, IR, JH, MIG
3) drafting a significant portion of the manuscript or figures: RG, JV, IR, MIG
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Rashid Ghaznawi https://orcid.org/0000-0002-6616-5276
Maarten HT Zwartbol https://orcid.org/0000-0001-5779-3150
Jeroen de Bresser https://orcid.org/0000-0003-0759-8407
Ina Rissanen https://orcid.org/0000-0002-6869-0437
References
- 1.Barnett HJM, Taylor DW, Haynes RB, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 2.Derdeyn CP Grubb RL Jr.andPowers WJ.. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999; 53: 251–259. [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Marko M, Ospel JM, et al. The risk of stroke and TIA in nonstenotic carotid plaques: a systematic review and meta-analysis. Am J Neuroradiol 2020; 41: 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard DPJ, Gaziano L, Rothwell PM. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. The Lancet Neurology 2021; 20: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the cardiovascular health study. The CHS collaborative research group. Stroke 1992; 23: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 6.Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. The Lancet Global Health 2020; 8: e721–e729. [DOI] [PubMed] [Google Scholar]
- 7.Sturlaugsdottir R, Aspelund T, Bjornsdottir G, et al. Prevalence and determinants of carotid plaque in the cross-sectional REFINE-Reykjavik study. BMJ Open 2016; 6: e012457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihle-Hansen H, Vigen T, Ihle-Hansen H, et al. Prevalence of carotid plaque in a 63- to 65-year-old Norwegian cohort from the general population: the ACE (Akershus cardiac examination) 1950 study. J Am Heart Assoc 2018; 7: e008562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasserman BA, Wityk RJ, Trout HH, et al. Low-grade carotid stenosis. Stroke 2005; 36: 2504–2513. [DOI] [PubMed] [Google Scholar]
- 10.Alhusaini S, Karama S, Nguyen TV, et al. Association between carotid atheroma and cerebral cortex structure at age 73 years. Ann Neurol 2018; 84: 576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerlings MI, Appelman AP, Vincken KL, et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis 2010; 210: 130–136. [DOI] [PubMed] [Google Scholar]
- 12.Ghaznawi R, Geerlings MI, Jaarsma-Coes M, et al. Association of white matter hyperintensity markers on MRI and long-term risk of mortality and ischemic stroke: the SMART-MR study. Neurology 2021; 96: e2172–e2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Hartog AG, Achterberg S, Moll FL, et al. Asymptomatic carotid artery stenosis and the risk of ischemic stroke according to subtype in patients with clinical manifest arterial disease. Stroke 2013; 44: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 14.Elgersma OE, van Leersum M, Buijs PC, et al. Changes over time in optimal duplex threshold for the identification of patients eligible for carotid endarterectomy. Stroke 1998; 29: 2352–2356. [DOI] [PubMed] [Google Scholar]
- 15.Goessens BM, Visseren FL, Kappelle LJ, et al. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke 2007; 38: 1470–1475. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anbeek P, Vincken KL, van Bochove GS, et al. Probabilistic segmentation of brain tissue in MR imaging. Neuroimage 2005; 27: 795–804. [DOI] [PubMed] [Google Scholar]
- 18.de Bresser J, Portegies MP, Leemans A, et al. A comparison of MR based segmentation methods for measuring brain atrophy progression. Neuroimage 2011; 54: 760–768. [DOI] [PubMed] [Google Scholar]
- 19.Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 1985; 112: 201–210. [DOI] [PubMed] [Google Scholar]
- 20.Lu PH, Boone KB, Cozolino L, et al. Effectiveness of the Rey-Osterrieth complex figure test and the Meyers and Meyers recognition trial in the detection of suspect effort. Clin Neuropsychol 2003; 17: 426–440. [DOI] [PubMed] [Google Scholar]
- 21.Robertson IH, Ward T, Ridgeway V, et al. The structure of normal human attention: the test of everyday attention. J Int Neuropsychol Soc 1996; 2: 525–534. [DOI] [PubMed] [Google Scholar]
- 22.Burgess PW, Shallice T. Bizarre responses, rule detection and frontal lobe lesions. Cortex 1996; 32: 241–259. [DOI] [PubMed] [Google Scholar]
- 23.Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia 1987; 25: 359–365. [DOI] [PubMed] [Google Scholar]
- 24.Benedictus MR, Leeuwis AE, Binnewijzend MA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer's disease. Eur Radiol 2017; 27: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivot A, Power MC, Glymour MM, et al. Jump, hop, or skip: modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol 2016; 183: 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizopoulos D. JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 2010; 35: 1–33.21603108 [Google Scholar]
- 27.Guarner V, Maria Esther R-R. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdisciplinary Topics in Gerontology and Geriatrics 2015; 40: 99–106. [DOI] [PubMed] [Google Scholar]
- 28.Gardener H, Caunca MR, Dong C, et al. Ultrasound markers of carotid atherosclerosis and cognition: the Northern Manhattan study. Stroke 2017; 48: 1855–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arntzen KA, Schirmer H, Johnsen SH, et al. Carotid atherosclerosis predicts lower cognitive test results: a 7-year follow-up study of 4,371 stroke-free subjects – the Tromso study. Cerebrovasc Dis 2012; 33: 159–165. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam scan study. Stroke 2008; 39: 2712–2719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221133859 for Low-grade carotid artery stenosis is associated with progression of brain atrophy and cognitive decline. The SMART-MR study by Rashid Ghaznawi, Jet MJ Vonk, Maarten HT Zwartbol, Jeroen de Bresser, Ina Rissanen, Jeroen Hendrikse and Mirjam I Geerlings in Journal of Cerebral Blood Flow & Metabolism