Abstract
Since their discovery in 1980, thymic nurse cells (TNCs) have been controversial. Questions pertaining to the existence of the TNC as a “unit” cell with thymocytes completely enclosed within its cytoplasm were the focus of initial debates. Early skeptics proposed the multicellular complex to be an artifact of the procedures used to isolate TNCs from the thymus. Since that time, TNCs have been found in fish, frogs, tadpoles, chickens, sheep, pigs, rats, mice, and humans. Their evolutionary conservation throughout the animal kingdom relieved most speculations about the existence of TNCs and at the same time demonstrated their apparent importance to the thymus and T-cell development. In this review we will discuss and debate reports that describe (i) the organization or structure of TNCs, (ii) the thymocyte subset(s) found within the cytoplasm of TNCs and their uptake and release, and (iii) the function of this fascinating multicellular interaction that occurs during the process of T-cell development. Discussions about the future of the field and experimental approaches that will lead to answers to remaining questions are also presented.
Much information has been published about the role of thymic stromal cells in the process of thymic education. The thymus is composed of bone marrow-derived macrophages and dendritic cells, as well as nonlymphoid epithelial cells (10). In mice, cortical epithelial cells are derived from the ectodermal branchial cleft, while medullary epithelium originates from endodermal cells of the third pharyngeal pouch (57). Epithelial cells of the cortex interact with the earliest lymphocyte immigrants. Immature thymocytes, both triple negatives (αβTCR− CD4− CD8−) and triple positives (αβTCR+ CD4+ CD8+), participate in intimate cortical lymphoepithelial complexes (7, 14, 16, 48). Most mature single-positive lymphocytes (CD4+ or CD8+) reside in the medulla. As thymocytes move through the thymus, from triple negatives to single positives, they produce a T-cell antigen receptor (αβTCR) on their cell surface (Fig. 1). The αβTCR is believed to participate in an interaction with self-peptides associated with major histocompatibility complex (MHC) antigens on the cell surface of epithelial cells, as well as dendritic cells and macrophages (29, 49). The nature of that interaction is proposed to be the crux of thymic education. Presently, the most scrutinized hypothesis proposes that thymocytes producing an αβTCR that binds tightly to self-peptide in association with MHC antigens are not allowed to mature. These cells are selectively deleted because they are potentially autoreactive if released from the thymus (Fig. 2). Thymocytes producing nonreactive αβTCRs are also deleted. On the other hand, thymocytes producing an αβTCR that binds self-peptide with low affinity are allowed to mature to the single-positive phenotype and are ultimately released from the thymus as functional T cells (30, 31). A major focus of many current studies is to define the thymic epithelial cells that participate in this interaction with developing T cells. In this review, we will discuss the investigative reports that examine the population of thymic cortical epithelial cells given the name “thymic nurse cells” (TNCs). More specifically, our review will focus on the structural nature of this multicellular complex and the immunological significance of this unique thymocyte–epithelial-cell interaction.
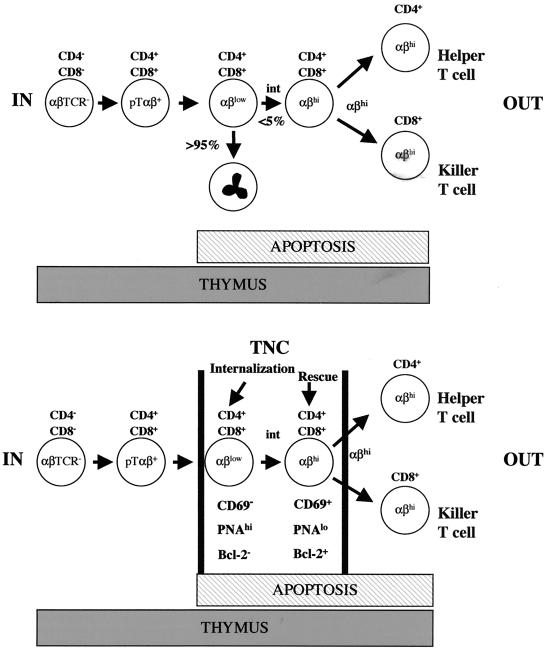
FIG. 1.
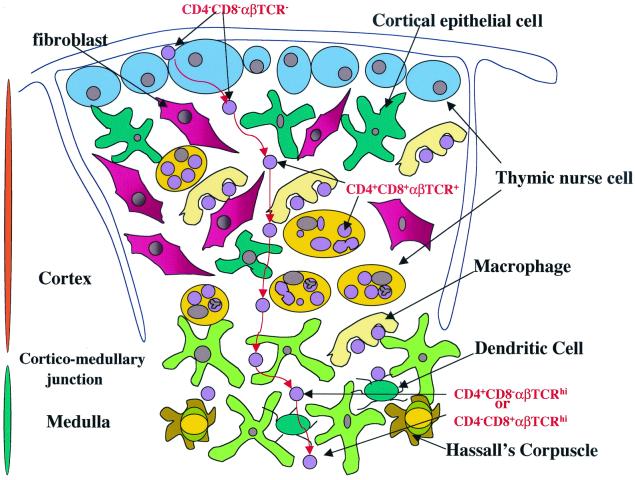
Schematic representation of T-cell migration and interactions with thymic stromal cells during development. The thymus is made up of a myriad of cell types. The thymic stroma consists of macrophages, fibroblasts, dendritic cells, and epithelial cells. These stromal cells define the matrix through which developing thymocytes migrate toward maturity. Developing thymocytes interact with cells of the thymic stroma to create a repertoire of mature T cells that recognize foreign antigens on the surface of self-cells when they exit the thymus. This differentiation pathway involves the removal of cells that have the potential to attack normal uninfected self-cells. Immunologists have been able to determine the sequence of events which lead to the maturation of T cells by using the expression pattern of CD4, CD8, and αβTCR. Early lymphoid immigrants do not express either of these antigens on their cell surface. These pre-T cells are termed triple negatives (αβTCR− CD4− CD8−) and are found near the subcapsular region of the thymic cortex. They then rearrange their antigen receptor genes to express an immature TCR (pre-TαβTCR) and are then termed double negatives. Each maturation event is accompanied by movement of developing thymocytes from the subcapsular region toward the medulla (red arrows). The completion of genetic rearrangement of the TCR results in the expression of a functional cell surface αβTCR. At the same time, both CD4 and CD8 are expressed on the plasma membrane. Now that all three antigens are expressed, the resulting subset of cells is called triple positive; these cells reside near the corticomedullary junction. After MHC restriction (the process that removes potentially autoreactive thymocytes and allows thymocytes that can recognize foreign antigen on the surface of self-cells to survive), mature T cells are visible in the medulla. Mature T cells express a functional αβTCR and either CD4 or CD8 and are termed single-positive cells. Single positives are then released from the thymus as functional T cells.
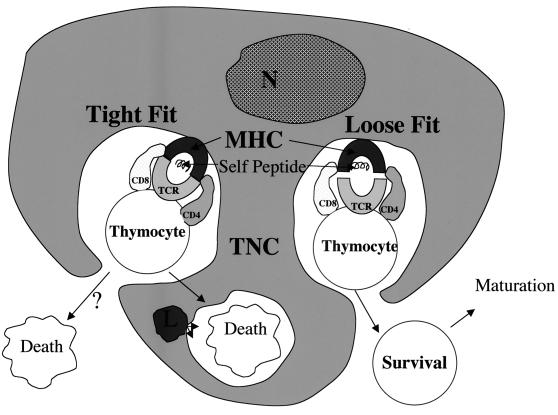
FIG. 2.
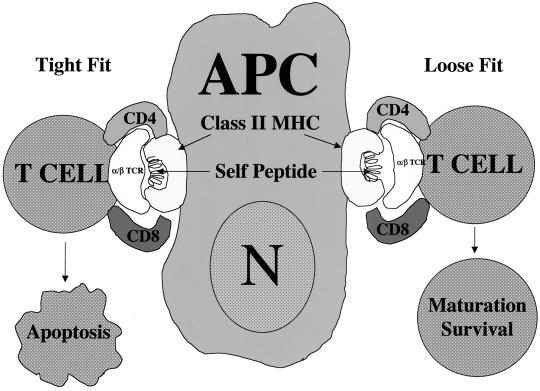
Schematic representation of MHC restriction during thymocyte development. MHC restriction, also referred to as thymic education, involves antigen-presenting cells (APC) and developing thymocytes. The antigen-presenting cells of the thymus are epithelial cells, macrophages, and dendritic cells. They express both class I and class II MHC antigens on their cell surface (only MHC class II antigen expression is shown in the figure). It is believed that self-antigens are uniquely combined with MHC antigens on the surface of antigen-presenting cells in a way that is recognizable by the αβTCR on developing T cells. The thymocytes that produce an αβTCR that binds tightly to self-peptide in the context of MHC on antigen-presenting cells are induced to undergo apoptosis because they are potentially autoreactive. The developing T cells that bind the MHC–self-peptide complex with low affinity are allowed to continue through the developmental pathway to maturity.
STRUCTURE OF TNCS
TNCs were discovered in mice by Wekerle and Ketelson in 1980 (58, 59). The initial report described TNCs as keratin-expressing cells containing several thymocytes completely enclosed within specialized cytoplasmic vacuoles. The number of thymocytes enclosed was reported to vary from about 7 to 50 (Fig. 3). TNCs were also shown to express both class I and class II MHC antigens on their cell surface as well as on the surface of the vacuoles surrounding internalized thymocytes. The expression of membrane class II MHC antigens is atypical for epithelial cells. The expression of class II antigens is generally thought to be restricted to cells of the immune system. Typically, epithelial cells do not function within the immune system. Following their initial discovery in mice, TNCs were isolated from the thymus of fish, frogs, chickens, sheep, pigs, rats, and humans (5, 19, 44, 58, 59). In mice, TNCs express thymic cortex-specific markers (1, 55, 60) and are not recognized by medullary-specific monoclonal antibodies (MAbs), nor are they recognizable by MAbs specific to macrophages, fibroblasts, or thymocytes (55). Two basic questions were spawned from these initial reports. (i) Are these complexes actually formed by the uptake of one cell by another cell in vivo? (ii) What is their exact location within the thymus?
FIG. 3.
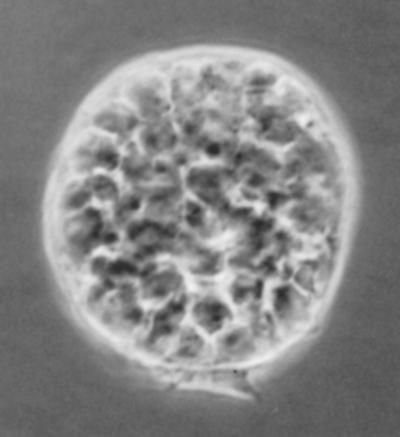
Light micrograph of a freshly isolated mouse TNC. TNCs have a unique morphology when recovered from the thymus. Several thymocytes are visible within the cytoplasm of each TNC. Its uniqueness caused many scientists to question whether the thymocytes were actually inside the epithelial cell or tightly bound to the cell surface. Magnification, ×200.
Micrographs of TNCs isolated from the thymus of all of the species mentioned above appeared to show thymocytes completely surrounded by membrane within the cytoplasm of a cell containing one large nucleus (Fig. 3) (43, 58, 59). However, it has been proposed that these structures result from incomplete enzymatic digestion of tightly bound cells during their isolation from the thymus (23, 52). This implies that the TNC complex develops from nonspecific wrapping of membrane fragments around tightly bound thymocytes in vitro rather than the in vivo membrane fusion event required for true vacuole formation within a cell. Although it is very difficult to show the intact structure of TNCs in vivo using frozen sections of the thymus, because of the very high density of cells within the thymic cortex, visualization of the entire membrane surrounding engulfed thymocytes has been presented. These experiments were done using human thymus tissue and staining with antibodies against keratins or MHC proteins (13, 44). Transmission electron microscopy (TEMs) of TNCs reveal a prominent nucleus within the cytoplasm containing enclosed thymocytes (Fig. 4). Cytoplasmic organelles, mitochondria, Golgi, and lysosomes have also been described within the membrane of TNCs (36). Other investigators used biochemical techniques to determine the integrity of this unusual structure (58). Extended treatment of TNCs with trypsin was used to determine the structural relationship between trapped thymocytes and TNCs. Extensive treatment with trypsin and collagenase does not dissociate the cells within the complex (M. Pezzano and J. Guyden, unpublished data). Further, the thymocyte subset within TNCs is inaccessible to thymocyte-specific antibodies before fixing and permeabilization with detergent or acetone (26, 58). Additional support for cytoplasmic enclosure of thymocytes by TNCs was provided with reports of the development of TNC lines (21, 31–33, 38, 42). The generation of TNC lines was important because prior to their development, the formation of this unique multicellular complex in culture had not been reported. Freshly isolated TNCs attach themselves to the bottom of tissue culture plates and release their enclosed thymocytes; however, the subsequent uptake of thymocytes by freshly isolated TNCs has not been reported. TNC cell lines were shown to be able to internalize thymocytes that were added separately to in vitro cultures. Data obtained from TEM and antibody-staining experiments have been presented to show multicellular complexes resulting from TNC internalization of added thymocytes. The internalization event has also been visualized using long-term video microscopy (42). In this presentation, internalization is defined as membrane sealed or complete separation from the extracellular environment.
FIG. 4.
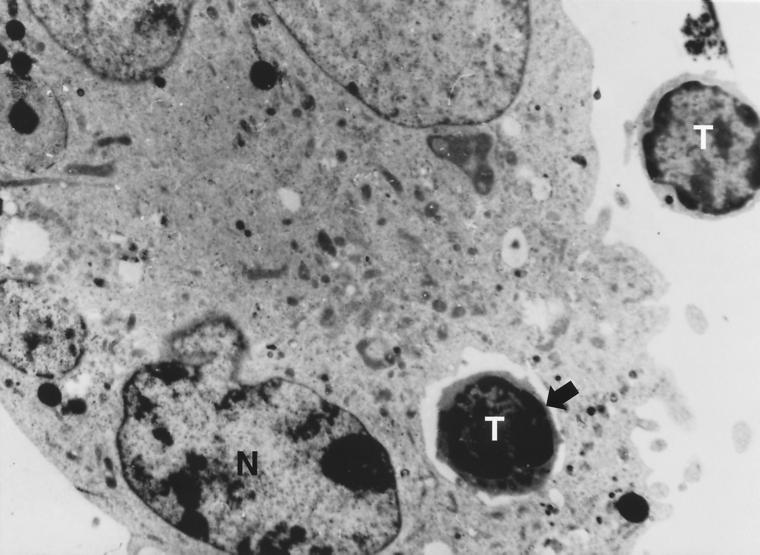
Transmission electron micrograph of an internalized thymocyte (42). Thymocytes were centrifuged onto monolayers of SVT-II2 cells (a TNC line) and incubated overnight before being prepared for examination. The micrograph shows one internalized thymocyte within the TNC. Cytoplasmic organelles are distinguishable. Another thymocyte can be seen outside the TNC cytoplasm. Magnification, 5,600.
The experiments reported above provided strong support for membrane separation between thymocytes inside of the TNC complex and those in the general thymic microenvironment. However, they do not define the nature of the resulting vacuole. These specialized structures could develop from tight membrane overlaps or from classical membrane fusion. Membrane fusion would produce vacuoles that are free to move within the cytoplasm. Vacuoles that result from overlapping membranes would not have free mobility because the membrane that generates their formation is contiguous with the plasma membrane. Overlapping membranes would create a seal that results in a tunnel-like structure along the surface of the plasma membrane rather than the “bag-within-a-bag” type structure of the more classical fused membrane vacuole. Although not definitive, more microscopic data exist to support the tunnel-like structure rather than the more classical vacuole. These data will be presented later in this review. As far as function is concerned, either vacuole type would suffice because both structures provide an isolated microenvironment for long-term contact and/or communication between enclosed thymocytes and thymic nurse cells.
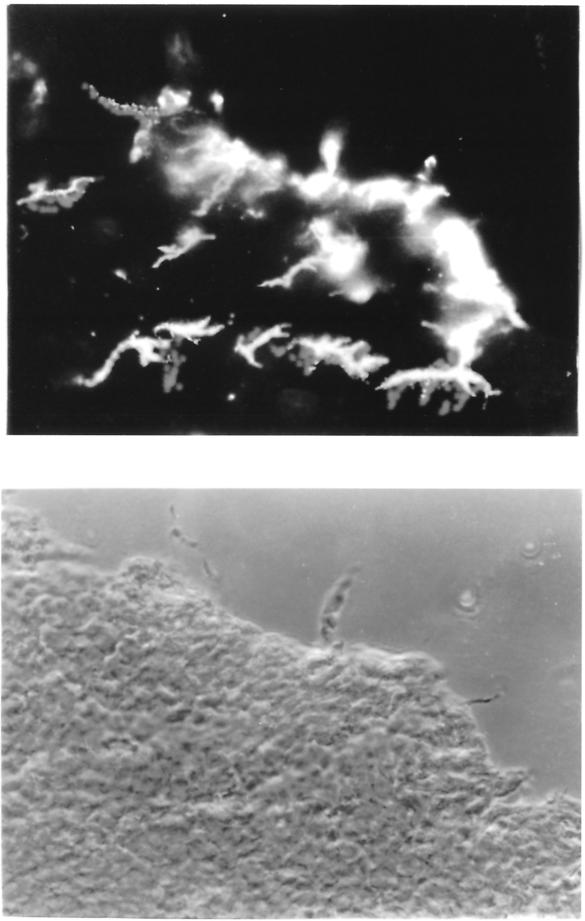
The second point of discussion, the location of TNCs within the thymus, was generated from a report by Kyewski and Kaplan (24). Although these studies defined TNCs as subcapsular cells, subsequent reports assigned their position to be more centrally located within the cortex (35). Finding their exact in vivo location is important because this information would aid in the determination of the subset of thymocytes that interacts with TNCs. Such information would subsequently shed light on TNC function during thymocyte development. To answer this question, Kyewski and Kaplan immersed the entire thymus in fluorescein isothiocyanate for increasing periods and analyzed the incorporation of fluorescein into TNC thymocytes versus free thymocytes. At each time point from 15 to 60 s, there was a higher percentage of fluoresceinated thymocytes associated with TNCs and an increase in the number of TNCs. They interpreted these results to demonstrate the presence of TNCs near the outer cortex of the thymus. These data never showed a comparative decrease in fluorescein uptake. The open-ended nature of these results did not define the limits of the compartment within the thymic cortex that house TNCs. However, in our laboratory, subsequent staining experiments using the TNC-specific monoclonal antibody ph91 revealed TNCs to be scattered throughout the cortex from the subcapsular region to the corticomedullary junction (Fig. 5) (41).
FIG. 5.
Immunofluorescence staining with MAb ph91 of mouse thymic sections (J. Guyden and M. Pezzano, unpublished data). (Top) Immunofluorescence microscopy; (bottom) phase microscopy. The TNC-specific MAb ph91 stains cells both in the subcapsular region and the region around the corticomedullary junction.
One way to localize TNCs within the thymus is to use an alternative approach, i.e., to define the thymocyte subset(s) enclosed within the cytoplasm of TNCs. Much has been reported to show that triple-positive thymocytes (αβTCR+ CD4+ CD8+) are more centrally located within the thymic cortex in mice while triple-negative thymocytes reside in the subcapsular region (35). Several coculture studies defined the TNC-internalized subset to be triple positive (20, 26, 32). However, conflicting evidence exists. Some reports show the TNC-interactive subset to have the triple-negative phenotype (18, 21). One TNC clone, TNCR3.1, establishes multicellular complexes in vitro with triple-negative thymocytes and supports growth and maturation of its interactive subset to the triple-positive window of development through the CD3− CD4+ intermediate pathway. Similarly, IT-79MTNC3, another mouse TNC clone, supports the growth and maturation of its interactive subset from the triple-negative window of development to the CD3− CD4+ intermediate stage. Internalization of thymocytes was not detected in IT-79MTNC3 cocultures. Support for the triple-positive phenotype of TNC thymocytes has been obtained through analysis of thymocytes enclosed within freshly isolated TNCs (15, 24). Such conflicting data create difficulty in assigning an exact thymic location for TNCs. Moreover, these opposing results initiated further controversy. It should be stated, however, that until TNCs containing triple-negative thymocytes are found in freshly isolated preparations, the development of epithelial cell lines that interact with triple-negative thymocytes may represent a thymic stromal cell population that is distinct from TNCs.
It must be stated that the studies described above were performed using mammalian cells. The cell surface phenotype of the thymocyte population within TNCs isolated from other classes varies significantly from those found in mice, rats, and humans.
THYMOCYTE INTERNALIZATION BY TNCS
The internalization of thymocytes by TNCs was captured through the addition of freshly isolated thymocytes to cultures containing cells derived from the immortalization of TNCs (20, 21, 32, 37, 42) (Fig. 6). Prior to those reports, it was difficult to determine the authenticity of these cell lines because no TNC-specific antigens had been defined. It should be made clear that at that time, the only TNC-specific characteristic observed was their ability to engulf immature thymocytes. In 1984 Jason and Janeway (22) produced short-term cultures of thymic epithelial cells with characteristics similar to TNCs. These cells were maintained in culture for approximately 6 weeks before fibroblast overgrowth occurred. Although some of the cells in their cultures were able to engulf thymocytes, they were not termed TNCs. Similarly, Nakashima et al. isolated a long-term thymic epithelial line (TEL-2) from BALB/c mice that form TNC-like complexes on incubation with normal mouse thymocytes (32). The cells of this line expressed none of the T-cell-specific antigens and were Mac-1−. TEL-2 cells express the 6C3 antigen, a cortical marker, and were MHC class I positive. Cell surface MHC class II antigen was not detectable. Again, these cells were not referred to as TNCs. However, recently three long-term lines were established and characterized as thymic nurse cells (21, 32, 38). Itoh et al. isolated an epithelial cell clone (IT-79MTNC3 [mentioned above]) from a spontaneous thymic tumor in a BALB/c mouse (21). These cells express Ia antigens only in the presence of gamma interferon. The nature of class I MHC antigen expression in these cells was not presented. IT-79MTNC3 cells support the proliferation of fetal thymocytes in the presence of recombinant interleukin-2 (rIL-2), IT-79MTNC3 cells develop complexes with thymocytes that are similar to those of freshly isolated TNCs. TEM studies showed thymocytes to be tightly bound to the surface of IT-79MTNC3 cells, but internalized thymocytes were not detected. Cells from another nurse cell line, termed B/c TEC-L1 (36), were shown to take up PNA+ lymphocytes in culture. PNA is expressed exclusively on triple-positive thymocytes (42). Complete internalization of thymocytes by B/c TEC-L1 cells was not described. Another TNC line was developed from the infection of freshly isolated TNCs (from C57BL/6 mice) with simian virus 40 (SV40) (38). Cells of two identical clones (SVT-MP5 and SVT-II2) were shown to internalize αβTCRlo CD4+ CD8+ thymocytes exclusively in vitro (38). Cells from both cell lines expressed cytokeratins and class I and class II MHC antigens. The long-term cultures of tsTNC-1 cells, another TNC line generated through SV40 transformation with freshly isolated thymocytes, resulted in the maturation of αβTCRlo CD69− triple positives into αβTCRhi CD69hi cells (39). SV40-derived TNC lines were also shown to express the neuroendocrine marker A2B5, which was previously shown to be a characteristic of freshly isolated TNCs. A2B5 colocalizes in TNCs along with the neuropeptides oxytocin, arginine-vasopressin, and their associated neurophysins (17, 38).
FIG. 6.
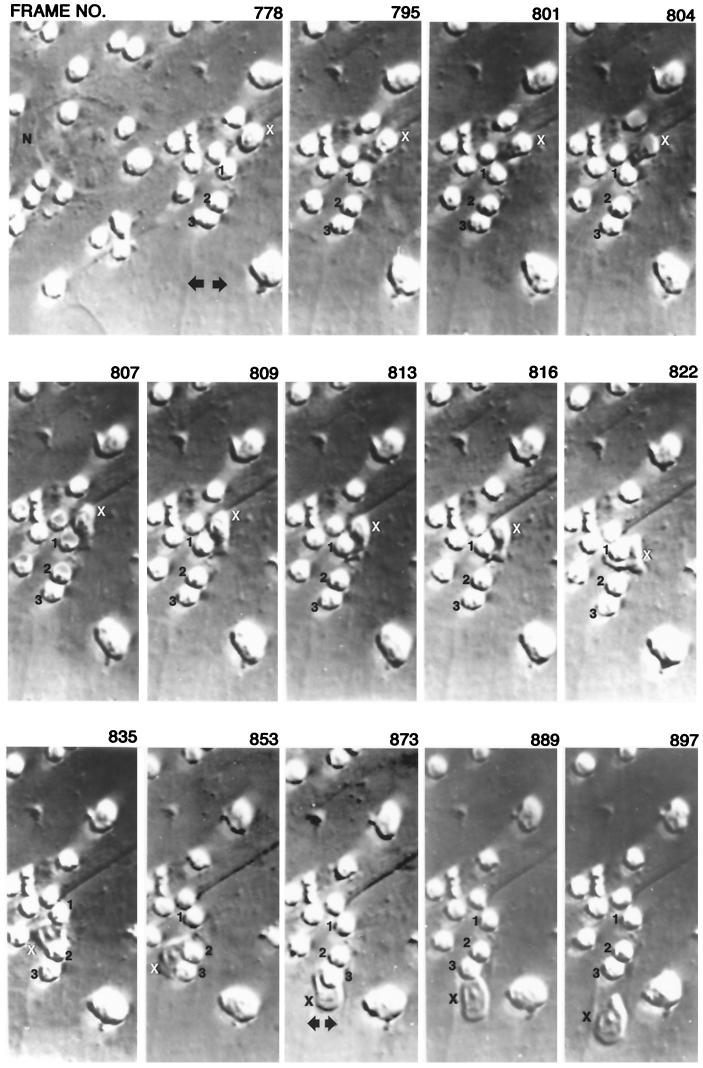
Dynamics of thymocyte internalization (42). Freshly isolated thymocytes were added to monolayers of SVT-II2 cells and incubated overnight. Unattached thymocytes were removed by washing, and the resulting cultures were observed using a Nikon Diaphat inverted microscope with Hoffman modulation contrast system. The microscope was attached to a Nikon CCD-72 camera. The samples were visualized on a Sony 19-in. color monitor coupled to a JVC 0.5-in. VCR. The thymocytes labeled 1, 2, and 3, are bound to the external surface of a TNC. The nucleus of the TNC is labeled N. The thymocyte labeled X is internalized in the series of micrographs. The arrows show a cytoplasmic channel that forms before the initiation of internalization. The thymic nurse cell cytoplasm extends beyond the frame of the photograph.
Long-term video microscopy was used to document the internalization event in SVT-II2 cells, one of the SV40-derived lines (42). Monolayers of SVT-II2 cells were exposed to freshly isolated thymocytes. Unbound thymocytes were removed, and the remaining cells were visualized over time. Immediately before an internalization event, membrane channels were detected within the TNC cytoplasm (Fig. 6). A thymocyte was marked and monitored throughout the internalization process. The marked thymocyte moved into one of the preformed channels beneath the surface of the TNC cytoplasmic membrane. It moved over 50 μm within the TNC membrane. These data suggested a cooperative mechanism between the two cell types that resulted in the movement of selected thymocytes from the cell surface into specialized structures within the TNC cytoplasm. Scanning electron microscopy studies showed the initial step of the internalization process to involve the production of fingerlike projections by the bound thymocytes that attach to the TNC cell surface.
Within an hour, membrane ruffling was detected. A wave of TNC membrane covered bound thymocytes and then reattached to complete the event. Fusion points were illustrated, but it was difficult to verify their authenticity using micrographic data. Some thymocytes were selectively excluded from the internalization event (Fig. 7). TEM of the multicellular complex that resulted from thymocyte uptake produced images identical to those detected in freshly isolated TNCs (32, 42). That is, thymocytes appeared to be isolated within membrane vacuoles. Some vacuoles were shown to contain more than one thymocyte. Movement of thymocytes around one another within one vacuole, along with unidirectional movement of several thymocytes within a single vacuole, was also documented. TEM performed by Philp et al. showed TNC membrane completely surrounding internalized thymocytes (42). In some studies, membrane thickening and unique “bridge-like” structures at contact points between TNCs and internalized thymocytes were described. These bridge-like structures are indicative of communicative apparatuses between cells (42, 58).
FIG. 7.
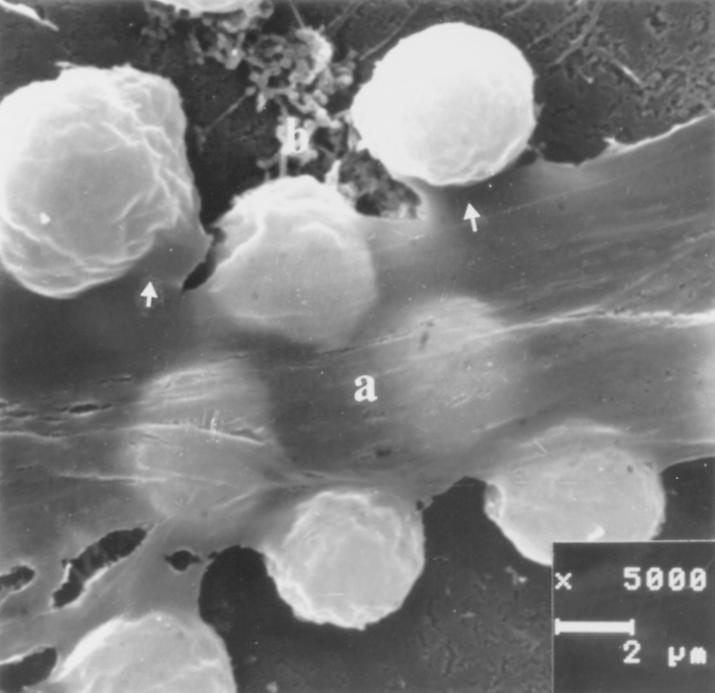
Scanning electron micrograph of thymocyte internalization (42). Thymocytes were centrifuged onto monolayers of SVT-II2 cells and prepared for examination after an incubation period of 4 h. Arrows show bound thymocytes that are selectively excluded from the internalization process. The TNC membrane above trapped thymocytes is labeled a, and the TNC beneath trapped thymocytes is labeled b.
Membrane fusion after thymocyte trapping is essential for classical vacuole formation. Postfusion vacuoles should have the ability to move within the TNC cytoplasm. If, however, the structure surrounding enclosed thymocytes was formed from a tight or close association of the two membrane surfaces without fusion, the vacuole-like compartment would be prevented from movement away from the surface of the plasma membrane. The data presented above support the formation of the attached, nonfused structure. TEM would not detect the lack of authentic vacuole formation without serial sectioning through the entire structure. Micrographs of sections taken above or below the point of attachment between the two membrane surfaces would produce images identical to authentic vacuoles. The scanning electron microscopy data described above show a wave of moving membrane, resembling waves that form in the ocean. On reattachment of the membrane, the enclosed thymocyte could move within the fold created by the overlapping membrane. This would account for the formation of channels or tunnels detected using long-term video microscopy (Fig. 6). The production of such a structure would also explain the long-distance movement described for internalized thymocytes and the existence of multiple thymocytes within one membrane compartment. These data show a unique relationship between the cells of this complex. Although debate concerning membrane fusion may continue, the experiments described above suggest that enclosed thymocytes are selectively and completely separated from surface-bound or unbound thymocyte populations.
Existing data define the expression of both class I and class II MHC antigens on the vacuole surface surrounding engulfed thymocytes of freshly isolated TNCs from mice and humans (12, 36, 58). Intercellular cell adhesion molecule 1 (ICAM-1) was also found on the TNC cell surface as well as on the specialized vacuoles surrounding enclosed thymocytes (8). The extracellular matrix proteins fibronectin, laminin, and type IV collagen have been reported to be constitutively produced by TNCs but were not localized within the vacuoles containing thymocytes (56). An unresolved molecular question pertains to the adhesion molecules responsible for the binding of thymocytes to TNCs. Many adhesion proteins and/or ligand complexes (e.g., Thy-1, neural cell adhesion molecule [NCAM], CD2/LFA-3, and LFA-1/ICAM-1) participate in the attachment of immature thymocytes to thymic epithelial cells (6, 9). An LFA-1-dependent interaction on thymic epithelial cells (not defined as TNCs) is restricted to a subpopulation of CD4+ CD8+ CD3lo thymocytes (25). Couture et al. reported another thymic epithelial cell molecule that also selectively binds triple-positive cells (9). This adhesion molecule contains two noncovalently associated glycoproteins with molecular masses of 23 and 45 kD. The subset of triple positives bound by this complex were reported to be precursors of mature single positives. However, this adhesion molecule appears to be specific to epithelial cells of the medulla, which suggested that a subset of αβTCR+ CD4+ CD8+ thymocytes that have undergone positive selection maintain their triple-positive phenotype until they interact with cells of the medulla. This is consistent with other reports showing that the switch to the mature T-cell phenotype occurs in the medulla (34) and not in the cortex, wherein TNCs reside.
None of the cell surface molecules described above have been definitively shown to facilitate the TNC-thymocyte interaction. Furthermore, inhibition of the binding of triple-positive thymocytes to TNCs was not detected using antibodies to CD4, CD8, CD3, αβTCR, MHC class I and II antigens, CD44, or CD45 (26). A partial inhibition was reported in the presence of anti-Ia antibody (11). In unpublished data from our laboratory, antibodies made against CD2, CD5, CD25, LFA-3, LFA-1, ICAM, and Thy-1 did not block or reduce TNC binding of thymocytes. Also, neither PNA nor antibodies against PNA inhibited the interaction. However, our laboratory has generated MAbs that are specific to the cell surface of TNCs (41). The MAb ph91 was shown to exclusively bind the surface of TNCs in vivo and the TNC line tsTNC-1 in culture. ph91 also recognized the multicellular complex that defines TNCs in the thymus. This MAb identified stromal cells in the thymic cortex but did not recognize cells of the medulla. ph91 also showed no specificity for T cells, developing thymocytes, or B cells. In tissue culture, preexposure of tsTNC-1 cells to ph91 significantly reduced the binding of the TNC-specific thymocyte subset (αβTCRlo CD4+ CD8+), as well as its subsequent internalization. In fetal organ culture, ph91 caused a 70% reduction of thymocyte viability. These data suggest that the protein (protein complex) recognized by ph91 participates in the binding interaction between thymocytes and TNCs and has an important function in the process of thymocyte development during the stage at which MHC restriction occurs.
IMMUNOLOGICAL FUNCTION OF THE THYMOCYTE-TNC INTERACTION
TNCs have the capacity to present antigen (20, 53). These results suggest that TNCs may play a role in MHC restriction. Information obtained from studies with chickens revealed that TNC thymocytes have the ability to recognize self-MHC (35). The authors proposed that thymocytes producing self reactive TCR are internalized to form the TNC complex. They also proposed that the internalization of these thymocytes would allow direct contact with numerous self-peptide–MHC complexes. It would then be possible for the long-term interactions that facilitate positive or negative selection to occur depending on the TCR binding affinity. Experiments designed to test hypothetical models for the mechanism used by TNCs to facilitate this selective process have so far been inconclusive (26).
Again, it must be stated that the studies described above were performed with chickens, which may have a significantly different pathway to the development of T cells than that within mammalian systems. However, Lorenz and Allen showed that TNCs isolated from mice also have the ability to present antigen (28, 29). The antigen-presenting capability of TNCs was shown to be deficient compared to that of macrophages or dendritic cells. This deficiency was overcome in the presence of IL-1β. Initially, these data were viewed with some skepticism because mature T-cell clones were used to perform the experiments. The data were supported in studies using freshly isolated thymocytes in coculture with TNC clones (39). Triple-positive thymocytes were rescued from apoptosis (a subset lived for over 4 days in coculture) in the presence of TNCs but maintained their immature phenotype in the absence of IL-1β. However, this rescued population matured to the αβTCRhi CD69+ phenotype when IL-1β was added to the cultures. The remaining thymocytes underwent apoptosis.
Collectively, these data support the involvement of TNCs in the process of MHC restriction, mainly because the TNC-interactive thymocyte population is the subset that participates in MHC restriction. However, controversy about the phenotype of TNC thymocytes remains. For instance, IT-79MTNC3 cells bind triple-negative thymocytes (21). This controversy can be settled (as far as IT-79MTNC3 cells are concerned) if we redefine IT-79MTNC3 cells as a cortical epithelial cell type distinct from that of TNCs. IT-79MTNC3 cells were shown not to have the capacity to internalize thymocytes, and their cell surface phenotype was inadequately described to determine their identity. To definitively show that TNCs participate in MHC restriction, one needs to demonstrate that TNC-thymocytes shift from the triple-positive phenotype to that of single positives. This has not been reported. However, as mentioned above, studies using clones of thymic nurse cells, which maintain the ability to selectively internalize immature αβTCRlo CD4+ CD8+ thymocytes in vitro, were used in long-term coincubation experiments to determine nurse cell function during the process of MHC restriction. The results of long-term coincubations produced apoptotic thymocytes and a subset of triple-positive thymocytes that were rescued from apoptosis (40). The thymocyte subset released from its association with TNCs contained both viable and apoptotic cells. The cells that remained within intracytoplasmic vacuoles died through the process of programmed cell death. The rescue activity of TNCs was drastically reduced with the addition of antibodies against either class I or class II MHC antigens to cocultures. A subset of the TNC-rescued population matured from the αβTCRlo CD69− phenotype to αβTCRhi CD69+-expressing cells in the presence of IL-1β. These results suggested that TNC rescue of early triple-positive thymocytes from apoptosis was associated with an interaction between the TCR and the MHC. Also, the shift from the αβTCRlo CD69− phenotype to αβTCRhi CD69+-expressing cells has been reported to be an early step in positive selection (40, 51). Long-term cocultures of freshly isolate αβTCRlo triple-positive thymocytes with TNCs resulted in high expression of the αβTCR and CD69, but single-positive thymocytes remained undetectable (40). The results obtained from these experiments suggested that TNCs may provide early signals required for thymocyte rescue and maturation within the triple-positive stage of development but that complete maturation occurs elsewhere. Again, these data are consistent with reports which propose that the switch to the mature single-positive window of development does not occur until thymocytes enter the medulla.
Evidence of thymocyte release from TNC intracytoplasmic compartments has been presented (42). Long-term video microscopy was used to capture the release of selected thymocytes. The percent release was not determined in that study, nor was their cell surface phenotype. Propidium iodide studies revealed that 4 to 11% of internalized thymocytes were deleted in SVT-II2 cultures, while the majority of the internalized thymocyte population remained viable. These reports indicate that TNCs may participate in the removal of thymocytes from the developmental process. If this interpretation is correct, it is then reasonable to propose that released thymocytes, which express αβTCRhi CD69hi, have been selected to continue through the developmental process. However, conclusive data have not yet been reported. The induction of negative selection by TNCs as well as other thymic cortical epithelial cells contradicts many reports that propose positive selection to be an exclusive function of cortical cells while negative selection is limited to the medulla (27).
Although much evidence has been reported to show an involvement of TNCs in MHC restriction, one report presented drastically different findings (3). The data presented suggested that cell surface expression of the αβTCR is not required for TNC formation. SCID mice, which do not express an αβTCR, have normal numbers of TNCs. In another experiment from that study, anti-CD3ɛ antibody was injected into BALB/C mice (2). Anti-CD3ɛ induces apoptosis in immature thymocytes. Mice receiving anti-CD3ɛ MAb showed an eightfold increase in TNC numbers per mouse thymus. The authors of this report proposed TNC function to be the clearance of nonfunctional, nonselected apoptotic thymocytes. Although many of the data presented in that study are difficult to reconcile with the bulk of the information described above, the authors suggested that thymocyte selection could occur on the outer surface of TNCs. This would allow positively selected thymocytes to continue their maturation while negatively selected cells would become internalized. This suggestion is consistent with studies that showed a subset of the TNC-bound thymocyte population to be selectively excluded from the internalization process (42). Assuming this explanation is correct, it will be important to define the compartment(s) from which TNC-thymocytes are released.
APOPTOSIS AND DESTRUCTION OF TNC-THYMOCYTES
Viable and apoptotic thymocytes have been detected within TNC vacuoles (4, 20, 39). Initial studies of apoptosis were performed using propidium iodide, DNA fragmentation ladders, or microscopic phenotypic characteristics (chromatin condensation) as markers for death (4). More recent experiments exploited the TUNEL assay, which results in terminal deoxynucleotidyl transferase-mediated labeling of DNA with numerous strand breaks as an indication of apoptosis. When the internalized population was examined using the TUNEL assay, apoptotic cells were detectable (39). There was a vast difference in the number of apoptotic thymocytes per TNC when freshly isolated thymocytes were examined; however, both apoptotic and nonapoptotic thymocytes were visible within each TNC (47). These data were consistent with earlier reports which suggested that TNCs have the ability to maintain or increase the viability of a subset of its interactive thymocyte population while selecting another subset to die through the process of programmed cell death (39). Studies of thymocyte apoptosis done in vitro supplied an added dimension to these investigations. With increasing time in coculture of freshly isolated thymocytes with TNC clones, apoptotic thymocytes within the specialized TNC vacuoles began to lyse until the structure of intact cells was no longer discernible. DNA fragments released from lysed thymocytes were then detected within the cytoplasm of the TNC, indicating total destruction of enclosed cells. A subsequent report using confocal microscopy showed degradation of thymocytes to result from the fusion of the specialized vacuoles containing apoptotic thymocytes with lysosomes (Fig. 8) (47). These findings may be correlated with the role of TNCs in negative selection.
FIG. 8.
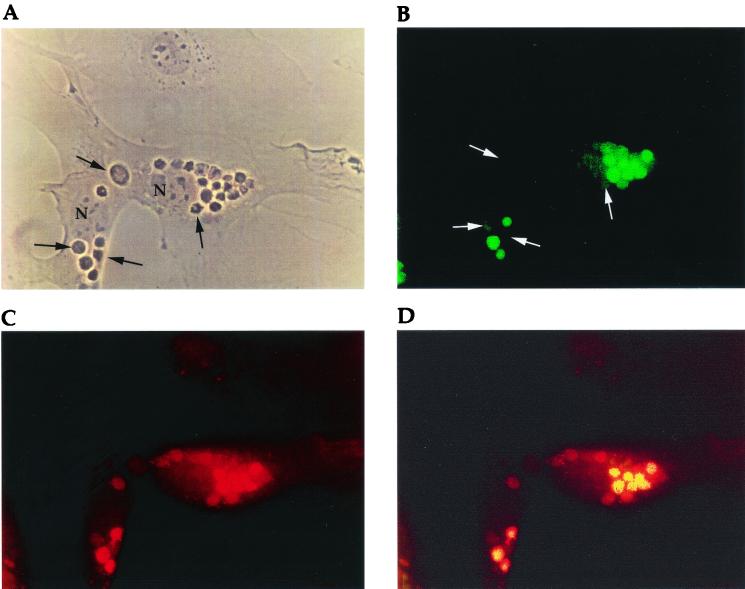
Confocal microscopic analysis of internalized thymocytes (47). Internalized thymocytes were incubated for 6 h before analysis. (A) Phase micrograph of TNCs containing internalized thymocytes. (B) TUNEL (fluorescein isothiocyanate) analysis of cytoplasmic thymocytes shown in panel A. (C) The TNCs shown in panel A stained with LAMP-1 antibody (tetramethylrhodamine isothiocyanate) to determine lysosome distribution. (D) Superimposed images of A and B. The yellow color indicates colocalization of lysosomes and apoptotic cells. Arrows indicate nonapoptotic thymocytes. N indicates nuclei of TNCs.
FUTURE STUDIES OF TNC FUNCTION
It should be obvious that much work has to be done before the precise role of TNCs in T-cell development is defined (Fig. 9). Many of the data described above suggest that TNCs function early during the MHC restriction process. These results described a TNC-interactive thymocyte population that is either induced to die or allowed to continue along the differentiation pathway (Fig. 10). The participation of triple-positive thymocytes in the formation of the TNC complex, evidence of MHC-TCR involvement in rescue from apoptosis or maturation, and the selective release or destruction of enclosed thymocytes support this contention. Whatever their function, the ability of TNCs to selectively engulf another cell type, alone, is a very unique and exciting biological phenomenon. Movement of thymocytes within the thymus is very important to the developmental process, yet little attention has been paid to this area of study. Future investigations of thymocyte movement toward, along the surface of, within, and/or out of TNCs should provide interesting information about T-cell development. Data collected from such studies should aid in understanding the developmental function of each maneuver. For instance, what is the difference between the triple-positive thymocytes that bind TNCs and those that do not bind? What is the difference between the population of bound thymocytes that are selectively internalized and those that are excluded from this process? As stated above, some studies report that all engulfed thymocytes are induced to die. If this is indeed the case, the thymocyte selection process reported to occur within TNCs may be restricted to the cell surface.
FIG. 9.
(Top) T-cell developmental pathway. The basic T-cell developmental pathway involves the expression pattern of CD4, CD8, and αβTCR. The thymocytes move from triple-negative cells to triple-positive cells, where they undergo MHC restriction. Thymocytes either die through the process of apoptosis or began to express intermediate and then high levels of the αβTCR and survive the MHC restriction process. The thymocyte subset that survives MHC restriction is allowed to mature to the single-positive stage of development. (Bottom) The same pathway shown above, with the proposed role of TNC during T-cell development. Collectively, the data presented in this review suggest that TNCs play a role in MHC restriction because they interact with triple-positive thymocytes. The TNC-interactive thymocyte subset expresses low levels of αβTCR and CD69. The increased expression of these cell surface antigens is an early indication of thymocyte survival and maturation through this stage of development. One report showed Bcl-2 activation to be associated with the thymocyte interaction with TNCs (39). It was shown that Bcl-2 expression does not guarantee survival to the single-positive stage. The remaining thymocytes are induced to die through the process of apoptosis. The process of apoptosis begins during the triple-positive stage of development and continues throughout thymic development, as represented by the hatched bar.
FIG. 10.
Proposed mechanism of TNC Function. TNCs selectively internalize immature αβTCRlo CD4+ CD8+ thymocytes. A subset of the TNC-interactive population matures from the αβTCRlo CD69− phenotype to αβTCRhi CD69+-expressing cells. Theoretically, the increased expression of the αβTCR and CD69 results from a low-affinity interaction between the TCR and MHC–self-peptide complex. Bcl-2 expression is turned on in these thymocytes but does not ensure their survival. Maturation of thymocytes within the TNC-rescued population requires the costimulatory effects of IL-1β. Thymocytes that have increased expression of αβTCR and CD69 also reduce cell surface PNA expression and are believed to be released from TNCs to continue the T-cell developmental pathway. High-affinity interactions within TNCs result in thymocyte apoptosis and destruction via lysosomes.
Future studies should reveal the complete molecular nature of the TNC-thymocyte interaction. It is important that both the receptors and ligands be identified. This interaction is key to understanding the involvement of TNCs in T-cell development. We should exhaustively describe the cell surface phenotype of thymocytes released from the TNC interaction. One way to obtain these data is the use of antigen-specific transgenic mice. With these animals, we should be able to pinpoint TNC involvement during MHC restriction. It should be fairly easy to determine developmental anomalies using fluorescence-activated cell sorter profiles of thymocytes isolated from αβTCR transgenic animals that have had their TNCs removed. TNCs can be deleted from the thymus in organ culture and in vivo using TNC-specific antibody treatment. Another approach would be to develop TNC lines that are specific to the αβTCR transgene for in vitro experiments. TNC lines that express an antigen recognized by an αβTCR would provide a system specifically designed to examine the role of TNCs during MHC restriction. Once such lines are developed, thymocytes from the transgenic animal can be easily isolated and exposed to the TNC clones in culture. Such a system would allow the determination of the fate of large numbers of the targeted thymocyte population during and after their interaction with TNCs.
We have recently proposed an interesting and easy set of experiments using the H-Y transgenic model (23, 54). The H-Y transgenic-mouse model was developed for in vivo study of MHC restriction. Each developing thymocyte in the transgenic animal produces a cell surface αβTCR that recognizes the male-specific H-Y antigen. In male animals having the selecting MHC background, the vast majority of male transgenic thymocytes are deleted through negative selection, while in female transgenic animals, above-normal positive selection (as determined by the increased percentage of the H-Y-specific single-positive subset) is detected. A simple experiment would be to quantitate the number of TNCs in male and in female H-Y transgenic mice. If TNC internalization is critical to MHC restriction, the female transgenic animals should have a large number of TNCs containing primarily viable thymocytes. Since female animals do not express the H-Y-antigen, virtually all of the thymocytes should receive the appropriate “low-affinity” selection signal through their transgenic TCR, necessary for positive selection. The male H-Y transgenic animals, which have the majority of their triple-positive thymocytes deleted, should have small numbers of TNCs, with the majority of internalized thymocytes being apoptotic. Alternatively, these animals may possess the normal complement of TNCs but virtually all of the thymocytes within them would be apoptotic. We expect to see small numbers of TNCs in male transgenic mice because the triple-positive subset of thymocytes known to interact with TNCs is drastically reduced in these animals. The thymocytes that make it to the triple-positive stage should produce a high-affinity interaction with TNCs and become apoptotic. A simple analysis of H-Y transgenic-mouse TNCs and their interactive thymocyte subset should soon reveal important information about TNC function.
Another area that should be further studied is the seemingly redundant role played by TNCs and macrophages. Thymocyte apoptosis and destruction within TNCs is not in agreement with results reported by Surh and Sprent (50), who showed most if not all thymocyte apoptosis to be associated with macrophages. Interestingly, Ezaki et al. found that in rats 15 to 30% of TNCs have macrophages enclosed within their cytoplasm (15). Unpublished data from our laboratory obtained using long-term video microscopy, showed the movement of macrophages into and out of TNCs in a dynamic fashion. These results leave certain issues unresolved. First, if such a dynamic interaction occurs between TNCs and macrophages, it is going to be difficult to determine which cell is ultimately responsible for the induction of apoptosis within this multicellular complex. On the other hand, such an association may provide interesting possibilities, as well as offering clues to answer questions generated from contradictory results. For example, as stated above, Surh and Sprent presented evidence to suggest that the site of apoptosis may be exclusively associated with macrophages (50). Apoptotic thymocytes were always colocalized with antibody staining specific to macrophages. However, if macrophages enclosed in TNCs cooperate in the induction of apoptosis in negative selection of thymocytes, this event could be located in the cytoplasm of TNCs. Future studies in this area will be important to further understand the relationship between TNCs, macrophages, and thymocyte apoptosis.
On the other hand, we may be looking under the lamppost to find something that was lost in the dark. Some investigators believe that the expression of neuropeptides (oxytocin and vasopressin) by TNCs (17) qualifies them to serve a neurohormonal function in the thymus. The fact that this report and others showing neuropeptide expression by TNCs (45, 46) have been essentially ignored may reflect our lack of knowledge about neurohormonal regulation within the thymus or the interrelationship between neuropeptide production and the immune system. However, if the experiments proposed above fail to shed light on TNC function, we may be forced to begin our search in the darkness of thymic neuroendocrinology.
ACKNOWLEDGMENTS
We thank Gregory Saunders for his technical aid.
This work was supported by NSF grant MCB-9602001 and NIH-RCMI grant G12RR-A103060. Michael Samms was supported by the NIH MBRS grant GM08168
REFERENCES
- 1.Adkins B, Tidmarsh G F, Wiessman I L. Normal thymic cortical epithelial cells developmentally regulate the expression of a β-lineage transformation-associated antigen. Immunogenetics. 1988;27:180–186. doi: 10.1007/BF00346584. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar L K, Agilar-Cordova E, Cartwright J, Jr, Belmont J W. Thymic nurse cells are sites of thymocyte apoptosis. J Immunol. 1994;152:2645–2651. [PubMed] [Google Scholar]
- 3.Andrew P, Boyd R L. The murine thymic nurse cell: an isolated thymic environment. Eur J Immunol. 1985;15:36–39. doi: 10.1002/eji.1830150108. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac A, Matzinger P, Seder R A, Paul W E, Schwartz R H. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd R, Oberhuber G, Hala K, Wick G. Obese strain (OS) chickens with spontaneous autoimmune thyroiditis have a deficiency in thymic nurse cells. J Immunol. 1984;132:718–724. [PubMed] [Google Scholar]
- 6.Brunet J F, Hirsh M P, Naquet P, Uberla K, Diamantstein T, Lipinski M, Goridis C. Developmentally regulated expression of the neural cell adhesion molecule (NCAM) by mouse thymocytes. Eur J Immunol. 1989;19:837–841. doi: 10.1002/eji.1830190509. [DOI] [PubMed] [Google Scholar]
- 7.Ceredig R, Dialynas D P, Fitch F W, Macdonald H R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency and quantitative recovery on a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983;158:1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordes U, Pedersen M, Bastholm L, Nielsen M, Werdelin O. Murine thymic nurse cells express ICAM-1 on caveolar and vacuolar membranes. Scand J Immunol. 1997;46:344–348. doi: 10.1046/j.1365-3083.1997.d01-134.x. [DOI] [PubMed] [Google Scholar]
- 9.Couture C, Patel P C, Potworowski E F. A novel thymic epithelial adhesion molecule. Eur J Immunol. 1990;20:2769–2763. doi: 10.1002/eji.1830201235. [DOI] [PubMed] [Google Scholar]
- 10.Crouse D A, Turpen J B, Sharp J G. Thymic non-lymphoid cells. Surv Immunol Res. 1985;4:120–134. doi: 10.1007/BF02918808. [DOI] [PubMed] [Google Scholar]
- 11.Defresne M P, Humblet C, Rongy A M, Greimers R, Boniver J. Effects of interferon and tumor necrosis factor-alpha on lymphoepithelial interactions within thymic nurse cells. Eur J Immunol. 1990;20:429–432. doi: 10.1002/eji.1830200229. [DOI] [PubMed] [Google Scholar]
- 12.De Waal Malefijt R, Leene W, Roholl P J M, Wormmeester J, Hoeben K A. T cell differentiation within thymic nurse cells. Lab Investig. 1986;55:25–34. [PubMed] [Google Scholar]
- 13.Dipasquale B, Tridente G. Immunohistochemical characterization of nurse cells in normal human thymus. Histochemistry. 1991;96:499–503. doi: 10.1007/BF00267075. [DOI] [PubMed] [Google Scholar]
- 14.Ewijk W V, Soest P L, van Engh G J. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subset defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, and T-200. J Immunol. 1981;127:2594–2604. [PubMed] [Google Scholar]
- 15.Ezaki T, Matsuno K, Kotani M. Thymic nurse cells (TNC) in spontaneous thymoma BUF/Mna rats as a model to study their role in T-cell development. Immunology. 1991;73:151–158. [PMC free article] [PubMed] [Google Scholar]
- 16.Fowlkes B J, Edison L, Mathieson B J, Chused T M. Early T lymphocytes. Differentiation in vivo of adult intrathymic precursor cells. J Exp Med. 1985;162:802–822. doi: 10.1084/jem.162.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geenen V, Defresne M, Robert F, Legros J, Franchimont P, Boniver J. The neurohormonal thymic microenvironment: immunocytochemical evidence that thymic nurse cells are neuroendocrine cells. Neuroendocrinology. 1988;47:365–368. doi: 10.1159/000124938. [DOI] [PubMed] [Google Scholar]
- 18.Goa X, Nishimura T, Takeuchi Y, Sudo T, Habu S. Thymic nurse cell clone supports the differentiation of CD4−8− thymocytes into CD4+8+ thymocytes in vitro. Immunol Lett. 1993;35:169–175. doi: 10.1016/0165-2478(93)90087-i. [DOI] [PubMed] [Google Scholar]
- 19.Hiramine C, Hojo K, Koseto M, Nakagawa T, Mukasa A. Establishment of a murine thymic epithelial cell line capable of inducing both thymic nurse cell formation and thymocyte apoptosis. Lab Investig. 1990;62:41–54. [PubMed] [Google Scholar]
- 20.Holtfreter H B, Cohen N. In vitro behavior of thymic nurse cell-like complexes from mechanically and enzymatically dissociated frog tadpole thymuses. Am J Anat. 1987;179:342–355. doi: 10.1002/aja.1001790405. [DOI] [PubMed] [Google Scholar]
- 21.Itoh T, Doi H, Chin S, Nishimura T, Kasahara S. Establishment of mouse thymic nurse cell clone from a spontaneous BALB/c thymic tumor. Eur J Immunol. 1988;18:821–824. doi: 10.1002/eji.1830180525. [DOI] [PubMed] [Google Scholar]
- 22.Jason J M, Janeway C A. In vitro cultivation of nonlymphoid thymic cells: morphological and immunological characterization. Clin Immunol Immunopathol. 1984;30:214–226. doi: 10.1016/0090-1229(84)90056-4. [DOI] [PubMed] [Google Scholar]
- 23.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;333:742–743. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 24.Kyewski B A, Kaplan H S. Lymphoepithelial interactions in the mouse thymus: phenotypic and kinetic on thymic nurse cells. J Immunol. 1982;128:2287–2294. [PubMed] [Google Scholar]
- 25.Lepesant H, Reggio H, Pierres M, Naquet P. Mouse thymic epithelial cell lines interact with and select a CD3low CD4+ CD8+ thymocyte subset through an LAF-1 dependent adhesion de-adhesion mechanism. Int Immunol. 1990;11:1021–1032. doi: 10.1093/intimm/2.11.1021. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Pezzano M, Philp D, Reid V, Guyden J. Thymic nurse cells exclusively bind and internalize CD4+ CD8+ thymocytes. Cell Immunol. 1992;140:495–506. doi: 10.1016/0008-8749(92)90214-a. [DOI] [PubMed] [Google Scholar]
- 27.Lo D, Reilly C R, Burkly L C, DeKoning J, Laufer T M, Glimcher L H. Thymic stromal cell specialization and the T-cell receptor repertoire. Immunol Res. 1997;16:3–14. doi: 10.1007/BF02786320. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz R G, Allen P M. Thymic cortical epithelial cells can present self-antigens in vivo. Nature. 1989;337:560–562. doi: 10.1038/337560a0. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz R G, Allen P M. Thymic cortical epithelial cells lack full capacity for antigen presentation. Nature. 1989;340:557–559. doi: 10.1038/340557a0. [DOI] [PubMed] [Google Scholar]
- 30.Marrack P, Kappler J. The T-cell repertoire for antigen and MHC. Immunol Today. 1988;9:308–315. doi: 10.1016/0167-5699(88)91324-2. [DOI] [PubMed] [Google Scholar]
- 31.McCormack J E, Wade T, Morales H, Kappler J, Marrack P. Analysis of class II MHC structure in thymic nurse cells. Cell Immunol. 1991;138:413–422. doi: 10.1016/0008-8749(91)90165-8. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima M, Mori K, Maeda K, Kishi H, Hirata K, Kawabuchi M, Watanabe T. Selective elimination of double-positive immature thymocytes by a thymic epithelial cell line. Eur J Immunol. 1990;20:47–53. doi: 10.1002/eji.1830200108. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura T, Takeuchi Y, Ichimura Y, Goa X, Akatsuka A, Tamaoki N, Yagita H, Okumura K, Habu S. Thymic stromal cell clone with nursing activity supports the growth and differentiation of murine CD4+8+ thymocytes in vitro. J Immunol. 1990;145:4012–4017. [PubMed] [Google Scholar]
- 34.Ohashi P S, Pircher H, Burki K, Zinkernagel R M, Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- 35.Penit C. In vitro thymocyte maturation. Budr labeling of cycling thymocytes and phenotypic analysis of their progeny support the single lineage model. J Immunol. 1986;137:2115–2121. [PubMed] [Google Scholar]
- 36.Penninger J, Rieker T, Roman N, Klima J, Salvenmoser W, Dietrich H, Stossel H, Wick G. Ultrastructural analysis of thymic nurse cell epithelium. Eur J Immunol. 1994;24:222–228. doi: 10.1002/eji.1830240135. [DOI] [PubMed] [Google Scholar]
- 37.Penninger J, Wick G. Thymic nurse cell lymphocytes react against self-major histocompatibility complex. Eur J Immunol. 1992;22:79–83. doi: 10.1002/eji.1830220113. [DOI] [PubMed] [Google Scholar]
- 38.Pezzano M, Li Y, Yang Y, Guyden J. The immortalization of thymic nurse cells by SV40 virus. Cell Immunol. 1991;133:434–445. doi: 10.1016/0008-8749(91)90116-s. [DOI] [PubMed] [Google Scholar]
- 39.Pezzano M, Philp D, Stephenson S, Li Y, Reid V, Maitta R, Guyden J C. Positive selection by thymic nurse cells requires IL-1 beta and is associated with an increased Bcl-2 expression. Cell Immunol. 1996;169:174–184. doi: 10.1006/cimm.1996.0108. [DOI] [PubMed] [Google Scholar]
- 40.Pezzano M, Li Y, Philp D, Omene C, Cantey M, Saunders G, Guyden J C. Thymic nurse cell rescue of early CD4+ CD8+ thymocytes from apoptosis. Cell Mol Biol. 1995;41:1099–1111. [PubMed] [Google Scholar]
- 41.Pezzano M, King K D, Philp D D, Adeyemi A, Gardiner B, Yang J, Samms M, Boto W, Guyden J C. A thymic nurse cell-specific monoclonal antibody. Cell Immunol. 1998;185:123–133. doi: 10.1006/cimm.1998.1279. [DOI] [PubMed] [Google Scholar]
- 42.Philp D, Pezzano M, Li Y, Omene C, Boto W, Guyden J C. The binding, internalization, and release of thymocytes by thymic nurse cells. Cell Immunol. 1993;148:301–315. doi: 10.1006/cimm.1993.1114. [DOI] [PubMed] [Google Scholar]
- 43.Rieker T, Penninger J, Roman N, Wick G. Chicken thymic nurse cells: an overview. Dev Comp Immunol. 1995;19:281–289. doi: 10.1016/0145-305x(95)00008-h. [DOI] [PubMed] [Google Scholar]
- 44.Ritter A, Suavage C A, Cotmore S F. The human thymus microenvironment: in vivo identification of thymic nurse cells and other antigenically-distinct subpopulations of epithelial cells. Immunology. 1981;44:439–446. [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts F, Geenen V, Schoenen J, Burgeon E, Defresne P, Legros J J, Franchimont P. Colocalization of immunoreactive oxytocin, vasopressin and interleukin-1 in human thymic epithelia neuroendocrine cells. Brain Behav Immunol. 1991;5:102–115. doi: 10.1016/0889-1591(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 46.Roberts F, Geenen V. Thymic neuropeptides and T-lymphocyte development. Ann N Y Acad Sci. 1992;650:99–104. doi: 10.1111/j.1749-6632.1992.tb49103.x. [DOI] [PubMed] [Google Scholar]
- 47.Samms M, Emanus F, Osuji O, Pezzano M, Guyden J C. Lysosomal-mediated degradation of apoptotic thymocytes within thymic nurse cells. Cell Immunol. 1999;197:108–115. doi: 10.1006/cimm.1999.1559. [DOI] [PubMed] [Google Scholar]
- 48.Scollay R, Shortman K. Identification of early stages of T lymphocyte development in the thymus cortex and medulla. J Immunol. 1985;134:3632–3642. [PubMed] [Google Scholar]
- 49.Schwartz R H. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 50.Surh C D, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 51.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 52.Tousaint-Demylle D, Scheiff J- M, Haumount S. Thymic nurse cells: morphological study during their isolation from murine thymus. Cell Tissue Res. 1990;261:115–123. doi: 10.1007/BF00329444. [DOI] [PubMed] [Google Scholar]
- 53.Toussaint-Demylle D, Scheiff J-M, Haumont S. Thymic accessory cell complexes in vitro and in vivo: morphological study. Cell Tissue Res. 1991;263:291–301. doi: 10.1007/BF00318771. [DOI] [PubMed] [Google Scholar]
- 54.Van Ewijk W, Kisielow P, Boehmer H. Immunohistology of T cell differentiation in the thymus of H-Y-specific T cell receptor alpha/beta transgenic mice. Eur J Immunol. 1990;20:129–137. doi: 10.1002/eji.1830200119. [DOI] [PubMed] [Google Scholar]
- 55.van Vliet E, Melis M, van Ewijk W. Immunohistology of thymic nurse cells. Cell Immunol. 1984;87:101–199. doi: 10.1016/0008-8749(84)90134-5. [DOI] [PubMed] [Google Scholar]
- 56.Villa-Verde D, Lagrota-Candido J M, Vannier-Santos M A, Chammas R, Brentani R R, Savino W. Extracellular matrix components of the mouse thymus microenvironment. IV. Modulation of thymic nurse cells by extracellular matrix ligands and receptors. Eur J Immunol. 1994;24:659–664. doi: 10.1002/eji.1830240326. [DOI] [PubMed] [Google Scholar]
- 57.Weissman I L. Thymic lymphocyte differentiation and thymic leukemogenesis. Int J Radia Oncol Biol Phys. 1985;11:57–64. doi: 10.1016/0360-3016(85)90362-1. [DOI] [PubMed] [Google Scholar]
- 58.Wekerle H, Ketelson U-P. Thymic nurse cells- Ia bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980;283:402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]
- 59.Wekerle H, Ketelson U-P, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J Exp Med. 1980;151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitlock C A, Tidmarsh G F, Muller-Sieburg C, Wiessman I L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987;48:1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]