Abstract
First carpometacarpal (CMC1) osteoarthritis can be accompanied by the collapse of the first ray, with hyperextension of the first metacarpophalangeal (MCP1) joint. It is suggested that failure to address substantial MCP1 hyperextension during CMC1 arthroplasty may diminish post-operative capability and increase collapse reoccurrence. An arthrodesis is recommended in case of severe MCP1 joint hyperextension (>400). We describe a novel combination of a volar plate advancement and abductor pollicis brevis tenodesis to address MCP1 hyperextension at the time of CMC1 arthroplasty as an alternative to joint fusion. In 6 women, mean MCP1 hyperextension with pinch before surgery was 450 (range 300-850) and improved to 210 (range 150-300) of flexion with pinch six months after surgery. No revision surgery has been necessary to date, and there were no adverse events. Long-term outcome data is needed to establish the longevity of this procedure as an alternative to joint fusion, but early results are promising.
Key Words: ABP tenodesis, Basal joint arthritis, CMC1 arthritis, MCP1 hyperextension, Volar plate advancement
Introduction
First carpometacarpal (CMC1) joint osteoarthritis is associated with the longitudinal collapse of the first ray. This collapse is characterized by dorsal subluxation and adduction of the first metacarpal and compensatory hyperextension of the first metacarpophalangeal (MCP1) joint [Figure 1]. It is suggested that failure to address substantial MCP1 hyperextension during CMC1 arthroplasty may diminish post-operative capability and increase collapse reoccurrence.1,2
Figure 1.
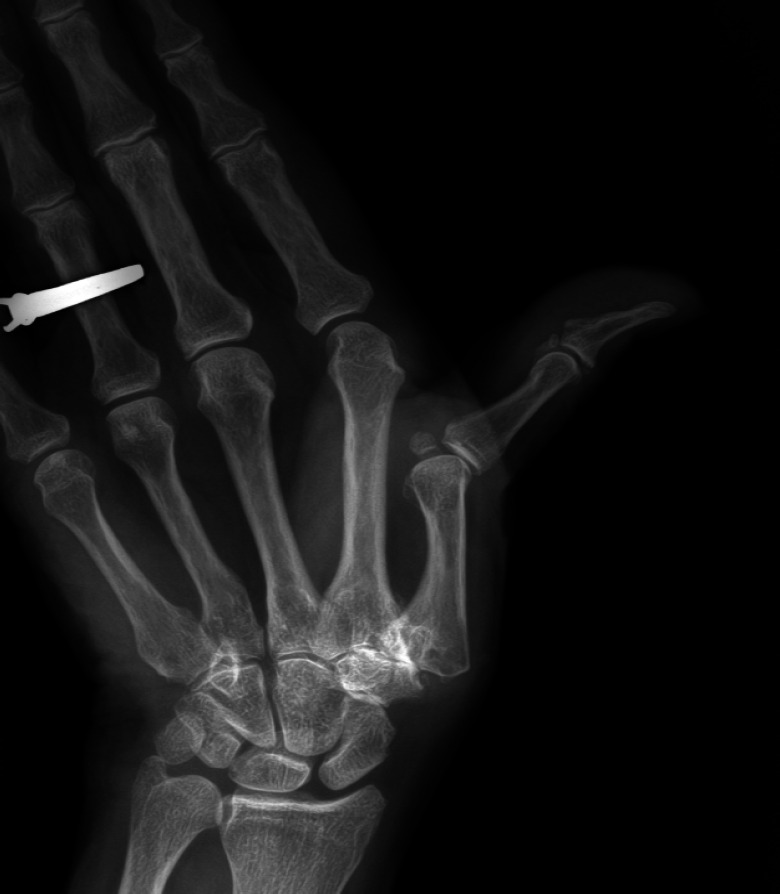
Radiograph of the collapse of the first ray in severe first carpometacarpal osteoarthritis
Based on wisdom, the following algorithm for the treatment of MCP1 hyperextension after CMC1 arthroplasty was proposed 1: (1) 00 to 100 of MCP1 hyperextension: casting in 200 flexion; (2) 100 to 200: MCP1 pinning, or extensor pollicis brevis transfer to radial and proximal on thumb metacarpal; (3) 200 to 400: volar capsulodesis, with or without sesamoidesis; (4) >400, or osteoarthritis, or fixed deformity: arthrodesis.
A potential limitation of this treatment algorithm is that volar capsulodesis risks injury to the digital neurovascular bundles to the thumb. Moreover, when patients present with more than 400 of MCP1 hyperextension, the recommended option is joint fusion. To address these limitations, we present a novel technique, combining a radial approach to volar plate advancement and abductor pollicis brevis tenodesis for the treatment of MCP1 hyperextension that can be performed on non-arthritic joints with substantial hyperextension (including >400 degrees) [Figure 2A; B]. This novel technique offers an alternative to joint fusion in people with pronounced MCP1 hyperextension.
Figure 2.
Drawing of the thumb metacarpal and phalanges
A: Proximal advancement of the volar plate. The original volar plate is outlined by the interrupted blue triangle. The advanced volar plate is outlined by the solid blue triangle. The direction of advancement is indicated by the interrupted black arrow.
B: Abductor pollicis brevis (APB) tenodesis. The interrupted blue triangle outlines part of the tendon to be moved volarly. The new volar location of the tendon is outlined by the solid blue triangle. The direction of advancement is indicated by the interrupted black arrow.
Technical note
Anatomy
The MCP1 joint consists of articulation of the first metacarpal head, proximal phalanx, and two sesamoid bones. The joint is stabilized by the dorsal capsule, ulnar and radial collateral ligaments, and volar plate.3 The sesamoid bones are embedded in the volar plate. The volar plate has a loose proximal attachment to the metacarpal neck and a strong distal attachment to the base of the proximal phalanx. The adductor pollicis muscle inserts on the ulnar sesamoid, while the flexor pollicis brevis and abductor pollicis brevis insert on the radial sesamoid. The digital nerves provide sensation to the thumb. The radial digital nerve passes volar to the flexor pollicis longus near the A1 pulley.
Indications/Contraindications
Since most people adapt to CMC1 osteoarthritis,4 we only offer CMC1 joint arthroplasty after an ineffective trial of non-operative treatment (adaptation, splinting, non-opioid pain killers, with or without intra-articular steroid injection). We discuss addressing MCP1 hyperextension in patients with the substantial longitudinal collapse of the first ray (also known as zig-zag deformity). It’s hard to provide an exact value to quantify “substantial” MCP1 hyperextension. As mentioned in the introduction, previous studies offer multiple cut-off values for addressing hyperextension at the MCP1 joint. It’s unclear if the cut-offs provided are determined at rest or with forceful pinch and the inter-observer variability of MCP1 hyperextension is unknown. Also, proposing one treatment to a patient with 390 of hyperextension, but proposing another to a patient with 410, seems arbitrary and makes exact cut-off values not useful in clinical practice. To date, no studies have assessed the effect of residual MCP1 joint hyperextension on pain intensity and disability after CMC1 joint arthroplasty. Also, no trials are comparing CMC1 arthroplasty with and without MCP1 joint hyperextension correction. However, because of the rationale that residual substantial MCP1 joint hyperextension might have a negative effect on the postoperative outcome, we offer to address the hyperextension in patients with substantial zig-zag deformity. Limited active motion at the MCP1 joint and osteoarthritis are contra-indications to our novel technique. Those patients would be offered an arthrodesis of the MCP1 joint.
Set-up and exposure
We use a standard hand surgery setup with an arm table, an upper arm tourniquet, and a brachial plexus block, with or without sedation. Our technique can be combined with any variation of CMC1 joint arthroplasty, which is outside the scope of this manuscript.
After CMC1 joint arthroplasty, a separate incision is made over the radial side of the MCP1 joint [Figure 3A]. The incision can be curved, angled, or straight. We dissect through the subcutaneous tissue to the fascia. Care is taken to identify and retract dorsal branches of the superficial radial nerve.
Figure 3.
Surgical technique in a cadaver
A: radial incision of the MCP.
B: volar plate to be advanced, outlined in the red interrupted square. P = proximal, D = distal, star = base of the first phalanx.
C: Volar plate (outlined in a red interrupted square) sutured down to bone anchor placed in the volar first metacarpal. P = proximal, D = distal.
D: part of the abductor pollicis brevis tendon sutured down volarly (outlined by red interrupted lines). P = proximal, D = distal.
Once the fascia is reached, skin flaps are developed to the volar and dorsal. Dorsally the complete insertion of the abductor pollicis brevis (APB) on the extensor hood is exposed, as well as the joint capsule and proximal phalanx.
While developing the volar skin flap, the radial digital nerve is identified and protected. This approach only puts the radial digital nerve at risk, not both digital nerves, as is the case during a standard volar capsulodesis. Next, the A1 pulley on the radial side is incised, and the flexor pollicis longus is retracted radially to expose the volar plate. The proximal volar plate is elevated off the metacarpal neck [Figure 3B].
Reconstruction
A 2.7 mm suture anchor is placed proximal to the metacarpal neck on the volar side, and the volar plate is sutured with the MCP1 joint slightly flexed, completing the volar plate advancement [Figure 3C]. Care is taken to ensure the suture anchor is proximal to the metacarpal neck to allow adequate volar plate advancement.
The volar half of the insertion of the APB is cut and brought volarly. This half of the APB tendon is sutured to the volar plate with a PDS 2.0 [Figure 3D]. We perform both the volar plate advancement and APB tenodesis with the MCPJ in 200 flexion. Twenty degrees is an arbitrary number, and others might consider somewhat more or less flexion. We used a k-wire in one of the first patients to temporarily reinforce MCP1 flexion, but we felt this did not add much to the hyperextension correction. In later cases, we no longer used a k-wire with similar results.
A tourniquet is deflated, and skin sutured closed with 4-0 nylon mattress sutures.
Rehabilitation
Patients are immobilized for two weeks with the MP joint in flexion and slight palmar abduction of the thumb. They are then changed to a removable brace for another four weeks. At six weeks after surgery, patients are provided with an at-home rehabilitation program, and they are offered formal hand therapy. When we used a k-wire, the person was immobilized for four weeks and we pulled the k-wire after four weeks.
Expected Outcomes
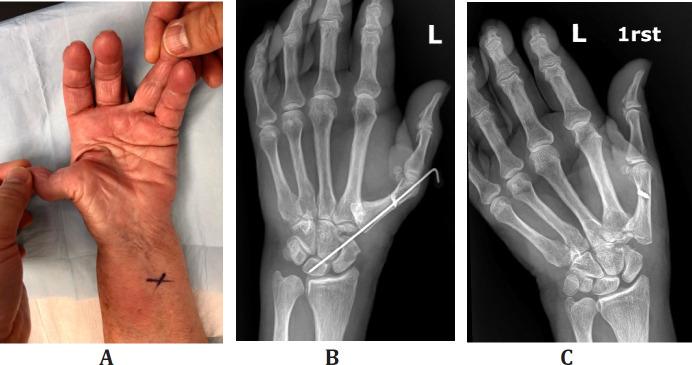
We performed this procedure in 6 females with CMC1 joint osteoarthritis, with continued pain after the non-operative treatment, with substantial first ray collapse. They were at a mean age of 68 (range 60-85) years. Mean MCP1 hyperextension with pinch before surgery was 450 (range 300-850) and improved to a post-operative pinch of 210 (range 150-300) of flexion. Final post-operative measures were taken at six months. Up to the final clinic visit one year after surgery, no revision surgery has been necessary. We noticed no reduction in palmar abduction of the thumb [Figure 4] [Table 1].
Figure 4.
A: pre-operative hyperextension at metacarpophalangeal joint;
B: post-operative extension block k-wire;
C: post-operative radiograph at six months;
Table 1.
Demographics and Results
| ID | Age | Sex | Pre-op degrees of hyperextension | Post-op degrees of flexion |
|---|---|---|---|---|
| 1 | 63 | Female | 35 | 20 |
| 2 | 85 | Female | 85 | 15 |
| 3 | 70 | Female | 30 | 30 |
| 4 | 66 | Female | 35 | 25 |
| 5 | 61 | Female | 45 | 15 |
| 6 | 71 | Female | 40 | 20 |
| Mean ±standard deviation | 69 ±8.6 | 45 ±20 | 21 ±5.8 |
Discussion
When CMC1 arthroplasty is considered for osteoarthritis, and there is severe MCP1 joint hyperextension (>400), some people advocate a concomitant MCP1 joint arthrodesis.1 We describe a novel technique combining a volar plate advancement and abductor pollicis brevis tenodesis that corrects MCP1 joint hyperextension and preserves some MCP1 joint motion. To date, this technique has shown reliable hyperextension correction in 6 people. Future studies should assess if this technique maintains long-term hyperextension correction in addition to determining which people benefit from correction of their hyperextension correction.
Potential adverse events
There were no adverse events reported. Potential adverse events included intra-operative nerve damage to branches of the superficial radial nerve and volar radial digital nerve. Intra-operative absence of the APB tendon is extremely unlikely, but there are reports of congenital absence.5 Congenital APB absence is accompanied by a flat thenar at birth and could be established pre-operatively. Two steps are particularly important in achieving adequate correction of MCP1 hyperextension: (1) placing the bone anchor proximal enough to hold the MCP1 joint in slight flexion; (2) tying the sutures of the anchor to the volar plate and from the APB to the volar plate. We aim for about 200 of flexion. Our main concern remains the loss of correction of hyperextension. If this happens, and it is thought to be associated with increased pain intensity and disability, MCP1 arthrodesis remains a good salvage option.
Long-term outcome data are needed to establish the longevity of this procedure as an alternative to joint fusion, but early results are promising.
References
- 1.Armbruster EJ, Tan V. Carpometacarpal joint disease: addressing the metacarpophalangeal joint deformity. Hand Clin. 2008;24(3):295–299, vii. doi: 10.1016/j.hcl.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Klinefelter R. Metacarpophalangeal hyperextension deformity associated with trapezial-metacarpal arthritis. J Hand Surg Am. 2011;36(12):2041–2; quiz 2043. doi: 10.1016/j.jhsa.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Imaeda T, An KN, Cooney WP 3rd. Functional anatomy and biomechanics of the thumb. Hand Clin. 1992;8(1):9–15. [PubMed] [Google Scholar]
- 4.Becker SJ, Briet JP, Hageman MG, Ring D. Death, taxes, and trapeziometacarpal arthrosis. Clin Orthop Relat Res. 2013;471(12):3738–3744. doi: 10.1007/s11999-013-3243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer KM, Stanley JK. Congenital absence of flexor pollicis brevis and abductor pollicis brevis. Hand. 1982;14(3):313–316. doi: 10.1016/s0072-968x(82)80067-3. [DOI] [PubMed] [Google Scholar]