Abstract
Objective
To estimate the effectiveness of maternal mRNA covid-19 vaccination during pregnancy against delta and omicron severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and hospital admission in infants.
Design
Test negative design study.
Setting
Community and hospital testing in Ontario, Canada.
Participants
Infants younger than six months of age, born between 7 May 2021 and 31 March 2022, who were tested for SARS-CoV-2 between 7 May 2021 and 5 September 2022.
Intervention
Maternal mRNA covid-19 vaccination during pregnancy.
Main outcome measures
Laboratory confirmed delta or omicron infection or hospital admission of the infant. Multivariable logistic regression estimated vaccine effectiveness, with adjustments for clinical and sociodemographic characteristics associated with vaccination and infection.
Results
8809 infants met eligibility criteria, including 99 delta cases (4365 controls) and 1501 omicron cases (4847 controls). Infant vaccine effectiveness from two maternal doses was 95% (95% confidence interval 88% to 98%) against delta infection and 97% (73% to 100%) against infant hospital admission due to delta and 45% (37% to 53%) against omicron infection and 53% (39% to 64%) against hospital admission due to omicron. Vaccine effectiveness for three doses was 73% (61% to 80%) against omicron infection and 80% (64% to 89%) against hospital admission due to omicron. Vaccine effectiveness for two doses against infant omicron infection was highest with the second dose in the third trimester (53% (42% to 62%)) compared with the first (47% (31% to 59%)) or second (37% (24% to 47%)) trimesters. Vaccine effectiveness for two doses against infant omicron infection decreased from 57% (44% to 66%) between birth and eight weeks to 40% (21% to 54%) after 16 weeks of age.
Conclusions
Maternal covid-19 vaccination with a second dose during pregnancy was highly effective against delta and moderately effective against omicron infection and hospital admission in infants during the first six months of life. A third vaccine dose bolstered protection against omicron. Effectiveness for two doses was highest with maternal vaccination in the third trimester, and effectiveness decreased in infants beyond eight weeks of age.
Introduction
Although most cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in infants present with mild or no signs, rates of admission to hospital and severe illness have been higher in infants compared with older children, especially when infection occurs during the first month of life or is complicated by other medical conditions.1 2 3 The increased severity in young infants might be related to their small airways and limited energy reserves, coupled with the naivety of the infant immune system.4 Covid-19 vaccines are highly effective against severe infection;5 6 however, these vaccines are not yet licensed for infants younger six months of age and the immunogenicity provided by new vaccine technologies in this age group is unknown.
Passive immunity for infants through the transfer of maternal antibodies after vaccination during pregnancy is well established for preventing infections such as pertussis, tetanus, and influenza.7 8 9 SARS-CoV-2 neutralising antibodies are present in umbilical cord blood, breastmilk, and serum specimens obtained from infants after natural maternal infection and vaccination during pregnancy.10 11 Additionally, emerging evidence suggests that maternal covid-19 vaccination during pregnancy might reduce the risk of SARS-CoV-2 infection and hospitalisation in infants.12 13 14 15 We sought to extend this evidence by evaluating the effectiveness of maternal vaccination with the primary (two doses) or primary plus booster (three doses) mRNA covid-19 vaccine series during pregnancy against delta and omicron SARS-CoV-2 infection and hospital admission of infants during their first six months of life.
Methods
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for case-control studies.16
Study design, setting, and participants
We conducted a population based, test negative design study in Ontario, Canada’s most populous province with 14.7 million residents and 140 000 births each year.17 18 We included infants younger than six months of age who were born between 7 May 2021 and 31 March 2022 and who were tested for SARS-CoV-2 between 7 May 2021 and 5 September 2022. We excluded infants who were part of a multiple birth, infants born at less than 20 weeks’ gestation or who weighed less than 500 g at birth, those with birth records that could not be linked to the administrative databases, those with missing gestational age, birthweight, or postal code, and infants of mothers younger than 12 years or older than 50 years of age. The unvaccinated group was defined as not having received a covid-19 vaccine dose up to 14 days before the infant’s SARS-CoV-2 test; we, therefore, excluded infants of mothers who received: one or two mRNA covid-19 vaccine doses before conception and no doses during pregnancy; a first or second mRNA covid-19 vaccine dose less than 14 days before delivery; or a first or second mRNA covid-19 vaccine dose postpartum and at least 14 days before the infant’s SARS-CoV-2 test. We also excluded infants of mothers who received a covid-19 vaccine that was viral vector-based or not Health Canada approved.
Data sources
We used data from ICES, which is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyse health care and demographic data, without consent, for health system evaluation and improvement. Databases contain clinical, laboratory, billing, and sociodemographic data. We linked databases using unique coded identifiers and analysed them at ICES. Information on all databases used in this study can be found in supplementary table S1, appendix page 3.
We identified maternal-newborn pairs using the Linked Delivering Mother and Newborns (MOMBABY) database, which contains deterministically linked maternal and newborn hospital delivery records from the Canadian Institute for Health Information Discharge Abstract Database.19 Greater than 99% of hospital birth records have been successfully linked in MOMBABY and fewer than 1% of records contain missing data.19
Measures
We derived information on maternal covid-19 vaccination from COVaxON, a centralised covid-19 vaccine registry that contains complete documentation of all covid-19 vaccination events in Ontario. BNT162b2 and mRNA-1273 became available to Ontario residents at high risk who were aged 18 years and older in December 2020.20 Pregnant women were prioritised for vaccination in phase 2 of the covid-19 vaccination programme, which began in April 2021; however, vaccines were available to pregnant women in earlier priority groups, such as healthcare or other frontline workers.21 Due to vaccine supply constraints, the interval between the first and second dose of the primary vaccine series varied over the study period from three to 16 weeks.22 23 Moreover, some people received a heterologous mRNA vaccine series due to fluctuating vaccine supplies.24 People who were immunocompromised were eligible for a third vaccine dose beginning in August 2021 and third dose eligibility gradually expanded to all adults, including pregnant women, in a stepwise manner based on risk in December 2021.25
We defined maternal vaccination with the primary covid-19 vaccine series during pregnancy as two vaccine doses administered up to 14 days before delivery, with at least one dose after the date of conception.14 We defined maternal vaccination with the primary plus booster covid-19 vaccine series as three vaccine doses administered up to 14 days before delivery, with at least one dose after the date of conception. Women were considered unvaccinated if they received no vaccine doses preconception, during pregnancy, or post partum between delivery and 14 days before their infant’s SARS-CoV-2 infection test.
Outcomes
We defined laboratory confirmed SARS-CoV-2 infection of the infant as a positive real-time polymerase chain reaction (PCR) test result on a respiratory specimen, irrespective of severity or the presence of signs. Omicron was first detected in Ontario on 22 November 2021 and circulation of delta after 2 January 2022 was limited. The prevalence of SARS-CoV-2 lineages in Ontario over the study period is shown in supplementary figure S1, appendix page 7. Whole genome sequencing and s-gene target failure screening were used to classify variants as omicron or delta during the six week transition period. If whole genome sequencing or s-gene target failure screening results were not available, all SARS-CoV-2 PCR tests before 3 December 2021 were classified as delta and all tests after 20 December 2021 were classified as omicron based on province-wide, representative surveillance (supplementary table S2, appendix page 4). We excluded positive tests from the transition period that could not be classified by whole genome sequencing, s-gene target failure screening, or dates. We obtained test results from the covid-19 Integrated Testing Dataset and identified variants using the Public Health Case and Contact Management Solution.
Between 31 May 2021 and 30 December 2021, SARS-CoV-2 PCR testing had no restrictions for infants.26 Due to high case volumes after this time, infant testing in the community was restricted to the following groups at high risk, beginning on 31 December 2021: infants in households with healthcare workers or staff working in other high risk settings; infants receiving emergency medical care or other outpatient medical care, at the discretion of the treating physician; First Nation, Inuit, or Métis infants; in the context of a confirmed or suspected outbreak; and within 24 h of birth and repeated at 48 h if negative at 24 h, for infants born to parents with confirmed covid-19 at the time of birth or if test results are pending at the time of hospital discharge.26 Inpatient SARS-CoV-2 PCR testing had no restrictions throughout the study period.
We considered infants who tested positive for delta or omicron to be cases and infants who tested negative to be controls. We used the date of respiratory specimen collection as the index date. For infants with multiple positive tests, which could represent a single episode of infection, we used the date of the first positive test as the index date. For infants with multiple negative tests, we included a randomly selected negative test, to reduce potential bias due to repeat asymptomatic screening in infants at high risk of exposure. Because the omicron and delta periods overlapped, some infants with negative tests served as controls in the vaccine effectiveness analyses for both variants.
We identified delta and omicron-related hospital admissions using reportable disease data in the Public Health Case and Contact Management Solution, which contains information on the clinical course of patients with a positive SARS-CoV-2 PCR test, including admissions to hospital because of covid-19.27 We excluded cases of nosocomial covid-19. We used the Canadian Institute for Health Information Discharge Abstract Database as an additional source to identify admissions to hospital with covid-19 as the most responsible diagnosis (ie, International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada (codes U07.1-U07.3)) and a positive SARS-CoV-2 PCR test within 14 days before up to three days after the date of admission.5
Covariates
We selected covariates a priori based on their potential association with study outcomes, covid-19 vaccination, or both.28 We extracted these covariates from the MOMBABY database: maternal age at birth, parity, calendar date of conception, and infant gestational age, birthweight, and sex. We ascertained the presence of maternal pre-pregnancy medical conditions (diabetes mellitus, hypertension, heart disease, asthma, autoimmune diseases, and immunosuppression) from multiple datasets using validated algorithms, as previously described.29 We categorised the adequacy of prenatal care into five groups (intensive, intermediate, adequate, inadequate, and no care) using the Revised Graduated Prenatal Care Index (R-GINDEX), which is derived from a combination of the gestational age at birth, the trimester of pregnancy that prenatal care began, and the number of prenatal visits.30 We extracted information to calculate the R-GINDEX from MOMBABY and the Ontario Health Insurance Plan databases. We identified positive maternal SARS-CoV-2 PCR test results using the Covid-19 Integrated Testing Dataset, which contains data on all SARS-CoV-2 PCR testing in Ontario. We obtained information on maternal influenza vaccination during the 2019-20 and 2020-21 influenza seasons as a proxy for health behaviour using the Ontario Health Insurance Plan and Ontario Drug Benefit databases. We obtained data for four variables at the level of the dissemination area (income quintile, proportion of the population who self-identify as a visible minority, proportion of the population employed in a high risk non-health occupation, and average number of people in each dwelling to account for transmission risk) from the 2016 Canadian census. Dissemination areas are the smallest census tract units and generally contain between 400 and 700 residents. We determined the public health unit of residence using a postal code conversion file from Statistics Canada and combined units into 10 larger regions.5 We also grouped testing into weekly intervals to capture changes in community covid-19 incidence, public health measures, and vaccine uptake.
Statistical analysis
We described the study participants using counts (percentages) for categorical data and medians (interquartile ranges) for continuous data. We used standardised differences to assess differences between cases and controls and vaccine exposed and unexposed groups, where an absolute standardised difference greater than 0.1 can indicate a potentially clinically important imbalance in the distribution between groups.31
We used multivariable logistic regression to estimate the adjusted odds ratio of maternal vaccination in delta or omicron cases who tested positive compared controls who tested negative and calculated vaccine effectiveness as (1–adjusted odds ratio) × 100%. We calculated vaccine effectiveness separately for the primary and primary plus booster vaccine series. We adjusted for the covariates described in the previous section (except we did not adjust for infant gestational age at birth or birthweight) and modelled week of testing using restricted cubic splines with five knots. We repeated the analysis for admissions to hospital that were related to delta and omicron by using infants who had negative tests during the relevant time periods as the control groups.
To assess possible waning of vaccine effectiveness for the primary series over time, we conducted additional analyses stratified by the trimester of pregnancy when the second vaccine dose was administered and by infant age when tested (ie, birth to 8 weeks, 9-16 weeks, and >16 weeks). Among infants of mothers who received the primary plus booster vaccine series, we evaluated vaccine effectiveness for the booster dose only during pregnancy (ie, doses one and two preconception) and the booster dose plus at least one primary series dose during pregnancy. We also conducted a secondary analysis estimating the effectiveness of only one vaccine dose during pregnancy (and no doses preconception or post partum between birth and 14 days before the infant’s test).
We conducted five sensitivity analyses against omicron infection for the primary vaccine series: before and after 31 December 2021, when changes to SARS-CoV-2 PCR testing eligibility were implemented; excluding infants of mothers who had a positive SARS-CoV-2 PCR test preconception or during pregnancy; excluding infants of immunosuppressed mothers who could have mounted an inadequate immune response with two vaccine doses; excluding infants of mothers who received one of their two vaccine doses preconception; and excluding infants tested during the first week of life when asymptomatic screening could have occurred for infants with extended birth admissions.
We conducted all analyses using SAS version 9.4. Tests were two sided with p<0.05 as the level of significance. We used 95% confidence intervals to show precision around point estimates.
Patient and public involvement
Although study participants contributed in important ways to this research, we did not involve them in the design, conduct, reporting, or dissemination plans of our study because of time limitations. Similarly, involvement of members of the public was not feasible.
Results
Study population
In total, 8809 infants met the eligibility criteria, including 99 delta cases compared with 4365 controls, and 1501 omicron cases compared with 4847 controls (fig 1). More infants were born to mothers who received the primary covid-19 vaccine series (ie, two doses; 4825 (54.8%) of 8809) than were born to mothers who received the primary plus booster vaccine series (ie, three doses; 691 (7.8%) of 8809; fig 2A-B). Baseline characteristics of infants and mothers are shown in table 1. Compared with infants in the test negative control group, a lower percentage of test positive cases were tested during the first eight weeks of life or born preterm. A lower percentage of mothers of infants in the case group had a positive SARS-CoV-2 PCR test during pregnancy but a higher percentage had a positive test post partum, compared with mothers of infants in the control group. Compared with vaccinated mothers, unvaccinated mothers were younger, less likely to have received an influenza vaccination during the two previous influenza seasons, and more likely to reside in areas with lower incomes and higher proportions of non-healthcare workers at high risk. The distributions of conception dates by case and control classification and vaccination status are shown in supplementary figure S2A-B, appendix page 8.
Fig 1.
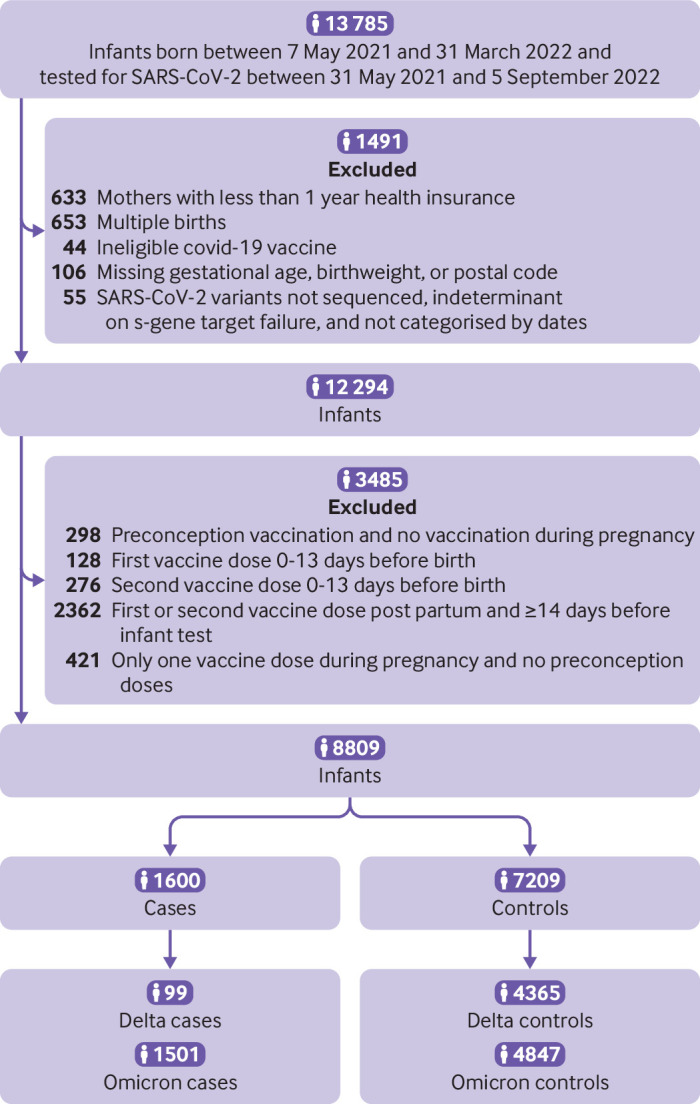
Summary of study sample selection
Fig 2.
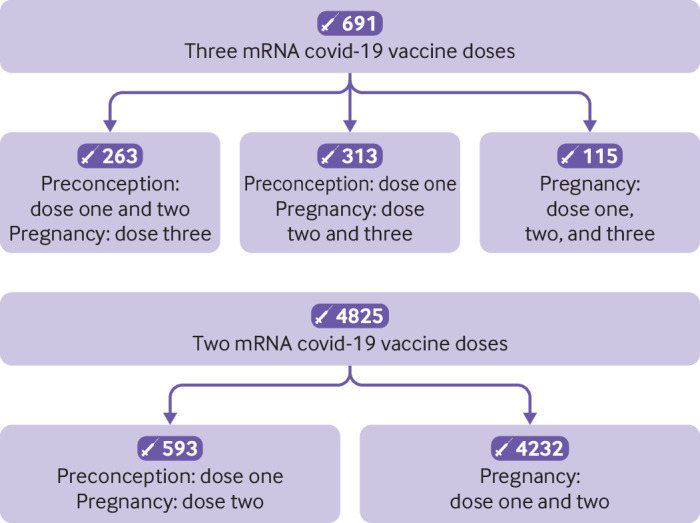
Maternal covid-19 vaccination timing in relation to pregnancy. (Top panel) Primary series plus booster (three doses). (Bottom panel) Primary series (two doses)
Table 1.
Characteristics of infants younger than six months of age tested for SARS-CoV-2 infection, May 2021 to September 2022, and their mothers, Ontario, Canada. Data are number (percentage), unless otherwise specified
| Characteristic | Infants (<6 months) tested for SARS-CoV-2 | Maternal covid-19 vaccination status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case (n=1600) | Control (n=7209) | SD* | Unvaccinated (n=3293) |
Primary and primary + booster† (n=5516) | SD* | Primary† (n=4825) | Primary plus booster† (n=691) | SD* | ||
| Infant sex, female | 742 (46.4) | 3161 (43.8) | 0.05 | 1506 (45.7) | 2397 (43.5) | 0.05 | 2111 (43.8) | 286 (41.4) | 0.05 | |
| Infant age when tested: | ||||||||||
| 0-8 weeks | 525 (32.8) | 4111 (57.0) | 0.50 | 1849 (56.1) | 2787 (50.5) | 0.11 | 2352 (48.7) | 435 (63.0) | 0.29 | |
| 9-16 weeks | 585 (36.6) | 1816 (25.2) | 0.25 | 802 (24.4) | 1599 (29.0) | 0.10 | 1435 (29.7) | 164 (23.7) | 0.14 | |
| >16 weeks | 490 (30.6) | 1282 (17.8) | 0.30 | 642 (19.5) | 1130 (20.5) | 0.02 | 1038 (21.5) | 92 (13.3) | 0.22 | |
| Gestational age at birth (weeks), median (interquartile range) | 39 (38-40) | 39 (37-39) | 0.23 | 39 (37-40) | 39 (38-39) | 0.01 | 39 (38-39) | 39 (38-39) | 0.02 | |
| Preterm birth‡ | 109 (6.8) | 985 (13.7) | 0.23 | 426 (12.9) | 668 (12.1) | 0.02 | 576 (11.9) | 92 (13.3) | 0.04 | |
| Low birthweight§ | 86 (5.4) | 813 (11.3) | 0.21 | 365 (11.1) | 534 (9.7) | 0.05 | 472 (9.8) | 62 (9.0) | 0.03 | |
| Mother's age at birth: | ||||||||||
| <25 years | 192 (12.0) | 703 (9.8) | 0.07 | 571 (17.3) | 324 (5.9) | 0.36 | 297 (6.2) | 27 (3.9) | 0.10 | |
| 25-29 years | 399 (24.9) | 1745 (24.2) | 0.02 | 956 (29.0) | 1188 (21.5) | 0.17 | 1076 (22.3) | 112 (16.2) | 0.15 | |
| 30-34 years | 591 (36.9) | 2768 (38.4) | 0.03 | 1038 (31.5) | 2321 (42.1) | 0.22 | 2009 (41.6) | 312 (45.2) | 0.07 | |
| 35-39 years | 346 (21.6) | 1641 (22.8) | 0.03 | 577 (17.5) | 1410 (25.6) | 0.20 | 1205 (25.0) | 205 (29.7) | 0.11 | |
| ≥40 years | 72 (4.5) | 352 (4.9) | 0.02 | 151 (4.6) | 273 (4.9) | 0.02 | 238 (4.9) | 35 (5.1) | 0.01 | |
| Nulliparous | 853 (53.3) | 4393 (60.9) | 0.15 | 1935 (58.8) | 3311 (60.0) | 0.03 | 2939 (60.9) | 372 (53.8) | 0.14 | |
| Pre-pregnancy maternal comorbidities: | ||||||||||
| Diabetes mellitus | 93 (5.8) | 447 (6.2) | 0.02 | 198 (6.0) | 342 (6.2) | 0.01 | 317 (6.6) | 25 (3.6) | 0.13 | |
| Hypertension | 42 (2.6) | 179 (2.5) | 0.01 | 81 (2.5) | 140 (2.5) | 0.01 | 118 (2.4) | 22 (3.2) | 0.04 | |
| Heart disease | ≤5 (≤0.3)¶ | ≤5 (≤0.1)¶ | 0-0.06¶ | ≤5 (≤0.2)¶ | ≤5 (≤0.1)¶ | 0-0.02¶ | ≤5 (≤0.1)¶ | 0 (0.0) | 0-0.01¶ | |
| Asthma | 307 (19.2) | 1498 (20.8) | 0.04 | 686 (20.8) | 1119 (20.3) | 0.01 | 981 (20.3) | 138 (20.0) | 0.01 | |
| Autoimmune disease | 46 (2.9) | 203 (2.8) | 0 | 72 (2.2) | 177 (3.2) | 0.06 | 148 (3.1) | 29 (4.2) | 0.06 | |
| Immunosuppression** | 31 (1.9) | 189 (2.6) | 0.05 | 72 (2.2) | 148 (2.7) | 0.03 | 127 (2.6) | 21 (3.0) | 0.02 | |
| Prenatal care index††: | ||||||||||
| Intensive/intermediate | 845 (52.8) | 3598 (49.9) | 0.06 | 1606 (48.8) | 2837 (51.4) | 0.05 | 2480 (51.4) | 357 (51.7) | 0.01 | |
| Adequate | 253 (15.8) | 1109 (15.4) | 0.01 | 483 (14.7) | 879 (15.9) | 0.04 | 767 (15.9) | 112 (16.2) | 0.01 | |
| Inadequate | 370 (23.1) | 1796 (24.9) | 0.04 | 872 (26.5) | 1294 (23.5) | 0.07 | 1120 (23.2) | 174 (25.2) | 0.05 | |
| No care | 132 (8.3) | 706 (9.8) | 0.05 | 332 (10.1) | 506 (9.2) | 0.03 | 458 (9.5) | 48 (6.9) | 0.09 | |
| Maternal influenza vaccine‡‡ | 381 (23.8) | 2095 (29.1) | 0.12 | 381 (11.6) | 2095 (38.0) | 0.64 | 1804 (37.4) | 291 (42.1) | 0.10 | |
| Maternal SARS-CoV-2 infection: | ||||||||||
| Pre-pregnancy | 28 (1.8) | 179 (2.5) | 0.05 | 80 (2.4) | 127 (2.3) | 0.01 | 100 (2.1) | 27 (3.9) | 0.11 | |
| During pregnancy | 69 (4.3) | 834 (11.6) | 0.27 | 425 (12.9) | 478 (8.7) | 0.14 | 381 (7.9) | 97 (14.0) | 0.20 | |
| Post partum | 324 (20.3) | 365 (5.1) | 0.47 | 309 (9.4) | 380 (6.9) | 0.09 | 348 (7.2) | 32 (4.6) | 0.11 | |
| Median household income quintile§§¶¶: | ||||||||||
| 1 (lowest) | 385 (24.1) | 1548 (21.5) | 0.06 | 1004 (30.5) | 929 (16.8) | 0.33 | 824 (17.1) | 105 (15.2) | 0.05 | |
| 2 | 348 (21.8) | 1406 (19.5) | 0.06 | 744 (22.6) | 1010 (18.3) | 0.11 | 894 (18.5) | 116 (16.8) | 0.05 | |
| 3 | 312 (19.5) | 1535 (21.3) | 0.04 | 645 (19.6) | 1202 (21.8) | 0.05 | 1057 (21.9) | 145 (21.0) | 0.02 | |
| 4 | 310 (19.4) | 1472 (20.4) | 0.03 | 539 (16.4) | 1243 (22.5) | 0.16 | 1066 (22.1) | 177 (25.6) | 0.08 | |
| 5 (highest) | 245 (15.3) | 1248 (17.3) | 0.05 | 361 (11.0) | 1132 (20.5) | 0.26 | 984 (20.4) | 148 (21.4) | 0.03 | |
| Self-identified visible minority quintile§§***: | ||||||||||
| 1 (lowest) | 245 (15.3) | 1225 (17.0) | 0.05 | 640 (19.4) | 830 (15.0) | 0.12 | 737 (15.3) | 93 (13.5) | 0.05 | |
| 2 | 246 (15.4) | 1260 (17.5) | 0.06 | 566 (17.2) | 940 (17.0) | 0 | 821 (17.0) | 119 (17.2) | 0.01 | |
| 3 | 273 (17.1) | 1343 (18.6) | 0.04 | 555 (16.9) | 1061 (19.2) | 0.06 | 890 (18.4) | 171 (24.7) | 0.15 | |
| 4 | 390 (24.4) | 1640 (22.7) | 0.04 | 660 (20.0) | 1370 (24.8) | 0.12 | 1198 (24.8) | 172 (24.9) | 0 | |
| 5 (highest) | 446 (27.9) | 1741 (24.2) | 0.08 | 872 (26.5) | 1315 (23.8) | 0.06 | 1179 (24.4) | 136 (19.7) | 0.11 | |
| Average number of people per dwelling quintile§§: | ||||||||||
| 1 (0-2.1) | 290 (18.1) | 1230 (17.1) | 0.03 | 659 (20.0) | 861 (15.6) | 0.12 | 732 (15.2) | 129 (18.7) | 0.09 | |
| 2 (2.2-2.4) | 236 (14.8) | 1345 (18.7) | 0.10 | 656 (19.9) | 925 (16.8) | 0.08 | 806 (16.7) | 119 (17.2) | 0.01 | |
| 3 (2.5-2.6) | 230 (14.4) | 1101 (15.3) | 0.03 | 473 (14.4) | 858 (15.6) | 0.03 | 756 (15.7) | 102 (14.8) | 0.03 | |
| 4 (2.7-3.0) | 397 (24.8) | 1817 (25.2) | 0.01 | 754 (22.9) | 1460 (26.5) | 0.08 | 1279 (26.5) | 181 (26.2) | 0.01 | |
| 5 (3.1-5.7) | 447 (27.9) | 1716 (23.8) | 0.09 | 751 (22.8) | 1412 (25.6) | 0.07 | 1252 (25.9) | 160 (23.2) | 0.06 | |
| High risk non-health occupation quintile§§†††: | ||||||||||
| 1 (0%-32.5%) | 235 (14.7) | 1238 (17.2) | 0.07 | 300 (9.1) | 1173 (21.3) | 0.34 | 997 (20.7) | 176 (25.5) | 0.11 | |
| 2 (32.5%–42.3%) | 344 (21.5) | 1538 (21.3) | 0 | 558 (16.9) | 1324 (24.0) | 0.18 | 1156 (24.0) | 168 (24.3) | 0.01 | |
| 3 (42.3%–49.8%) | 333 (20.8) | 1479 (20.5) | 0.01 | 701 (21.3) | 1111 (20.1) | 0.03 | 988 (20.5) | 123 (17.8) | 0.07 | |
| 4 (50.0%–57.5%) | 350 (21.9) | 1493 (20.7) | 0.03 | 796 (24.2) | 1047 (19.0) | 0.13 | 919 (19.0) | 128 (18.5) | 0.01 | |
| 5 (57.5%–100%) | 338 (21.1) | 1461 (20.3) | 0.02 | 938 (28.5) | 861 (15.6) | 0.31 | 765 (15.9) | 96 (13.9) | 0.06 | |
| Public health unit region: | ||||||||||
| Central East | 84 (5.3) | 432 (6.0) | 0.03 | 197 (6.0) | 319 (5.8) | 0.01 | 270 (5.6) | 49 (7.1) | 0.06 | |
| Central West | 338 (21.1) | 1532 (21.3) | 0 | 739 (22.4) | 1131 (20.5) | 0.05 | 978 (20.3) | 153 (22.1) | 0.05 | |
| Durham | 89 (5.6) | 353 (4.9) | 0.03 | 148 (4.5) | 294 (5.3) | 0.04 | 254 (5.3) | 40 (5.8) | 0.02 | |
| Eastern | 56 (3.5) | 502 (7.0) | 0.16 | 220 (6.7) | 338 (6.1) | 0.02 | 300 (6.2) | 38 (5.5) | 0.03 | |
| Northern | 121 (7.6) | 337 (4.7) | 0.12 | 182 (5.5) | 276 (5.0) | 0.02 | 242 (5.0) | 34 (4.9) | 0 | |
| Ottawa | 61 (3.8) | 514 (7.1) | 0.15 | 125 (3.8) | 450 (8.2) | 0.18 | 399 (8.3) | 51 (7.4) | 0.03 | |
| Peel | 197 (12.3) | 746 (10.3) | 0.06 | 378 (11.5) | 565 (10.2) | 0.04 | 510 (10.6) | 55 (8.0) | 0.09 | |
| South West | 237 (14.8) | 1200 (16.6) | 0.05 | 634 (19.3) | 803 (14.6) | 0.13 | 716 (14.8) | 87 (12.6) | 0.07 | |
| Toronto | 299 (18.7) | 1175 (16.3) | 0.06 | 484 (14.7) | 990 (17.9) | 0.09 | 844 (17.5) | 146 (21.1) | 0.09 | |
| York | 118 (7.4) | 418 (5.8) | 0.06 | 186 (5.6) | 350 (6.3) | 0.03 | 312 (6.5) | 38 (5.5) | 0.04 | |
| Covid-19 vaccine dose 2 or 3 during pregnancy | 906 (56.6) | 4610 (63.9) | 0.15 | 0 (0.0) | 5516 (100.0) | — | 4825 (100.0) | 691 (100.0) | 0 | |
| Covid-19 vaccine dose 3 during pregnancy | 111 (6.9) | 580 (8.0) | 0.04 | 0 (0.0) | 691 (12.5) | — | 0 (0.0) | 691 (100.0) | — | |
| Maternal covid-19 vaccine dose 1 and 2: | ||||||||||
| BNT162b2-BNT162b2 | 650 (40.6) | 3155 (43.8) | 0.06 | 0 (0.0) | 3805 (69.0) | — | 3320 (68.8) | 485 (70.2) | 0.03 | |
| mRNA-1273-mRNA-1273 | 154 (9.6) | 800 (11.1) | 0.05 | 0 (0.0) | 954 (17.3) | — | 850 (17.6) | 104 (15.1) | 0.07 | |
| Heterologous‡‡‡ | 102 (6.4) | 655 (9.1) | 0.10 | 0 (0.0) | 757 (13.7) | — | 655 (13.6) | 102 (14.8) | 0.03 | |
| Timing of maternal covid-19 vaccination: | ||||||||||
| First dose | ||||||||||
| Preconception | 215 (13.4) | 954 (13.2) | 0.01 | 0 (0.0) | 1169 (21.2) | — | 593 (12.3) | 576 (83.4) | 2.02 | |
| First trimester | 239 (14.9) | 913 (12.7) | 0.07 | 0 (0.0) | 1152 (20.9) | — | 1051 (21.8) | 101 (14.6) | 0.19 | |
| Second trimester | 346 (21.6) | 1942 (26.9) | 0.12 | 0 (0.0) | 2288 (41.5) | — | 2274 (47.1) | 14 (2.0) | 1.23 | |
| Third trimester | 106 (6.6) | 801 (11.1) | 0.16 | 0 (0.0) | 907 (16.4) | — | 907 (18.8) | 0 (0.0) | 0.68 | |
| Second dose | ||||||||||
| Preconception | 40 (2.5) | 223 (3.1) | 0.04 | 0 (0.0) | 263 (4.8) | — | 0 (0.0) | 263 (38.1) | 1.11 | |
| First trimester | 185 (11.6) | 723 (10.0) | 0.05 | 0 (0.0) | 908 (16.5) | — | 577 (12.0) | 331 (47.9) | 0.85 | |
| Second trimester | 411 (25.7) | 1576 (21.9) | 0.09 | 0 (0.0) | 1987 (36.0) | — | 1891-1895 (39.2-39.3)¶ | 92-96 (13.3)-13.9)¶ | 0.60-0.62 | |
| Third trimester | 270 (16.9) | 2088 (29.0) | 0.29 | 0 (0.0) | 2358 (42.7) | — | 2353-2357 (48.8-48.8)¶ | ≤5 (≤0.7)¶ | 1.34-1.38 | |
| Third dose | ||||||||||
| Second trimester | 12 (0.8) | 49 (0.7) | 0.01 | 0 (0.0) | 61 (1.1) | — | 0 (0.0) | 61 (8.8) | — | |
| Third trimester | 99 (6.2) | 531 (7.4) | 0.05 | 0 (0.0) | 630 (11.4) | — | 0 (0.0) | 630 (91.2) | — | |
| Interval between covid-19 vaccine doses 1 and 2: | ||||||||||
| <35 days | 226 (14.1) | 904 (12.5) | 0.05 | 0 (0.0) | 1130 (20.5) | — | 1004 (20.8) | 126 (18.2) | 0.06 | |
| 35-55 days | 296 (18.5) | 1627 (22.6) | 0.10 | 0 (0.0) | 1923 (34.9) | — | 1668 (34.6) | 255 (36.9) | 0.05 | |
| ≥56 days | 384 (24.0) | 2079 (28.8) | 0.11 | 0 (0.0) | 2463 (44.7) | — | 2153 (44.6) | 310 (44.9) | 0 | |
| Interval between covid-19 vaccine doses 2 and 3: | ||||||||||
| <168 days | 25 (1.6) | 117 (1.6) | 0 | 0 (0.0) | 142 (2.6) | — | 0 (0.0) | 142 (20.5) | — | |
| ≥168 days | 86 (5.4) | 463 (6.4) | 0.04 | 0 (0.0) | 549 (10.0) | — | 0 (0.0) | 549 (79.5) | — | |
Absolute standardised difference. Values greater than 0.10 indicate a potentially clinically important difference in the distribution between groups.
Primary covid-19 vaccination series defined as two doses up to 14 days before delivery with at least one dose after conception. Primary plus booster covid-19 vaccination series defined as three doses up to 14 days before delivery with at least one dose after conception. Unvaccinated defined as no covid-19 vaccine doses preconception, during pregnancy, and postpartum up to 14 days before the infant’s SARS-CoV-2 polymerase chain reaction test.
Preterm birth: birth at <37 weeks’ gestation.
Low birthweight: birthweight <2500 g.
In accordance with Ontario’s privacy legislation, ICES data privacy policy prohibits us from reporting cells with fewer than five people. Ranges reported in adjacent cells are to prevent back calculation of small cells.
Immunosuppression defined as solid organ or stem cell transplant, active cancer, sickle cell anaemia, HIV infection, immunosuppressing therapies, and other immune system disorders.
Adequacy of prenatal care characterised with the Revised-Graduated Prenatal Care Utilisation Index (R-GINDEX). Intensive and intermediate categories were combined due to small cells.
Maternal influenza vaccination during the 2019-20 and/or 2020-21 influenza seasons.
Dissemination area level variable.
Household income quintile has variable cut-off values in different cities; census areas to account for cost of living. A dissemination area being in quintile 1 means it is among the lowest 20% of dissemination areas in its city by income.
Percentage of people in the area who self-identified as a visible minority. Census counts for people are randomly rounded up or down to the nearest number divisible by five, which causes some minor imprecision.
Percentage of people in the area working in the following occupations: sales and service; trades, transport, and equipment operators, and related occupations; natural resources, agriculture, and related production occupations; and manufacturing and utilities. Census counts for people are randomly rounded up or down to the nearest number divisible by five, which causes some minor imprecision.
Heterologous: mRNA-1273 followed by BNT162b2 or vice versa.
Vaccine effectiveness
Vaccine effectiveness for the primary vaccine series was 95% (95% confidence interval 88% to 98%) against delta infection and 45% (37% to 53%) against omicron infection (table 2). Vaccine effectiveness for the primary plus booster vaccine series was 73% (61% to 80%) against omicron infection. We could not estimate vaccine effectiveness for the primary plus booster vaccine series against delta infection because only nine mothers were triple vaccinated in the period with the delta variant dominant. Although confidence intervals overlapped, vaccine effectiveness for the primary vaccine series (ie, two doses) against omicron infection was higher when the second dose was given in the third (53% (42% to 62%)) compared with first (47% (31% to 59%)) or second (37% (24% to 47%)) trimesters of pregnancy (fig 3A, and supplementary table S3, appendix page 5). Effectiveness decreased in a step wise manner by infant age at the time of testing (57% (44% to 66%) between birth and eight weeks to 40% (21% to 54%) after 16 weeks of age (fig 3B and supplementary table S3, appendix page 5). Vaccine effectiveness of the primary plus booster vaccine series against omicron infection was similar in infants of mothers who received only the booster dose during pregnancy (75% (57% to 85%)) or the booster dose plus at least one primary series dose during pregnancy (72% (60% to 81%)).
Table 2.
Effectiveness of maternal mRNA covid-19 vaccination during pregnancy at any gestational time against delta and omicron infection and admission to hospital in infants younger than six months of age in Ontario, Canada. Data are number of infants of vaccinated mothers/total number of infants for each analysis(%) or percentage (95% confidence interval)
| Infants in case group | Infants in control group | Effectiveness of maternal vaccination | |
|---|---|---|---|
| Infection | |||
| Delta variant, primary vaccine series*† | ≤5/99 (≤5.1) | 2549/4357 (58.5) | 95 (88 to 98) |
| Omicron variant, primary vaccine series*‡ | 790/1390 (56.8) | 2880/4267 (67.5) | 45 (37 to 53) |
| Omicron variant, primary+booster vaccine seriesठ| 111/711 (15.6) | 580/1967 (29.5) | 73 (61 to 80) |
| Admission to hospital | |||
| Delta variant, primary vaccine series*† | ≤5/29 (≤17.2) | 2549/4357 (58.5) | 97 (73 to 100) |
| Omicron variant, primary vaccine series*‡ | 160/309 (51.8) | 2880/4267 (67.5) | 53 (39 to 64) |
| Omicron variant, primary+booster vaccine seriesठ| 21/170 (12.4) | 580/1967 (29.5) | 80 (64 to 89) |
Vaccine effectiveness estimated as one minus the adjusted odds ratio of maternal vaccination in cases versus controls times 100%. Multivariable logistic regression adjusted for the following variables: calendar week of testing, infant age in weeks when tested (continuous), infant sex, mother’s age at birth in years (continuous), parity, maternal SARS-CoV-2 infection preconception or during pregnancy, maternal influenza vaccination during the 2019/2020 or 2020/2021 influenza season, maternal pre-pregnancy comorbidities (diabetes mellitus, hypertension, heart disease, asthma, autoimmune disease), maternal immunosuppression, adequacy of prenatal care (Revised Graduated Prenatal Care Index), four census dissemination area-level variables (income quintile, proportion of the population who self-identify as a visible minority, proportion of the population employed in a high-risk non-health occupation, and average number of people in each dwelling to account for transmission risk), and public health unit region.
Primary covid-19 vaccination series defined as two doses up to 14 days before delivery with at least one dose after conception.
Infants tested between 31 May 2021 and 2 January 2022.
Infants tested between 22 November 2021 and 5 September 2022.
Primary plus booster covid-19 vaccination series defined as three doses up to 14 days before delivery with at least one dose after conception.
Fig 3.
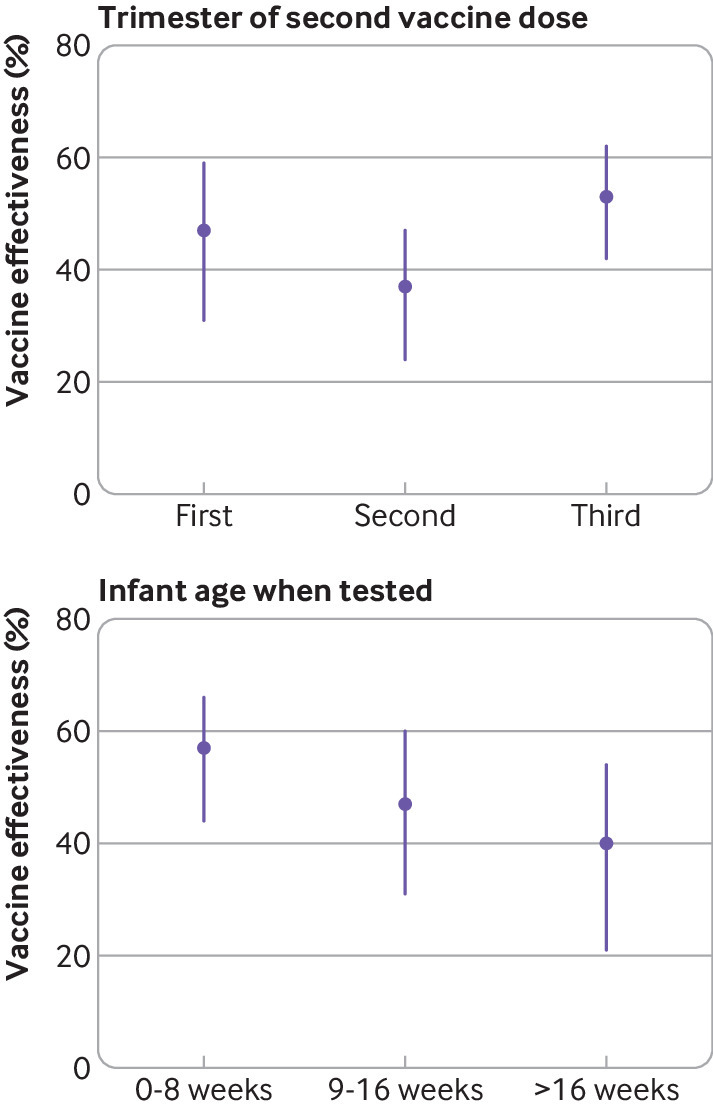
Effectiveness of the primary mRNA covid-19 vaccine series during pregnancy against omicron infection in infants younger than six months of age. (Top panel) By trimester of pregnancy of second vaccine dose. (Bottom panel) By infant age at the time of testing
In total, 29 (29%) of 99 infants were admitted to hospital because of a delta infection and 330 (22%) of 1501 infants were admitted to hospital because of an omicron infection. Against admission to hospital, vaccine effectiveness for the primary vaccine series was 97% (73% to 100%) against the delta variant and 53% (39% to 64%) for the omicron variant. Vaccine effectiveness for the primary plus booster vaccine series was 80% (64% to 89%) against admission to hospital related to the omicron variant.
In addition to the 8809 infants in the main analyses, 421 infants were born to mothers who received only one mRNA covid-19 vaccine dose during pregnancy, and no doses preconception or post partum between birth and 14 days before the infant’s test. Seventy five percent (314/421) of the vaccinations occurred in the third trimester. Vaccine effectiveness of one dose was 81% (33% to 95%) against delta and 30% (–9.6% to 55%) against an omicron infection.
Results were robust in sensitivity analyses with vaccine effectiveness for the primary vaccine series against omicron infection ranging from 44% to 52% (supplementary table S4, appendix page 6).
Discussion
mRNA vaccines are highly effective at preventing severe infection in pregnant women, whom have an elevated risk of covid-19 complications compared with their non-pregnant counterparts.32 33 34 Moreover, epidemiological studies suggest that adverse pregnancy outcomes, including spontaneous abortion, stillbirth, and preterm birth, after a covid-19 vaccination during pregnancy are similar to or lower than background rates in the general population.35 36 37 38 39 In this study, we show that maternal covid-19 vaccination during pregnancy might have dual benefits by also conferring protection to their infants.
Main findings
Using a test-negative study design, we estimated high (≥95%) vaccine effectiveness for maternal vaccination with the primary mRNA covid-19 vaccine series during pregnancy against delta infection and admission to hospital in infants younger than six months of age. Vaccine effectiveness against omicron infection (45%) and admission to hospital (53%) was moderate, but improved with receipt of a booster dose during pregnancy (73% and 80%, respectively). Our results also suggest waning protection with two doses over time against omicron infection. The greatest protection was noted during the first eight weeks of life and when maternal vaccination with the second dose occurred in the third trimester of pregnancy. The clinical implications of this finding on maternal vaccination need to be weighed against the risks of delaying vaccination to the pregnant woman and the fetus. Additionally, receipt of only the first vaccine dose during pregnancy offered less protection than completion of the two or three dose series.
Comparison with other studies
Our findings are broadly consistent with three recent studies that reported that maternal mRNA covid-19 vaccination during pregnancy was associated with reductions in SARS-CoV-2 infection and the number of infants admitted to hospital during periods of delta and omicron predominance. In a Norwegian population based cohort study including 21 643 newborns, maternal receipt of a second or third mRNA covid-19 vaccine dose during the second or third trimester of pregnancy was associated with a reduced hazard of SARS-CoV-2 infection in the first four months of life of 71% (56% to 81%) during delta predominant periods and 33% (21% to 43%) during omicron periods.12 Similar to the dose-response effect observed in our study, the risk of a positive test was lower for infants of mothers who received a third dose compared with a second dose in the omicron dominant period. In a US based, prospective, test-negative design study, including 1049 infants, the effectiveness of maternal mRNA covid-19 vaccination with two doses during pregnancy against admission to hospital related to covid-19 among infants younger than six months of age was 80% (60% to 90%) during the period of delta predominance and 38% (8% to 58%) during the period of omicron predominance.14 Estimates of vaccine effectiveness were higher when vaccination occurred after 20 weeks’ gestation compared with the first 20 weeks, during both delta and omicron predominant periods.14 Lastly, vaccine effectiveness of a second or third BNT162b2 dose in the second or third trimester of pregnancy against admission to hospital related to covid-19 during a period of delta predominance among infants younger than six months of age (n=464) was evaluated in a test-negative design study at three paediatric hospitals in Israel.15 Estimated vaccine effectiveness was 62% (32% to 78%) with the highest protection during the first two months of life.15 Only six mothers in this study received a third vaccine dose during pregnancy, precluding analysis of the effect of booster doses.15
Strengths and limitations
Our study has several strengths. We used deterministically linked, population based databases within a publicly funded healthcare system, which allowed us to identify all hospital deliveries and SARS-CoV-2 PCR test results in Ontario during the study period. A high percentage of women in our study were vaccinated during pregnancy and detailed information on vaccination status through the centralised COVaxON data system assured highly accurate classification of maternal vaccination status. Additionally, because gestational age is derived from early ultrasound assessment for most births in Ontario,19 the accuracy of gestational timing of maternal vaccination was likely to be high. We also used a test-negative design, which reduces bias due to differential access to testing, healthcare seeking behaviour, and case ascertainment.40
Our study also has limitations. We adjusted for multiple potential confounders, however, we were limited by the information available in the databases and unmeasured potential confounders, such as breastfeeding, could have differed between infants of vaccinated and unvaccinated mothers. Testing eligibility varied over the study period, and although we accounted for weekly testing periods in our analyses, we did not have information on home rapid antigen test results. Due to small case numbers, we could not evaluate the impact of waning vaccine effectiveness against delta infection or admissions to hospital or waning effectiveness after third doses. We were limited to the assessment of mRNA vaccines because very few pregnant women received viral vector-based covid-19 vaccines during the study period in Ontario. Additionally, maternal vaccination during pregnancy might protect infants from infection through antibody transfer across the placenta and through breastmilk, as well as via reduced exposure to maternal infection; our study was not designed to answer the question of which mechanism, or combination of mechanisms, was responsible for our findings.
Conclusions
In this population based study, maternal vaccination with the primary mRNA covid-19 vaccine series was highly effective against delta and moderately effective against omicron infection and admission to hospital in infants during the first six months of life. Three vaccine doses improved protection against omicron infection and admission to hospital. Vaccination during the third trimester of pregnancy provided the greatest protection, and effectiveness was highest in infants between birth and eight weeks of age.
What is already known on this topic
SARS-CoV-2 neutralising antibodies are present in umbilical cord blood, breastmilk, and infant serum specimens following both maternal infection and vaccination during pregnancy
Emerging evidence suggests maternal covid-19 vaccination during pregnancy may reduce the risk of SARS-CoV-2 infection and hospital admission in infants
What this study adds
Vaccination with a second mRNA covid-19 vaccine dose during pregnancy was highly effective against delta and moderately effective against omicron infection and hospital admission in infants younger than six months of age; receipt of a third dose bolstered protection against omicron
Vaccine effectiveness of two doses decreased over time against omicron infection with the greatest protection during the first eight weeks of life and when maternal vaccination occurred in the third trimester of pregnancy
Acknowledgments
We would like to acknowledge Public Health Ontario for access to vaccination data from COVaxON case-level data from the Public Health Case and Contact Management Solution and covid-19 laboratory data, as well as assistance with data interpretation. We also thank the staff of Ontario’s public health units who are responsible for covid-19 case and contact management and data collection within the Public Health Case and Contact Management Solution. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data or information compiled and provided by: Ontario Ministry of Health, Canadian Institutes of Health Research, Statistics Canada, and IQVIA Solutions Canada Inc. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Adapted from Statistics Canada, Canadian Census 2016. This does not constitute an endorsement by Statistics Canada of this product. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributions: SCJJ, DBF, and JCK conceived the study. SCJJ, DBF, PCA, RD, AG, and JCK developed the study design and analytical approach, in consultation with other project team members. AH linked the data sources. SCJJ performed the statistical analyses, which were supervised by DBF and JCK. SCJJ drafted the initial version of the manuscript. All authors contributed to the interpretation of the findings and reviewed and edited the manuscript for intellectual content. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work. JCK is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and no others meeting criteria have been omitted.
Funding: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long term Care. This study also received funding from: the Canadian Immunization Research Network through a grant from the Public Health Agency of Canada and the Canadian Institutes of Health Research (CNF 151944), the Public Health Agency of Canada, through the Vaccine Surveillance Working Party and the covid-19 Immunity Task Force and the Ontario Health Data Platform, a Province of Ontario initiative to support Ontario’s ongoing response to covid-19 and its related impacts. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the Ontario Health Data Platform, its partners, or the Province of Ontario is intended or should be inferred. Funders had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. JCK is supported by a Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine. RD and AG are supported by Canada Research Chairs. SCJJ is supported by a trainee grant from the Canadian Immunization Research Network.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Public Health Agency of Canada, the Canadian Institutes of Health Research, and the Ontario Ministry of Health and the Ministry of Long term Care for the submitted work; KW is chief executive officer of CANImmunize and serves on the data safety board for the Medicago covid-19 vaccine trial.
The corresponding author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results of this study have been made available to the public on a preprint server and shared with the Ontario Ministry of Health and ICES. Following peer review publication, they will be further disseminated by ICES through news media and social media. It is not possible to send study results to participants because all personal identifying information has been removed from the dataset.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
ICES is a prescribed entity under Ontario’s Personal Health Information Protection Act. Section 45 of this act authorises ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to or planning for all or part of the health system. Projects that use data collected by ICES under section 45 of Personal Health Information Protection Act, and use no other data, are exempt from research ethics board review. The use of the data in this project is authorised under section 45 and approved by ICES’ Privacy and Legal Office.
Data availability statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (eg, healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. Correspondence and requests for materials should be addressed to JCK.
References
- 1. Marks KJ, Whitaker M, Agathis NT, et al. COVID-NET Surveillance Team . Hospitalization of Infants and Children Aged 0-4 Years with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 2020-February 2022. MMWR Morb Mortal Wkly Rep 2022;71:429-36. 10.15585/mmwr.mm7111e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gale C, Quigley MA, Placzek A, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child Adolesc Health 2021;5:113-21. 10.1016/S2352-4642(20)30342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piché-Renaud PP, Panetta L, Farrar DS, et al. Canadian Paediatric Surveillance Program COVID-19 Study Team . Clinical manifestations and disease severity of SARS-CoV-2 infection among infants in Canada. PLoS One 2022;17:e0272648. 10.1371/journal.pone.0272648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010;23:74-98. 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung H, He S, Nasreen S, et al. Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators . Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 2021;374:n1943. 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nasreen S, Febriani Y, Velásquez García HA, et al. Effectiveness of COVID-19 vaccines against hospitalization and death in Canada: A multiprovincial test-negative design study. Clin Infect Dis 2022;ciac634. 10.1093/cid/ciac634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol 2010;39(Suppl 1):i102-9. 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlaudecker EP, Steinhoff MC, Omer SB, et al. IgA and neutralizing antibodies to influenza a virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One 2013;8:e70867. 10.1371/journal.pone.0070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Switzer C, D’Heilly C, Macina D. Immunological and Clinical Benefits of Maternal Immunization Against Pertussis: A Systematic Review. Infect Dis Ther 2019;8:499-541. 10.1007/s40121-019-00264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jorgensen SCJ, Burry L, Tabbara N. Role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacotherapy 2022;42:58-70. 10.1002/phar.2649. [DOI] [PubMed] [Google Scholar]
- 11. Nir O, Schwartz A, Toussia-Cohen S, et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am J Obstet Gynecol MFM 2022;4:100492. 10.1016/j.ajogmf.2021.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlsen EO, Magnus MC, Oakley L, et al. Association of COVID-19 vaccination during pregnancy with incidence of SARS-CoV-2 infection in infants. JAMA Intern Med 2022;182:825-31. 10.1001/jamainternmed.2022.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Halasa NB, Olson SM, Staat MA, et al. Overcoming COVID-19 Investigators. Overcoming COVID-19 Network . Effectiveness of maternal vaccination with mRNA covid-19 vaccine during pregnancy against covid-19-associated hospitalization in infants aged <6 months - 17 States, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:264-70. 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halasa NB, Olson SM, Staat MA, et al. Overcoming Covid-19 Investigators . Maternal vaccination and risk of hospitalization for covid-19 among infants. N Engl J Med 2022;387:109-19. 10.1056/NEJMoa2204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danino D, Ashkenazi-Hoffnung L, Diaz A, et al. Effectiveness of BNT162b2 vaccination during pregnancy in preventing hospitalization for Severe Acute Respiratory Syndrome Coronavirus 2 in infants. J Pediatr 2022;S0022-3476(22)00896-4. 10.1016/j.jpeds.2022.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573-7. 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 17.Statistics Canada. Table 13-10-0428-01 Live births and fetal deaths (stillbirths), by type of birth (single or multiple). 10.25318/1310042801-eng. [DOI]
- 18.Statistics Canada. Table 17-10-0005-01 Population estimates on July 1st, by age and sex. 10.25318/1710000501-eng. [DOI]
- 19.MOMBABY. ICES data dictionary (ICES, Toronto) https://datadictionary.ices.on.ca/Applications/DataDictionary/Library.aspx?Library=MOMBABY.
- 20.Ontario Ministry of Health. Ontario’s COVID-19 vaccination plan. https://covid-19-ontario-ca.myaccess.library.utoronto.ca/ontarios-covid-19-vaccination-plan#our-three-phased-vaccination-plan.
- 21. BORN Ontario . Report #5: COVID-19 vaccination during pregnancy in Ontario, December 14, 2020 to May 31, 2022. https://www.bornontario.ca/en/whats-happening/resources/Documents/COVID-19-Vaccination-during-pregnancy-in-Ontario_Report_Dec2020-May2022.pdf. 2022.
- 22.Vaccine Clinical Advisory Group (VCAG) Recommendations on Exceptions to Extended Dose Intervals for COVID-19 vaccines. Ontario Ministry of Health 2021. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID_19_medical_exceptions_vaccine_dose_intervals.pdf. Accessed 9 September 2022.
- 23.Ontario. High-risk healthcare workers eligible to receive a second dose of the COVID-19 vaccine at shortened intervals. May 2021. https://news.ontario.ca/en/backgrounder/1000089/high-risk-health-care-workers-eligible-to-receive-a-second-dose-of-the-covid-19-vaccine-at-a-shortened-interval. Accessed 9 September 2022.
- 24.Summary of National Advisory Committee on Immunization statement of June 17, 2021. National Advisory Committee on Immunization. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-statement-june-17-2021.html Accessed 9 September 2022.
- 25.Government of Canada. Vaccination in specific populations: immunocompromised persons. COVID-19 vaccine: Canadian immunization guide. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html#a6.4 Accessed 9 September 2022.
- 26.Government of Ontario. COVID-19 testing and treatment. 3 October 3 2022. https://www.ontario.ca/page/covid-19-testing-and-treatment#:~:text=There%20are%20two%20main%20publicly,rapid%20antigen%20tests
- 27.Case and Contact Management (CCM). ICES data dictionary (ICES, Toronto) https://datadictionary.ices.on.ca/Applications/DataDictionary/Library.aspx?Library=CCM Accessed 9 September 2022.
- 28. Fell DB, Dimitris MC, Hutcheon JA, et al. Guidance for design and analysis of observational studies of fetal and newborn outcomes following COVID-19 vaccination during pregnancy. Vaccine 2021;39:1882-6. 10.1016/j.vaccine.2021.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kwong JC, Buchan SA, Chung H, et al. Can routinely collected laboratory and health administrative data be used to assess influenza vaccine effectiveness? Assessing the validity of the Flu and Other Respiratory Viruses Research (FOREVER) Cohort. Vaccine 2019;37:4392-400. 10.1016/j.vaccine.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 30. Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep 1996;111:408-18, discussion 419. [PMC free article] [PubMed] [Google Scholar]
- 31. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228-34. 10.1080/03610910902859574. [DOI] [Google Scholar]
- 32. Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA 2021;326:728-35. 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magnus MC, Håberg SE, Carlsen EØ, et al. Pregnancy status at the time of COVID-19 vaccination and incidence of severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis 2023;76:57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Allotey J, Stallings E, Bonet M, et al. for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following covid-19 vaccination during pregnancy. JAMA 2021;326:1629-31. 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zauche LH, Wallace B, Smoots AN, et al. CDC v-safe Covid-19 Pregnancy Registry Team . Receipt of mRNA covid-19 vaccines and risk of spontaneous abortion. N Engl J Med 2021;385:1533-5. 10.1056/NEJMc2113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N Engl J Med 2021;385:2008-10. 10.1056/NEJMc2114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. JAMA 2022;327:1478-87. 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fell DB, Dimanlig-Cruz S, Regan AK, et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study. BMJ 2022;378:e071416. 10.1136/bmj-2022-071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill 2013;18:20585. 10.2807/1560-7917.ES2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (eg, healthcare organisations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. Correspondence and requests for materials should be addressed to JCK.


