Abstract
Background
The COVID-19 pandemic has severely affected the entire world, especially sub-Saharan Africa. As a result, researchers and government agencies are working to create effective COVID-19 vaccinations. While vaccination campaigns are moving rapidly in high-income nations, COVID-19 is still ruthlessly affecting people in low-income nations. However, this difference in the spread of the disease is not because of a lack of a COVID-19 vaccine but mainly due to people's reluctance. As a result, this review summarized the data on COVID-19 vaccination adoption and factors related among nations in sub-Saharan Africa.
Method
Comprehensive searches were conducted using PubMed, Embase, Medline, Web of Science, Google Scholar, and the Cochrane Library databases. The risk of bias and methodological quality of each published article that fit the selection criteria were evaluated using Critical Appraisal Checklist tools. All statistical analysis was done by STATA 16.
Results
This review was based on 29 studies with 26,255 participants from sub-Saharan Africa. Using a random-effects model, the pooled prevalence of COVID-19 vaccine acceptance among study participants was 55.04% (95 % CI: 47.80–62.27 %), I2 = 99.55%. Being male [POR = 1.88 (95% CI: 1.45, 2.44)], having a positive attitude toward the COVID-19 vaccine [POR = 5.56 (95% CI: 3.63, 8.51)], having good knowledge in the COVID-19 vaccine [POR = 4.61 (95% CI: 1.24, 8.75)], having government trust [POR = 7.10 (95% CI: 2.37, 21.32)], and having undergone COVID-19 testing in the past [POR = 4.41 (95%CI: (2.51, 7.75)] were significant predictor variables.
Conclusion
This analysis showed that respondents had a decreased pooled prevalence of COVID-19 vaccination acceptance. Sex, attitude, knowledge, government trust, and COVID-19 testing were statistically significantly correlated characteristics that affected the acceptability of the COVID-19 vaccine. All stakeholders should be actively involved in increasing the uptake of the COVID-19 vaccine and thereby reducing the consequences of COVID-19. The acceptance of the COVID-19 vaccination can be increased by using this conclusion as an indicator for governments, healthcare professionals, and health policymakers in their work on attitude, knowledge, government trust, and COVID-19 testing.
Keywords: COVID-19 vaccine acceptance, associated factors, systematic review, meta-analysis, sub-Saharan Africa, COVID-19
Background
Coronavirus disease 2019 (COVID-19) is a dangerous communicable disease caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). COVID-19 was first pronounced in Wuhan, China, in December 2019 (2). It has taken an enormous toll on the world's population through its mortality, morbidity, and impact on mental health and quality of life (3). Due to COVID-19's dissemination throughout the globe speedily, the World Health Organization (WHO) officially stated a pandemic and a public health emergency of international concern (PHEIC) (3). Even if the COVID-19 epicenters were in China, some European countries, and the United States (4), all other parts of the world, including Sub-Saharan Africa (SSA), are affected directly and indirectly by the pandemic (5). This is due to the large volumes of air transportation between these nations and Africa during the spread of COVID-19 (6). Besides, fragile health facility infrastructure, low clinician–population, inadequate laboratory competence, malnutrition, anemia, HIV/AIDs, and chronic respiratory diseases (7–9) led to worries that SSA could be hit hard. According to the WHO as of 22 June 2021, Africa has scored above 5.1 million cases with over 137,000 mortality (10). SSA is one of the least affected regions, with 28,848 diseased cases and 1,112 mortality recorded as of 27 April 2020 (11).
Consequently, enormous efforts by scholars, NGOs, and governments were absorbed in the development of successful and harmless vaccines for COVID-19 (12). Vaccination movements in high-income countries are fast-tracking in the world, but the population in low-income countries continues to be grievously exposed to COVID-19 (13). This gap is not only due to the inaccessibility of vaccines for COVID-19 but also due to the different perceptions of the community about the COVID-19 vaccine. Approximately 36% of South Africans (14), 6% of Ethiopians, and 41% of the population in the Democratic Republic of Congo are reluctant to be vaccinated against COVID-19 (15).
Many studies on COVID-19 vaccine acceptance and associated factors have been conducted in different parts of the world, including sub-Saharan African countries. These findings showed a variation in the magnitude of COVID-19 vaccine acceptance and its associated factors. Some shreds of evidence showed that COVID-19 vaccine acceptance was very low. There is a need to find reasons why COVID-19 vaccine acceptance differs in various sub-Saharan African countries, which enables stakeholders to formulate strategies for promoting COVID-19 vaccines in these regions. Therefore, this systematic review and meta-analysis were designed to estimate the pooled effect size and its predictor variables.
Method
Search strategies
The search results were reported based on the Preferred Reporting Items for Systematic Review and Meta-analysis statement (PRISMA) guideline (14).
PubMed, Embase, MEDLINE, Web of Science, Google Scholar, and Cochrane Library databases were used in comprehensive searching. The search words included “COVID-19” OR “SARS-CoV-2” OR “coronavirus” OR “novel coronavirus” OR “SARS-CoV-2” AND “vaccines” OR “vaccination” OR “COVID-19 Vaccines” OR “Vaccine acceptance” OR “level of COVID-19 acceptance”, “vaccination perception” OR “vaccine hesitancy” AND “associated factors” OR “Determinants” AND “Sub-Sahara African countries.” A manual search of the reference lists was also done to find possible important articles. All published articles were included.
Screening of eligible studies
Two reviewers (EA and ME) conducted the screening using titles and abstracts of the articles independently. Any discrepancies in screening results were resolute by discussion between reviewers and, if needed, the overall team to reach a consensus. All screened studies were exported into EndNote version 8.1, and duplication records were removed. Then, these reviewers retrieved all the involved full-text articles. Then, the overlapping articles that both assessors chose were considered for the succeeding phase without any doubt, and disparities were solved with the help of the reviewers (JA and DZ).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) cross-sectional studies conducted among sub-Saharan African countries; (2) studies published in English language articles; (3) studies that investigated COVID-19 vaccine acceptance and/or associated factors; and (4) studies that used a standardized and validated tool. The exclusion criteria were as follows: (1) nonrelevant articles; (2) not fully reported; (3) low-quality studies; and (4) studies with an unsatisfactory value (0–4 points) were considered as low quality and excluded from the final systematic review and meta-analysis.
Study quality assessment and data extraction
All studies were assessed for methodological quality by two independent reviewers using the reference to the following 10 items (clear statement of the aims of the research, methodology appropriateness, research design appropriateness, recruitment strategy, way of data collection, the relationship between researcher and participants, ethical issues, data analysis, statement of findings, and valuably of the research), from the Newcastle-Ottawa Scale (NOS) for analytical cross-sectional studies (15). The methodological quality of each included study was valued as very good quality (9–10 points), good quality (7–8 points), satisfactory (5–6 points), and unsatisfactory (0–4 points). The modified NOS for cross-sectional studies was used to include studies with ≥5 out of 10, which is considered a high-quality score (16). Based on the criteria, only high-quality studies were included.
Upon choosing the articles and appraising their quality, data in Table 1 were extracted. Author name(s), publication year, sample size, study population, study setting, outcomes, study design, and associated factors (pieces of information) were extracted from the included studies using Microsoft Excel 2016 (Table 1). Finally, the data were imported to STATA software version 14 for analysis.
Table 1.
Characteristics of studies included for the final systematic review and meta-analysis of COVID-19 vaccine acceptance and/or associated factors (n = 29).
| SN. | References | SS | Country | V A (%) | Study setting | Target population | Study design | The final score of NOS |
|---|---|---|---|---|---|---|---|---|
| 1 | Nzaji et al., 2020 (17) | 613 | Congo | 27.7 | Health facilities | HCWs | CS | 7 |
| 2 | Ditekemena et al., 2021 (18) | 4,131 | Congo | 55.9 | Community-based | General Population | CS | 8 |
| 3 | Ataboho et al.„ 2021 (19) | 703 | Congo | 45.9 | Community-based | General Population | CS | 8 |
| 4 | Tlale et al., 2022 (20) | 5,300 | Botswana | 73.4 | Community-based | General Population | CS | 7 |
| 5 | Ayele et al., 2021 (21) | 422 | Ethiopia | 45.3 | Health facilities | HCWs | CS | 7 |
| 6 | Berihun et al., 2021 (22) | 416 | Ethiopia | 59.4 | Health facilities | Chronic disease | CS | 7 |
| 7 | Mose et al., 2021 (23) | 396 | Ethiopia | 70.7 | Health facilities | Antenatal Care | CS | 8 |
| 8 | Taye et al., 2021 (24) | 423 | Ethiopia | 69.3 | School | Students | CS | 8 |
| 9 | Boche et al., 2022 (25) | 319 | Ethiopia | 73 | Health facilities | HCWs | CS | 8 |
| 10 | Agyekum et al., 2021 (26) | 234 | Ghana | 39.3 | Health facilities | HCWs | CS | 8 |
| 11 | Manan et al.„ 2022 (27) | 348 | Ghana | 35 | Community-based | General Population | CS | 7 |
| 12 | Lamptey et al., 2021 (28) | 1,000 | Ghana | 54.1 | Community-based | General Population | CS | 8 |
| 13 | Stephen et al., 2021 (29) | 267 | Ghana | 41.95 | Community-based | General Population | CS | 7 |
| 14 | Echoru et al., 2021 (30) | 1,067 | Uganda | 53.6 | Community-based | General Population | CS | 8 |
| 15 | Kanyike et al., 2022 (31) | 600 | Uganda | 37.3 | School | Students | CS | 7 |
| 16 | Yassin et al., 2022 (32) | 793 | Nigeria | 97.3 | Health facilities | HCWs | CS | 8 |
| 17 | Mustapha et al., 2021 (33) | 440 | Nigeria | 40 | School | Students | CS | 7 |
| 18 | Allagoa et al., 2021 (34) | 1,000 | Nigeria | 24.6 | Health facilities | Patients | CS | 9 |
| 19 | Yassin et al., 2022 (32) | 400 | Sudan | 63.8 | Health facilities | HCWs | CS | 7 |
| 20 | Raja et al., 2022 (35) | 217 | Sudan | 55.8 | School | Students | CS | 8 |
| 21 | Hoque et al., 2020 (36) | 346 | South Africa | 63.3 | Health facilities | Women | CS | 8 |
| 22 | Adeniyi et al., 2021 (37) | 1,308 | South Africa | 90.1 | Health facilities | HCWs | CS | 9 |
| 23 | Ngasa et al., 2021 (38) | 371 | Cameroon | 45.38 | Health facilities | HCWs | CS | 7 |
| 24 | Dula et al., 2021 (39) | 1,878 | Mozambique | 71.4 | Community-based | General Population | CS | 8 |
| 25 | McAbee et al., 2021 (40) | 551 | Zimbabwe | 55.7 | Community-based | General Population | CS | 7 |
| 26 | Mudenda et al., 2021 (41) | 677 | Zambia | 33.4 | Community-based | General Population | CS | 8 |
| 27 | Mudenda et al., 2022 (42) | 326 | Zambia | 24.5 | School | Students | CS | 7 |
| 28 | Kivuva et al., 2021 (43) | 659 | Kenya | 51.3 | Community-based | General Population | CS | 7 |
| 29 | Carpio et al., 2021 (44) | 1,050 | Kenya | 96 | Community-based | General Population | CS | 8 |
SN, Serial number; PI, primary investigator; PY, publication year; SS, sample size; VA, Vaccine acceptance; HCWs, Healthcare workers; CS, Cross-sectional.
Statistical analysis
COVID-19 vaccine acceptance and odds ratios of associated factors found in each article were aggregated after changing the original appraisals. To measure COVID-19 vaccine acceptance, point estimates of effect size, odds ratios (ORs), and 95% confidence intervals (95% CI) were calculated. Due to the variability of factors and heterogeneity of studies, the random effect model is more suitable for calculating the combined effects of COVID-19 vaccine acceptance (45).
Sub-group analysis also was done based on country, study setting, sample size, and year of publication to identify sources of heterogeneity. The degree of heterogeneity of these studies was measured qualitatively (forest plot) and quantitatively (I2 statistics, Cochrane's Q statistics). Then, values of I2 > 50% were considered an indication of high heterogeneity (46). Egger's weighted regression method was implemented for calculating the presence and effect of publication bias. All the analyses were conducted using statistical packages in STATA version 16.
Protocol registration
The review protocol was registered with the PROSPERO database through a registration number (PROSPERO-CRD42022330781).
Results
Search results
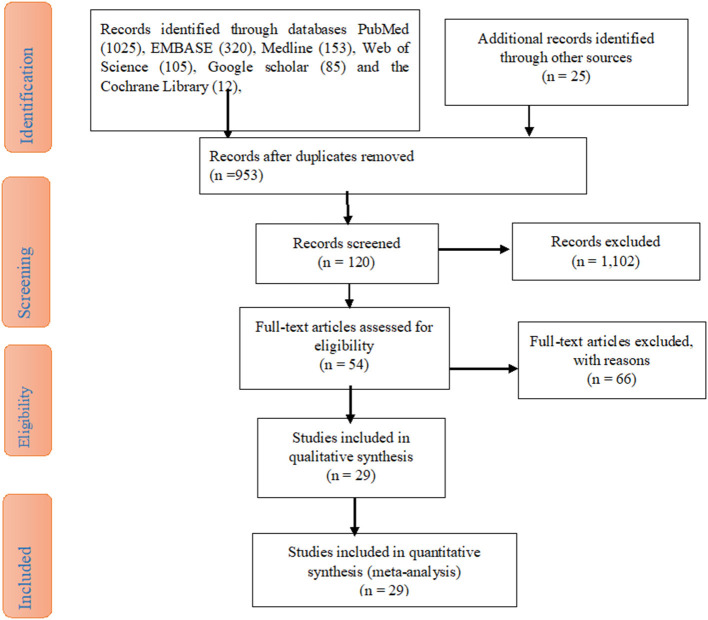
The study search for the articles' results was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) statement. After a compressive search through electronic databases such as PubMed (1,025), Embase (320), Medline (153), Web of Science (105), Google scholar (85), and the Cochrane Library (12), studies were recorded. Due to duplication, 953 records were removed. Through the assessment of the titles and abstracts, 1,102 articles were removed. Then, 120 studies were retrieved and 66 articles were excluded. Finally, 29 studies were included due to their eligibility criteria (Figure 1).
Figure 1.
A PRISMA diagram of the study selection procedure on the acceptance of the COVID-19 vaccine in sub-Saharan Africa Countries.
Characteristics of included articles
Approximately 29 articles met the selection criteria and were included in this systematic review and meta-analysis. All articles had a cross-sectional design with data collected using face-to-face interviews, telephone, or online.
Five studies were from Ethiopia, four from Ghana, three from Congo, three from Nigeria, two from Uganda, Sudan, Zambia, Kenya, and South Africa, and one from Botswana, Cameroon, Mozambique, and Zimbabwe. These studies had 26,255 study participants ranging from 217 to 5,300, and the level of COVID-19 vaccine acceptance ranged from 24.5% (41) to 97.3% (47). The majority of the selected studies were community-based (18, 20, 28–30, 39, 40, 42–44), followed by the health institution (17, 21–23, 25, 26, 32, 34, 36–38, 47), whereas the remaining were school-based (24, 31, 33, 35, 41), considering the study setting. All of these studies were cross-sectional in study design (Table 1).
Meta-analysis
Prevalence of COVID-19 vaccine acceptance
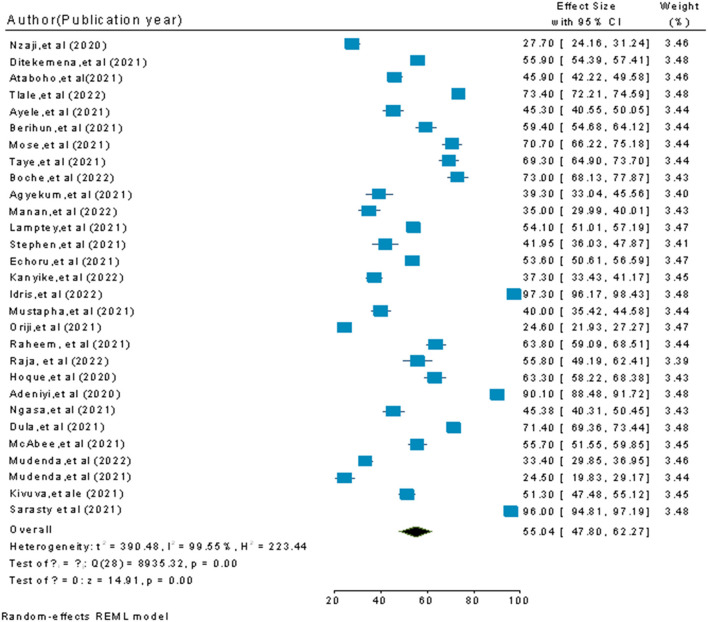
Since high heterogeneity was observed, a random-effects meta-analysis was conducted to assess the differences from an inverse-variance model. The pooled estimate of COVID-19 vaccine acceptance prevalence was 55.04% (95% CI: 47.80–62.27; P-value < 0.001) at a higher degree of heterogeneity (I2 = 99.55%) (Figure 2).
Figure 2.
A pooled estimate of the COVID-19 vaccine acceptance rate among participants in sub-Saharan African Countries.
Publication bias
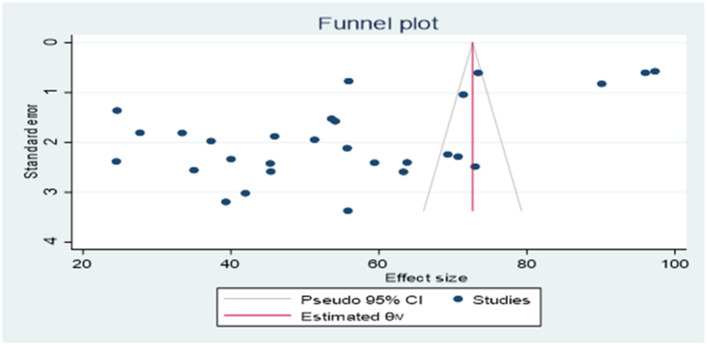
Publication bias was observed based on the results of asymmetric examination using the funnel plot (Figure 3) followed by Egger's regression check (P = 0.0035).
Figure 3.
Assessment of publication bias.
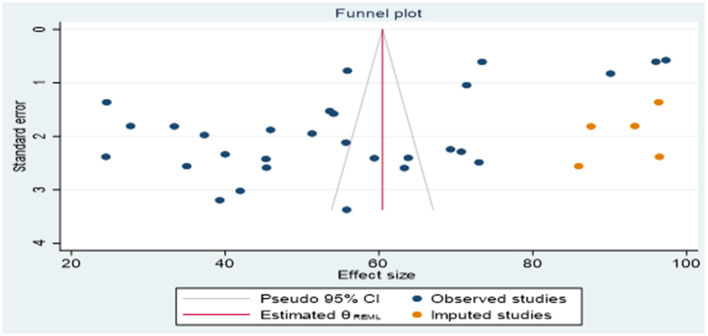
The result of the non-parametric trim-and-fill method indicated that, in addition to the 29 observed articles, there were an extra five important studies that were not observed because of publication bias (Figure 4). Therefore, our corrections change the number of significant results from imputing on the right from 55.04 to 60.453. However, there was no change from imputing on the right.
Figure 4.
Funnel plot for publication bias after adjustment.
Sub-group analysis of COVID-19 vaccination acceptance
Since the included studies varied in the study population, study period, study setting, effect of COVID-19, vaccine awareness, education status, and geographical areas, high heterogeneity was observed (I2 = 99.7%). Hence, subgroup analysis was done to find the source of heterogeneity. This was performed based on country, sample size, study setting, and publication year.
In the country-based sub-group analysis, the pooled estimate of COVID-19 vaccine acceptance among study participants was [43.22%, 95% CI (27.02, 59.42)] in DR Congo, [63.55%, 95% CI (53.53, 73.57)] in Ethiopia, [42.80%, 95% CI (34.44, 51.17)] in Ghana, [45.50%, 95% CI (29.52, 61.47)] in Uganda, and [53.99%, 95% CI (10.60, 97.39)] in Nigeria.
The prevalence of acceptance of the COVID-19 vaccine among study participants from health institutions was [58.36%, 95% CI (45.40, 71.33)], from community-based studies was [55.73%, 95% CI, (45.72, 65.74)], and from school-based studies was [45.34%, 95% CI (30.07, 60.62)].
The prevalence of acceptance toward the COVID-19 vaccine among studies published in 2020 was [60.39%, 95% CI (24.92, 95.86)], in 2021 was [53.12%, 95% CI (42.43, 60.81)], and in 2022 was [57.94%, 95% CI (39.80, 76.08)].
The prevalence of acceptance of the COVID-19 vaccine among the respondents in studies with a sample size of < 319 was [52.58%, 95% CI (37.34, 67.83)], in studies with a sample size of 319–551 was [52.05%, 95% CI (42.16, 60.94)], in studies with a sample size of 552–1000 was [46.48%, 95% CI (30.39, 62.57)], and in studies with a sample size ≥1,000 was [73.42%, 95% CI (59.60, 87.24)] (Figure 5).
Figure 5.
Forest plot showing the pooled prevalence of COVID-19 vaccine acceptance among the study population through subgroup analysis by sample size, country, study setting, and publication year.
Analysis of factors associated with COVID-19 vaccine acceptance
Sex, attitude, knowledge, government trust, and testing for COVID-19 were statistically significantly associated variables in COVID-19 vaccine acceptance.
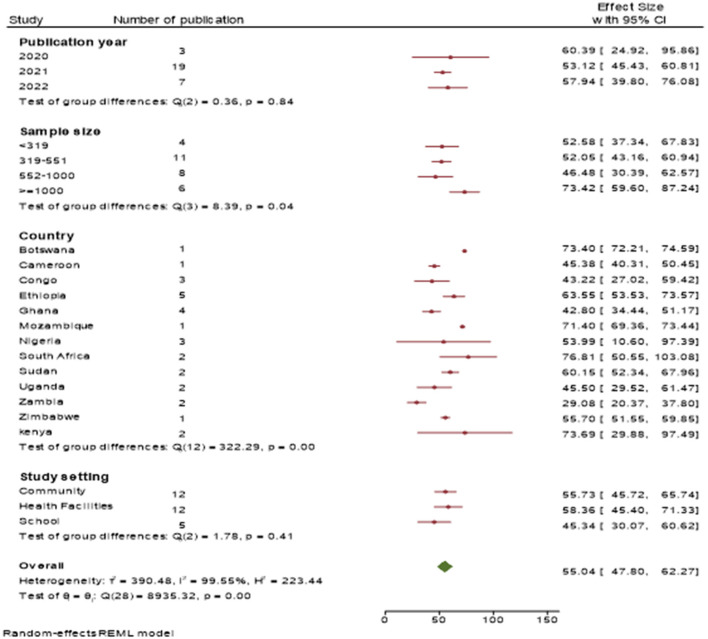
The odds of accepting the COVID-19 vaccine in men were 1.88 [POR = 1.88 (95% CI: 1.45, 2.44)] times more likely compared to women (Figure 6).
Figure 6.
Forest plot revealing the relationship between sex and COVID-19 vaccine acceptance among the study population.
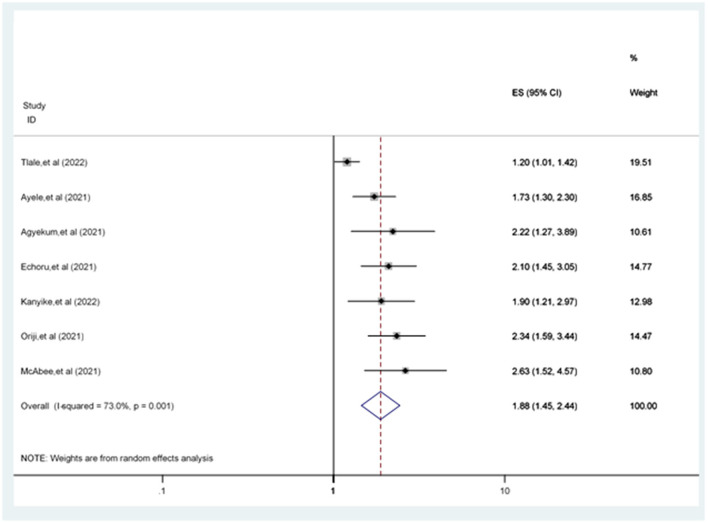
Participants having a positive attitude toward the COVID-19 vaccine were 5.56 [POR = 5.56 (95% CI: 3.63, 8.51)] times more likely to accept the COVID-19 vaccine compared to participants who have a poor attitude (Figure 7).
Figure 7.
Forest plot revealing the relationship between attitude and COVID-19 vaccine acceptance among the study population.
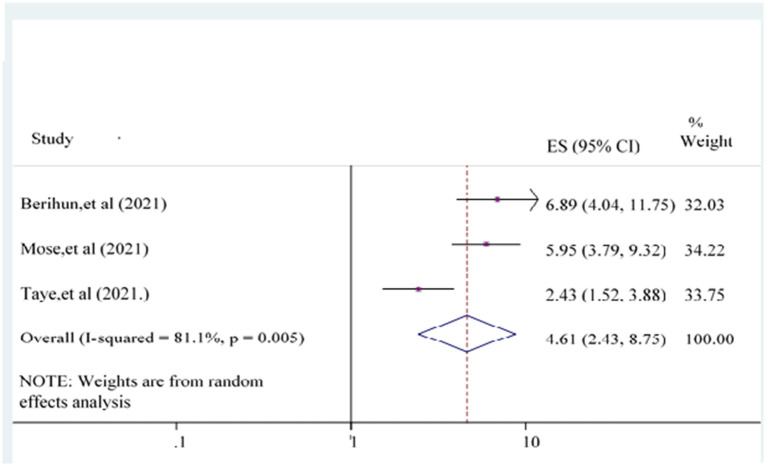
The odds of having good knowledge of the COVID-19 vaccine {POR = 4.61 [95% CI: (2.43, 8.75)]} was 4.61 times more likely than poor knowledge counterpart participants (Figure 8).
Figure 8.
Forest plot showing the relationship between knowledge and COVID-19 vaccine acceptance among the study population.
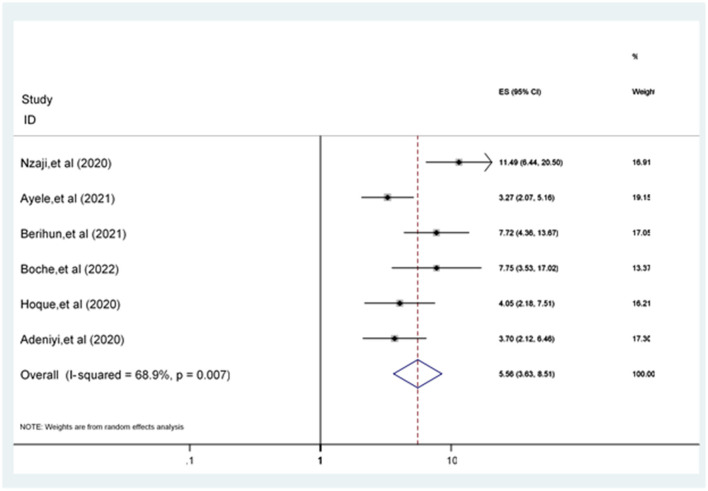
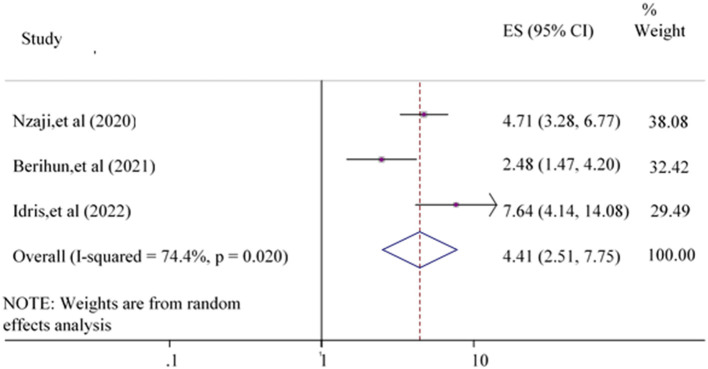
Whereas, participants previously tested for COVID-19 were 4.41 {POR = 4.41 [95% CI: (2.51, 7.75)]} times more likely to accept the COVID-19 vaccine compared to non-teased participants (Figure 9).
Figure 9.
Forest plot showing the relationship between testing for COVID-19 and COVID-19 vaccine acceptance among the study population.
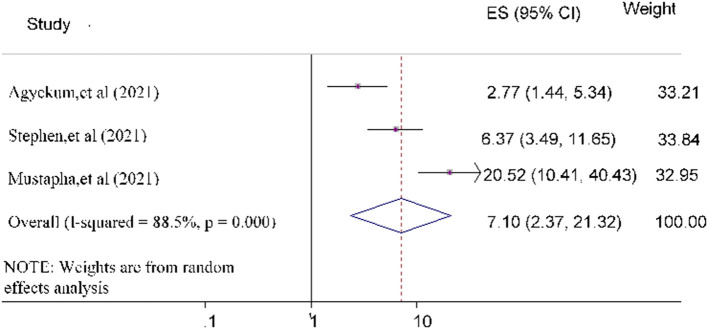
Finally, the odds of government trust toward COVID-19 vaccine acceptance was 7.10 [POR = 7.10 (95% CI: 2.37, 21.32)] times more likely compared to study subjects who did not trust the government (Figure 10).
Figure 10.
Forest plot depicting the relationship between government trust and COVID-19 vaccine acceptance among the study population.
Discussion
This systematic review and meta-analysis assessed COVID-19 vaccine acceptance and associated factors among sub-Saharan African countries. The pooled estimated prevalence of COVID-19 vaccine acceptance among sub-Saharan African countries was 55.04% (95% CI: 47.80–62.27%).
Our review findings showed that the acceptance of COVID-19 vaccines was lower than what was observed in previous national phone survey studies done among sub-Saharan African countries (87.6%) (48). This variation could be because the study subject chosen in the phone survey might not address the low-income people, people with no education, and others far from the media. This indicated that the phone surveys lacked representativeness from sub-Saharan African countries. Besides, the acceptance in this review was lower than the studies conducted on the global population (81.65%) (49), China (91.3%) (50), and Indonesia (93.3%) (51) and a review study (63.5%) (52). A possible explanation for this difference could be due to variations in the study setting, sociocultural characteristics, and study period.
However, this finding was better than the previous reviews on the pooled prevalence of COVID-19 vaccine acceptance in Africa (48.93%) (53). This disparity could be due to the dissimilarities in socio-culture, economic, level of awareness of the study participants, study setting, sample size, and study design toward the COVID-19 vaccine. This finding is comparable with low-income and lower-middle-income countries (58.5%) (3) and the global review (61.74%) (54) on COVID-19 vaccine acceptance.
This systematic review and meta-analysis indicated substantial variation in COVID-19 vaccine acceptance prevalence among study participants due to country, study setting, and year of publication difference. The subgroup analysis of this review showed that the highest COVID-19 vaccine acceptance prevalence was detected in South Africa (76.81%), while the lowest was observed in Zambia (29.08%). This lowest value of COVID-19 vaccine acceptance in Zambia is due to the uptake of the COVID-19 vaccine remaining unknown until 2021 (55). Another possible explanation for these differences could be the variation in media exposure, study setting, sample size, and sociodemographic profiles of the respondents.
The other characteristic used for subgroup analysis was the study setting. This subgroup analysis revealed that the highest COVID-19 vaccine acceptance was scored at health institutions [58.36%, 95% CI (45.40, 71.33)], followed by community-level study settings [55.73%, 95% CI (45.72, 65.74)], and the lowest COVID-19 vaccine acceptance prevalence was observed at school study setting [45.34%, 95% CI (30.07, 60.62)]. This disparity could be due to health institutions' study settings being more focused on healthcare workers who were more passionate about a COVID-19 vaccine than the general community and schools. This issue was supported by previous studies, which indicated that self-protection and readiness to safeguard families, friends, and sick people were significant predictors of overdue healthcare workers receiving vaccinations (56, 57). Meanwhile, healthcare workers have a more detailed understanding of COVID-19; consequently, they might be more likely to get the COVID-19 vaccine than the general population and school participants (55).
The pooled prevalence of COVID-19 vaccine acceptance among study participants in sub-Saharan African countries in 2020 was [60.39%, 95% CI (24.92, 95.86)], in 2021 was [53.12 %, 95% CI (42.43, 60.81)], and in 2022 was [57.94%, 95% CI (39.80, 76.08)]. This finding revealed that recent studies have higher COVID-19 vaccine acceptance. This difference might be due to the increased time people may be familiar with the COVID-19 vaccine through different communication media.
This review revealed a difference between men and women in COVID-19 vaccine acceptance rates. Based on seven studies (20, 21, 30, 31, 34, 40, 58), the odds of being male were higher in COVID-19 vaccine acceptance among study participants compared to the female participants. This finding is supported by earlier study evidence (49, 59). However, this finding contradicted a previous study (60). The possible explanation for this disparity might be that male participants are less familiar with protective and hygienic actions and are more exposed to different media, including Facebook. Male participants are more interactive with others, enhancing their information about COVID-19 vaccines, leading them to accept COVID-19 vaccines more than female participants (60).
Six studies (17, 21, 25, 26, 36, 37) examined attitude as a factor associated with accepting the COVID-19 vaccine. The pooled odds of a positive attitude toward the COVID-19 vaccine were a statistically significant predictor of COVID-19 vaccine acceptance. This result was in line with the studies conducted in Ethiopia (61), Korea (62), Indonesia (63), and China (50). Participants' positive attitude toward the safety and effectiveness of the COVID-19 vaccine looks to be the solution to the COVID-19 pandemic, especially the positive attitude concerning vaccination is essential (52).
Three studies (22–24) evaluated knowledge as a predictor of the acceptance of the COVID-19 vaccine. Participants with good knowledge about the COVID-19 vaccine were another significantly associated independent variable with COVID vaccine acceptance. This finding is supported by previous studies and surveys carried out in Ethiopia (61), England (64), and Southeast Asia (65). The possible explanation for this finding is that participants have good knowledge of using the COVID-19 vaccine and could accept the COVID-19 vaccine. The other reason might be that participants with good knowledge would be more informed and concerned about their health and wellbeing as a consequence of enhanced as well as becoming more concerned about life accomplishments that might affect them (55). Knowledge is the way for the directorial announcement in the strategies of escalating COVID-19 vaccine acceptance and for understanding means of organizing for future health crises (66).
Based on the evidence of three studies (26, 29, 33), government trust was a significant predictive factor in accepting the COVID-19 vaccine. Study participants with the highest confidence level in the national government had higher acceptance of the COVID-19 vaccine than their counterparts with the least trust. This finding is in line with other previous studies conducted worldwide (66–70). Political influences play a most important share in determining attitudes toward COVID-19 vaccination. Another possible explanation could be that trusting the government's competence in confirming that the COVID-19 vaccine may have fewer side effects and is effective, increasing their acceptance of the COVID-19 vaccination (71).
Finally, three articles (17, 22, 47) on the previous testing for COVID-19 were evaluated as a predictor of the acceptance of the COVID-19 vaccine. The odds of accepting the COVID-19 vaccine were higher among respondents who were tested for COVID-19 previously compared to participants who did not test for COVID-19. The possible reason for this difference might be that those respondents who participated in COVID-19 testing could create awareness of the COVID-19 vaccine and the consequences if they, their families, and the community at large are not vaccinated. Another possible explanation is that COVID-19 testing is not only for checking but also for educating the public about COVID-19 costs and the implications of the COVID-19 vaccine.
Limitations of the review
First, the effect of the latent variable in the primary studies was not assessed, affecting the interpretation of the predictor variable in this review. Second, the other limitation, some important covariates, such as age, level of education, and comorbidity, were not reported sufficiently in many studies to include in this review. Third, the COVID-19 infection status during the review was not accessed.
Conclusion
This review indicated that the pooled prevalence of COVID-19 vaccine acceptance was lower among respondents. Sex, attitude, knowledge, government trust, and testing for COVID-19 were statistically significantly associated factors toward COVID-19 vaccine acceptance; and they were also determinant factors in COVID-19 vaccine acceptance. All stakeholders should be vigorously involved to increase the acceptance level of the COVID-19 vaccine to prevent the impacts of COVID-19. This conclusion might be used as an indicator for governments, healthcare workers, and health policymakers in evaluating attitude, knowledge, government trust, and testing for COVID-19, which can improve COVID-19 vaccine acceptance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Data curation: JA, EA, and ME. Formal analysis, software, and writing: JA. Investigation, validation, methodology, and visualization: JA, DZ, EA, and ME. Writing–review and editing: JA, DZ, EA, ME, and VC. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama. (2020) 324:782–93. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 2.Dan-Nwafor C, Ochu CL, Elimian K, Oladejo J, Ilori E, Umeokonkwo C, et al. Nigeria's public health response to the COVID-19 pandemic: January to May 2020. J Global Health. (2020) 10:9. 10.7189/jogh.10.020399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patwary MM, Alam MA, Bardhan M, Disha AS, Haque MZ, Billah SM, et al. COVID-19 vaccine acceptance among low-and lower-middle-income countries: a rapid systematic review and meta-analysis. Vaccines. (2022) 10:427. 10.3390/vaccines10030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thekkur P, Tweya H, Phiri S, Mpunga J, Kalua T, Kumar A, et al. Assessing the Impact of COVID-19 on TB and HIV programme services in selected health facilities in lilongwe, malawi: operational research in real time. Trop Med Infect Dis. (2021) 6:81. 10.3390/tropicalmed6020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassegn A, Endris E. Review on socio-economic impacts of ‘Triple Threats' of COVID-19, desert locusts, and floods in East Africa: Evidence from Ethiopia. Cogent Soc Sci. (2021) 7:1885122. 10.1080/23311886.2021.1885122 [DOI] [Google Scholar]
- 6.Gilbert M, Pullano G, Pinotti F, Valdano E, Poletto C, Boëlle P-Y, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. (2020) 395:871–7. 10.1016/S0140-6736(20)30411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sadr WM, Justman J. Africa in the path of Covid-19. New England J Med. (2020) 383:e11. 10.1056/NEJMp2008193 [DOI] [PubMed] [Google Scholar]
- 8.Peter T, Keita M-S, Nkengasong J. Building laboratory capacity to combat disease outbreaks in Africa. African J Labor Med. (2016) 5:1–2. 10.4102/ajlm.v5i3.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker T, Schell CO, Petersen DB, Sawe H, Khalid K, Mndolo S, et al. Essential care of critical illness must not be forgotten in the COVID-19 pandemic. Lancet. (2020) 395:1253–4. 10.1016/S0140-6736(20)30793-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachega JB, Sam-Agudu NA, Siedner MJ, Rosenthal PJ, Mellors JW, Zumla A, et al. Prioritizing pregnant women for coronavirus disease 2019 vaccination in African countries. Clin Infect Dis. (2022). 10.1093/cid/ciac362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguimkeu P, Tadadjeu S. Why is the number of COVID-19 cases lower than expected in Sub-Saharan Africa? A cross-sectional analysis of the role of demographic and geographic factors. World Development. (2021) 138:105251. 10.1016/j.worlddev.2020.105251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte C, Sogni F, Affanni P, Veronesi L, Argentiero A, Esposito S. Vaccines against coronaviruses: the state of the art. Vaccines. (2020) 8:309. 10.3390/vaccines8020309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill I, Schellekens P. COVID-19 is a Developing Country Pandemic. Washington, WA, USA: Future Development; The Brookings Institution. (2021). [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Modesti P, Reboldi G, Cappuccio F. Newcastle-Ottawa Quality Assessment Scale (adapted for cross sectional studies). PLoS ONE. (2016) 11:e0147601.26808317 [Google Scholar]
- 16.Ungar OJ, Baris H, Oron Y, Shilo S, Handzel O, Warshavsky A, et al. Meta-analysis of time to extrusion of tympanostomy tubes by tympanic membrane quadrant. Clin Otolaryngol. (2021) 46:1165–71. 10.1111/coa.13843 [DOI] [PubMed] [Google Scholar]
- 17.Nzaji MK, Ngombe LK, Mwamba GN, Ndala DBB, Miema JM, Lungoyo CL, et al. Acceptability of vaccination against COVID-19 among healthcare workers in the Democratic Republic of the Congo. Pragm Observ Res. (2020) 11:103. 10.2147/POR.S271096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ditekemena JD, Nkamba DM, Mavoko AM, Hypolite M, Siewe Fodjo JN, Luhata C, et al. COVID-19 vaccine acceptance in the Democratic Republic of Congo: a cross-sectional survey. Vaccines. (2021) 9:153. 10.3390/vaccines9020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atabobho EE, Ngambou MN. Determinants of COVID-19 vaccination acceptance among workers at an oil company, Pointe-Noire (Congo). Curr J Appl Sci Technol. (2021) 40:1–8. 10.9734/CJAST/2021/v40i4231608 [DOI] [Google Scholar]
- 20.Tlale LB, Gabaitiri L, Totolo LK, Smith G, Puswane-Katse O, Ramonna E, et al. Acceptance rate and risk perception towards the COVID-19 vaccine in Botswana. PLoS ONE. (2022) 17:e0263375. 10.1371/journal.pone.0263375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayele AD, Ayenew NT, Tenaw LA, Kassa BG, Yehuala ED, Aychew EW, et al. Acceptance of COVID-19 vaccine and associated factors among health professionals working in Hospitals of South Gondar Zone, Northwest Ethiopia. Hum Vaccin Immunother. (2021) 17:4925–33. 10.1080/21645515.2021.2013082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berihun G, Walle Z, Berhanu L, Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting Dessie Comprehensive Specialized Hospital, Northeastern Ethiopia. Patient Prefer Adherence. (2021) 15:1795. 10.2147/PPA.S324564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mose A, Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in southwest ethiopia: institutional-based cross-sectional study. Int J Gen Med. (2021) 14:2385. 10.2147/IJGM.S314346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taye BT, Amogne FK, Demisse TL, Zerihun MS, Kitaw TM, Tiguh AE, et al. Coronavirus disease 2019 vaccine acceptance and perceived barriers among university students in northeast Ethiopia: A cross-sectional study. Clin Epidemiol Global Health. (2021) 12:100848. 10.1016/j.cegh.2021.100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boche B, Kebede O, Damessa M, Gudeta T, Wakjira D. Health Professionals' COVID-19 vaccine acceptance and associated factors in tertiary hospitals of south-west ethiopia: a multi-center cross-sectional study. INQUIRY. (2022) 59:00469580221083181. 10.1177/00469580221083181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agyekum MW, Afrifa-Anane GF, Kyei-Arthur F, Addo B. Acceptability of COVID-19 vaccination among health care workers in Ghana. Adv Public Health. (2021) 2021:9998176. 10.1101/2021.03.11.21253374 [DOI] [Google Scholar]
- 27.Abdul-Manan S, Abdullai AI, Yussif BG. Determinants of COVID-19 vaccine acceptance in Ghana. Int J Health Sci Res. (2022) 12:175–87. 10.52403/ijhsr.2022012435802742 [DOI] [Google Scholar]
- 28.Lamptey E, Serwaa D, Appiah AB, A. nationwide survey of the potential acceptance and determinants of COVID-19 vaccines in Ghana. Clin Exp Vaccine Res. (2021) 10:183. 10.7774/cevr.2021.10.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephen AH, Manortey S. Determinants of COVID-19 Vaccine Acceptance in the Ashaiman Municipality of the Greater Accra Region in Ghana. In: International Journal of Science and Research. (2020). [Google Scholar]
- 30.Echoru I, Ajambo PD, Keirania E, Bukenya EE. Sociodemographic factors associated with acceptance of COVID-19 vaccine and clinical trials in Uganda: a cross-sectional study in western Uganda. BMC Public Health. (2021) 21:1–8. 10.1186/s12889-021-11197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanyike AM, Olum R, Kajjimu J, Ojilong D, Akech GM, Nassozi DR, et al. Acceptance of the coronavirus disease-2019 vaccine among medical students in Uganda. Trop Med Health. (2021) 49:1–11. 10.1186/s41182-021-00331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yassin EOM, Faroug HAA, Ishaq ZBY, Mustafa MMA, Idris MMA, Widatallah SEK, et al. COVID-19 vaccination acceptance among healthcare staff in Sudan, 2021. J Immunol Res. (2022) 2022:1–11. 10.1155/2022/3392667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mustapha M, Lawal BK, Sha'aban A, Jatau AI, Wada AS, Bala AA, et al. Factors associated with acceptance of COVID-19 vaccine among University health sciences students in Northwest Nigeria. PLoS ONE. (2021) 16:e0260672. 10.1371/journal.pone.0260672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allagoa DO, Oriji PC, Tekenah ES, Obagah L, Njoku C, Afolabi AS, et al. Predictors of acceptance of Covid-19 vaccine among patients at a tertiary hospital in South-South Nigeria. Int J Community Med Public Health. (2021) 8:2165–72. 10.18203/2394-6040.ijcmph20211733 [DOI] [Google Scholar]
- 35.Raja SM, Osman ME, Musa AO, Hussien AA, Yusuf K. COVID-19 vaccine acceptance, hesitancy, and associated factors among medical students in Sudan. PLoS ONE. (2022) 17:e0266670. 10.1371/journal.pone.0266670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoque A, Buckus S, Hoque M, Hoque M, Van Hal G. COVID-19 vaccine acceptability among pregnant women at a primary health care facility in Durban, South Africa. Eur J Med Health Sci. (2020) 2:493. 10.24018/ejmed.2020.2.5.493 [DOI] [Google Scholar]
- 37.Adeniyi OV, Stead D, Singata-Madliki M, Batting J, Wright M, Jelliman E, et al. Acceptance of COVID-19 vaccine among the healthcare workers in the Eastern Cape, South Africa: a cross sectional study. Vaccines. (2021) 9:666. 10.3390/vaccines9060666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngasa NC, Ngasa SN, Tchouda LAS, Tanisso E, Abanda C, Dingana TN. Spirituality and other factors associated with COVID-19 Vaccine Acceptance amongst Healthcare Workers in Cameroon. (2021). 10.21203/rs.3.rs-712354/v1 [DOI] [Google Scholar]
- 39.Dula J, Mulhanga A, Nhanombe A, Cumbi L, Júnior A, Gwatsvaira J, et al. COVID-19 vaccine acceptability and its determinants in Mozambique: An online survey. Vaccines. (2021) 9:828. 10.3390/vaccines9080828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAbee L, Tapera O, Kanyangarara M. Factors associated with COVID-19 vaccine intentions in eastern Zimbabwe: a cross-sectional study. Vaccines. (2021) 9:1109. 10.3390/vaccines9101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mudenda S, Mukosha M, Meyer JC, Fadare J, Godman B, Kampamba M, et al. Awareness and Acceptance of COVID-19 Vaccines among Pharmacy Students in Zambia: The Implications for Addressing Vaccine Hesitancy. (2021). 10.21203/rs.3.rs-651501/v1 [DOI] [Google Scholar]
- 42.Mudenda S, Hikaambo CNa, Daka V, Chileshe M, Mfune RL, Kampamba M, et al. Prevalence and factors associated with COVID-19 vaccine acceptance in Zambia: a web-based cross-sectional study. Pan African Med J. (2022) 41:31219. 10.11604/pamj.2022.41.112.31219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kivuva EW. Assessing the relation between covid-19 perception and acceptance of the COVID-19 vaccine In Kenya. (2021). 10.31730/osf.io/4gdu2 [DOI] [Google Scholar]
- 44.Carpio CE, Sarasty O, Hudson D, Macharia A, Shibia M. The demand for a COVID-19 vaccine in Kenya. Hum Vaccin Immunother. (2021) 17:3463–71. 10.1080/21645515.2021.1938494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. Bmj. (1997) 315:1533–7. 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 47.Abubakar AT, Suleiman K, Ahmad SI, Suleiman SY, Ibrahim UB, Suleiman BA, et al. Acceptance of COVID-19 vaccine among healthcare workers in Katsina state, Northwest Nigeria. medRxiv. (2022). 10.1101/2022.03.20.22272677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanyanda S, Markhof Y, Wollburg P, Zezza A. Acceptance of COVID-19 vaccines in sub-Saharan Africa: Evidence from six national phone surveys. BMJ Open. (2021) 11:e055159. 10.1136/bmjopen-2021-055159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Yang L, Jin H, Lin L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prevent Med. (2021) 150:106694. 10.1016/j.ypmed.2021.106694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Jing R, Lai X, Zhang H, Lyu Y, Knoll M, et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines (Basel). (2020) 8:482. 10.3390/vaccines8030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health. (2020) 8:381. 10.3389/fpubh.2020.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Intention of healthcare workers to accept COVID-19 vaccination and related factors: A systematic review and meta-analysis. Asian Pac J Trop Med. (2021) 14:543. 10.4103/1995-7645.332808 [DOI] [Google Scholar]
- 53.Wake AD. The acceptance rate toward COVID-19 vaccine in Africa: a systematic review and meta-analysis. Global Pediatric Health. (2021) 8:2333794X211048738. 10.1177/2333794X211048738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahmud S, Mohsin M, Hossain S, Islam MM, Muyeed A. The acceptance of COVID-19 vaccine: a global rapid systematic review and meta-analysis. (2021). 10.2139/ssrn.385598735335059 [DOI] [Google Scholar]
- 55.Nindrea RD, Usman E, Katar Y, Sari NP. Acceptance of COVID-19 vaccination and correlated variables among global populations: A systematic review and meta-analysis. Clin Epidemiol Global Health. (2021) 12:100899. 10.1016/j.cegh.2021.100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasilevska M, Ku J, Fisman DN. Factors associated with healthcare worker acceptance of vaccination: a systematic review and meta-analysis. Infect Control Hospital Epidemiol. (2014) 35:699–708. 10.1086/676427 [DOI] [PubMed] [Google Scholar]
- 57.Nguyen TTM, Lafond KE, Nguyen TX, Tran PD, Nguyen HM, Do TT, et al. Acceptability of seasonal influenza vaccines among health care workers in Vietnam in 2017. Vaccine. (2020) 38:2045–50. 10.1016/j.vaccine.2019.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalis M, Hatim A, Elmouden L, Diakite M, Marfak A, Ait El Haj S, et al. Acceptability of COVID-19 vaccination among health care workers: A cross-sectional survey in Morocco. Hum Vaccin Immunother. (2021) 17:5076–81. 10.1080/21645515.2021.1989921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: a cross-sectional study from Jordan. PLoS ONE. (2021) 16:e0250555. 10.1371/journal.pone.0250555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kazeminia M, Afshar ZM, Rajati M, Saeedi A, Rajati F. Evaluation of the acceptance rate of Covid-19 vaccine and its associated factors: A systematic review and meta-analysis. J Prevention. (2022) 43:421–467. 10.1007/s10935-022-00684-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mekonnen BD, Mengistu BA. COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis. Clinical Epidemiology and Global Health. (2022) 14:101001. 10.1016/j.cegh.2022.101001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya SR, Moon DH, Shin YC. Assessing Attitude Toward COVID-19 Vaccination in South Korea. Front Psychol. (2021) 12:694151. 10.3389/fpsyg.2021.694151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Willingness-to-pay for a COVID-19 vaccine and its associated determinants in Indonesia. Hum Vaccin Immunother. (2020) 16:3074–80. 10.1080/21645515.2020.1819741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherman SM, Smith LE, Sim J, Amlôt R, Cutts M, Dasch H, et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. (2021) 17:1612–21. 10.1080/21645515.2020.1846397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. (2021) 49:21–9. 10.1016/j.ajic.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindholt MF, Jørgensen F, Bor A, Petersen MB. Public acceptance of COVID-19 vaccines: cross-national evidence on levels and individual-level predictors using observational data. BMJ Open. (2021) 11:e048172. 10.1136/bmjopen-2020-048172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callaghan T, Moghtaderi A, Lueck JA, Hotez P, Strych U, Dor A, et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc Sci Med. (2021) 272:113638. 10.1016/j.socscimed.2020.113638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith DT, Attwell K, Evers U. Support for a COVID-19 vaccine mandate in the face of safety concerns and political affiliations: An Australian study. Politics. (2021) 42:480–91. 10.1177/02633957211009066 [DOI] [Google Scholar]
- 69.Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of US adults. Ann Intern Med. (2020) 173:964–73. 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin C, Tu P, Beitsch L. Confidence and receptivity for COVID-19 vaccines: A rapid systematic review. Vaccines. (2020) 9:16. 10.3390/vaccines9010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooper S, van Rooyen H, Wiysonge CS. COVID-19 vaccine hesitancy in South Africa: how can we maximize uptake of COVID-19 vaccines? Expert Rev Vaccines. (2021) 20:921–33. 10.1080/14760584.2021.1949291 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.