Summary
Background
Estimates show that breast cancer, the leading cause of cancer death in females worldwide, will continue to increase in incidence, highlighting the need for increased treatment capacity. While postoperative radiation therapy (RT) is commonly used to reduce recurrence and mortality, research has shown that moderately hypofractionated radiation therapy (HFRT) and 5-fraction HFRT are equally safe and effective and can reduce treatment costs. This study aimed to compare the cost of conventional RT (50Gy/25), moderately HFRT (40.05Gy/15), and 5-fraction HFRT (26Gy/5) for breast cancer patients in Brazil.
Methods
The cost of each RT regimen was calculated using the International Atomic Energy Agency's Radiotherapy Cost Estimator Tool. The potential annual savings were then estimated by applying the cost of each regime to the 2020 Brazilian cancer incidence rates.
Findings
The average costs per patient for 25 fractions, 15 fractions, and 5 fractions are $2,699.20, $1,711.98, and $929.81, respectively. The annual cost savings associated with treating 70% of patients with 15 fraction HFRT and 30% of patients with 5 fraction HFRT as compared to treating all patients with 25 fraction RT is $72,929,315.40. The estimated annual productivity of 1 LINAC machine for 25 fractions, 15 fractions, and 5 fractions is 338, 647, and 1,310 patients, respectively.
Interpretation
The cost analysis revealed decreased patients’ costs and potential for increased EBRT access associated with HFRT in the Brazilian perspective.
Funding
None.
Keywords: Radiation therapy, Breast cancer, Brazil, Hypofractionated, Costs
Research in context.
Evidence before this study
Moderately hypofractionated radiation therapy (HFRT) involves delivering 40.05 Gy to 42.5Gy over 15 to 16 fractions. In breast cancer patients, the safety and efficacy surrounding moderately HFRT is well understood, and safety and efficacy surrounding ultra HFRT (26 Gy over the course of only 5 consecutive fractions) is being increasingly supported, there are key components of these hypofractionated regimens that make them promising therapies. These key components include convenience, increased patient access to treatment, and reduced cost for both patients and healthcare service providers.
Added value of this study
The current study is the first to report the costs related to the HFRT in the Brazilian context. Our findings indicate that the cost analysis revealed decreased patients’ costs and the potential to increase external beam radiation therapy access associated with HFRT in the Brazilian perspective.
Implications of all the available evidence
The present cost analysis found that HFRT is associated with reduced average estimated costs per patient, with conventional fractionation costing $2,669.20, moderately HFRT costing $1,7111.98, and ultra HFRT costing $929.81. Additionally, this paper demonstrated the significantly a potential increased access to treatment associated with HFRT. Although we considered an increase in patient output for hypofractionated regiments, it is questionable if radiation departments in Brazil can deal with logistic issues regarding this increased patient turnover. It is important to have adequate numbers of personnel, adequate transportation services, and adequate referral structure in order to make it possible.
Alt-text: Unlabelled box
Introduction
Brazil is one of the 159 countries where breast cancer is the most commonly diagnosed type of cancer in women, with an estimated 66,280 new cases and 16,724 deaths in the 2020-2022 triennium.1 Furthermore, there are a variety of treatment-related problems in Brazil, particularly concerning radiation therapy (RT). Currently, the Brazilian public health system is unable to meet the national RT demand, meaning that the shortage of RT has led to thousands of preventable deaths.2 As such, given the alarmingly high incidence of breast cancer in Brazil, the future projections of increasing trends, and the shortage of treatment capacity, it is crucial to consider strategies to improve the treatment capacity for Brazilian breast cancer patients.
Overall, the main goals for treating non-metastatic breast cancer are to eliminate the tumour from the breast as well as the regional lymph nodes and prevent metastatic recurrence and death.3,4 A key contributor to accomplishing these goals is the delivery of postoperative external beam radiation therapy (EBRT).4 Historically, the standard EBRT dose administered to breast cancer patients ranges between 50 to 50.4 Gray (Gy) over 25 to 28 fractions over the course of 5 to 6 weeks.5 There is a significant amount of literature supporting the safety and efficacy of conventional fractionation RT (CFRT) in reducing breast cancer recurrence and mortality, which is why it has historically been the standard dose delivered to breast cancer patients.6, 7, 8, 9, 10 However, in recent years there has been increasing evidence in support of a shorter course of RT which delivers a higher dose per fraction. This type of EBRT schedule is referred to as hypofractionated RT (HFRT).
Moderately HFRT involves delivering approximately 40.05 Gy to 42.5Gy over 15 to 16 fractions. Long-term studies investigating the safety and efficacy of moderately HFRT have found that it is a non-inferior treatment option as compared to the conventional 5-week schedule.5,11, 12, 13, 14, 15, 16, 17, 18, 19
More recently, an ultra-hypofractionated EBRT (ultra HFRT) schedule has been investigated which delivers a total of 26 Gy throughout only 5 consecutive fractions. The investigators of the UK-based FAST-Forward multicentre, phase 3 non-inferiority trial randomly allocated breast cancer patients to either a moderately HFRT regimen of 40 Gy over 15 fractions (3 weeks), an ultra HFRT regimen of 27 Gy over 5 fractions (1 week), and another ultra HFRT regimen of 26 Gy over 5 fractions.20 The investigators found that the ultra HFRT, consisting of 26 Gy over 5 fractions, is a non-inferior form of treatment, as compared to the recent standard of moderately HFRT, consisting of 40.05 Gy over 15 fractions.20
While the safety and efficacy surrounding moderately HFRT is well understood, and the safety and efficacy surrounding ultra HFRT is being increasingly supported, there are key components of these hypofractionated regimens that make them promising therapies. These key components include convenience, increased patient access to treatment, and reduced cost for both patients and healthcare service providers.21,22 It is important to point out that there is less evidence regarding safety with 5-fraction RT for patients with advanced tumours, as well as when lymphatic drainage irradiation is indicated since nodal irradiation was excluded from the first part of the FAST-Forward trial.
Cost-analysis studies are a crucial element to guide the implementation of technology or intervention into clinical practice. The primary aims of this paper are to estimate and compare the cost of CFRT, defined as delivering 50 Gy over 25 fractions, moderately HFRT, defined as delivering 40.05 Gy over 15 fractions, and ultra HFRT, defined as delivering 26 Gy over 5 fractions. The cost analysis is conducted in the Brazilian context to elucidate the financial implications and access to treatment associated with adopting HFRT in Brazil.
Methods
Based on the 2020 Brazilian cancer incidence rates, an estimated 61,178 patients were identified who were expected to receive EBRT, considering early-stage breast cancer.22
IAEA model calculations
As we were focused on costs from infrastructure and staff, i.e., medical salary, working hours, and cost of EBRT machines, the International Atomic Energy Agency's (IAEA) EBRT Cost Estimator Tool23 was used to perform the analysis.
In order to perform the cost analysis, the cost of each regimen was calculated using the International Atomic Energy Agency's (IAEA) EBRT Cost Estimator Tool.23 This tool allowed for the input of various parameters, such as staff salary, working hours, cost of EBRT machines, and EBRT protocols, which were adjusted based on a review of the literature and guidance from a radiation oncologist in Brazil. A review of the literature also determined that the annual capacity of 1 LINAC machine is to deliver 9,700 fractions.24 This total number of fractions was used in combination with the number of fractions associated with each regimen to calculate the annual capacity of 1 LINAC machine in terms of patients, rather than fractions. When delivering CFRT regimens of 25 fractions, a total of 388 patients can be treated, as per the calculation of 9,700 patients divided by 25 fractions per patient. When utilizing the moderately HFRT regimen, however, the annual capacity increases to 647 patients, as per the calculation of 9,700 patients divided by 15 fractions per patient. However, for the ultra HFRT regimen, the number of total fractions cannot just be divided by 5 fractions per patient, because this crude calculation would not account for the various inefficiencies such as scheduling difficulties and the additional time used for using image-guided RT. To account for this, a crude calculation was conducted, which divided the total 9,700 fractions by 5 fractions per patient, leading to 1,940 patients. This number was then multiplied by an estimation factor of 0.9 to account for logistical inefficiencies related to ultra HFRT, and then further multiplied by 0.75 to account for the additional time needed to use image-guided RT at every fraction. After taking these factors into account, the annual productivity of 1 LINAC machine using an ultra HFRT regimen is estimated to be 1,310 patients. After calculating the annual productivity of a LINAC machine for each RT regimen, the respective number of patients was inputted into the Cost Estimator Tool and various parameters were adjusted. First, the baseline cost of a LINAC machine was increased by 40%, from US$1 million to $1.4 million, to account for importation taxes in Brazil. Additionally, the baseline number of LINACs at 1 facility was increased from 1 to 2, since that is the standard in Brazil. The monthly salary of a radiation oncologist was changed from an outdated $3000 to $6000. Additionally, the minimum number of medical physicists per facility was increased from 1 to 2, as per the oncology criteria in Brazil. Similarly, the number of senior radiation technologists was changed from 1 to 3 in all regimens, the number of junior radiation oncologists was changed from 3 to 4 in all fractions, the number of radiation oncologists was changed from 3 to 4 in the ultra HFRT regimen, the number of nurses was changed from 1 to 2 in the ultra HFRT regimen, and the number of administrative personnel was changed from 2 to 3 in the ultra HFRT regimen as per the oncology criteria in Brazil. Based on these inputs, the Cost Estimator Tool provided a breakdown of all operating costs, capital costs, department costs, and the average cost per patient, for each RT regimen.
Details about the calculation behind the IAEA tool are available at https://humanhealth.iaea.org/HHW/RadiationOncology/Makingthecaseforradiotherapyinyourcountry/Roleofradiotherapyincancercare/Staffingandcostcalculation/RTE_User_Manual1_z.pdf.
Cost savings analysis
To obtain an estimate of the annual cost savings, the average cost per patient of each RT regimen was applied to the 2020 Brazilian cancer incidence rates. This was done for 4 different scenarios, the cost of treating all patients with CFRT, the cost of treating all patients with moderately HFRT, the cost of treating all patients with ultra HFRT, and the cost of treating all patients with a realistic model based on the Brazilian data regarding the prevalence of breast cancer stages. According to 2019 Brazilian data on the prevalence of breast cancer stages, 23.3% of patients were diagnosed with stage I breast cancer, 53.5% of patients were diagnosed with stage II breast cancer, and 23.3% of patients were diagnosed with stage III breast cancer. Based on the FAST-Forward trial, the patients diagnosed with stage I breast cancer and a subset of eligible patients diagnosed with stage II breast cancer, represent the early-stage breast cancer patients who are eligible for ultra HFRT.20 As such, in this realistic model, 30% of all patients will receive ultra HFRT, while the remaining 70% received moderately HFRT. To obtain the cost savings associated with adopting HFRT, the total cost of various regimens were compared, such as the total annual cost of CFRT vs. moderately HFRT, moderately HFRT vs. ultra HFRT, CFRT vs ultra HFRT, CFRT vs. the realistic mode, and moderately HFRT vs. the realistic model.
Role of the funding source
This research did not receive any specific grant from funding agencies.
Results
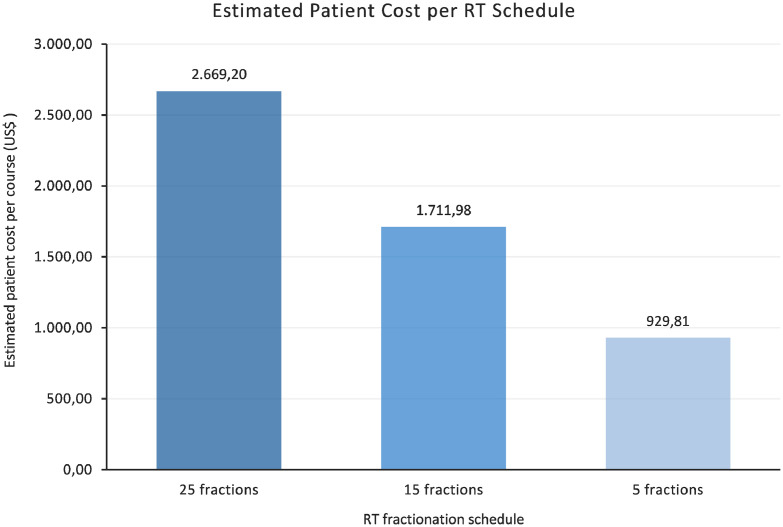
As shown in Figure 1, the average cost per patient for a 25 fraction CFRT regimen is $2,669.20. The average cost per patient for a 15-fraction moderately HFRT regimen is $1,711.98, representing a reduction of 35.9% compared to the cost of the CFRT regimen. Lastly, for a 5-fraction ultra HFRT schedule, the average cost per patient is $929.81, representing a reduction of 65.1% compared to the cost of the CFRT regimen. Table 1 details a cost breakdown of the estimated annual operating costs, capital costs, annual departmental costs, and average cost per patient, associated with each RT regimen. Table 2 lists the number of personnel required for each RT regimen, which led to changes in the annual operating costs associated with each EBRT regimen.
Figure 1.
Estimated average patient costs associated with CFRT (25 fractions), moderately HFRT (15 fractions), and ultra HFRT (5 fractions).
Table 1.
Cost breakdown of estimated annual operating costs, capital costs, annual department costs, and average patient cost associated with each EBRT regimen.
| Cost breakdown | 25 fractions | 15 fractions | 5 fractions |
|---|---|---|---|
| Operating cost/year | $975,782 | $1,047,782 | $1,158,182 |
| Personnel cost | $367,200 | $439,200 | $549,600 |
| Building and equipament amortization | $303,507 | $303,507 | $303,507 |
| Building and equipment calibration and maintenance | $305,075 | $305,075 | $305,075 |
| Capital cost | $4,920,350 | $4,920,350 | $4,920,350 |
| Departament cost/year | $1,035,648 | $1,107,648 | $1,218,048 |
| Average cost per patient | $2,669,20 | $1,711,98 | $929,81 |
Table 2.
Breakdown of number of personnel required for each EBRT regimen.
| Number of personnel required | 25 fractions | 15 fractions | 5 fractions |
|---|---|---|---|
| Radiation oncologists | 2 | 3 | 4 |
| Medical physicists | 2 | 2 | 2 |
| Senior radiation technologists | 3 | 3 | 3 |
| Junior radiation technologists | 4 | 4 | 4 |
| Nurse | 1 | 1 | 2 |
| Administrative personnel | 1 | 1 | 3 |
When these estimated patient costs are applied to all 61,178 patients, we can see a clear decrease in the annual estimated cost associated with switching from CFRT to moderate HFRT and a further decrease associated with switching to ultra HFRT. The estimated cost of treating all patients with CFRT is $163,296,318, while the cost of treating all patients with moderately HFRT and ultra HFRT is $104,735,512 and $56,883,916.20, respectively. An important consideration, however, is that not all patients would be eligible for ultra HFRT, since it is generally only used in early-breast cancer patients and caution is still needed for patients who have breast implants. As such, we created a realistic model based on the Brazilian data regarding the prevalence of breast cancer stages. According to this model, the cost of treating 30% of patients with ultra HFRT and 70% of patients with moderately HFRT is $90,387,002.60. Based on these estimated annual costs, the cost savings associated with switching from CFRT to moderately HFRT is $58,560,805.20, the cost savings associated with switching from moderately HFRT to ultra HFRT is $47,851,596.30, the cost savings associated with switching from CFRT to ultra HFRT is $106,412,401, the cost savings associated with switching from CFRT to the realistic model is $72,929,315.40, and lastly, the cost savings associated with switching from moderately HFRT to the realistic model is $14,368,509.40.
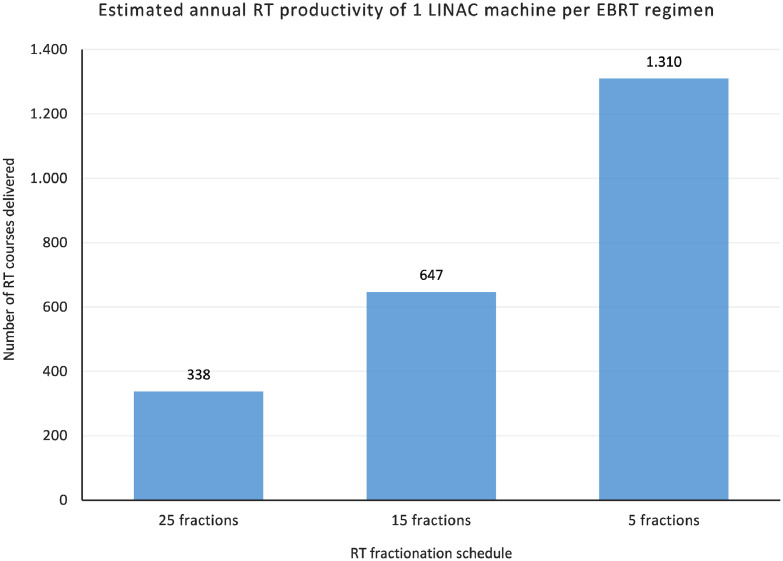
Regarding access to treatment, Figure 2 depicts the estimated annual productivity of 1 LINAC machine. This graph demonstrates a significant increase in the numbers of patients that can be treated with the adoption of HFRT, with annual LINAC productivity of 388 patients for CFRT, 647 patients for moderately HFRT, and 1,310 patients for ultra HFRT.
Figure 2.
Estimated Annual EBRT Productivity of 1 LINAC machine per RT regimen, in terms of number of EBRT courses delivered (1 EBRT course = 1 patient).
Discussion
The present cost analysis found that HFRT is associated with reduced average estimated costs per patient, and a significantly increased access to treatment associated with HFRT, even after accounting for inefficiencies, such as increased first counselling sessions and scheduling issues, and additional time needed for image-guided RT. Although we considered an increase in patient output for hypofractionated regiments, it is questionable if radiation departments in Brazil can deal with logistic issues regarding this increased patient turnover. It is important to have adequate numbers of personnel, adequate transportation services, and an adequate referral structure in order to make it possible.25,26
The estimated costs determined in this cost analysis are supported by a 2021 study conducted by Yaremko et al.27, which found similar estimated per-patient costs of CA$851.77 for the ultra HFRT FAST-Forward 1 regimen and $1,339.75 for the moderately HFRT regimen. However, many of the patient costs reported in other studies are significantly higher. This is primarily because many of these studies conducted their investigations using data from the US, which has higher patient costs, as compared to other upper-middle-income countries, and significantly higher patient costs compared to low-income countries and lower-middle-income countries. For example, a US-Based Time-Driven Activity-Based Costing analysis conducted by Dziemianowicz et al.25 found that accelerated whole breast irradiation, a form of HFRT which delivers 42.5 Gy in 16 fractions + a boost of 10 Gy in 4 fractions, costs a total of US$6,965, compared to $9,267 for CFRT. Unsurprisingly, the investigators found that a majority (86%) of the difference in cost between HFRT and CFRT was due to the lower cost of fewer daily fractions. When we compare countries with different levels of development, the personnel salaries also play a more important role in differences in costs. Overall, while the estimated costs of each regimen vary significantly depending on the country of interest, the overall trend of significantly lower costs associated with HFRT, as compared to CFRT, is consistent among the literature.
In respect to the reviewed literature relating to cost considerations, a majority of the publications reported that HFRT was associated with significantly reduced patient and/or healthcare costs, supporting the findings of this present cost analysis.28, 29, 30 However, while the cost analysis reveals promising cost savings for patients and the healthcare system, there are some concerns regarding the adoption of HFRT given current reimbursement models, which would reduce the lead to a revenue loss for oncology departments.26 Marta et al.26 found that 77% of the countries investigated would experience a revenue loss through per-patient income ranging from 5%-40%. However, the adoption of HFRT is not only an evidence-based and international guideline-supported treatment but also holds the potential to drastically improve access to treatment, which is critical in emerging economies and resource-poor settings that are unable to meet their RT demand. Given these large-scale benefits, withholding evidence-based treatments such as HFRT, due to a potential loss of income for healthcare providers, would be highly unethical. As such, it is recommended that further cost investigations must be conducted to analyze the integration of HFRT to improve the uptake of HFRT from a financial perspective.
While this paper is important in conducting a cost analysis to compare the cost of various regimes in Brazil, it does have certain limitations. A limitation regarding the cost analysis is the lack of use of a stringent model, such as a time-driven activity-based costing model or a Markov chain model. The use of such models has been supported to aid in estimating the total cost of delivering RT based on the cost of various resources, such as personnel, space, equipment, materials, and utilities. However, this present cost analysis used the IAEA's EBRT Cost Estimator tool, which is a comprehensive and reliable tool that considers personnel salaries, working arrangements, treatment protocols and building and equipment costs.23 Thus, the article is interesting in that it raises awareness of the topic and tries to evaluate it in terms of cost. Nevertheless, these numbers/models, as stated earlier, are crude and may not reflect the reality even though the model works well.
Overall, the findings presented in the paper are significant because they indicate that the adoption of HFRT could save significant healthcare costs, which is advantageous for all economies, but especially crucial for emerging economies. This is especially important in Brazil, whose public health system is currently unable to meet the national RT demand, meaning that the shortage of RT has led to thousands of preventable deaths.2 Importantly, this present analysis is unique in that it not only compares CFRT and moderately HFRT but also includes the more novel ultra HFRT regimen. Previous cost analyses, such as the one conducted by Irabor et al.21, are limited to consider the cost considerations of adopting moderately HFRT, rather than both moderately HFRT and ultra HFRT. Additionally, the findings also indicate that the adoption of HFRT can drastically improve access to RT treatment for breast cancer patients, both directly, through shorter treatments, and indirectly through reduced costs. While CFRT takes, on average, 5 weeks to complete treatment, moderately HFRT can complete this in only 3 weeks, and ultra HFRT can do so in only 1 week. As such, with significantly shorter treatment times, radiation oncology clinics can treat a significantly increased number of patients, as demonstrated by the findings of this paper. It is also important to discuss that, from the patient side, this reduces the number of trips to the clinics- which will have a tremenduous impact for people needind to travel long distances (sometimes to other states) to access treatment.
Given the results of the analysis, due to the cost and access benefits associated with HFRT, radiation oncology departments should consider adopting the use of this shorter course of treatment for eligible breast cancer patients. The adoption of HFRT could significantly decrease the number of preventable deaths attributed to the shortage of RT in Brazil. Additionally, while Brazil was the country of focus in this present study, this cost analysis methodology could be replicated and extrapolated to various countries. There are many regions of the world which are struggling to meet their national RT demand, and as such, it is recommended that further studies should be conducted to investigate the local implications of adopting HFRT, to facilitate the uptake of evidence-informed treatment strategies that hold the potential to improve healthcare capacity.
In conclusion, the adoption of HFRT is associated with significant reductions in cost and treatment time, leading to a significant increase in RT access and treatment capacity. In combination with the previously established clinical safety and efficacy of HFRT for breast cancer patients, the positive cost and access implications suggest that the adoption of HFRT presents a promising solution to improve Brazilian breast cancer treatment capacity.
Contributors
All authors above contributed to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Data sharing statement
Not applicable.
Declaration of interests
The authors have declared no conflicts of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ministério da Saúde . Incidência de Câncer no Brasil; Rio de Janeiro: 2020. Instituto Nacional do Câncer. Estimativa 2020. [Google Scholar]
- 2.Mendez LC, Moraes FY, Fernandes G dos S, Weltman E. Cancer deaths due to lack of universal access to radiotherapy in the Brazilian Public Health System. Clin Oncol. 2018;30:e29–e36. doi: 10.1016/j.clon.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Stebbing J, Delaney G, Thompson A. Breast cancer (non-metastatic) BMJ Clin Evid. 2007;2007 [PMC free article] [PubMed] [Google Scholar]
- 4.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 5.Marta GN, Coles C, Kaidar-Person O, et al. The use of moderately hypofractionated post-operative radiation therapy for breast cancer in clinical practice: a critical review. Crit Rev Oncol Hematol. 2020;156 doi: 10.1016/j.critrevonc.2020.103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe O, Abe R, Enomoto K, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet North Am Ed. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.Liljegren G, Holmberg L, Adami HO, Westman G, Graffman S, Bergh J. Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. J Natl Cancer Inst. 1994;86:717–722. doi: 10.1093/jnci/86.9.717. [DOI] [PubMed] [Google Scholar]
- 8.Liljegren G, Holmberg L, Bergh J, et al. 10-year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/jco.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 9.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 10.Malmström P, Holmberg L, Anderson H, et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: a randomised clinical trial in a population with access to public mammography screening. Eur J Cancer. 2003;39:1690–1697. doi: 10.1016/S0959-8049(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 11.Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/nejmoa0906260. [DOI] [PubMed] [Google Scholar]
- 12.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 13.Bentzen S, Agrawal R, Aird E, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal RK, Aird EGA, Barrett JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet North Am Ed. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaitelman SF, Lei X, Thompson A, et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: results of a randomized, noninferiority clinical trial. J Clin Oncol. 2018;36:3495. doi: 10.1200/JCO.18.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Fang H, Hu C, et al. Hypofractionated versus conventional fractionated radiotherapy after breast-conserving surgery in the modern treatment era: a multicenter, randomized controlled trial from China. J Clin Oncol. 2020;38:3604–3614. doi: 10.1200/JCO.20.01024. [DOI] [PubMed] [Google Scholar]
- 17.Chua BH, Link E, Kunkler I, et al. Abstract GS2-04: a randomized phase III study of radiation doses and fractionation schedules in non-low risk ductal carcinoma in situ (DCIS) of the breast (BIG 3-07/TROG 07.01) Cancer Res. 2021;81 doi: 10.1158/1538-7445.SABCS20-GS2-04. [DOI] [Google Scholar]
- 18.Offersen B v., Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III Trial: the DBCG HYPO trial. J Clin Oncol. 2020;38(31):3615–3625. doi: 10.1200/JCO.20.01363. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Fang H, Song Y, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20:352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 20.Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet North Am Ed. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irabor OC, Swanson W, Shaukat F, et al. Can the adoption of hypofractionation guidelines expand global radiotherapy access? An analysis for breast and prostate radiotherapy. JCO Glob Oncol. 2020:667–678. doi: 10.1200/JGO.19.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delaney G, Barton M, Jacob S. Estimation of an optimal radiotherapy utilization rate for breast carcinoma. Cancer. 2003;98:1977–1986. doi: 10.1002/CNCR.11740. [DOI] [PubMed] [Google Scholar]
- 23.International Atomic Energy Agency. Staffing and cost calculation 2008. https://humanhealth.iaea.org/HHW/RadiationOncology/Makingthecaseforradiotherapyinyourcountry/Roleofradiotherapyincancercare/Staffingandcostcalculation/index.html. Accessed 14 July 2021.
- 24.Zubizarreta E, Fidarova E, Healy B, Rosenblatt E. Need for radiotherapy in low and middle income countries – the silent crisis continues. Clin Oncol (R Coll Radiol) 2015;27:107–114. doi: 10.1016/J.CLON.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Dziemianowicz M, Burmeister J, Dominello M. Basic Original Report Examining the Financial Impact of Altered Fractionation in Breast Cancer: An Analysis Using Time-Driven Activity-Based Costing. Pract Radiat Oncol. 2021;11(4):245–251. doi: 10.1016/j.prro.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Marta GN, Ramiah D, Kaidar-Person O, et al. The financial impact on reimbursement of moderately hypofractionated postoperative radiation therapy for breast cancer: an international consortium report. Clin Oncol. 2021;33:322–330. doi: 10.1016/j.clon.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Yaremko HL, Locke GE, Chow R, Lock M, Dinniwell R, Yaremko BP. Cost minimization analysis of hypofractionated radiotherapy. Curr Oncol. 2021;28(1):716–725. doi: 10.3390/curroncol28010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marta GN, Riera R, Pacheco RL, et al. Moderately hypofractionated post-operative radiation therapy for breast cancer: Systematic review and meta-analysis of randomized clinical trials. Breast. 2022;62:84–92. doi: 10.1016/j.breast.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meattini I, Becherini C, Boersma L, et al. European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23(1 January):e21–e31. doi: 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
- 30.Najas GF, Stuart SR, Marta GN, et al. Hypofractionated radiotherapy in breast cancer: a 10-year single institution experience. Rep Pract Oncol Radiother. 2021 Dec 30;26(6):920–927. doi: 10.5603/RPOR.a2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]