Abstract
Viscoelastic testing (VET), including thromboelastography and thromboelastometry, provides a rapid and comprehensive picture of whole blood coagulation dynamics and hemostasis that can be reviewed and evaluated at the point-of-care. This technology is over 50 years old; however, over the past few years, there has been a significant increase in research examining the use of VET. Best practice guidelines for the use of VET exist in both the United States and Europe, particularly for elective cardiac surgery, although recommendations for implementation are somewhat limited in some clinical areas by the lack of studies constituting high-grade evidence. Other challenges to implementation surround validation of the technology in some care settings as well as lack of training. Nevertheless, there is a wide range of potential clinical applications, such as treating coagulopathies in liver disease and transplant surgery, critical care, as well as within obstetrical hemorrhage. In this illustrated review, we provide an overview of viscoelastic testing technology (also called viscoelastic hemostatic assays) and describe how the assays can be used to provide a broad overview of hemostasis from clot formation to clot lysis, while highlighting the contribution of coagulation factors and platelets. We then summarize the major clinical applications for viscoelastic testing, including more recent applications, such as in COVID-19. Each section describes the clinical context, and key publications, followed by a representative algorithm and key guidelines
Keywords: blood coagulation, clinical applications, fibrinolysis, guidelines, hemostasis, ROTEM, TEG, thromboelastography, thromboelastometry, viscoelastic testing
Essentials
-
•
Viscoelastic testing (VET) provides a full hemostasis overview from a patient whole blood sample.
-
•
VET can be rapidly reviewed and assessed at the point-of-care and acted upon in real-time.
-
•
Clinical use cases area established in trauma, cardiovascular surgery, and liver transplant areas.
-
•
More high-quality data is needed to identify and further establish clinical benefit areas with VET.
Acknowledgments
The authors thank Meridian HealthComms, Plumley, UK for providing medical writing support, in particular assistance with the illustrations, which was funded by Haemonetics S.A., Signy, Switzerland in accordance with Good Publication Practice (GPP4).
Author contributions
All authors contributed equally to the conception and writing of the review; all authors reviewed the manuscript and approved for it to be submitted.
Relationship Disclosure
J.H. is an employee of Haemonetics. D.H. is a member of TMSCC for AABB and has received meeting attendance/travel support from the St. Louis University Department of Pathology. J.H.L. has participated on a data safety monitoring boards for Merck and Octapharma and advisory boards for Leading Biosciences and Werfen.
Footnotes
Handling Editor: M Sholzberg
References
- 1.Hartmann J., Murphy M., Dias J.D. Viscoelastic hemostatic assays: moving from the laboratory to the site of care-a review of established and emerging technologies. Diagnostics (Basel) 2020;10:118. doi: 10.3390/diagnostics10020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carll T., Wool G.D. Basic principles of viscoelastic testing. Transfusion. 2020;60:S1–S9. doi: 10.1111/trf.16071. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann J., Mason D., Achneck H. Thromboelastography (TEG) point-of-care diagnostic for hemostasis management. Point Care J Near Patient Test Technol. 2018;17:15–22. [Google Scholar]
- 4.Abdelfattah K., Cripps M.W. Thromboelastography and rotational thromboelastometry use in trauma. Int J Surg. 2016;33:196–201. doi: 10.1016/j.ijsu.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Cohen T., Haas T., Cushing M.M. The strengths and weaknesses of viscoelastic testing compared to traditional coagulation testing. Transfusion. 2020;60:S21–S28. doi: 10.1111/trf.16073. [DOI] [PubMed] [Google Scholar]
- 6.Curry N.S., Davenport R., Pavord S., Mallett S.V., Kitchen D., Klein A.A., et al. The use of viscoelastic haemostatic assays in the management of major bleeding: a British Society for Haematology Guideline. Br J Haematol. 2018;182:789–806. doi: 10.1111/bjh.15524. [DOI] [PubMed] [Google Scholar]
- 7.Selby R. “TEG talk”: expanding clinical roles for thromboelastography and rotational thromboelastometry. Hematology Am Soc Hematol Educ Program. 2020;2020:67–75. doi: 10.1182/hematology.2020000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartert H. Blood clotting studies with thrombus stressography; a new investigation procedure. Klin Wochenschr. 1948;26:577–583. doi: 10.1007/BF01697545. [DOI] [PubMed] [Google Scholar]
- 9.Hardaway R., Bredenberg C. Monitoring hematology laboratory values; Kansas: 1988. Care of wounded in Vietnam. Manhatten. [Google Scholar]
- 10.Shore-Lesserson L., Manspeizer H.E., DePerio M., Francis S., Vela-Cantos F., Ergin M.A. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Kang Y.G., Martin D.J., Marquez J., Lewis J.H., Bontempo F.A., Shaw B.W., Jr., et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 12.Serraino G.F., Murphy G.J. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth. 2017;118:823–833. doi: 10.1093/bja/aex100. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S., Johnson R.I., Shaw M. Preoperative point-of-care platelet function testing in cardiac surgery. J Cardiothorac Vasc Anesth. 2015;29:333–341. doi: 10.1053/j.jvca.2014.06.025. [DOI] [PubMed] [Google Scholar]
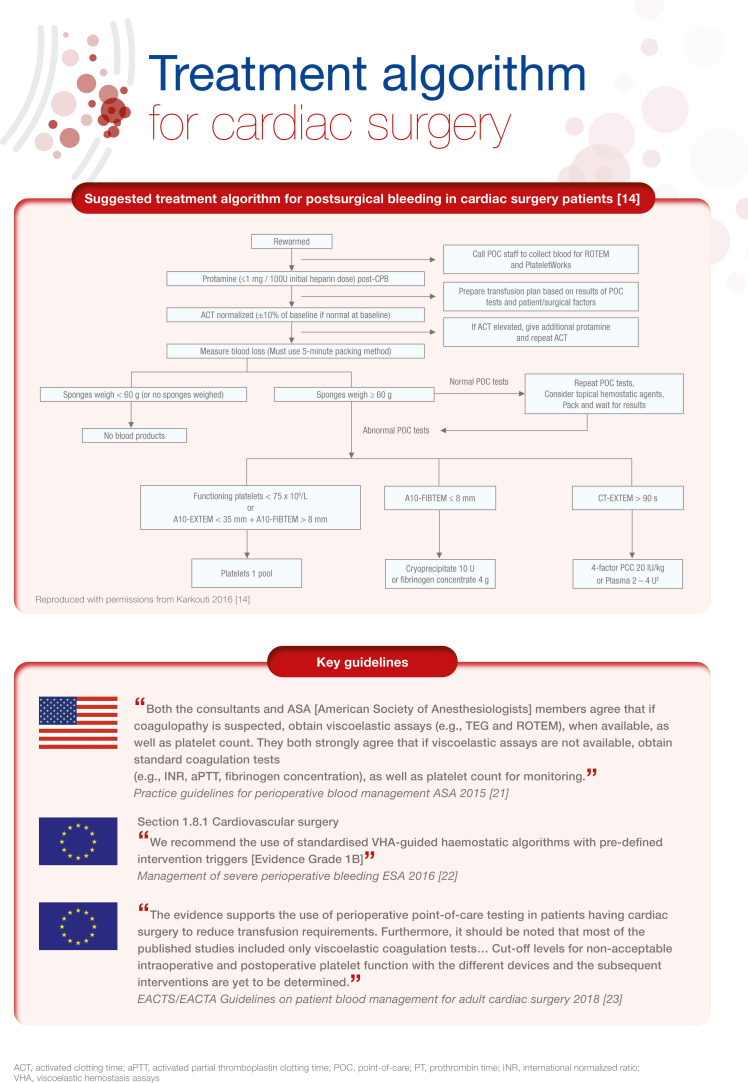
- 14.Karkouti K., Callum J., Wijeysundera D.N., Rao V., Crowther M., Grocott H.P., et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation. 2016;134:1152–1162. doi: 10.1161/CIRCULATIONAHA.116.023956. [DOI] [PubMed] [Google Scholar]
- 15.Weber C.F., Görlinger K., Meininger D., Herrmann E., Bingold T., Moritz A., et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–547. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 16.Santos A.S., Oliveira A.J.F., Barbosa M.C.L., Nogueira J.L.D.S. Viscoelastic haemostatic assays in the perioperative period of surgical procedures: systematic review and meta-analysis. J Clin Anesth. 2020;64 doi: 10.1016/j.jclinane.2020.109809. [DOI] [PubMed] [Google Scholar]
- 17.Meco M., Montisci A., Giustiniano E., Greco M., Pappalardo F., Mammana L., et al. Viscoelastic blood tests use in adult cardiac surgery: meta-analysis, meta-regression, and trial sequential analysis. J Cardiothorac Vasc Anesth. 2020;34:119–127. doi: 10.1053/j.jvca.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Li C., Zhao Q., Yang K., Jiang L., Yu J. Thromboelastography or rotational thromboelastometry for bleeding management in adults undergoing cardiac surgery: a systematic review with meta-analysis and trial sequential analysis. J Thorac Dis. 2019;11:1170–1181. doi: 10.21037/jtd.2019.04.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deppe A.C., Weber C., Zimmermann J., Kuhn E.W., Slottosch I., Liakopoulos O.J., et al. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 2016;203:424–433. doi: 10.1016/j.jss.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Wikkelsø A., Wetterslev J., Møller A.M., Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;2016:CD007871. doi: 10.1002/14651858.CD007871.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists Task Force on Perioperative Blood Management Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management∗. Anesthesiology. 2015;122:241–275. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 22.Kozek-Langenecker S.A., Ahmed A.B., Afshari A., Albaladejo P., Aldecoa C., Barauskas G., et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34:332–395. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 23.Pagano D., Milojevic M., Meesters M.I., Benedetto U., Bolliger D., von Heymann C., et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:79–111. doi: 10.1093/ejcts/ezx325. [DOI] [PubMed] [Google Scholar]
- 24.Sibbing D., Aradi D., Alexopoulos D., Ten Berg J., Bhatt D.L., Bonello L., et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. doi: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Bliden K.P., DiChiara J., Tantry U.S., Bassi A.K., Chaganti S.K., Gurbel P.A. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49:657–666. doi: 10.1016/j.jacc.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 26.Valgimigli M., Bueno H., Byrne R.A., Collet J.P., Costa F., Jeppsson A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 27.Xu O., Hartmann J., Tang Y.D., Dias J. The use of thromboelastography in percutaneous coronary intervention and acute coronary syndrome in East Asia: a systematic literature review. J Clin Med. 2022;11:3652. doi: 10.3390/jcm11133652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y.D., Wang W., Yang M., Zhang K., Chen J., Qiao S., et al. Randomized comparisons of double-dose clopidogrel or adjunctive cilostazol versus standard dual antiplatelet in patients with high posttreatment platelet reactivity: results of the CREATIVE trial. Circulation. 2018;137:2231–2245. doi: 10.1161/CIRCULATIONAHA.117.030190. [DOI] [PubMed] [Google Scholar]
- 29.Wang X.D., Zhang D.F., Zhuang S.W., Lai Y. Modifying clopidogrel maintenance doses according to vasodilator-stimulated phosphoprotein phosphorylation index improves clinical outcome in patients with clopidogrel resistance. Clin Cardiol. 2011;34:332–338. doi: 10.1002/clc.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cochrane C., Chinna S., Um J.Y., Dias J.D., Hartmann J., Bradley J., et al. Site-of-care viscoelastic assay in major trauma improves outcomes and is cost neutral compared with standard coagulation tests. Diagnostics (Basel) 2020;10:486. doi: 10.3390/diagnostics10070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
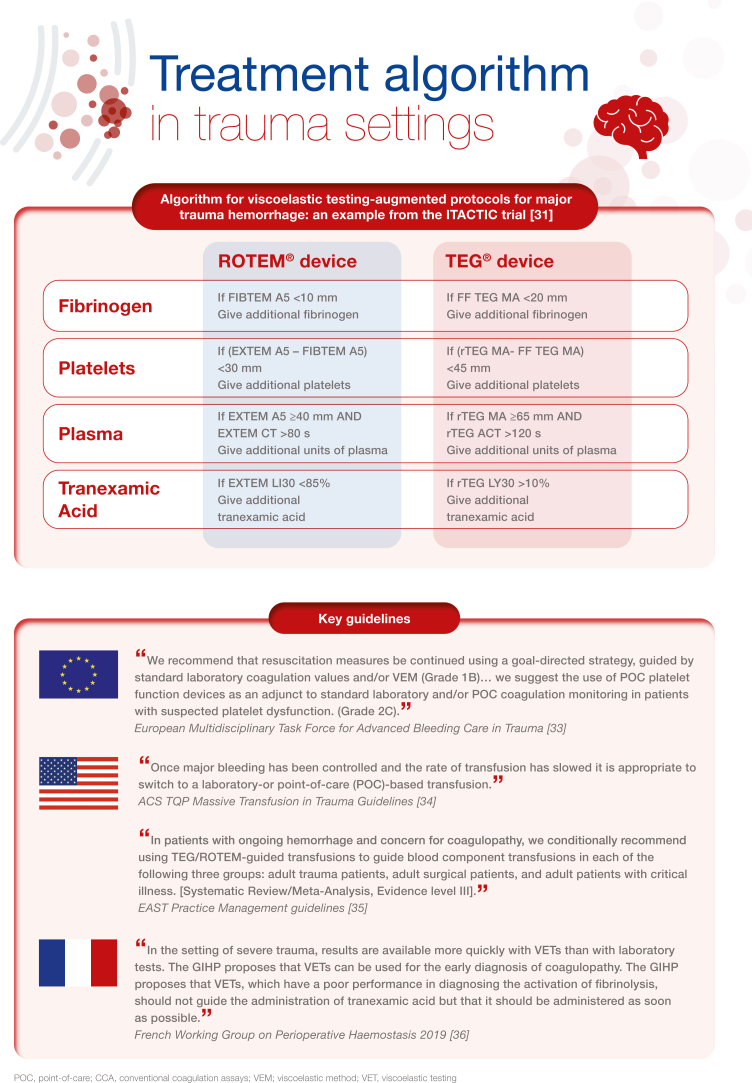
- 31.Baksaas-Aasen K., Gall L.S., Stensballe J., Juffermans N.P., Curry N., Maegele M., et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 2021;47:49–59. doi: 10.1007/s00134-020-06266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez E., Moore E.E., Moore H.B., Chapman M.P., Chin T.L., Ghasabyan A., et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263:1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spahn D.R., Bouillon B., Cerny V., Duranteau J., Filipescu D., Hunt B.J., et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23:98. doi: 10.1186/s13054-019-2347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American College of Surgeons ACS TQIP Massive transfusion in trauma guidelines. https://www.facs.org/media/zcjdtrd1/transfusion_guildelines.pdf; 2014 [accessed September 2022]
- 35.Bugaev N., Como J.J., Golani G., Freeman J.J., Sawhney J.S., Vatsaas C.J., et al. Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy: practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;89:999–1017. doi: 10.1097/TA.0000000000002944. [DOI] [PubMed] [Google Scholar]
- 36.Roullet S., de Maistre E., Ickx B., Blais N., Susen S., Faraoni D., et al. Position of the French Working Group on Perioperative Haemostasis (GIHP) on viscoelastic tests: what role for which indication in bleeding situations? Anaesth Crit Care Pain Med. 2019;38:539–548. doi: 10.1016/j.accpm.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Kovalic A.J., Khan M.A., Malaver D., Whitson M.J., Teperman L.W., Bernstein D.E., et al. Thromboelastography versus standard coagulation testing in the assessment and reversal of coagulopathy among cirrhotics: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:291–302. doi: 10.1097/MEG.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 38.Tangcheewinsirikul N., Moonla C., Uaprasert N., Pittayanon R., Rojnuckarin P. Viscoelastometric versus standard coagulation tests to guide periprocedural transfusion in adults with cirrhosis: a meta-analysis of randomized controlled trials. Vox Sang. 2022;117:553–561. doi: 10.1111/vox.13225. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann J., Dias J.D., Pivalizza E.G., Garcia-Tsao G. Thromboelastography-guided therapy enhances patient blood management in cirrhotic patients: a metaanalysis based on randomized controlled trials. Semin Thromb Hemost. 2022 doi: 10.1055/s-0042-1753530. 2022 [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Pietri L., Bianchini M., Montalti R., De Maria N., Di Maira T., Begliomini B., et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63:566–573. doi: 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
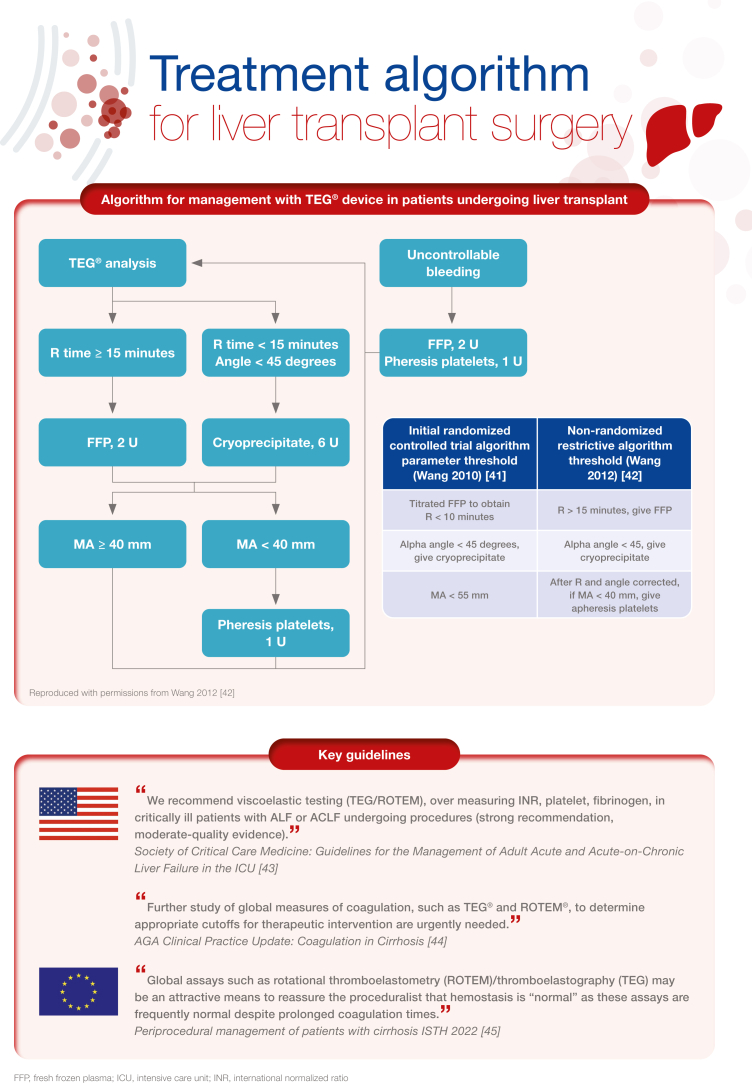
- 41.Wang S.C., Shieh J.F., Chang K.Y., Chu Y.C., Liu C.S., Loong C.C., et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590–2593. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 42.Wang S.C., Lin H.T., Chang K.Y., Mandell M.S., Ting C.K., Chu Y.C., et al. Use of higher thromboelastogram transfusion values is not associated with greater blood loss in liver transplant surgery. Liver Transpl. 2012;18:1254–1258. doi: 10.1002/lt.23494. [DOI] [PubMed] [Google Scholar]
- 43.Nanchal R., Subramanian R., Karvellas C.J., Hollenberg S.M., Peppard W.J., Singbartl K., et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48:e173–e191. doi: 10.1097/CCM.0000000000004192. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary J.G., Greenberg C.S., Patton H.M., Caldwell S.H. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157:34–43.e1. doi: 10.1053/j.gastro.2019.03.070. [DOI] [PubMed] [Google Scholar]
- 45.Roberts L.N., Lisman T., Stanworth S., Hernandez-Gea V., Magnusson M., Tripodi A., et al. Periprocedural management of abnormal coagulation parameters and thrombocytopenia in patients with cirrhosis: guidance from the SSC of the ISTH. J Thromb Haemost. 2022;20:39–47. doi: 10.1111/jth.15562. [DOI] [PubMed] [Google Scholar]
- 46.Zhou J., Xin Y., Ding Q., Jiang L., Chen Y., Dai J., et al. Thromboelastography predicts risks of obstetric complication occurrence in (hypo)dysfibrinogenemia patients under non-pregnant state. Clin Exp Pharmacol Physiol. 2016;43:149–156. doi: 10.1111/1440-1681.12509. [DOI] [PubMed] [Google Scholar]
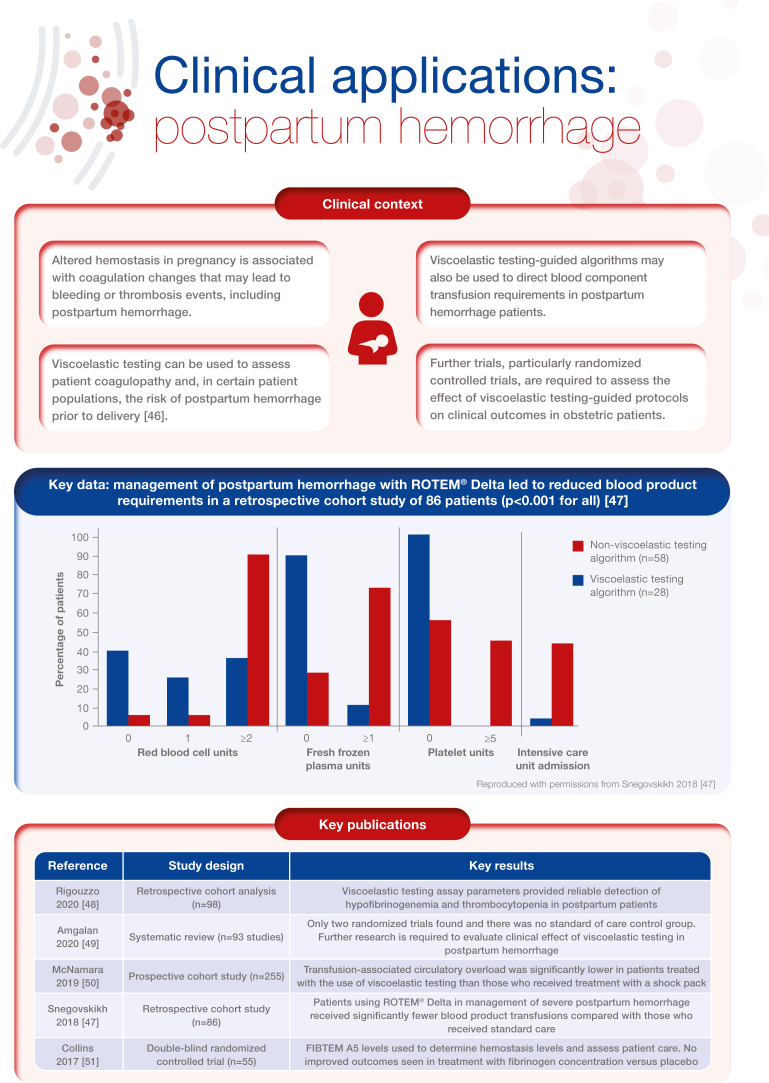
- 47.Snegovskikh D., Souza D., Walton Z., Dai F., Rachler R., Garay A., et al. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50–56. doi: 10.1016/j.jclinane.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Rigouzzo A., Louvet N., Favier R., Ore M.V., Piana F., Girault L., et al. Assessment of coagulation by thromboelastography during ongoing postpartum hemorrhage: a retrospective cohort analysis. Anesth Analg. 2020;130:416–425. doi: 10.1213/ANE.0000000000004422. [DOI] [PubMed] [Google Scholar]
- 49.Amgalan A., Allen T., Othman M., Ahmadzia H.K. Systematic review of viscoelastic testing (TEG/ROTEM) in obstetrics and recommendations from the women’s SSC of the ISTH. J Thromb Haemost. 2020;18:1813–1838. doi: 10.1111/jth.14882. [DOI] [PubMed] [Google Scholar]
- 50.McNamara H., Kenyon C., Smith R., Mallaiah S., Barclay P. Four years’ experience of a ROTEM® -guided algorithm for treatment of coagulopathy in obstetric haemorrhage. Anaesthesia. 2019;74:984–991. doi: 10.1111/anae.14628. [DOI] [PubMed] [Google Scholar]
- 51.Collins P.W., Cannings-John R., Bruynseels D., Mallaiah S., Dick J., Elton C., et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br J Anaesth. 2017;119:411–421. doi: 10.1093/bja/aex181. [DOI] [PubMed] [Google Scholar]
- 52.Bell S.F., Kitchen T., John M., Scarr C., Kelly K., Bailey C., et al. Designing and implementing an all Wales postpartum haemorrhage quality improvement project: OBS Cymru (the Obstetric Bleeding Strategy for Wales) BMJ Open Qual. 2020;9 doi: 10.1136/bmjoq-2019-000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dias J.D., Butwick A.J., Hartmann J., Waters J.H. Viscoelastic haemostatic point-of-care assays in the management of postpartum haemorrhage: a narrative review. Anaesthesia. 2022;77:700–711. doi: 10.1111/anae.15662. [DOI] [PubMed] [Google Scholar]
- 54.Collins P., Abdul-Kadir R., Thachil J. Subcommittees on Women’s Health Issues in Thrombosis and Haemostasis and on Disseminated Intravascular Coagulation. Management of coagulopathy associated with postpartum hemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:205–210. doi: 10.1111/jth.13174. [DOI] [PubMed] [Google Scholar]
- 55.Muñoz M., Stensballe J., Ducloy-Bouthors A.S., Bonnet M.P., De Robertis E., Fornet I., et al. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 2019;17:112–136. doi: 10.2450/2019.0245-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S.M., Kim S.I., Yu G., Kim J.S., Hong S.I., Chae B., et al. Role of thromboelastography in the evaluation of septic shock patients with normal prothrombin time and activated partial thromboplastin time. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartmann J., Ergang A., Mason D., Dias J.D. The role of teg analysis in patients with COVID-19-associated coagulopathy: a systematic review. Diagnostics (Basel) 2021;11:172. doi: 10.3390/diagnostics11020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maatman T.K., Jalali F., Feizpour C., Douglas A., 2nd, McGuire S.P., Kinnaman G., et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. 2020;48:e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright F.L., Vogler T.O., Moore E.E., Moore H.B., Wohlauer M.V., Urban S., et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203.e1. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kruse J.M., Magomedov A., Kurreck A., Münch F.H., Koerner R., Kamhieh-Milz J., et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. 2020;24:676. doi: 10.1186/s13054-020-03401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaudhary R., Kreutz R.P., Bliden K.P., Tantry U.S., Gurbel P.A. Personalizing antithrombotic therapy in COVID-19: role of thromboelastography and thromboelastometry. Thromb Haemost. 2020;120:1594–1596. doi: 10.1055/s-0040-1714217. [DOI] [PubMed] [Google Scholar]
- 63.Bareille M., Hardy M., Douxfils J., Roullet S., Lasne D., Levy J.H., et al. Viscoelastometric testing to assess hemostasis of COVID-19: a systematic review. J Clin Med. 2021;10:1740. doi: 10.3390/jcm10081740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S.M., Kim S.I., Yu G., Kim Y.J., Kim W.Y. Which septic shock patients with non-overt DIC progress to DIC after admission? Point-of-care thromboelastography testing. Shock. 2022;57:168–174. doi: 10.1097/SHK.0000000000001847. [DOI] [PubMed] [Google Scholar]
- 65.Lee H., Martin-Stone S., Volod O. Diagnosis and management of new onset severe coagulopathy during after-hours with thromboelastography. Am J Hematol. 2022;97:375–382. doi: 10.1002/ajh.26435. [DOI] [PubMed] [Google Scholar]
- 66.Ramiz S., Hartmann J., Young G., Escobar M.A., Chitlur M. Clinical utility of viscoelastic testing (TEG and ROTEM analyzers) in the management of old and new therapies for hemophilia. Am J Hematol. 2019;94:249–256. doi: 10.1002/ajh.25319. [DOI] [PubMed] [Google Scholar]
- 67.Raza I., Davenport R., Rourke C., Platton S., Manson J., Spoors C., et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11:307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 68.Gurbel P.A., Bliden K.P., Tantry U.S., Monroe A.L., Muresan A.A., Brunner N.E., et al. First report of the point-of-care TEG: a technical validation study of the TEG-6S system. Platelets. 2016;27:642–649. doi: 10.3109/09537104.2016.1153617. [DOI] [PubMed] [Google Scholar]
- 69.Lloyd-Donald P., Churilov L., Cheong B., Bellomo R., McCall P.R., Mårtensson J., et al. Assessing TEG6S reliability between devices and across multiple time points: a prospective thromboelastography validation study. Sci Rep. 2020;10:7045. doi: 10.1038/s41598-020-63964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dias J.D., Pottgiesser T., Hartmann J., Duerschmied D., Bode C., Achneck H.E. Comparison of three common whole blood platelet function tests for in vitro P2Y12 induced platelet inhibition. J Thromb Thrombolysis. 2020;50:135–143. doi: 10.1007/s11239-019-01971-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dias J.D., Shafizadeh E., Leiriao J., Hartmann J. Global coagulation assays to measure in vitro fibrinolysis. Thromb Update. 2021;4 [Google Scholar]
- 72.Neal M.D., Moore E.E., Walsh M., Thomas S., Callcut R.A., Kornblith L.Z., et al. A comparison between the TEG 6s and TEG 5000 analyzers to assess coagulation in trauma patients. J Trauma Acute Care Surg. 2020;88:279–285. doi: 10.1097/TA.0000000000002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Theusinger O.M., Nürnberg J., Asmis L.M., Seifert B., Spahn D.R. Rotation thromboelastometry (ROTEM) stability and reproducibility over time. Eur J Cardiothorac Surg. 2010;37:677–683. doi: 10.1016/j.ejcts.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 74.Gillissen A., van den Akker T., Caram-Deelder C., Henriquez D.D.C.A., Bloemenkamp K.W.M., Eikenboom J., et al. Comparison of thromboelastometry by ROTEM® Delta and ROTEM® Sigma in women with postpartum haemorrhage. Scand J Clin Lab Invest. 2019;79:32–38. doi: 10.1080/00365513.2019.1571220. [DOI] [PubMed] [Google Scholar]
- 75.Kuiper G.J., Kleinegris M.C., van Oerle R., Spronk H.M., Lancé M.D., Ten Cate H., et al. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016;14:1. doi: 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schöchl H., Nienaber U., Hofer G., Voelckel W., Jambor C., Scharbert G., Kozek-Langenecker S., et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]