Summary
While the use of medical and recreational cannabis is rapidly expanding under state jurisdiction, the convolution of federal regulations is obstructing research progress to the detriment of healthcare equity and the protection of vulnerable populations, such as the underaged. U.S. Senate bill S.253 is designed to accelerate the development of trusted preclinical and clinical principles based on scientific data to guide physicians in their daily practice, inform lawmakers, and thereby protect public health. This goes together with a reinforcement of the legal protection that practitioners have acquired over years of litigation with the federal government when working with their patients. S.253 supports open communication between physicians and their patients when discussing cannabis as a treatment option. The bill passed the U.S. Senate on March 24, 2022.
Funding
This work was supported by intramural funding (NYIT College of Osteopathic Medicine) to the corresponding author, Dr. Joerg R. Leheste.
Keywords: Health policy, US congress, Marijuana, US senate bill S235, The cannabidiol and marijuana research expansion act (S235), Public health, Research, Medicinal marijuana, Recreational marijuana, Developmental delays and damages, psychosis, Access of care issue, Quality of care issue
Introduction
Recently, the United States has experienced groundbreaking shifts in policy and public opinion regarding the promotion and use of cannabis and its compounds.1 With medical cannabis now legal in most states and a rising number of states permitting adult recreational use,2 a clash exists between state and federal law. Cannabis remains illegal at the Federal level under the Controlled Substances Act (CSA) as a Schedule I drug, without currently accepted medical use, a high abuse potential, and a lack of accepted safety for use under medical supervision.3 While cannabis and its medicinal derivatives appear to be relatively safe, clinical outcomes are often insufficient in establishing efficacy requiring further investigation. This is true, for example, for various types of chronic pain, affecting its suitability as an adjunct or replacement for opioids.4 On the other hand, data on the use of cannabinoids with chronic neuropathic pain and spasticity associated with multiple sclerosis (MS), are strongly supporting its safety, efficacy, and therapeutic use.5, 6, 7 Clinical application of the non-psychoactive cannabidiol (CBD) for treatment-resistant epilepsy has produced the most convincing results, especially in children.8 To this day, the Food and Drug Administration (FDA) has only approved one cannabis-derived and three cannabis-related drugs for specific uses, including chemotherapy-induced or HIV/AIDS-associated nausea and vomiting and treatment-resistant epilepsy associated with three rare pediatric disorders.9 In the face of rapidly expanding state-legal access, regulatory barriers and funding biases limit the investigation into cannabis-derived drugs, creating substantial gaps in knowledge surrounding their potential role in treating a number of diseases. Many from the scientific community argue that the deficit of rigorous research is primarily a result of the burdensome regulations associated with obtaining a Schedule I research license through the DEA. Additionally, there are increasing reports of individuals obtaining cannabis to treat or palliate serious medical conditions for which there is little, if any, evidence of benefit.10, 11, 12 Due to increased availability, there is also an increasing number of individuals using cannabis who are particularly vulnerable to the known short-term and long-term adverse effects, including pregnant women,13 adolescents,14 and individuals with psychosis.15 The Cannabidiol and Marijuana Research Expansion Act (S.253) seeks to simplify the Schedule I research registration process, streamline the development of new FDA-approved medications, and protect recommending physicians from professional liability.16
Funding & focus
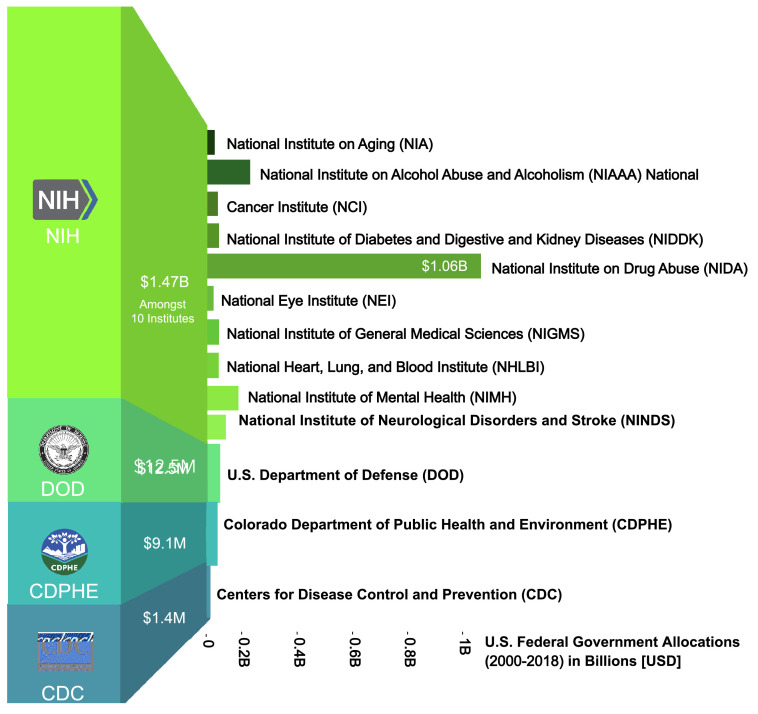
The US is amongst the nations that have expanded federal research funding considerably over the past two decades; however, the primary federal research focus remains locked on abuse rather than therapeutic potential. Between 2000 and 2018, roughly $1.49 billion were granted to researchers in the US, and a majority of those funds, about $1.47B, were distributed through the National Institutes of Health (NIH), which comprise 27 institutes and centers with various distinct focuses. Among these, the three largest funders were the National Institute on Drug Abuse (NIDA) ($1.06B), National Institute on Alcohol Abuse and Alcoholism (NIAAA) ($133M), and National Institute of Mental Health (NIMH) ($64.9M).17 Studying the priority focus areas and research goals of these institutes, clearly indicates a clinical award focus on drug abuse and adverse mental health effects and not on the medicinal potential of cannabis (Figure 1). Analysis of project grants awarded by NIDA from 2000 to 2018 reveals that the three most funded areas of research were related to the physical, psychological, and social effects of cannabis, the treatment of cannabis use or abuse, and the endocannabinoid system. Although there has been an upward trend in federal funding for research examining the therapeutic use of cannabis there is an emphasis on its negative health effects. Projections by the computerized reporting process of the NIH, the Research, Condition, and Disease Categorization (RCDC) for 2021 and 2022, are indicating small increases in cannabinoid research funding clearly earmarked for therapeutic purposes. However, the limited stratification of the RCDC precludes any further analysis along the same lines as above.18 While this research is irrefutably necessary, it dwarfs a clinical research focus including science-driven prescribing practices informing therapeutic recommendations.
Figure 1.
Top U.S. funders of cannabis research (2000–2018).
The federal government awarded approximately $1.49 billion to scientists for cannabis research. Most funds focus on drug abuse and adverse mental effects. The top three funding institutions include the National Institute on Drug Abuse (NIDA; $1.06B), National Institute on Alcohol Abuse and Alcoholism (NIAAA; $133.0M), and National Institute of Mental Health (NIMH; $64.9M). Funds with a focus on the therapeutic effect of cannabinoids include the Department of Defense (DOD, Focus: PTSD, mental health, traumatic brain injury; $12.5M), Colorado Department of Public Health and Environment (CDPHE, Focus: Therapeutic effects of whole plant vs. purified/synthetic cannabinoids; $9.1M), Centers for Disease Control and Prevention (CDC; Most awards granted to research on cannabis regulation; $1.4M).
The data gathered so far also lacks practical value because the widely used strains and potencies that are available through the state dispensaries differ significantly from what has been the sole federal source of cannabis for research purposes for the past 50 years, until recent policy changes which took effect on January 19, 2021.19,20 Further funding analysis also revealed that most of the research investigating its therapeutic effects utilize purified or synthesized cannabinoids versus all the plant's promising constituents, including major and minor cannabinoids, terpenes, and flavonoids.21, 22, 23
Regulatory process
The Drug Enforcement Agency (DEA) oversees the process through which scientists are certified to conduct research with cannabis and other controlled substances. With Schedule I substances in particular, the researcher registration process involves joint review of an application and research protocol by the DEA, an agency of the Department of Justice (DOJ), and the Department of Health and Human Services (HHS), acting through the FDA. These government agencies strictly enforce the CSA and the Food, Drug, and Cosmetics Act (FD&C), respectively, to prevent the diversion of controlled substances and ensure public safety.
Current policy dictates that to initiate a Schedule I research registration involving human subjects, an investigator must first fulfill the requirements set forth by their respective state authority (e.g., State Department of Health) and an institutional review board (IRB) at the researching institution.24,25 The DEA encourages investigators to contact local Diversion Field Offices in order to verify state requirements, as state laws and regulations vary across jurisdictions. Investigators conducting in-vitro laboratory studies or research using animal subjects are not reported to encounter the same administrative burdens or registration delays, as these protocols are subject to less scrutiny and requirements compared with those involving human subjects.
As with any other drug being studied in a clinical trial, researchers must initiate an investigational new drug (IND) application with the FDA Center for Drug Evaluation and Research (CDER), Office of New Drugs (OND), in which the study drug pharmacology, preclinical data, manufacturing information, and protocol design are assessed along with investigator qualifications to ensure that the research does not pose unreasonable risks on human subjects.26 The FDA recommends requesting a pre-IND meeting with CDER for guidance before submitting an IND, as well as the CDER Botanical Review Team (BRT). Investigators are also encouraged to consider submitting drug master files (DMFs), which are submissions to FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. DMFs are not required by statute or regulation but allow parties to reference material without disclosing DMF contents to those parties, particularly proprietary information about the product.
If the desired cultivar, or plant variation, is available through the NIDA DSP or another DEA-approved manufacturer, the investigator can request information on that particular study drug in order to fulfill the IND application requirements. In the case that a DMF for the drug is available, the researcher may request a Letter of Authorization (LOA) from the manufacturer or DMF-holder, which permits the FDA to review relevant drug information in support of an IND, or New Drug Application (NDA).27 The FDA can then perform a technical review of the protocol and study drug with reference to the DMF. The FDA will determine if the DMF is adequate or inadequate in support of the research protocol in relation to an IND or NDA application. A researcher may proceed with a protocol 30 calendar days after the submission of an application unless otherwise notified, as in the case of a clinical hold if there are deficiencies identified in any component of the application. In lieu of a DMF, during this time, FDA has an opportunity to review the IND for safety to assure that research subjects will not be subjected to unreasonable risk. If a clinical hold is imposed, an investigator may address discrepancies or safety concerns. The FDA will respond within 30 days as to whether the deficiencies are resolved and the study can proceed. In the circumstance that a study sponsor or investigator is not granted an LOA, or if a detailed analysis for a specific strain is not available, researchers must first apply for a DEA registration in order to procure the drug product for the purpose of chemistry, manufacturing, and controls analysis. After the IND application process, a researcher can then apply for a DEA Registration to conduct a clinical investigation of the drug.
Once the DEA receives a complete application for a Schedule I research registration, containing investigator information, the research protocol, facility storage and security provisions, as well as notice of institutional and IND approval, an assessment of the protocol, facility, and investigator credentials can proceed. However, if the DEA administrator identifies discrepancies in the application or protocol that require the submission of supplemental information, there are no subsequent measures in place that would ensure swift remedial processing (Figure 2). These complexities involving multiple protocol reviews and uncertainties regarding timelines put a bias on all Schedule I drug research which impedes scientific progress. For instance, the urgent need for more research on fentanyl, in the context of the opioid overdose crisis, was outlined in congressional testimony by Nora Volkow, MD, Director of NIDA, in which she described the Schedule I research registration process as problematic, in need of an overhaul, and confusing to even experienced researchers.28
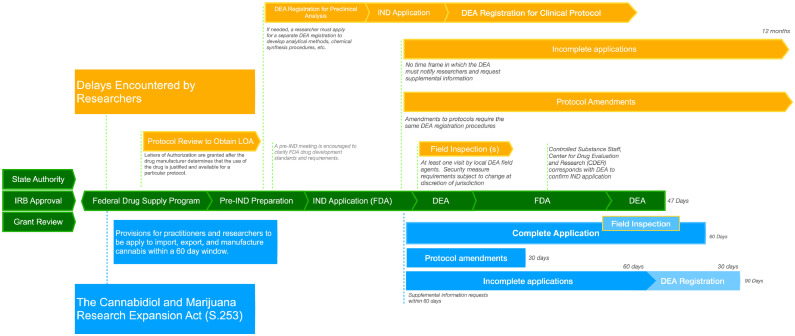
Figure 2.
Regulatory processes and DEA registration to conduct clinical research on cannabis in the US.
The total registration process is currently divided into a pre-registration (left) and a registration process (right). Prior to initiating a Schedule I research application with the DEA, investigators must obtain approval and documentation from their respective state authority and IRB. A researcher who seeks federal funds to conduct a study will often submit their protocol to be assessed by one of the science-focused federal institutions as part of a grant review.9 Protocols may also be reviewed by NIDA DSP or another federally approved manufacturer to obtain an LOA, which allows the FDA to reference detailed information on a particular cultivar, or strain, including chemistry, manufacturing, and controls during the review of an IND application. Obtaining drug product and a Letter of Authorization (LOA) has been a notable barrier for researchers as cannabis from NIDA DSP, the single federal source of cannabis before a recent DEA policy change, has been described as poor quality, of limited variety, and often available in limited or insufficient quantities. As of May 2022, an additional six manufacturers have been licensed to manufacture cannabis for research purposes. S.253 provides an avenue for new cannabis manufacturing registration applications to be processed in a timely manner, requiring the Attorney General to approve eligible new manufacturers within 60 days (blue). Regulatory hurdles and delays have been reported at every step of these processes (yellow), well before the DEA will consider an applicant.
Currently, as the DEA evaluates the safeguards in place to prevent drug diversion, the protocol is forwarded within seven days after receipt to CDER. During this FDA review, the CDER Controlled Substances Staff will correspond with the respective division of the FDA Office of New Drugs (OND) to verify the status of the associated IND authorization. Within 30 days the CDER is expected to report back to the DEA and provide commentary on the merits of the protocol and researcher qualifications, as well as a recommendation as to whether a Schedule I research registration should be issued. The DEA will conduct one or more site inspections to ensure that adequate security measures are in place to prevent the diversion of cannabis. These visits are reported to create further delays in obtaining a research registration, as local field agents assess security measures at their own discretion, which may result in unforeseen modification requests.29
S.253 would establish clear standards for drug storage, requiring an adequately constructed and securely locked cabinet consistent with practitioner storage requirements for other Schedule I and II controlled substances that pose similar public safety and diversion concerns.
Once all statutory responsibilities delineated by the CSA and FD&C are satisfied, a Schedule I research registration to conduct clinical research is issued by the DEA 10 days following the FDA recommendation. The average time for a complete application to be approved by the Attorney General (AG) is 52 days; however, only 30% of applications are complete at the time of submission.30 If an application is deemed incomplete or other complications arise, the process is further delayed and can take up to a year.31
S.253 aims to streamline the workflow between federal agencies by requiring transparent and binding timeframes for new protocols. If enacted, the DEA will be bound by a 60-day window in which federal agency review, supplement requests, and researcher certification must come to completion. Furthermore, once any supplemental materials are received, the AG would be required to issue or deny the registration within 30 days. If an application is rejected, S.253 would also require a written explanation from the AG within the timeframe outlined.
Under current policy, any research protocol amendments to the quantity, source, or conditions for storage, tracking, or administration required submission of supplemental documentation “shall be processed and approved or denied in the same manner as the original research protocol,” meaning a time-intense repetition of the initial registration process. This is another source of concern for investigators, as it creates delays during active and ongoing research or clinical studies. S.253 would guarantee an expeditious and reliable timeframe for protocol amendments (Figure 3).
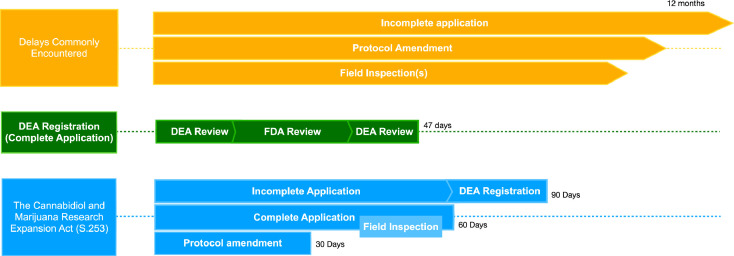
Figure 3.
DEA registration for interventional research with cannabis.
Only approximately 30% of researcher registration applications are complete at the time of submission. In this case, a registration would be issued within 47 days (green). However, the DEA reports an average processing time of 52 days once an application is complete.31 Typical delays (orange) are reported in the 6 – 12-month range. S.253 (blue) would mandate a total timeframe of no more than 60 days for complete applications. Any supplemental material must be requested by the DEA within 60 days. Upon receipt of a supplemental application, the DEA would be required to grant a registration or denial with a written explanation within 30 days. Protocol amendments are processed and assumed to be accepted within 30 days of receipt by the DEA unless they involve changes in drug quantity, source, or storage conditions. If any of the latter is required, the researcher may provide supplemental information detailing the necessary safeguards taken. In these circumstances, the Attorney General (AG) must provide written approval or instructions to modify the protocol accordingly within 10 days of receiving an amendment.
Despite these hurdles, there has been a steep increase (+149%) in the number of active cannabis researchers registered with the DEA from 2014 to 2020.32 During that time frame, studies involving cannabis extracts and derivatives accounted for 72% of the DEA's total Schedule I research registrations (589 out of 808). Although the DEA reports having decreased the whole approval time, including other federal and local protocol reviews, from 161 days in 2013 to 105 days in 2019, researchers continue to encounter regulatory obstacles from every angle of oversight. In February 2018, the DEA implemented an electronic application service, to which they attribute the shorter processing time of complete registration applications. In 2019, the DEA Chief of Policy Section at the Diversion Control Division, Loren Miller, referred to these research barriers described by scientists as “misinformation” during an oral presentation and declared a “straightforward” registration process. Instead, the presentation cited a lack of appropriate state approval, clerical discrepancies, and record-keeping errors as the primary causes of registration delays, thereby discounting any need for reform. The increase in registrants and decreased protocol approval time may, in part, be a result of the enactment of the Improving Regulatory Transparency for New Medical Therapies Act (H.R. 639) in 2015, which, in addition to Schedule I drug development policy changes and timeline impositions on the DEA to issue registrations to manufacture other controlled substances, eliminated the duplicative Public Health Service (PHS) protocol review, an appraisal that was required for non-federally funded research on Schedule I substances from 1999 - 2015.33 S. 253 contains a provision that prohibits the reinstatement of the PHS review. Concerning redundant protocol reviews, S.253 would require a registration to be issued if the protocol is approved by the DEA and HHS, or another federal entity that funds federal research. Researchers have reported that the NIH, FDA, and DEA have been overly critical in reviewing study protocols, often requesting extraneous supplemental information, protocol modifications, and costly adjustments, such as instrument calibrations.
Under the United Nations Single Convention on Narcotic Drugs of 1961, only one federal agency can oversee the production and distribution of cannabis in the United States.34 The National Center for Natural Products Research (NCNPR) at the University of Mississippi, which is federally contracted by NIDA's Drug Supply Program (DSP), has been the only source of cannabis for research and licit purposes in the United States until recent changes to the longstanding policy. Scientists have continually expressed that cannabis from NIDA DSP has been of limited value, as its constituents and pharmacologic properties do not represent the multitude of cannabis strains and formulations available to patients and consumers throughout the states. NIDA DSP has increased the maximum available concentration of THC to approximately 12% over recent years, yet the potency of strains currently available through state dispensaries is, on average, 15-25%.35 With these concentrations trending upward,36 reaching concentrations of 35% and as high as 80% in popular cannabis concentrates, the unknown cognitive and psychomotor impact of highly potent strains is of growing public health concern.37 In 2021, genomic comparison of cannabis available through state dispensaries and federally produced cannabis confirmed the significant variance between products obtained by these means.38 Beyond the concern of not being able to mimic the reality of what is being sold and consumed, S.253 will enable clinical researchers to properly establish efficacy data not only for THC and CBD but eventually the other phytocannabinoids, terpenes, and flavonoids that may hold therapeutic potential and act as contributors to the distinguishable effects observed with different strains.39 Complicating matters further, bulk cannabis from the University of Mississippi may be frozen and stored for many years before being distributed. In the past, this has led to spoilage due to microbial contamination and additional constraints for researchers.40 The legal gap between federal and state laws imposes additional hurdles and other deterrents to scientists, as well as their associated institutions, who can be withheld federal funding if cannabis is obtained for research from state-legal facilities.
In 2016, the DEA issued a statement outlining a new approach to increase the number of federally authorized cannabis growers in the US. In December 2020, after several years of interjection from the DOJ Office of Legal Counsel over concerns of treaty and CSA obligations, the DEA published a final ruling indicating that the agency would begin granting registrations to additional manufacturers for research purposes.41 In compliance with the United Nations Single Convention on Narcotic Drugs, the DEA will purchase and acquire all cannabis from manufacturers, to serve as the single federal source through which researchers can obtain cannabis. In response to public concerns that there will be registration delays due to over 50 pending applications, the DEA administrator indicated that because of the applicant review process required for diversion control, the agency will not adhere to a time frame; at the same time where a need for further reform has been denied, as previously described. Additionally, under the new policy, there are concerns that the DEA will deny the applications of capable and experienced manufacturers who supplied state-legal dispensaries in the past. The DEA stated that such prior illegal conduct will be weighed, on a case-by-case basis, when assessing the diversion risks and trustability of a facility and its operators. To date, six applicants have been registered to bulk manufacture cannabis for research purposes.42
Accordingly, there are provisions in S.253 that aim to streamline the manufacturing application process, requiring the agency to grant a manufacturing license or request supplemental information within 60 days of receiving an application, as well as approve or deny an applicant 30 days after receiving supplement material. The Senate bill focus will also require the Attorney General to evaluate the supply of cannabis to ensure that adequate quantities and varieties are available to investigators. The recent policy changes implemented by the DEA do not remove or lessen the current federal regulatory obstacles or delays that researchers must navigate in order to obtain Schedule I research registration.
Having access to relevant strains may offer incentive for researchers who are otherwise deterred by the burdensome registration process, though the researcher registration process remains status quo for now. S.253 would also mandate federal agencies to investigate the research barriers encountered by states that have decriminalized or legalized its use and make recommendations on how any research barriers may be overcome, with consideration of potential public-private partnerships and Federal-State research partnerships for the purpose of enhancing access to strains and formulations of more variety.
The enactment of the Agricultural Improvement Act 2018 (Farm Bill) defined hemp, under federal law, as “the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.” The CSA was amended correspondingly to exclude hemp from the legal definition of marijuana, effectively eliminating DEA oversight of its cultivation and marketing. The 2018 bill also contains provisions that permit the use of hemp as a source of cannabis and cannabis-derived compounds, with less than 0.3 percent delta-9 tetrahydrocannabinol concentration, for clinical research and drug development without the need for a Schedule I research registration.43
In 1999, the National Academy of Sciences Institute of Medicine made a call for NIH-funded clinical trials on the medicinal effects of cannabis.44 In 2019, Kent Hutchinson, professor of psychology and neuroscience at the University of Colorado in Boulder and the Founder of the Center for Research and Education Addressing Cannabis and Health (CU REACH), reviewed registered clinical trials from 1999 to 2018 to determine if this call was met by the NIH and the associated scientific community, specifically for the treatment of pain-related conditions, seizure disorders, and depression or anxiety — common conditions for which medical cannabis is used.45 By 2018, there were only five NIH-funded trials studying the clinical utility of cannabis as an analgesic (3 randomized trials, 197 subjects) versus 25 randomized trials for opioids (7096 subjects), or 1.5% and 12.5% of all registered pain control trials, respectively. Of the 222 antidepressant trials and 30 antiepileptic trials, there were none examining the efficacy of cannabis or cannabinoids. Since this publication, RCTs investigating the safety and efficacy of cannabidiol in the treatment of certain seizure disorders have been executed resulting in the FDA approval of epidiolex.
Healthcare practitioners
Federal law, specifically the CSA, prohibits healthcare providers from prescribing cannabis, as this would align with aiding or abetting the acquisition of a schedule I controlled substance. In November 1996, the passage of Proposition 215 (The Compassionate Use Act)46 in California and Proposition 200 (Drug Medicalization, Prevention, and Control Act)47 in Arizona decriminalized the physician-recommended use or cultivation of cannabis for patients with serious illnesses. Within weeks after these initiatives were enacted, the Director of the Office of National Drug Control Policy promulgated the federal government's policy in a statement declaring that recommending or prescribing Schedule I controlled substances is a violation of federal law that will result in the revocation of physicians' DEA licenses.48 After a class-action lawsuit brought by physicians, advocacy groups, and patients, the Ninth Circuit of Appeals opined that open doctor-patient communications are integral to medical practice and protected by the Free Speech Clause of the First Amendment, including the discussion of treatment options (Conant v. Walters, 309 F.3d 629 (9th Cir. 2002)).49 While the permanent injunction affirmed by the 9th Circuit of Appeals generally protects physicians who recommend cannabis, practitioners must carefully consider their state's cannabis legislation and the current legal landscape that often changes with new administrations and court decisions. For instance, the DOJ adopted starkly different policies contrasting the Obama and Trump administrations regarding actively enforcing federal cannabis policy in states that permit its use for medicinal or recreational purposes.50 The DEA has a record of launching investigations on physicians who have engaged in activities considered unlawful outside of the discussed federal legal bounds. The agency has also launched investigations and revoked the DEA licenses of physicians who issued too many certifications for medical cannabis or recommendations for high plant counts.51 Physicians enter a legal ‘gray area' when taking administrative, advisory, or board positions with dispensaries and are urged to consult with legal experts if doing so. In 2014, after the Ogden memo was released indicating that the Obama Administration would not interfere with state cannabis policy or prosecute individuals compliant with state law, Massachusetts physicians who were affiliated with cannabis dispensaries were targeted by the DEA and presented with the ultimatum to either sever ties with dispensaries or risk revocation of their DEA license, a consequence that could severely limit a clinician's practice.52
To expand treatment equity and uphold the doctor-patient relationship, S.253 reinforces the Conant v. Walters permanent injunction, creating another layer of protection for physicians who choose to recommend medical cannabis.
State medical boards overseeing medical cannabis programs often provide practitioners with guidelines that aim to prevent exposure to federal law. Physicians are urged to exercise caution regarding their dialogue and recommendation of cannabis. The Medical Board of California, like other state government agencies, lays out the guidelines for physicians participating in medical cannabis programs, including qualifying conditions, informed and shared decision making, physician conflict of interest, and more.53 In contrast to the guidelines that practitioners are accustomed to for all other purposes, they are instructed to not steer patients toward a specific product or service because of the legal challenges involving cannabis-based products. Additionally, a recommendation should never contain any instruction that directs a dispensing facility to prepare or dispense the product to their patient. Many physicians find these restrictions to be contrary to the principles and methods enforced by their medical training and often cite this discrepancy as a primary reason for not considering medical cannabis treatment options for their patients.
Along with the legal and procedural hurdles practitioners encounter in these circumstances, the clinical data that could provide guidance regarding optimal and safe dosing, routes of administration, and appropriate formulations for specific conditions remains scarce. Obtaining informed consent indicating that the potential side effects, including psychomotor and cognitive impairment, have been thoroughly discussed with the patient is highly encouraged by legal experts to protect physicians against malpractice liability.54 A recent study amongst pediatric oncologists and their perspectives on considering medical cannabis in children with cancer revealed that only 5% were familiar with state-specific regulations and 59% with its continued prohibition by federal law. This research also revealed that beyond concerns of substance abuse potential and federal prosecution, the absence of clear guiding standards detailing formulations, potency, and dosing were the greatest barriers to recommending medical cannabis to their patients (46%).55 A scoping review of medical and allied health students’ education on cannabis found that most trainees are unprepared to counsel patients on cannabis use or medical cannabis.56 To what extent medical education and residency training will adapt in the coming years remains unknown. Recently, there has been more of a legislative focus to enhance the doctor-patient relationship, standard of care, and medical evaluation for medical cannabis patients, particularly in jurisdictions with legal programs. Over time, states have been implementing mandatory training courses for physicians who wish to certify patients for medical cannabis. The New York State Department of Health (NYSDOH), for instance, requires practitioners to complete a single 2-4 hour course before they can certify patients or work at a dispensing facility.57 A systematic review examining practitioners’ self-perceived knowledge of cannabis suggests that they consider their legislative and medical knowledge of cannabis poor, often acquiring information through their own clinical experience, the internet, and news media.58 Likewise, surveyed medical cannabis patients have expressed low levels of confidence in their doctor to integrate cannabis as a treatment, and many report withholding their cannabis use and medication substitution to their primary care provider (PCP).59,60 In fact, an analysis of Medicare D enrollee prescriptions in states with medical cannabis programs has shown that FDA-approved medications for a range of conditions significantly decline after the enactment of state cannabis laws.61,62 These data present a public health concern, as cannabis has not been proven efficacious in treating most of the conditions identified in these studies, such as glaucoma and depression, and may even exacerbate underlying conditions. As medical knowledge on the pharmacology of cannabis and its clinical applications increases, it will be vital to step up and extend proper training and certification not only to physicians but also to all allied healthcare trainees to ensure a continuum of knowledge and good clinical decision making.63
S.253 aims to equip those at the forefront of patient care with knowledge and tools to effectively counsel and treat patients, as well as enrich a diminished doctor-patient relationship in the age of state cannabis legalization. At the same time, it will be important to continue to ensure best practice and patient safety by continuing to investigate physicians who may have acted in their own rather than their patient's interest by granting access to medical cannabis without fulfilling basic responsibilities as physicians.64
Policymakers
The negative health effects of cannabis have been studied to a much greater extent but even so, the accepted assessment of potential harms may be an underestimate due to the less potent variety of cannabis reserves used for research. Acute adverse effects of cannabis use include altered sensorium, cognitive deficits, psychomotor delay, tachycardia, and paranoia or psychosis.65 Chronic frequent use has been linked with decreased cognitive abilities, as well as a higher risk of substance use disorders and mental illness, including depressive and psychosis-related disorders. Individuals who begin using cannabis during adolescence are more vulnerable to these adverse effects and may have lower psychosocial and educational outcomes later in life. Research suggests that cannabis use may increase the risk of myocardial infarction and stroke.66 More research is needed on both short-term and long-term effects of cannabis use to identify associated risks of developing various cancers, pulmonary disease, and metabolic disorders.67
Cannabis use prior to operating a vehicle or machinery is known to alter a driver's abilities and judgment, increasing the risk of motor vehicle accidents – a fact that is especially problematic in young drivers.68,69 Standardized reporting systems and reliable measures of impairment across jurisdictions are currently needed to implement effective nationwide countermeasures.
Edible products available through state markets often resemble common snacks, such as brownies and fruit chews. The packaging and branding containing these commonly sought products are also known to mimic that of common foods and candies.70 This, along with increasingly potent products and inaccurate labeling, has likely contributed to an increase in emergency room visits and poison control calls for cases involving cannabinoid hyperemesis syndrome and accidental ingestion in states with medical and recreational cannabis dispensaries.71
While the official federally sanctioned application process is slow and full of hurdles, state-level decisions on medical and recreational use of cannabis are guided significantly by popular perception and demand, leaving many individuals uninformed of the associated risks and vulnerable to negative sequelae. Due to the indecision associated with data collection across states, surveillance capacity has been constrained, as well as the reach of policymaking to mitigate outcomes such as motor vehicle crashes, psychosocial disparities, and economic burdens. Title IV of the Senate bill specifically addresses these public health concerns, mandating federal health and science agencies to research and report on the upward trend in THC potency and the adverse health effects of cannabis, particularly on adolescents and developing fetuses, as well as motor vehicle or heavy machinery operators. The critical data obtained from this mandated research can guide federal and state government officials and law enforcement agencies in establishing effective regulations and harm reduction strategies such as potency caps, labeling, and packaging requirements, and national highway safety regulations.
Contributors
J.M.P.: Writing - original draft, writing - review and editing, figures creation, data collection, formal analysis, interpretation, literature search. T.M.P.: Data interpretation, writing - review and editing. J.R.L.: Conceptualization, supervision, writing - review and editing, figures review and editing, data analysis and interpretation, literature search.
Declaration of interests
The authors declared no conflicts of interest.
Funding
This work was supported by intramural funding (NYIT College of Osteopathic Medicine) to the corresponding author, Dr. Joerg R. Leheste.
References
- 1.Chiu V, Leung J, Hall W, Stjepanović D, Degenhardt L. Public health impacts to date of the legalisation of medical and recreational cannabis use in the USA. Neuropharmacology. 2021;193 doi: 10.1016/j.neuropharm.2021.108610. [DOI] [PubMed] [Google Scholar]
- 2.21 U.S. Code § 812 - schedules of controlled substances. Legal Information Institute. https://www.law.cornell.edu/uscode/text/21/812. Accessed 11 May 2022.
- 3.National Conference of State Legislatures . Ncsl.org; 2019. State Medical Marijuana Laws.https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx Updated November 29, 2021. Accessed 7 December 2021. [Google Scholar]
- 4.Pantoja-Ruiz C, Restrepo-Jimenez P, Castañeda-Cardona C, Ferreirós A, Rosselli D. Cannabis and pain: a scoping review. Braz J Anesthesiol. 2022;72(1):142–151. doi: 10.1016/j.bjane.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudroff T, Honce JM. Cannabis and multiple sclerosis-the way forward. Front Neurol. 2017;8:299. doi: 10.3389/fneur.2017.00299. Published 2017 June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fragoso YD, Carra A, Macias MA. Cannabis and multiple sclerosis. Expert Rev Neurother. 2020;20(8):849–854. doi: 10.1080/14737175.2020.1776610. [DOI] [PubMed] [Google Scholar]
- 7.Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules. 2019;24(8):1459. doi: 10.3390/molecules24081459. Published 2019 April 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration . Food and Drug Administration Website; 2020. FDA and Cannabis: Research and Drug Approval Process.https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process Accessed 2 February 2021. [Google Scholar]
- 10.Wallis D, Coatsworth JD, Mennis J, et al. Predicting self-medication with cannabis in young adults with hazardous cannabis use. Int J Environ Res Public Health. 2022;19(3):1850. doi: 10.3390/ijerph19031850. Published 2022 Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jugl S, Okpeku A, Costales B, et al. A mapping literature review of medical cannabis clinical outcomes and quality of evidence in approved conditions in the USA from 2016 to 2019. Med Cannabis Cannabinoids. 2021;4(1):21–42. doi: 10.1159/000515069. Published 2021 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montero-Oleas N, Arevalo-Rodriguez I, Nuñez-González S, Viteri-García A, Simancas-Racines D. Therapeutic use of cannabis and cannabinoids: an evidence mapping and appraisal of systematic reviews. BMC Complement Med Ther. 2020;20(1):12. doi: 10.1186/s12906-019-2803-2. Published 2020 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine JD, Moreau AL, Karcher NR, et al. Association of prenatal cannabis exposure with psychosis proneness among children in the adolescent brain cognitive development (ABCD) study. JAMA Psychiatry. 2019;76(7):762–764. doi: 10.1001/jamapsychiatry.2019.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186–2193. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427–436. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2021. Cannabidiol and Marihuana Research Expansion Act. S. 253. 117th Congress (2021-2022)https://www.congress.gov/bill/117th-congress/senate-bill/253/text Accessed 7 February 2021. [Google Scholar]
- 17.Hudson J. Hellth.com Website; 2020. Funding for Cannabis Research.http://www.hellth.com Accessed 2 February 2021. [Google Scholar]
- 18.Report. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). National Institutes of Health. https://report.nih.gov/funding/categorical-spending#/. Accessed 11 May 2022.
- 19.Muro A., Cladellas R., Castellà J. Cannabis and its different strains: do they have differential effects on time perception? Experiment Psychol. 2021;68(2):57–66. doi: 10.1027/1618-3169/a000510. [DOI] [PubMed] [Google Scholar]
- 20.Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain. 2018;19(1):37. doi: 10.1186/s10194-018-0862-2. Published 2018 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of cannabis sativa L. Prog Chem Org Nat Prod. 2017;103:1–36. doi: 10.1007/978-3-319-45541-9_1. [DOI] [PubMed] [Google Scholar]
- 22.LaVigne JE, Hecksel R, Keresztes A, Streicher JM. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci Rep. 2021;11(1):8232. doi: 10.1038/s41598-021-87740-8. Published 2021 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferber SG, Namdar D, Hen-Shoval D, et al. The “Entourage Effect”: terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr Neuropharmacol. 2020;18(2):87–96. doi: 10.2174/1570159×17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.21 U.S. Code § 823(f) - Registration Requirements. Legal Information Institute. Cornell Law School website. www.law.cornell.edu/uscode/text/21/823. Accessed 8 December 2021.
- 25.Schedule 1 Researcher Pre-application Checklist. CSA Registration Tools. Drug Enforcement Agency Diversion Control website. https://apps.deadiversion.usdoj.gov/webforms2/spring/main?execution=e1s2. Accessed 24 May 2022.
- 26.The Food and Drug Administration . U.S. Food and Drug Administration; 2020. FDA and Cannabis: Research and Drug Approval Process.https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process Accessed 11 May 2022. [Google Scholar]
- 27.Center for Drug Evaluation and Research . U.S. Food and Drug Administration; 2019. Drug Master Files: Guidance for Industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-master-files-guidance-industry Accessed 11 May 2022. [Google Scholar]
- 28.NIDA . National Institute on Drug Abuse website; 2021. The Overdose Crisis: Interagency Proposal to Combat Illicit Fentanyl-Related Substances.https://nida.nih.gov/about-nida/legislative-activities/testimony-to-congress/2021/the-overdose-crisis-proposal-to-combat-illicit-fentanyl Accessed 8 May 2022. [Google Scholar]
- 29.Evaluating the therapeutic potential of cannabinoids workshop summary . 2018. Evaluating the Therapeutic Potential of Cannabinoids: How To Conduct Research Within the Current Regulatory Framework.https://files.nccih.nih.gov/s3fs-public/Evaluating%20the% 20Therapeutic%20Potential%20of%20Cannabinoids%20Work shop%20 Summary [2].pdf?9aWvuFL_LeB0oOj5NZqaek37ZUBk_aus Accessed 12 May 2022. [Google Scholar]
- 30.Cannabis Policy: Public Health and Safety Issues and Recommendations . 2021. United States Senate Caucus on International Narcotics Control website.https://www.drugcaucus.senate.gov/wp-content/uploads/2021/08/02-March-2021-Cannabis-Policy-Report-Final.pdf Accessed March 2021. [Google Scholar]
- 31.NIDA . National Institute on Drug Abuse website; 2020. Hearing on Cannabis Policies for the New Decade.https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2020/hearing-on-cannabis-policies-for-the-new-decade Accessed 24 November 2021. [Google Scholar]
- 32.Miller L. Drug Enforcement Administration Diversion Control Division website; 2019. Research and the DEA Registration.https://www.deadiversion.usdoj.gov/mtgs/researcher_train/conf_2019/feb_2019/miller.pdf Accessed June 2021. [Google Scholar]
- 33.2015. Improving Regulatory Transparency for New Medical Therapies Act. H.R.639. 114th Congress (2015-2016)https://www.congress.gov/bill/114th-congress/house-bill/639/text Accessed 1 April 2021. [Google Scholar]
- 34.Single Convention on Narcotic Drugs . United Nations Treaty Collection website; 1961. Chapter VI Narcotic Drugs and Psychotropic Substances.https://treaties.un.org/Pages/ViewDetails.aspx?src= IND&mtdsg_no=VI-15&chapter=6&clang=_en Published March 30, 1961. Accessed 3 March 2021. [Google Scholar]
- 35.Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep. 2017;7:46528. doi: 10.1038/srep46528. Published 2017 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A comprehensive review of cannabis potency in the United States in the last decade. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):603–606. doi: 10.1016/j.bpsc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Meier MH, Docherty M, Leischow SJ, Grimm KJ, Pardini D. Cannabis concentrates use in adolescents. Pediatrics. 2019;144(3) doi: 10.1542/peds.2019-0338. [DOI] [PubMed] [Google Scholar]
- 38.Vergara D, Huscher EL, Keepers KG, et al. Genomic evidence that governmentally produced cannabis sativa poorly represents genetic variation available in state markets. Front Plant Sci. 2021;12 doi: 10.3389/fpls.2021.668315. Published 2021 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amin MR, Ali DW. Pharmacology of medical cannabis. Adv Exp Med Biol. 2019;1162:151–165. doi: 10.1007/978-3-030-21737-2_8. [DOI] [PubMed] [Google Scholar]
- 40.Newman H. Food and Drug Law Institute website; 2019. Cannabis Clinical Investigations in Colorado.https://www.fdli.org/2019/09/food-and-drug-law-journal-symposium-spotlight-cannabis-clinical-investigations-in-colorado-2019/ Accessed 10 March 2021. [Google Scholar]
- 41.Controls To Enhance the Cultivation of Marihuana for Research in the United States. Drug Enforcement Administration . 2020. Federal Register. Vol. 85, No. 244/Friday. Rules and Regulations - Document 85 FR 82333. 82333-82355 (23 pages). CFR: Codified 21 CFR 1301, 21 CFR 1318.https://www.federalregister.gov/d/2020-27999 Accessed 1 March 2021. [Google Scholar]
- 42.Marihuana Growers Information. Marihuana growers information. https://www.deadiversion.usdoj.gov/drugreg/marihuana.htm. Accessed 11 May 2022.
- 43.2018. H.R.2 - 115th Congress (2017-2018): Agriculture Improvement Act of 2018.https://www.congress.gov/bill/115th-congress/house-bill/2/text Accessed 4 May 2022. [Google Scholar]
- 44.Institute of Medicine (US) In: Marijuana and Medicine: Assessing the Science Base. Joy JE, Watson SJ Jr., Benson JA Jr., editors. National Academies Press (US); Washington, DC: 1999. [PubMed] [Google Scholar]
- 45.Hutchison KE, Bidwell LC, Ellingson JM, Bryan AD. Cannabis and health research: rapid progress requires innovative research designs. Value Health. 2019;22(11):1289–1294. doi: 10.1016/j.jval.2019.05.005. Epub 2019 Oct 18. PMID: 31708066. [DOI] [PubMed] [Google Scholar]
- 46.California Legislature . California Legislative Information website; 1996. Article 2. Cannabis [11357 - 11362.9]https://leginfo.legislature.ca.gov/faces/codes_displaySection.xhtml?sectionNum=11362.5.&lawCode=HSC Accessed 4 July 2021. [Google Scholar]
- 47.Ballotpedia . Ballotpedia.org website; 1996. Arizona Use or Possession of Controlled Substances, Proposition 200.https://ballotpedia.org/Arizona_Use_or_Possession_of_Controlled_Substances,_Proposition_200_(1996) Accessed 4 July 2021. [Google Scholar]
- 48.62 FR 6164 - Administration Response to Arizona Proposition 200 and California Proposition 215. Drug Enforcement Administration. Federal Register. 1997;62(28) Accessed 17 January 2022. [Google Scholar]
- 49.Conant v. Walters, 309 F.3d 629(9th Cir. 2002). Casetext website. https://casetext.com/case/conant-v-walters. Accessed 1 July 2021.
- 50.The U.S. Attorney General Revokes Previous Guidance on Federal Enforcement of State Marijuana Laws. 2018 https://guinncenter.org/u-s-attorney-general-revokes-previous-guidance-federal-enforcement-state-marijuana-laws/ Accessed 27 December 2021. [Google Scholar]
- 51.Ingold J. Dea Pulls Certificates for Two Colorado Doctors in Medical Marijuana Controversy. 2017 https://www.denverpost.com/2017/02/06/dea-pulls-doctors-certificates-medical-marijuana/ Accessed 27 December 2021. [Google Scholar]
- 52.Lazar K, Murphy S. BostonGlobe.com; 2014. DEA Targets Doctors Linked to Medical Marijuana - The Boston Globe.https://www.bostonglobe.com/metro/2014/06/05/drug-enforcement-administration-targets-doctors-associated-with-medical-marijuana-dispensaries-physicians-say/PHsP0zRlaxXwnDazsohIOL/story.html Accessed 27 December 2021. [Google Scholar]
- 53.Brown E, GnanaDev D, Kirchmeyer K., Medical Board of California . Medical Board of California; 2018. Medical Board of California's Guidelines for the Recommendation of Cannabis for Medical Purposes.https://www.mbc.ca.gov/Download/Publications/guidelines-cannabis-recommendation.pdf Accessed 11 January 2022. [Google Scholar]
- 54.Susman E. AAPM: Docs Get Advice on Medical Marijuana. 2011 https://www.medpagetoday.com/meetingcoverage/aapm/25601 Accessed 27 December 2021. [Google Scholar]
- 55.Ananth P, Ma C, Al-Sayegh H, et al. Provider perspectives on use of medical marijuana in children with cancer. Pediatrics. 2018;141(1) doi: 10.1542/peds.2017-0559. Epub 2017 Dec 12. PubMed PMID: 29233937; PubMed Central PMCID: PMC5744275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zolotov Y, Metri S, Calabria E, Kogan M. Medical cannabis education among healthcare trainees: a scoping review. Complement Ther Med. 2021;58 doi: 10.1016/j.ctim.2021.102675. [DOI] [PubMed] [Google Scholar]
- 57.New York Department of Health. Practitioner Information for the Medical Marijuana Program. https://www.health.ny.gov/regulations/medical_marijuana/practitioner/. Accessed 16 January 2022.
- 58.Gardiner KM, Singleton JA, Sheridan J, Kyle GJ, Nissen LM. Health professional beliefs, knowledge, and concerns surrounding medicinal cannabis - a systematic review. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0216556. PMID: 31059531; PMCID: PMC6502454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azcarate PM, Zhang AJ, Keyhani S, Steigerwald S, Ishida JH, Cohen BE. Medical reasons for marijuana use, forms of use, and patient perception of physician attitudes among the US population. J Gen Intern Med. 2020;35(7):1979–1986. doi: 10.1007/s11606-020-05800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boehnke K.F., Litinas E., Worthing B., et al. Communication between healthcare providers and medical cannabis patients regarding referral and medication substitution. J Cannabis Res. 2021;3:2. doi: 10.1186/s42238-021-00058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradford AC, Bradford WD, Abraham A, Bagwell Adams G. Association between US state medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Intern Med. 2018;178(5):667–672. doi: 10.1001/jamainternmed.2018.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradford AC, Bradford WD. Medical marijuana laws reduce prescription medication use in medicare part D. Health Aff. 2016;35(7):1230–1236. doi: 10.1377/hlthaff.2015.1661. [DOI] [PubMed] [Google Scholar]
- 63.Sajdeya R, Shavers A, Jean-Jacques J, et al. Practice patterns and training needs among physicians certifying patients for medical marijuana in Florida. J Prim Care Community Health. 2021;12 doi: 10.1177/21501327211042790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glickman A, Sisti D. Prescribing medical cannabis: ethical considerations for primary care providers. J Med Ethics. 2020;46(4):227–230. doi: 10.1136/medethics-2019-105759. [DOI] [PubMed] [Google Scholar]
- 65.NIDA . National Institute on Drug Abuse website; 2019. Cannabis (Marijuana) DrugFacts.https://nida.nih.gov/publications/drugfacts/cannabis-marijuana Accessed 8 May 2022. [Google Scholar]
- 66.Marijuana and Public Health . 2021. Centers for Disease Control and Prevention.https://www.cdc.gov/marijuana/index.htm Accessed 11 May 2022. [Google Scholar]
- 67.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice . National Academies Press (US); Washington (DC): 2017. Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. [PubMed] [Google Scholar]
- 68.Hammond D, Goodman S, Wadsworth E, Rynard V, Boudreau C, Hall W. Evaluating the impacts of cannabis legalization: The International Cannabis Policy Study. Int J Drug Policy. 2020;77 doi: 10.1016/j.drugpo.2020.102698. [published online ahead of print, 2020 February 26] [DOI] [PubMed] [Google Scholar]
- 69.Pearlson GD, Stevens MC, D'Souza DC. Cannabis and driving. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.689444. Published 2021 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ompad DC, Snyder KM, Sandh S, et al. Copycat and lookalike edible cannabis product packaging in the United States. Drug Alcohol Depend. 2022;235 doi: 10.1016/j.drugalcdep.2022.109409. [published online ahead of print, 2022 March 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mycyk MB, Routsolias JC. Growth in recreational cannabis markets and burden on emergency departments. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.25275. [DOI] [PubMed] [Google Scholar]