Summary
Background
An up-to-date analysis of gastric cancer mortality among Hispanic/Latino populations is required for estimating disease burden and assessing the effectiveness of clinical and preventive strategies.
Methods
We retrieved gastric cancer deaths between 1997 and 2017 (as available) from the Surveillance, Epidemiology, and End Results Program (United States Hispanics) and the World Health Organization databases (Puerto Rico, 16 Latin American and Caribbean countries). Joinpoint regression analysis was used to examine trends in age-standardized mortality rates (ASMR; per 100 000 person-years) and calculate average annual percent changes (AAPCs) by country (or territory), age group (25–49 and ≥50 years), and sex. Trends were compared to assess slope parallelism.
Findings
In 2017, Chile (31·8), Colombia (24·3) and Costa Rica (24·3) had the highest ASMR of gastric cancer for men, while Guatemala (17·2), Peru (13·5), and Costa Rica (13·3) had the highest ASMR for women. Small-to-moderate mortality declines (AAPCs ranged −4 to −0.5%) were observed between 1997 and 2017. In almost all countries, trends decreased among individuals aged ≥50 years. However, age-specific trends were not parallel (p-values <0.05) in Brazil, Colombia, Mexico, the United States, and Venezuela for both men and women, and in five additional countries for only women; with a few countries showing stable or slightly increasing trends for individuals aged 25–49 years.
Interpretation
Overall gastric cancer mortality rates in Hispanics/Latinos declined in the last two decades. However, there was a notable variation in trends by country, sex, and age group. Continued and targeted prevention efforts are needed to reduce the disease burden in these vulnerable populations.
Funding
Universidad Cientifica del Sur, Peru, and National Cancer Institute, United States.
Keywords: Gastric cancer, Hispanics, Latinos, Mortality trends
Research in context.
Evidence before this study
Gastric cancer is one of the leading causes of cancer mortality globally, affecting disproportionally Hispanic/Latino populations. Coverage and quality of cancer incidence registration vary across geographic regions, with only ∼20% of the Latin American population covered by population-based registration. In most of the Americas, gastric cancer incidence rates are similar to mortality rates due to the lack of screening and limited successful treatment options. Temporal trends of mortality are important indicators of health progress. Previous studies of gastric cancer mortality trends in Hispanic/Latino populations have evaluated a limited number of populations and short periods of time.
Added value of this study
We used data from the Surveillance, Epidemiology, and End Results Program and the World Health Organization to provide the most up-to-date estimates on gastric cancer mortality trends at a country-specific level for Hispanic/Latino populations in the United States, Latin America, and the Caribbean. We present sex- and age-specific mortality rates, as well as trends from 1997 to 2017. We also predicted the burden of gastric cancer deaths by 2030. Although overall mortality rates are declining, the magnitude of change varies across Hispanic/Latino populations. Notably, over the last two decades, mortality rates of gastric cancer have been stable or increasing in a few young populations, particularly women.
Implications of all the available evidence
A comprehensive gastric cancer control program requires substantial public health measures, and possibly screening and surveillance by use of upper gastrointestinal endoscopy in certain high-risk populations. Gastric cancer is and will continue to be one of the top health concerns for several Hispanic/Latino populations. This study provides a baseline for the evaluation of future activities as decision-makers design interventions to reduce the mortality of gastric cancer in the region. In addition, further research into the causes of gastric cancer and its evolving nature in these populations is warranted.
Alt-text: Unlabelled box
Introduction
Gastric cancer incidence and mortality rates have been declining over the last 30 years.1 Nevertheless, this neoplasia is currently the fourth leading cause of cancer death worldwide, after lung, colorectal, and liver cancers.2 Gastric cancer risk widely varies by geographical region,1 with Eastern Asia exhibiting the highest rates worldwide. The substantial global reduction in gastric cancer incidence and mortality is attributed to decreasing prevalence of Helicobacter pylori infection and other well-recognized risk factors (i.e., smoking and high salt intake), as well as the implementation of successful screening programs in high-risk East Asian countries.3
Important advances have been made in cancer registration in Latin America. However, the proportion of the population covered by the existing cancer registries in the region is ∼20%, with high-quality information coverage estimated at 7·1%.4 In the absence of prevention strategies, gastric cancer is a lethal disease. In most of the Americas, gastric cancer incidence and mortality rates are similar because of lack of screening and limited successful treatment options. In the Latin American region, the mortality burden of gastric cancer in men aged 25 years or older represents 81% of its estimated incidence (age-adjusted rates: 21 vs. 17 per 100 000 population, respectively; GLOBOCAN 2020). In the United States, racial/ethnic minority populations, including Hispanics/Latinos have an increased risk of developing and dying from gastric cancer as compared to non-Hispanics whites.5 Latin America and the Caribbean is a region with low-to-moderate burden of gastric cancer. Previous studies of incidence and mortality of gastric cancer in Hispanic/Latino populations have been limited to short periods of time or a restricted number of countries.6, 7, 8 Temporal trends in disease mortality are key indicators of health system performance and may guide decision-making regarding public health interventions. Therefore, our study aimed to evaluate gastric cancer national mortality trends over 20 years for several Hispanic/Latino populations in the United States, Latin America and the Caribbean, and predict their future burden.
Methods
Population and setting
We used mortality data from the Surveillance, Epidemiology, and End Results (SEER) Program database for the United States (Hispanic/Latino populations only) and from the World Health Organization database for Puerto Rico (territory of the United States) and 16 Latin American and Caribbean countries. We retrieved mortality data for the period 1997 (or the first year available) to 2017 (or the last year available). The SEER database was accessed through SEER*Stat software (version 8.3.9.2), and the data were suppressed by default where counts were fewer than ten. Deaths from gastric cancer were identified with the code C16 (malignant neoplasm of the stomach) of the International Classification of Diseases 10th revision. Our analysis was restricted to individuals diagnosed at ages 25 years or older. The thirteen 5-year age groups (i.e., 25-29, …, 80-84, and ≥85) were further collapsed into two groups, 25-49 and ≥50 years. Projected population estimates (medians) of the study countries (or territories) were obtained from the United Nations World Population Prospects database. Institutional review board approval was not applicable for this study.
Statistical analyses
We estimated annual age-standardized mortality rates (ASMRs) per 100 000 person-years for 21 years (1997 to 2017) by the direct method using Segi's world standard population. We calculated male-to-female ratios of ASMRs for 1997 and 2017, and tested if there was a statistically significant difference between both years by Z test statistic. Age- and sex-specific mortality trends by country were estimated using Poisson models with the Joinpoint Regression Program version 4.8.0.1. We estimated country-specific average annual percentage change (AAPC) and the corresponding 95% confidence intervals (CI). AAPCs were considered statistically significant at a p-value <0.05. We performed pairwise tests of parallelism (based on segmented line regression models) to compare trends by age group (25–49 vs. ≥50 years) and sex (men vs. women). A set of two trends was considered not parallel if the p-value was <0.05.
Mortality rate predictions through 2030 were computed using the Nordpred package in R software.9 Briefly, an age-period-cohort model was fitted to the observed data (2003–2017), and then the Segi world-standardized mortality rates were calculated for the thirteen five-year age groups (i.e., 25–49, …, 80–84, ≥85), further collapsed into two groups (i.e., 25–49 and ≥50 years). An extrapolation of the trends based on the observed rates (i.e., 2003–2007, 2008–2012, and 2013–2017) was carried out for three 5-year calendar periods (i.e., 2018–2022, 2023–2027, and 2028–2032). For the predicted period, we used the median population of the year representing half of the five-year periods (i.e., 2020, 2025, and 2030). The method of cut trend was used to attenuate the linear drift by 0%, 25%, and 50%, at the first to third 5-year periods, respectively. The estimates for the year 2030 were calculated as the middle point for the last period 2028–2032. The numbers of predicted deaths due to changes in demographics (i.e., population growth and aging) were calculated using the observed rates, and the numbers predicted due to risk change were estimated as the difference between the total and those due to demographic changes.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to publish.
Results
In the 18 countries (or territories) included in this analysis, men had higher ASMRs than women. The male-to-female ratios for 2017 (or the most recent year) ranged from 1·1 in Guatemala to 2·7 in Chile (Supplementary Table 1).
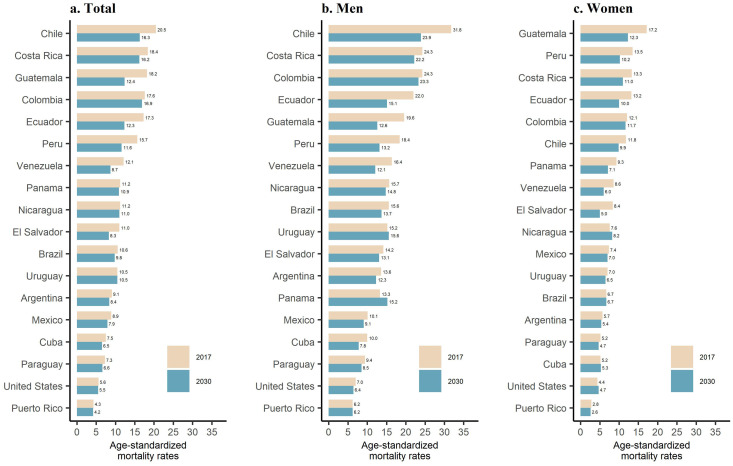
Among men, Puerto Rico (6·2 per 100 000), the United States (7·0), and Paraguay (9·4) had the lowest ASMR in 2017, while Chile (31·8), Costa Rica (24·3), and Colombia (24·3) had the highest rates. Most countries are expected to experience a decrease in their mortality rates by 2030 (Figure 1). Among women, the lowest ASMRs in 2017 were observed in Puerto Rico (2·8 per 100 000), the United States (4·4), Paraguay (5·2), and Cuba (5·2), while the highest were in Guatemala (17·2), Peru (13·5), and Costa Rica (13·3).
Figure 1.
Age-standardized (SEGI's world standard population) gastric cancer mortality rates per 100 000 persons-years in 2017* and predicted 2030** among Hispanic/Latino populations aged 25 and older in the United States, Latin America, and the Caribbean. *Data from 2014 for Venezuela and 2015 for El Salvador; **Age-standardized rate represents the mid-point for the projected period 2028–2032.
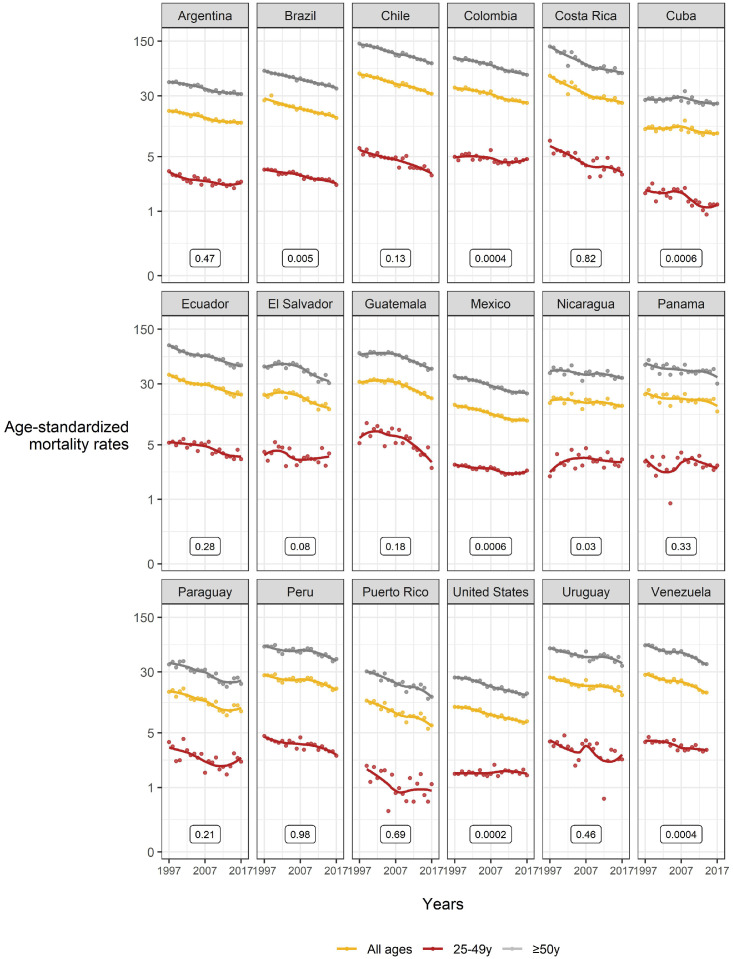
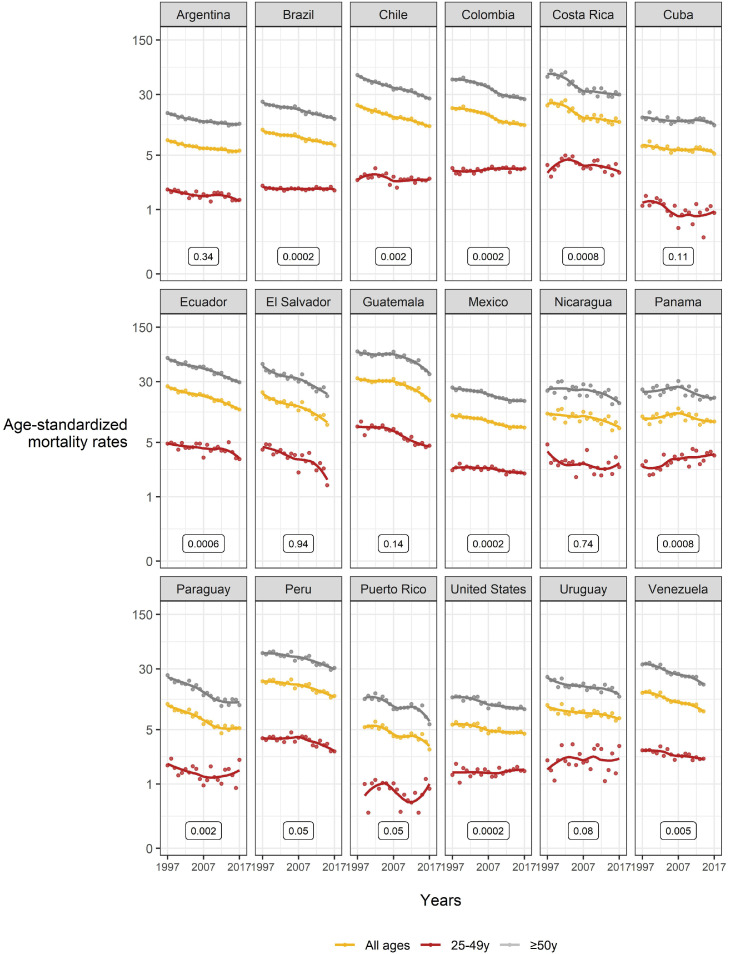
Figures 2 and 3 show the overall and age-specific 1997-2017 mortality trends of gastric cancer for men and women, respectively. For both sexes, we observed downward trends in the overall population and the older age group (≥50 years). On the contrary, ASMRs in the young group (25–49 years) were less precise with a few countries showing stable or slightly increasing trends. In men (Figure 2), age-specific trends were not parallel in Brazil, Colombia, Cuba, Mexico, Nicaragua, the United States, and Venezuela. In women (Figure 3), age-specific trends were not parallel in Brazil, Chile, Colombia, Costa Rica, Ecuador, Mexico, Panama, Paraguay, the United States, and Venezuela.
Figure 2.
Gastric cancer mortality trends (rates per 100 000 persons-years; all ages, 25-49, and ≥50 years) 1997*-2017** among Hispanic/Latino males in the United States, Latin America, and the Caribbean. *Data from 1999 for Puerto Rico; **Data from 2014 for Venezuela and 2015 for El Salvador. P-values represent the difference for statistical parallelism tests (based on segmented line regression models) between 25–49 years and ≥50 years.
Figure 3.
Gastric cancer mortality trends (rates per 100 000 persons-years; all ages, 25–49, and ≥50 years) 1997–2017 among Hispanic/Latino females in the United States, Latin America, and the Caribbean. *Data from 1999 for Puerto Rico; **Data from 2014 for Venezuela and 2015 for El Salvador. P-values represent the difference for statistical parallelism tests (based on segmented line regression models) between 25–49 years and ≥50 years.
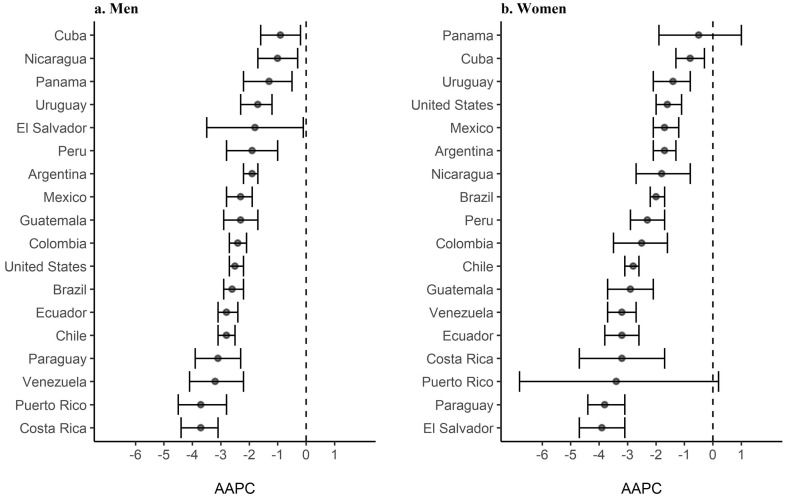
Regarding the overall (all ages) AAPC, all countries exhibited downward trends for both sexes (Figure 4 and Supplementary Table 1). In men, the countries with the highest changes were Costa Rica and Puerto Rico (AAPC, −3·7% for both countries), while those with the smallest changes were Cuba (AAPC, −0·9%) and Nicaragua (−1·0%). In women, the countries with the highest changes in trends were El Salvador (AAPC, −3·9%) and Paraguay (−3·8%), while those with the smallest changes were Panama (AAPC, −0·5%) and Cuba (AAPC, −0·8%).
Figure 4.
Average annual percent change (AAPC) and 95% confidence interval (CI) for gastric cancer age-adjusted mortality rates among Hispanic/Latino populations in the United States, Latin America, and the Caribbean by sex, 1997*–2017**. *Data from 1999 for Puerto Rico; **Data from 2014 for Venezuela and 2015 for El Salvador.
In the older age group (≥50 years), all ASMRs in men had significant downward trends (supplementary table 2), with the most remarkable changes in Costa Rica and Puerto Rico (AAPCs, −3·7% in both countries). For women, ASMRs are significantly declining in most countries, except for Panama and Puerto Rico. In contrast, widely variable trends were observed in the young group (25-49 years) (Supplementary Table 3). In men, AAPCs ranged from −3·8% in Guatemala to 0·2% in the United States. In women, AAPCs ranged from −3·9% in El Salvador to 2·1% in Panama. Male-to-female ratios for the most recent year declined for some countries (supplementary table 3), but the results were not statistically significant (p-values >0.05).
According to the age-period-cohort predictions for 2030, in almost all countries there should be a gastric cancer mortality reduction in men, with an increase in population size and a decrease in change due to risk (Table 1). In women, there would also be a mortality reduction from gastric cancer in most countries (Table 2). However, in Puerto Rico and El Salvador, the reduction in the risk of death would exceed the increase due to changes in the population size and structure.
Table 1.
Number of gastric cancer deaths, age-standardized mortality rates, and percentage change in cases due to population growth and risk among Hispanic/Latino men in the United States, Latin America, and the Caribbean, 2017 and predicted 2030.a
| Country (or territory) | Male population aged 25 and older (annual million) |
Number of deaths in men aged 25 and older |
Age-standardized mortality rates |
Total change, % | Change due to population, % | Change due to risk, % | |||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2030 | 2017 | 2030 | 2017 | 2030 | ||||
| Argentina | 12·8 | 14·6 | 1898 | 2162 | 13·6 | 12·3 | 15·5 | 29·7 | −14·2 |
| Brazil | 65·0 | 73·7 | 9188 | 12575 | 15·6 | 13·7 | 36·9 | 70·4 | −33·5 |
| Chile | 6·2 | 6·6 | 2191 | 2480 | 31·8 | 23·9 | 13·7 | 66·0 | −52·3 |
| Colombia | 14·8 | 17·1 | 3188 | 4892 | 24·3 | 23·3 | 57·6 | 79·6 | −22·0 |
| Costa Rica | 1·6 | 1·8 | 398 | 585 | 24·3 | 22·2 | 48·8 | 76·0 | −27·2 |
| Cuba | 4·0 | 4·1 | 560 | 578 | 10·0 | 7·8 | 8·5 | 34·3 | −25·8 |
| Ecuador | 4·7 | 5·8 | 953 | 1029 | 22·0 | 15·1 | 14·1 | 70·4 | −56·3 |
| El Salvador | 1·5 | 1·8 | 221 | 251 | 14·2 | 13·1 | 7·7 | 32·9 | −25·2 |
| Guatemala | 3·4 | 5·3 | 558 | 555 | 19·6 | 12·6 | −5·7 | 64·9 | −70·6 |
| Mexico | 34·8 | 41·3 | 3282 | 4108 | 10·1 | 9·1 | 31·1 | 51·5 | −20·4 |
| Nicaragua | 1·6 | 2·0 | 186 | 276 | 15·7 | 14·8 | 51·0 | 65·9 | −14·9 |
| Panama | 1·2 | 1·4 | 184 | 281 | 13·3 | 15·2 | 41·8 | 65·4 | −23·6 |
| Paraguay | 1·9 | 2·3 | 154 | 195 | 9·4 | 8·5 | 30·7 | 57·9 | −27·2 |
| Peru | 9·9 | 11·0 | 1536 | 1908 | 18·4 | 13·2 | 27·1 | 86·0 | −58·9 |
| Puerto Rico | 0·9 | 1·0 | 102 | 127 | 6·2 | 6·2 | 8·3 | 31·5 | −23·2 |
| Uruguay | 1·0 | 1·1 | 204 | 260 | 15·2 | 15·6 | 7·0 | 23·4 | −16·4 |
| United States | 16·7 | 23·3 | 1059 | 1642 | 7·0 | 6·4 | 69·1 | 92·1 | −23·0 |
| Venezuela | 7·7 | 9·9 | 1107 | 1289 | 16·4 | 12·1 | 16·5 | 60·6 | −44·0 |
Age-standardized rate represents the mid-point for the projected period 2028–2032.
Table 2.
Number of gastric cancer deaths, age-standardized mortality rates, and percentage change in cases due to population and risk among Hispanic/Latino women in the United States, Latin America, and the Caribbean, 2017 and predicted 2030.a
| Country (or territory) | Female population aged 25 and older (annual million) |
Number of deaths in women aged 25 and older |
Age-standardized mortality rates |
Total change, % | Change due to population, % | Change due to risk, % | |||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2030 | 2017 | 2030 | 2017 | 2030 | ||||
| Argentina | 14·2 | 16·1 | 1087 | 1252 | 5·7 | 5·4 | 17·6 | 27·2 | −9·6 |
| Brazil | 70·2 | 79·9 | 5082 | 7399 | 6·7 | 6·7 | 45·0 | 64·1 | −19·1 |
| Chile | 6·6 | 7·0 | 1106 | 1198 | 11·8 | 9·9 | 8·6 | 47·4 | −38·8 |
| Colombia | 16·1 | 18·5 | 1991 | 2849 | 12·1 | 11·7 | 46·5 | 77·8 | −31·3 |
| Costa Rica | 1·6 | 1·9 | 252 | 321 | 13·3 | 11·0 | 34·8 | 74·9 | −40·1 |
| Cuba | 4·1 | 4·2 | 334 | 474 | 5·2 | 5·3 | 33·9 | 39·9 | −6·0 |
| Ecuador | 4·9 | 6·0 | 654 | 759 | 13·2 | 10·0 | 11·7 | 71·4 | −59·7 |
| El Salvador | 2·0 | 2·3 | 177 | 158 | 8·4 | 5·0 | −19·2 | 51·7 | −70·9 |
| Guatemala | 3·9 | 6·4 | 620 | 699 | 17·2 | 12·3 | 1·4 | 69·7 | −68·3 |
| Mexico | 38·6 | 45·6 | 2863 | 3786 | 7·7 | 7·0 | 38·0 | 58·9 | −20·9 |
| Nicaragua | 1·8 | 2·2 | 118 | 195 | 7·6 | 8·2 | 52·9 | 69·7 | −16·8 |
| Panama | 1·2 | 1·5 | 118 | 141 | 9·3 | 7·1 | 23·9 | 68·1 | −44·2 |
| Paraguay | 1·8 | 2·3 | 87 | 111 | 5·2 | 4·7 | 32·1 | 57·9 | −25·8 |
| Peru | 9·9 | 11·5 | 1103 | 1687 | 13·5 | 10·2 | 25·6 | 73·0 | −47·4 |
| Puerto Rico | 1·1 | 1·2 | 64 | 78 | 2·8 | 2·6 | −5·3 | 33·8 | −39·1 |
| Uruguay | 1·2 | 1·3 | 141 | 162 | 7·0 | 6·5 | 8·8 | 16·9 | −8·1 |
| United States | 16·6 | 23·1 | 812 | 1314 | 4·4 | 4·7 | 77·8 | 80·9 | −3·1 |
| Venezuela | 8·3 | 10·6 | 720 | 764 | 8·6 | 6·0 | 6·7 | 61·2 | −54·4 |
Age-standardized rate represents the mid-point for the projected period 2028–2032.
Discussion
Hispanics/Latinos have low-to-moderate gastric cancer mortality rates. Our study represents the most comprehensive analysis of gastric cancer mortality in these populations. Although there was a substantial variation in ASMRs across countries (up to ∼4-fold overall), we found that in all Hispanic/Latino populations in the United States, Latin America, and the Caribbean overall gastric cancer mortality has declined in the last two decades. Notably, mortality rates are declining in older populations but stable or increasing in a few groups of individuals aged <50 years. Moreover, in these young groups, the classic male predominance of gastric cancer seems to be changing in some countries.
The most common (>90%) anatomical subtype of gastric cancer among Hispanic/Latino populations in the United States and Latin America is H. pylori-associated noncardia.10, 11, 12 Declining mortality rates of gastric cancer in older Hispanics/Latinos may reflect decreases in incidence related to a reduction in H. pylori acquisition during childhood as well as favorable changes in other risk factors. However, the reasons for discrepant trends in younger individuals are unclear. We and others have reported that the incidence of gastric cancer (mainly noncardia) appears to be leveling off or even increasing among young birth cohorts in the United States and other Western countries,13, 14, 15 potentially unrelated to H. pylori infection. In particular, our analysis shows a modest rising in noncardia cancer incidence among United States Hispanics/Latinos aged <50 years (estimated annual percentage change for men and women combined, 0·44%; p<0.05).15 In the United States, H. pylori seroprevalence among Hispanics/Latinos remains high (57%) and differed significantly by background and nativity.16 Studies of H. pylori infection in Hispanics/Latinos in Latin America show a sustained prevalence higher than 50%.17 In addition, H. pylori infection resistance to antibiotics is a growing problem in the Americas.18,19 The control of H. pylori infection is a cornerstone to gastric cancer risk reduction, which in turn would decrease mortality. Several randomized trials have shown that H. pylori eradication significantly reduces gastric cancer incidence and mortality.20 Also, H. pylori test-and-treat strategies targeting adults at moderate-to-high gastric cancer risk are likely to be cost-effective.21
In most of the Americas, gastric cancer incidence and mortality are similar because of a lack of screening and limited successful treatment options. Unfortunately, diagnosis of gastric cancer at advanced-stage is common in Hispanic/Latino populations, particularly in Latin America where access to specialized oncology centers is in short supply.22 Besides incidence, mortality trends are affected by changes in survival. A few studies have addressed gastric cancer survival in Hispanics/Latinos. In line with delayed diagnosis, a population-based study in Colombia showed a low 5-year overall survival (16% for men and 18% for women) for the period 1994–2004.23 In the US, Liu et al., reported slightly higher 5-year survival estimates of 37·6% for Hispanics in Florida and 33·5% for Hispanics in SEER for the 2006-2016 period.24
Mortality differences across countries could be explained by variations in prevention strategies, access to healthcare services, health insurance coverage, and patient care practices. Except for Costa Rica, no other country in this study has implemented secondary prevention programs.25 Gastric cancer is a multistep process with well-defined premalignant histological stages that allow a wide window of intervention. As recommended by international guidelines,26 endoscopy surveillance of patients with severe gastric atrophy could provide an opportunity to control gastric cancer. In particular, high-risk countries in the Latin American region should consider a consistent follow-up of patients with advanced intestinal metaplasia (i.e., corpus extension). This secondary prevention strategy would allow identification of treatable gastric lesions associated with good prognosis. Future prospective studies should define the optimal time for endoscopy and the identification of non-invasive biomarkers of severe gastric atrophy that can be combined with circulating pepsinogens.
As expected, our study showed that South and Central American countries, Chile, Guatemala, Costa Rica, Colombia, Ecuador, and Peru in particular, had the highest mortality rates of gastric cancer, while North American countries and those in the Caribbean had the lowest rates. Studies in the Pacific rim and Andean regions have suggested that a geographic clustering, coupled with low-economic income settings, poor education, and limited access to healthcare, creates an environment that allows the spread of risk factors that limit the control of mortality from gastric cancer.27
Most countries in our analysis had mortality reductions between 2 to 3% per year during the study period, and the projected ASMR for 2030 would yield similar or even smaller declines. These estimates are lower than those observed in other populations without screening programs. Major European countries had downward trends between 3 to 4% for the 1980–2005 period,28 probably related to an earlier reduction in gastric cancer risk factors and better access to health care services.
Our analysis confirmed an overall male predominance in gastric cancer mortality. Although the reasons underlying this sex difference is likely complex and multifactorial, men may have a higher prevalence of gastric cancer risk factors (i.e., poor hygiene, lower consumption of fruits or vegetables, and higher tobacco consumption)29 that are being reduced over time. Nevertheless, while women are more attentive to health-related information and behaviors that potentially affect health, men are prone to avoid healthcare visits, which increases the risk of detecting gastric cancer in late stages and, thus, the mortality risk.
The ∼60 million Hispanics/Latinos in the United States represent 18% of the country's total population in 2020. This population group deserves special public health attention given its aging and rapid growth. The United States Hispanics/Latinos represent a heterogeneous population of United States- and foreign-born immigrants mainly from Mexico, Central America, and the Caribbean. Our study found that for 2017 ASMRs in the United States were lower (7·0 per 100 000 for men and 4·4 for women) than most Latin American countries. Mortality in United States Hispanics/Latinos could be influenced by migration patterns, entailing a socio-cultural behavioral difference between immigrants and non-Hispanic whites. These disparities of cancer mortality are important hurdles to reduce the burden of gastric cancer for which population tailored approaches are needed, as well as increasing access to healthcare services.
This study has the usual caveats of a cancer registry analysis, such as missing data, lack of specific information on tumor characteristics (i.e., histology and anatomical subsite), limited individual-level information, and variation in death registration completeness and quality. Ongoing initiatives to establish population-based cancer incidence registries in the Central America Four region (comprising Guatemala, Honduras, El Salvador, and Nicaragua)30 are expected to improve cancer information and amend for the potential underreporting of available mortality data. Unfortunately, we could not retrieve mortality information from some countries in the region, including Honduras and Bolivia. Torres et al. reported an ASMR of 15·1 per 100 000 for men and 13 for women in Bolivia.27 The GLOBOCAN 2020 (Latin America Hub) ASMRs for men (ages ≥25 years) are 20·5 and 14·6 for Honduras and Bolivia, respectively. The absence of gastric cancer mortality data could limit public health interventions in these least developed Latin American countries.
In conclusion, although Hispanic/Latino populations in the United States, Latin America, and the Caribbean have overall downward mortality trends of gastric cancer and ASMRs are predicted to decrease further, this geographic region still has one of the highest mortality rates globally. Future research should explore factors underlying the stable or increasing mortality rates in some young population groups. Further surveillance of age-specific mortality and incidence trends will be informative. Our findings emphasize the need for timely and effective control and prevention strategies in these populations to ensure that these unfavorable trends do not persist.
Contributors
JS Torres-Roman and MC Camargo conceived the study. JS Torres-Roman and P Guerra-Canchari extracted and organized the mortality data. DLB de Souza provided guidance on methods. JS Torres-Roman, CS Alvarez, C Alves Santos, SCM Soares and DLB de Souza analyzed the data. B Valcarcel was responsible for data visualization. JS Torres-Roman, CS Alvarez, JF Martinez-Herrera, and MC Camargo wrote the original draft. All authors reviewed and edited the manuscript. All authors had full access to the data and accept responsibility to submit for publication.
Data sharing statement
Data are available in an open access repository and can be accessed and downloaded at: https://www-dep.iarc.fr/whodb/whodb.htm.
Declaration of interests
All authors declare that they have no conflict of interest with this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100376.
Appendix. Supplementary materials
References
- 1.Ferro A, Peleteiro B, Malvezzi M, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Suh YS, Yang HK. Screening and early detection of gastric cancer: east versus west. Surg Clin North Am. 2015;95(5):1053–1066. doi: 10.1016/j.suc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Pineros M, Abriata MG, Mery L, Bray F. Cancer registration for cancer control in Latin America: a status and progress report. Rev Panam Salud Publica. 2017;41:e2. doi: 10.26633/RPSP.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer. 2006;107(8):1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 6.Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in central and South America. Cancer Epidemiol. 2016;44:S62–S73. doi: 10.1016/j.canep.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz EF, Torres-Roman JS, Servan SA, et al. Trends and geographic pattern of stomach cancer mortality in Peru. Cancer Epidemiol. 2019;58:193–198. doi: 10.1016/j.canep.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 8.del Pilar Diaz M, Icaza G, Nuñez L, Pou SA. Gastric cancer mortality trends in the southern cone: disentangling age, period and cohort patterns in Argentina and Chile. Sci Rep. 2020;10(1):1–8. doi: 10.1038/s41598-020-58539-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Møller B, Fekjær H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22(17):2751–2766. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 10.Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control. 2013;24(2):217–231. doi: 10.1007/s10552-012-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 12.Long Parma D, Schmidt S, Muñoz E, Ramirez AG. Gastric adenocarcinoma burden and late-stage diagnosis in Latino and non-Latino populations in the United States and Texas, during 2004–2016: a multilevel analysis. Cancer Med. 2021;10(18):6468–6479. doi: 10.1002/cam4.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merchant SJ, Kim J, Choi AH, Sun V, Chao J, Nelson R. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer. 2017;20(2):226–234. doi: 10.1007/s10120-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo G, Zhang Y, Guo P, Wang L, Huang Y, Li K. Global patterns and trends in stomach cancer incidence: age, period and birth cohort analysis. Int J Cancer. 2017;141(7):1333–1344. doi: 10.1002/ijc.30835. [DOI] [PubMed] [Google Scholar]
- 15.Anderson WF, Rabkin CS, Turner N, Fraumeni JF, Jr, Rosenberg PS, Camargo MC. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110(6):608–615. doi: 10.1093/jnci/djx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsang SH, Avilés-Santa ML, Abnet CC, et al. Seroprevalence and determinants of helicobacter pylori infection in the hispanic community health study/study of Latinos. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curado MP, de Oliveira MM, de Araújo Fagundes M. Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: a systematic review and meta-analysis. Cancer Epidemiol. 2019;60:141–148. doi: 10.1016/j.canep.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Camargo MC, García A, Riquelme A, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109(4):485. doi: 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JY, Dunbar KB, Mitui M, et al. Helicobacter pylori clarithromycin resistance and treatment failure are common in the USA. Dig Dis Sci. 2016;61(8):2373–2380. doi: 10.1007/s10620-016-4091-8. [DOI] [PubMed] [Google Scholar]
- 20.Ford AC, Yuan Y, Forman D, Hunt R, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev. 2020;2020(7) doi: 10.1002/14651858.CD005583.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beresniak A, Malfertheiner P, Franceschi F, Liebaert F, Salhi H, Gisbert JP. Helicobacter pylori “Test-and-Treat” strategy with urea breath test: a cost-effective strategy for the management of dyspepsia and the prevention of ulcer and gastric cancer in Spain—Results of the Hp-Breath initiative. Helicobacter. 2020;25(4):e12693. doi: 10.1111/hel.12693. [DOI] [PubMed] [Google Scholar]
- 22.Calderón-Aparicio A, Orue A. Precision oncology in Latin America: current situation, challenges and perspectives. Ecancermedicalscience. 2019;13:920. doi: 10.3332/ecancer.2019.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo LE, García LS, Collazos PA. Cancer survival in Cali, Colombia: a population-based study, 1995-2004. Colomb Med. 2014;45(3):110–116. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Medina H, Reis IM, Sussman DA, Pinheiro PS. Disadvantages for non-Hispanic whites in gastric carcinoma survival in Florida. Cancer causes & control : CCC. 2020;31:815–826. doi: 10.1007/s10552-020-01320-1. [DOI] [PubMed] [Google Scholar]
- 25.Rosero-Bixby L, Sierra R. X-ray screening seems to reduce gastric cancer mortality by half in a community-controlled trial in Costa Rica. Br J Cancer. 2007;97(7):837–843. doi: 10.1038/sj.bjc.6603729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (maps II): European society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and sociedade portuguesa de endoscopia digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 27.Torres J, Correa P, Ferreccio C, et al. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24(2):249–256. doi: 10.1007/s10552-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125(3):666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 29.Baker AH, Wardle J. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40(3):269–275. doi: 10.1016/s0195-6663(03)00014-x. [DOI] [PubMed] [Google Scholar]
- 30.Pineros M, Frech S, Frazier L, et al. Advancing reliable data for cancer control in the central America four region. J Glob Oncol. 2018;4:1–11. doi: 10.1200/JGO.2016.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.