Summary
Background
Results from numerous clinical trials have led to a consensus that moderately hypofractionated radiation therapy is the ideal postoperative irradiation treatment plan in patients with breast cancer (BC). However, there are specific situations such as chest wall (with or without breast reconstruction) and regional node irradiation that still face obstacles in its widespread use. There is a lack of evidence supporting the use of moderately hypofractionated irradiation from the Latin American context. This study aims to describe the profile and clinical outcomes of patients treated with moderate hypofractionation for both early-stage (Stage I and II) and locally advanced BC (Stage III) regardless of the type of surgery in a Brazilian Oncology Center.
Methods
All patients with non-metastatic BC who were treated with moderately hypofractionated schedules of 40Gy in 15 fractions or 42.4Gy in 16 fractions between 2010 to 2019 at Hospital Sírio-Libanês, Brazil were retrospectively analyzed. The rates of local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), distance recurrence-free survival (DRFS) and overall survival (OS) were estimated. Acute and late toxicity profiles were accessed for the entire cohort.
Findings
A total of 670 patients were included. The median age was 57 years and the median follow-up time was 31 months. Most of the patients had stage I and II breast cancer, and 81.6% underwent breast-conserving surgery. Of the 123 women who underwent mastectomy treatment, 29% (n = 37) had immediate reconstruction with implants and 28% (n = 35) with autologous tissue. Seventy-one per cent of the patients presented luminal subtype tumour and 84.3% received adjuvant hormonal therapy. Chemotherapy was administered to almost half of the patients and all 80 patients with Her-2 positive disease received trastuzumab-based systemic therapy. One-third of patients received regional node irradiation; boost was performed in 41.1% of treatments. The 5-year LRFS, RRFS, DRFS and OS was 95.6%, 97.6%,92.2% and 95.9%, respectively. Acute and late side effects profile were mild and only 2.9% of patients developed grade 3 dermatitis. Among patients with breast implants, 11.4% had capsular contracture.
Interpretation
In this Brazilian institution experience, moderately hypofractionated irradiation to the breast, chest wall (with or without breast reconstruction), and regional lymph nodes was safe and with an acceptable toxicity profile.
Funding
None.
Keywords: Breast cancer, Radiation therapy, Hypofractionation
Research in context.
Evidence before this study
Results from numerous clinical trials have led to a consensus that moderately hypofractionated radiation therapy is the ideal postoperative irradiation treatment plan in patients with breast cancer. However, specific situations such as chest wall (with or without breast reconstruction) and regional node irradiation still face obstacles in its widespread use worldwide. Moreover, there is a lack of evidence supporting the use of moderately hypofractionated irradiation from the Latin American context.
Added value of this study
Our study demonstrated that moderately hypofractionated irradiation to the breast, chest wall (with/without breast reconstruction), and regional lymph nodes were safe with an acceptable toxicity profile.
Implications of all the available evidence
Our study can contribute to providing more evidence in support of using moderately hypofractionated irradiation more extensively in clinical practice, especially in Brazil and Latin America.
Alt-text: Unlabelled box
Introduction
Breast cancer is the most common malignancy impacting women worldwide.1 In Brazil, breast cancer represented approximately 30% of all female cancers diagnosed in 2020.2 Radiation therapy is a common postoperative treatment aimed at reducing local recurrence and, ultimately, improving overall survival rates.3,4 Historically, total doses ranging from 45 to 50Gy were divided into daily doses of 1.8 to 2Gy, resulting in long treatment durations.5,6 Recently, moderately hypofractionated radiation therapy schemes ranging from 13 to 16 daily fractions were evaluated in breast cancer treatment cohorts. Acceptable clinical outcomes compared with conventionally fractionated regimens have been observed.7 Potential benefits of using moderately hypofractionated irradiation may include improved adherence due to the reduction in the number of fractions and a potential reduction in treatment-related costs.8, 9, 10
Several randomized phase III trials showed similar rates of local control and survival in early breast cancer with moderately hypofractionated radiation therapy. They found interesting toxicity and cosmetic profiles, which were often better than conventional schedules.11,12 More recently, a Chinese non-inferiority randomized phase III trial demonstrated that postmastectomy moderately hypofractionated irradiation to the chest wall and regional lymph nodes in patients with locally advanced diseases was comparable to conventionally fractionated regimens in terms of local control, overall survival and side effects.13 Despite these results, the widespread use of moderately hypofractionated irradiation as standard fractionation faces barriers in patients where regional node irradiation is required and/or after breast reconstruction.7
The majority of studies informing the safety and efficacy of moderately hypofractionated radiation therapy have come from European, American and Canadian cohorts. There is little literature in Latin America. Our study aims to describe the demographics and clinical outcomes of patients treated with moderate hypofractionation for both early-stage and locally advanced breast cancer in a Brazilian Oncology Center.
Patients and methods
Patient population and study variables
Our cohort included adult patients (≥ 18 years) with non-metastatic breast cancer treated with moderately hypofractionated irradiation schedules of 40Gy in 15 fractions or 42.4Gy in 16 fractions between January 2010 and December 2019 at the Hospital Sirio-Libanês in Sao Paulo and Brasília, Brazil. Patients received 16 fractions from 2010 to 2013 and 15 fractions from 2014 to 2019.
Patients were excluded if they had atypical histology such as sarcoma, metaplastic, neuroendocrine or clear cells carcinoma; re-irradiation to breast, chest wall and/or regional lymph nodes, previous diagnosis of any other malignancy or were missing required data on medical records.
Clinical data related to the disease, patient characteristics, treatment and outcomes were collected retrospectively. Collected data included: age, histology, tumour grade, molecular subtype, clinical and pathological stage (AJCC 7th edition), type of breast and axillary surgery, use of mammary reconstruction (implants and autologous tissue), systemic therapy (chemotherapy, hormonotherapy and anti-Her 2 agents), dose fractionation and target volume of radiation, use of boost and breast clinical target volume (CTV).
All patients underwent computed tomography-based simulation and three-dimensional conformal planning with tangential field-in-field technique as the standard. Volumetric modulated arc therapy (VMAT) was allowed. We used the following dose constraints: lung V16.8Gy ≤ 12%; V8.8Gy ≤ 16%; V4.5Gy ≤ 25%; mean dose ≤ 6 Gy, heart V16.8Gy ≤ 4%; V8.8Gy ≤ 6%; mean dose ≤ 3.6 Gy, contralateral breast D2% ≤ 2.8Gy. 40Gy in 15 fractions or 42.4Gy in 16 fractions was used on the breast/chest wall and regional lymph nodes when indicated.
This study was approved by the local institutional ethical review committee - Hospital Sírio-Libanês – Sao Paulo, Brazil - number: 32320720.3.0000.5461.
Outcomes
The primary outcome was local recurrence-free survival (LRFS). LRFS was defined as the time interval between the date of radiation therapy completion to the date of local recurrence. Secondary outcomes included regional recurrence-free survival (RRFS) (defined as the time from the date of radiation therapy completion to the date of regional recurrence), metastasis-free survival (MFS) (defined as the date of radiation therapy completion to the date of the occurrence of distant metastases), and overall survival (OS) (defined as the date of radiation therapy completion to the date of death from any cause).
Acute and late side effects were evaluated based on CTCAE 5.0 graduation.13 We defined acute toxicity assessment as up to three months and late toxicity as more than three months after radiation therapy completion.
Statistical analyses
Descriptive and frequencies analysis were performed with calculation of mean, minimum and maximum values, standard deviation, and median values with interquantiles ranges, absolute and relative frequencies (percentage). The cumulative incidence of the outcomes was calculated using the Kaplan-Meier method.
The inferential analyzes used in order to confirm or refute evidence found in the descriptive analysis were done with the Log-Rank test, Pearson's Chi-Square and Fisher's Exact test. For regression analyses was used de Cox regression model. In all conclusions obtained through the inferential analysis, an alpha significance level of 5% was set.
Statistical analyses were performed with SPSS 24.0 IBM®.
Role of funding source
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Results
Study population
A total of 670 patients with breast cancer were included with a median follow-up time of 31 months (range 22–45). The clinical and disease characteristics of the cohort are described in Table 1. The median age was 57 years (range 48–65). Most of the patients had the early-stage disease (T1-2/ N0-N1) and 81.6% of them underwent breast-conserving surgery. Ninety-one patients had ductal carcinoma “in situ” (DCIS). Of the 123 women who submitted to mastectomy, 71 had immediate reconstruction (29% with implants and 28% with autologous tissue).
Table 1.
Patients and tumor characterists.
| Characteristics | N | |
|---|---|---|
| Age | 670 | 57 years (27–87 years) |
| % | ||
| Histology | ||
| Invasive ductal | 512 | 76.4 |
| Invasive lobular | 59 | 8.8 |
| DCIS | 91 | 13.5 |
| Other | 8 | 1.2 |
| Histology grade | ||
| 1 | 98 | 14.6 |
| 2 | 314 | 46.9 |
| 3 | 198 | 29.5 |
| Unknow | 60 | 9.0 |
| Clinical Tumor Stage | ||
| Tis | 91 | 13.6 |
| T1 | 350 | 52.3 |
| T2 | 168 | 25 |
| T3 | 46 | 6.9 |
| T4 | 14 | 2.1 |
| Tx | 1 | 0.1 |
| Clinical Nodal Stage | ||
| N0 | 540 | 80.6 |
| N1 | 105 | 15.7 |
| N2 | 20 | 3.0 |
| N3 | 4 | 0.6 |
| Nx | 1 | 0.1 |
| Clinical Stage (AJCC 7th edition) | ||
| II | 113 | 16.9 |
| III | 63 | 9.4 |
| Unkown | 2 | 0.4 |
| Breast Surgery | ||
| Conserving surgery | 547 | 81.6 |
| Mastectomy | 123 | 18.4 |
| Reconstruction (mastectomy patients only/ n = 123) | ||
| Implants | 35 | 28.4 |
| Autologous tissue | 36 | 29.2 |
| None | 52 | 42.4 |
| Axillary surgery | ||
| SNB | 443 | 66.1 |
| Axillary dissection | 183 | 27.4 |
| None | 44 | 6.5 |
| Pathological Stage | ||
| 0 | 116 | 17.6 |
| I | 368 | 54.9 |
| II | 121 | 18.0 |
| III | 62 | 9.2 |
| Unknown | 3 | 0.3 |
| Margin status | ||
| Positive | 11 | 1.7 |
| Negative | 550 | 82.0 |
| Unknown | 109 | 16.3 |
| Subtype (only invasive carcinoma n = 579) | ||
| Luminal A | 171 | 29.5 |
| Luminal B | 215 | 37.2 |
| Her 2 + | 80 | 13.8 |
| Triple negative | 72 | 12.5 |
| Unknown | 41 | 7.0 |
| Chemotherapy | ||
| No | 359 | 53.6 |
| Adjuvant | 176 | 26.3 |
| Neoadjuvant | 135 | 20.1 |
| Hormone Therapy | ||
| No | 104 | 15.7 |
| Yes | 565 | 84.3 |
Note: DCIS = ductal carcinoma “in situ”; SNB = sentinel node biopsy.
Seventy per cent of the patients had luminal-like subtype tumours and 84.3% received adjuvant hormone therapy. Chemotherapy was administered to almost half of the patients. ACT (adriamycin, cyclophosphamide and taxane) was the most frequently used treatment scheme. All 80 patients with Her-2 positive diseases received trastuzumab-based therapy.
Radiation treatment
The majority of radiation therapy planning was three-dimensional conformal tangential fields with field-in-field technique and only 5% underwent VMAT planning. The median CTV volume was 867.9 cm3 (range 602.7 cm3 – 1079.6 cm3). Most patients (85.6%) received 40Gy in 15 fractions; regional node irradiation was performed in 31.8% of the patients. Boost was executed in 275 patients and a hypofractionated scheme of 8.01Gy in 3 fractions was used in 90.5% of them (Table 2).
Table 2.
Radiation therapy charactheristics.
| Radiation therapy charactheristics | N | % |
|---|---|---|
| Dose | ||
| 15 × 2.67Gy | 574 | 85.7 |
| 16 × 2.65Gy | 96 | 14.3 |
| Boost | ||
| None | 395 | 58.9 |
| 3 × 2.67Gy | 249 | 37.2 |
| 5 × 2Gy | 26 | 3.9 |
| Regional node irradiation | ||
| None | 457 | 68.3 |
| Supraclavicular fossa only | 111 | 16.6 |
| Axilla only | 3 | 0.4 |
| Supraclavicular fossa + Axilla | 29 | 4.3 |
| Supraclavicular fossa + Internal mamamary | 70 | 10.4 |
| Technique | ||
| 3D conformal | 636 | 95.0 |
| VMAT | 34 | 5.0 |
Note: VMAT = volumetric arc therapy.
Survival outcomes
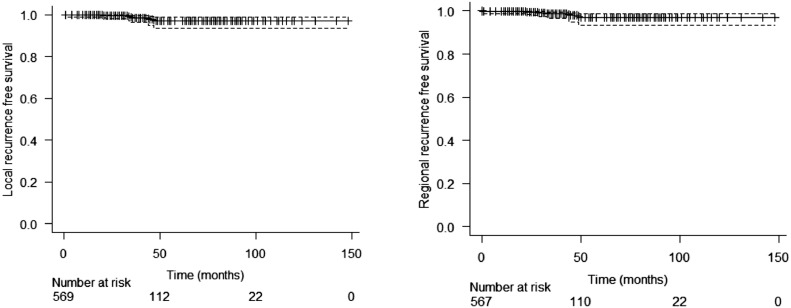
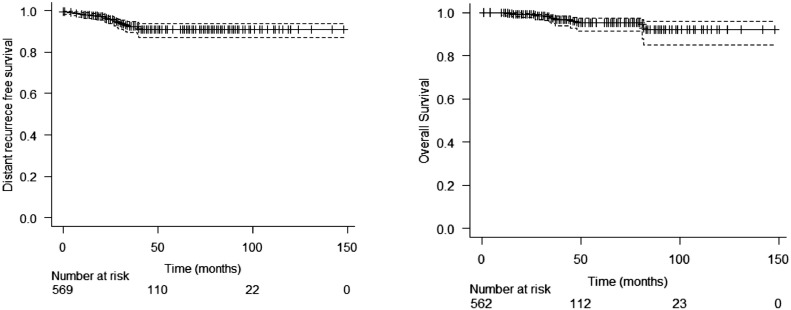
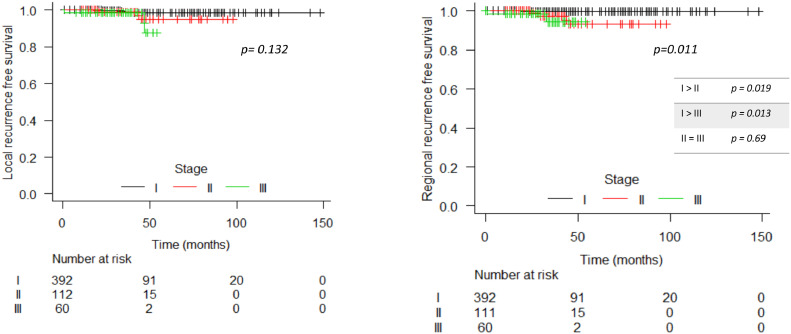
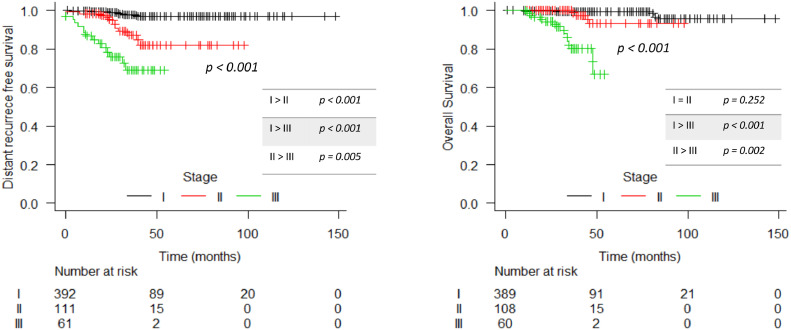
In the entire cohort, there were 13 (1.94%) local recurrences: 7 in patients with invasive carcinoma and 6 in DCIS population. Between patients with invasive disease, the 3- and 5-year estimated LRFS was 98.5% (CI 97.2 – 99.9%) and 97.1% (CI 94.7 – 99.5%), respectively (Figure 1). Rates of estimated regional, distance recurrence-free survival and OS at 3 years were 98.6% (CI 97.4 – 99.9%), 92.4% (CI 89.8 – 95.1%) and 97.3% (CI 95.5 – 99.1%), respectively, and at 5 years were 97.1% (CI 94.7 – 99.6%), 90.8% (CI 87.7 – 94%) and 95.3% (CI 92.4 – 98.2%), respectively (Figure 2). LRFS was better in stage I patients compared to stage II and III (p= 0.019 and p= 0.013; respectively). Generally, MFS and OS were worse among stage III patients. In DCIS patients, the 3-year and 5-year LRFS were 99.4% and 88.3% respectively. In the regression analyses for local recurrence, triple-negative was found as a variable with a higher risk (HR 10.2 / p: 0.013; CI 1.63 – 64.0) of relapse than luminal tumours (Supplement 1). The survivals according to the stage for invasive disease and ductal carcinoma in situ (DCIS) are shown in Figures 3 and 4 and Supplement 2.
Figure 1.
Local and regional recurrence free survival in invasive disease population.
Figure 2.
Distant recurrence free survival and overall survival in invasive disease population.
Figure 3.
Local and regional recurrence free survival per stage in invasive disease population.
Figure 4.
Distant recurrence free survival and overall survival per stage in invasive disease population.
Side effects
Radiation dermatitis was the most frequent acute toxicity, and 2.9% had grade 3 reactions. One-fifth (n = 137) of patients experienced acute fatigue. Dysphagia was a rare acute event, occurring in only 2.1% of the entire cohort, and was associated with regional node irradiation, specifically supraclavicular fossa treatment (7.9% regional node irradiation versus 0% non-regional node irradiation; p< 0.001).
Few late toxicity adverse events were observed. The most frequent events were hyperpigmentation and mild fibrosis, which were not related to boost use (8.1% boost versus 4.1% non-boost; p= 0.094). More details of toxicity profile with the use of boost in Supplement 3. No rib fracture or brachial plexopathy was observed in this population. Capsular contracture occurred in 4 of 35 patients (11%) who underwent immediate breast reconstruction with implants. More details of acute and late toxicity profiles are listed in Tables 3 and 4. Radiation therapy was also well-tolerated in patients who received regional lymph nodes irradiation and those who underwent breast reconstruction (Supplements 4 and 5).
Table 3.
Acute toxicity.
| Acute toxicity | N | % |
|---|---|---|
| Skin reactions (dermatitis) | ||
| None | 146 | 21.8 |
| Grade 1 | 345 | 51.4 |
| Grade 2 | 160 | 23.9 |
| Grade 3 | 19 | 2.9 |
| Itching | ||
| None | 631 | 94.3 |
| Mild | 38 | 5.6 |
| Moderate | 1 | 0.1 |
| Dysphagia | ||
| None | 655 | 97.9 |
| Grade 1 | 14 | 2.0 |
| Grade 2 | 1 | 0.1 |
| Fatigue | ||
| None | 533 | 79.6 |
| Mild | 135 | 20.1 |
| Moderate | 2 | 0.3 |
| Skin retraction | ||
| No | 668 | 99.7 |
| Yes | 2 | 0.3 |
Table 4.
Late toxicity.
| Late toxicity | N | % |
|---|---|---|
| Skin reactions (dermatitis) | ||
| None | 647 | 96.5 |
| Grade 1 | 23 | 3.5 |
| None | 666 | 99.5 |
| Mild | 4 | 0.5 |
| Dysphagia | ||
| None | 669 | 99.9 |
| Fatigue | ||
| None | 666 | 99.5 |
| Mild | 4 | 0.5 |
| No | 661 | 98.7 |
| Yes | 9 | 1.3 |
| Fibrosis | ||
| Yes | 25 | 3.7 |
| Hyperpigmentation | ||
| No | 631 | 94.2 |
| Yes | 39 | 5.8 |
| Pneumonitis | ||
| No | 668 | 99.7 |
| Yes | 2 | 0.3 |
| Symptomatic pulmonary fibrosis | ||
| No | 669 | 99.9 |
| Yes | 1 | 0.1 |
| Ischemic cardiac event | ||
| No | 669 | 99.9 |
| Yes | 1 | 0.1 |
| Capsular contracture (N = 35) | ||
| No | 31 | 89.6 |
| Yes | 4 | 11.4 |
Discussion
Following breast-conserving surgery, moderately hypofractionated radiation therapy is the standard schedule for the treatment of early breast cancer patients.7,14,15 Some specific situations such as chest wall (with or without breast reconstruction) and regional node irradiation still face difficulties in its worldwide widespread use.16,17
To our knowledge, this is the largest cohort study on the use of moderately hypofractionated radiation therapy among breast cancer patients in Latin America. In early 2021, Najas G et al. published long-term data from a single Brazilian institution's experience with moderately hypofractionated radiation therapy. They included 394 breast cancer patients with primarily early-stage breast cancer. Breast reconstruction and regional irradiation were performed in 6 and 14 women only. During the 5-year, the LRFS and OS were 99% and 96%, respectively.17 In our study, we found a very similar 5-year LRFS of 96%, which was also comparable to the rates of local control in main large randomized trials of stage I and II breast cancer.10,18,19 In a Canadian trial, only patients with early-stage breast cancer with negative lymph nodes after breast-conserving surgery were enrolled and the 5-year LRFS in the hypofractionated arm was 97%.10 START A and B trials enrolled patients with higher risk factors for recurrence, such as lymph node-positive and grade 3 disease. Rates of 5-year local control were above 95% with hypofractionated schedules.11,18
In both UK trials,11,18 a significant portion of patients received a boost of 10Gy in five daily fractions as part of the radiation therapy schedule, similar to our cohort wherein 41.8% received a boost, but, mostly with a hypofractionated regimen. It is important to recognise that the EORTC 22881/10882 clinical trial showed that a boost dose to the tumour cavity after breast-conserving surgery and whole-breast irradiation was associated with a reduction in local relapse rates while increasing the risk of breast fibrosis.20 Our study demonstrated that the use of boost is related to an absolute increase in the incidence of late breast fibrosis, but interestingly, this was not statistically significant. This might be due to the short follow-up of our study or even to the modulated whole breast external beam technique.
DCIS was not included in the initial phase III trials and was first evaluated in a Danish randomized trial, where it was represented by 13% of the studied population. No difference in 9-year locoregional recurrence was observed between conventional and hypofractionated schemes. Eight of 123 (6.5%) patients with DCIS treated with hypofractionated radiation therapy had a local recurrence.21 The high rates of local recurrence rates among DCIS patients (3-year 99%, 5-year 88%) in our study may be explained by the presence of risk factors in our population, such as high grade, tumour size or age. Moreover, the absence of a boost could also explain the higher rates of local recurrence in this subgroup. Although moderate hypofractionated radiation therapy for DCIS is already accepted in clinical practice in the vast majority of oncology centres,7 the BIG 3-07/TROG 07-01 trial will probably bring more evidence in support the use of this regimen with or without boost.22
Regional node irradiation was performed in only 14.7% in the UK trials, corresponding to 864 patients.11,18,23 After 10 years of follow-up, the cumulative incidence rates of both physician- and patient- evaluated moderate and marked toxicities were similar for both groups (moderately hypofractionated irradiation and conventionally fractionated regimen). Moreover, similar outcomes were observed in a population-based cohort of four thousand women where hypofractionated regional node irradiation was evaluated.24 With long-term follow-up, it was demonstrated that locoregional radiation therapy with moderately hypofractionated offers similar breast cancer-specific outcomes compared with conventionally fractionated doses.25
In a non-inferiority trial of post-mastectomy patients, Wang S et al. found suitable results with the use of moderately hypofractionated irradiation to the chest wall and regional nodes area in more advanced stage disease. The 5-year locoregional cumulative incidence of recurrence was 8% and the 5-year OS was 84%. In our study, patients with stage III disease did not reach 60 months of follow-up; only 3-year rates were calculated with 98% of LRFS and 83% of OS, which are very comparable to Wang et al's results.12
Concerns still exist due to the prolonged period of time that radiation toxicities may appear, particularly regarding to nerve tissue, lung function and heart. Some authors argue that moderate hypofractionation for regional nodal irradiation must be used cautiously until more data from randomized trials are available. Likewise, additional concerns exist once chemotherapy was used in only 20% of patients in the UK trials,11,18,23 with most patients undergoing a non-standard systemic therapy schedule. Nevertheless, a standard chemotherapy schedule was used in the MD Anderson trial, the Chinese trial and also in our study, with acceptable side effects.12,26 The START trials indicated extremely low rates of lung fibrosis, brachial plexopathy and ischemic heart disease.27 Breast cancer patients rarely develop pulmonary or cardiac disorders that demand medical intervention.28, 29, 30 The side effects associated with radiation therapy are likely related to the older radiation therapy technique than to the dose regimen. This theory has been established in different countries, including the UK, the Netherlands and Italy where moderately hypofractionated irradiation has been the standard for nearly all patients with breast cancer for many years.31,32 Our study, added to others,33, 34, 35, 36 suggests that moderately hypofractionated irradiation to the breast and or chest wall, with or without regional nodal irradiation, is well-tolerated for the real-life practice.
There is a paucity of data available supporting the use of moderately hypofractionated irradiation after breast reconstruction. Past retrospective data show variable rates of implant complications with conventional fractionation breast irradiation. Any grade of capsular contracture can occur in almost 70% of patients, but only a few patients developed a serious form (grade IV) of contracture (1.2% to 6.9%).37, 38, 39 A retrospective study of 223 patients with immediate implants treated with hypofractionation schemes, only 1.4% developed major contracture over 2 years.40 Indeed, radiation therapy may increase the rate of complications involving reconstruction failures and capsular contracture.41, 42, 43, 44 In our series, 11% of any grade of capsular contracture was observed, none of them severe. We may suggest that after breast reconstruction, patient satisfaction with moderately hypofractionated irradiation will be comparable to conventional fractionation because most breast-associated toxicities that are related to the treatment (e.g., breast shrinkage, fibrosis, and skin retraction) tend to be less severe and less common in patients who received hypofractionation regiment.7
This study is limited by its retrospective design and this may lead to an underreporting of events, mainly related to toxicity. Moreover, because of the short follow-up, more long-term outcomes and patient-reported toxicity were not available. It is important to recognize that toxicity assessment may be affected by the lack of adjustment for follow-up time/patient. Additionally, locally advanced disease (stage III) and post-mastectomy scenarios were underrepresented in this cohort. However, our study can contribute to providing more evidence in support of using moderately hypofractionated irradiation more extensively in clinical practice.
In conclusion, in this Brazilian institution's experience, moderately hypofractionated irradiation to the breast, chest wall (with/without breast reconstruction), and regional lymph nodes were safe and effective with an acceptable toxicity profile.
Contributors
G.N.M. and G.S.M.S. conceived the project; G.S.M.S., S.A.H., L.F.M., F.A.M., H.A.C. and G.N.M. performed the literature search; all authors contributed to the literature analysis and synthesis of data; G.N.M. and G.S.M.S. created the figures and tables; G.N.M. and G.S.M.S. wrote the review; all authors were involved in further editing and finalising the manuscript.
Data sharing statement
The datasets generated during and/or analysed during the current study are not publicly available due to Brazilian data protection law but are available from the corresponding author on reasonable request.
Declaration of interests
The authors have declared no conflicts of interest.
Funding
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2022.100323.
Appendix. Supplementary materials
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed]
- 2.De Oliveira Santos M. Estimativa/2020 – Incidência de Câncer no Brasil. Rev. Bras. Cancerol. 20° de março de 2020 [citado 17° de julho de 2022];66(1):e-00927. Disponível em: https://rbc.inca.gov.br/index.php/revista/article/view/927.
- 3.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haffty BG. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Breast Dis. 2014;25:343–344. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 7.Marta GN, Coles C, Kaidar-Person O, et al. The use of moderately hypofractionated post-operative radiation therapy for breast cancer in clinical practice: a critical review. Crit Rev Oncol Hematol. 2020;156 doi: 10.1016/j.critrevonc.2020.103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marta GN, Ramiah D, Kaidar-Person O, et al. The financial impact on reimbursement of moderately hypofractionated postoperative radiation therapy for breast cancer: an international consortium report. Clin Oncol. 2021;33(5):322–330. doi: 10.1016/j.clon.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Fang M, Marta GN. Hypofractionated and hyper-hypofractionated radiation therapy in postoperative breast cancer treatment. Rev Assoc Med Bras. 2020;66(9):1301–1306. doi: 10.1590/1806-9282.66.9.1301. [DOI] [PubMed] [Google Scholar]
- 10.Viani GA, Gouveia AG, Bratti VF, et al. Prioritising locations for radiotherapy equipament in Brazil: a cross-sectional, population-based study and development of a LINAC shortage index. Lancet Oncol. 2022;23(4):531–539. doi: 10.1016/S1470-2045(22)00123-1. [DOI] [PubMed] [Google Scholar]
- 11.Whelan TJ, Pignol JP, Levine MN, et al. Long term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2020;362(6):513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 12.Bentzen S, Agrawal RK, Aird EGA, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2019;20(3):352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 14.Marta GN, Riera R, Pacheco RL, et al. Moderately hypofractionated post-operative radiation therapy for breast cancer: systematic reviem and meta-analysis of randomized clinical trials. Breast. 2022;62:84–92. doi: 10.1016/j.breast.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meattini I, Becherini C, Boersma L, et al. European Society os Radiotherapy and Oncology Advisory Committe in Radiation Oncology Pratice consensus recommendations on patiet selection and dose and fractionation for external beam radiotherapy in early breast cancer. Lancet Oncol. 2022;23(1):e21–e31. doi: 10.1016/S1470-2045(21)00539-8. [DOI] [PubMed] [Google Scholar]
- 16.Woodward SG, Varshney K, Anne PR, George BJ, Willis AI. Trends in use of hypofractionated whole breast radiation in breast cancer: an analysis of the national cancer database. Int J Radiat Oncol Biol Phys. 2021;109(2):449–457. doi: 10.1016/j.ijrobp.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Freitas NMA, Rosa AA, Marta GN, et al. Recommendations for hypofractionated whole-breast irradiation. Rev Assoc Med Bras. 2018;64(9):770–777. doi: 10.1590/1806-9282.64.09.770. [DOI] [PubMed] [Google Scholar]
- 18.Najas GF, Stuart SR, Marta GN, et al. Hypofractionated radiotherapy in breast cancer: a 10-year single institution experience. Rep Pract Oncol Radiother. 2021;26(6):920–927. doi: 10.5603/RPOR.a2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal RK, Aird EGA, Barrett JM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 21.Vrieling C, Van Werkhoven E, Maingon P, et al. Prognostic factors for local control in breast cancer after long-term follow-up in the EORTC boost vs no boost trial: a randomized clinical trial. JAMA Oncol. 2017;3(1):42–48. doi: 10.1001/jamaoncol.2016.3031. [DOI] [PubMed] [Google Scholar]
- 22.Offersen BV., Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase III trial: the DBCG HYPO trial. J Clin Oncol. 2020;38(31):3615–3636. doi: 10.1200/JCO.20.01363. [DOI] [PubMed] [Google Scholar]
- 23.King MT, Link EK, Whelan TJ, et al. Quality of life after breast-conserving therapy and adjuvant radiotherapy for non-low-risk ductal carcinoma in situ (BIG 3-07/TROG 07.01): 2-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(5):685–698. doi: 10.1016/S1470-2045(20)30085-1. [DOI] [PubMed] [Google Scholar]
- 24.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 25.Koulis TA, Nichol AM, Truong PT, et al. Hypofractionated adjuvant radiation therapy is effective for patients with lymph node–positive breast cancer: a population-based analysis. Int J Radiat Oncol Biol Phys. 2020;108(5):1150–1158. doi: 10.1016/j.ijrobp.2020.07.2313. [DOI] [PubMed] [Google Scholar]
- 26.Leong N, Truong PT, Tankel K, Kwan W, Weir L, Olivotto IA. Hypofractionated nodal radiation therapy for breast cancer was not associated with increased patient-reported arm or brachial plexopathy symptoms. Int J Radiat Oncol Biol Phys. 2017;99(5):1166–1172. doi: 10.1016/j.ijrobp.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Shaitelman SF, Lei X, Thompson A, et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: Results of a randomized, noninferiority clinical trial. J Clin Oncol. 2018;36(35):3495–3503. doi: 10.1200/JCO.18.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haviland JS, Mannino M, Griffin C, et al. Late normal tissue effects in the arm and shoulder following lymphatic radiotherapy: Results from the UK START (Standardisation of Breast Radiotherapy) trials. Radiother Oncol. 2018;126(1):155–162. doi: 10.1016/j.radonc.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liss AL, Marsh RB, Kapadia NS, et al. Decreased lung perfusion after breast/chest wall irradiation: quantitative results from a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2017;97(2):296–302. doi: 10.1016/j.ijrobp.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbanck S, Hanon S, Schuermans D, et al. Mild lung restriction in breast cancer patients after hypofractionated and conventional radiation therapy: a 3-year follow-up. Int J Radiat Oncol Biol Phys. 2016;95(3):937–945. doi: 10.1016/j.ijrobp.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Chan EK, Woods R, Virani S, et al. Long-term mortality from cardiac causes after adjuvant hypofractionated vs. conventional radiotherapy for localized left-sided breast cancer. Radiother Oncol. 2015;114(1):73–78. doi: 10.1016/j.radonc.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Marta GN, Poortmans P. Moderately hypofractionated breast radiation therapy: is more evidence needed? Lancet Oncol, 2019;20(5):e226. doi: 10.1016/S1470-2045(19)30078-6. [DOI] [PubMed]
- 33.Bloomfield DJ. Development of postoperative radiotherapy for breast cancer: UK Consensus Statements — a model of patient, clinical and commissioner engagement? Clin Oncol. 2017;29(10) doi: 10.1016/j.clon.2017.06.011. 639–641. [DOI] [PubMed] [Google Scholar]
- 34.Sayan M, Yehia ZA, Ohri N, Haffty BG. Hypofractionated postmastectomy radiation therapy. Adv Radiat Oncol. 2021;6(1) doi: 10.1016/j.adro.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin SM, No HS, Vega RM, et al. Breast, chest wall, and nodal irradiation with prone set-up: Results of a hypofractionated trial with a median follow-up of 35 months. Pract Radiat Oncol. 2016;6(4):e81–e88. doi: 10.1016/j.prro.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Bellefqih S, Elmajjaoui S, Aarab J, et al. Hypofractionated regional nodal irradiation for women with node-positive breast cancer. Int J Radiat Oncol Biol Phys. 2017;97(3):563–570. doi: 10.1016/j.ijrobp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Khan AJ, Poppe MM, Goyal S, et al. Hypofractionated postmastectomy radiation therapy is safe and effective: First Results from a prospective phase II trial. J Clin Oncol. 2017;35(18):2037–2043. doi: 10.1200/JCO.2016.70.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: An analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134(4):588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 39.Hughes K, Brown C, Perez V, et al. The effect of radiotherapy on implant-based breast reconstruction in the setting of skin-sparing mastectomy: clinical series and review of complications. Anticancer Res. 2012;32(2):553–557. [PubMed] [Google Scholar]
- 40.Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113(3):877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 41.Kim DY, Park E, Heo CY, et al. Hypofractionated versus conventional fractionated radiotherapy for breast cancer in patients with reconstructed breast: Toxicity analysis. Breast. 2021;55:37–44. doi: 10.1016/j.breast.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowen D, Gross E, Rouannet P, et al. Immediate post-mastectomy breast reconstruction followed by radiotherapy: risk factors for complications. Breast Cancer Res Treat. 2010;121(3):627–634. doi: 10.1007/s10549-010-0791-5. [DOI] [PubMed] [Google Scholar]
- 43.Anderson PR, Freedman G, Nicolaou N, et al. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant-is there a difference in complication rates? Int J Radiat Oncol Biol Phys. 2009;74(1):81–85. doi: 10.1016/j.ijrobp.2008.06.1940. [DOI] [PubMed] [Google Scholar]
- 44.Tallet AV., Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57(1):136–142. doi: 10.1016/s0360-3016(03)00526-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.