Summary
Background
Hypophosphatemic rickets is a rare, genetic syndrome with multisystem involvement. It causes skeletal abnormalities, painful enthesopathies, increased risk of fracture, and short stature; leading to a substantial burden of disease, disability, and worsening of quality of life. To improve health conditions of people living with this disease, it is essential to know its prevalence which is currently unknown in Colombia. This study aimed to estimate the prevalence of hypophosphatemic rickets in Colombia by using a mathematical model and national statistic records.
Methods
We executed a model to estimate probabilities of transitions between health, disease, and death states (Markov chains). The model was fed with international prevalences taken from original studies (systematic review) and administrative records’ data from SISPRO (a national health information system) using the International Classification of Diseases (ICD-10) E833 code, vital statistics, and census data. World Health Organization's (WHO) DISMOD II software was used to develop the model.
Findings
The estimated overall prevalence of hypophosphatemic rickets in Colombia in 2018 was 2·03 cases per 100 000 people (981 affected people), with a sensitive range of 1·97 to 2·09. The estimated prevalence by sex was 2·61 (645 people) and 1·43 (336 people) cases per 100 000 women and men, respectively.
Interpretation
Our overall estimated prevalence shows consistency with original international data. This is the first prevalence estimation of hypophosphatemic rickets in Colombia and will be relevant to support public health decisions for rare diseases and to provide a pre-test probability framework in clinical practice. DISMOD II and the model are useful tools to estimate the prevalence of rare and orphan diseases, when probabilistic studies cannot be carried out. There are limited bibliographic resources worldwide reporting prevalence values supported by original studies. Our study can be used as a cost-effective methodology reference in this regard, especially for Latin America.
Funding
Ultragenyx Pharmaceutical, as a donation.
Keywords: Rickets, Hypophosphatemic; Osteomalacia; prevalence; Rare diseases; Models, Statistical; Colombia
Research in context.
Evidence before this study
The model used to estimate the hypophosphatemic rickets prevalence in Colombia was developed with World Health Organization (WHO)’s statistical software DISMOD II. Data required to feed the software and adjust the model were obtained from the national population census, vital statistics, administrative records, and prevalences of hypophosphatemic rickets from original international studies and national case reports. Those studies were retrieved through the systematic literature search and review in the EMBASE, PubMed, Bireme (LILACS, SciELO), and Orphanet databases. The search strategy was performed in 2019 with the population, intervention, control, and outcomes (PICO) methodology, and in the broadest possible way to improve the sensitivity of the search (no limits on the date of publication, any type of original epidemiological study, and any language but whose abstracts were available in Spanish or English). Eleven original studies were retrieved with information on population frequency measures. However, most were related to the distribution of mutations in the population with hypophosphatemic rickets diagnosed by clinical and laboratory tests. Only three studies had prevalence estimates of hypophosphatemic rickets. No original studies on the prevalence of hypophosphatemic rickets were found in Colombia or Latin America.
Added value of this study
This study provides the first prevalence estimate of hypophosphatemic rickets in Colombia, even the first original estimate in a Latin American country. The estimate is representative at a national level due to the characteristics of the sources used. In addition, it was generated with the most recent data available (not only for children but for the general population) and provides a cost-effective alternative to estimate the prevalence of rare diseases.
Implications of all the available evidence
Prevalence is necessary to support decisions of high impact in public health, it also generates a pre-test probability framework in the clinical setting. However, there are limited original studies worldwide on the prevalence of hypophosphatemic rickets. Our study can be a reference for estimating the prevalence of rare and orphan diseases when it cannot be obtained from probabilistic studies. Additional studies (using the analysis of each case through clinical and biochemical records, and molecular confirmations) are required to deepen our outcomes. Comparisons of other original studies with our research should be cautious due to the divergence in study populations, methods, and sources of information. To ease international comparisons, more collaborative work is needed to standardize the registration and validation of cases of hypophosphatemic rickets.
Alt-text: Unlabelled box
Introduction
Hypophosphatemic rickets (or familial hypophosphatemia) is a rare, genetic, renal phosphate wasting disorder with multisystem involvement. It is characterized by hypophosphatemia, phosphaturia, normocalcemia, osteomalacia, and failure (or delay) in endochondral calcification of the growth plates of long bones.1 It causes skeletal abnormalities, painful enthesopathies, increased risk of fracture, and short stature.2
These alterations are produced mainly by serum elevation of fibroblast growth factor 23 (FGF23) protein,3 caused by pathological variants of genes such as the phosphate regulating neutral endopeptidase (PHEX), FGF23, dentin matrix acidic phosphoprotein 1 (DMP1), and ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1). Its inheritance can be autosomal dominant (ADHR), autosomal recessive (ARHR), or (much more frequently) x-linked (x-linked hypophosphatemia [XLH]).4, 5, 6, 7 Other mutations described are the sodium phosphate cotransporter protein 2C (SLC34A3) gene that encodes a sodium-phosphate cotransporter, and the chloride voltage-gated channel 5 (CLCN5) gene that encodes a voltage-gated chloride ion channel. These genes do not induce an elevation of FGF23, but are affected in hypophosphatemic rickets with hypercalciuria.5
Treatment to mitigate complications and control symptoms consists of a regular and early supply of calcitriol, phosphate salts, or FGF23 neutralizing antibody (burosumab; more recent and effective but only for XLH).6,8,9
Hypophosphatemic rickets generates a substantial economic and healthcare burden, morbidity, disability, and worsening of quality of life. However, given its irreversibility and low mortality,10, 11, 12 it behaves like a chronic disease that demands greater health resources.
Prevalence is a fundamental measure in epidemiology as it quantifies the incidence of a disease in a population in a particular location and at a given time point. Knowing the disease prevalence builds a body of evidence and recognition of the pathology importance, allows for allocating health resources, planning costs and evaluating the impact on public health.13,14 It also illuminates doctors by providing a pre-test probability framework to the diagnostic process (according to the clinical picture and disease frequency in its influenced area).
The Colombian government recognizes patients with rare diseases as especially protected people with prioritized healthcare.15 Rare diseases are defined as those with a prevalence of less than 1 per 5 000 people, chronically debilitating, and life-threatening.16 Consequently, to improve health policies, health care conditions, and treatment designation for people with hypophosphatemic rickets, it is essential to know the prevalence of the disease, which is currently unknown in Colombia.
National population health surveys (NPHS) with probabilistic sampling are frequently used to estimate disease prevalence, however, they are large in scale, technically complex and costly. If the phenomenon under study is of low frequency and a genetic disease, the technical difficulties will be higher due to fewer cases which are harder to find. Thus, NPHSs are inefficient for rare diseases prevalence studies.17
As an alternative, the prevalence might be determined using the disease mean duration and the new cases (incidence) detected at birth,18 but hypophosphatemic rickets is not usually identified at birth.
The objective of this study was to estimate the prevalence of hypophosphatemic rickets in Colombia using a mathematical model and national statistics.
Methods
Study design and statistical analysis
We used a mathematical health state transitional model supported by a system of differential equations. The probability of moving from one health state to another is proportional to the number of people in each state, and the probability of an event depends only on the immediately preceding event (Markov Chain). Markov model has been used in epidemiological research of noncommunicable chronic diseases.19, 20, 21 The model was developed with WHO's statistical software DISMOD II,22 which has been used to identify epidemiologic characteristics of rare orphan diseases.23, 24, 25 DISMOD II must be fed with at least three of the possible four states that model the course of the disease: healthy (remission cases included), sick from the specific disease, dead from the specific disease, and dead from a different cause.26 Therefore, relative risks of mortality, specific mortality rates, general mortality rates (from all causes), prevalence (or incidence) are required. Population data by age, sex, and remission cases are required as well.
DISMOD II generated the mathematical model that produced the punctual estimates of the prevalence for both sexes and by sex. We also used DISMOD II to run the sensitivity analysis; which was calculated for this prevalence, producing a sensitive range. We used Microsoft Excel® and R statistical software to prepare data and graphs.
Data sources
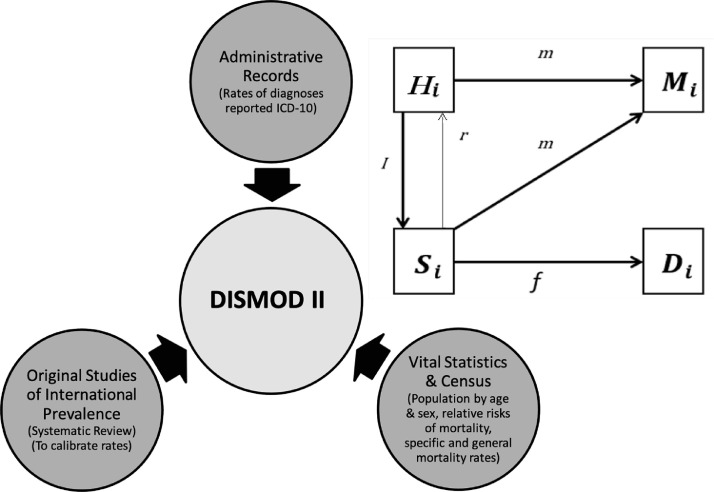
Colombia has publicly available population census data and vital statistics27 available at Departamento Administrativo Nacional de Estadística (DANE), and administrative records available at Sistema Integrado de Información de la Protección Social (SISPRO). All of these are of national coverage, unified, captured by legal norm, and continuously improved per processes.1,28 Those sources that contained data on users and health services were analyzed and selected to generate the epidemiological information (Figure 1).
Figure 1.
Sources and method.
Di=specific deaths by age; f= case fatality; Hi=healthy people by age (people without the disease under consideration); ICD=International Classification of Diseases; l=incidence rate; Mi=general deaths by age; m=mortality rate; r=not applicable for this study; Si=sick people by age., Note: Adapted from Barendregt JJ, et al 26
We used Colombian demographic information and population structure from the last housing population census (2018) to feed the developed model. Hypophosphatemic rickets was assumed to be an irreversible disease, thus, there are no remission cases.
We used mortality information from the last vital statics (2017). We calculated both adjusted five-year and sexes mortality rates of all causes, general mortality rates, and specific mortality rate. General mortality rates are defined as how many people died in one year in Colombia in one sex and in a single five-year age group, divided by the population of the same sex and five-year age group. The specific mortality rate is defined as how many people died with a specific diagnostic (ICD-10 code), divided by the population alive in Colombia within the same sex and same age group. DISMOD II used the specific mortality rates from the E833 ICD-10code. The source of the vital statistics and the populations was DANE.
We tabulated nationwide rates by five-year age groups and gender groups from diagnosed cases reported in medical records (diagnoses with ICD-10 code E833), considering that these diagnoses are cases from people who were still alive. The source of this data was Sistema Integrado de Información de la Protección Social (SISPRO).
Hypophosphatemic rickets’ cases were defined using the specific code from the International Classification of Diseases, 10th revision (ICD-10). There is no specific ICD-10 code for hypophosphatemic rickets, therefore, we reviewed the ICD-10 codes and selected 23 of them (E55, E83, M21, M41, M83, Q75, Q77, Q78, Q87 group-related codes) corresponding to diseases considered differential diagnoses because they could have been used to code a case under study. We analyzed each of the codes and selected E833 “Disorders of phosphorus metabolism and phosphatases” because it is the most accurate to record the diagnosis and has been used in studies of hypophosphatemic rickets.29
Systematic review of the literature
We conducted a systematic review of the literature to identify original studies containing prevalence estimates and used these data to adjust the model.
Articles were selected using the following criteria: 1) Their prevalence estimation was original (calculated from population databases; population surveys; and cross-sectional, cohort, and ecological studies); 2) Study population was people with hypophosphatemic rickets; and 3) The outcome was a measure of frequency (incidence, prevalence, frequency, or distribution by demographic characteristics). Articles were excluded if: 1) Their population had other types of rickets (different than hypophosphatemic rickets); 2) Their results presented secondary frequency estimations (from other studies such as narrative articles or systematic reviews); or 3) The methodology to estimate the frequency values was not clearly specified. Studies were retrieved through systematic literature review and cases reports. A systematic literature search was carried out in the EMBASE, PubMed, Bireme (LILACS, SciELO), and Orphanet databases. The search strategy was performed in 2019 with the population, intervention, control, and outcomes (PICO) methodology, and in the broadest possible way to improve the sensitivity of the search (no limits on the date of publication, any type of original epidemiological study, and in any language but whose abstracts were available in Spanish or English). An operational definition of hypophosphatemic rickets’ case was established for the selection (Table 1).
Table 1.
Operational definition of hypophosphatemic rickets’ case.
| Probable Case | Conditions |
|---|---|
| Patient with short stature and one or more of the following conditions (appeared between 1 and 15 years): |
|
| |
| |
| In adults, in addition to the previous conditions (without being exclusive to configure a probable case of hypophosphatemic rickets) it can be observed: |
|
| |
|
Role of the funding source
The funding source had no role in study design, data collection, data analysis, results interpretation, or writing of the report.
Results
Systematic review and Model Adjustment
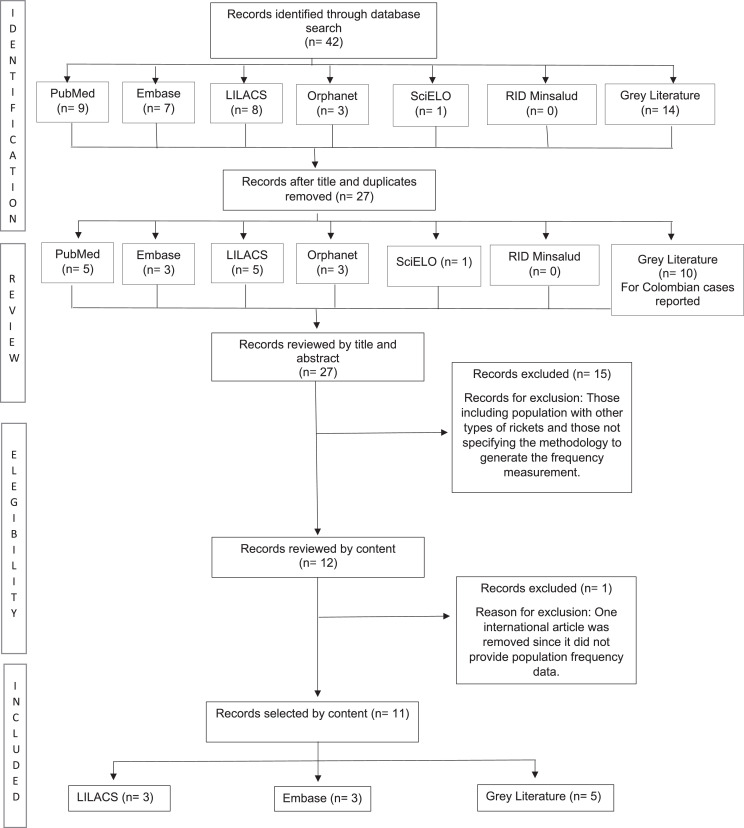
Eleven original studies were retrieved with information on population frequency measures (Figure 2). However, most were related to the distribution of mutations in the population with hypophosphatemic rickets diagnosed by clinical and laboratory tests. Only three studies had nationwide prevalence estimates of hypophosphatemic rickets, with values ranging between 0·65 and 4·8 cases per 100 000 people. None of them were carried out in Colombia or in Latin America.
Figure 2.
Systematic review search algorithm.
RID=Institutional Digital Repository.
Three original studies of hypophosphatemic rickets in Colombia were found,30, 31, 32 but none of them had an original frequency measurement. Original studies found in Colombia reported prevalence only as data retrieved from other review articles.
Estimated prevalence in Colombia
Our model estimated, for 2018, a prevalence of hypophosphatemic rickets in Colombia of 2·03 cases per 100 000 inhabitants (equivalent to 0·0203 cases per 1 000 inhabitants). According to the results of the 2018 population census, Colombia has 48 258 494 inhabitants, with 51·2% being women.27 Based on this population, it is inferred that there exist 981 cases of living people with hypophosphatemic rickets, 336 would correspond to men and 645 to women (65·8%).
DISMOD II allows to do sensitivity exercises of the estimates. Thus, a simulation was performed using a uniform distribution in the estimation parameters. A uniform distribution (U [a * 0·97, a * 1·03]) was used around each of the parameters; that means that a uniform distribution of 6% amplitude was assumed. DISMOD II performed a Monte Carlo simulation to produce a sensitive interval on prevalence.
Overall, the estimated prevalence for diagnoses of hypophosphatemic rickets (E833 ICD-10 code) was 2·03 cases per 100 000 inhabitants, with a sensitive range of 1·98 to 2·09 per 100 000 inhabitants. When translated into number of cases (using the 2018 population census), between 954 and 1 008 cases of hypophosphatemic rickets are estimated in Colombia. The estimated prevalence and corresponding sensitive range were 2·61 (2·53 – 2·68) rate per 100 000 inhabitants for women, and 1·43 (1·39 – 1·46) rate per 100 000 inhabitants for men.
Discussion
We calculated the prevalence estimation of hypophosphatemic rickets in Colombia from current, nationwide, and real-world data because of the characteristics of the sources used. Furthermore, this becomes the first nationally-representative estimated prevalence of familial rickets. This estimation generates a pre-test probability framework useful for the clinical practice. Also, as a frequency measure of a chronic disease, it allows to make national or international temporal comparisons.
Prevalences of less than 1 case per 1 000 000 inhabitants have been reported for autosomal dominant hypophosphatemic rickets and hypophosphatemic rickets with hypercalciuria.33,34 The worldwide prevalence of XLH rickets is considered to be 1 to 9 cases per 1 000 000 inhabitants.35 In Latin America, one case is reported for every 20 000 children.31,36 However, these data correspond to subject reviews and not to original studies.
Beck-Nielsen et al. used population databases to estimate the prevalence and to define the disease case in southern Denmark. They studied a population-based retrospective cohort by reviewing medical records, and estimated a prevalence of hypophosphatemic rickets of 4·8 per 100 000 inhabitants aged 0 to 14·9 years.37
Rafaelsen et al. also used national databases and the E833 ICD-10 code to estimate hypophosphatemic rickets prevalence. They studied a cohort of children in Norway with 28 cases of diagnosed hypophosphatemic rickets (21 of them confirmed by genetic study), and estimated a prevalence of 1 in 60 000, equivalent to 1·67 cases per 100 000 inhabitants.29
For the United Kingdom, a prevalence of 0·65 cases per 100 000 inhabitants was preliminarily estimated using clinical databases and a probabilistic case classification.38 After our systematic search, a study was published with an adjustment of the prevalence estimate to 1·5 cases per 100 000 inhabitants.39
Colombia is the most populous nation of Spanish-speakers in South America and is almost five times bigger than the United Kingdom. Also, it is nine times more populated than Norway or Denmark. A strength of our study is the potential generalization of the findings, considering the population and characteristics of the sources used (nationwide, comprehensive census data).
Population analysis of heritage genetic diseases may show heterogeneous distribution among different regions because of race-specific biological predisposition, selection pressure, and inbreeding or consanguinity behavior. Usually, familial hypophosphatemia has no selection pressure.
Latin American population shows extensive genetic and phenotypic diversity, and Colombia is not an exception. European and African ancestries (Europe 61·9%, America 29·2%, Africa 9·6%) are slightly more significant than those in most of the Latin American countries.40 Colombian population displays consanguinity rates similar to those in the countries that we used for comparison of hypophosphatemic rickets’ prevalence. In fact, consanguinity rates are less than 5% than those in China, Russia, Australia, Europe, and all-America.41,42 Consequently, the hypophosphatemic rickets’ prevalence should not have many variations within Colombia, even in Latin America. Although some endogamic point into each country may be possible.
Familial hypophosphatemia affects both males and females. In our estimation, 65·8% of living people with hypophosphatemic rickets correspond to women (Colombian women are 51·2% of the population). XLH is the most common form of heritable rickets, while related disorders (ADHR and ARHR), are encountered far less frequently. Therefore, an excess of females is expected from an X-linked disorder which is consistent with other studies.39
DISMOD II is a useful tool to assess disease duration, disability, burden of disease, prognosis, economic evaluation, and provision of health resources.19,20,24 Furthermore, it allows us to estimate epidemiological parameters of rare diseases such as in our research.23, 24, 25 Estimations produced with DISMOD II have limited capacity to detect changes over time or to make estimates in the medium or long term. In addition, they show relative instability in the age subgroups with a small number of cases because few cases in a specific age group can cause a significant change in the prevalence estimate. Therefore, we recommend our prevalence estimate to be considered at a national level, and to be used for a specific point in time and not for forecast.
Few original studies with prevalence estimations of this disease have been published worldwide. Our prevalence is higher than the usual one reported, but it is consistent with other prevalences from the original studies based on the population database Table 2. Thus, we can outline the hypothesis that the prevalence of hypophosphatemic rickets might be higher than the one traditionally reported. This could be explained by a greater access to medical services, greater recognition of the disease among professionals, or a better quality of coding and registration.
Table 2.
Comparison of the estimated prevalence's of hypophosphatemic rickets. Colombia versus world estimates.
| Country | Prevalence (Rate per 100 000 inhabitants) |
|---|---|
| Global (usually reported) | 0·1 - 0·9 |
| United Kingdom | 1·5 |
| Norway | 1·67 |
| Colombia | 2·03 (1·98 – 2·09) |
| Denmark | 4·8 |
Note: After our systematic search, a study was published with adjustment of the information for the United Kingdom. It reported a prevalence of XLH (with 2016 estimates) in childhood and adulthood of 15·1 (95% CI = 11·3-20·1) and 15·7 (95% CI = 11·8-20·9) per million, respectively. 33 This is equivalent to 1·5 cases per 100 000 inhabitants.
The E833 ICD-10 code includes phosphorus metabolism disorders which correspond to hypophosphatemic rickets and closely related diseases. Causes of hypophosphatemia include deficiency of phosphorus in the diet, its malabsorption, iatrogenic disease, and increase in the loss of phosphorus (such as in hypophosphatemic rickets).43 Hypophosphatasia is also a phosphorus metabolism disorder, but with a different clinical presentation.44 All these situations contribute to an overestimation of the hypophosphatemic rickets’ prevalence.
Our estimation is subject to underreporting of cases due to cases of hypophosphatemic rickets in the process of diagnostic confirmation from differential diagnoses, cases with a confirmed diagnosis that were not registered in the clinical records, deaths due to hypophosphatemic rickets that were not registered in the vital statistics, and cases that were registered with a cause of death other than the E833 ICD-10 code.
Acquired causes of renal phosphate wasting disorders are seldom considered as part of hypophosphatemic rickets. They rarely appear and are usually caused by a humoral factor such as FGF23 produced by benign mesenchymal tumors. This phenomenon is best known as tumor-induced osteomalacia.6 Nevertheless, there is a low probability that doctors coded this diagnosis using E833 ICD-10 because it is classified as a mixed connective tissue tumor (M83.8 ICD-10 code: “Other adult osteomalacia”).45
The differential main diagnoses of hypophosphatemic rickets are osteochondrodysplasias (pathological growth arrest in growth plates), bone fibrous dysplasia, and Fanconi renal syndrome.46 However, it is unlikely to code these diagnoses using E833 ICD-10 because the dysplasias have similar clinical manifestations (especially at initial stages) but no renal phosphate waste. Therefore, clinicians use Q78 ICD-10 “Other osteochondrodysplasias” related code.47 On the other hand, Fanconi renal syndrome is an acidification disorder characterized by renal proximal tubule dysfunction with chronic kidney disease degree. Thus, E72 ICD-10 “Other disorders of amino-acid metabolism” or N25 ICD-10 “Disorders resulting from impaired renal tubular function” related codes48 are more feasible to be used.
Due to the Colombia's proximity to the equator, its weather is tropical and generally isothermal (without any real seasons change). Hence, there is sunlight exposition throughout the year. Rickets may occur as a result of Vitamin D deficiency, usually by nutritional insufficiency in combination with a lack of sunlight exposure.49 In Colombia, this situation is unlikely; but it would be a consideration as differential diagnoses in other countries with less sunlight.
The best-adjusted prevalences for rare and genetic diseases must consider molecular confirmation, but it is not always available. To improve estimations of hypophosphatemic rickets, unique and different codes must be created for hypophosphatemic rickets and other phosphorus metabolism disorders. This would be particularly important to consider for the new ICD-11.50
Population surveys are probabilistic epidemiological studies suitable to estimate prevalence. However, they are not feasible in rare diseases due to the characteristics of these diseases.51 Hence, this study shows an alternative with a cost-efficient methodology in that regard. Nevertheless, comparisons of other original studies with our research should be cautious on the divergence in study populations, methods, and sources of information. To easily make international comparisons, more collaborative work is needed to generate patient registry and validation of cases of hypophosphatemic rickets. Also, more studies with cases confirmed by molecular methods are needed.
In conclusion, our study provides the first prevalence estimate of hypophosphatemic rickets in Colombia, and the first original estimate in Latin American. It shows consistency with international original studies as well. Hence, our estimation can be a reference for analytical studies aiming at rare diseases and is suitable for Latin America because it provides a lower-cost methodology to estimate prevalences. However, further analytical studies are required to deepen our outcomes, using the analysis of each case (through clinical and biochemical records, and molecular confirmations).
Contributors
JAHQ conceptualized the study, developed the research questions, managed and supervised the research, drafted the manuscript and figures with critical revisions by all other authors. JAHQ and HMVP defined the hypophosphatemic rickets’ case, selected the ICD-10 differential diagnoses of hypophosphatemic rickets after review and consensus, and carried out the analysis. DACO developed the mathematical model and carried out the statistical analysis. NLT performed the systematic literature review. All authors had full access to the data, reviewed and approved the final version of the manuscript. The principal investigator had the final decision to submit for publication.
Data sharing statement
Data used in this study are freely available from the Colombian Sistema Integrado de Información de la Protección Social (SISPRO) and Departamento Administrativo Nacional de Estadística (DANE).
Declaration of Competing Interest
We declare no competing interests.
Acknowledgements
This study was funded with a donation from Ultragenyx Pharmaceutical. We thank Julián Barrera (editing and proofreading of the manuscript) and Odds Epidemiology (epidemiological consulting services).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lana.2021.100131.
Appendix. Supplementary materials
References
- 1.Ruppe M, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K AA. X-Linked Hypophosphatemia. GeneReviews. 2012;1 http://www.ncbi.nlm.nih.gov/pubmed/22319799 Published online February 9Accessed November 8, 2018. [Google Scholar]
- 2.Cheung M, Roschger P, Klaushofer K, Veilleux LN, Roughley P, Glorieux FH RF. Cortical and Trabecular Bone Density in X-Linked Hypophosphatemic Rickets. J Clin Endocrinol Metab. 2013;98(5):E954–E961. doi: 10.1210/jc.2012-4133. [DOI] [PubMed] [Google Scholar]
- 3.White KE, Evans WE, O'Riordan JLH, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 4.Ruppe MD, Brosnan PG, Au KS, Tran PX, Dominguez BW, Northrup H. Mutational analysis of PHEX, FGF23 and DMP1 in a cohort of patients with hypophosphatemic rickets. Clin Endocrinol (Oxf) 2011;74(3):312–318. doi: 10.1111/j.1365-2265.2010.03919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guven A, Al-Rijjal RA, BinEssa HA, et al. Mutational analysis of PHEX, FGF23 and CLCN5 in patients with hypophosphataemic rickets. Clin Endocrinol (Oxf) 2017;87(1):103–112. doi: 10.1111/cen.13347. [DOI] [PubMed] [Google Scholar]
- 6.Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A Clinical and Molecular Genetic Study of Hypophosphatemic Rickets in Children. Pediatr Res. 2005;58(2):329–333. doi: 10.1203/01.PDR.0000169983.40758.7B. [DOI] [PubMed] [Google Scholar]
- 7.Capelli S, Donghi V, Maruca K, et al. Clinical and molecular heterogeneity in a large series of patients with hypophosphatemic rickets. Bone. 2015;79:143–149. doi: 10.1016/J.BONE.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Imel EA, Biggin A, Schindeler A, Munns CF. FGF23, Hypophosphatemia, and Emerging Treatments. JBMR Plus. 2019;3(8):e10190. doi: 10.1002/jbm4.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416–2427. doi: 10.1016/S0140-6736(19)30654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jüppner H. Krzywica Hipofosfatemiczna- ORPHA437. 2012 https://www.orpha.net/data/patho/PL/Krzywicahipofosfatemiczna-PLplAbs160.pdf Accessed November 12, 2017. [Google Scholar]
- 11.Forestier-Zhang L, Watts L, Turner A, et al. Health-related quality of life and a cost-utility simulation of adults in the UK with osteogenesis imperfecta, X-linked hypophosphatemia and fibrous dysplasia. Orphanet J Rare Dis. 2016;11(1):1–9. doi: 10.1186/s13023-016-0538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Canadian Agency for Drugs and Technologies in Health (CADTH) Pharmacoeconomic Review Report - Burosumab. 2020 https://www.ncbi.nlm.nih.gov/books/NBK565319/ [PubMed] [Google Scholar]
- 13.Bonita R, Beaglehole R. Organization WH. 2nd editio. World Heal Organ; 2006. Basic epidemiology; p. 226.http://books.google.it/books?id=AAZGobMNTXgC&dq=basic+epidemiology+beaglehole&printsec=frontcover&source=bn&hl=en&ei=dG9US57XLaXUmgO668GfCg&sa=X&oi=book_result&ct=result&resnum=4&ved=0CB8Q6AEwAw#v=onepage&q=&f=false Suppl 2. [Google Scholar]
- 14.Mirón Canelo JA, Alonso Sardón M. Medidas de frecuencia, asociación e impacto en investigación aplicada. Med Segur Trab (Madr) 2008;54(211):93–102. http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0465-546X2008000200011 Accessed March 5, 2019. [Google Scholar]
- 15.República de Colombia- Gobierno Nacional Ley 1751 de 2015. Por Medio de La Cual Se Regula El Derecho Fundamental a La Salud y Se Dictan Otras Disposiciones. 2015 https://www.minsalud.gov.co/Normatividad_Nuevo/Ley%201751%20de%202015.pdf [Google Scholar]
- 16.República de Colombia Ley 1438 Del 19 de Enero de 2011: Por Medio de La Cual Se Reforma El Sistema General de Seguridad Social En Salud y Se Dictan Otras Disposiciones. 2011 http://www.alcaldiabogota.gov.co/sisjur/normas/Norma1.jsp?i=41355 [Google Scholar]
- 17.Bowling A, Ebrahim S. Open University Press; 2005. Handbook of Health Research Methods: Investigation, Measurement and Analysis. [Google Scholar]
- 18.Orphanet. Prevalencia de las enfermedades raras : Datos bibliográficos. In: Nguengang Wakap S, ed. Informes Periódicos de Orphanet, Serie Enfermedades Raras. Joint Action; update 2021:13 24. Accessed November 12, 2017.
- 19.Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 20.Cho SW, Kim SH, Kim Y-E, Yoon S-J, Jo M-W. Estimating Lifetime Duration of Diabetes by Age and Gender in the Korean Population Using a Markov Model. J Korean Med Sci. 2019;34(Suppl 1) doi: 10.3346/jkms.2019.34.e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begun A, Morbach S, Rümenapf G, Icks A. Study of disease progression and relevant risk factors in diabetic foot patients using a multistate continuous-time Markov chain model. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barendregt J, World Health Organization. WHO | Software tools. Health statistics and information systems .
- 23.Villaverde-Hueso A, Posada de la Paz M, Martín-Arribas MC, Sánchez-Valle E, Ramírez-González A BA. Prevalence of scleroderma in Spain: an approach for estimating rare disease prevalence using a disease model. Pharmacoepidemiol Drug Saf. 2008;17(11):1100–1107. doi: 10.1002/pds.1660. [DOI] [PubMed] [Google Scholar]
- 24.Chung S-E, Cheong H-K, Park J-H, Kim HJ. Burden of Disease of Multiple Sclerosis in Korea. Epidemiol Health. 2012;34 doi: 10.4178/epih/e2012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Cui Y, Li Y, Wang C, Zhao H, Han J. Using inpatient data to estimate the prevalence of Wegener's granulomatosis in China. Intractable Rare Dis Res. 2016;5(1):31–35. doi: 10.5582/irdr.2015.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barendregt JJ, GJ VO, T V, Murray CJ. A generic model for the assessment of disease epidemiology: The computational basis of DisMod II. Popul Health Metr. 2003;1(1):4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Departamento Administrativo Nacional de Estadística (DANE. Estadísticas Vitales de Colombia. December 29, 2018 https://www.dane.gov.co/index.php/poblacion-y-demografia/nacimientos-y-defunciones/118-demograficas/estadisticas-vitales/2877-defunciones-no-fetales Accessed. [Google Scholar]
- 28.Rivillas García JC, Huertas Quintero JA, Montaño Caicedo JI, Ospina ML. Progresos en eSalud en Colombia : adopción del Sistema de Información Nacional en Cáncer. Rev Panam salud pública. 2014;35(5/6):442–448. http://www.scielosp.org/pdf/rpsp/v35n5-6/22.pdf [PubMed] [Google Scholar]
- 29.Rafaelsen S, Johansson S, Ræder H BR. Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol. 2016;174(2):125–136. doi: 10.1530/EJE-15-0515. https://eje.bioscientifica.com/view/journals/eje/174/2/125.xml Accessed November 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglesias Gamarra A, Peña Cortés M, Restrepo Suárez JF, Rondón Herrera F, Sánchez Contreras A, Iglesias Rodríguez A, Lacouture Gómez M, Giraldo Ríos A. Calvo Páramo E CC. Osteomalacia y raquítismo. Análisis y estudio en diferentes periodos historicos en Colombia. REEMO. 2000;9(6):207–250. http://www.elsevier.es/es-revista-reemo-70-articulo-osteomalacia-y-raquitismo-analisis-y-10021833?referer=buscador Accessed November 24, 2018. [Google Scholar]
- 31.Quintana López G, Restrepo JF, Sánchez Á, Calvo E, Fernández A, Iglesias Gamarra A. Raquitismo hipofosfatémico ligado al X (XLH): Presentación de una familia, asociado a una osteoartritis prematura y simulando además una espondiloartropatía seronegativa. Rev Colomb Reumatol. 2008;15(2):117–122. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-81232008000200006&lng=en&tlng=en Accessed March 14, 2019. [Google Scholar]
- 32.Tiempo El, Correa M. En cauca, caso único en el mundo de raquitismo. El Tiempo - Archivo Digital de Noticias de Colombia y el Mundo desde 1.990. August 16, 1991 http://www.eltiempo.com/archivo/documento/MAM-137669 Published. [Google Scholar]
- 33.Orphanet. Raquitismo hipofosfatémico autosómico dominante. Orphanet Portal de información de enfermedades raras y medicamentos huérfanos. June 28, 2019 https://www.orpha.net/consor4.01/www/cgi-bin/Disease_Search.php?lng=ES&data_id=11912&Disease_Disease_Search_diseaseGroup=Raquitismo-hipofosfatemico&Disease_Disease_Search_diseaseType=Pat&Enfermedad(es)/grupodeenfermedades=Raquitismo-hipofosfatemico- Published 2017Accessed. [Google Scholar]
- 34.Orphanet. Raquitismo hipofosfatémico hereditario con hipercalciuria. Orphanet Portal de información de enfermedades raras y medicamentos huérfanos. June 28, 2019 https://www.orpha.net/consor4.01/www/cgi-bin/Disease_Search.php?lng=ES&data_id=17137&Disease_Disease_Search_diseaseGroup=Raquitismo-hipofosfatemico&Disease_Disease_Search_diseaseType=Pat&Enfermedad(es)/grupodeenfermedades=Raquitismo-hipofosfatemico- Published 2017Accessed. [Google Scholar]
- 35.Orphanet. Hipofosfatemia ligada al X. Orphanet Portal de información de enfermedades raras y medicamentos huérfanos. June 28, 2019 https://www.orpha.net/consor4.01/www/cgi-bin/Disease_Search.php?lng=ES&data_id=11911&Disease_Disease_Search_diseaseGroup=Raquitismo-hipofosfatemico&Disease_Disease_Search_diseaseType=Pat&Enfermedad(es)/grupodeenfermedades=Hipofosfatemia-ligada-al-X& Published 2017Accessed. [Google Scholar]
- 36.Sakihara Asato G, Choqque Tacca H, Mendoza Bedriñaña A, López Córdova V, Pimentel Klose G, Encinas Arana M AZA. Raquitismo Renal Hipofosfatémico: Experiencia en 10 Años. Rev peru pediatr. 2014;67(1):22–30. https://docplayer.es/21725928-Revista-peruana-de-pediatria-publicacion-oficial-de-la-sociedad-peruana-de-pediatria.html Accessed November 14, 2018. [Google Scholar]
- 37.Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160(3):491–497. doi: 10.1530/EJE-08-0818. [DOI] [PubMed] [Google Scholar]
- 38.Javaid MK, Delmestri A, Shaw N, Prieto-Alhambra D, Cooper C, Pinedo Villanueva R. World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (WCO-IOF-ESCEO 2018) Vol. 29. Springer; London: 2018. X-linked hypophosphataemia: burden of disease using United Kingdom primary care data; p. S277.https://ora.ox.ac.uk/objects/uuid:ae89f3cd-c254-4288-9775-39da3b488914 [Google Scholar]
- 39.Hawley S, Shaw NJ, Delmestri A, et al. Prevalence and Mortality of Individuals With X-Linked Hypophosphatemia: A United Kingdom Real-World Data Analysis. J Clin Endocrinol Metab. 2020;105(3):e871. doi: 10.1210/clinem/dgz203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adhikari K, Mendoza-Revilla J, Chacón-Duque JC, Fuentes-Guajardo M, Ruiz-Linares A. Admixture in Latin America. Curr Opin Genet Dev. 2016;41:106–114. doi: 10.1016/j.gde.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Hamamy H. Impact on Health and Counseling- Training Course in Sexual and Reproductive Health Research. Consanguineous Marriages Trends. 2015 https://gfmer.ch/SRH-Course-2015/community-genetics/pdf/Consanguineous-marriages-Hamamy-2015.pdf Accessed September 22, 2021. [Google Scholar]
- 42.Liascovich R, Rittler M, Castilla EE. Consanguinity in South America: Demographic aspects. Hum Hered. 2001;51:27–34. doi: 10.1159/000022956. https://www.jstor.org/stable/48506484?refreqid=excelsior%3Af264eaaf3ed89743b6f432b65384468c Accessed September 22, 2021. [DOI] [PubMed] [Google Scholar]
- 43.De Menezes Filho H, De Castro LCG, Damiani D. Hypophosphatemic rickets and osteomalacia. Arq Bras Endocrinol Metabol. 2006;50(4):802–813. doi: 10.1590/s0004-27302006000400025. [DOI] [PubMed] [Google Scholar]
- 44.Whyte MP. Hypophosphatasia-aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233–246. doi: 10.1038/nrendo.2016.14. [DOI] [PubMed] [Google Scholar]
- 45.Cesca L, Nijenhuis T, Orphanet Walsh S. The portal for rare diseases and orphan drugs. Oncogenic osteomalacia. 2019 https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=352540 PublishedAccessed October 7, 2021. [Google Scholar]
- 46.Pavone V, Testa G, Gioitta Iachino S, Evola FR, Avondo S, Sessa G. Hypophosphatemic rickets: etiology, clinical features and treatment. Eur J Orthop Surg Traumatol. 2015;25(2):221–226. doi: 10.1007/s00590-014-1496-y. [DOI] [PubMed] [Google Scholar]
- 47.Chapurlat R. Orphanet:The portal for rare diseases and orphan drugs. Fibrous dysplasia of bone. October 7, 2021 https://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=GB&Expert=249 Published 2019Accessed. [Google Scholar]
- 48.Walsh S. Orphanet:The portal for rare diseases and orphan drugs. Fanconi syndrome. October 7, 2021 https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=12113&Disease(s)_concerned=Fanconi-syndrome&title=Fanconi-syndrome&search=Disease_Search_Simple Published 2019Accessed. [Google Scholar]
- 49.Carpenter TO. National Organization for Rare Disorders (NORD) Familial Hypophosphatemia Rare disease database. 2019 https://rarediseases.org/rare-diseases/familial-hypophosphatemia/ PublishedAccessed September 22, 2021. [Google Scholar]
- 50.Releases new International Classification of Diseases (ICD 11) World Health Organization; 2018. World Health Organization.http://www.who.int/news-room/detail/18-06-2018-who-releases-new-international-classification-of-diseases-(icd-11) PublishedAccessed August 2, 2018. [Google Scholar]
- 51.Colombia. Ministerio de Salud y Protección Social. Dirección de Epidemiología y Demografía. Sistema Nacional de Estudios y Encuestas Poblacionales Para La Salud. Imprenta Nacional. 2013 https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/guia-estudios-poblacionales.pdf Accessed February 17, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.