Summary
Background
Gabapentin, opioids, and/or benzodiazepines are commonly prescribed for a variety of pain and psychiatric conditions. Despite the high likelihood of co-prescription of these medications, little is known about co-utilization of gabapentin (GABA), opioids (OP), and benzodiazepines (BZD) and associated public health outcomes.
Methods
Using Medicare CCW Data, 2013-2016, we conducted a nested case-control study to examine the association between concurrent utilization of GABA, OP, and BZD and respiratory depression, opioid, and substance-related overdose among Medicare disabled beneficiaries. Cases and controls were Fee-for-service disabled beneficiaries who had a diagnosis of acute pain (AP), chronic pain (CP) or mental health conditions (MH) and received GABA, OP or BZD. Cases with respiratory depression, opioid or substance-related overdose were matched with up to 4 controls on socio-demographics, year of cohort entry and disease risk score. Primary exposure was concurrent medication utilization defined as an overlap of at least one day in prescriptions for GABA, OP and BZD.
Findings
Across all cohorts, the majority of cases and controls were under 65, female, dually eligible and had prior histories of pain and mental health conditions. GABA+OP+BZD use was associated with increased odds of respiratory depression [AOR(95%CI)―AP: 1.35 (1.19-1.52), CP:1.24 (1.11-1.38) and MH: 1.16 (1.02-1.32) vs. OP only], opioid-related overdose [AP: 1.43 (1.04-1.98), CP: 1.47 (1.07-2.00) and MH: 1.44 (1.04-2.00) vs. OP only], and substance-related overdose [AP: 1.77 (1.26-2.50), CP: 1.70 (1.24-2.34) and MH: 1.92 (1.31-2.82) vs. GABA only]. While there were cohort differences in the association between GABA+OP and both respiratory depression and opioid-related overdose, GABA+OP and GABA+BZD use were associated with significantly higher odds of substance-related overdose across all clinical cohorts.
Interpretation
Among Medicare disabled beneficiaries, concurrent utilization of gabapentin, opioids, and benzodiazepines is associated with multiple adverse outcomes. Given this, it is imperative that the benefits and risks of co-prescribing these medications be comprehensively examined.
Funding
None.
Keywords: Gabapentin, Opioids, Benzodiazepine, Pain, Mental health
Research in context.
Evidence before this study
We searched PubMed for relevant articles published in English from 2015 using the following search terms ‘gabapentin’ AND ‘opioid’ AND ‘benzodiazepines’ AND ‘overdose’ OR ‘respiratory depression.’ We identified three articles which examined gabapentin and overdose. One of these articles was a Canadian based study which noted higher rates of fatal opioid overdose when gabapentin was used concomitantly with opioids. The other two studies were based in the United States and suggested that combination(s) of gabapentinoids with opioids were associated with higher adverse outcomes, specifically overdose and health care utilization.
Added value of this study
While the majority of prior studies focused on examining gabapentinoids (gabapentin and pregabalin) and opioids, our study focused on teasing out the relationship between gabapentin use in combination with opioids and/or benzodiazepines and several adverse outcomes in a high-risk Medicare disabled population. Using a methodologically rigorous approach which accounted for multiple confounders, our study noted that individuals who utilized gabapentin, opioids, and benzodiazepines were at significantly higher risk of respiratory depression, opioid and overall substance related overdose. Further, we noted that utilization of gabapentin and opioids only as well as gabapentin and benzodiazepines only was associated with a significantly higher risk of substance-related overdose.
Implications of all the evidence available
Our study suggests that there is a need for additional research on the combined use of gabapentin, opioids, and benzodiazepines in other study populations. Additionally, we highlight the need for physicians to weigh the benefits and risks associated with prescribing these medications concomitantly.
Alt-text: Unlabelled box
Introduction
Within the last two decades, drug-related overdose deaths have quadrupled in the United States.1, 2, 3, 4 In 2019 alone, these overdoses accounted for over 70,000 deaths―more than half of which involved an opioid.1,2 Concomitant utilization of CNS depressants such as benzodiazepines with opioids is a known risk factor for respiratory depression and associated opioid-related overdose.5, 6, 7, 8 Focused attention has been devoted to this increased overdose risk associated with concurrent benzodiazepine use among opioid users; in 2016, the FDA issued a black box warning concerning concurrent utilization of both medications. Despite modest decreases in concurrent opioid and benzodiazepine utilization following this warning, up to 11,537 opioid-related overdose deaths in 2017 involved benzodiazepines (both pharmaceutical and illicit sources).9,10
Gabapentin utilization is common among opioid and/or benzodiazepine users.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 While only FDA approved for partial seizures and post-herpetic neuralgia, gabapentin has become an essential component of several multimodal pain management strategies utilized by physicians.11,12 Not only that, gabapentin is also prescribed for the management of several psychiatric conditions.13, 14, 15, 16, 17, 18, 19, 20 Widespread off-label gabapentin utilization, especially for pain and psychiatric conditions, has been linked to its previously perceived benign safety profile; however, recently, several studies have linked its utilization with opioids to several adverse outcomes including overdose and adverse drug-related events.21, 22, 23, 24, 25, 26, 27, 28, 29
In one of such studies, a Canadian population-based case-control study, Gomes et al. investigated the risk of opioid-related death among the publicly insured. They found the rate of unintentional fatal opioid overdose was 49% higher among individuals with concomitant prescription opioid and gabapentin use.28 Similarly, Zhou and colleagues noted that in the Medicare population, consistent gabapentinoid and opioid use (regardless of dose) was associated with a two-fold increased risk of drug overdose.29
Despite emerging safety concerns regarding gabapentin, little is known about adverse consequences associated with gabapentin in combination with other CNS depressants such as benzodiazepines. In addition, despite the high likelihood of gabapentin, opioid and benzodiazepine co-prescription, no studies have examined the additional impact of benzodiazepines on gabapentin and opioid utilization, especially given continued opioid and benzodiazepine concurrent utilization. A majority of the prior studies that have examined gabapentin utilization have used a composite gabapentinoid measure which also captures pregabalin; little is known about the specific association between gabapentin alone and many of these adverse outcomes previously examined.27, 28, 29
Given this, the goal of our study was to quantify the associations between concurrent therapy involving gabapentin, opioids, and benzodiazepines and public health outcomes ―respiratory depression events, opioid and substance-related overdose events among Social Security Disability Insurance (SSDI)-eligible Medicare beneficiaries. This specific population provides access to a cohort of individuals who, due to disability, are more likely to be prescribed gabapentin, benzodiazepines, and/or opioids.
Methods
Data source
We utilized a 5% random sample of 2013-2016 Chronic Conditions Data Warehouse (CCW) Medicare data from the Center for Medicare & Medicaid Services (CMS). Based on the rule of thumb for sample size requirements (10 events per variable), our 5% random sample exceeded this requirement.30 We obtained demographic and enrollment information from the Master Beneficiary Summary File. Data on health care utilization were obtained from Medicare Parts A and B files, and comprehensive prescription drug information was derived from Medicare Part D files.
Identification of cohort and outcomes
We conducted a nested case-control study among SSDI-eligible Medicare beneficiaries―SSDI eligibility was defined using the original reason for entitlement in Medicare flag and included both those younger than 65 years old and those 65 years old and older who originally entered Medicare based on disability. Cohort entry date was defined as the date of the earliest prescription fill for gabapentin, opioids, and benzodiazepines. To address confounding by indication related to these medications, we also required beneficiaries to have diagnoses of acute pain (AP), chronic pain (CP), or mental health (MH) conditions in the 6-months prior to receipt of any of our medications of interest.
To ensure availability of beneficiaries’ health care utilization and prescription drug information, we excluded beneficiaries without continuous Medicare Parts A, B and D coverage and those with Medicare Advantage coverage in the 6-months prior to and 12-months following the cohort entry date. Because patterns of medication utilization among individuals with cancer/hospice benefits potentially differ from those of the general Medicare population, we also excluded beneficiaries with these conditions during the entire study period.31,32 Prior evidence suggests that chronic kidney disease increases the risk of gabapentin related respiratory depression33; hence, we excluded beneficiaries diagnosed with this condition during our study period. Additionally, because prior events increase the risk for subsequent outcome/events, we completed analyses in three distinct cohorts for each outcome ―in each cohort, we excluded beneficiaries diagnosed with the outcome of interest in the 6-months prior to cohort entry. Disabled beneficiaries diagnosed with AP, CP or MH within our nested cohort were followed for 12-months following cohort entry to ascertain outcome and exposure status.
The primary outcomes of interest were respiratory depression, opioid and substance-related overdose, defined using the International Classification of Disease, Ninth and Tenth Revision, clinical codes. Substance-related overdose events included opioid, sedative and/or epileptic poisoning and opioid-related overdose events included opioid-related poisonings. Overdose events included both intentional and unintentional poisonings. We excluded overdose events related to heroin to focus specifically on overdose events related to prescription opioids. Cases were beneficiaries within the nested cohort with at least one inpatient claim or two outpatient claims for an outcome of interest in the 12-months post cohort entry. The index date for cases was assigned as the date of the first outcome event. Controls were beneficiaries within the nested cohort without any of the outcomes of interest within the 12-month follow-up period and their index dates were randomly assigned based on the index dates for cases.
Independent variable
The primary independent variable was concurrent medication utilization. Beneficiaries with an overlap of at least one day in the days supplied of prescriptions for gabapentin, opioids, and benzodiazepines were considered GABA+OP+BZD users. Those with an overlap in prescriptions for gabapentin and opioids but not benzodiazepines were considered GABA+OP users and those with only opioid prescriptions were grouped as OP only users. A similar definition applied to GABA+BZD users and GABA only users. Exposure categories differed based on the specific outcomes examined. For respiratory depression and opioid-related overdose events, we included GABA+OP+BZD users, GABA+OP users and OP only users. For substance-related overdose events, we included GABA + OP + BZD users, GABA+OP users, GABA+BZD users and GABA only users. Since the risks for adverse outcomes associated with concurrent opioid and benzodiazepine utilization are well documented, we did not include OP+BZD only in our analysis. Additionally, given the objective of our study was to understand the additional impact of opioid and/or benzodiazepine use with gabapentin, we did not examine BZD only users. Concurrent medication utilization was assessed in the 90 days before the index date for cases and controls. This 90-day time window was selected based on current guidelines which suggest that pain therapies should be assessed after 90 days.34
Covariates
Demographic characteristics included age, race/ethnicity, and sex. Health insurance factors included Medicaid-Medicare dual eligibility. Clinical factors such as co-morbidities, service utilization and pharmacologic variables were assessed. Co-morbidities captured included chronic lung disease, diabetes, myocardial infarction, congestive heart failure, peripheral vascular disease, connective tissue disease, cerebrovascular disease, peptic ulcer disease, liver disease, hypertension, hypothyroidism, seizure disorder, substance-related disorders (alcohol, opioid and non-opioid) and dementia. Additionally, we included a measure for the number of inpatient and outpatient visits to properly adjust for health care utilization patterns in our analyses. Based on the distribution of these visits, we categorized the number of inpatient visits into four categories (none, 1-5, 6-10, >10) and the number of inpatient visits into three categories (none, 1, and >1.) These covariates were measured in the 6-month baseline period prior to cohort entry for cases and controls.
Statistical analyses
We utilized a disease risk score to summarize the relationship between covariates and outcome. A disease risk score is particularly useful in our analysis because highly evolving prescriber preferences related to our exposure categories make a propensity score relatively unstable.35, 36, 37, 38 In addition, we had multilevel exposure categories.35, 36, 37, 38 This score was constructed by fitting a logistic regression model linking confounders and outcomes in the full cohort (with exposure set as zero).35, 36, 37, 38 The estimated probability of disease occurrence (under the assumption of no exposure) for each beneficiary within our cohort was used as the assigned disease risk score for that beneficiary.35, 36, 37, 38 We constructed separate disease risk scores for each outcome within separate cohorts of beneficiaries diagnosed with AP, CP, and MH.
To increase comparability between cases and controls, incident density sampling was used to match each case with up to four controls on the following characteristics: disease risk score, age, race, dual eligibility status, sex, and cohort entry year. The calliper used for matching disease risk scores was 0.03 for respiratory depression, and 0.008 for opioid and substance-related overdose. When a full number of matches could not be found, we matched as many available controls to cases.
Descriptive statistics were used to compare baseline characteristics between cases and controls. We used multivariable conditional logistic regression (PROC LOGISTIC using STRATA option) to account for matching in estimating the association between outcomes and concurrent medication use. Selected reference categories for comparison in logistic regression analysis differed based on the specific outcome. For respiratory depression and opioid-related overdose, the reference category was OP only users and for overall substance-related overdose, GABA only users was the selected reference category. We estimated odds ratios (OR) and 95% confidence intervals for all comparisons and adjusted for any covariates not previously matched on. There was no missing data. All analyses were completed using SAS v.9.4. A 2-sided p<0.05 was considered statistically significant.
To account for varying interval periods of gabapentin, opioid, and benzodiazepine utilization, we varied the definitions of concurrent use. Tighter definitions requiring overlap of at least 7 days for days supplied of all medications, and continuous use of all medications for at least 30 days (without the requirement of overlap) were incorporated. Looser definitions including any gabapentin, opioid and benzodiazepine exposure in the pre-index period (without requirement of overlap) were examined.
Results
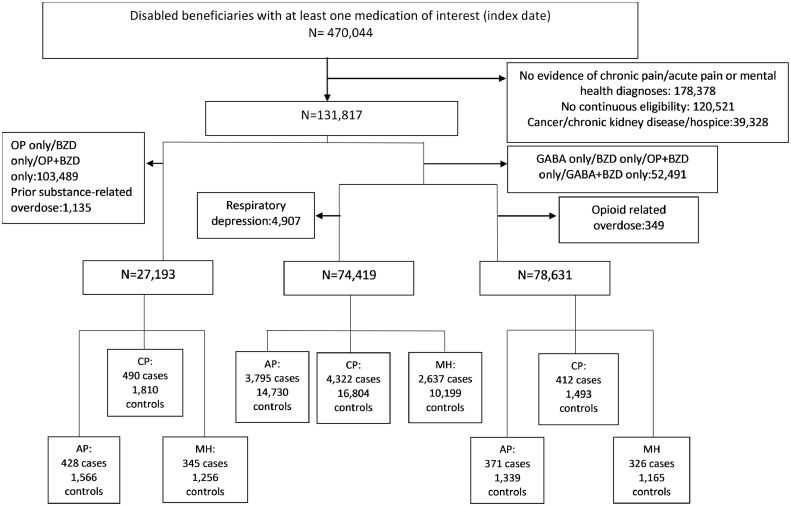
470,044 disabled beneficiaries received at least one prescription for gabapentin, opioids or benzodiazepines during the entire study period. Of these, 131,817 beneficiaries met additional inclusion criteria for the overall nest cohort. There were 74,419 (56,595 with CP, 47,834 with AP, and 29,176 with MH) beneficiaries in our cohort for examining respiratory depression, 78,631 (58,709 with CP, 50,256 with AP and 30,907 with MH) beneficiaries in our opioid-related overdose cohort and 27,193 (20,520 with CP, 16,998 with AP and 12,252 with MH) beneficiaries in our substance-related overdose cohort. The final analytic cohort for respiratory depression analyses included 4322 cases matched to 16,804 controls (CP cohort), 3795 cases and 14,730 controls (AP cohort) and 2637 cases and 10,199 controls (MH cohort); for opioid-related overdose― 412 cases matched to 1493 controls (CP cohort), 371 cases and 1339 controls (AP cohort) and 326 cases and 1165 controls (MH cohort) and for substance-related overdose 490 cases were matched to 1810 controls (CP cohort), 428 cases were matched to 1566 controls (AP cohort) and in our MH cohort, 345 cases were matched to 1256 controls (Figure 1).
Figure 1.
Selection criteria for cases and controls.
Baseline characteristics of cases and controls included in each sub-cohort are presented in Table 1, Table 2, Table 3. Across all sub-cohorts, the majority of cases and controls were younger than 65, female and dually eligible. In addition, cases and controls tended to have been previously diagnosed with pain and mental health conditions and this pattern was consistent across all sub-cohorts examined. Overall, baseline characteristics did not differ significantly between cases and controls. Across all sub-cohorts, cases tended to utilize higher doses of opioids when compared to controls.
Table 1.
Baseline characteristics of matched cases and controls in cohort for assessing respiratory depression.
| Characteristic | Acute pain |
Chronic pain |
Mental Health |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases N(%) | Controls N (%) | p-value | Cases N (%) | Controls N (%) | p-value | Cases N (%) | Controls N (%) | p-value | |
| Age | 0.57 | 0.51 | 0.6 | ||||||
| <65 | 2524 (66.5) | 9869 (67) | 2839 (65.7) | 11,128(66.2) | 1877 (71.2) | 7318 (71.8) | |||
| >65+ | 1271 (33.5) | 4861 (33) | 1483 (34.3) | 5676 (33.8) | 760 (28.8) | 2881 (28.3) | |||
| Gender | 0.69 | 0.62 | 0.6 | ||||||
| Females | 2352 (62) | 9181 (62.3) | 2639 (61.1) | 10,328(61.5) | 1743 (66.1) | 6800 (66.7) | |||
| Males | 1443 (38) | 5549 (37.7) | 1683 (40) | 6476 (38.5) | 894 (33.9) | 3399 (33.3) | |||
| Ethnicity | 0.35 | 0.45 | 0.6 | ||||||
| White | 3027 (79.8) | 111,879(80.6) | 3458 (80) | 13,562(80.7) | 2218 (84.1) | 8649 (84.8) | |||
| Black | 572 (15.1) | 2162 (14.7) | 651 (15.1) | 24,80 (14.8) | 309 (11.7) | 1155 (11.3) | |||
| Others | 196 (5.2) | 689 (4.7) | 213 (4.9) | 762 (4.5) | 110 (4.2) | 395 (3.9) | |||
| Dual eligibility | 2517 (66.3) | 9792 (66.5) | 0.9 | 2823 (65.3) | 11,008(65.5) | 0.81 | 1846 (70) | 7159 (70.2) | 0.8 |
| Chronic pain | 2960 (78) | 11,357 (77.1) | 0.7 | –––– | ––––– | –– | 2471 (93.7) | 9539 (93.5) | 0.7 |
| Acute pain | ––– | ––– | –– | 3614 (83.6) | 13,747(81.8) | 0.06 | 2219 (84.2) | 8465 (83) | 0.07 |
| Mental Health conditions | 2230 (58.8) | 8454 (57.4) | 0.06 | 2501 (57.9) | 9410 (56) | 0.07 | ––––– | –––– | –– |
| Substance Use disorder | 297 (7.8) | 1081 (7.3) | 0.3 | 325 (7.5) | 1228 (7.3) | 0.6 | 241 (9.1) | 856 (8.4) | 0.2 |
| Chronic lung disease | 1346 (35.5) | 4992 (33.9) | 0.06 | 1527 (35.3) | 5713 (31.6) | 0.07 | 1013 (38.4) | 3733 (34.2) | 0.05 |
| Diabetes | 1403 (37) | 5216 (35.4) | 0.07 | 1582 (36.6) | 5881 (36) | 0.07 | 914 (34.7) | 3365 (33) | 0.06 |
| Myocardial Infarction | 120 (3.2) | 389 (2.6) | 0.07 | 133 (3.1) | 422 (2.5) | 0.08 | 82 (3.1) | 283 (2.8) | 0.4 |
| Congestive Heart Failure | 430 (11.3) | 1338 (9.1) | 0.06 | 481 (11.1) | 1512 (9) | 0.05 | 273 (10.4) | 857 (8.4) | 0.05 |
| Peripheral Vascular disease | 351 (9.3) | 1309 (8.9) | 0.5 | 383 (8.9) | 1428 (8.5) | 0.4 | 213 (8.1) | 774 (7.6) | 0.4 |
| Connective tissue disease | 234 (6.2) | 966 (6.6) | 0.4 | 263 (6.1) | 1094 (6.5) | 0.31 | 133 (5) | 499 (4.9) | 0.7 |
| Cerebrovascular disease | 291 (7.7) | 1056 (7.2) | 0.3 | 326 (7.5) | 1187 (7.1) | 0.28 | 212 (8.0) | 749 (7.3) | 0.2 |
| Peptic ulcer disease | 45 (1.2) | 142 (1) | 0.3 | 44 (1) | 168 (1) | 0.7 | 30 (1.1) | 91 (0.9) | 0.2 |
| Liver disease | 197 (5.2) | 606 (4.1) | 0.08 | 206 (4.8) | 729 (4.3) | 0.2 | 138 (5.2) | 425 (4.2) | 0.07 |
| Hypertension | 1693 (44.6) | 6341 (43.1) | 0.08 | 1905 (44.1) | 7124 (42.4) | 0.06 | 1167 (44.3) | 4366 (42.8) | 0.2 |
| Hypothyroidism | 473 (12.5) | 1866 (12.7) | 0.7 | 507 (11.7) | 2021 (12) | 0.6 | 334 (12.7) | 1324 (13) | 0.7 |
| Seizures | 224 (5.9) | 670 (4.6) | 0.07 | 238 (5.5) | 765 (4.6) | 0.1 | 182 (6.9) | 558 (5.5) | 0.07 |
| Dementia | 73 (1.9) | 307 (2.1) | 0.5 | 73 (1.9) | 307 (2.1) | 0.5 | 55 (2.1) | 214 (2.1) | 0.9 |
| Muscle relaxants | 992 (26.1) | 3808 (25.9) | 0.7 | 1146 (26.5) | 4260 (25.4) | 0.1 | 759 (28.8) | 2883 (28.3) | 0.6 |
| Benzodiazepines | 1167 (30.8) | 4256 (28.) | 0.06 | 1341 (31) | 4957 (29.5) | 0.07 | 1063 (40.3) | 3908 (38.3) | 0.06 |
| Gabapentin | 986 (26) | 3535 (24) | 0.05 | 1113 (25.8) | 4054 (24.1) | 0.06 | 749 (28.4) | 2727 (26.7) | 0.08 |
| Pregabalin | 324 (8.5) | 1226 (8.3) | 0.7 | 362 (8.4) | 1354 (8.1) | 0.5 | 232 (8.8) | 876 (8.6) | 0.7 |
| Non-benzodiazepine sedatives | 575 (15.2) | 2104 (14.3) | 0.2 | 651 (15.1) | 2388 (14.2) | 0.2 | 486 (18.4) | 1731 (17) | 0.08 |
| Opioids | 2594 (68.4) | 9869 (67) | 0.06 | 3003 (69.5) | 11,427 (68) | 0.06 | 1906 (72.3) | 7241 (71) | 0.08 |
| Opioid dose | <.01 | <.01 | <.01 | ||||||
| <50MME | 2702 (71.8) | 11,127 (76.2) | 3063 (71.4) | 12,657(75.7) | 1815 (69.3) | 7.574 (74.6) | |||
| 51-90MME | 608 (16.2) | 2058 (14.1) | 691 (6.1) | 2281 (13.6) | 456 (17.4) | 1491 (14.7) | |||
| 91-150MME | 238 (6.3) | 813 (5.6) | 279 (6.5) | 972 (5.8) | 182 (7) | 599 (5.9) | |||
| >150MME | 217 (5.8) | 614 (4.2) | 257 (6) | 808 (4.8) | 166 (6.3) | 491 (4.8) | |||
| Number of Outpatient visits | 0.06 | 0.06 | 0.03 | ||||||
| None | 15 (0.4) | 72 (0.5) | 19 (0.4) | 90 (0.5) | –––– | 36 (0.4) | |||
| 1-5 | 254 (6.7) | 1093 (7.4) | 364 (8.4) | 1596 (9.5) | –––– | 744 (7.3) | |||
| 6-10 | 510 (13.4) | 2171 (14.7) | 649 (15) | 2705 (16) | 324 (12.3) | 1359 (13.3) | |||
| >10 | 3016 (79.5) | 11,394 (77.4) | 3290 (76.1) | 12,434 (74) | 2127 (80.7) | 8060 (79) | |||
| Number of Inpatient visits | 0.06 | 0.06 | 0.2 | ||||||
| None | 2990 (78.8) | 11,912 (80.9) | 3460 (80.1) | 13,779 (82) | 2045 (77.6) | 8066 (79.1) | |||
| 1 | 563 (14.8) | 2128 (14.5) | 609 (14.1) | 2178 (13) | 412 (15.6) | 1508 (14.8) | |||
| >1 | 242 (6.4) | 690 (4.7) | 253 (5.9) | 840 (5) | 180 (6.8) | 625 (6.1) | |||
Table 2.
Baseline characteristics of matched cases and controls in cohort for assessing opioid-related overdose.
| Characteristic | Acute pain |
Chronic pain |
Mental Health |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases N (%) | Controls N (%) | p-value | Cases N (%) | Controls N (%) | p-value | Cases N (%) | Controls N (%) | p-value | |
| Age | 0.8 | 0.9 | 0.5 | ||||||
| <65 | 308 (83) | 1120 (83.6) | 345 (83.7) | 1254 (84) | 276 (84.7) | 1003 (86.1) | |||
| >65+ | 63 (17) | 219 (17) | 67 (16.3) | 5239 (16) | 50 (15.3) | 162 (13.9) | |||
| Gender | 0.7 | 0.9 | 0.8 | ||||||
| Females | 230 (62) | 842 (62.9) | 259 (62.9) | 1935 (62.6) | 206 (63.2) | 744 (63.9) | |||
| Males | 141 (38) | 497 (37.1) | 153 (37.1) | 558 (37.4) | 120 (36.8) | 421 (36.1) | |||
| Ethnicity | 0.6 | 0.6 | 0.9 | ||||||
| White | 326 (87.9) | 1201 (89.7) | 361 (87.6) | 1331 (89.2) | 297 (91.1) | 1085 (84.8) | |||
| Black | ––––– | 110 (8.2) | –––– | 132 (8.8) | 29 (8.9) | 80 (16.2) | |||
| Others | ––––– | 28 (2.1) | –––– | 30 (2) | –––– | –––– | |||
| Dual eligibility | 261 (70.4) | 956 (71.4) | 0.7 | 283 (68.7) | 1046 (70.1) | 0.6 | 239 (73.3) | 858 (73.7) | 0.8 |
| Chronic pain | 360 (97) | 1291 (96.4) | 0.6 | –––– | –––– | –– | 307 (94.2) | 1070 (92) | 0.2 |
| Acute pain | ––––– | ––––– | 364 (88.4) | 1290 (86.4) | 0.3 | 288 (88.3) | 988 (84.8) | 0.1 | |
| Mental Health conditions | 282 (76) | 951 (71) | 0.08 | 301 (73.1) | 1054 (70.5) | 0.2 | –––– | –––– | – |
| Substance Use disorder | 142 (38.3) | 428 (32) | 0.07 | 155 (37.6) | 507 (33.6) | 0.08 | 133 (40.8) | 419 (36) | 0.08 |
| Chronic lung disease | 110 (29.7) | 367 (27.4) | 0.4 | 119 (28.9) | 367 (24.6) | 0.07 | 102 (31.3) | 322 (27.6) | 0.2 |
| Diabetes | 102 (27.5) | 329 (24.6) | 0.3 | 108 (26.2) | 390 (26.1) | 0.9 | 83 (25.5) | 281 (24.1) | 0.6 |
| Myocardial Infarction | –––– | 30 (2.2) | 0.6 | ––––– | 30 (2) | 0.6 | –––– | 30 (2.6) | 0.9 |
| Congestive Heart Failure | 25 (6.7) | 83 (6.2) | 0.7 | 28 (6.8) | 105 (7) | 0.9 | 23 (7.1) | 100 (8.6) | 0.8 |
| Peripheral Vascular disease | 21 (5.7) | 69 (5.2) | 0.7 | 25 (6.1) | 86 (5.8) | 0.8 | 17 (5.2) | 41 (3.5) | 0.2 |
| Connective tissue disease | 16 (4.3) | 63 (4.7) | 0.8 | 16 (3.9) | 76 (5.1) | 0.3 | 13 (4) | 64 (5.5) | 0.3 |
| Cerebrovascular disease | 20 (5.4) | 72 (5.4) | 0.9 | 22 (5.3) | 61 (4.1) | 0.3 | 14 (4.3) | 56 (4.8) | 0.7 |
| Peptic ulcer disease | –––– | 17 (1.3) | 0.2 | 44 (1) | 168 (1) | 0.9 | –––– | 18 (1.6) | 0.2 |
| Liver disease | 24 (6.5) | 93 (7) | 0.7 | 23 (5.6) | 83 (5.6) | 0.9 | 20 (6.1) | 74 (6.4) | 0.9 |
| Hypertension | 153 (41.2) | 485 (36.2) | 0.08 | 153 (40) | 553 (37) | 0.4 | 130 (40) | 434 (37.3) | 0.4 |
| Hypothyroidism | 47 (12.8) | 181 (13.5) | 0.7 | 52 (12.6) | 199 (13.3) | 0.7 | 45 (13.8) | 155 (13.3) | 0.8 |
| Seizures | 24 (6.5) | 71 (5.3) | 0.4 | 20 (4.9) | 66 (4.4) | 0.7 | 19 (5.9) | 56 (4.9) | 0.5 |
| Dementia | ––––– | ––––– | 0.9 | –––– | –––– | 0.1 | –––– | ––––– | ––– |
| Muscle relaxants | 155 (41.8) | 511 (38.2) | 0.2 | 174 (42.2) | 553 (37) | 0.06 | 133 (40.8) | 408 (35) | 0.05 |
| Benzodiazepines | 187 (50.4) | 603 (45) | 0.07 | 209 (50.7) | 684 (45.8) | 0.06 | 181 (55.5) | 593 (50.9) | 0.1 |
| Gabapentin | 131 (35.3) | 409 (30.6) | 0.08 | 139 (33.7) | 435 (29.1) | 0.07 | 107 (32.8) | 333 (28.6) | 0.1 |
| Pregabalin | 36 (9.7) | 139 (10.4) | 0.7 | 40 (9.7) | 150 (10.1) | 0.8 | 36 (11) | 110 (9.4) | 0.4 |
| Non-benzodiazepine sedatives | 83 (22.4) | 244 (18.2) | 0.07 | 89 (21.6) | 259 (17.4) | 0.05 | 83 (25.5) | 281 (24.1) | 0.6 |
| Opioids | 301 (81.1) | 1053 (78.6) | 0.3 | 335 (81.3) | 1167 (78.2) | 0.2 | 261 (80.1) | 899 (77.2) | 0.3 |
| Opioid dose | <.01 | <.01 | 164 (51.6) | 791 (70) | <.01 | ||||
| <50MME | 174 (47.3) | 928 (69.7) | 197 (48.3) | 1009 (68.1) | 70 (22) | 179 (15.8) | |||
| 51-90MME | 79 (21.5) | 220 (16.5) | 89 (21.8) | 254 (17.1) | 46 (14.5) | 82 (7.2) | |||
| 91-150MME | 59 (16) | 105 (7.9) | 62 (15.2) | 118 (8) | 38 (12) | 82 (7.2) | |||
| >150MME | 56 (15.2) | 79 (5.9) | 60 (14.7) | 101 (6.8) | |||||
| Number of Outpatient visits | 0.06 | 0.06 | 0.2 | ||||||
| None | ––––– | ––––– | –––– | –––– | –––– | –––– | |||
| 1-5 | ––––– | ––––– | 29 (7) | 136 (9.1) | –––– | –––– | |||
| 6-10 | 27 (7.3) | 147 (11) | 37 (9) | 179 (12) | 27 (8.3) | 139 (11.9) | |||
| >10 | 321 (86.5) | 1085 (81) | 345 (83.7) | 1194 (80) | 278 (85.3) | 944 (81) | |||
| Number of Inpatient visits | 0.06 | 0.06 | 0.4 | ||||||
| None | 266 (71.7) | 1037 (77.5) | 302 (73.3) | 1183 (79.2) | 230 (70.6) | 868 (74.5) | |||
| 1 | 71 (19.1) | 209 (15.6) | 74 (18) | 224 (13.3) | 64 (19.6) | 196 (16.8) | |||
| >1 | 34 (9.2) | 93 (7) | 36 (8.7) | 112 (7.5) | 32 (9.8) | 101 (8.7) | |||
Table 3.
Baseline characteristics of matched cases and controls in cohort for assessing substance-related overdose.
| Characteristic | Acute pain |
Chronic pain |
Mental Health |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases N(%) | Controls N(%) | p-value | Cases N(%) | Controls N(%) | p-value | Cases N(%) | Controls N(%) | p-value | |
| Age | 0.8 | 0.9 | 0.9 | ||||||
| <65 | 365 (85.3) | 1326 (84.7) | 416 (84.9) | 1533 (84.7) | 297 (86.1) | 1081 (86.1) | |||
| >65+ | 63 (14.7) | 240 (15.3) | 74 (15.1) | 277 (15.3) | 48 (13.9) | 175 (13.9) | |||
| Gender | 0.9 | 0.9 | 0.8 | ||||||
| Females | 274 (64) | 1006 (64.2) | 309 (63.1) | 1138 (62.9) | 218 (63.2) | 802 (63.9) | |||
| Males | 154 (36) | 560 (35.8) | 181 (36.9) | 672 (37.1) | 127 (36.8) | 454 (36.2) | |||
| Ethnicity | 0.9 | 0.9 | 0.9 | ||||||
| White | 381 (89) | 1392 (88.9) | 439 (89.6) | 1619 (89.5) | 316 (91.6) | 1155 (91.9) | |||
| Black | 33 (7.7) | 125 (8) | 33 (6.7) | 125 (6.9) | 19 (5.5) | 67 (5.3) | |||
| Others | 14 (3.3) | 49 (3.1) | 18 (3.7) | 66 (3.7) | 10 (2.9) | 34 (2.7) | |||
| Dual eligibility | 333 (77.8) | 1221 (78) | 0.9 | 369 (75.3) | 1364 (75.4) | 0.9 | 270 (78.3) | 986 (78.5) | 0.9 |
| Chronic pain | 412 (96.3) | 1492 (95.3) | 0.4 | –––– | ––––– | –– | 331 (95.9) | 1177 (93.7) | 0.1 |
| Acute pain | ––––– | ––––– | –– | 418 (85.3) | 1494 (82.5) | 0.1 | 335 (97.9) | 1210 (96.3) | 0.1 |
| Mental Health conditions | 344 (80.4) | 1238 (79.1) | 0.6 | 393 (80.2) | 1415 (78.2) | 0.3 | –––– | –––– | –– |
| Substance Use disorder | 83 (19.4) | 251 (16) | 0.06 | 92 (18.8) | 291 (16.1) | 0.1 | 70 (20.3) | 203 (16.2) | 0.07 |
| Chronic lung disease | 126 (29.4) | 442 (28.2) | 0.6 | 139 (28.4) | 502 (27.7) | 0.8 | 111 (32.2) | 365 (29.1) | 0.3 |
| Diabetes | 121 (28.3) | 490 (31.3) | 0.2 | 137 (28) | 519 (28.7) | 0.8 | 89 (25.1) | 311 (24.8) | 0.7 |
| Myocardial Infarction | 12 (2.8) | 40 (2.6) | 0.8 | 14 (2.9) | 59 (3.3) | 0.7 | 9 (2.6) | 38 (3) | 0.7 |
| Congestive Heart Failure | 26 (6.1) | 99 (6.3) | 0.9 | 27 (5.5) | 112 (6.2) | 0.6 | 21 (6.1) | 73 (5.8) | 0.9 |
| Peripheral Vascular disease | 24 (5.6) | 88 (5.6) | 0.9 | 25 (5.1) | 97 (5.4) | 0.8 | 15 (4.6) | 44 (3.5) | 0.5 |
| Connective tissue disease | 17 (4) | 59 (3.8) | 0.8 | 20 (4.1) | 73 (4.) | 0.9 | 12 (3.5) | 51 (4.1) | 0.6 |
| Cerebrovascular disease | 26 (6.1) | 101 (6.5) | 0.8 | 32 (6.5) | 118 (6.5) | 0.9 | 23 (6.7) | 75 (6) | 0.6 |
| Peptic ulcer disease | 8 (1.9) | 20 (1.3) | 0.4 | 8 (1.6) | 27 (1.5) | 0.8 | 6 (1.7) | 16 (1.3) | 0.5 |
| Liver disease | 27 (6.3) | 80 (5.1) | 0.3 | 30 (6.1) | 100 (5.5) | 0.6 | 22 (6.4) | 68 (5.4) | 0.5 |
| Hypertension | 161 (37.6) | 607 (38.8) | 0.7 | 185 (37.8) | 677 (37.4) | 0.9 | 135 (39.1) | 450 (35.8) | 0.3 |
| Hypothyroidism | 59 (13.8) | 196 (12.5) | 0.5 | 64 (13.1) | 246 (13.6) | 0.8 | 50 (14.5) | 149 (11.9) | 0.2 |
| Seizures | 48 (11.2) | 141 (9) | 0.2 | 58 (11.8) | 199 (11) | 0.1 | 58 (13.7) | 146 (11.7) | 0.1 |
| Dementia | 8 (1.9) | 31 (2) | 0.9 | 9 (1.84) | 32 (1.77) | 0.9 | 6 (1.7) | 28 (2.2) | 0.6 |
| Muscle relaxants | 173 (40.4) | 596 (38.1) | 0.4 | 200 (40.8) | 688 (38) | 0.3 | 138 (40) | 480 (38.2) | 0.6 |
| Benzodiazepines | 226 (52.8) | 786 (50.2) | 0.3 | 260 (53.1) | 928 (51.3) | 0.5 | 191 (55.4) | 676 (53.9) | 0.6 |
| Gabapentin | 305 (71.3) | 1080 (69) | 0.4 | 347 (70.8) | 1216 (67.2) | 0.1 | 248 (71.9) | 880 (70.1) | 0.5 |
| Gabapentin dose | 0.03 | 0.2 | 0.5 | ||||||
| <900mg | 183 (43.3) | 781 (50.5) | 216 (44.5) | 888 (49.6) | 191 (45.7) | 770 (50.1) | |||
| 901-1799mg | 123 (29.1) | 356 (23) | 129 (26.6) | 435 (24.3) | 105 (25.1) | 347 (22.6) | |||
| 1800-2699mg | 87 (20.6) | 320 (20.7) | 103 (21.2) | 358 (20) | 95 (22.7) | 334 (21.7) | |||
| >2700mg | 30 (7.1) | 90 (5.8) | 37 (7.6) | 109 (6.1) | 27 (6.5) | 86 (5.6) | |||
| Pregabalin | 21 (4.9) | 65 (4.2) | 0.5 | 22 (4.5) | 86 (4.8) | 0.8 | 14 (4.06) | 45 (3.6) | 0.7 |
| Non-benzodiazepine sedatives | 84 (19.6) | 266 (17) | 0.2 | 95 (19.4) | 311 (17.2) | 0.3 | 71 (20.6) | 244 (19.4) | 0.6 |
| Opioids | 323 (75.5) | 1147 (73.2) | 0.4 | 359 (73.3) | 1259 (69.6) | 0.1 | 260 (75.4) | 894 (71.2) | 0.1 |
| Number of Outpatient visits | 0.9 | 0.9 | 0.6 | ||||||
| None | 1 (0.2) | 6 (0.4) | 1 (0.2) | 4 (0.2) | ––– | ––– | |||
| 1-5 | 19 (4.4) | 68 (4.3) | 23 (4.7) | 89 (4.9) | –––– | 58 (4.6) | |||
| 6-10 | 48 (11.2) | 191 (12.2) | 57 (11.6) | 219 (12.1) | 30 (8.7) | 128 (10.2) | |||
| >10 | 360 (84.1) | 1301 (83.1) | 409 (83.5) | 1498 (82.8) | 303 (87.8) | 1069 (85.1) | |||
| Number of Inpatient visits | 0.8 | 0.6 | 0.4 | ||||||
| None | 325 (75.93) | 1207 (77.08) | 375 (76.5) | 1417 (78.3) | 253 (73.3) | 961 (76.5) | |||
| 1 | 67 (15.65) | 237 (15.13) | 73 (14.9) | 259 (14.3) | 61 (17.7) | 205 (16.3) | |||
| >1 | 36 (8.41) | 122 (7.79) | 42 (8.6) | 134 (7.4) | 31 (9) | 90 (7.2) | |||
Among disabled Medicare beneficiaries with CP, AP, and MH in our cohorts for respiratory depression assessment, 486 (11.2%), 437 (11.5%) and 377 (14.3%) cases and 9.5%, 9.3% and 12.8% of controls had concurrent utilization of GABA+OP+BZD, respectively. Similarly, 667 (15.4%), 568 (15%) and 382 (14.5%) cases had concurrent utilization of GABA+OP when compared to 15.1%, 14.3% and 15% of controls, respectively. Across the CP, AP, and MH cohorts for opioid-related overdose analyses, 81 (19.7%), 78 (21%) and 74 (22.7%) cases had concurrent utilization of GABA+OP+BZD compared to 15.4%, 15.9%, and 17.8% of controls, respectively; 68(16.5%), 63 (16.9%) and 48 (14.7%) cases had concurrent utilization of GABA+OP when compared to 14.7%, 15.3%, and 12.9% of controls, respectively. When compared to 33.6%, 35%, and 40% of controls, 190 (38%), 167 (39%), and 143 (41.4%) cases in the CP, AP and MH cohort for substance-related overdose assessment had concurrent utilization of GABA+OP+BZD; 154 (31.4%), 140 (32.7%) and 99 (28.7%) of cases and 32.4%, 30.6%, and 28.1% of controls had concurrent utilization of GABA+OP. Similarly, 58 (11.8%), 49 (11.5%), and 42 (12.2%) cases had concurrent utilization of GABA+BZD when compared to 10%, 8.2% and 11.7% of controls, respectively.
Across all cohorts, GABA+OP+BZD use was associated with significantly higher odds of respiratory depression (Table 4, AOR[95%CI] AP: 1.35[1.19-1.52]; CP:1.24[1.11-1.38]; MH: 1.16[1.02-1.32]), opioid (AP: 1.43[1.04-1.98]; CP:1.47[1.07-2.00]; MH: 1.44[1.04-2.00]) and substance-related overdose (AP: 1.77[1.26-2.50]; CP:1.70[1.24-2.34]; MH: 1.92[1.31-2.82]). However, we noted subgroup differences in the odds of respiratory depression and opioid-related overdose among GABA+OP users (when compared to opioid only)―GABA+OP use was associated with higher odds of respiratory depression in the AP and CP cohort but was only associated with higher odds of an opioid-related overdose in the CP cohort. When compared to GABA only use, GABA+OP and GABA+BZD use were associated with higher odds of substance-related overdose, and this was consistent across cohorts.
Table 4.
Association between concurrent medication utilization and adverse outcomes.
| Respiratory depression |
Opioid related overdose |
Substance related overdose |
||||
|---|---|---|---|---|---|---|
| Medication category | Unadjusted OR 95%CI | Adjusted OR* 95%CI | Unadjusted OR 95%CI | Adjusted OR* 95%CI | Unadjusted OR 95%CI | Adjusted OR* 95%CI |
| AP | ||||||
| GABA+OP +BZD |
1.62 (1.45-1.80) | 1.35 (1.19-1.52) | 3.51 (2.75-4.47) | 1.43 (1.04-1.98) | 3.28 (2.52-4.27) | 1.77 (1.26-2.50) |
| GABA+OP | 1.23 (1.12-1.34) | 1.10(1.03-1.18) | 1.60 (1.23-2.08) | 1.21 (0.90-1.70) | 1.98 (1.36-2.06) | 1.74 (1.25-2.41) |
| GABA+BZD | –––––– | –––––– | –––––– | –––––– | 2.79 (1.97-3.97) | 2.19 (1.43-3.34) |
| OP only | reference | reference | reference | reference | –––––– | –––––– |
| GABA only | –––––– | –––––– | –––––– | –––––– | reference | reference |
| CP | ||||||
| GABA+OP +BZD |
1.56 (1.41-1.72) | 1.24 (1.11-1.38) | 3.24 (2.56-4.09) | 1.47 (1.07-2.00) | 3.16 (2.49-4.03) | 1.70 (1.24-2.34) |
| GABA+OP | 1.22 (1.21-1.33) | 1.06 (1.01-1.12) | 1.63 (1.27-1.95) | 1.23 (1.04-1.48) | 1.43 (1.11-1.83) | 1.39 (1.03-1.89) |
| GABA+BZD | –––––– | –––––– | –––––– | –––––– | 2.76 (2.00-3.82) | 1.68 (1.14-2.47) |
| OP only | reference | reference | reference | reference | –––––– | –––––– |
| GABA only | –––––– | –––––– | –––––– | –––––– | reference | reference |
| MH | ||||||
| GABA+OP +BZD |
1.37 (1.22-1.54) | 1.16 (1.02-1.32) | 2.56 (1.99-3.28) | 1.44 (1.04-2.00) | 2.04 (1.60-2.61) | 1.92 (1.31-2.82) |
| GABA+OP | 1.27 (1.13-1.42) | 0.98 (0.86-1.12) | 1.48 (1.10-2.00) | 1.29 (0.90-1.85) | 1.81 (1.34-2.08) | 1.63 (1.03-2.58) |
| GABA+BZD | –––––– | –––––– | –––––– | –––––– | 1.65 (1.20-2.27) | 1.64 (1.12-2.39) |
| OP only | reference | reference | reference | reference | –––––– | –––––– |
| GABA only | –––––– | –––––– | –––––– | –––––– | reference | reference |
AP, acute pain; CP, chronic pain; MH, Mental Health; OR, Odds Ratio.
–––––– N/A.
Significant findings highlighted in bold.
Adjusted for all variables previously listed in tables 1 through 3.
In sensitivity analyses involving continuous medication use for 30 days, the results for respiratory depression were similar to the main analyses. When any medication use (without overlap requirement) was assessed, GABA+OP use was associated with significantly higher odds of opioid-related overdose among beneficiaries with AP and MH. Additionally, continuous 30-day medication use and 7-day medication overlap of GABA+OP were associated with higher odds of an opioid-related overdose in the MH cohort.
Discussion
In this study, we noted that in the Medicare disabled population, concurrent utilization of gabapentin, opioids and benzodiazepines is associated with an almost 2-fold increased risk of substance-related overdose (compared to gabapentin only users). In addition, we noted greater than 40% and 15% increased odds of opioid-related overdose and respiratory depression among beneficiaries who utilized gabapentin, opioids, and benzodiazepines concurrently (compared to opioid only users). Concurrent utilization of gabapentin and opioids or gabapentin and benzodiazepines also increased the potential for substance-related overdose in this population. Although we noted no association between concurrent use of gabapentin and opioids with respiratory depression and opioid-related overdose within some of our cohorts, when we varied our definition of concurrent use, we noted an increased risk of both outcomes within this population.
Although our findings regarding overdose are consistent with previous studies that have examined the association between gabapentinoids and overdose, the magnitude of noted associations differs across studies. This is likely due to heterogeneity in exposure and outcome assessments. The previous study conducted in the Medicare population examined gabapentin using a composite gabapentinoid category which also captures pregabalin. Several adverse outcomes associated with pregabalin have been well documented; in fact, pregabalin is a schedule V substance (i.e. drugs with lower potential for abuse) in the United States. Given this, the inclusion of pregabalin with gabapentin potentially masks the true association between gabapentin and overdose. Though results from a Canadian based study focused primarily on gabapentin, it only assessed fatal overdose. Since prior non-fatal overdoses increase the risk of future overdoses, a comprehensive examination of all overdose events ―both fatal and non-fatal―is essential to quantifying the risk associated with these medications. Our study builds upon these studies and is among the first to examine the additional impact of benzodiazepine use among concurrent gabapentin and opioid users.
Our finding that any gabapentin, opioid and/or benzodiazepine utilization across cohorts of beneficiaries with AP, CP and MH was more strongly associated with opioid-related overdose than concurrent utilization is important because it suggests that any co-utilization of these medications ― even without overlapping prescriptions - significantly increases the risk for potential overdose events. In addition, our finding that prescription overlap of at least seven days or continuous use for at least 30 days was strongly associated with a higher risk of opioid-related overdose among beneficiaries with MH suggests that long term use of these medications especially within this population should be monitored and avoided when possible.
The mechanism through which gabapentin potentially increases opioid-related overdose is not fully understood; however, it has been linked to increased respiratory depression resulting from gabapentin's potentiation of opioid analgesia through a shared physiologic pathway.27,28 This is supported by our results which indicate that GABA+OP and GABA+OP+BZD use were associated with a higher risk of respiratory depression in this population. Differences noted in the associations for opioid-related overdose and those for respiratory depression are potentially related to their definitions. Our measure of overdose excludes poisonings related to heroin; since gabapentin and opioids are often used illicitly with other substances, it is plausible that our definition underestimates the overdose risk associated with these medications. Similarly, our definition of respiratory depression captures non-specific symptoms including hypoxemia, apnea, and acute and chronic respiratory failure which may not be directly related to overdose.
Our study is among the first to examine the association between concurrent gabapentin, opioids, and benzodiazepines and substance-related overdose. While previous studies have focused on gabapentin use with opioids, our finding that the odds of substance-related overdose was especially high among GABA+BZD users suggests that gabapentin and benzodiazepine users are also a high-risk group who are potentially also susceptible to adverse outcomes. Not only that, since there has been limited research on this population, they represent a subgroup among whom further research is warranted.
Our study had some limitations. Firstly, since we did not have indications for any of our medications of interest, there is a potential for confounding by indication which we addressed by conducting analyses in separate cohorts of individuals diagnosed with AP, CP and MH. Additionally, we did not have a measure of pain severity. It is plausible that this is an unmeasured confounder in our analyses; however, since individuals with more severe pain are more likely to seek treatment, we used previous health care utilization as a proxy for pain severity in our analyses. There is a potential for selection bias resulting from capturing only those who seek care and missing any beneficiaries who obtained prescriptions for any of our medications of interest via cash, other payors, or illicit sources. However, since beneficiaries who obtain these medications illicitly potentially utilize these medications differently from those with prescriptions, we focused specifically on adverse outcomes associated with prescription medications.
To address the potential for detection bias, we used an opioid only or gabapentin only reference group rather than a no use category. In addition, since gabapentin utilization has only recently become scrutinized, it is likely that prescriber monitoring/ follow up was similar across our exposure groups. Although the preferred approach for DRS estimation is using a time period prior to therapy introduction, this approach was not feasible for our study given data constraints. Nevertheless, full cohort DRS estimation has been noted to perform well (minimal bias) in simulation studies, even in settings with a moderate association between covariates and exposure.37,38 Benzodiazepine reimbursement under Medicare began in 2013; as a result, this limits the number of available data years. Finally, our study focused on the Medicare disabled population and as such, additional research may be needed to validate these results in other study populations.
Amid the opioid epidemic, efforts which hope to curb this ongoing crisis must incorporate measures which address concurrent gabapentin, opioids, and benzodiazepine utilization. At the practice level, there is a need for increased education of providers/clinicians on the risks associated with the combined use of these medications. In addition, in the instances of prescribing, increased monitoring of high-risk sub-groups especially those with pain and mental health conditions should be considered. At the state and national levels, several states have adopted regulations, legislation, and monitoring requirements for gabapentin. At the moment, Kentucky, Michigan, West Virginia, Virginia, North Dakota, Alabama, and Tennessee remain the only states where gabapentin is classified as a Schedule V medication; however, twelve other states require mandated gabapentin reporting to prescription drug monitoring programs.39,40 While our findings support the recent calls for more stringent legislation focused on gabapentin prescribing within the nation – including a change in gabapentin scheduled status or perhaps, more widespread incorporation of gabapentin in prescription drug monitoring programs, our results should be interpreted within the context of the discussed limitations.27,29,39,40
Among Medicare disabled beneficiaries, concurrent utilization of gabapentin, opioids and/or benzodiazepines was associated with an increased risk of multiple adverse outcomes including respiratory depression, opioid and substance-related overdose. Given the widespread incorporation of gabapentin into several pain management protocols and further, its increasing utilization among those with psychiatric conditions, the benefits, and risks of gabapentin co-prescribing with opioids and/or benzodiazepines should be weighed by clinicians in these settings. Further, additional research focused on examining the outcomes associated with the concomitant use of these medications in other populations is warranted.
Contributors
All authors were involved in data interpretation. A.O., W.C.C., D.Q. and L.W. were involved in study design. A.O. and L.W. were also involved in conceptualization and data analyses.
Data sharing statement
Data is provided by CMS. Data access is prohibited for all individuals not included in the Data Use Agreement. For specific information regarding data, please contact the corresponding author.
Declaration of interests
AO, AA, FP and LS have nothing to disclose. WCC received funding from PhRMA for work unrelated to this study. DQ received funding from the Food and Drug Administration for work unrelated to this study. LW received funding from the National Institute on Aging for work unrelated to this study.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.National Institute on Drug Abuse; 2021. Overdose Death Rates | National Institute on Drug Abuse.https://www.drugabuse.gov/drug-topics/trends-statistics/overdose-death-rates Accessed 3 October 2021. [Google Scholar]
- 2.Hedegaard H, Miniño A, Warner M. Drug overdose deaths in the United States, 1999–2019. NCHS Data Brief. 2020;394 [PubMed] [Google Scholar]
- 3.Wilson N, Kariisa M, Seth P, Smith H, Davis N. Drug and opioid-involved overdose deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 2020;69(11):290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2014. Information Sheet on Opioid Overdose [Internet]http://www.who.int/substance_abuse/information- sheet/en/ Accessed 24 June 2021. [Google Scholar]
- 5.CDC . Centers for Disease Control and Prevention; 2016. Concurrent Use of Opioids and Benzodiazepines in a Medicare Part D Population. [Google Scholar]
- 6.Sun EC, Humphreys K, Dixit A, et al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis: Sun EC, Dixit A, Humphreys K, et al. BMJ. 2017;356:760–767. doi: 10.1136/bmj.j760. Journal of Emergency Medicine (0736-4679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez Immaculada HM, Maria B., Yuting Z. Exposure Response Association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in medicare part D beneficiaries. JAMA. 2018;1(2) doi: 10.1001/jamanetworkopen.2018.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350(8012):1. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cdc.gov.https://www.cdc.gov/nchs/data/nhsr/nhsr137-508.pdf. Published 2021. Accessed 10 September 2021.
- 10.National Institute on Drug Abuse; 2021. Benzodiazepines and Opioids|National Institute on Drug Abuse.https://www.drugabuse.gov/drug-topics/opioids/benzodiazepines-opioids Accessed 10 September 2021. [Google Scholar]
- 11.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952. [DOI] [PubMed] [Google Scholar]
- 12.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(4):434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Peckham AM, Evoy KE, Ochs L, Covvey JR. Gabapentin for off-label use: evidence-based or cause for concern? Subst Abuse. 2018;12 doi: 10.1177/1178221818801311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed S, Bachu R, Kotapati P, et al. Use of gabapentin in the treatment of substance use and psychiatric disorders: a systematic review. Front Psychiatry. 2019;10:228. doi: 10.3389/fpsyt.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–1174. doi: 10.1111/add.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verduin ML, McKay S, Brady KT. Gabapentin in comorbid anxiety and substance use. Am J Addict. 2007;16(2):142–143. doi: 10.1080/10550490601186238. [DOI] [PubMed] [Google Scholar]
- 17.Van Ameringen M, Mancini C, Pipe B, Bennett M. Antiepileptic drugs in the treatment of anxiety disorders: role in therapy. Drugs. 2004;64(19):2199–2220. doi: 10.2165/00003495-200464190-00004. [DOI] [PubMed] [Google Scholar]
- 18.Markota M, Morgan RJ. Treatment of generalized anxiety disorder with gabapentin. Case Rep Psychiatry. 2017;2017 doi: 10.1155/2017/6045017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mersfelder TL, Nichols WH. Gabapentin: abuse, dependence, and withdrawal. Ann Pharmacother. 2016;50(3):229–233. doi: 10.1177/1060028015620800. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen T, Li Y, Refua S. Gabapentin: a surprising drug with many uses. J Pharm Pract Res. 2017;47:304–307. [Google Scholar]
- 21.Evoy KE, Covvey JR, Peckham AM, Ochs L, Hultgren KE. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: an analysis of the food and drug administration adverse events reporting system (FAERS) Res Social Adm Pharm. 2019;15(8):953–958. doi: 10.1016/j.sapharm.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185–1215. doi: 10.1016/j.euroneuro.2017.08.430. [DOI] [PubMed] [Google Scholar]
- 23.Kim S. The unsuspected threat of three opioid-like substitutes. Arch Psychiatr Nurs. 2019;33(4):325–328. doi: 10.1016/j.apnu.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Tharp AM, Hobron K, Wright T. Gabapentin-related deaths: patterns of abuse and postmortem levels. J Forensic Sci. 2019;64(4):1105–1111. doi: 10.1111/1556-4029.14021. [DOI] [PubMed] [Google Scholar]
- 25.Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend. 2018;186:80–85. doi: 10.1016/j.drugalcdep.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Häkkinen M, Vuori E, Kalso E, Gergov M, Ojanperä I. Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci Int. 2014;241:1–6. doi: 10.1016/j.forsciint.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Peckham AM, Fairman KA, Sclar DA. All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: a retrospective cohort analysis of a commercially insured US population. Drug Saf. 2018;41(2):213–228. doi: 10.1007/s40264-017-0595-1. [DOI] [PubMed] [Google Scholar]
- 28.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med. 2017;14(10) doi: 10.1371/journal.pmed.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou L, Bhattacharjee S, Kwoh CK, et al. Dual-trajectories of opioid and gabapentinoid use and risk of subsequent drug overdose among Medicare beneficiaries in the United States: a retrospective cohort study. Addiction. 2021;116(4):819–830. doi: 10.1111/add.15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley RD, Snell KIE, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: Part II -binary and time-to-event outcomes. Stat Med. 2019;38 doi: 10.1002/sim.7992. 1276–1296.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557–564. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olopoenia A, Onukwugha E, Simoni-Wastila L, et al. Patterns of prescription opioid utilization among adolescents and adults with comorbid chronic pain and mental health diagnosis. Pain. 2020;161(10):2299–2307. doi: 10.1097/j.pain.0000000000001934. [DOI] [PubMed] [Google Scholar]
- 33.Savelloni J, Gunter H, Lee KC, et al. Risk of respiratory depression with opioids and concomitant gabapentinoids. J Pain Res. 2017;10:2635–2641. doi: 10.2147/JPR.S144963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowell D, Haegerich TM, Chou R. CDC Guidelines for prescribing opioids for chronic pain―United States, 2016. MMWR Recomm Rep. 2016;65(No. RR-1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen O. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104(6):609–620. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 36.Cadarette SM, Gagne JJ, Solomon DH, Katz JN, Stürmer T. Confounder summary scores when comparing the effects of multiple drug exposures. Pharmacoepidemiol Drug Saf. 2010;19(1):2–9. doi: 10.1002/pds.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai RJ, Glynn RJ, Wang S, Gagne JJ. Performance of disease risk score matching in nested case-control studies: a simulation study. Am J Epidemiol. 2016;183(10):949–957. doi: 10.1093/aje/kwv269. [DOI] [PubMed] [Google Scholar]
- 38.Arbogast P.G., Ray W.A. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174(5):613–620. doi: 10.1093/aje/kwr143. [DOI] [PubMed] [Google Scholar]
- 39.Peckham AM, Ananickal Maria J. Sclar David A. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy. 2018;11:109–116. doi: 10.2147/RMHP.S168504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell L, Coomer T, Jacob G, Lenz R. Gabapentin controlled substance status. J Am Pharm Assoc. 2021;61(4) doi: 10.1016/j.japh.2021.01.025. [DOI] [PubMed] [Google Scholar]