Summary
Background
Motoric Cognitive Risk Syndrome (MCR) is a predementia stage where slow gait speed and subjective memory complaints are present. The purpose of this study was to estimate the prevalence of MCR and assess its relationship with sociodemographic factors and chronic conditions.
Methods
This is a secondary analysis of the SABE Colombia study conducted in 2015. The analytic sample consisted of 17·577 participants. After determining MCR prevalence, logistic regression was performed to examine the correlates of MCR.
Findings
The prevalence of MCR was 10·71 %. The median age was 71 years and women composed 74·63 % of the MCR group. After adjusting for confounding variables MCR was associated with increasing age (OR 1·69, CI 1·43 - 1·92), no or low education (OR 1·99, CI 1·67- 2·37), MMSE (OR 0·93, CI 0·91 - 0·95) and chronic conditions such as mental disorders (OR 1·36, CI 1·11-1·67), history of myocardial infarction (OR 1·24, CI 1·04 - 1·47), hypertension (OR 1·23, CI 1·08 - 1·40) and diabetes (OR 1.18, CI 1.01 – 1.37).
Interpretation
This study found a prevalence of 10·71 % of MCR in Colombian older adults. Additionally, MCR was associated with chronic conditions and sociodemographic factors identified in prior studies. These results increase the awareness of a novel predementia stage whose identification can be performed by clinicians in the outpatient clinic, minimizing the cost of a full neuropsychologic evaluation performed in a memory clinic.
Funding
Funded by the Administrative Department of Science, Technology and Innovation (Colciencias) and the Ministry of Health and Social Protection of Colombia.
Keywords: Cognitive Dysfunction, Dementia, Gait, Memory, Prevalence, Risk Factors, Skeletal Muscle
Research in Context.
Evidence before this study
Before undertaking the analysis, we read the first paper that validated the Motoric Cognitive Risk (MCR) syndrome in nondemented older individuals with cognitive complaints, this article was published by Joe Verghese in the Journal of Gerontology in 2013. Later, we did a quick review on other multicentric studies that included this syndrome predicting a higher risk of developing dementia, particularly vascular dementia, and other geriatric outcomes such as frailty, disability, falls and, overall mortality. We also did a review in other available articles to understand the pathophysiology and the neurological basis of this cognitive and motor decline. The reading about this MCI subtype filled our interest given its feasible identification in the outpatient clinic. While doing the review we found out that Aguilar-Navarro et. al. studied the prevalence and risk factors associated with MCR in a Mexican cohort. These findings motivated us to study this syndrome in a similar Latin America population, thus we decided to use the data from SABE Colombia 2015, a cross-sectional study that included adults over 60 years of age who lived in community.
Most of the evidence was taken out from articles of scientific journals, and most of these papers were longitudinal and descriptive analysis, rather than metanalysis. We used PubMed, Scielo and Embase databases as our searching tools. The terms included were “Motoric Cognitive Risk Syndrome”, “Cognitive performance”, “Gait speed”, “Older adults”.
Added value of this study
This article increases the awareness of this pre-dementia stage and to our knowledge this is the first time this syndrome is studied in this population. MCR can be used as a screening tool for cognitive impairment given that a gait assessment, a cognitive complaint questionary, and a functional inquire can be performed by clinicians in the outpatient clinic. The foregoing, in relation with the existing evidence regarding pre-dementia stages, can help clinicians detect people at risk to develop dementia and make interventions that can slow down disease progression.
Implication of all the available evidence
MCR is a prevalent condition among the elderly, with even higher rates in Latin-American countries, its early identification can help establish preventive strategies based on risk assessments. This approach is particularly helpful for low-and middle-income countries since it could reduce the costs associated with a full neuropsychologic assessment or biomarkers such as PET imaging, offering an easy-to-apply and accessible tool even for remote populations.
Alt-text: Unlabelled box
Background
Dementia is an increasing global health challenge. Care support of patients with dementia has a wide-ranging impact for families, health-care systems and society in general.1 This is a condition with high prevalence and incidence, with even higher rates in Latin-American countries.2 Under these circumstances, it would be very useful to have an estimate of the population at risk of cognitive decline and dementia, but clinical assessments are rare in resource-limited countries, such as Colombia.3
The identification of preclinical stages of dementia are important for preventive measures because it reduces the burden of the disease and related costs.4 Traditionally, identification of predementia syndromes is based on neuropsychological tests, blood/CSF biomarkers and neuroimaging.5 It is not feasible to use this approach to identify predementia syndromes in large populations, however, particularly those of low- and middle-income countries where access to clinical settings is very limited.
Recent evidence shows that slower gait-speed (GS) precedes cognitive decline by several years independently of other factors such as ageing or co-morbidities.6, 7, 8, 9 One study found that an deacceleration in gait speed decline can occur as much as 12 years prior to the onset of Mild Cognitive Impairment (MCI),7 our group previous published results relating GS and hand-grip strength with cognition in Colombian Older adults.10,11 Although these changes are due to several factors, the presence of slow GS in older adults has been shown to predict dementia independently of the underlying etiology.6 GS has been proposed as an important parameter during older adult cognitive assessment.
The Motoric Cognitive Risk Syndrome (MCR) was described by Verghese et. al.12 as a preclinical stage for dementia. MCR is defined as the presence of slow GS and subjective memory complaints, without cognitive or functional impairment.12 MCR is associated with increased incidence of dementia, particularly Alzheimer's Disease (AD) and Vascular Dementia.5,12 In addition to dementia, MCR increases the risk of other geriatric outcomes including frailty, disability, falls and, mortality.5,13,14
Multiple comorbidities have been associated with MCR, and some of the risk factors for this condition vary depending on the economic situation of the countries. In countries like United States, Japan, France, Ireland, Canada and China, the comorbidities with the greatest association are diabetes, sedentariness, depression, low educational level, cardiovascular disease and cerebrovascular disease. However, in countries such as Malaysia, in addition to the aforementioned factors, an association has been found with rural residential area, having cancer, and low socioeconomic status.3,5,6,12,15,16 Still, the strongest risk factors are diabetes, hypertension, stroke and coronary disease, which are also associated with vascular changes and white matter integrity.3
To the date, there are no studies describing the prevalence or correlates of MCR in Colombia. A novel measure like MCR can help estimate the population at risk for preclinical dementia and can be used even with limited resources. This study objective is to estimate the prevalence of MCR and assess its relationship with cognitive risk factors in Colombia.
Methods
Study design
This is a secondary analysis of the SABE Colombia study (Health, Well-Being, and Aging) conducted in 2015. This study included a nationally representative sample of 23·694 older adults living in the community aged 60 years or more. The sample was probabilistic, clustered, stratified/multi-staged by urban and rural areas. The methods and procedures conducted in the study were based on those used in the SABE international study for comparability; however, it was modified and adapted for the context of Colombia.17 The information was integrated within the general framework of the Colombia National Surveys System. Other technical details of the SABE Colombia survey can be found elsewhere.18,19
Sample selection
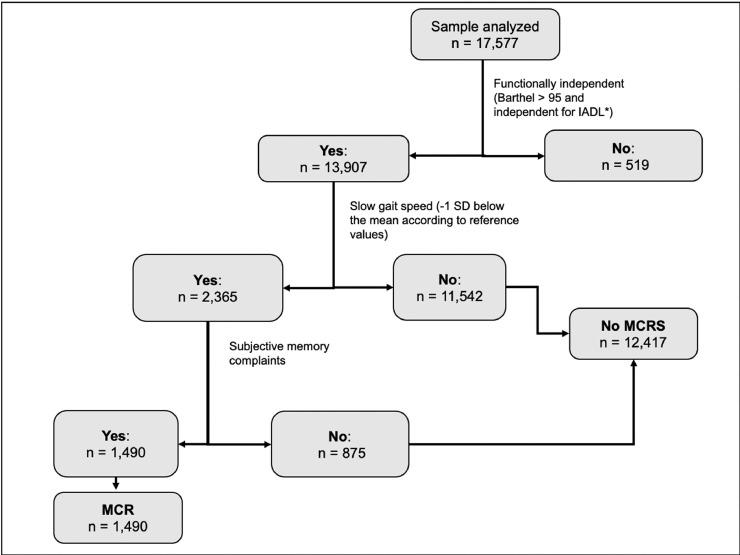
Figure 1. shows the criteria used to select participants for inclusion in the analysis. We included those individuals with complete data in the gait speed, cognitive function, and subjective memory assessment. Exclusion criteria included values of less than 13 points in the Abbreviated Mini-Mental State Examination, as these individuals were considered cognitively impaired.
Figure 1.
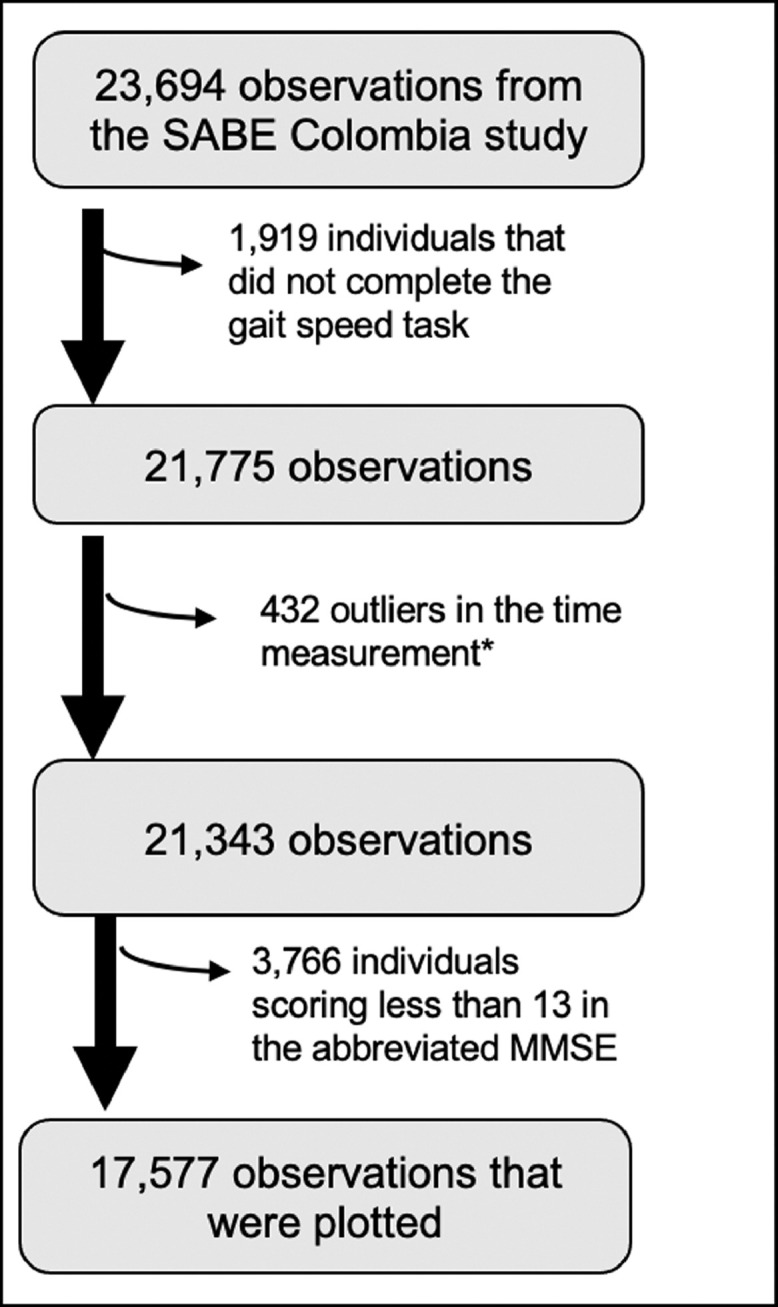
Flow-chart of the study sample selection.
* Values below the p1 and above the p99 on the time spent walking 3 m were excluded from the analysis
A recent cross-sectional study, carried out with a population with similar characteristics, found out that in the 4 m gait speed test the mean speed was 1·11 m/s with a range between 0·24 – 1·92 m/s.20 Similarly, it has been described that in individuals ages 60 years or more the mean gait speed is between 1·2 m/s to 0·9 m/s for women and 1·3 m/s to 0·9 m/s for men.21 Therefore, we excluded outliers data bellow 0·06 m/s and above 1·45 m/s, corresponding to values below the 1st percentile, or above the 99th percentile, as this might correspond to individuals that ran during the test or erroneously recorded data. The final study sample was 17·577 participants.
Variables
Dependent variable: Motoric Cognitive Risk Syndrome
People with slow gait speed and subjective memory complaints, without cognitive or functional impairment were categorized as having MCR (Figure 2).
-
1.
Gait: GS as a variable was created using the GS sub test in the Short Physical Performance Battery (SPPB), previously validated in Colombian populations and applied in the SABE Colombia survey.22 The participants were asked to walk 3 meters at their regular pace two times from a standing position. The best of both trials was used to determine GS. Individuals were considered as having a slow gait if they were 1.0 standard deviation or more below the mean for their age group and sex (Table 1), using age group cut points determined in a validation study among Colombian older adults.23
-
2.
Absence of dementia based on their cognitive status: To exclude cognitive impairment, individuals included had a score greater or equal to 13 in the abbreviated version of the MMSE.24
-
3.
Subjective memory complaints: We consider individuals to have self-reported memory complaints if they reported their memory as being "fair", "bad" or "really bad".
-
4.
Functional assessment: Subjects were classified as functionally independent if their Barthel scale score was greater than 95 and if they did not have difficulty performing any instrumental activities of daily living (IADL) including preparing a hot meal, shopping for groceries, managing money and taking medications.3
Figure 2.
Creation of the MCR variable. Four criteria were taken into account: slow gait speed, subjective memory complaints, no objective cognitive impairment or functional dependency.
*IADL: Instrumental activities of daily living. For more information regarding which IADL see methods
**Cutoff point defined as -1 SD below the mean according to the previously validated reference values of GS in Colombian population (See Table 1)
Table 1.
Gait speed cutoff points by age and sex*.
| Age group | Mean | Standard deviation (SD) | Cutoff point* |
|---|---|---|---|
| Males | m/s | m/s | m/s |
| 60-69 | 0.88 | 0.29 | 0.59 |
| 70-79 | 0.79 | 0.23 | 0.56 |
| 80 + | 0.66 | 0.21 | 0.45 |
| Females | |||
| 60-69 | 0.77 | 0.24 | 0.53 |
| 70-79 | 0.69 | 0.22 | 0.47 |
| 80 + | 0.58 | 0.15 | 0.43 |
*Cutoff points were set based on the previously validated reference values for the Short Physical Performance Battery (SPPB) in the Colombian Population. Adapted from: Ramírez-Vélez R, Pérez-Sousa MA, Venegas-Sanabria LC, Cano-Gutierrez CA, Hernández-Quiñonez PA, Rincón-Pabón D, et al. Normative Values for the Short Physical Performance Battery (SPPB) and Their Association With Anthropometric Variables in Older Colombian Adults. The SABE Study, 2015. Front Med. 2020 Feb 20;7.
Independent Variables
We included sociodemographic variables such as age, sex, and level of education (categorized as none, elementary or middle school, high school, or higher education).
Regarding chronic conditions, the history of hypertension, coronary artery disease, stroke, cancer, diabetes, and mental disorders, were analyzed based on self-reported medical history (if they had ever been told by a doctor or a nurse that they suffer from this medical conditions). Obesity was defined as having a body mass index greater or equal than 30 kg/m2 (WHO classification). Respondents were considered to have depressive symptoms if they had a score greater or equal to 6 points on the Yesavage or Geriatric Depression Scale (GDS).25
Statistical Analysis
A cross sectional analysis was performed. We first present the characteristics of the total sample and the subsamples with and without MCR. We examined differences in sociodemographic factors, cognitive function, and comorbidities by MCR status using Chi-squared tests for categorical variables and Wilcoxon Ranksum tests for continuous variables. In variables with more than 2 categories statistical significance was tested using Kruskal – Wallis with Dunn poshoc.
Next, we used logistic regression to examine associations of respondent characteristics with MCR. We first report the odds ratios (ORs), 95% CIs and p-values from models of the bivariate association of each characteristic with MCR. We then present results from a fully adjusted model, including socioeconomic status, functionality, chronic conditions, and related geriatric conditions such as frailty and polypharmacy. Frailty was defined with a score equal or less than six on the SPPB and polypharmacy was defined as the consumption of 5 or more medications at the time of the survey. All statistical analyses were performed with Stata 16. Statistical significance was defined with an alpha value of 5%, and a confidence interval was set at 95%.
Ethics approval and consent to participate
Two universities involved in developing the SABE Colombia study (University of Caldas, ID protocol CBCS-021-14, and University of Valle, ID protocol 09-014 and O11-015) reviewed and approved the study protocol, and written informed consent was obtained from everyone before inclusion and completion of the first examination (including permission to use secondary data and blood samples). The study protocol to the secondary analysis was approved by The Human Subjects Committee at the Pontificia Universidad Javeriana (ACTA ID 20/2017- 2017/180, FM-CIE-0459-17).
Role of the funding source
The database used of this study is part of a larger project funded by the Administrative Department of Science, Technology, and Innovation (Colciencias) and the Ministry of Health and Social Protection of Colombia (SABE Study 2015, ID No. 764).
Results
Of the 17·577 participants from the SABE Colombia survey included in the analysis (Figure 1), 1·490 met the criteria of MCR (median age of 71, IQR 11), the overall prevalence of MCR was 10·71%. Sociodemographic characteristics are shown in Table 2. There were more women than men in the MCR group (74·63 % vs. 55·75%) and slightly more of the older adults in the MCR group had no education (21·54% vs. 19·76%). The median MMSE score of those in the MCR group was 26, one point below the No MCR group. Similarly, comorbidities such as obesity, mental disorders, history of stroke or myocardial infarction, hypertension, and diabetes were more frequent in the MCR group.
Table 2.
Sociodemographic, measurements and clinical factors by groups.
| Total | No MCR | MCR | p value | |
|---|---|---|---|---|
| n = 13,907 | n= 12,417 (89.29 %) | n= 1,490 (10.71 %) | ||
| Sociodemographics | ||||
| Age | ||||
| Median (IQR) | 67 (10) | 68 (9) | 71 (11) | 0.127 |
| Sex | ||||
| Female | 8,034 (57.77 %) | 6,922 (55.75 %) | 1,112 (74.63 %) | < 0.001 |
| Level of education | ||||
| None | 2,774 (19.95%) | 2,453 (19.76%) | 321 (21.54%) | < 0.001 |
| Elementary or middle school | 7,926 (56.99%) | 6,947 (55.95%) | 979 (65.7%) | < 0.001 |
| High school - Higher education | 3,207 (23.06%) | 3,017 (24.3%) | 190 (12.5%) | < 0.001 |
| Measurements | ||||
| MMSE | ||||
| Median (IQR) | 27 (4) | 27 (4) | 26 (4) | < 0.001 |
| Chronic conditions | ||||
| Obesity | ||||
| 4,203 (30.22%) | 3,663 (29.5%) | 540 (36.24%) | < 0.001 | |
| Depression | ||||
| 5,149 (37.02%) | 4,617 (37.18%) | 532 (35.7%) | 0.264 | |
| Major mental disorder | ||||
| 926 (6.67%) | 783 (6.32%) | 143 (9.6%) | < 0.001 | |
| History of stroke | ||||
| 364 (2.62%) | 299 (2.41 %) | 65 (4.37%) | < 0.001 | |
| History of myocardial infarction | ||||
| 1,592 (11.45%) | 1,365 (11%) | 227 (15.23%) | < 0.001 | |
| Hypertension | ||||
| 6,999 (50.38%) | 6,108 (49.24%) | 891 (59.88%) | < 0.001 | |
| Diabetes | ||||
| 2,148 (15.47%) | 1,844 (14.87 %) | 304 (20.46%) | < 0.001 | |
| History of cancer | ||||
| 554 (3.99%) | 479 (3.86%) | 75 (5.04%) | 0.028 |
The results from the logistic regression models are shown in Table 3. The first column shows the unadjusted associations of each variable with MCR. We found MCR was associated with higher age, female sex, lower level of education, lower MMSE, and higher risk of obesity, mental disorders, history of stroke, myocardial infarction, hypertension, diabetes, and cancer. The second column shows the same associations adjusted for all other respondent characteristics. The MCR group was more likely to have lower MMSE score (OR 0·93, CI 0·91 - 0·95; p < 0·001). Also, MCR was associated with age above eighty years (OR 1·69, 1·43 - 1·92; p < 0·001), no or low educational attainment (OR 1·99, CI 1·67- 2·37; p < 0·001), MMSE (OR 0·93, CI 0·91 - 0·95; p < 0·001) and chronic conditions such as mental disorders (OR 1·36, CI 1·11-1·67; p 0·003), history of myocardial infarction (OR 1·24, CI 1·04 - 1·47; p 0·012), hypertension (OR 1·23, CI 1·08 - 1·40; p < 0·001) and diabetes (OR 1·18, CI 1.01 – 1·37; p 0·034).
Table 3.
Multivariable analysis for variables associated with MCR.
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| OR (CI 95%) | P value | OR (CI 95%) | P value | |
| Sociodemographics | ||||
| Age | ||||
| 70-79 | 2.05 (1.83-2.30) | < 0.001 | 1.50 (1.32 - 1.70) | < 0.001 |
| 80+ | 3.15 (2.65 - 3.76) | < 0.001 | 1.69 (1.43 - 1.92) | < 0.001 |
| Sex | ||||
| Female | 2.33 (2.06 - 2.63) | < 0.001 | 1.75 (1.53 - 1.99) | < 0.001 |
| Level of education | ||||
| None | 2.23 (1.9 - 2.62) | < 0.001 | 1.99 (1.67- 2.37) | < 0.001 |
| Elementary or middle school | 2.07 (1.7 - 2.58) | < 0.001 | 1.68 (1.38 - 2.06) | < 0.001 |
| Measurements | ||||
| MMSE | ||||
| 0.88 (0.86 - 0.90) | < 0.001 | 0.93 (0.91 - 0.95) | < 0.001 | |
| Chronic conditions | ||||
| Obesity | ||||
| 1.35 (1.21 - 1.52) | < 0.001 | 1.07 (0.95 - 1.21) | 0.225 | |
| Depression | ||||
| 0.93 (0.83 - 1.04) | 0.260 | 0.97 (0.86 - 1.10) | 0.703 | |
| Major mental disorder | ||||
| 1.57 (1.30 - 1.89) | < 0.001 | 1.36 (1.11-1.67) | 0.003 | |
| History of Stroke | ||||
| 1.84 (1.40-2.43) | < 0.001 | 1.29 (0.95 - 1.76) | 0.095 | |
| History of myocardial infarction | ||||
| 1.45 (1.24 - 1.69) | < 0.001 | 1.24 (1.04 - 1.47) | 0.012 | |
| Hypertension | ||||
| 1.53 (1.37 - 1.71) | < 0.001 | 1.23 (1.08 - 1.40) | 0.001 | |
| Diabetes | ||||
| 1.47 (1.28 - 1.68) | < 0.001 | 1.18 (1.01 - 1.37) | 0.034 | |
| History of Cancer | ||||
| 1.32 (1.03 - 1.69) | 0.03 | 1.26 (0.96 - 1.66) | 0.093 | |
*Model adjusted by socioeconomic status, functionality, and related geriatric conditions such as frailty and polypharmacy.
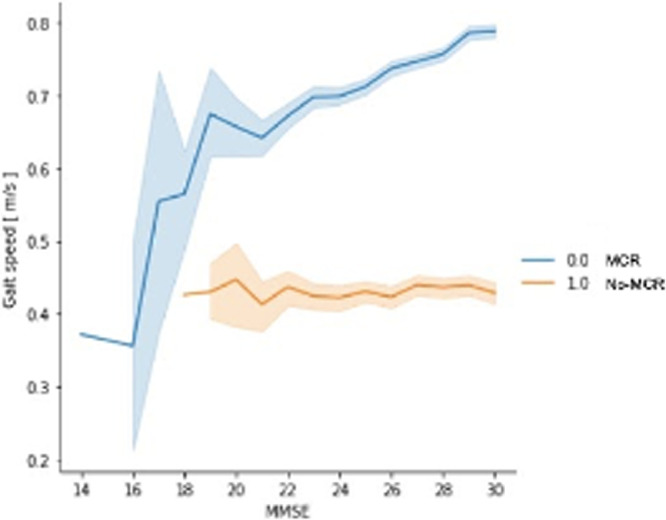
Figure 3 shows the association of GS and the MMSE score comparing individuals with and without MCR. When plotted, individuals without MCR displayed a higher GS as MMSE increased, which is different from the MCR group exhibiting a flatter curve.
Figure 3.
Comparison of GS and MMSE in individuals with and without MCR. The blue line (0.0) shows individuals without MCR and the orange line (1.0) shows individuals with MCR.
Discussion
In this study, we report a prevalence of 10·71% for MCR in Colombian community dwelling-older adults. To our knowledge this is the first time this syndrome is studied in this population Previous studies have reported similar prevalence of MCR: 14·3% in México, 8·0% in Europe, 7·0 % in Americans and 6·3% in Japanese older adults.3,26 Given differences in source populations, sample sizes, protocols and cutoff points in GS, variability between regions is not unexpected. This reflects not only differences in GS measurement, but also variation in cognitive tests across populations.
Regarding the GS assessment, the Mexican study used 0.8 m/s as the cutoff point to define slow GS, in contrast with our lower cutoff point. This can explain why we have a smaller prevalence, but it is important to mention that they also had a much smaller sample size compared to our number of participants. Another relevant point to highlight is the fact that most of these studies used a different GS test, the 4-meter and 6-meter timed walk. The 3-meter GS test might be less reliable in comparison to those who used longer distance. Studies have shown a higher reliability in the 10-meter test than in the 4-meter and 6-meter test.27
A review of MCR epidemiology compared the prevalence of this syndrome among different countries. Canada reported a prevalence of 7%, France 9·9%, Ireland 2·56%, and India 27·3%. These studies also reported different risk factors for MCR in their populations: In Canada they found that younger age, being a woman and some comorbidities such as obesity and hypertension were risk factors; France included waist-hip ratio, hypertension, and diabetes and Ireland included hypertension, poor vision, and other comorbidities.16
The differences in prevalence in those countries, compared to the data we report in Colombian older adults may be attributed to the way MCR criteria are operationalized, for example the heterogeneity in subjective memory complain assessment.16 Nevertheless, our results are in line with a multicentric study that estimates the prevalence of MCR raging from 5·3% to 15·6% in low – middle income countries.5 Even so, given SABE Colombia characteristics, determining a prevalence in this more selective sample likely does not reflect the real prevalence of the Colombian older adults, thus it does not help in decision making at population level. Neverthless, there is major relevance in the clinical practice if the definition criteria can be applied in any population.
Traditionally, MCI has been proposed as the main predementia stage; nevertheless, motor dysfunction has been described as a useful marker that could identify individuals at high risk for dementia.28 Also, slowing GS is thought to be a transition period that can occur concurrently with cognitive decline; and can predict cognitive loss and progression to MCI.
Because MCR combines both cognitive and gait criteria, it has been proposed that they share common brain regions. The premotor and prefrontal cortices, and specifically its dorsolateral segment, have shown a smaller volume in individuals with MCR.29, 30, 31 The dorsolateral segment is a key region in executive functions as it mediates memory process and gait control. The latter has been supported by imaging studies that have shown that individuals with gait disturbances and cognitive impairment have a reduced volume in the prefrontal and frontoparietal cortex.32 So, it has been suggested that MCR may predict with more accuracy neurodegenerative dementias of the cortical type rather than subcortical dementias.29
It would be mistaken to attribute the incidence of MCR to just the premotor and prefrontal cortex because cognition and gait control are a complex processes that involve many neural pathways and regions. Gait, for example, requires integration of attention, sensory input, motor planning and executive function, as well as an optimal musculoskeletal and cardiovascular system. Thus, basal ganglia and cerebellum has been proposed as important regions in planning and execution of purposeful locomotion.32 Other studies have shown that MCR are associated with lacunar infarcts, white matter hyperintensities and cortical strokes.33,34
In our analysis there were statistical differences in terms of cognitive performance in individuals with MCR compared to those without MCR (p value < 0.001). Nevertheless, the MCR definition as a predementia stage excludes those individuals with significant cognitive impairment, and we hypothesize that motor symptoms and cognitive complaints, both included in the MCR definition, may occur years before the objective cognitive decline detected through traditional neuropsychological evaluation.35 Given that MCR is an early subtype of MCI, individuals with subjective memory complaints might have lower scores in the cognitive screening test but do not classify as being cognitively impaired. Some authors even consider a pre-MCI stage the presence of subjective memory complaints.36,37 Several studies have reported the likelihood of developing cognitive impairment and dementia in individuals with MCR. In a multicentric study individuals with MCR had a HR of 2·0 (95% CI 1·7–2·4) for developing dementia.5 Similarly, another study reported a 2·4-fold increased risk of cognitive impairment.3 Moreover, a U.S. cohort that included 997 individuals with MCR strongly predicted the risk of vascular dementia (HR = 12·81).12
On the other hand, we found an association between age, sex, level of education, mental disorders, history of myocardial infarction, and MCR. Various studies have reported that increased age is related to higher prevalence of MCR. In a prevalence study involving 1,366 participants from Malaysia the prevalence of this syndrome was higher in older ages and in women (16.33%). Similarly, a cross-sectional study done in Japanese older adults reported a higher prevalence with advancing age.15 Regarding sex, being a female was associated with MCR; this can be explained because women have lower muscle mass and bone mineral density putting them at a higher risk of becoming frail and consequently, having gait disturbances.38
The lack of education was found to be associated with MCR; cognitive reserve has been reported as a protective factor for cognitive decline as it helps slow the progression seen in neurodegenerative diseases by preserving the metabolism and increasing the connectivity in temporal and frontal brain areas.4 Regarding the association with comorbidities like hypertension, diabetes, and history of myocardial infarction, it can be explained by the fact that these are vascular risk factors that contribute to microdamage of the vessels of various regions of the cerebral cortex such as the prefrontal cortex, affecting both cognition and motor function.34 On the other hand, mental disorders were also associated with MCR. There are some descriptions that point out that gait control needs for the recruitment of specific brain areas in the prefrontal cortex and cerebellum, the same brain areas can be affected in patients with mental disease.39,40 This information provides us insight about the brain substrate of this association.
Limitations
SABE Colombia is a community-dwelling cross-sectional study, so causality cannot be established between MCR and sociodemographic factors or chronic conditions; instead, it helps characterize this syndrome in Colombian population. In addition, the MMSE has limitations detecting individuals with subtle changes in cognition and the 3m GS test might underestimate the real GS; thus we may slightly underestimate the prevalence of MCR. Nevertheless, similar prevalence results have been reported worldwide. Also, missing data in sociodemographic characteristics and chronic conditions, affect the quality of the evidence, can bias the results, and can distort the internal and external validity of results.
Conclusions
This study found a prevalence of 10·71 % of MCR in Colombian older adults. We found an association between cognitive performance (MMSE) and MCR. In addition, an association was identified between chronic conditions and sociodemographic factors previously identified as risk factors for MCR. This is the first paper describing this motor syndrome in the Colombian population. Our results increase the awareness of a novel predementia syndrome that has been shown to predict the risk of developing cognitive impairment and dementia. Despite MCR having a low prevalence in Colombian older adults, its identification can be used as a screening tool for cognitive impairment given that a gait assessment, a cognitive complaint questionnaire, and a functional assessment can be performed by clinicians in the outpatient clinic. This approach may reduce the costs carried out by a full neuropsychologic evaluation, can be a practical approach for primary care physicians, and can be applied in low-and middle-income countries such as Colombia, where it can be difficult to access a memory clinic. Nevertheless, much more research is needed to determine if MCR is a cost-effective screening tool for cognitive impairment.
Contributors
Elkin García-Cifuentes conceived the presented idea and performed the statistical analysis with Isabel Márquez, Felipe Ramirez Velandia and Ana María Saavedra. It was supervised and adjusted with the guidance of Jennifer Ailshire and Margarita Osuna.
Isabel Márquez, Felipe Ramirez Velandia and Ana María Saavedra helped draft the manuscript with supervision of Elkin Garcia-Cifuentes, Miguel German Borda, Margarita Osuna and Jennifer Ailshire. Angela Iragorri and Carlos Cano-Gutierrez helped supervise the whole project. All authors discussed the results and contributed to the final manuscript.
Data sharing statement
Will individual participant data be available (including data dictionaries)? Yes
What data in particular will be shared? All of the individual participant data collected during the trial, after de-identification
What other documents will be available? Study protocol, statistical analysis plan, informed consent form, clinical study report, analytic code
When will data be available (start and end dates)? Immediately following publication; no end date
With whom? Anyone who wishes to access the data
For what types of analyses? Any purpose
By what mechanism will data be made available? Data are available indefinitely at https://drive.google.com/file/d/1CEsd1g7xgxwQpFR0xKqCa7CE8ckvoxn-/view?usp=drivesdk
Declaration of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict.
References
- 1.GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar-Navarro SG, Mimenza-Alvarado AJ, Aguilar-Esquivel JE, Yeverino-Castro SG, Juárez-Cedillo T, Mejía-Arango S. Motoric Cognitive Risk Syndrome: Prevalence and Risk of Cognitive Impairment in a Population Studied in the Mexican Health and Aging Study 2012–2015. J Nutr Heal Aging [Internet] 2019;23(3):227–231. doi: 10.1007/s12603-019-1160-7. https://pubmed.ncbi.nlm.nih.gov/30820509/ [cited 2020 Jul 19] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission [Internet] The Lancet. Lancet Publishing Group. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [cited 2020 Oct 15]Available from: https://doi.org/10.1016/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, et al. Motoric cognitive risk syndrome Multicountry prevalence and dementia risk. Neurology. 2014;83(8):718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese J, Wang C, Bennett DA, Lipton RB, Katz MJ, Ayers E. Motoric cognitive risk syndrome and predictors of transition to dementia: A multicenter study. Alzheimer's Dement. 2019;15(7):870–877. doi: 10.1016/j.jalz.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buracchio T, Dodge H, Howieson D, Al E. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2016;67:980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mielke M, Roberts R, Savica R, et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68A:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumurgier J, Artaud F, Touraine C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci. 2017;72A:655–661. doi: 10.1093/gerona/glw110. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cifuentes E, David-Pardo DG, Borda MG, Pérez-Zepeda MU, Cano CA. TWO-WAY BRIDGE BETWEEN MUSCULAR DYSFUNCTION AND COGNITIVE IMPAIRMENT: SECONDARY ANALYSIS OF SABE – BOGOTA STUDY. The Journal of Frailty & Aging©. 2017;6 doi: 10.14283/jfa.2017.17. Barcelona -Spain. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Cifuentes E, Márquez I, Vasquez D, Aguillon D, Borda MG, Lopera F, et al. The Role of Gait Speed in Dementia: A Secondary Analysis from the SABE Colombia Study. Dement Geriatr Cogn Disord. 2020:1–8. doi: 10.1159/000510494. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Wang C, Lipton R, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayers E, Verghese J. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement. 2016;12:556–564. doi: 10.1016/j.jalz.2015.08.167. [DOI] [PubMed] [Google Scholar]
- 14.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T. Motoric cognitive risk syndrome: Association with incident dementia and disability. J Alzheimers Dis. 2017;59(1):77–84. doi: 10.3233/JAD-170195. [DOI] [PubMed] [Google Scholar]
- 15.T D, J V, H S, H M, K T, R H, et al. Motoric Cognitive Risk Syndrome: Prevalence and Risk Factors in Japanese Seniors. J Am Med Dir Assoc [Internet] 2015;16(12):1103.e21–1103.e25. doi: 10.1016/j.jamda.2015.09.003. https://pubmed.ncbi.nlm.nih.gov/26476498/ [cited 2021 Sep 25]Available from. [DOI] [PubMed] [Google Scholar]
- 16.Meiner Z, Ayers E, Verghese J. Motoric Cognitive Risk Syndrome: A Risk Factor for Cognitive Impairment and Dementia in Different Populations. Ann Geriatr Med Res. 2020;24(1):3–14. doi: 10.4235/agmr.20.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albala C, Lebrão M, León Díaz E, Ham-Chande R, Hennis A, Palloni A. The Health, Well-Being, and Aging survey: methodology applied and profile of the study population. Rev Panam Salud Publica. 2005;17(5–6):307–322. doi: 10.1590/s1020-49892005000500003. [DOI] [PubMed] [Google Scholar]
- 18.Gomez F, Corchuelo J, Curcio CL, Calzada MT, Mendez F. SABE Colombia: Survey on Health, Well-Being, and Aging in Colombia - Study Design and Protocol. Curr Gerontol Geriatr Res. 2016;2016 doi: 10.1155/2016/7910205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuellar Segura CM. Documento Metodológico: Encuesta Nacional de Salud, Bienestar y Envejecimiento SABE Colombia [Internet] Ministerio de Salud y Protección Social de Colombia. 2021 Oct 2 https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/ED/GCFI/doc-metodologia-sabe.pdf [cited]. Available from: [Google Scholar]
- 20.Krumpoch S, Lindemann U, Rappl A, Becker C, Sieber CC, Freiberger E. The effect of different test protocols and walking distances on gait speed in older persons. Aging Clin Exp Res. 2020;(0123456789) doi: 10.1007/s40520-020-01703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohannon RW, Andrews AW. Normal walking speed: a descriptive meta-analysis. Physiother. 2011;97:182–189. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Gómez Montes J, Curcio C, Alvarado B, Zunzunegui M, Guralnik J. Validity and reliability of the Short Physical Performance Battery (SPPB): a pilot study on mobility in the Colombian Andes. Colomb Med. 2013;44(3):165–171. [PMC free article] [PubMed] [Google Scholar]
- 23.Ramírez-Vélez R, Pérez-Sousa MA, Venegas-Sanabria LC, Cano-Gutierrez CA, Hernández-Quiñonez PA, Rincón-Pabón D, et al. Normative Values for the Short Physical Performance Battery (SPPB) and Their Association With Anthropometric Variables in Older Colombian Adults. The SABE Study. 2015 doi: 10.3389/fmed.2020.00052. Front Med. 2020 20;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folstein M, Folstein S, McHugh P. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 26.Maggio M, Lauretani F. Prevalence, incidence, and clinical impact of cognitive-motoric risk syndrome in Europe, USA, and Japan: facts and numbers update 2019. J Cachexia Sarcopenia Muscle. 2019;10(5):953–955. doi: 10.1002/jcsm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hee-jae K, Lhyoek P, Joo LH, Lee O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J Exerc Nutr Biochem. 2016;20(3):046–050. doi: 10.20463/jenb.2016.09.20.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, et al. The motor signature of mild cognitive impairment: Results from the gait and brain study. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69(11):1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beauchet O, Allali G, Annweiler C, Verghese J. Association of Motoric Cognitive Risk Syndrome With Brain Volumes: Results From the GAIT Study. Journals Gerontol - Ser A Biol Sci Med Sci. 2016;71(8):1081–1088. doi: 10.1093/gerona/glw012. [DOI] [PubMed] [Google Scholar]
- 30.Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J. A Gray Matter Volume Covariance Network Associated with the Motoric Cognitive Risk Syndrome: A Multicohort MRI Study. J Gerontol A Biol Sci Med Sci [Internet] 2019;74(6):884–899. doi: 10.1093/gerona/gly158. https://pubmed.ncbi.nlm.nih.gov/29985983/ [cited 2021 Dec 5]Available from: [DOI] [PubMed] [Google Scholar]
- 31.Blumen HM, Schwartz E, Allali G, Beauchet O, Callisaya M, Doi T, et al. Cortical Thickness, Volume, and Surface Area in the Motoric Cognitive Risk Syndrome. J Alzheimers Dis [Internet] 2021;81(2):651–665. doi: 10.3233/JAD-201576. https://pubmed.ncbi.nlm.nih.gov/33867359/ [cited 2021 Dec 5]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Low L, Schwenk M, Mills N, Gwynn J, Clemson L. Review of Gait, Cognition, and Fall Risks with Implications for Fall Prevention in Older Adults with Dementia. Dement Geriatr Cogn Disord. 2019;48(1–2):17–29. doi: 10.1159/000504340. [DOI] [PubMed] [Google Scholar]
- 33.Mergeche J, Verghese J, Allali G, Wang C, Beauchet O, Pradeep Kumar V, et al. White Matter Hyperintensities in Older Adults and Motoric Cognitive Risk Syndrome. J Neuroimaging Psychiatry Neurol. 2016;1(2):73–78. doi: 10.17756/jnpn.2016-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang N, Allali G, Kesavadas C, Noone ML, Pradeep VG, Blumen HM, et al. Cerebral Small Vessel Disease and Motoric Cognitive Risk Syndrome: Results from the Kerala-Einstein Study. J Alzheimers Dis [Internet] 2016;50(3):699–707. doi: 10.3233/JAD-150523. https://pubmed.ncbi.nlm.nih.gov/26757037/ [cited 2021 Dec 5]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease [Internet] Expert Review of Neurotherapeutics. 2011;11:665–676. doi: 10.1586/ern.11.57. [cited 2020 Oct 12]Available from: /pmc/articles/PMC3121966/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choe YM, Byun MS, Lee JH, Sohn BK, Lee DY, Kim JW. Subjective memory complaint as a useful tool for the early detection of Alzheimer's disease. Neuropsychiatr Dis Treat. 2018;14:2451–2460. doi: 10.2147/NDT.S174517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 38.Janssen I, Heymsfield S, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 39.Sekhon H, Allali G, Beauchet O. The association of anxio-depressive disorders and depression with motoric cognitive risk syndrome: results from the baseline assessment of the Canadian longitudinal study on aging. GeroScience [Internet] 2019;41(4):409–418. doi: 10.1007/s11357-019-00093-z. https://pubmed.ncbi.nlm.nih.gov/31463648/ [cited 2021 Dec 5]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lallart E, Jouvent R, Herrmann FR, Perez-Diaz F, Lallart X, Beauchet O, et al. Gait control and executive dysfunction in early schizophrenia. J Neural Transm [Internet] 2014;121(4):443–450. doi: 10.1007/s00702-013-1111-0. https://pubmed.ncbi.nlm.nih.gov/24201834/ [cited 2021 Dec 5]Available from: [DOI] [PubMed] [Google Scholar]