Summary
Background
Hepatitis C is a preventable and treatable disease that has been declared a public health problem. In 2012, the prevalence of HCV serum anti-bodies in the Mexican adult population aged 20 to 49 years was 0·30%.
Methods
We randomly selected a probabilistic sub-sample of 12,389 adults (20+ years) from adults participating in the National Health and Nutrition Survey (ENSANUT) 2018 who provided a venous blood sample. Anti-HCV antibodies and HCV RNA were determined for this sub-sample. We estimated the national prevalence of anti-HCV antibodies and the proportion with viral RNA detection and evaluated their association with sociodemographic characteristics for all adults and with sexual behaviours in those aged 20 to 49 years using logistic regression.
Findings
The national prevalence of anti-HCV antibodies in serum was 0·38% (95%CI 0·24, 0·59) in the population aged 20 years and older; 14·9% of them had viral RNA. In the population aged 20 to 49 years antibody prevalence was 0·23% (95%CI 0·11, 0·48), being higher for males and people living in urban areas. In the population aged 50 years and older, the prevalence was 0·59% (95%CI 0·34, 1·06).
Interpretation
The prevalence of antibodies anti-HCV in people aged 20 to 49 years was similar in 2018 than in 2012, suggesting that the prevalence of HCV has remained stable. ENSANUT is a household study and could underestimate the prevalence of HCV. Further efforts must be made to identify cases in non-household populations.
Funding
National Institute of Statistics and Geography and National Institute of Public Health of Mexico [CIEE/1807].
Keywords: hepatitis C, hepatitis C virus antibodies, HCV antibodies, prevalence, epidemiology
Research in context.
Evidence before this study
Liver cirrhosis is the fourth leading cause of death and one of the main causes of disability in Mexico. Hepatitis C infection (HCV) is an important contributor to cirrhosis and other hepatic diseases. Before 1993, blood transfusion was the most common risk factor for HCV in Mexico. The last nationally representative estimates of anti-HCV antibodies in Mexico were produced in 20212, when 0·30% of the adult population aged 20 to 49 years was seropositive.
Added value of this study
This information is particularly important given the commitment of countries to eradicate hepatitis by 2030 and the need to identify critical groups for treatment. In Mexico, the national prevalence of hepatitis C has remained stable; however, antibodies continue to be higher in men compared to women and in urban compared to rural areas, showing health inequities and differential exposure.
Implications of all the available evidence
Identifying vulnerable groups/treatment groups in the population can be beneficial to focus public health interventions. Given the paucity of hepatitis C studies in middle-income countries, our paper can play an important role by indicating variables associated to HCV that are common to other countries in the region, making this information valuable for the Americas.
Alt-text: Unlabelled box
Introduction
Worldwide, around 71 million people live with chronic hepatitis C virus infection (HCV).1 Hepatitis C is a preventable and treatable disease that has been declared a public health problem.2 The advent of new treatments that can eliminate HCV infection has opened the discussion to develop short-term plans to reduce its burden, an objective that is part of the 2030 Sustainable Development Agenda (SDG).3 The World Health Organization (WHO) has proposed global strategies to prevent and control viral hepatitis4 that have been echoed by several countries.5
The last nationally representative seroprevalence of HCV in Mexican adults was estimated in 2012, finding that 0·30% of adults 20 to 49 years were seropositive to HCV.6 No other representative estimates have been published to date, yet, in high-risk subpopulations the pooled HCV prevalence has been estimated to be 84·2%.7 HCV infections can lead to liver cirrhosis, cancer, and hepatocellular carcinoma.1 Liver cirrhosis is the fourth leading cause of death and one of the main causes of disability in Mexico.8 Before 1993, blood transfusion was the most common risk factor for HCV in Mexico.9 Since then, the Mexican government implemented official standards for blood donation and a specific action program for the prevention, diagnosis, and treatment of hepatitis C for 2016-2018, aiming to reduce morbidity and mortality.10 Currently, intravenous drug use, multiple sexual partners, unsafe tattoos, and perinatal transmission are the most usual ways of transmission.8 In 2018, direct-acting antivirals were introduced by public providers as part of their basic drug formulary11 to help eliminate hepatitis. Treatment is freely available through the National Program of Hepatitis C Elimination, which includes access to screening and diagnostic tests, as well as treatment with a primary care focus.12
We aimed to estimate the 2018 national prevalence of anti-HCV antibodies and HCV RNA among seropositive cases in Mexico. While anti-HCV will provide us with an estimate of prior infection, the estimate of HCV RNA will allow us to identify active cases. We analyzed data from a probabilistic sample of adults aged 20 years and older, who provided blood samples to the National Health and Nutrition Survey (ENSANUT 2018-19, for its Spanish acronym).
Methods
Sampling design of ENSANUT
ENSANUT 2018-19 is a nationally representative survey of inhabitants of community households; the sampling design is probabilistic, stratified, and uses a two-stage cluster sampling. The design and the sampling methodology of the survey have been described elsewhere.13
ENSANUT visited 6,268 primary sampling units, contacting 44,612 households and obtaining a response to the survey from 43,070 adults (20+ years). A random sample of 27,640 adults was asked to provide a blood sample for biochemical analysis (26·9% of the adults in ENSANUT household); a total of 16,584 adults agreed to participate in the procedure (60% participation rate). The minimum sample size to estimate the prevalence of hepatitis C with a semi-amplitude of the interval of 0·15% was calculated with the following assumptions: , with p=0·3% (previous estimate of HCV), Deff=2 (usual design effect of ENSANUT surveys), δ=0·15% (half the value of the previous estimate). Then the minimum sample size was n= 10,213, yet our financial resources allowed us to include 12,389 randomly selected blood samples to detect HCV antibodies; seropositive samples were further submitted to RNA quantification.
Sampling weights of ENSANUT were calculated based on probabilities of selection, probabilities of response, and official estimates of the population. Compared to survey response, a lower proportion of men and participants aged 20 to 34 years provided a blood sample; therefore, the sampling weights for the 12,389 venous samples were estimated to adjust for differences in age and sex considering the low participation rate (Supplementary Table 2). From the 12,389 selected venous samples, 12,335 had completed data on necessary variables from the individual questionnaire. The fieldwork was done by INEGI (National Institute of Geography and Statistics). Informed consent was obtained from all participants. ENSANUT was approved by the Ethical, Research, and Biosecurity Committee of the National Institute of Public Health.
Blood sample collection
A five milliliter blood sample was collected in vacutainer SST tubes (Becton Dickinson and Company, New Jersey, USA) with eight hours of fasting according to the technique described by the WHO.14 Afterwards, the sample was centrifugated at 3,500 rpm for 15 minutes. Finally, the serum was stored in a cryovial in liquid nitrogen (-80°C) until analysis.
Anti-HCV antibodies and HCV RNA determination in serum
The determination of anti-HCV antibodies was performed with the ARCHITECT Anti-HCV (6C37, Abbot, Germany) assay, a two-step immunoassay that uses chemiluminescent technology for the qualitative detection of anti-HCV with a sensitivity of 99·1% and a specificity of 99·6%. HCV RNA was quantified among participants seropositive to anti-HCV antibodies. For HCV RNA quantification, we used the RealTime HCV assay (1N30, Abbot, Germany), which uses a reverse transcription-polymerase chain reaction (RT-PCR) to generate an amplified product from the RNA genome of HCV. All assays were performed at the National Institute of Medical Sciences and Nutrition Salvador Zubirán's Central Laboratory in Mexico City.
Questionnaire
Individual questionnaires were obtained from to participants selected to provide a venous blood sample, including sociodemographic characteristics and risk factors for viral hepatitis. Age in years was categorized in decades, from 20 to 70 years and more. Marital status was categorized as single, cohabitating—free union or legally or religiously married —, separated —from a union or divorced—, or widowed. Education was categorized as elementary or less, middle/high school, and college/postgraduate. The country was divided into four regions: Center, North, Mexico City, and South.13 Area of residence was categorized as rural (<2,500 inhabitants) and urban (≥2,500 inhabitants). Socioeconomic status was constructed as in the ENSANUT 2012, generating an index through principal component analysis using household characteristics and assets, then, tertiles of the index were used to divide the population in three strata representing low, middle and high socioeconomic status.
With the question “Have you ever been diagnosed with hepatitis C by a physician?” we obtained the self-reported hepatitis C infection. The history of blood transfusion was obtained with the question “Did you receive a blood transfusion before 1995?” For comparability with ENSANUT 2012, the population aged 20 to 49 years responded to additional questions about sexual behaviours. Onset of sexual life (“At which age did you have your first sexual intercourse?”) was categorized into “not initiated”, “initiated before age 18”, “initiated after age 18”. We obtained data about condom use with the question “The first time/last time you had sexual intercourse: what did you or your partner use to avoid pregnancy or a sexually transmitted infection?”.
Statistical analysis
The primary outcome was the presence of anti-HCV antibodies, and the secondary outcome was HCV RNA among seropositive cases. Both were treated as dichotomous: present or absent. The national weighted prevalence was estimated for anti-HCV antibodies as follows:
Where n is the sample size, wi is the sampling weight of i-unit, and yi indicates if the anti-HCV antibodies are positive; variance of estimators was estimated using the variability between primary sampling units as described by Cochran.15
Chi-square test was used to evaluate the association between anti-HCV antibodies and sociodemographic characteristics in the adult population, and additionally sexual behaviours available for the population aged 20 to 49 years. The prevalence of HCV RNA was estimated with 95% CI, and the distribution of sociodemographic characteristics and sexual behaviours amongst those positive to anti-HCV antibodies were described using percentages. Data were analysed considering the sampling design (weights, strata, clusters) in STATA v14 (College Station, TX: StataCorp LP) and a predefined alpha value of 0.05.
Ethical approval
The present study was approved by the Ethical, Research, and Biosecurity Committee of the National Institute of Public Health and all participants signed an informed consent.
Role of funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data, the corresponding author had the final responsibility for the decision to submit for publication.
Results
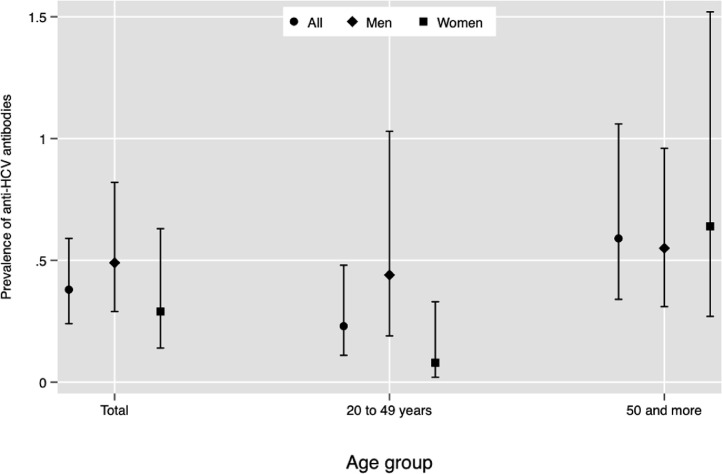
Figure 1 describes the weighted prevalence of HCV by age group. The national prevalence was 0·38% (95% CI 0·24, 0·59%), equivalent to 307 000 inhabitants (95%CI 171 000, 443 000) positive to anti-HCV antibodies in serum. In the population aged 20 to 49 years the prevalence was 0·23% (95% CI 0·11, 0·48%), equivalent to 113 000 inhabitants; in this age group, the prevalence was higher for men. In participants aged 50 years and older, the prevalence was 0·59% (95% CI 0·34, 1·06); women had a slightly higher prevalence than men.
Figure 1.
National prevalence of anti-HCV in serum in the Mexican adult population (aged 20 years and more). ENSANUT 2018.
Table 1 describes the prevalence of anti-HCV antibodies in participants aged 20 to 49 years. Men had a higher prevalence compared to women (p=0·02), while participants with middle/high school education had a lower prevalence than those with elementary school or less, and college and higher; the prevalence in rural areas was lower than in urban areas (p<0·01). Participants who reported having used a condom in the last sexual intercourse had a higher prevalence than those who did not.
Table 1.
National prevalence of anti-HCV antibodies by sociodemographic characteristics in Mexican population aged 20 to 49 years. ENSANUT 2018.
| Prevalence (95% CI) | P-value | Estimated cases (n/N) | |
|---|---|---|---|
| Age group (years) | 0·52 | ||
| 20-29 | 0·12 (0·03, 0·46) | 3/21000 | |
| 30-39 | 0·34 (0·09, 1·29) | 5/49000 | |
| 40-49 | 0·25 (0·10, 0·64) | 6/43000 | |
| Marital status | 0·75 | ||
| Single | 0·17 (0·05, 0·64) | 3/21000 | |
| Cohabitating | 0·23 (0·09, 0·63) | 7/73000 | |
| Separated or widowed | 0·36 (0·10, 1·28) | 4/18000 | |
| Education | 0·06 | ||
| Elementary or less | 0·44 (0·17, 1·17) | 5/42000 | |
| Middle/High school | 0·08 (0·03, 0·19) | 6/23000 | |
| College and higher | 0·47 (0·11, 1·94) | 3/48000 | |
| Country region | 0·43 | ||
| Center | 0·40 (0·15, 1·07) | /637000 | |
| North | 0·13 (0·05, 0·33) | 5/521000 | |
| Mexico Citya | 0·43 (0·06, 2·96) | 1/32000 | |
| South | 0·14 (0·03, 0·58) | 2/22000 | |
| Sex | 0·02 | ||
| Men | 0·45 (0·19, 1·03) | 12/89000/ | |
| Women | 0·08 (0·02, 0·33) | 2/23000 | |
| Area | <0·01 | ||
| Rural | 0·02 (0·00, 0·14) | 1/2000 | |
| Urban | 0·29 (0·14, 0·61) | 13/110000 | |
| Socioeconomic status | 0·75 | ||
| Low | 0·15 (0·04, 0·54) | 3/22000 | |
| Medium | 0·30 (0·12, 0·71) | 7/49000 | |
| High | 0·23 (0·05, 1·11) | 4/41000 | |
| Condom use: last intercourse | 0·01 | ||
| No | 0·11 (0·04, 0·30) | 5/35000 | |
| Yes | 0·56 (0·21, 1·51) | 8/73000 | |
| Did not answer | 0·13 (0·11, 0·48) | 1/4000 | |
| Condom use: first intercourse | 0·60 | ||
| No | 0·29 (0·11, 0·77) | 8/74000 | |
| Yes | 0·17 (0·06, 0·49) | 5/34000 | |
| Did not answer | 0·13 (0·11, 0·48) | 1/4000 | |
| Onset of active sexual life | 0·66 | ||
| Have not initiated | 0·15 (0·02, 1·03) | 1/4000 | |
| <18 years | 0·20 (0·07, 0·51) | 6/38000 | |
| ≥18 years | 0·30 (0·11, 0·85) | 7/70000 | |
| Did not answer | 0·00 (0·00, 0·00) | 0/0 | |
| Prior HCV diagnosis | 0·81 | ||
| No | 0·23 (0·11, 0·49) | 13/110000 | |
| Yes | 0·29 (0.04, 2·07) | 1/2000 | |
| Does not know/remember | 0·00 (0·00, 0·00) | 0/0 | |
| Did not answer | 0·00 (0·00, 0·00) | 0/0 | |
| Blood transfusion before 1995 | 0·82 | ||
| No | 0·24 (0·10, 0·56) | 10/92000 | |
| Yes | 0·00 (0·00, 0·00) | 0/0 | |
| Does not know/remember | 0·25 (0·05, 1·28) | 2/13000 | |
| Did not answer | 0·23 (0·06, 0·91) | 2/7000 | |
Sample size: 7,454 which represents 48 million 979 thousands inhabitants aIncludes surrounding urban municipalities. N is weighted sample and n is the study sample. N may not add up to 113 000 due to rounding.
Table 2 describes the characteristics associated with anti-HCV antibodies in the population aged 50 years and older. Age was the only variable associated with anti-HCV antibodies in serum (p=0·03). Prevalence increased with age, being 0·16% among participants aged 50-59 years, 0·81% in those aged 60-69 years, and 1·07% among people aged 70 and more.
Table 2.
National prevalence of anti-HCV antibodies by sociodemographic characteristics in Mexican population aged 50 years and more. ENSANUT 2018.
| Prevalence (95% CI) | P-value | Estimated cases (n/N) | |
|---|---|---|---|
| Age group (years) | 0·03 | ||
| 50-59 | 0·16 (0·07, 0·38) | 7/22000 | |
| 60-69 | 0·81 (0·41, 1·59) | 14/83000 | |
| 70 or more | 1·07 (0·38, 2·98) | 14/90000 | |
| Marital status | 0·20 | ||
| Single | 0·75 (0·23, 2·37) | 4/23000 | |
| Cohabitating | 0·43 (0·24, 0·76) | 22/88000 | |
| Separated | 0·37 (0·11, 1·31) | 4/12000 | |
| Widowed | 1·29 (0·35, 4·64) | 5/72000 | |
| Education | 0·90 | ||
| Elementary or less | 0·65 (0·30, 1·41) | 25/124000 | |
| Middle/High school | 0·52 (0·20, 1·33) | 7/52000 | |
| College and higher | 0·57 (0·17, 1·84) | 3/19000 | |
| Country region | 0·7 | ||
| Centre | 0·32 (0·10, 0·98) | 5/23000 | |
| North | 0·55 (0·25, 1·22) | 12/53000 | |
| Mexico Citya | 0·73 (0·10, 4·96) | 1/44000 | |
| South | 0·79 (0·42, 1·49) | 17/74000 | |
| Sex | 0·75 | ||
| Men | 0·55 (0·31, 0·96) | 20/77000 | |
| Women | 0·64 (0·27, 1·51) | 15/118000 | |
| Area | 0·69 | ||
| Rural | 0·71 (0·30, 1·65) | 11/45000 | |
| Urban | 0·57 (0·29, 1·14) | 24/150000 | |
| Socioeconomic status | 0·78 | ||
| Low | 0·54 (0·28, 1·06) | 15/52000 | |
| Medium | 0·50 (0·22, 1·14) | 9/51000 | |
| High | 0·72 (0·25, 2·03) | 11/92000 | |
| Prior HCV diagnosis | 0·84 | ||
| No | 0·62 (0·35, 1·08) | 35/195000 | |
| Yes | 0·00 (0·00, 0·00) | 0/0 | |
| Does not know/remember | 0·00 (0·00, 0·00) | 0/0 | |
| Did not answer | 0·00 (0·00, 0·00) | 0/0 | |
| Blood transfusion before 1995 | 0·67 | ||
| No | 0·66 (0·36, 1·21) | 29/171000 | |
| Yes | 0·00 (0·00, 0·00) | 0/0 | |
| Does not know/remember | 0·72 (0·12, 4·08) | 3/20 | |
| Did not answer | 0·13 (0·04, 0·44) | 3/3000 |
Sample size: 4935 which represents 32 million 466 thousand inhabitants. aIncludes surrounding urban municipalities. N is weighted sample and n is the study sample. N may not add up to 195 000 due to rounding.
Of the total population estimated with anti-HVC antibodies, 14·91% (95% CI 11·21, 19·55%) had viral RNA, equivalent to 46 000 inhabitants with active HCV infection. Table 3 shows the distribution of sociodemographic characteristics and sexual behaviours in those positive to RNA. The majority of cases positive to RNA among seropositive participants were aged 40 to 59 years, had elementary school or less, and lived in the Central region (no active cases were detected in Mexico City). The sex distribution was similar among active cases. The majority of cases lived in urban areas, had medium socioeconomic status, and reported not wearing a condom in their last or their first sexual intercourse. Among participants with an active infection, 55·86% began sexual activity before 18 years of age. In addition, all cases of RNA positive cases referred not having received a diagnosis of hepatitis C.
Table 3.
Sociodemographic characteristic of inhabitants with HCV RNA infection and positive anti-HCV antibodies. ENSANUT 2018.
| % (95% CI) | Estimated cases (thousands) | |
|---|---|---|
| Age group (years) | ||
| 20-29 | 5·15 (0·63, 31·75) | 3 |
| 30-39 | 15·23 (2·02, 61·02) | 7 |
| 40-49 | 44·25 (12·62, 81·35) | 20 |
| 50-59 | 35·37 (10·36, 72·17) | 16 |
| Marital status | ||
| Single | 23·45 (5·95, 59·73) | 11 |
| Cohabitating | 66·73 (31·38, 89·79) | 31 |
| Separated or widowed | 9·82 (2·01, 36·58) | 4 |
| Education | ||
| Elementary or less | 48·31 (15·38, 82·77) | 22 |
| Middle/High school | 36·61 (11·18, 72·59) | 17 |
| College and higher | 15·09 (2·00, 60·76) | 7 |
| Country region | ||
| Centre | 50·61 (16·80, 83·87) | 23 |
| North | 38·27 (9·14, 79·26) | 18 |
| Mexico Citya | 0.00 (0.00, 0.00) | 0 |
| South | 11·12 (1·42, 52·05) | 5 |
| Sex | ||
| Men | 51·69 (17·23, 84·62) | 24 |
| Women | 48·31 (15·38, 82·77) | 22 |
| Area | ||
| Rural | 21·39 (5·70, 55·05) | 10 |
| Urban | 78·61 (44·95, 94·30) | 36 |
| Socioeconomic status | ||
| Low | 22·15 (6·01, 55·87) | 10 |
| Medium | 69·08 (34·13, 90·60) | 32 |
| High | 8·77 (1·10, 45·38) | 4 |
| Condom use: last intercourseb | ||
| No | 51·18 (17·60, 83·72) | 24 |
| Yes | 13·44 (2·72, 46·34) | 6 |
| Did not answer | 35·37 (10·47, 71·94) | 16 |
| Condom use: first intercourseb | ||
| No | 59·95 (24·63, 87·27) | 28 |
| Yes | 4·67 (0·57, 29·63) | 2 |
| Did not answer | 35·37 (10·47, 71·94) | 16 |
| Onset of active sexual lifeb | ||
| <18 years | 55·86 (21·03, 85·74) | 26 |
| ≥18 years | 8·77 (1·12, 44·99) | 4 |
| Did not answer | 35·37 (10·47, 71·94) | 16 |
| Blood transfusion before 1995 | ||
| No | 80·48 (48·73, 94·71) | 37 |
| Does not know/remember | 7·93 (1·55, 32·04) | 4 |
| Did not answer | 11·58 (2·13, 44·03) | 5 |
Includes surrounding urban municipalities.
Includes only 20- to 49-year-olds.
Discussion
This paper aimed to estimate the national prevalence of anti-HCV antibodies and HCV RNA in Mexico. We found that the prevalence of serum anti-HCV antibodies was 0·38% across the whole population, being 0·59% among those 50 and older and 0·23% in those aged 20 to 49 years; in the latter group, the prevalence of active infection was 14·9%.
Around the world, the prevalence of hepatitis C ranges from 0·5 to 2·3% depending on the region.16 The United States reported a prevalence of 1·7% between 2013 and 2016.17 The prevalence in Latin America is very heterogeneous and ranges from 0·9% in Brazil to 2·0% in Costa Rica, Ecuador, and others.18 Our results showed that in Mexico, adults aged 20 years and older presented a prevalence of 0·38% of anti-HCV antibodies. The highest prevalence was found among participants aged 50 years of age and older (0·59%). This is expected since blood transfusion was the main source of infection until 1993 when widespread screening for hepatitis C virus in blood donors was implemented in Mexico.19 In people aged 20 to 49 years, we observed a prevalence of 0.23%, which represents a 0·07 percentage point reduction in the prevalence compared to 2012 (0·30%); however, the confidence intervals for 2018 and 2012 for this age group overlap.6
In Mexico, hepatitis C antibodies continue to be higher in men compared to women (0·49, 95% CI 0·24, 0·59, vs. 0·29%, 95% CI 0·14, 0·63, respectively). We found a higher male to female ratio compared with the results in 2012 (5·6 in 2018 and 4·5 in 2012).6 This could be explained by changes in the patterns of risk behaviours in men.20 In addition, we found that the use of condoms in the last sexual intercourse was associated with a higher prevalence of anti-HCV antibodies. Since this is a cross-sectional survey, it is likely that this finding is due to reverse causality, and that people who know that they are HCV positive take extra precautions to reduce contagion of their sexual partners; among people unaware of their HCV status, counselling for other sexually transmitted infection could lead to increased condom use.
Comparisons with other reports are difficult because of the sample framework. Most studies available use convenience samples that are not nationally representative and are prone to self-selection. Selection bias could overrepresent populations with higher seroprevalence compared to the general population. A recent meta-analysis in Mexico included studies from 2008 to 2019 and estimated a pooled prevalence of 1·58% (95%CI 1·52, 1·64) in hospital out-patients and 0·64% (95%CI 0·63, 0·64) in blood donors, yet they observed high levels of heterogeneity (97%), signalling to the inclusion of groups at different levels of HCV risk.7
The presence of anti-HCV antibodies in serum provides no information about active infection. It has been reported that antibodies can remain in serum for up to 10 years after infection has been cleared.21 Thus, RNA must be traced to determine an active infection of hepatitis C.22 We found that 14·9% of those with anti-HCV antibodies were positive to viral RNA, compared to 35·7% in ENSANUT 2000.23 This apparent reduction could be explained by ageing and survival. No active cases were observed in people aged 60 years and older, although this group constituted more than 50% of seropositive cases. Yet, the largest proportion of RNA-positive cases was observed in people aged 40 to 49 years with 44·25% and people aged 50 to 59 with 35·37%, followed by lower proportions at younger ages.
While the very small number of RNA-positive cases precludes any meaningful conclusion, it is likely that chronic HCV infection is resulting in a high lethality, leading to few active cases being observed in those over 60 years of age. The proportion of RNA-positive cases in Mexico is low compared to developed countries such as the United States, where 57·5% of active infection was reported among people with anti-HCV antibodies in serum from 2013 to 2016.17 However, this finding should be interpreted with caution, considering that the design of the ENSANUT is representative of the non-institutionalized civilian population and, therefore, many key groups are not represented. Also, for this study, the sample size was calculated to estimate the prevalence of anti-HCV antibodies but not of HCV RNA which could lead to imprecision.
From all the people identified with an active hepatitis C infection, none of them reported having received a diagnosis. This is of great concern since not being aware of their hepatitis C status turns them into an active source of dissemination of the infection. We found that the urban population had a higher prevalence of antibodies, yet, no active infections in Mexico City were observed. The lack of cases in Mexico City could reflect better access to preventive strategies, diagnosis and treatment, given that it is the only city with two specialized clinics for the diagnosis and treatment of sexually transmitted infections, and that it concentrates 33·3% of the country's tertiary care centres.24 Among active cases, 93% did not wear a condom in their last sexual encounter—contrary to the higher antibody prevalence in those who wore a condom—which might reflect high-risk sexual behaviours, consistent with known risk-factors for men.25
The present study has some limitations that should be addressed. First, the sample for this survey was not calculated to be representative of high-risk groups and the real prevalence of hepatitis C could be underestimated. Specific subgroups known to be at a higher risk of HCV are not represented, which could underestimate the true national prevalence. Previous reports in Mexico have reported a higher prevalence in persons who are imprisoned (3·3%),26 living with HIV (12·1%)27, and among intravenous drug users (94·6%).20 However, this nationally representative sample allowed us to compare the 2018 estimates with those of 2012, which followed the same methodology and sampling procedures. Second, the study lacks detailed information about some important practices that have been previously linked to HCV transmissions, such as drug injection, unsafe tattoos, and high-risk sexual practices, which limits the information available for public health decision-making for key groups. Nevertheless, this study determined the Anti-HCV antibodies and HCV RNA in serum, which provides much more robust results compared to studies relying on self-reported testing and diagnosis.
The prevalence of anti-HCV antibodies has remained stable in the Mexican population. Our results could help identify subpopulations with a high prevalence of past infection and potentially in need ot treatment for active HCV infection. Mexico is potentially on track to accomplish the elimination goal of the SDG Agenda by 2030, yet, prevention, detection and treatment efforts need to be strengthened through a more comprehensive and targeted national program.
Contributors
MC: Formal analysis, Visualization, Writing of original draft. TBG: Methodology, Formal analysis, Visualization, Writing review & editing. DVP: Formal analysis verification, Writing review & editing. MRM: Methodology, Formal analysis verification, Writing review & editing. MCMB: Writing review & editing. EGP: Writing review & editing. RFS: Validation, Writing review & editing. DK: Writing review & editing. CAA: Writing review & editing. ELP: Writing review & editing. TSL: Conceptualization, Methodology, Supervision, and Writing review & editing. All authors approved the version to be published.
Funding
This study was funded in part by the National Institute of Statistics and Geography (INEGI) of Mexico and by the National Institute of Public Health (INSP) [CIEE/1807].
Data sharing statement
Data collected with HCV results and sociodemographic characteristics without participant identifiers can be available from the corresponding author upon reasonable request.
Declaration of interests
None declared
Acknowledgments
To all the staff of the National Institute of Statistics and Geography and the Evaluation and Survey Research Center who performed the field work. Thank you to Sergio Rivera at the National Institute of Medical Sciences and Nutrition Salvador Zubirán's Central Laboratory in Mexico City for technical and analytical support. We are thankful to Lianca Sartoris Ayala for technical editing of the manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lana.2021.100165.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; 2017. Global hepatitis report 2017.https://apps.who.int/iris/handle/10665/255016 Licence: CC BY-NC-SA 3.0 IGO [accessed 20 October 2021] [Google Scholar]
- 2.World Health Organization . 2010. Sixty-third World Health Assembly closes after passing multiple resolutions.https://www.who.int/news/item/11-12-2010-sixty-third-world-health-assembly-closes-after-passing-multiple-resolutions [accessed 16 June 2021] [Google Scholar]
- 3.World Health Organization . 2015. UN Sustainable Development Summit.https://apps.who.int/mediacentre/events/meetings/2015/un-sustainable-development-summit/en/index.html 2015 [accessed 20 March 2020] [Google Scholar]
- 4.World Health Organization . 2016. Global Health Sector Strategy on Viral Hepatitis 2016-2021. Towards ending viral hepatitis.https://apps.who.int/iris/handle/10665/246177 [accessed 20 March 2020] [Google Scholar]
- 5.Action plan for the health sector response to viral hepatitis in the WHO European Region. http://www.euro.who.int/__data/assets/pdf_file/0017/318320/European-action-plan-HS-viral-hepatitis.pdf?ua=1; [accessed 13 May 2020].
- 6.Gutiérrez JP, Sucilla-Pérez H, Conde-González CJ, Izazola JA, Romero-Martínez M, Hernández-Ávila M. Decrease of HCV seroprevalence in Mexico: results from the National Health and Nutrition Survey 2012. Salud Publica Mex. 2016;58:25–32. doi: 10.21149/spm.v58i1.7664. [DOI] [PubMed] [Google Scholar]
- 7.Sedeño-Monge V, Laguna-Meraz S, Santos-López G, et al. A comprehensive update of the status of hepatitis C virus (HCV) infection in Mexico-A systematic review and meta-analysis (2008-2019) Ann Hepatol. 2021;20 doi: 10.1016/j.aohep.2020.100292. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Figueroa S, Ceballos-Salgado E, Santillán-Arreygue L, Miranda-García M, Rubio-Lezama M, Garduño-García JJ. Risk factors associated with hepatitis C virus infection in an urban population of the State of Mexico. Arch Virol. 2012;157(2):329–332. doi: 10.1007/s00705-011-1149-y. [DOI] [PubMed] [Google Scholar]
- 9.Sosa-Jurado F, Santos-López G, Guzmán-Flores B, et al. Hepatitis C virus infection in blood donors from the state of Puebla, Mexico. Virol J. 2010;7:18. doi: 10.1186/1743-422X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sectoral Health Program. Specific Action Program. Prevention, diagnosis and treatment of hepatitis C 2016. https://www.gob.mx/cms/uploads/attachment/file/327907/SS_2016_PAE_Hepatitis_C.pdf; [accessed 20 March 2020].
- 11.General Health Council. Seventh update of the 2017 edition of the basic frame and medication catalogue. http://www.csg.gob.mx/descargas/pdf/priorizacion/cuadro-basico/med/actualizaciones/2018/7a_Act_Ed_2017_CByCM_12.septiembre.2018.pdf; [accessed 13 May 2020].
- 12.Secretary of Health. Press release 150. https://www.gob.mx/salud/prensa/150-mexico-lanza-el-primer-programa-nacional-de-eliminacion-de-la-hepatitis-c-del-continente-americano [accessed 27 October 2021] [Spanish]
- 13.Romero-Martínez M, Shamah-Levy T, Vielma-Orozco E, Heredia-Hernández O, Mojica-Cuevas-Nasu L, Rivera-Dommarco J. National Health and Nutrition Survey 2018-19: methodology and perspectives. Salud Publica Mex. 2019;61:917–923. doi: 10.21149/11095. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Geneva, Switzerland; 2010. WHO guidelines on drawing blood: best practices in phlebotomy.https://www.euro.who.int/__data/assets/pdf_file/0005/268790/WHO-guidelines-on-drawing-blood-best-practices-in-phlebotomy-Eng.pdf [PubMed] [Google Scholar]
- 15.Cochran WG. 3rd ed. Wiley; New York: 1977. Sampling Techniques. [Google Scholar]
- 16.World Health Organization . 2020. Hepatitis C.https://www.who.int/es/news-room/fact-sheets/detail/hepatitis-c [accessed 16 June 2021] [Google Scholar]
- 17.Hofmeister MG, Resenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69:1020–1031. doi: 10.1002/hep.30297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabo SM, Bibby M, Yuan Y, et al. The epidemiologic burden of hepatitis C virus infection in Latin America. Ann Hepatol. 2012;11(5):623–635. doi: 10.1016/J.JVAL.2011.08.1655. [DOI] [PubMed] [Google Scholar]
- 19.NORMA Oficial Mexicana NOM-003-SSA2-1993. Para la disposición de sangre humana y sus componentes con fines terapéuticos. http://www.salud.gob.mx/unidades/cdi/nom/003ssa23.html [accessed 16 June 2021].
- 20.White EF, Garfein RS, Brouwer KC, et al. Prevalence of hepatitis C virus and HIV infection among injection drug users in two Mexican cities bordering the U.S. Salud Publica Mex. 2007;49(3):165–172. doi: 10.1590/s0036-36342007000300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyoda H, Kumada T, Kiriyama S, et al. Changes in hepatitis C virus (HCV) antibody status in patients with chronic hepatitis C after eradication of HCV infection by interferon therapy. Clin Infect Dis. 2005;40(6):e49–e54. doi: 10.1086/428128. [DOI] [PubMed] [Google Scholar]
- 22.Alter MJ, Kuhnert WL, Finelli L, Centers forDisease Control and Prevention Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52(RR-3):1–13. doi: 10.1086/428128. [DOI] [PubMed] [Google Scholar]
- 23.Valdespino JL, Conde-González CJ, Olaiz-Fernández G, et al. Seroprevalencia de la hepatitis C en adultos de México: ¿un problema de salud pública emergente? Salud Publica Mex. 2007;49(Suppl: 3):395–403. [Google Scholar]
- 24.Catálogo CLUES. http://www.dgis.salud.gob.mx/contenidos/intercambio/clues_gobmx.html [Accessed 29 October 2021]
- 25.Webster DP, Klenerman P, Dusheiko GM., Hepatitis C. Lancet. 2015;385(9973):1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverman-Renata O, Serván-Mori E, McCoy SI, Larney S, Bautista-Arredondo S. Hepatitis C antibody prevalence among Mexico City prisoners injecting legal and illegal substances. Drug Alcohol Depend. 2017;181:140–145. doi: 10.1016/j.drugalcdep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Rivas-Estilla AM, Ramírez-Valles E, Martínez-Hernández R, et al. Hepatitis C virus infection among HIV-1 infected individuals from northern Mexico. Hepatol Res. 2007;37(5):311–316. doi: 10.1111/j.1872-034X.2007.00035.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.