Abstract
Background:
Leisure-time physical activity(LTPA) is associated with a reduced risk of breast cancer, but this has less been investigated by cancer subtypes in Africans living in Sub-Saharan Africa(SSA). We examined the associations between LTPA and breast cancer including its subtypes in Nigerian women and explored the effect modification of body size on such associations.
Methods:
The sample included 508 newly diagnosed primary invasive breast cancer cases and 892 controls from the Nigerian Integrative Epidemiology of Breast Cancer(NIBBLE) Study. Immunohistochemical(IHC) analysis was available for 294 cases. Total metabolic equivalents(METs) per hour/week of LTPA were calculated and divided by quartiles(Q1 <3.75, Q2:3.75–6.69, Q3:6.70–14.74, Q4:14.75 ≤). We applied logistic regressions to estimate the adjusted Odds Ratios(ORs) between LTPA and breast cancer and by its molecular subtypes and whether age-adjusted associations are modified by BMI.
Results:
The mean age(Mean±SD) of cases vs. controls(45.5 ± 11.1vs.40.1 ± 9.0) was higher, and the mean total METs hour/week was higher in controls vs. cases(11.9 ± 14.9vs.8.3 ± 11.1,p-value<0.001). Overall, 43.2%(N = 127/294) were classified as HRP, and 41.8%(N = 123/294) as TNBC. Women in the higher LTPA quartiles(Q3-Q4) vs. Q1 had lower odds of having breast cancer(ORQ4vs.Q1=0.51,95%CI:0.35–0.74) and TNBC(ORQ4vs.Q1=0.51, 95%CI:0.27–0.96), but not HRP(ORQ4vs.Q1=0.61,95%CI:0.34–1.09) after adjusting for age, age at first menarche, body size, breastfeeding, menopausal, parity, contraceptives, demographics, alcohol, smoking, and physical activity at home and work. Lastly, LTPA and its age-adjusted association with breast cancer was more pronounced in women with BMI< 30 vs. BMI 30 +.
Conclusions:
LTPA may reduce the risk of breast cancer, especially TNBC, which is the more aggressive and prevalent molecular subtype of breast cancer in SSA.
Keywords: Breast cancer, Triple-negative, Leisure-time physical activity, Nigeria, Sub-Saharan Africa
1. Introduction
The incidence of breast cancer in Sub-Saharan Africa(SSA) is rising, and it is the most common cancer in Nigerian women [1]. In Nigeria, which constitutes nearly 52% of the population of West Africa and 16.7% of all Africans, breast cancer incidence increased by approximately 25% per decade from the estimated age-standardized incidence rate(ASR) of 13.7/100,000 in 1960–1969–41.7/100,000 in 2018 [2]. In 2020, the estimated amount of newly diagnosed breast cancers in Nigeria was 28,380 which is 39.4% of all new cancers in Nigerian women and 15.2% of all new breast cancer cases in Africa [1,3].
Several factors are responsible for the rising rates of breast cancer in SSA. These include increased life expectancy thereby increasing the number of women growing into cancer-bearing old age, reduced risk of death from competing causes such as infections, social-economic development [4], lifestyle changes including older age at first birth, reduced parity, and reduced duration of breastfeeding, as well as a higher prevalence of obesity and physical inactivity [1,4–9]. In Abuja, an urban city in Nigeria, a survey reported that most Nigerian women (74%) were overweight or obese [10].
Furthermore, the prevalence of physical inactivity in SSA has increased recently as the population transitioned from a predominantly rural and agrarian culture to more developed urban, socioeconomic systems [4–6]. In Nigeria, more than 80% of urbanized adult women are physically inactive and do not meet the World Health Organization’s (WHO) criteria for minimum levels of leisure time physical activity needed to maintain a healthy lifestyle. This places Nigerian women at a higher risk for chronic diseases including breast cancer [5,6,11–14].
Leisure time physical activity (LTPA) can be associated with a reduced risk of breast cancer through several biological mechanisms. Women with high levels of LTPA have lower serum estradiol and higher sex hormone-binding globulin levels regardless of obesity [15,16]. Exercise may influence breast cancer risk by inducing a systemic anti-inflammatory effect, which may be mediated through a reduction in visceral fat mass [16–18]. Acute physical activity is associated with oxidative stress, human adaptation to repeated exercise leads to the development of a protective anti-oxidant effect associated with reduced cancer progression and metastasis [16,19]. In addition, physical activity reduces insulin resistance and circulating leptin and insulin levels, while increasing adiponectin, Insulin-like growth factor binding protein (IGFBP)− 1, and IGFBP-3 levels [18–22]. These influence the associations between the insulin pathway and breast cancer development and progression [12,17,18,23–26].
Recent studies also show that LTPA is associated with various molecular subtypes of breast cancer [27–29]. In a matched case-control study among 698 pairs of Spanish women, those who report adherence to international physical activity recommendations entail a significant decrease in the risk for all pathologic breast cancer subtypes(e.g., hormone receptor-positive(HRP) and human epidermal growth factor 2(HER2)+ tumors) [27]. In the European Prospective Investigation into Cancer and Nutrition(EPIC) study, during 11.6 years of median follow-up, moderate to high levels of total physical activity reduced the risk for breast cancer by 8–13%, and for combined recreational and household physical activity(active vs. not active), the strongest association was observed for HRP with adjusted hazard ratios(HR)= 0.84%, and 95% confidence interval(CI):0.74–0.96)(p-trend=0.02) [29].
In SSA, however, only a few studies examined the relationships between LTPA and breast cancer risk including its molecular subtypes in African women [30–32], and to the best of our knowledge, no study has focused on triple-negative breast cancer(TNBC), the more aggressive and prevalent molecular subtype of breast cancer in SSA [30,31].
Since LTPA is one of the potentially modifiable risk factors for reducing the risk of breast cancer, studies of its prevalence and association with breast cancer risk are likely to be informative and contributory to public policy [33]. Therefore, this study aimed to examine the associations between LTPA and breast cancer risks as well as with its molecular subtypes e.g., HRP and TNBC and to explore whether such association is modified by body size in Nigerian women.
2. Materials and Methods
2.1. Study design and setting
We studied women who enrolled in the Nigerian Integrative Epidemiology of Breast Cancer(NIBBLE) Study, a case-control study of female breast cancer. The study recruited participants at six government hospitals in Nigeria, five of whom are located in Abuja(% of recruited participants by hospital)(National Hospital(24.1%), University of Abuja Teaching Hospital Gwagwalada(42.3%), Asokoro District Hospital (12.1%), Garki Hospital(5.7%) and Wuse General Hospital(1.7%)) and the sixth hospital, the University of Nigeria Teaching Hospital(14.1%), in Enugu, between 1/2014–7/2016. The details of the study design and setting have been previously published [2].
2.2. Participants
Overall, 508 newly diagnosed patients with primary invasive breast cancer aged > 25 years were identified at their first clinic visit. Research nurses informed potential participants about the study and obtained informed consent from most of them(94.0%). Age-matched hospital-based controls(N = 892) who did not have cancer or endocrine diseases and were within ± 5 years of the age of specific breast cancer patients enrolled within one month in the same hospital(Fig. 1). Research nurses conducted face-to-face interviews in medical clinics for controls(98.5%) as well as for cases(80.6%) or in medical wards(19.4%) in English (70.6%) or local Nigerian language(29.4%) according to the patient’s preference.
Fig. 1.
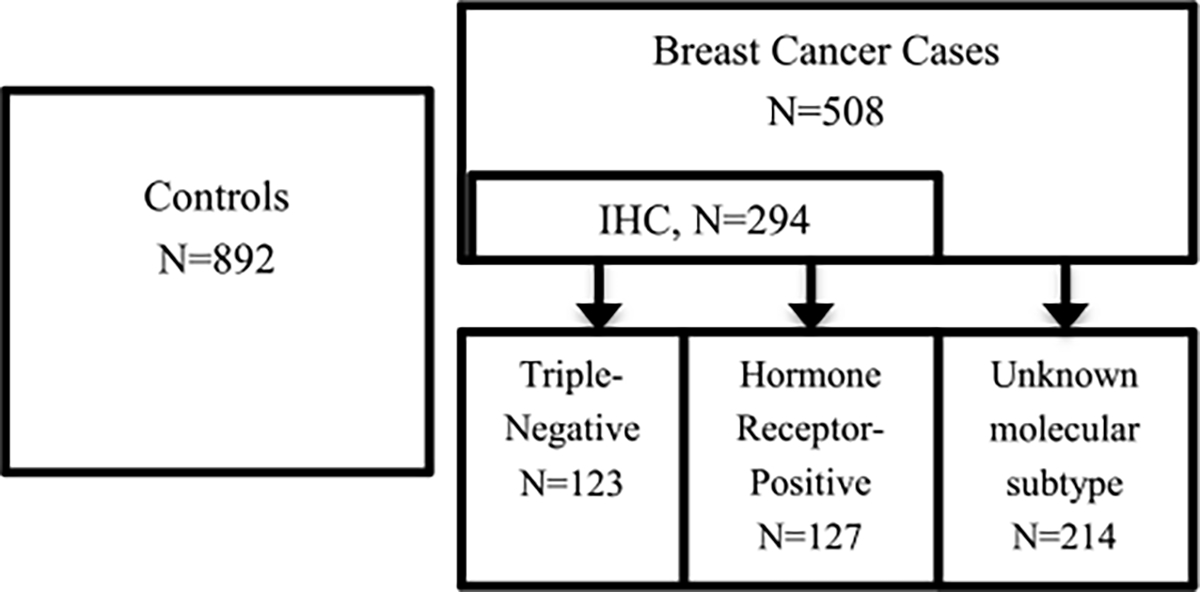
Flowchart describing the study samples by controls, breast cancer cases, and its molecular subtypes, the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
2.3. Breast cancer ascertainment
Needle core biopsies were performed using Bard Magnum Biopsy Gun®. Breast specimens were fixed in 10% neutral buffered formalin and processed within 48 h of fixation with a minimum fixation time of 8 h in Leica® automatic tissue processors at the African Collaborative Center for Microbiome and Genomics Research(ACCME) Laboratory at the Institute of Human Virology, Nigeria.
2.3.1. Histology
Sections of Paraffin-embedded blocks were cut at 3–4 μm and stained with hematoxylin and eosin stains. Histological features were classified according to 2003 WHO classification of breast diseases and graded using the Nottingham modification of the Bloom-Richardson grading [35]. The 508 cases had final histologic confirmation of breast cancer and were included in the final study sample [2].
2.3.2. Immunohistochemistry(IHC)
Histologically confirmed invasive breast tumors were stained by immunohistochemical techniques using the Thermo Scientific Lab Vision primary antibodies(clones ER-SP1;PR-SP2;Her2-SP3) and Thermo Scientific™ Ultra Vision™ Quanto HRP DAB detection kit according to the manufacturer’s recommended protocol. More details on the IHC procedures are provided in the supplement(S1) [36,37]. IHC was done specifically for this study using a more rigorous and standardized approach [2]. However, we planned to perform IHC for all participants in the NIBBLE study but since in some cases the core tissue biopsies were too small the IHC was not feasible.
2.3.3. Ascertainment of breast cancer molecular subtypes
We classified breast cancer subtypes using combinations of the IHC markers as follows(a) HRP were tumors that had positive estrogen(ER) or progesterone(PR) tests and(b) TNBC were tumors that lacked all 3 markers [38].
2.4. Primary exposure
For the LTPA assessment, we used a modification of the Untied States (U.S.) Nurses’ Health Study(NHS) II physical activity questionnaire [4]. The questionnaire measures the average amount of time spent per week on moderate and vigorous leisure-time activities. Participants reported the average time per week spent on each of the following activities, in the past year: walking, hiking, jogging, running, bicycling, dancing, playing tennis, soccer, squash, golf, swimming, aerobics, weightlifting or resistance exercise. We calculated participants’ metabolic equivalents (METs)-hour/week of total LTPA by multiplying the number of hours per week of each activity with its corresponding MET values and then summarized all the MET values [39]. The final METs score was used to create two categories of LTPA: those who met the WHO recommendations of at least 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic, or an equivalent combination vs. those who did not [34]. In addition, we created categories of LTPA in quartiles of METs(Q1 <3.75, Q2:3.75–6.69, Q3:6.70–14.74, Q4:14.75 ≤) based on the LTPA distribution among controls.
2.5. Covariates
We collected information on age, levels of education completed, marital status, occupation, PA at work and home, smoking experience, alcohol use, age at menarche, parity, ever use of oral contraceptives, menopausal status, and breastfeeding experience of more than one month. Body Mass Index(BMI) kg/m2 was categorized into < 25, 25–29.99, ≥ 30) and Waist-Hip Ratio(WHR) into ≤ 0.85, and > 0.85. Extreme values of WHR < 0.7/ > 1.6 or BMI < 10 kg/m2/ > 50 kg/m2 were excluded(N = 41) [40]. To compute socioeconomic status, we calculated the ‘wealth index’ using the following variables - house ownership and type of house owned(e.g. home, apartment, house, or duplex); source of drinking water(e.g. from outside, well, borehole, piped or bottled); type of cooking fuel; use of a separate room for cooking; type of toilet; and ownership of household goods including car and refrigerator. We used Principal Component Analysis(PCA) with varimax rotation to compute factor scores based on the sum of responses to these variables weighted by their factor loading. We used the first component in the PCA that explained(35%) of the variations in the data, to generate a wealth index [41]. The wealth index variable was used to classify participants into low socioeconomic status(<40%), middle (40–70%) and high(>70%).
2.6. Statistical analysis
To examine bivariate associations between independent variables, primary exposure(LTPA) by cases(Total cases, N = 508; HRP, N = 127; TNBC, N = 123; Cases with unknown subtype, N = 214) vs. controls (N = 892), we implemented Pearson’s chi-square(X2) for categorical variables and Student’s t-test for continuous variables. For the multivariable analysis, logistic regression models were constructed while selecting independent variables with a p-value < 0.20 in the bivariate analysis to develop full models. To test the significance of the primary coefficients(non-zero slope) between LTPA and breast cancer(Total, HRP, TNBC, Unknown subtype, separately), we used the Wald test statistic and the 95% confidence interval(95% CI). Wald tests were also used to remove covariates demonstrating the least significant association with total and molecular subtypes of breast cancer to eventually create the most parsimonious models. Lastly, we constructed an interaction term between LTPA and BMI to explore the effect modification of body size has on the age-adjusted association between LTPA and breast cancer. Multiple imputation technique was used to impute missing values of the independent variables after conducting missing completely at random test (MCAR) (p-value= 0.579). We present the crude and adjusted odds ratios(ORs) and 95%CIs of LTPA and PA recommendations with total breast cancer cases and by its subtypes e.g., HRP and TNBC, and unknown subtype. A type I error(α) level of 0.05 was considered as significant for testing the study’s hypotheses. All analyses were performed using STATA SE version 15.1(College Station, Texas).
3. Results
Overall, 57.9 % of the total breast cancer cases(N = 294/508) had ER, PR, and HER2 test results, and were classified as HRP(43.2%, N = 127/294) and TNBC(41.8%,N = 123/294), and 42.1 % (N = 214/508) as unknown subtype(Fig. 1). There was no difference in the wealth index between breast cancer cases with vs. without IHC(p-value >0.425) (data not shown).
The characteristics of the study population by total cases of breast cancer, HRP, and TNBC compared to controls are shown in Table 1. Women with breast cancer were older(45.5 ± 11.1) vs. controls(40.1 ± 9.0) regardless of the molecular subtypes. In addition, breast cancer cases vs. controls were more likely to have lower educational attainment, non-professional jobs and be either separated, divorced, or widowed as well as they were more likely to be postmenopausal and have high parity. Breast cancer cases were similar to controls with regards to the age at first menstrual period, use of oral contraceptives, breastfeeding experiences, alcohol use, and smoking status.
Table 1.
Descriptive characteristics of the study population by breast cancer cases and its molecular subtypes compared to controls, the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
| Breast Cancer Cases |
|||||||
|---|---|---|---|---|---|---|---|
| N = 1400 | Controls N = 892 N (%) / Mean ± SD |
Total Cases N = 508 N (%) / Mean ± SD |
p-value a | Hormone Receptor Positive (HRP) (ER+/PR+) N = 127 N (%) / Mean ± SD |
p-value a | Triple-Negative (TNBC) (ER−, PR−, HER2−) N = 123 N (%) / Mean ± SD |
p-value a |
|
| |||||||
| Age, years | 40.1 ± 9.0 | 45.5 ± 11.1 | < 0.001 | 45.5 ± 8.9 | < 0.001 | 46.7 ± 12.6 | < 0.001 |
| Age at first menstrual period, years | 14.4 ± 2.0 | 14.3 ± 1.7 | 0.450 | 14.3 ± 1.6 | 0.713 | 14.2 ± 1.7 | 0.231 |
| Education | < 0.001 | < 0.001 | < 0.001 | ||||
| Elementary≤ | 136(15.2) | 146(28.7) | 39(30.7) | 42(34.2) | |||
| Completed HS | 239(26.8) | 103(20.3) | 18(14.2) | 21(17.1) | |||
| Post HS no University | 209(23.4) | 125(24.6) | 37(29.1) | 29(23.6) | |||
| Completed University | 304(34.1) | 115(22.6) | 29(22.8) | 29(23.6) | |||
| Missing | 4(0.5) | 19(3.7) | 4(3.2) | 2(1.5) | |||
| Marital status | < 0.001 | 0.017 | < 0.001 | ||||
| Married | 633(71.0) | 351(69,1) | 91(71.7) | 88(71.6) | |||
| Single | 157(17.6) | 54(10.6) | 11(8.6) | 10(8.1) | |||
| Separated/Divorced/Widowed | 100(11.2) | 84(16.6) | 21(16.5) | 23(18.7) | |||
| Missing | 2(0.2) | 19(3.7) | 4(3.2) | 2(1.6) | |||
| Occupation | < 0.001 | < 0.001 | < 0.001 | ||||
| Self-Employed | 97(10.9) | 135(26.6) | 29(22.8) | 42(34.2) | |||
| Unskilled Manual | 483(54.2) | 203(40.0) | 49(38.6) | 46(37.4) | |||
| Skilled Manual | 150(16.8) | 116(22.8) | 32(25.2) | 28(22.8) | |||
| Professional/Executive | 127(14.2) | 24(4.7) | 6(4.7) | 3(2.4) | |||
| Missing | 35(3.9) | 30(5.9) | 11(8.7) | 4(3.2) | |||
| Wealth Index | 0.001 | 0.011 | 0.543 | ||||
| Low | 356(39.9) | 190(37.4) | 45(35.4) | 55(44.7) | |||
| Middle | 232(26.0) | 171(33.7) | 47(37.1) | 42(34.2) | |||
| High | 293(32.9) | 123(24.2) | 29(22.8) | 22(17.9) | |||
| Missing | 11(1.2) | 24(4.7) | 6(4.7) | 4(3.3) | |||
| Ever used oral contraceptives | 0.127 | 0.138 | 0.154 | ||||
| Yes | 298(33.4) | 144(28.4) | 33(26.0) | 33(26.8) | |||
| No | 584(65.5) | 340(66.9) | 89(70.0) | 88(71.6) | |||
| Missing | 10(1.1) | 24(4.7) | 5(4.0) | 2(1.6) | |||
| Parity | 0.009 | 0.110 | 0.074 | ||||
| 0 | 117(13.1) | 50(9.8) | 10(7.9) | 14(11.4) | |||
| 1–2 | 204(22.9) | 86(16.9) | 21(16.5) | 25(20.3) | |||
| 3–5 | 409(45.9) | 237(46.7) | 62(48.8) | 48(39.0) | |||
| ≥ 6 | 155(17.4) | 111(21.9) | 28(22.1) | 33(26.8) | |||
| Missing | 7(0.8) | 24(4.7) | 6(4.7) | 3(2.5) | |||
| Menopausal status | < 0.001 | < 0.001 | < 0.001 | ||||
| Postmenopausal | 179(20.1) | 185(36.4) | 42(33.1) | 51(41.6) | |||
| Premenopausal | 708(79.4) | 304(59.8) | 81(63.8) | 70(56.9) | |||
| Missing | 5(0.5) | 19(3.8) | 2(2.1) | 2(1.5) | |||
| Ever breastfed more than one month | 0.332 | 0.404 | 0.653 | ||||
| Yes | 677(75.9) | 386(76.0) | 100(78.7) | 95(77.2) | |||
| No | 191(21.4) | 95(18.7) | 23(18.1) | 24(19.5) | |||
| Missing | 24(2.7) | 27(5.3) | 4(3.2) | 4(3.3) | |||
| BMI kg/m2 | 0.096 | 0.808 | 0.272 | ||||
| < 25 | 240(26.9) | 154(30.3) | 35(27.6) | 31(25.2) | |||
| 25–29.99 | 305(34.2) | 171(33.7) | 38(29.9) | 45(36.6) | |||
| ≥ 30 | 324(36.3) | 153(30.1) | 45(35.4) | 44(35.8) | |||
| Missing | 23(2.6) | 30(5.9) | 9(7.1) | 2(2.4) | |||
| WHR | 0.004 | 0.095 | 0.565 | ||||
| High, > 0.85 | 558(62.6) | 347(68.4) | 86(67.7) | 80(65.0) | |||
| Normal≤ 0.85 | 299(33.5) | 130(25.5) | 32(25.2) | 38(30.9) | |||
| Missing | 35(3.9) | 31(6.1) | 9(7.1) | 5(4.1) | |||
| Smoke | 0.714 | 0.972 | 0.334 | ||||
| Yes | 7(0.8) | 3(0.6) | 1(0.7) | 0(0) | |||
| No | 879(98.5) | 485(95.5) | 121(95.3) | 121(98.4) | |||
| Missing | 6(0.7) | 20(3.9) | 5(4.0) | 2(1.6) | |||
| Alcohol use | 0.507 | 0.107 | 0.037 | ||||
| Yes | 178(20.0) | 105(20.7) | 17(13.3) | 35(28.5) | |||
| No | 708(79.4) | 381(75.0) | 105(82.7) | 86(69.9) | |||
| Missing | 6(0.6) | 22(4.3) | 5(4,0) | 2(1.6) | |||
| Total METs hour/week | 11.9 ± 14.9 | 8.3 ± 11.1 | < 0.001 | 10.6 ± 16.3 | 0.369 | 7.8 ± 9.4 | 0.003 |
| Physical activity at home (Standing hour/week) | 0.051 | 0.025 | 0.093 | ||||
| 0–5 | 291(32.6) | 191(37.6) | 30(23.6) | 48(39.0) | |||
| 6–10 | 323(36.2) | 164(32.3) | 40(31.6) | 47(38.2) | |||
| ≥ 10 | 273(30.6) | 131(25.8) | 52(40.9) | 26(21.2) | |||
| Missing | 5(0.6) | 22(4.3) | 5(3.9) | 2(1.6) | |||
| Physical activity at work (Standing hour/week) | < 0.001 | 0.208 | 0.007 | ||||
| 0–5 | 185(20.7) | 156(30.7) | 34(28.8) | 40(32.5) | |||
| 6–10 | 276(30.9) | 158(31.1) | 33(26.0) | 36(29.3) | |||
| ≥ 10 | 426(47.8) | 173(34.1) | 55(43.3) | 45(36.6) | |||
| Missing | 5(0.6) | 21(4.1) | 5(3.9) | 2(1.6) | |||
| Leisure-time physical activity (meet the WHO recommendations) | < 0.001 | 0.134 | < 0.001 | ||||
| Physical active | 246(27.7) | 84(16.6) | 28(22.1) | 19(15.4) | |||
| Physical inactive | 616(69.1) | 374(73.6) | 93(73.2) | 97(78.9) | |||
| Missing | 30(3.2) | 50(9.8) | 6(4.7) | 7(5.7) | |||
| Leisure-time physical activity by METs h/w | < 0.001 | 0.032 | < 0.001 | ||||
| < 3.75 | 208(23.3) | 202(39.8) | 48(37.8) | 56(45.5) | |||
| 3.75–6.69 | 222(24.9) | 100(19.7) | 28(22.0) | 26(21.1) | |||
| 6.70–14.74 | 216(24.2) | 80(15.7) | 20(15.8) | 15(12.2) | |||
| 14.75 ≤ | 216(24.2) | 76(15.0) | 25(19.7) | 19(15.5) | |||
| Missing | 30(3.4) | 50(9.8) | 6(4.7) | 7(5.7) | |||
BMI, Body Mass Index; HS, High School; METs, Metabolic Equivalents; SD, Standard Deviation; WHR, Waist-Hip Ratio; h, hour; w, week.
cases(Total, hormone receptor positive, triple-negative) are compared to controls.
Prevalence of abdominal fat measured by WHR(>0.85) was higher in women with breast cancer(68.4 vs.62.6%,p-value=0.004); but it did not differ across its molecular subtypes, e.g., TNBC(p-value=0.565). However, the prevalence of obesity measured by BMI 30 + was marginally lower in cases vs. controls (30.1 vs.36.3%, p-value=0.096), but not across subtypes.
The most common LTPAs were walking, dancing, jogging/running, and hiking(Fig. 2). Women in the control vs. case groups had higher mean total METs hour/week(11.9 ± 14.9 vs.8.3 ± 11.1,p-value<0.001) and vs. TNBC(11.9 ± 14.9 vs.7.8 ± 9.4,p-value=0.003) but not vs. HRP (11.9 ± 14.9 vs.10.6 ± 16.3,p-value=0.369). Women with breast cancer vs. controls were more likely to be physically inactive(METs<3.75) (39.8 vs.27.4%,p-value<0.001) and were less likely to follow the PA WHO recommendations and be more physically inactive(73.6 vs.69.1%, p-value<0.001)(Table 1).
Fig. 2.
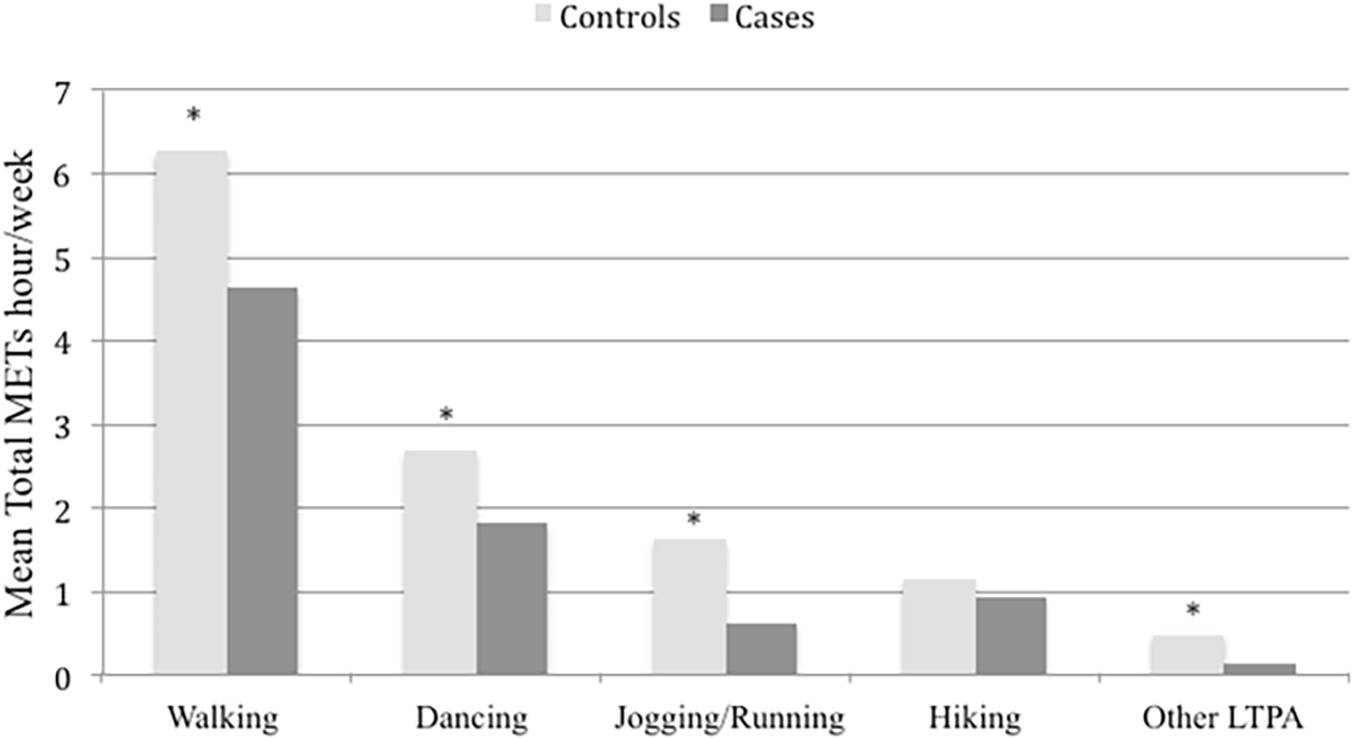
Mean of total metabolic equivalents (METs) per hour/week by types of leisure-time physical activity(LTPA) while comparing breast cancer cases vs. controls, the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
There was no age difference between LTPA categories(p-value=0.396), as well as no parity difference(p = 0.091) and menopausal status(p = 0.157). However, women at the Q4 vs.Q1 of LTPA were more likely to have unskilled manual jobs(69.9 vs.39.0%,p-value<0.001), low wealth index(46.3 vs. 30.4%,p-value=0.005), were less likely to be obese(35.9 vs.46.8%,p-value=0.004), and be more physically active at work(61.6 vs.43.3%,p-value<0.001) (Table 2).
Table 2.
Leisure-time physical activity by selected study variables among women in the control group. The Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
| Leisure-Time Physical Activity (LTPA) by METs h/wa |
|||||
|---|---|---|---|---|---|
| < 3.75 N = 208 N (%) /Mean ± SD |
3.75–6.69 N = 222 | 6.70–14.74 N = 216 | 14.75 ≤ N = 216 | p-value | |
|
| |||||
| Age, years | 39.9 ± 9.8 | 39.7 ± 9.3 | 41.0 ± 8.4 | 40.1 ± 8.6 | 0.396 |
| Occupation | < 0.001 | ||||
| Self-Employed | 45(22.6) | 21(9.7) | 18(8.7) | 9(4.4) | |
| Unskilled Manual | 78(39.0) | 142(65.7) | 105(50.5) | 144(69.9) | |
| Skilled Manual | 36(18.0) | 30(14.8) | 46(22.1) | 30(14.6) | |
| Professional/Executive | 41(20.4) | 21(9.7) | 39(18.7) | 23(11.1) | |
| Wealth Index | 0.005 | ||||
| Low | 63(30.4) | 101(45.9) | 82(38.3) | 99(46.3) | |
| Middle | 57(27.5) | 58(26.4) | 57(26.6) | 57(26.6) | |
| High | 87(42.5) | 61(27.7) | 75(35.1) | 58(27.1) | |
| Parity | 0.091 | ||||
| 0 | 32(15.5) | 34(15.3) | 22(10.2) | 28(13.1) | |
| 1–2 | 51(24.8) | 52(23.4) | 52(24.1) | 42(19.6) | |
| 3–5 | 99(48.1) | 100(45.1) | 102(47.2) | 92(43.0) | |
| ≥ 6 | 24(11.6) | 36(16.2) | 40(18.5) | 52(24.3) | |
| Menopausal status | 0.157 | ||||
| Postmenopausal | 39(18.8) | 36(16.3) | 47(21.9) | 53(24.5) | |
| Premenopausal | 169(81.2) | 185(83.7) | 168(78.1) | 163(75.5) | |
| BMI kg/m2 | 0.004 | ||||
| < 25 | 38(18.9) | 79(36.1) | 59(27.3) | 57(27.3) | |
| 25–29.99 | 69(34.3) | 68(31.1) | 80(37.0) | 77(38.8) | |
| ≥ 30 | 94(46.8) | 72(32.8) | 77(35.7) | 75(35.9) | |
| Physical activity at home(Standing hour/week) | 0.016 | ||||
| 0–5 | 59(28.4) | 85(38.3) | 82(38.0) | 53(24.7) | |
| 6–10 | 77(37.0) | 81(36.5) | 73(33.8) | 87(40.5) | |
| ≥ 10 | 72(34.6) | 56(25.2) | 61(28.2) | 75(34.8) | |
| Physical activity at work(Standing hour/week) | < 0.001 | ||||
| 0–5 | 68(32.7) | 42(19.0) | 37(17.2) | 29(14.4) | |
| 6–10 | 50(24.0) | 90(40.5) | 75(34.9) | 54(25.0) | |
| ≥ 10 | 90(43.3) | 90(40.5) | 103(47.9) | 133(61.6) | |
BMI, Body Mass Index; METs, Metabolic Equivalents, SD, Standard Deviation; h, hour; w, week.
Complete case analysis.
The crude and adjusted multivariable models showed that there is a significant inverse association between LTPA (quartiles, WHO recommendations) and the risk for total breast cancer (Table 3). Compared to the lowest LTPA quartile(Q1) women in the higher quartiles(Q3-Q4) had 49% lower odds of having breast cancer (ORQ4vs.Q1=0.51,95% CI:0.35–0.74). Also, women who met the WHO recommendations had 37% lower odds of having breast cancer than those who did not (OR=0.63, 95%CI:0.47–0.85). The significant findings included adjustment for age, age at first menstrual period, WHR, BMI, breastfeeding experience, menopausal status, parity, use of oral contraceptives, wealth index, occupation, marital status, education, alcohol use, smoking status, and physical activity at home and at work.
Table 3.
Crude and adjusted multivariable models of leisure-time physical activity and risk of total breast cancer cases in the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
| Breast Cancer |
|||
|---|---|---|---|
| Crude Model | Multivariable Modela | ||
| OR (95% CI) | OR (95% CI) | ||
|
| |||
| Leisure-time physical activityb (meet the WHO recommendations) | Case/Controlc | ||
| Physical inactive | 374/616 | 1.00 | 1.00 |
| Physical active | 84/246 | 0.56 (0.42–0.74) | 0.63 (0.47–0.85) |
| P-value | < 0.001 | 0.003 | |
| Leisure-time physical activity by METs h/w d | |||
| < 3.75 | 202/208 | 1.00 | 1.00 |
| 3.75–6.69 | 100/222 | 0.68 (0.50–0.91) | 0.77 (0.55–1.07) |
| 6.70–14.74 | 80/216 | 0.44 (0.32–0.61) | 0.51 (0.35–0.72) |
| 14.75 ≤ | 76/216 | 0.42 (0.30–0.58) | 0.51 (0.35–0.74) |
| P-trend | < 0.001 | < 0.001 | |
BMI, Body Mass Index; CI, Confidence Interval; METs, Metabolic Equivalents; OR, Odds Ratio; WHR, Waist-Hip Ratio; h, hour; w, week.
Models were adjusted for age, age at first menstrual period, WHR, BMI, breastfeeding experience, menopausal status, parity, use of oral contraceptives, wealth index, occupation, marital status, education, alcohol use, smoking status, physical activity at home and physical activity at work.
Reference category- leisure-time physical inactive, those who did not meet the WHO recommendations for physical activity.
Multiple imputation was done for variables with missing values.
Reference category- leisure-time physical activity of < 3.75 METs h/w.
By breast cancer molecular subtypes, women in LTPA (Q3-Q4) vs. (Q1) had 49–60% lower odds of having TNBC (ORQ4vs.Q1=0.51, 95% CI:0.27–0.96). Women who met the PA WHO recommendations vs. did not, had 44% lower odds of having TNBC (OR=0.56, 95% CI:0.33–0.98) (Table 4). LTPA Q3 vs. Q1 was only associated with HRP(ORQ3vs.Q1=0.48,95%CI:0.26–0.87) but not with LTPA Q4 vs. Q1 (ORQ4vs.Q1=0.61,95%CI:0.26–1.09) and not with WHO recommendations (OR=0.80, 95% CI:0.49–1.29) after adjustment for covariates, (Table 4). Among cases with unknown molecular subtypes (Table S2, supplement), the final models showed reduced risks of breast cancer by 41–73% associated with LTPA > 3.75 METs vs. below and reduced risks by 60% related with WHO recommendations vs. did not.
Table 4.
Crude and adjusted multivariable models of leisure-time physical activity and risk of breast cancer molecular subtypes(HRP, TNBC) in the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
| HRP/Controla | Hormone Receptor Positive (HRP) (ER+/PR+) |
TNBC/Controla | Triple-Negative (TNBC) (ER−, PR−, HER2−) |
|||
|---|---|---|---|---|---|---|
| Crude Model OR (95% CI) | Multivariable Modelb OR (95% CI) | Crude Model OR (95% CI) | Multivariable Modelb OR (95% CI) | |||
|
| ||||||
| Leisure-time physical activityc (meet the WHO recommendations) | ||||||
| Physical inactive | 93/616 | 1.00 | 1.00 | 97/616 | 1.00 | 1.00 |
| Physical active | 28/246 | 0.75(0.48–1.17) | 0.80(0.49–1.29) | 19/247 | 0.49(0.29–0.82) | 0.56(0.33–0.98) |
| P-trend | 0.205 | 0.351 | 0.006 | 0.041 | ||
| Leisure-time physical activity by METs h/w d | ||||||
| < 3.75 | 48/208 | 1.00 | 1.00 | 56/208 | 1.00 | 1.00 |
| 3.75–6.69 | 28/222 | 0.68(0.42–1.11) | 0.74(0.43–1.26) | 26/222 | 0.63(0.39–1.01) | 0.83(0.48–1.42) |
| 6.70–14.74 | 20/216 | 0.44(0.25–0.77) | 0.48(0.26–0.87) | 15/216 | 0.29(0.16–0.54) | 0.40(0.21–0.77) |
| 14.75 ≤ | 25/216 | 0.56(0.32–0.93) | 0.61(0.34–1.09) | 19/216 | 0.37(0.21–0.65) | 0.51(0.27–0.96) |
| P-trend | 0.011 | 0.037 | < 0.001 | 0.006 | ||
BMI, Body Mass Index; CI, Confidence Interval; ER, Estrogen Receptor; HER2, Human Epidermal Growth Factor-2; HRP, Hormone Receptor Positive; METs, Metabolic Equivalents; OR, Odds Ratio; PR, Progesterone Receptor; TNBC, Triple-Negative; WHR, Waist-Hip Ratio; h, hour; w, week.
Multiple imputation was done for variables with missing values.
Models were adjusted for age, age at first menstrual period, WHR, BMI, breastfeeding experience, menopausal status, parity, use of oral contraceptives, wealth index, occupation, marital status, education, alcohol use, smoking status, physical activity at home and physical activity at work.
Reference category-leisure-time physical inactivity, did not meet the WHO recommendations for physical activity.
Reference category-leisure-time physical activity of < 3.75 METs h/w.
Lastly, the effect modification of body size e.g., BMI has on the target association wax explored (Figs. 3 and 4). LTPA was associated with breast cancer only among women with BMI< 30 and not among women with BMI 30 + (p-value=0.043). By breast cancer molecular subtypes, the age-adjusted interaction term between LTPA and BMI was marginally significant in HRP (p-values=0.072) and TNBC (p-value= 0.156).
Fig. 3.
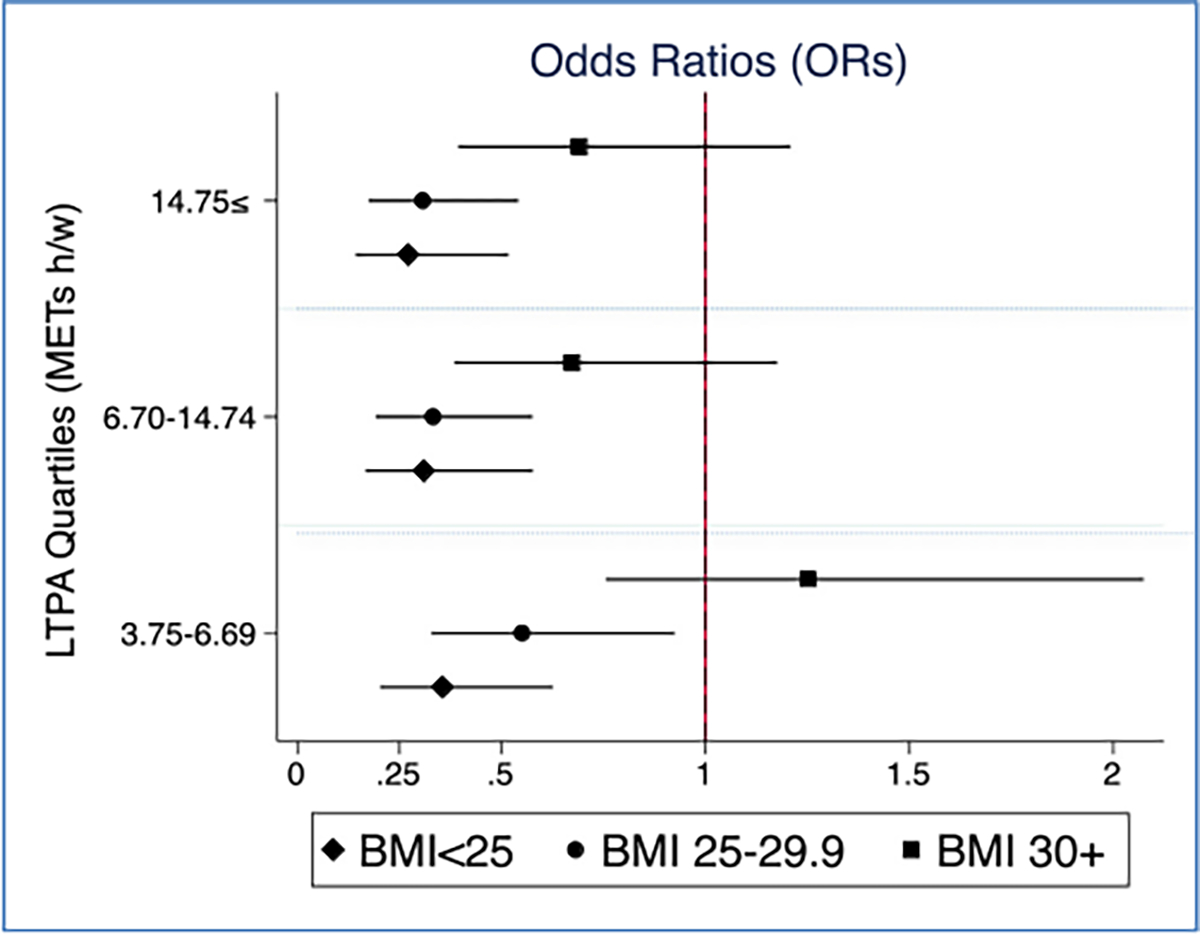
Age- adjusted ORs between LTPA (METs hour/week) and total breast cancer stratified by BMI, the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
Fig. 4.
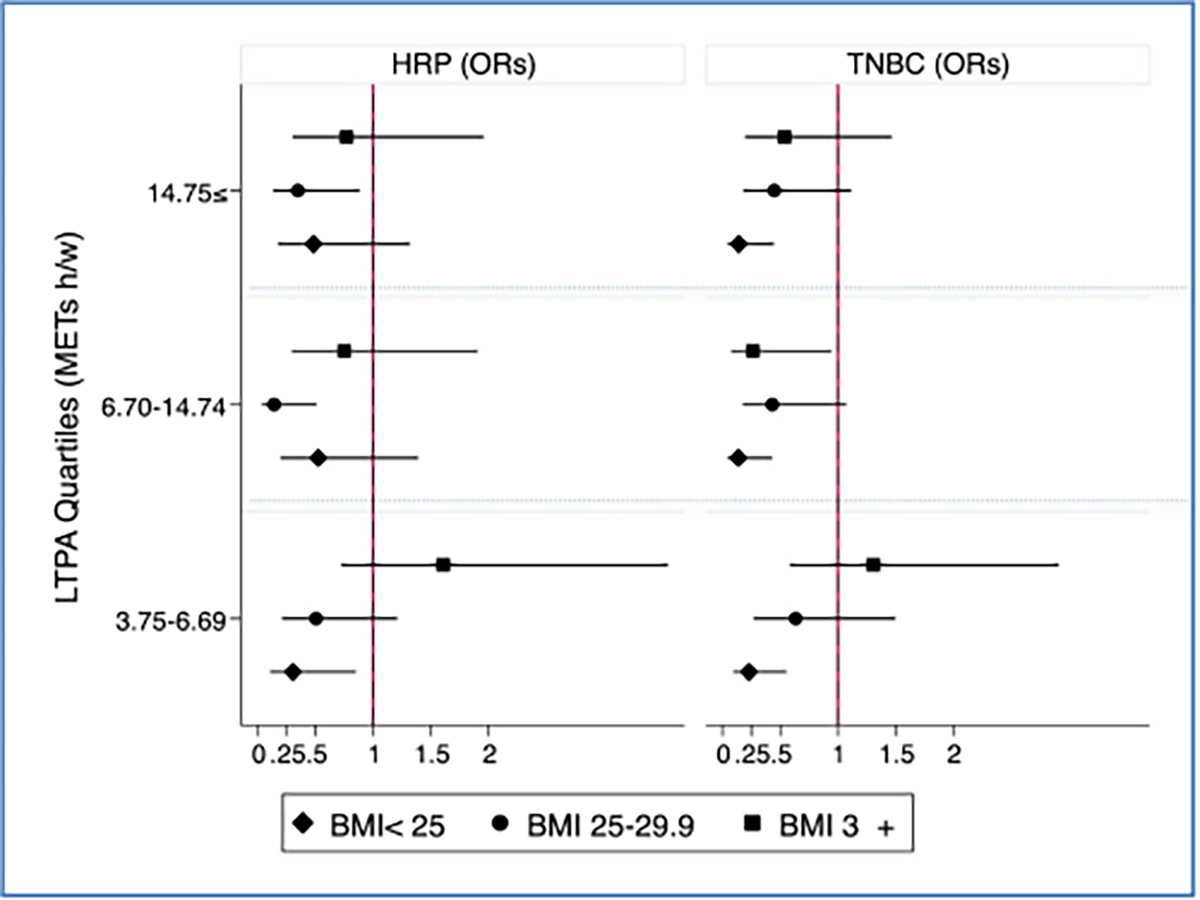
Age- adjusted ORs between LTPA (METs hour/week) and breast cancer subtypes, HRP and TNBC, stratified by BMI, the Nigerian Integrative Epidemiology of Breast Cancer Study(NIBBLE), years 2014–2016.
4. Discussion
In this case-control study of 1400 women utilizing data from the NIBBLE Study, we confirmed the inverse association between higher LTPA and reduced risk of breast cancer in African women. Specifically, the study showed that LTPA of > 6.70 METs hour per week or following the WHO recommendations is associated with a reduction of up to 49% in the odds of having breast cancer among Nigerian women. The study also showed that the target associations of LTPA varied between molecular subtypes, e.g., HRP, and TNBC, which indicate hormone-related mechanisms.
Our result is consistent with findings from other breast cancer studies on African women [11–13]. For example, a multi-country case-control study of 558 cases and 1014 controls from Nigeria, Cameroon, and Uganda who completed culturally tailored physical activity questionnaires showed that physical activity was significantly associated with up to 60% risk reduction in breast cancer [11]. Another case-control study conducted in Tunisia among 800 participants aged 25–75 years showed significant risk reductions of 58–73% in breast cancer between the highest vs. the lowest level of lifetime history of physical activity [13].
In the current study, the inverse association of LTPA (e.g., quartiles and WHO PA recommendations) was more pronounced in women with TNBC and less so in women with HRP. Examining TNBC in relation with LTPA is the first study in African women and similar to findings in other populations [14,18,27–29,42,43]. For example, a multi-center population-based case-control study of young women in the U.S. showed a 27% reduction in the risk of TNBC [44]. As opposed to our finings regarding the relationship between LTPA and HRP, a study utilizing data from the Women’s Health Initiative examined the baseline recreational physical activity and the risk of breast cancer subtypes, eight years later. Women in the highest recreational physical activity category had a significantly reduced risk of triple-negative and estrogen receptor-positive breast cancer compared to women who reported no recreational physical activity [19].
Our study also explored the role of BMI in physical activity’s association with breast cancer. Only in non-obese women i.e., BMI< 30, higher LTPA was associated with a reduced risk for breast cancer. This points to a potential mechanism linking LTPA to breast cancer regardless to its effect on weight loss, another cancer-relevant risk factor [10,45]. In obese women i.e., BMI 30 +, however, no such association has been observed suggesting that a higher level of LTPA may be needed to see a decrease in cancer incidence or with a combination of weight loss. Overall, increasing LTPA among Nigerian may be an effective strategy since the burden of cancer attributed to overweight and obesity in Nigeria is relatively small [10]. Further research is needed to examine the mechanism by which LTPA is linked to breast cancer beyond its effect on weight loss in African women [14].
In the current study, the majority of the participants(69.1–73.6%) did not meet the PA WHO recommendations of minimum levels of physical activity and this is similar to the findings from our previous study of LTPA among urbanized Nigerians [5]. Compared with the global average, where only 1 in 4 adult does not meet the WHO recommendation, the prevalence of physical inactivity in urbanized Nigerian women is significantly high [46]. Furthermore, the median LTPA in our overall study sample(N = 1400) was a total of 6.0 METs-hour/week, which is lower than the median activity level of 8.0 METs-hour/week found in studies done in the US and Europe [14].
In this study sample, the commonest LTPAs were walking, dancing, jogging/running, and hiking. This is similar to the findings of other studies in Nigeria [5]. In Africa, dancing most frequently occurred during religious observances, therefore intervention programs to encourage uptake of physical activity should consider approaches that enhance and promotes current, culturally relevant practices [5].
Numerous case-control and cohort studies suggest there is an overall average of 20–25% reduction in risk of breast cancer associated directly with increased physical activity [6,7,47,48]. Although our findings are similar to these previous studies, the potential risk reduction in our population is much higher after controlling for obesity, lifestyle factors, fertility covariates, other physical activities including at home and work, and socio-demographic factors. This is because of the currently high levels of physical inactivity in Nigerian women. Although the incidence of breast cancer in Nigeria is lower than in high-income countries, increasing LTPA may attain some reduction in the rising incidence of breast cancer in this population.
The study limitations include recall bias, the potential impact of breast cancer on levels of LTPA, and the use of a self-reported questionnaire [49]. Previous studies of LTPA in Nigerian women without breast cancer, however, showed similar results to our study [5]. We did not adjust for family history of breast cancer, but previous studies showed a low prevalence of this risk factor in Nigerian breast cancer patients. We also did not adjust for foods and nutrients, but we did adjust for BMI, WHR, and alcohol intake, the dietary factors most consistently associated with breast cancer risk [10,50]. The study used a modification of the U.S.(NHS) II physical activity questionnaire, which has not been extensively validated in African populations. However, the questionnaire included the option to add other activities that were not specified in the questionnaire e.g. yoga. The study covers the most common LTPAs among Nigerian women, which are walking, dancing, jogging/running, and hiking [5].
Despite these limitations, the strengths of our study include histological and IHC confirmation of breast cancer and its molecular subtypes, a large sample size with sufficient power to detect significant results, the inclusion of a broad range of well-established covariates and confounders such as fat mass, fertility factors, demographic variables, types of occupation, and other PA e.g., at work and home, as well as relative homogeneity of the study population [11,28,51].
5. Conclusions
LTPA is associated with a reduced risk for breast cancer including its molecular subtype, TNBC, which is a more aggressive and prevalent in women living in SSA. In low- and middle-income countries where the incidence of breast cancer is rising, increasing LTPA may be an effective strategy for addressing this growing public health concern.
Supplementary Material
Acknowledgments
We acknowledge the staff of the Research Department of the Institute of Human Virology, Nigeria, and the clinical sites where participants were enrolled.
Funding
The project described was supported by the Training Program in Nigeria for Non-communicable Diseases Research (TRAPING NCD, D43TW009106 from the Fogarty International Centre; the African Female Breast Cancer Epidemiology (AFBRECANE, U01HG009784), and the African Collaborative Center for Microbiome and Genomics Research grants (ACCME U54HG006947) from the NIH Office of the Director/NHGRI.
Additional support was received from the Maryland Department of Health’s Cigarette Restitution Fund, the University of Maryland Greenebaum Comprehensive Cancer Center Support Grant (P30CA134274), and the American Cancer Society Institutional Research Grant IRG-18-160-16.
The funding agencies did not play any role in data collection, analysis, or publication.
Abbreviations:
- ACCME
African Collaborative Center for Microbiome and Genomics Research
- ASCO
American Society of Clinical Oncology
- ASR
Age-Standardized incidence Rate
- BMI
Body Mass Index
- CAP
College of American Pathologists
- CI
Confidence Interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- ER
Estrogen Receptor
- HER2
Human Epidermal Growth Factor 2
- HR
Hazard Ratios
- HRP
Hormone Receptor-Positive Breast Cancer
- IHC
Immunohistochemistry
- IGFBP
Insulin-Like Growth Factor Binding Protein
- LTPA
Leisure-Time Physical Activity
- METs
Metabolic Equivalents
- NHS
Nurses’ Health Study
- NIBBLE
Nigerian Integrative Epidemiology of Breast Cancer
- MCAR
Missing Completely at Random
- OR
Odds Ratio
- PA
Physical Activity
- PCA
Principal Component Analysis
- PR
ProgesteroneReceptor
- SSA
Sub-Saharan Africa
- TNBC
Triple-Negative Breast Cancer
- U.S
Untied States
- WHO
World Health Organization
- WHR
Waist-Hip Ratio
Footnotes
Declaration of Competing Interest
CAA had the idea for the NIBBLE study, designed it, obtained funding, supervised implementation, and data management. SA contributed to the study design and data management. GB conducted all the statistical analyses, generated the first draft of the manuscript, which was revised by the co-authors, and finalized the manuscript. TY, MY, OO, OB, EE, IS, EM, IA, and BA enrolled participants, conducted biopsies, data collection, and ensured data quality. AF and BA conducted the laboratory diagnosis all authors contributed to drafting the manuscript, provided critical revisions, and approved the final draft.
Ethical approval and consent to participate
Ethical approval was obtained from the National Health Research Ethics Committee of Nigeria, Health Research Ethics Committees in each participating hospital, and the institutional ethics committees at the University of Maryland School of Medicine, Baltimore(US). All participants gave written informed consent following the Declaration of Helsinki and the Nigerian National Code for Health Research Ethics.
Credit authorship contribution statement
Clement Adebamowo: Conceptualization, Methodology, Software, Investigation, Funding acquisition. Sally N. Adebamowo: Validation, Data curation. Galya Bigman: Formal analysis, Writing – original draft preparation, Writing – review & editing. Ayo Famooto and Benjamin Achusi: Project administration. King-David Terna Yawe, Monday Yilkudi, Oluwole Olaomi, Olawale Badejo, Emmanuel Ezeome, Iliya Karniliyus Salu, Elijah Miner, Ikechukwu Anosike, and Benjamin Achusi: Investigation.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.canep.2022.102195.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, Accessed: April 18, 2022, CA Cancer J. Clin. 71 (209) (2021) 249, 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Jedy-Agba E, McCormack V, Olaomi O, Badejo W, Yilkudi M, Yawe T, et al. , Determinants of stage at diagnosis of breast cancer in Nigerian women: sociodemographic, breast cancer awareness, health care access and clinical factors, Cancer Causes Control 28 (7) (2017) 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization, Accessed: April 18, International Agency for Research on Cancer,, 2022, https://gco.iarc.fr/. [Google Scholar]
- [4].Pervaiz R, Faisal F, Breast cancer outcome in Africa is associated with socioeconomic development and health care setups, WCRJ 4 (2) (2017), e890. [Google Scholar]
- [5].Akarolo-Anthony SN, Adebamowo CA, Prevalence and correlates of leisure-time physical activity among Nigerians, BMC Public Health 14 (2014) 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brinton LA, Figueroa JD, Awuah B, Yarney J, Wiafe S, Wood SN, et al. , Breast cancer in Sub-Saharan Africa: opportunities for prevention, Breast Cancer Res. Treat. 144 (3) (2014) 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. , Physical activity and breast cancer: a systematic review, Epidemiology 18 (1) (2007) 137–157. [DOI] [PubMed] [Google Scholar]
- [8].Parkin DM, Bray F, Ferlay J, Jemal A, Cancer in Africa 2012, Cancer Epidemiol. Biomark. Prev 23 (6) (2014) 953–966. [DOI] [PubMed] [Google Scholar]
- [9].Odutola MK, Olukomogbon T, Igbinoba F, Otu TI, Ezeome E, Hassan R, Jedy-Agba E, Adebamowo SN, Cancers attributable to overweight and obesity from 2012 to 2014 in Nigeria: a population-based cancer registry study, Jun 11, Front. Oncol 9 (2019) 460, 10.3389/fonc.2019.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Akarolo-Anthony SN, Willett WC, Spiegelman D, Adebamowo CA, Obesity epidemic has emerged among Nigerians, BMC Public Health 14 (2014. 15) 455, 10.1186/1471-2458-14-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hou N, Ndom P, Jombwe J, Ogundiran T, Ademola A, Morhason-Bello I, et al. , An epidemiologic investigation of physical activity and breast cancer risk in Africa, Cancer Epidemiol. Biomark. Prev 23 (12) (2014) 2748–2756. [DOI] [PubMed] [Google Scholar]
- [12].Wu Y, Zhang D, Kang S, Physical activity and risk of breast cancer: a meta-analysis of prospective studies, Breast Cancer Res. Treat. 137 (3) (2013) 869–882. [DOI] [PubMed] [Google Scholar]
- [13].Awatef M, Olfa G, Rim C, Asma K, Kacem M, Makram H, et al. , Physical activity reduces breast cancer risk: a case-control study in Tunisia, Cancer Epidemiol 35 (6) (2011) 540–544. [DOI] [PubMed] [Google Scholar]
- [14].Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. , Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults, JAMA Intern. Med. 176 (6) (2016) 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Friedenreich CM, Physical activity and breast cancer risk: the effect of menopausal status, Exerc. Sport Sci. Rev 32 (4) (2004) 180–184. [DOI] [PubMed] [Google Scholar]
- [16].de Boer MC, Worner EA, Verlaan D, van Leeuwen PAM, The mechanisms and effects of physical activity on breast cancer, Clin. Breast Cancer 17 (4) (2017) 272–278. [DOI] [PubMed] [Google Scholar]
- [17].McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity(Silver Spring). 2006;14(9):1662–77. [DOI] [PubMed] [Google Scholar]
- [18].Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, et al. , Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer, Cancer Epidemiol. Biomark. Prev 20 (3) (2011) 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yeo S, Davidge ST, Possible beneficial effect of exercise, by reducing oxidative stress, on the incidence of preeclampsia, J. Women’S. Health Gend. -Based Med 10 (10) (2001) 983–989. [DOI] [PubMed] [Google Scholar]
- [20].Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA, The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease, Nat. Rev. Immunol. 11 (9) (2011) 607–615. [DOI] [PubMed] [Google Scholar]
- [21].Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, et al. , Plasma adiponectin concentrations and risk of incident breast cancer, J. Clin. Endocrinol. Metab. 92 (4) (2007) 1510–1516. [DOI] [PubMed] [Google Scholar]
- [22].Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB, Plasma leptin levels and risk of breast cancer in premenopausal women, Cancer Prev. Res 4 (9) (2011) 1449–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wolf I, Rubinek T, Diabetes mellitus and breast cancer. Diabetes and Cancer. Epidemiological Evidence andMolecular Links, in: Masur K, Thévenood F, Zänker KS (Eds.), Front Diabetes, Basel, Karger, 2008, pp. 97–113. [Google Scholar]
- [24].Neil-Sztramko SE, Boyle T, Milosevic E, Nugent SF, Gotay CC, Campbell KL, Does obesity modify the relationship between physical activity and breast cancer risk? Breast Cancer Res Treat. 166 (2) (2017) 367–381. [DOI] [PubMed] [Google Scholar]
- [25].McTiernan A, Mechanisms linking physical activity with cancer, Nat. Rev. Cancer 8 (3) (2008) 205–211. [DOI] [PubMed] [Google Scholar]
- [26].Hans-Olov Adami DJH, Lagiou Pagona, and Mucci Lorelei. Textbook of Cancer Epidemiology Third edition: Oxford university press; 2018. [Google Scholar]
- [27].Lope V, Martin M, Castello A, Casla S, Ruiz A, Baena-Canada JM, et al. , Physical activity and breast cancer risk by pathological subtype, Gynecol. Oncol. 144 (3) (2017) 577–585. [DOI] [PubMed] [Google Scholar]
- [28].Park B, Choi JY, Sung HK, Ahn C, Hwang Y, Jang J, et al. , Attribution to heterogeneous risk factors for breast cancer subtypes based on hormone receptor and human epidermal growth factor 2 receptor expression in Korea, Medicine 95 (14) (2016), e3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Steindorf K, Ritte R, Eomois PP, Lukanova A, Tjonneland A, Johnsen NF, et al. , Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition, Int. J. Cancer 132 (7) (2013) 1667–1678. [DOI] [PubMed] [Google Scholar]
- [30].Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, et al. , Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer, J. Clin. Oncol. 27 (27) (2009) 4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wright N, Rida P, Rakha E, Agboola A, Aneja R, Panoptic overview of triple-negative breast cancer in Nigeria: current challenges and promising global initiatives, J. Glob. Oncol. 4 (2018) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zheng Y, Walsh T, Gulsuner S, Casadei S, Lee MK, Ogundiran TO, et al. , Inherited breast cancer in Nigerian women, J. Clin. Oncol. 36 (28) (2018) 2820–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ligibel JA, Basen-Engquist K, Bea JW, Weight management and physical activity for breast cancer prevention and control, Am. Soc. Clin. Oncol. Educ. Book 39 (2019) e22–e33. [DOI] [PubMed] [Google Scholar]
- [34].Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. Accessed: April 20, 2022: https://www.ncbi.nlm.nih.gov/books/NBK305057/. [PubMed] [Google Scholar]
- [35].Christgen M, Langer F, Kreipe H, [Histological grading of breast cancer], Pathologe 37 (4) (2016) 328–336. [DOI] [PubMed] [Google Scholar]
- [36].Hammond ME, Hayes DF, Dowsett M, et al. , American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer(unabridged version), Arch. Pathol. Lab. Med. 134 (7) (2010) e48–e72, 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- [37].Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. , American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer, Arch. Pathol. Lab. Med. 131 (1) (2007) 18–43. [DOI] [PubMed] [Google Scholar]
- [38].Waks AG, Winer EP, Breast cancer treatment: a review, JAMA 321 (3) (2019) 288–300. [DOI] [PubMed] [Google Scholar]
- [39].Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. , Compendium of physical activities: an update of activity codes and MET intensities, Med Sci. Sport. Exerc 32 (9) (2000) S498–S504. [DOI] [PubMed] [Google Scholar]
- [40].Adebamowo CA, Ogundiran TO, Adenipekun AA, Oyesegun RA, Campbell OB, Akang EE, et al. , Waist-hip ratio and breast cancer risk in urbanized Nigerian women, Breast Cancer Res. 5 (2) (2003) R18–R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Filmer D, Pritchett LH, Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India, Demography 38 (1) (2001) 115–132. [DOI] [PubMed] [Google Scholar]
- [42].Ellingjord-Dale M, Vos L, Hjerkind KV, Hjartaker A, Russnes HG, Tretli S, et al. , Alcohol, physical activity, smoking, and breast cancer subtypes in a large, nested case-control study from the norwegian breast cancer screening program, Cancer Epidemiol. Biomark. Prev 26 (12) (2017) 1736–1744. [DOI] [PubMed] [Google Scholar]
- [43].Ma H, Xu X, Clague J, Lu Y, Togawa K, Wang SS, et al. , Recreational physical activity and risk of triple negative breast cancer in the California Teachers Study, Breast Cancer Res. 18 (1) (2016) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, et al. , The epidemiology of triple-negative breast cancer, including race, Cancer Causes Control 20 (7) (2009) 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM, Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention, CA Cancer J. Clin 67 (5) (2017) 378–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Global action plan on physical activity 2018–2030: more active people for a healthier world. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. Accessed: March 25, 2021: https://apps.who.int/iris/bitstream/handle/10665/272722/9789241514187-eng.pdf. [Google Scholar]
- [47].Kruk J, Lifetime physical activity and the risk of breast cancer: a case-control study, Cancer Detect Prev. 31 (1) (2007) 18–28. [DOI] [PubMed] [Google Scholar]
- [48].Lynch BM, Neilson HK, Friedenreich CM, Physical activity and breast cancer prevention, Recent Results Cancer Res 186 (2011) 13–42. [DOI] [PubMed] [Google Scholar]
- [49].Dallal CM, Brinton LA, Matthews CE, Lissowska J, Peplonska B, Hartman TJ, et al. , Accelerometer-based measures of active and sedentary behavior in relation to breast cancer risk, Breast Cancer Res. Treat. 134 (3) (2012) 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shield KD, Soerjomataram I, Rehm J, Alcohol use and breast cancer: a critical review, Alcohol Clin. Exp. Res. 40 (6) (2016) 1166–1181, 10.1111/acer.13071. Epub 2016 Apr 30. [DOI] [PubMed] [Google Scholar]
- [51].Kakugawa Y, Tada H, Kawai M, Suzuki T, Nishino Y, Kanemura S, et al. , Associations of obesity and physical activity with serum and intratumoral sex steroid hormone levels among postmenopausal women with breast cancer: analysis of paired serum and tumor tissue samples, Breast Cancer Res. Treat. 162 (1) (2017) 115–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


