Abstract
Background:
Impairment in initiating joint attention (IJA) is associated with autism spectrum disorder (ASD) in children, although it is unclear when impairments arise. Due to the early development of IJA use and late diagnosis of ASD, groups at high-risk of ASD, such as infants with an older sibling with ASD (ASIBs) and infants with fragile X syndrome (FXS), provide opportunities to study early IJA behaviours for children who are later diagnosed with ASD. This study analysed these two groups to determine if IJA use differed compared to typically developing (TD) peers at 12 months and whether IJA was associated with later ASD outcomes.
Method:
An experimental attention task was used to analyse IJA gaze shifts and gestures in the high-risk groups. Clinical best estimate diagnoses were given to each participant to compare IJA behaviours to ASD severity.
Results:
No differences in the frequency of IJA gaze shifts and gestures were found between 12-month-old ASIBs and TD controls, but infants with FXS demonstrated a significantly reduced range of IJA gaze shifts relative to TD controls. Additionally, ASD outcomes at 24 months were related to IJA use for infants with FXS at 12 months, but not infant ASIBs, though these findings were explained by differences in nonverbal cognitive development.
Conclusions:
Although previous studies have reported delays in IJA use in children with FXS and ASIBs at ages 21 and 14 months, respectively, our results suggest IJA behaviours for these high-risk groups are not distinct from TD children at 12 months. When differences were found at 12 months, they were explained by nonverbal cognitive development, particularly for infants with FXS. Differences in IJA use at 12 months in this study were too small to serve as a potential indicator of later ASD.
Keywords: Joint attention, social communication, gesture, autism, fragile X syndrome
Initiating Joint Attention in Infants with Fragile X and those at Familial High-Risk for ASD
Initiating Joint Attention: Developmental Course and Importance
Joint attention (JA) is a nonverbal social communicative tool used to direct another’s attention to an object or event using behaviours including gaze shifts or gestures (Poon, Watson, Baranek, & Poe, 2012). Infants first learn to respond to others’ JA and then begin to initiate JA (IJA) themselves using actions including three-point gaze shifts or gestures to direct attention (Cassel et al. 2007; Crais, Douglas, & Campbell, 2004; Winder, Wozniak, Parlade, & Iverson, 2013; Watson, Crais, Baranek, Dykstra, & Wilson, 2013). Acquisition and mastery of IJA as an early developmental and social communicative tool is associated with later vocabulary growth, social attention, and understanding of shared experiences with others for typically-developing (TD) children (Poon et al. 2012; Beuker, Rommelse, Donders & Buitelaar, 2013; Mundy et al. 2007). Furthermore, TD infants who use more IJA behaviours, such as showing and pointing gestures, have been found to have better communication and executive functioning skills later in development (Kuhn, Willoughby, Wilbourn, Vernon-Feagans, & Blair, 2014). These studies highlight the critical role that early emerging IJA skills have on the development of communication competency. Therefore, IJA behaviours are important to analyse in the context of disorders with social communication and language impairments, such as autism spectrum disorder (ASD). Groups known for their high-risk of developing ASD due to unknown familial risk, such as siblings of children diagnosed with ASD (ASIBs), or neurogenetic syndromes, such as fragile X syndrome (FXS) provide opportunities to examine IJA use early in development and compare that development to later ASD outcomes.
Initiating Joint Attention in Populations with ASD or at High-Risk for ASD
Non-syndromic ASD.
Autism spectrum disorder is a developmental disorder defined by deficits in social communication, restricted interests, and the presence of repetitive and stereotyped behaviours (American Psychiatric Association, 2013). ASD affects about one in 68 children (CDC, 2014). Extensive research has been conducted analysing social communicative profiles of children with ASD compared to TD peers. Veness et al. (2014) used retrospective video analyses to determine that children with ASD make fewer attempts to gain another person’s attention using gestures at 8 months of age compared to TD infants. During the second year of life, children later diagnosed with ASD use significantly less IJA gaze shifts (Ibañez, Grantz, & Messinger, 2013; Landa, Gross, Stuart, & Faherty, 2013). Compared to TD peers, infants diagnosed with ASD also show lower frequencies of IJA gestures, such as pointing and showing from 12 to 24 months (Veness et al. 2012; Watson et al. 2013). Initiating JA gesture use at 24 months has been found to be a strong predictor of later ASD diagnosis (Barbaro & Dissanayake, 2013; Veness, Prior, Eadie, Bavin, & Reilly, 2014). Overall, research suggests that there are differences in IJA behaviours in the second year of life that are linked to ASD outcomes, making them important to study in groups who can be identified early as high-risk, such as ASIBs.
Siblings of children with ASD.
Although precise genetic factors associated with non-syndromic ASD are not known, there is a strong heritability of ASD as ASIBs have over ten times higher risk of ASD than TD children without siblings diagnosed with ASD (Ozonoff et al. 2011). While some research studies consider ASIBs as a high-risk group only, others categorise ASIBs by their diagnostic outcomes (e.g., ASD, developmental delay, typical).
Some research has shown that ASIBs, regardless of diagnostic outcome, use fewer IJA behaviours than TD children at 14 to 18 months (Landa, Holman, & Garrett-Mayer, 2007; Stone, McMahon, Yoder, & Walden, 2007). However, this age range may be a developmental turning point for IJA use in ASIBs as Cassel et al. (2007) found that infant ASIBs younger than 14 months used similar amounts of IJA to their TD peers. In relation to later ASD outcomes, several studies show that infant ASIBs later diagnosed with ASD use fewer IJA gestures and gaze shifts than infant ASIBs who are not later diagnosed with ASD and TD infants at 14 to 17 months (Landa et al. 2007; Ibanez et al. 2013). Rozga et al. (2011) also found that ASIBs who were later diagnosed with ASD used fewer IJA gestures than TD infants at 12 months, whereas ASIBs who did not have a later diagnosis of ASD did not significantly differ from TD peers. The lack of consensus on whether ASIBs differ from typical development in IJA use prior to 14 months highlights a need to further analyse IJA use in this group. The present study considers both positions by investigating ASIBs as a high-risk group independent of diagnostic outcomes and also examining how their diagnostic outcomes relate to their IJA behaviours during infancy.
Fragile X syndrome.
Fragile X syndrome is the most common inheritable genetic cause of intellectual disability that affects approximately one in 3500 males (Coffee et al. 2009). The syndrome is caused by a mutation on the FMR1 gene that causes impaired production of fragile X mental retardation protein. There is high comorbidity of FXS and ASD with about 50–75% of children with FXS meeting criteria of ASD (Clifford et al. 2007; Lee, Martin, Berry-Kravis, & Losh, 2016); thus, the heightened risk of ASD for individuals with FXS compared to other genetic syndromes makes it an important group to investigate.
Prior research on social communication in children with FXS has focused on language outcomes using visual attention (Kover, McCary, Ingram, Hatton, & Roberts, 2015), preschool-aged children and adolescents rather than infants with FXS (Rogers, Hepburn, Stackhouse, & Wehner, 2003; Flenthrope & Brady, 2010), other forms of JA (Marschik et al. 2014; Hahn, Brady, McCary, Rague, & Roberts, 2017), or restricted use of gestures in general rather than focusing solely on IJA gestures (Flenthrope & Brady, 2010). To our knowledge, there are no studies focusing specifically on IJA gaze shifts in infants with FXS and only one study analysing IJA gesture use in preschoolers with FXS. Roberts, Mirrett, Anderson, Burchinal, and Neebe (2002) found that a sample of 22 preschool-aged boys with FXS showed significantly delayed development of IJA gesture use compared to TD peers. However, the study did not test whether other factors, such as nonverbal cognitive development or ASD status, were associated with the delay in gesture use. Thus, additional studies are needed to examine early IJA gaze shift and gesture use in FXS. The presence of comorbid ASD in individuals with FXS is etiologically distinct from non-syndromic groups (e.g. ASIBs), making it important to study the early social communicative profiles of children with FXS in comparison to children with non-syndromic ASD.
Present Study
There is consensus that early IJA behaviours are important in early identification efforts of ASD, a disorder with impairment in social communication (Schietecatte, Roeyers, & Warreyn, 2012; Poon et al. 2012). Although ASD can be reliably diagnosed in children at two years old, most children with ASD are diagnosed at three or four years of age (CDC, 2014; Charman & Baird, 2002; Shattuck et al. 2009). The increased risk of ASD for ASIBs and children with FXS, the delay in diagnosing ASD, and the impact of poor development of IJA on later outcomes suggests a need for refined early identification of IJA deficits and ultimately ASD. Furthermore, limited studies have analysed IJA behaviours early in development or in relation to later ASD symptomology for infants with FXS. The present study analyses IJA gaze shifts and gesture behaviours within two samples at high-risk for developing ASD, 12-month-old ASIBs and infants with FXS, compared to their TD peers. The first research question examined whether the frequencies of specific IJA behaviours at 12 months, gaze shifts and gesture use, differed between groups at high risk for ASD independent of later ASD outcomes. The second research question examined whether the frequencies of IJA gaze shifts and gestures differed between groups based on whether they were later diagnosed with ASD. The third research question examined whether there is a relationship between overall IJA use at 12 months and ASD outcomes at 24 months; gaze shifts and gestures were combined into an overall composite IJA score to answer this question.
Method
Participants
Fifty-seven 12-month-old males participated in this study. Due to differing clinical and cognitive profiles in males and females with ASD or FXS (Rinehart, Cornish, & Tonge, 2011), only males were investigated in the current study. The ASIB group (mean chronological age (CA)=12.55, SD=0.67) was comprised of 21 infants with an older sibling with a confirmed diagnosis of ASD. The FXS group (CA=12.83, SD=1.11) was comprised of 18 infants with a FXS diagnosis through genetic testing. The control group (CA=12.22, SD=0.46) was comprised of 18 TD infants with no history of developmental delays or a family history of ASD and average nonverbal cognitive abilities. Confirmation of nonverbal cognitive abilities and lack of ASD symptomology was verified through study participation. For descriptive statistics, see Table 1. All participants were recruited as a part of a larger longitudinal study at the University of South Carolina focusing on early indicators of ASD in high-risk infant populations and were seen within three months of their first birthday. Families were recruited using listservs for parents of children with FXS and families receiving services for ASD, flyers in the local area, and word of mouth.
Table 1.
Descriptive Statistics of ASIB, FXS, and TD Variables
| TD (n = 18) | ASIB (n = 21) | FXS (n = 18) | Group Differences | |
|---|---|---|---|---|
| Age (months) | ||||
| Mean | 12.22 | 12.55 | 12.83 | p = .081 |
| SD | 0.46 | 0.67 | 1.11 | |
| Nonverbal Cognition | ||||
| Mean | 56.28 | 53.76 | 35.67 | p = .000a |
| SD | 7.68 | 9.79 | 10.67 | |
| ADOS-2 CSS | ||||
| Mean | 1.50 | 4.19 | 4.95 | p = .000b |
| SD | 0.71 | 2.04 | 1.90 | |
| SES (dollars) | ||||
| Mean | 70,352 | 78,287 | 74,818 | p = .875 |
| SD | 31,426 | 41,441 | 54,891 | |
| Ethnicity (%) | ||||
| African-American | 11.1 | 4.8 | 11.1 | p = .364 |
| Caucasian | 89.9 | 95.2 | 83.3 | |
| Asian/Pacific Islander | - | - | 5.6 |
Note. TD is typically developing controls; ASIB is younger siblings of children with ASD; FXS is infants with fragile X syndrome; SD is standard deviation; ADOS-2 CSS is Autism Diagnosis Observation Scale Second Edition calibrated severity score; SES is socio-economic status
Group differences found on nonverbal cognition (FXS < ASIB/TD)
Group differences found on ADOS-2 CSS (TD < ASIB/FXS)
Measures
Laboratory Temperament Assessment Battery (Lab-TAB).
The Lab-TAB (Goldsmith & Rothbart, 1996) is a standardised assessment used to observe differences in temperament that includes a variety of tasks. The present study focused on IJA using an unstructured attention task during which the participants were given a set of button-activated noise-making keys to independently play with for three minutes while seated on their mothers’ laps. The examiner and infant’s mother were seated at the table and remained neutral. A second examiner videotaped the task for later offline behavioural coding. Although the original aim of the task was to study attention (Roberts, Hatton, Long, Anello, & Colombo, 2012; Kover et al. 2015), it was expanded for the present study to look at IJA gaze shifts and gestures.
Gaze shifts.
Initiating JA gaze shifts were defined as three-point gaze shifts to direct the examiner or parent’s attention to an object. Initiation of three-point gaze shifts began with the participant looking at the keys in the play task, then shifting gaze to make eye contact with the examiner or parent, and returning gaze to the toy keys in under two seconds (Cassel et al. 2007; Clifford & Dissanayake, 2008). Gaze shifts were coded only when contact between the infant’s eyes and either the mother’s or examiner’s eyes was visible on the video.
Gestures.
Gestures were defined using the Communication and Symbolic Behaviour Scales (CSBS; Wetherby & Prizant, 2002), a developmental measure that assesses socially communicative behaviours, as a guide for coding gestures. Gestures are hand movements or manipulation of an object to direct another person’s attention to an object. Similar to previous studies, the CSBS was used as a guide to create categories of gestures used for IJA: showing, giving, and pointing. Eye gaze was only mandatory for coding showing gestures to confirm the communicative intent of the gesture (e.g. showing versus visual examination of the toy). For definitions on the three types of IJA gestures analysed, see Table 2.
Table 2.
Definitions of IJA Gesture Categories
| Gesture Category | Definition |
|---|---|
| Show | The participant presents an object by extending it in the direction of a person. To exclude nonsocial behaviours, the gesture must be accompanied by gaze towards the person being shown the object. |
| Give | The participant hands off the toy to another person in an effort to give away the object. The participant must fully let go of the object and place it in the direction of the examiner or parent to qualify as giving. Due to setup of the task, the participant often cannot reach the examiner; therefore, the definition was adapted to include throwing or pushing the object toward the examiner. |
| Point | The participant uses his/her index finger to point at an object and direct attention to it. To exclude nonsocial behaviours, the participant could not make contact with the keys for the gesture to be considered pointing. |
Autism Diagnostic Observation Scale – Second Edition (ADOS-2).
The ADOS-2 (Lord et al. 2012) is a semi-structured assessment of social interaction and repetitive behaviours in children that calculates severity of ASD symptoms. Observations and scores from the ADOS-2 were used to inform clinical best estimate (CBE) outcomes for participants at 24 months. In addition, the calibrated severity score (CSS) was used as a scale of ASD symptom severity to determine if ASD symptom severity correlated with overall IJA use. The CSS is derived from ADOS-2 raw scores and ensures uniformity of ASD severity across ADOS-2 modules (Gotham, Pickles, & Lord, 2009).
Clinical best estimate outcome.
The CBE process mirrors the gold standard in clinical practice, where ASD diagnoses are made by a team comprised of a licensed psychology and two staff with research reliability on the ADOS-2. As opposed to a single ADOS-2 test score, all available evidence was used to determine if a participant had an outcome of ASD, including evidence form parent interviews, developmental measures, and direct observation. The CBE process is valid and reliable as determined by Lord et al. (2012). As recommended (Havdahl et al. 2016), a differential diagnostic approach was applied with a diagnosis of ASD assigned only when the data were consistent with DSM-5 criteria and another diagnosis did not better account for the symptoms (e.g., developmentally delayed versus ASD). Ten ASIBs and eight infants with FXS were diagnosed with ASD using the CBE process. For descriptive statistics on groups based on CBE outcomes, see Table 3. Additionally, CBE outcomes were utilised to exclude non-typically developing children from the TD group prior to data analyses.
Table 3.
Descriptive Statistics of TD, FXS, FXS+ASD, ASIB, and ASIB+ASD Variables
| TD (n = 18) | FXS (n = 10) | FXS+ASD (n = 8) | ASIB (n = 11) | ASIB+ASD (n = 10) | Group Differences | |
|---|---|---|---|---|---|---|
| Age (months) | ||||||
| Mean | 12.22 | 12.88 | 12.76 | 12.54 | 12.55 | p = .283 |
| SD | 0.46 | 1.41 | 0.73 | 0.76 | 0.60 | |
| Nonverbal | ||||||
| Cognition | ||||||
| Mean | 56.28 | 35.90 | 35.38 | 52.82 | 54.80 | p = .000a |
| SD | 7.68 | 10.70 | 11.35 | 11.75 | 7.58 | |
| ADOS-2 CSS | ||||||
| Mean | 1.50 | 2.80 | 7.63 | 2.82 | 5.70 | p = .000b |
| SD | 0.71 | 2.15 | 1.60 | 1.25 | 2.91 | |
| SES (dollars) | ||||||
| Mean | 70,352 | 69,888 | 81,157 | 68,331 | 87,000 | p = .878 |
| SD | 10,671 | 14,666 | 16,629 | 16,629 | 15,555 | |
| Ethnicity (%) | ||||||
| Caucasian | 88.9 | 70.0 | 100.0 | 90.9 | 100.0 | p = .119 |
| African-American | 11.1 | 20.0 | - | 9.1 | - | |
| Asian/Pacific Islander | - | 10.0 | - | - | - |
Note. TD is typically developing controls; FXS is infants with fragile X syndrome; FXS+ASD is infants with fragile X syndrome later diagnosed with ASD; ASIB is younger siblings of children with ASD; ASIB+ASD is ASIBs who are later diagnosed with ASD; ADOS-2 CSS is Autism Diagnosis Observation Scale Second Edition calibrated severity score; SES is socio-economic status
Group differences found on nonverbal cognition (TD/ASIB/ASIB+ASD > FXS, TD/ASIB/ASIB+ASD > FXS+ASD)
Group differences found on ADOS-2 CSS (TD/ASIB/FXS < FXS+ASD, TD/ASIB/FXS < ASIB+ASD, ASIB+ASD < FXS)
Mullen Scales of Early Learning (MSEL).
The MSEL (Mullen, 1995) is a standardised assessment of language, visual reception, and motor skills for young children aged birth to 68 months. The MSEL was collected at participants’ 12-month assessments. A nonverbal cognitive composite score for each participant was calculated by averaging the visual reception and fine motor domain t-scores. T-scores of 50 represent the population mean (population SD = 10) and t-scores ranging from 40 to 60 are considered average. Nonverbal cognitive composite scores were entered as a covariate during analyses to control for nonverbal cognitive differences between groups and were also used to exclude non-typically developing children from the TD group prior to data analysis (nonverbal cognition t-score < 40).
Procedure
The MSEL, Lab-TAB, and ADOS-2 measures were administered and scored by research-reliable, trained examiners as part of a larger standardised battery. Participants’ families traveled to USC for their 12-month assessments where the MSEL and Lab-TAB were administered. Examiners traveled to participants’ homes for their 24-month assessments to administer the ADOS-2. Initiating JA gaze shifts and gestures were coded using the computer-based coding software Noldus Observer XT 10.5 and a coding manual defining these behaviours. To establish coding reliability, two researchers coded videos together and then separately until a Cohen’s kappa coefficient of ≥ .80 on three consecutive videos was achieved, which has been accepted as a suitable measurement of overall interrater agreement (Warrens et al. 2014). One researcher coded all videos; a random sample of 20% of all videos were double coded by the second researcher. Cohen’s kappa coefficient of 0.90 was achieved for both codes indicating reliability of codes was maintained.
Data analysis.
Preliminary analyses were conducted to examine normality and homogeneity of residuals. Due to the relatively small sample size and observed positive skew in the IJA variables, we used nonparametric analyses to address our research questions. To answer the first and second research questions, Kruskal-Wallis tests (non-parametric analog to ANOVA with >2 groups) were conducted to evaluate differences in the distributions of frequencies of IJA gaze shifts and gestures among groups. The first question analysed differences in the three groups regardless of later ASD outcomes (infant ASIBs, infants with FXS, and TD infants) and the second question analysed differences in groups based on later ASD outcomes (i.e., TD infants, infants with FXS alone, infants with FXS and ASD [FXS+ASD], infant ASIBs without a later diagnosis of ASD, and infant ASIBs later diagnosed with ASD [ASIB+ASD]).
Additionally, Quade’s rank analysis of covariance tests (non-parametric analog to ANCOVA) were used for to analyse mean differences in the frequencies of IJA gaze shifts and gestures for both sets of groups in the first and second research questions after controlling for nonverbal cognitive skills. To answer the third research question about the relationship between overall IJA use at 12 months and later ASD outcomes, a composite IJA use score was calculated, equal to the sum of gaze shifts and gestures for each participant. Gaze shifts and gestures that occurred at the same time were counted only once in the composite IJA score. Kruskal-Wallis test and Quade’s rank analysis of covariance were conducted to analyse IJA composite scores based on diagnostic outcomes at 24 months.
Results
IJA Gaze Shifts and Gesture Use at 12 Months for High-Risk Groups
IJA Gaze Shifts.
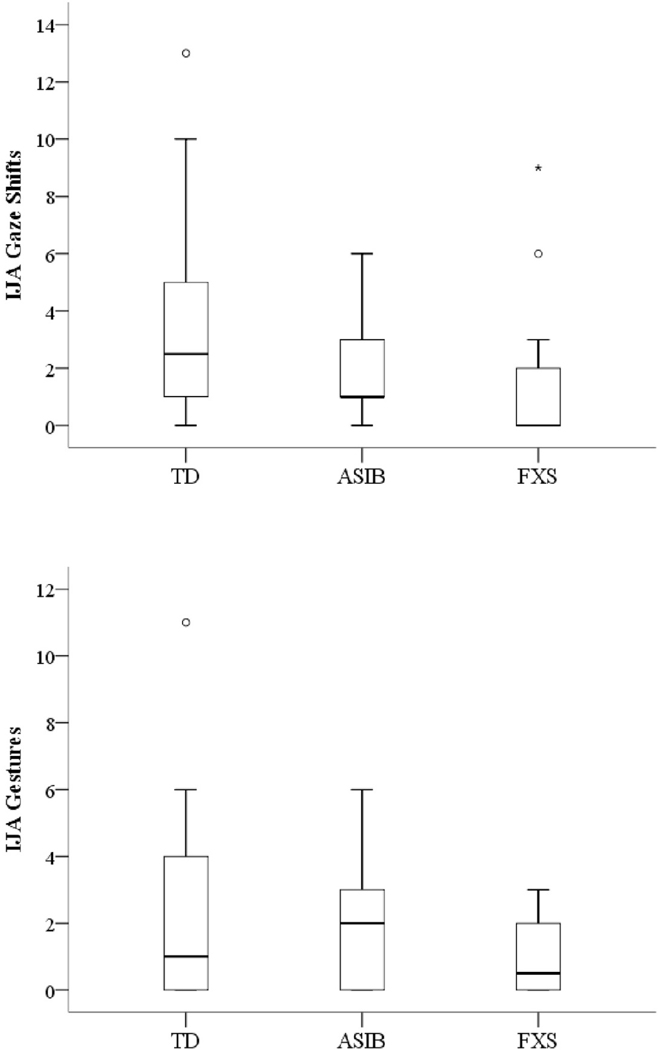
Boxplots indicating distribution of gaze shifts by the three groups (FXS, ASIBs, TD) are displayed in Figure 1, revealing positively skewed distributions for all three groups. Kruskal-Wallis results indicated group differences in the distributions of IJA gaze shifts (X2(2)=8.93, p=.011). The frequency of gaze shifts in infants with FXS was significantly lower than TD infants, U=75.5, p=.005. However, ASIBs did not significantly differ from infants with FXS, U=129, p=.080, or TD infants, U=130, p=.091. Quade’s rank analysis of covariance test indicated no significant between-group differences in frequencies of IJA gaze shifts between groups after controlling for the influence of nonverbal cognition, F(2, 54)=1.91, p=.158.
Figure 1.
Boxplots depicting distributions of IJA gaze shifts and IJA gestures for high-risk groups. * Significant outlier; Ο Outlier; TD is typically developing infants; ASIB is younger siblings of children with autism; FXS is infants with FXS.
Gesture Use.
Figure 1 displays boxplots indicating the distribution of gesture use for each group, revealing positively skewed, and largely overlapping, distributions for all three groups. The Kruskal-Wallis test indicated no significant differences between group distributions, X2(2)=3.52, p=.172, and Quade’s rank analysis of covariance revealed no significant differences after controlling for nonverbal cognition, F(2, 54)=.854, p=.431.
IJA Gaze Shifts and Gesture Use at 12 Months for Groups Subdivided by ASD Outcomes
IJA Gaze Shifts.
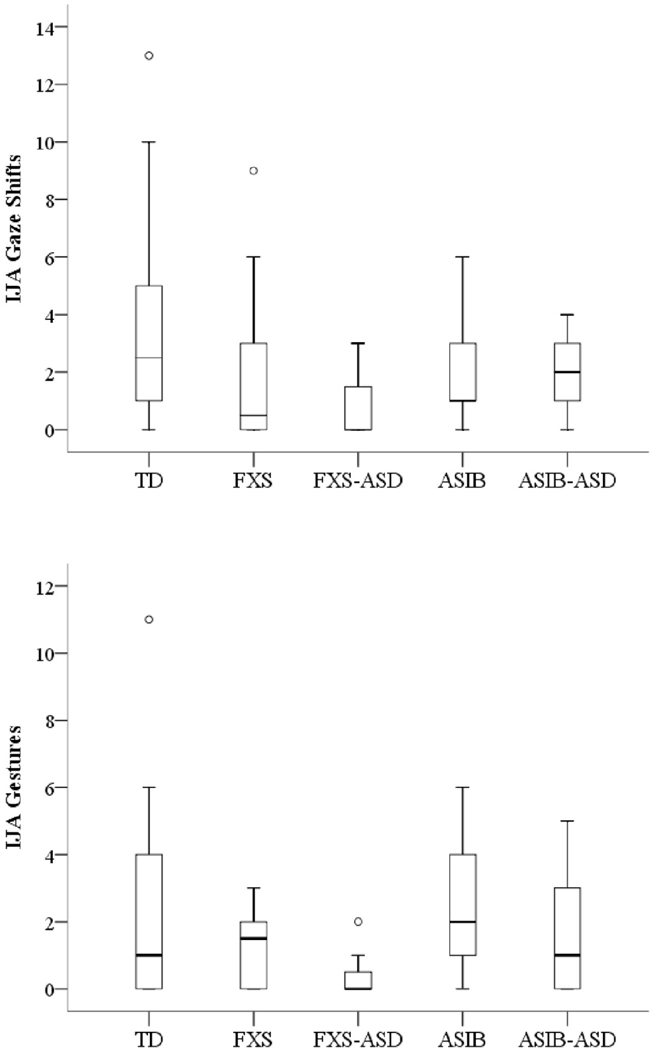
Boxplots indicating distribution of gaze shifts by the five groups subdivided by their later ASD outcomes at 24 months are displayed in Figure 2. Kruskal-Wallis results indicated group differences in the distributions of IJA gaze shifts, X2(4)=9.82, p=.044. Pairwise comparisons indicated that the frequency of gaze shifts in infants with FXS+ASD was significantly lower than TD infants, U=22.50, p=.005. Quade’s rank analysis of covariance revealed no significant differences between the groups after controlling for nonverbal cognition, F(4, 52)=1.17, p=.333.
Figure 2.
Boxplots depicting distributions of IJA gaze shifts and IJA gestures for groups subdivided by later ASD outcomes at 24 months. Ο Outlier; TD is typically developing infants; FXS is infants with FXS alone; FXS+ASD is infants with FXS later diagnosed with ASD; ASIB is younger siblings of children with autism; ASIB+ASD is infant ASIBs later diagnosed with ASD.
Gesture Use.
Figure 2 displays boxplots indicating the distribution of gesture use for the groups subdivided by their later ASD outcomes. The Kruskal-Wallis test indicated no significant differences between groups, X2(4)=7.76, p=.101 and Quade’s rank analysis of covariance revealed no significant differences after controlling for nonverbal cognition, F(4, 52)=1.59, p=.192.
Composite IJA Use at 12 Months for Groups Subdivided by ASD Outcomes
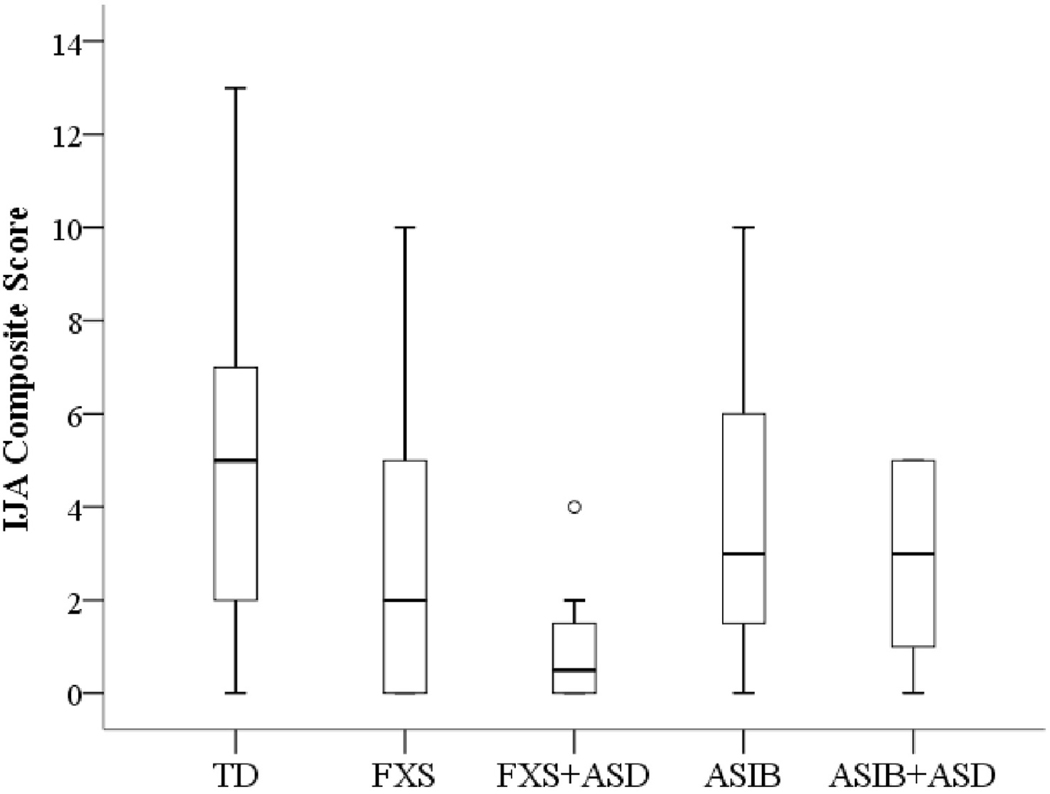
Figure 3 displays boxplots indicating distribution of IJA composite scores (equal to the sum of gaze shifts and gestures) for analysis of the five subgroups defined by ASD outcome at 24 months (FXS, FXS+ASD, ASIBs, ASIB+ASD, TD). Boxplots revealed positively skewed distributions for the FXS and FXS+ASD groups and a negatively skewed distribution for the ASIB+ASD group. The range of scores for the FXS+ASD group was particularly restricted. The distribution was more uniform for the TD and ASIB groups. A Kruskal-Wallis test indicated significant group differences in distributions of IJA composite scores (X2(4)=12.49, p=.014) across the five diagnostic outcomes at 24 months. Pairwise comparisons indicated that infants with FXS+ASD used less IJA behaviours than both TD infants,_U=15.5, p=.002, and ASIB infants, U=16, p=.019. Infants with FXS+ASD also had a lower composite IJA score than ASIB+ASD infants, U=18.5, p=.050. Quade’s rank analysis of covariance test, however, revealed no significant group differences in IJA composite scores after accounting for group differences in nonverbal cognition, F(4, 52)=2.03, p=.103.
Figure 3.
Boxplot depicting the group distributions of IJA Composite at 12 months by later ASD outcomes at 24 months. Ο Outlier; TD is typically developing infants; FXS is infants with FXS alone; FXS+ASD is infants with FXS later diagnosed with ASD; ASIB is younger siblings of children with autism; ASIB+ASD is infant ASIBs later diagnosed with ASD.
Given that IJA composite scores were significantly different depending on the presence of ASD symptoms in the FXS group, correlations were run between IJA composite scores and ADOS-2 CSS across all participants in the study (n = 57). Analyses revealed a significant correlation between IJA composite scores and ADOS-2 CSS, rs=−.294, p=.026. This finding suggests that as ADOS-2 severity scores increased, IJA composite scores decreased across groups.
Discussion
The present study used a prospective longitudinal design and examined early IJA behaviours within two etiologically-distinct samples of infants at high-risk for ASD (infant ASIBs and infants with FXS) in comparison to same-aged TD peers. First, we examined differences in specific IJA behaviours, gaze shifts and gestures across three groups including infants with FXS, ASIBs, and TD controls independent of ASD outcomes. Previous research has shown that high-risk groups for ASD generally have lower frequencies of IJA gaze shifts and gestures than TD peers starting around 15 months and continuing throughout the second year of life regardless of ASD outcomes (Goldberg et al. 2005; Cassel et al. 2007). We were interested in whether these patterns were present at 12 months of age and whether the profiles differed in two distinct groups at risk for ASD.
Our results suggest that 12-month-old infants with FXS, but not infant ASIBs, use less IJA gaze shifts in comparison to their age-matched TD peers with no differences appearing between infants with FXS and infant ASIBs. Differences between infants with FXS and TD infants appeared to be associated with nonverbal cognitive development as the groups did not differ in IJA gesture use controlling for nonverbal cognition. These findings indicate that ASIBs are not impaired relative to TD infants in IJA behaviours, and that patterns of IJA gaze shifts and gesture use do not differ between two etiologically-distinct 12-month-old groups at risk for ASD (ASIBs and FXS) when accounting for nonverbal cognitive development.
Our second research question investigated whether differences in IJA behaviours differed in these risk groups when they were further subdivided based on ASD outcomes at 24 months. Relative to the other groups, children with FXS and ASD exhibited a restricted range for both gaze shifts and gestures, but the only statistically significant difference was observed between the FXS and ASD group and the TD group for the frequency of gaze shifts. Likewise with the difference in IJA gaze shifts between the TD and FXS group in first research question, this significant difference disappears after accounting for nonverbal cognitive development.
A global measure of IJA use was also examined using an IJA composite (sum of gaze shifts and gestures) and parsing apart groups based on their later ASD outcomes at 24 months (FXS with and without ASD, ASIBs with and without ASD and TD controls). The group with FXS and ASD had the lowest median value and most restricted distribution of IJA scores contrasted to all other groups. Descriptively, infants with FXS and ASD used fewer IJA behaviours than infants with FXS alone, whereas infant ASIBs with and without ASD did not appear to differ in their IJA composite (the medians were equivalent although the range of scores observed in ASIBs was wider than that of ASIB+ASD). This finding indicates that there may be a relationship between IJA use in infants with FXS and later ASD outcome. These descriptive profiles were supported by analyses that show only the FXS and ASD group differed from any other group with a more restricted range than the TD group. However, differences based on ASD outcome in the FXS group were no longer significant after accounting for nonverbal cognitive development. Despite these differences in IJA composite scores being accounted for by nonverbal cognition, a general relationship between ASD symptom severity and IJA use was found across all of the study groups. As expected and in line with previous literature (Veness et al. 2014; Watson et al. 2013), a relationship between elevated ASD symptom severity and IJA use was found. Overall, our findings suggest the developmental trajectory of IJA and its relation to ASD outcomes throughout the first two years of life may differ between the two high-risk groups. Considering the sparse amount of research conducted on social communicative behaviours for children with FXS and ASD during infancy and early childhood, these results underscore a clear need for further exploration of IJA use in comorbid FXS and ASD.
The lack of a significant difference in IJA use between TD infants and infant ASIBs at 12 months in the current study aligns with previous literature finding that ASIBs do not use fewer IJA behaviours than their TD peers prior to 14 months of age (Cassel et al. 2007). Our results correspond with previous studies suggesting that participants’ IJA use may be appropriate to developmental expectations at 12 months, regardless of group status. In this study, ASIBs and TD infants presented overlapping distributions of IJA gaze shifts with floor effects present in both groups. Thus, at 12 months of age, ASIBs do not appear to be experiencing deficits in IJA use in this study. Findings of differences in IJA behaviour use between TD infants and ASIBs after 14 months of age may be related to a reduced floor effect in the TD group and IJA use increases for TD infants between 12 to 14 months.
The present study sought to analyse IJA use in infants with FXS at a time point in which many TD children are beginning to master IJA use. To the authors’ knowledge, this study is the first to analyse IJA behaviours in infants with FXS earlier than 21 months, and the results add to the current understanding of the FXS social communicative profile. In this study, TD infants were quite heterogeneous with regard to IJA behaviour frequency. Whereas IJA behaviours were quite frequent in some TD children, other TD children showed complete absence of IJA gaze shifts or gestures. Our results suggest that infants with FXS have a restricted distribution of IJA behaviours as a group compared to TD infants. These findings are particularly interesting, because they pinpoint a period in which one aspect of social communicative development in infants with FXS appears delayed in comparison to same-aged TD children but is within overall developmental expectations when nonverbal cognition is controlled. These findings of IJA as a generalised delay, rather than as a specific communication impairment is consistent with previous literature for preschool-aged males with FXS (Roberts et al. 2002). Future work is important to extend these initial findings with larger samples to determine if IJA is a salient marker of social communication impairments in signaling ASD in FXS.
Limitations and Future Directions.
The primary limitation of this preliminary study is its small sample size. However, the sample size is comparatively robust in relation to existing studies of infants with FXS (Kover et al. 2015; Mirrett et al. 2004). A second limitation was the Lab-TAB task used to measure frequencies of IJA behaviours at 12 months which constrained the infants’ initiated behaviours to be captured in an object-focused context. Use of the task serves both as a strength in providing a check on the generalisability of past findings and a limitation in that it is much shorter and requires more neutrality from the examiner. The findings of the present study and limited research on IJA for infants with FXS suggest it is important to investigate how the development of IJA behaviours changes over time and how those trajectories might signal risk for ASD. Future studies should include a larger sample and contrast the impact of low and high opportunity for social interaction, as well as investigate other IJA behaviours in these groups to determine if differences in these skills arise. Finally, future research could expand the range of outcomes, such as language, that IJA might predict in addition to ASD symptom severity.
Summary and Implications.
Our results suggest that IJA is still emerging in TD children at 12 months of age, and thus between-group differences in IJA may be too subtle at 12 months of age to provide discrimination of groups at high risk for ASD from TD controls. Despite finding increased ASD symptom severity related to decreased IJA use, differences in IJA use within and across the high-risk groups were accounted for by nonverbal cognitive development. More work is needed to determine whether a more sensitive measure of IJA would yield larger group differences. Such work is important to understanding the phenotypes specific to these genetically-distinct, high-risk groups as well as to the development of indices of later-emerging ASD features. Furthermore, impairment in IJA has been shown to impact later language and social outcomes (Poon et al. 2012; Mundy et al. 2007) and to indicate later ASD symptoms (Barbaro & Dissanayake, 2013; Veness et al. 2014; Ibanez et al. 2013). The ability to identify the beginning of delays in IJA would allow for earlier entry into targeted intervention to increase these skills and prevent impairment of later skills, such as language and social skills. Future research on the developmental trajectory and predictive power of IJA in the first two years for later ASD symptoms is needed to address the gap between identification of ASD and services and to provide better strategies to target problem areas and maximise positive outcomes for these groups in early development.
Footnotes
Publisher's Disclaimer: This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/jir.12539
References
- American Psychiatric Association. (2013) Diagnostic and statistical manual of mental disorders (5th ed.), Washington, DC. [Google Scholar]
- Bailey DB, Raspa M, Bishop E, & Holiday D. (2009) No change in the age of diagnosis for fragile X syndrome: findings from a national parent survey. Pediatrics, 124(2), 527–533. DOI: 10.1542/peds.2008-2992. [DOI] [PubMed] [Google Scholar]
- Barbaro J, & Dissanayake C. (2013) Early markers of autism spectrum disorders in infants and toddlers prospectively identified in the social attention and communication study. Autism: The International Journal of Research & Practice, 17(1), 64–86. DOI: 10.1177/1362361312442597. [DOI] [PubMed] [Google Scholar]
- Bates E, & Dick F. (2002) Language, gesture and the developing brain. Dev. Psychobiol, 40, 293–310. [DOI] [PubMed] [Google Scholar]
- Beuker KT, Rommelse N, Donders R, & Buitelaar JK (2013) Development of early social communication skills in the first two years of life. Infant Behaviour and Development, 36, 71–83. [DOI] [PubMed] [Google Scholar]
- Brady NC, Marquis J, Fleming K, & McLean L. (2004) Prelinguistic predictors of language growth in children with developmental disabilities. Journal of Speech, Language & Hearing Research, 47(3), 663–677. DOI: 10.1044/1092-4388(2004/051). [DOI] [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, & Buchman AC (2007) Early social and emotional communication in the infant siblings of children with autism spectrum disorders: an examination of the broad phenotype. Journal of Autism & Developmental Disorders, 37(1), 122–132. DOI: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2014) Prevalence of autism spectrum disorders among children aged 8 years – autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report (MMWR), 63(2), 1–21. [PubMed] [Google Scholar]
- Charman T., & Baird G. (2002) Practitioner review: diagnosis of autism spectrum disorder in 2-and 3-year-old children. Journal of Child Psychology & Psychiatry & Allied Disciplines, 43(3) 289. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Macari S, Powell K, DiNicola L, & Shic F. (2016) Enhanced social attention in female infant siblings at risk for autism. Journal of the American Academy of Child & Adolescent Psychiatry, 55(3), 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007) Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism & Developmental Disorders, 37(4) 738–747. DOI: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Clifford SM, & Dissanayake C. (2008) The early development of joint attention in infants with autistic disorder using home video observations and parental interview. Journal of Autism & Developmental Disorders, 38(5), 791–805. DOI: 10.1007/s10803-007-0444-7. [DOI] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, et al. (2009) Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American Journal of Human Genetics, 85(4), 503–514. DOI: 10.1016/j.ajhg.2009.09007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crais E, Douglas DD, & Campbell CC (2004) The intersection of the development of gestures and intentionality. Journal of Speech, Language & Hearing Research, 47(3), 678–694. DOI: 10.1044/1092-4388(2004/052). [DOI] [PubMed] [Google Scholar]
- Flenthrope JL, & Brady NC (2010) Relationships between early gestures and later language in children with fragile X syndrome. American Journal of Speech-Language Pathology, 19(2), 135–142. DOI: 10.1044/1058-0360(2009/09-0018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg WA, Jarvis KL, Osann K, Laulhere TM, Straub C, Thomas E, et al. (2005) Brief report: early social communication behaviours in the younger siblings of children with autism. Journal of Autism & Developmental Disorders, 35(5), 657–664. DOI: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, & Rothbart MK (1996) The laboratory temperament assessment battery, Madison, University of Wisconsin, Department of Psychology. [Google Scholar]
- Gotham K, Pickles A, & Lord C. (2009) Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism & Developmental Disorders, 39(5), 693–705. DOI: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn LJ, Brady NC, McCary L, Rague L, & Roberts JE (2017) Early social communication in infants with fragile X syndrome and infant siblings of children with autism spectrum disorder. Research in Developmental Disabilities, 71, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havdahl KA., Bal VH., Huerta M., Pickles A., Øyen A-S., Stoltenberg C., … Bishop SL. (2016). Multidimensional influences on autism symptom measures: Implications for use in etiological research. Journal of the American Academy of Child and Adolescent Psychiatry, 55(12), 1054–1063.e3. 10.1016/j.jaac.2016.09.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez LV, Grantz CJ, & Messinger DS (2013) The development of referential communication and autism symptomatology in high-risk infants’. Infancy, 18(5), 687–707. DOI: 10.1111/j.1532-7078.2012.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover ST, McCary LM, Ingram AM, Hatton DD, & Roberts JE (2015) Language development in infants and toddlers with fragile x syndrome: change over time and the role of attention. American Journal on Intellectual and Developmental Disabilities, 120(2), 125–144. DOI: 10.1352/1944-7558-120.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn LJ, Willoughby MT, Wilbourn MP, Vernon-Feagans L, & Blair CB (2014) Early communicative gestures prospectively predict language development and executive function in early childhood. Child Development, 85(5), 1898–1914. DOI: 10.1111/cdev.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, & Faherty A. (2013) Developmental trajectories in children with and without autism spectrum disorders: the first 3 years. Child Development, 84(2), 429–442. DOI: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. (2007) Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch. Gen. Psychiatry 64, 853–864. [DOI] [PubMed] [Google Scholar]
- Lee M, Martin GE, Berry-Kravis E, & Losh M. (2016). A developmental, longitudinal investigation of autism phenotypic profiles in fragile X syndrome. Journal of Neurodevelopmental Disorders, 8, 47. 10.1186/s11689-016-9179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin D. … Risi S. (2012) A multisite study of the clinical diagnosis of different autism spectrum disorders. Archives of General Psychiatry, 69(3), 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL … Guthrie W. (2012), Autism Diagnostic Observation Schedule, Second Edition, Western Psychological Services, Torrance, CA. [Google Scholar]
- Marschik PB, Bartl-Pokorny KD, Sigafoos J, Urlesberger L, Pokorny F, Didden R, … & Kaufmann WE (2014). Development of socio-communicative skills in 9-to 12-month-old individuals with fragile X syndrome. Research in developmental disabilities, 35(3), 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D., Young G., Ozonoff S., Dobkins K., Carter A., Zwaigenbaum L., …Sigman (2013). Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry, 52(3), 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrett PL, Bailey DB, Roberts JE, & Hatton DD (2004) Developmental screening and detection of developmental delays in infants and toddlers with fragile x syndrome. Journal of Developmental and Behavioural Pediatrics, 25(1), 21–27. [DOI] [PubMed] [Google Scholar]
- Mullen EM (1995) Mullen Scales of Early Learning: AGS Edition, American Guidance Service, Circle Pines, MN. [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Vaughan Van Hecke A, & Parlade MV (2007). Individual differences and the development of joint attention in infancy. Child Development, 78(3), 938–954. 10.1111/j.1467-8624.2007.01042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Delgado C, Block J, Venezia M, Hogan A, Seibert J. (2003) A manual for the abridged early social communication scales (ESCS), Coral Gables, FL, University of Miami, Psychology Department. [Google Scholar]
- National Research Council (2001) Educating children with autism, Lord C, & McGhee JP (Eds.), National Academy Press, Washington, DC. [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L. et al. (2011) Recurrence risk for autism spectrum disorders: a baby sibling research consortium study. Pediatrics, 128, e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Watson L, Baranek G, & Poe M. (2012) To what extent do joint attention, imitation, and object play behaviours in infancy predict later communication and intellectual functioning in ASD? Journal of Autism & Developmental Disorders, 42(6), 1064–1074. DOI: 10.1007/s10803-011-1349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart NJ, Cornish KM, & Tonge BJ (2011). Gender differences in neurodevelopmental disorders: autism and fragile X syndrome. Current Topics in Behavioural Neuroscience, 8, 209–229. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Anderson K, Burchinal M, & Neebe E. (2002) Early communication, symbolic behaviour, and social profiles of young males with fragile X syndrome. American Journal of Speech-Language Pathology, 11(3), 295. [Google Scholar]
- Roberts J, Hatton D, Long A, Anello V, & Colombo J. (2012) Visual attention and autistic behaviour in infants with fragile X syndrome. Journal of Autism & Developmental Disorders, 42(6), 937–946. DOI: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ., Hepburn SL., Stackhouse T., & Wehner E. (2003) Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology & Psychiatry & Allied Disciplines, 44(5), 763–781. DOI: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rozga A, Hutman T, Young GS, Rogers SJ, Ozonoff S, Dapretto M, et al. (2011) Behavioural profiles of affected and unaffected siblings of children with autism: contribution of measures of mother-infant interaction and nonverbal communication. Journal of Autism & Developmental Disorders, 41(3), 287–301. DOI: 10.1007/s10803-010-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salley B, Miller A, & Bell MA (2013) Associations between temperament and social responsiveness in young children. Infant & Child Development, 22(3), 270–288. DOI: 10.1002/icd.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietecatte I, Roeyers H, & Warreyn P. (2012) Can infants’ orientation to social stimuli predict later joint attention skills? British Journal of Developmental Psychology, 30(2), 267–282. DOI: 10.1111/j.2044-835X.2011.02039.x. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. (2009) Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. Journal of the American Academy of Child & Adolescent Psychiatry, 48(5), 474–483. DOI: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway S, Farmer C, Thurm A, Joseph L, Black D, & Golden C. (2012) The ADOS calibrated severity score: relationship to phenotypic variables and stability over time. Autism Research: Official Journal of the International Society for Autism Research, 5(4), 267–276. 10.1002/aur.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA (2007) Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Arch Peiatr Adolesc Med, 161(4), 384–390. DOI: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Veness C, Prior M, Bavin E, Eadie P, Cini E, & Reilly S. (2012) Early indicators of autism spectrum disorders at 12 and 24 months of age: A prospective, longitudinal comparative study. Autism: The International Journal of Research & Practice, 16(2), 163–177. DOI: 10.1177/1362361311399936. [DOI] [PubMed] [Google Scholar]
- Veness C, Prior M, Eadie P, Bavin E, & Reilly S. (2014) Predicting autism diagnosis by 7 years of age using parent report of infant social communication skills. Journal of Paediatrics & Child Health, 50(9), 693–700. DOI: 10.1111/jpc.12614. [DOI] [PubMed] [Google Scholar]
- Warrens MJ. (2014) New interpretations of cohen’s kappa. Journal of Mathematics, 1–9. DOI: 10.1155/2014/203907. [DOI] [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Dykstra JR, & Wilson KP (2013) Communicative gesture use in infants with and without autism: A retrospective home video study. American Journal of Speech-Language Pathology, 22(1), 25–39. DOI: 10.1044/1058-0360(2012/11-0145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A, & Prizant B. (2002) Communication and Symbolic Behaviour Scales Developmental Profile-first normed edition, Brookes Publishing, Baltimore, MD. [Google Scholar]
- Winder BM, Wozniak RH, Parlad MV, & Iverson JM (2013) Spontaneous initiation of communication in infants at low and heightened risk for autism spectrum disorders. Developmental Psychology, 49(10), 1931–1942. DOI: 10.1037/30031061. [DOI] [PMC free article] [PubMed] [Google Scholar]