Abstract
Background
Measures taken to address the COVID-19 pandemic interrupted routine diagnosis and care for breast cancer. The aim of this study was to characterize the effects of the pandemic on breast cancer care in a statewide cohort.
Patients and Methods
Using data from a large health information exchange, we retrospectively analyzed the timing of breast cancer screening, and identified a cohort of newly diagnosed patients with any stage of breast cancer to further access the information available about their surgical treatments. We compared data for four subgroups: pre-lockdown (preLD) 25 March to 16 June 2019; lockdown (LD) 23 March to 3 May 2020; reopening (RO) 4 May to 14 June 2020; and post-lockdown (postLD) 22 March to 13 June 2021.
Results
During LD and RO, screening mammograms in the cohort decreased by 96.3% and 36.2%, respectively. The overall breast cancer diagnosis and surgery volumes decreased up to 38.7%, and the median time to surgery was prolonged from 1.5 months to 2.4 for LD and 1.8 months for RO. Interestingly, higher mean DCIS diagnosis (5.0 per week vs. 3.1 per week, p < 0.05) and surgery volume (14.8 vs. 10.5, p < 0.05) were found for postLD compared with preLD, while median time to surgery was shorter (1.2 months vs. 1.5 months, p < 0.0001). However, the postLD average weekly screening and diagnostic mammogram did not fully recover to preLD levels (2055.3 vs. 2326.2, p < 0.05; 574.2 vs. 624.1, p < 0.05).
Conclusions
Breast cancer diagnosis and treatment patterns were interrupted during the lockdown and still altered 1 year after. Screening in primary care should be expanded to mitigate possible longer-term effects of these interruptions.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-023-13119-w.
After Indiana’s first case of coronavirus disease 2019 (COVID-19) was confirmed on 6 March 2020, a public health emergency was declared by the Indiana State Department of Health.1,2 With the pandemic’s rapid progression, a stay-at-home order (“lockdown”) was enforced from 25 March to 1 May 2020 (38 days) together with mitigation strategies including limiting large gatherings and taking social distancing measures recommended by the US Centers for Disease Control and Prevention and the Indiana State Department of Health. To respond to the pandemic’s unprecedented demands on health care, health care routines including cancer care were disrupted in many ways. Health care systems needed to deploy staff and capacity away from non-emergency services, such as breast cancer screening and diagnosis; and, to make beds and services available for patients with COVID-19, the federal government asked hospitals to limit non-essential, elective adult surgeries and procedures during lockdowns.3
Breast cancer is one of the most diagnosed cancers, representing 30% of female cancers in the USA.4 Screening, diagnosing, and treating breast cancer involve a series of health services, including screening and diagnostic mammograms, diagnosis consultation, surgery, and nonsurgical treatments. The timeliness of these services is crucial in the cancer care continuum. Nowadays, breast cancers are caught at earlier stages thanks to the wide adoption of early screening. Ductal carcinoma in situ (DCIS) and early-stage breast cancer (ESBC, stages I, IIA, and IIB with T2N1) account for the largest part of all newly diagnosed breast neoplastic lesions.5,6 For the majority of patients with DCIS and ESBC who are hormone receptor positive, HER-2 negative, surgery is the first definitive treatment. The time waiting for surgery after diagnosis (time to surgery) is another timeliness measure that is significantly associated with overall survival and disease-specific survival.7–9
During the pandemic lockdown, DCIS and ESBC surgeries were not considered urgent, and thus many were postponed. Many national and international professional societies published recommendations and deferral strategies to manage patients with breast cancer during the pandemic.10–13 With an option of initiating neoadjuvant endocrine therapy (NET; selective estrogen receptor modulators such as tamoxifen or aromatase inhibitors such as anastrozole, letrozole, and exemestane) for DCIS and hormone-receptor-positive (HR+) ESBC while awaiting surgery and monitoring disease progression, recommendations for the delay of surgery varied: 3–6 months by the Society of Surgical Oncology11 and 6–12 months by the American Society of Breast Surgeons and the COVID-19 Pandemic Breast Cancer Consortium.10,12 For the general population, deferring routine screening for 6–12 months until the pandemic subsided was deemed reasonable by the COVID-19 Pandemic Breast Cancer Consortium.10
However, disruptions to oncology services were reported to be a source of psychological stress for patients diagnosed with breast cancer.1 The stress was compounded by concerns about poorer cancer outcomes from care delays as well as poorer COVID-19 outcomes for patients with an active cancer. To expand on previous findings about the long-term consequences of delayed treatment and the stress burden on patients with breast cancer,8,9,14–18 it is important to understand the actual course and extent of the pandemic’s collateral damage on breast cancer diagnosis and treatment. This understanding can help guide current treatment of patients with breast cancer as well as helping prepare the health care profession for potential future disruptions of care. Although delays in breast cancer screening, diagnosis, and treatment have varied geographically given local situations and responses to COVID-19, the experience of a large cohort in one area of the USA can provide insights that will be broadly useful in breast cancer care. The aim of this study was to analyze and quantify the short-term effects of the COVID-19 pandemic on breast cancer screening, diagnosis, surgery, and other treatment in a cohort of patients in a large healthcare system.
Patients and Methods
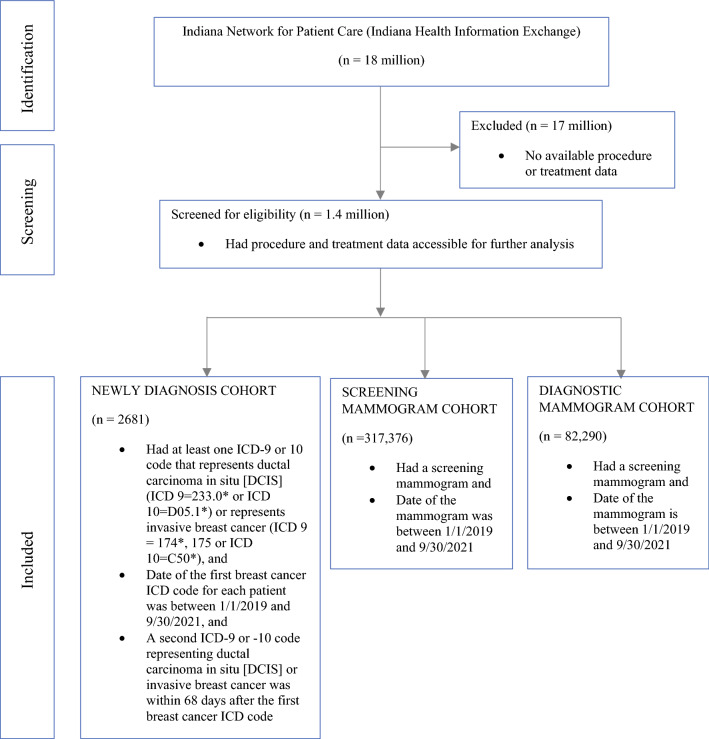
The Indiana Network for Patient Care (INPC), also called Indiana Health Information Exchange, is one of the largest health information exchanges in the USA, receiving clinical data from over 100 separate healthcare entities in Indiana and covering over 18 million patients. To examine patient’s procedure and treatment, we narrowed down to a subset of INPC patients that have their treatment files accessible. The INPC cohort used for this study consisted of patients newly diagnosed with breast cancer at any stage between 1 January 2019 and 30 September 2021. As initial criteria, we used one ICD-9 or ICD-10 code that represents DCIS or invasive breast cancer. To be considered a true breast cancer diagnosis, a repeated breast cancer ICD code within 68 days is required, considering a 30-day window accepted as a routine lag and the 38-day lockdown period. Patients with their first breast cancer ICD codes in the longitudinal data between 1 January 2019 and 30 September 2021 and with a second breast cancer ICD code within 68 days were included. In addition, to provide screening and diagnostic mammogram cohorts for comparison purposes, we ran a query of unique patients who had an internal code in the INPC data between 1 January 2019 and 30 September 2021. Consistently with the lockdown order, radiology scheduling administration restricted screening mammograms at the end of March 2020 and then started to resume this service at limited capacity (25%) at the beginning of May. Thus, we identified four time periods to compare services delivered for the newly diagnosed and mammogram cohorts: (1) lockdown (LD) from 23 March to 3 May 2020; (2) reopening (RO) from 4 May to 14 June 2020; and the corresponding periods a year before and after: (3) pre-lockdown (preLD) from 25 March to 16 June 2019 and (4) post-lockdown (postLD) from 22 March to 13 June 2021 (Fig. 1).
Fig. 1.
Flow diagram of the cohort selection process
We then reviewed data for the newly diagnosed cohort patients who had surgeries (mastectomy or lumpectomy) after the diagnosis date (defined as the date of their first breast cancer ICD code). The diagnosis date was also used to stratify the newly diagnosed cohort into LD, RO, preLD, and postLD subgroups for analysis. Kaplan–Meier survival curves were used to estimate the median time to surgery and 95% confidence interval. Time to surgery was defined as the interval between diagnosis date and first breast cancer surgery date. Log-rank test was used to compare potential delays of time to surgery in the four subgroups. Categorical variables were summarized by frequency and percentage; continuous variables were summarized by median, lower quartile, and upper quartile. Comparisons between the four subgroups were made by using chi-square test or Fisher’s exact test for the exact test for categorical variables and Kruskal–Wallis test for continuous variables. We took a few additional steps to expand understanding of the cohort characteristics. To look for patients’ preliminary staging characteristics at their initial diagnosis, we used an in-house natural language processing tool dedicated to cancer stage and found their first cancer stage after their diagnosis date to record.
The statuses of estrogen receptor, human epidermal growth factor receptor 2, and triple-negative breast cancer were extracted from the unstructured data in the database. These unstructured characteristics were not manually validated after the machine capture. Nonsurgery breast cancer treatment after diagnosis was also reported; this included endocrine therapy (anastrozole, tamoxifen, letrozole, and exemestane), chemotherapy, and radiation therapy. The neoadjuvant and adjuvant status of the nonsurgery treatment was inferred by comparing surgery date and nonsurgery treatment date, and the patients with incomplete dates were reported as “Others.”
All data used in this study were de-identified and met the criteria for exempt review by the Indiana University Institutional Review Board (IRB 2004400414). All statistical analyses were conducted using SAS v9.4 (Cary, NC), and RStudio (Version 1.1.463) was used to do the line plots (ggplot2 package); p < 0.05 was considered statistically significant.
Results
Newly Diagnosed Breast Cancer Cohort
From 1 January 2019, to 30 September 2021, a total of 2681 patients were newly diagnosed with breast cancers. Of these, 240 were diagnosed in the pre-lockdown (preLD) period, 79 and 68 were in the lockdown (LD) and reopening (RO) periods, and 264 were in the post-lockdown (postLD) group (Table 1). The median age was 62.0 years [51.0–70.0 (25th–75th percentile)]. The racial distribution in each period was not statistically different from the overall cohort, with white making up 83.3%, Black 11.14%, and Asian 1.88%. In this cohort, 83.23% were identified as estrogen-receptor positive, and 15.63% were triple-negative breast cancers. A higher proportion of the newly diagnosed patients in the LD group than in the other groups was identified as having triple-negative breast cancers (31.65%, p = 0.0017). Of these patients, 222 (8.28%), 1080 (40.28%), 372 (13.88%), 290 (10.82%), and 136 (5.07%) were identified as having stage 0, I, II, III, and IV at diagnosis, respectively; stage information for 581 (21.67%) remained undetected by algorithm. Also, 968 (36.11%) did not have a surgery captured within the healthcare system, while 1223 (45.62%) and 490 (18.28%) underwent breast-conserving surgery (BCS) and mastectomy, respectively.
Table 1.
Patient characteristics in the newly diagnosed breast cancer cohort
| Patient characteristic | Full cohort (1 January2019 to 30 September 2021) N = 2681 |
Subgroup | |||||
|---|---|---|---|---|---|---|---|
| Four subgroups overall N = 651 |
Pre-lockdown (25 March 2019 to 16 June 2019) N = 240 |
Lockdown (23 March 2020 to 3 May 2020) N = 79 |
Reopening (4 May 2020 to 14 June 2020) N = 68 |
Post-lockdown (22 March 2021 to 13 June 2021) N = 264 |
p-value | ||
| Age at diagnosis by median (25th, 75th percentiles) | 62.0 (51.0, 70.0) | 62.0 (51.0, 70.0) | 63.0 (52.0, 70.0) | 60.0 (49.0, 67.0) | 59.0 (49.0, 67.0) | 63.0 (52.0, 71.0) | 0.0371* |
| Sex | 0.5108 | ||||||
| Female | 2636 (99.21%) | 637 (98.61%) | 236 (98.74%) | 79 (100.0%) | 65 (97.01%) | 257 (98.47%) | |
| Male |
21 (0.79%) |
9 (1.39%) |
3 (1.26%) |
2 (2.99%) |
4 (1.53%) |
||
| Race | 0.4278 | ||||||
| White | 2215 (83.33%) | 532 (82.23%) | 198 (82.85%) | 62 (78.48%) | 52 (77.61%) | 220 (83.97%) | |
| Black | 296 (11.14%) | 88 (13.60%) | 33 (13.81%) | 12 (15.19%) | 13 (19.40%) | 30 (11.45%) | |
| Others (Asian, American Indian/Alaska Native, Native Hawaiian/Pacific Islander) |
91 (3.42%) |
17 (2.63%) |
7 (2.93%) |
2 (2.53%) |
1 (1.49%) |
7 (2.67%) |
|
| Unknown or refused |
91 (3.42%) |
17 (2.63%) |
7 (2.93%) |
2 (2.53%) |
1 (1.49%) |
7 (2.67%) |
|
| Ethnicity | 0.8760 | ||||||
| Hispanic or Latino |
52 (1.96%) |
20 (3.09%) |
7 (2.93%) |
3 (3.80%) |
3 (4.48%) |
7 (2.67%) |
|
| - Not Hispanic or Latino | 2545 (95.82%) | 617 (95.36%) | 227 (94.98%) | 76 (96.20%) | 63 (94.03%) | 251 (95.80%) | |
| Declined or unknown |
59 (2.22%) |
10 (1.55%) |
5 (2.09%) |
1 (1.49%) |
4 (1.53%) |
||
| Estrogen receptor status | 0.3224 | ||||||
| Negative | 383 (14.29%) | 117 (17.97%) | 39 (16.25%) | 19 (24.05%) | 14 (20.59%) | 45 (17.05%) | |
| Positive | 1901 (70.91%) | 448 (68.82%) | 164 (68.33%) | 49 (62.03%) | 43 (63.24%) | 192 (72.73%) | |
| Unknown | 397 (14.81%) | 86 (13.21%) | 37 (15.42%) | 11 (13.92%) | 11 (16.18%) | 27 (10.23%) | |
| Human epidermal growth factor receptor-2 status | 0.1530 | ||||||
| Negative | 1572 (58.64%) | 386 (59.29%) | 148 (61.67%) | 39 (49.37%) | 44 (64.71%) | 155 (58.71%) | |
| - Positive | 323 (12.05%) | 79 (12.14%) | 27 (11.25%) | 17 (21.52%) | 7 (10.29%) | 28 (10.61%) | |
| Unknown | 786 (29.32%) | 186 (28.57%) | 65 (27.08%) | 23 (29.11%) | 17 (25.00%) | 81 (30.68%) | |
| Triple-negative breast cancer | 0.0017* | ||||||
| No | 2262 (84.37%) | 530 (81.41%) | 191 (79.58%) | 54 (68.35%) | 55 (80.88%) | 230 (87.12%) | |
| Yes | 419 (15.63%) | 121 (18.59%) | 49 (20.42%) | 25 (31.65%) | 13 (19.12%) | 34 (12.88%) | |
| Charlson Comorbidity Index | 2.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 2.0 (0.0, 2.0) | 1.0 (0.0, 2.0) | 0.6168 |
| Insurance | 0.0018* | ||||||
| Managed care | 1104 (41.66%) | 270 (41.80%) | 79 (33.05%) | 38 (48.10%) | 34 (50.75%) | 119 (45.59%) | |
| Medicaid | 129 (4.87%) | 27 (4.18%) | 17 (7.11%) | 3 (3.80%) | 5 (7.46%) | 2 (0.77%) | |
| Medicare | 1199 (45.25) | 291 (45.05%) | 116 (48.54%) | 32 (40.51%) | 25 (37.31%) | 118 (45.21%) | |
| Others | 218 (8.23%) | 58 (8.98%) | 27 (11.30%) | 6 (7.59%) | 3 (4.48%) | 22 (8.43%) | |
| Stage at diagnosis** | 199 | 190 | 0.0505 | ||||
| 0 | 222 (8.28%) | 53 (8.14%) | 15 (6.25%) | 6 (7.59%) | 4 (5.88%) | 28 (10.61%) | |
| I | 1080 (40.28%) | 248 (38.10%) | 97 (40.42%) | 29 (36.71%) | 22 (32.35%) | 100 (37.88%) | |
| II | 372 (13.88%) | 91 (13.98%) | 36 (15.00%) | 15 (18.99%) | 13 (19.12%) | 27 (10.23%) | |
| III | 290 (10.82%) | 78 (11.98%) | 32 (13.33%) | 9 (11.39%) | 12 (17.65%) | 25 (9.47%) | |
| IV | 136 (5.07%) | 37 (5.68%) | 19 (7.92%) | 5 (6.33%) | 3 (4.41%) | 10 (3.79%) | |
| Unknown | 581 (21.67%) | 144 (22.12%) | 41 (17.08%) | 15 (18.99%) | 14 (20.59%) | 74 (28.03%) | |
| Surgery | 0.9220 | ||||||
| Conserving surgery (BCS) | 1223 (45.62%) | 296 (45.47%) | 117 (48.75%) | 35 (44.30%) | 28 (41.18%) | 116 (43.94%) | |
| Mastectomy | 490 (18.28%) | 126 (19.35%) | 43 (17.92%) | 15 (18.99%) | 15 (22.06%) | 53 (20.08%) | |
| No surgery or no further encounter | 968 (36.11%) | 229 (35.18%) | 80 (33.33%) | 29 (36.71%) | 25 (36.76%) | 95 (35.98%) | |
| Nonsurgery treatment | |||||||
| Endocrine therapy | 886 | 205 | 109 | 40 | 29 | 27 | |
| Neoadjuvant | 15 (1.69%) | 5 (2.44%) | 2 (1.83%) | 1 (2.50%) | 1 (3.45%) | 1 (3.70%)) | |
| Adjuvant endocrine therapy | 650 (73.36%) | 144 (70.24%) | 83 (76.15%) | 21 (52.50%) | 24 (82.76%)) | 16 (59.26%) | |
| Neoadjuvant + adjuvant endocrine therapy |
10 (1.13%) |
4 (1.95%)) |
0 (0.00%) |
4 (10.00%) |
0 (0.00%) |
0 (0.00%) |
|
| Status unknown | 211 (23.81%) | 52 (25.37%) | 24 (22.02%) | 14 (35.00%) | 4 (13.79%) | 10 (37.04%) | |
| Chemotherapy | 1219 | 303 | 129 | 55 | 42 | 77 | |
| Neoadjuvant chemotherapy | 106 (8.70%) | 27 (8.91%) | 11 (8.53%) | 2 (3.64%) | 6 (14.29%) | 8 (10.39%) | |
| Adjuvant chemotherapy | 636 (52.17%) | 143 (47.19%) | 71 (55.04%) | 20 (36.36%) | 20 (47.62%) | 32 (41.56%) | |
| Neoadjuvant + adjuvant endocrine therapy | 111 (9.11%) | 33 (10.89%) | 15 (11.63%) | 13 (23.64%) | 5 (11.90%) | 0 (0.00%) | |
| Status unknown | 366 (30.02%) | 100 (33.00%) | 32 (24.81%) | 20 (36.36%) | 11 (26.19%) | 37 (48.05%) | |
| Radiation | 227 | 47 | 22 | 4 | 5 | 16 | |
| Neoadjuvant chemotherapy |
6 (2.64%) |
2 (4.26%) |
2 (9.09%) |
0 (0.00%) |
0 (0.00%) |
0 (0.00%) |
|
| Adjuvant chemotherapy | 153 (67.40%) | 32 (68.09%) | 12 (54.55%) | 2 (50.00%) | 3 (60.00%) | 15 (93.75%) | |
| Neoadjuvant + adjuvant endocrine therapy |
0 (0.00%) |
0 (0.00%) |
0 (0.00%) |
0 (0.00%) |
0 (0.00%) |
0 (0.00%) |
|
| Status unknown | 68 (29.96%) | 13 (27.66%) | 8 (36.36%) | 2 (50.00%) | 2 (40.00%) | 1 (6.25%) | |
Values are expressed as n (%) or median (25th, 75th percentiles). The p-value comparisons across subgroup categories are based on chi-square test or Fisher’s exact test or Monte Carlo estimate for the exact test for categorical variables; p-values for continuous variables are based on Kruskal–Wallis test for median
*Significant at p < 0.05
**An in-house natural language processing tool (staging state machine) dedicated to cancer stage identification in the unstructured electronic health record data was used to search among a variety of breast cancer-related notes (clinical, radiology, pathology, operational, radiation, etc.). The algorithm extracted stage groups I–IV and TNM staging information separately. The TNM stages were later consolidated into stage groups I–IV for reporting. The staging state machine did not differentiate whether the stage was clinical or pathologic nor which staging system
Screening Mammogram, Diagnostic Mammogram, Diagnosis, and Surgery Trends
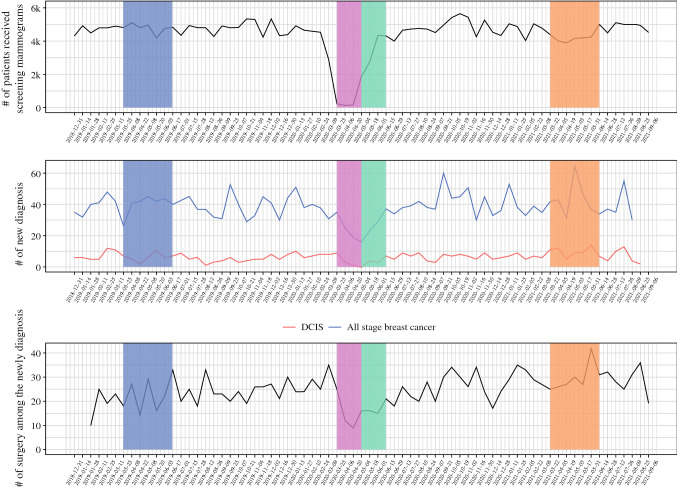
In Fig. 2, over 21,000 fewer patients received a screening mammogram in 2020 compared with 2019 (103,310 vs. 124,647) in the entire INPC health information exchange. Weekly screening mammograms continued to be lower in the postLD than the preLD. Roughly 3000 fewer patients received a screening mammogram between 1 January and 30 September 2021, compared with the same time of 2019 (89,419 vs. 92,481). Interestingly, in the final 12 weeks of 2021 captured in this study (14 June to 5 September), the number of screening mammograms returned to the preLD level with an average of 2413 patients per week, compared with an average of 2397 patients per week in 2019.
Fig. 2.
Time series of patients who received a screening mammogram, a new breast cancer diagnosis, and surgery among the newly diagnosed patients within the health system
As summarized in Table 2, the average weekly numbers of screening and diagnostic mammograms (standard deviation) in preLD were 2326.2 (183.0) and 624.1 (67.7), respectively, compared with 87.2 (37.2) and 195.2 (65.2) in LD, 1484.7 (565.6) and 437.2 (88.3) in RO, and 2055.3 (149.5) and 574.2 (41.4) in postLD. In the newly diagnosed cohort, there were 20.0 (5.4) new breast cancer diagnoses per week in preLD compared with 13.2 (4.0) in LD, 11.3 (3.4) in RO, and 22.0 (6.5) in postLD. The postLD subgroup had a statistically higher number and percentage of DCIS diagnoses than the other subgroups with 5.0 (1.9) diagnoses per week, constituting 23.9% (10.0%) of all breast cancer diagnoses. Also, there were more surgeries per week in the postLD newly diagnosed group compared with the LD and RO subgroups, with 14.8 (6.1) surgeries per week compared with 7.7 (4.4) and 7.8 (3.0), respectively. While significantly more patients received a breast cancer surgery in postLD (14.8 patients per week) than in preLD (10.5 patients per week), there were two fewer surgeons (17 vs. 19) who performed a breast cancer surgery in postLD. The average surgeries each of those surgeons performed during the 6-week windows in preLD and postLD were 7.2 (7.2) versus 11.9 (13.2), not a significant difference (p = 0.5631).
Table 2.
Summary of weekly average patients who received screening mammogram, diagnostic mammogram, a new breast cancer diagnosis, and surgery performed among the newly diagnosed patients within the health system during the pre-lockdown, lockdown, reopening, and post-lockdown periods
| Procedure, diagnosis, or treatment | Overall p-value | Pre-lockdown | Lockdown | Reopening | Post-lockdown | |
|---|---|---|---|---|---|---|
| 25 March 2019 to 16 June 2019 | 23 March 2020 to 3 May 2020 | 4 May 2020 to 14 June 2020 | 22 March 2021 to 13 June 2021 | |||
| Weeks 13–24 | Weeks 13–18 | Weeks 19–24 | Weeks 12–23 | |||
| Screening mammogram (patients per week) | Mean (SD) | < 0.0001† | 2326.2 (183.0) |
87.2 (37.2) |
1484.7 (565.6) |
2055.3 (149.5) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.0042 | 0.0079 | 0.0143 | |
| Diagnostic mammogram (patients per week) | Mean (SD) | < 0.0001† |
624.1 (67.7) |
195.2 (65.2) |
437.2 (88.3) |
574.2 (41.4) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.0041 | 0.0144 | 0.0426 | |
| All-stage new diagnosis (patients per week) | Mean (SD) | 0.0012† |
20.0 (5.4) |
13.2 (4.0) |
11.3 (3.4) |
22.0 (6.5) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.1192 | 0.0325 | 0.954 | |
| DCIS new diagnosis (patients per week) | Mean (SD) | 0.0027‡ |
3.1 (2.4) |
2.2 (2.1) |
1.2 (1.2) |
5.0 (1.9) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.3672 | 0.0648 | 0.0256 | |
| DCIS of all-stage new diagnosis (%) | Mean (SD) | 0.0367† |
15.0 (10.8) |
14.2 (11.0) |
9.6 (11.0) |
23.9 (10.0) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.9997 | 0.7304 | 0.16 | |
| Surgery (patients per week) | Mean (SD) | 0.0079‡ |
10.5 (3.3) |
7.7 (4.4) |
7.8 (3.0) |
14.8 (6.1) |
| Versus pre-LD, pairwise p-value | N/A | N/A | 0.2271 | 0.255 | 0.0306 |
Normality assumption was tested via Kolmogorov–Smirnov test; † indicates Kruskal–Wallis test; ‡indicates using the linear regression model
SD standard deviation, Pre-LD pre-lockdown, DCIS ductal carcinoma in situ
Time to Surgery
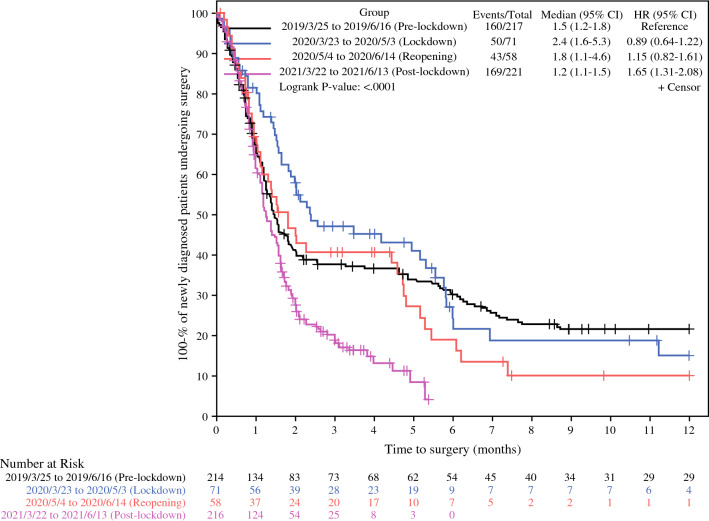
We also evaluated the time to surgery (TTS) for all subgroups of the newly diagnosed cohort patients who received a surgery in the health system. Compared with the TTS in preLD (1.2–1.8 months, 95% CI), the ranges of TTS during LD (1.6–5.3) and DO (1.1–4.6) were wider (Fig. 3). Interestingly, the postLD subgroup had a significantly shorter TTS than the preLD subgroup (p < 0.0001). At month 2 after diagnosis, 41.3% of patients in preLD were still actively seeking care without a recorded surgery, compared with 29.2% in postLD. The median TTS for DCIS patients did not fluctuate significantly among the subgroups (p > 0.05).
Fig. 3.
Time to surgery among the newly diagnosed patients with any-stage breast cancer using Kaplan–Meier method
Discussion
As a routine preventive care service, the volume of screening mammograms in our study decreased dramatically during the LD by 96.6%. The remainder of patients with underlying breast cancers presented with symptoms and received a diagnosis, prompting an in-person evaluation, physical examination, and diagnostic mammogram. Though radiology services were allowed to gradually increase from 25% of normal capacity at around the beginning of RO, screening mammograms reached 63% throughout RO. Although diagnostic mammograms were not restricted, they fell to 31.3% and 70.1% in LD and RO from their preLD rates, likely in part because fewer screening examinations were performed. According to the Breast Cancer Surveillance Consortium, generally 4.8 breast cancers were detected per 1000 screening mammograms prior to the pandemic, and 78.6% of those were stage 0 or I.19 With the lockdown and the pandemic more generally, patterns of screening and diagnostic mammograms were altered significantly in the preLD, LD, RO, and postLD subgroups in our study (p < 0.0001). While the long-term effects remain unknown, Sud et al. predicted that a 3-month delay in diagnosis would result in a decrease in 10-year survival of 2.14% to 7.70% across all age groups.20 Despite a precipitous drop in screening mammogram services in our cohort, the diagnosis volume of any stage of breast cancer and DCIS as well as the volume of breast surgery was less impacted during LD and RO. Still, there were fewer diagnoses of any stage cancer in the RO (11.3 per week vs. 20.0 per week, p = 0.03), which correlates with a decrease in screening during the LD. Many of these diagnosed patients likely underwent a primary diagnostic mammogram, which was considered an essential service during the pandemic for patients with symptoms such as palpable lumps. Our study did not determine whether the diagnosis of breast cancer was from a screening or a diagnostic mammogram.
After diagnosis, surgery is the primary definitive treatment for most early-stage breast cancers and DCIS. Newly diagnosed patients are often in emotional distress under any conditions,15 and during the pandemic lockdown, uncertainties and delays in treatment greatly increased. Though the weekly volume of surgery for the newly diagnosed patients with breast cancer remained at a similar level during LD (7.8 ± 4.4 per week) and RO (7.7 ± 3.0 per week) to the preLD (10.5 ± 3.3 per week), individual patient’s surgery was delayed to various degrees. The TTSs for all stages of breast cancer varied significantly among the subgroup but not the TTSs for the DCIS. Longer median TTSs with wider 95% CI ranges of 2.4 (1.6–5.3) months and 1.8 (1.1–4.6) months were observed in LD and RO, compared with 1.5 (1.2–1.8) months and 1.2 (1.1–1.5) months in preLD and postLD. Their 95% CI all stayed within the 3–6-month delay recommended by the Society of Surgical Oncology.11 The long-term impact of TTS delays during the pandemic remains unclear. In Italy, Vanni et al. reported an increase in lymph node involvement after a delay during the first COVID-19 lockdown.21 Recent studies investigating the historical data have contributed to understanding the ramifications of delays in TTS. Minami et al. found increased TTS was associated with a small rise in pathological upstaging in DCIS patients in the National Cancer Database 2010–2016.7 Sud et al. reported a delay of 3 and 6 months in TTS would mitigate 19% and 43% of life-years gained from cancer surgery for England in 2013–2017.20
At baseline, 21.6% of any-stage patients diagnosed in preLD in our study did not have a captured breast surgery event at month 12 while they were still under care for breast cancers (Fig. 3). This finding has several potential explanations. Some patients may require neoadjuvant treatment or may not be surgical candidates, as over 20% of preLD patients were stage III or IV and the stage was not detectable in 17.08%. Additionally, there may be patients who were diagnosed or received a second opinion at the eligible breast care clinics but received treatment from unaffiliated surgeons. Compared with the preLD, lower percentages of patients diagnosed during the LD (15.2%) and RO (10.2%) did not have captured surgeries, and RO patients seemed to be diagnosed at more advanced stages (Table 1). A number of these patients would likely receive neoadjuvant treatment for their more advanced stage. However, some patients may have postponed surgery to avoid clinical settings or opted to receive care in less populated, rural settings to avoid travelling to urban referral centers with higher population density and COVID-19 transmission rates.
As of mid-2022, after the USA had entered a less acute phase of the pandemic, over 2 years had passed since the beginning of the historic COVID-19 pandemic in early 2020, during which variant spikes were seen around the world.22 Although only one lockdown was imposed in Indiana, the years-long pandemic radically changed the behaviors of patients with cancer and the delivery of health services, which will likely continue to impact cancer care in the back-to-normal stage.23 Even though scheduling restrictions were totally lifted in August 2020, the volume of screening was still at 88.4% (2055.3 per week) of the preLD average (2326.2 per week) in postLD. If the average number of screening mammograms had been at the 2019 pre-pandemic level (2397 per week) from 1 January 2020 through 30 September 2021, an additional 25,403 screening mammograms would have been performed, demonstrating the accumulated backlog that may be presently impacting the population. Not only are there undiagnosed patients still waiting for their screenings, but cancer survivors need access to mammograms for active surveillance. Despite the mammogram backlog, diagnosis of any-stage cancer and the percentage of DCIS returned to preLD levels during postLD. Significant increases in postLD from the preLD levels were observed in diagnosis of DCIS (5.0 per week vs. 3.1 per week) as well as the volume of breast surgeries (14.8 per week vs. 10.5 per week), which implies a late impact from the screening backlog resulting in a catch-up period.
Noticeably, in postLD, not only the TTS for any-stage breast cancers (1.6 months) is shorter, but patients seeking care are more likely to complete a surgery within the INPC healthcare system (see the post-lockdown curve in Fig. 3). Interestingly, although the availability of surgery was challenged by hospitals’ COVID-positive patients and nursing shortages, the weekly surgery volume (standard deviation) went up to 14.8 (6.1) in postLD compared with 10.5 (3.3) in preLD (p = 0.03). This could be explained by the improved availability of operating rooms and providers resulting from fewer meetings, business trips, and other elective operations, considering there were two fewer surgeons in postLD. It could also correlate with a potentially increased patient demand observed from a higher DCIS diagnosis level (postLD: 5.0 ± 1.9 vs. preLD: 3.1 ± 2.4, p = 0.03) and an earlier stage distribution among the stage-detectable patients in postLD. A potential change in patients’ behaviors could also contribute to the shorter TTS in postLD as patients might seek care and treatment when they are able to or continue cancer treatment within the same healthcare system given the high unpredictability during the pandemic. Providers reacted to the delay of surgery by offering neoadjuvant endocrine therapy. Besides that, providing mental health support to diagnosed patients and maintaining a professional communication channel to support their primary care providers were encouraged to help patients and providers adapt to the pandemic conditions and strengthen community resilience.17,24 Screening mammogram, diagnostic mammogram, all-stage diagnosis, DCIS diagnosis, percentage of DCIS diagnosis, and surgery all showed statistically significant alterations in their patterns during the pandemic.
In postLD, the significantly higher level of DCIS diagnosis, together with higher average all-stage diagnosis (22.0 ± 6.5 vs. 20.0 ± 5.4 in preLD, p = 0.95) and average percentage of DCIS diagnosis (23.9 ± 10.0% vs. 15.0 ± 10.8%, p = 0.16), poses questions about whether they are early signs of worsening breast cancer incidence since fewer screening (88.4% of preLD) and diagnostic mammograms (92.0% of preLD) were provided in postLD. Our study could not investigate this question due to no direct linkage between the screening mammogram and diagnosis data. While the changes in diagnosis results warrant further monitoring, we should not wait to take action until the impact becomes evident. We advocate expanding screening in primary care to mitigate the possible longer-term effects.
Our study had several limitations. The mammogram data were extracted at a population level in the INPC as upstream to understand the interruption of diagnosis and treatment in the healthcare system. The patients could have received a screening mammogram and sought cancer care in different healthcare systems, whose data are not captured by our approach. For this reason, we could not assess potential linkages between screening and surgery in the cohorts, so the effects of the pandemic on individual patients cannot be ascertained from the roughly 25,000-mammogram deficit between 1 January 2020 and 30 September 2021. Moreover, due to limitations in the dataset, we were unable to obtain a structured stage or hormone receptor status for all patients. Attempting to enhance the capture and exchange of high-quality oncological data in a standardized way, such as Minimal Common Oncology Data Elements,25 would be crucial to further the results of studies like ours. Deeper investigation into the subpopulations of hormone-receptor-positive patients who received neoadjuvant endocrine therapy and evaluations for discrepancies between pathology of the initial biopsy and surgical excision (i.e., the rate of upstaging DCIS due to the delay in care) are warranted and would provide interesting insights into breast cancer biology and the consequences of delay in care.
Conclusions
In this study, numbers of screening mammograms, diagnostic mammograms, all-stage diagnoses, DCIS diagnoses, percentage of DCIS diagnoses, and surgeries declined during the pandemic. While screening decreased by 96.3% during the LD and 36.2% during the RO, the overall breast cancer diagnosis and surgery volumes decreased up to 38.7%, and the time to surgery was prolonged. One year after the lockdown, the numbers of screening and diagnostic mammograms had not fully recovered to pre-pandemic levels. The postLD subgroup had a higher DCIS diagnosis and surgery volume than preLD and a shorter TTS. Because reduced levels of diagnosis and treatment may have long-term impacts on patients with breast cancer, additional screening in primary care should be conducted to mitigate the possible downstream effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
We gratefully acknowledge the enthusiastic help from Anna Roberts and Regenstrief Institute Inc. on sourcing of data for this project, Carlee Johnson from Health System Patient Access Center, and the tremendous support of Lynn Whittaker for editing and formatting the manuscript.
Disclosure
The authors certify that they have no commercial interest in the subject of study. The research for this publication was supported by the Translational Science Initiative at the Indiana University School of Medicine funded by Lilly Endowment Incorporated (grant number LILLY END/20091568000) and the US National Library of Medicine (grant number T15LM012502).
Ethics approval and consent to participate
This was a retrospective cohort study using de-identified data from the Indiana Health Information Exchange database and the Indiana University Health data warehouse. Therefore, consent for patient participation and study publication was not required. The study was approved for exempt review by the Indiana University Institutional Review Board (IRB 2004400414).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Indiana Governor Eric J. Holcomb: 2020 Executive Orders. https://www.in.gov/gov/newsroom/executive-orders/2020-executive-orders/. 2020. Accessed February 13, 2022.
- 2.Indiana State Department of Health. Novel Coronavirus (COVID-19). https://www.coronavirus.in.gov/. 2022. Accessed 21 August 2022.
- 3.Centers for Medicare & Medicaid Services. CMS Adult Elective Surgery and Procedures Recommendations. March 2020. https://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf. Accessed May 14, 2020.
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 6.Breast Cancer: Statistics. https://www.cancer.net/cancer-types/breast-cancer/statistics. January 2020. Accessed May 15, 2020.
- 7.Minami CA, Kantor O, Weiss A, Nakhlis F, King TA, Mittendorf EA. Association between time to operation and pathologic stage in ductal carcinoma in situ and early-stage hormone receptor-positive breast cancer. J Am Coll Surg. 2020;231(4):434–447.e2. doi: 10.1016/j.jamcollsurg.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hills N, Leslie M, Davis R, et al. Prolonged time from diagnosis to breast-conserving surgery is associated with upstaging in hormone receptor-positive invasive ductal breast carcinoma. Ann Surg Oncol. 2021;28(11):5895–5905. doi: 10.1245/s10434-021-09747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–339. doi: 10.1001/jamaoncol.2015.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 Pandemic Breast Cancer Consortium. Recommendations for Prioritization, Treatment and Triage of Breast Cancer Patients During the COVID-19 Pandemic. National Comprehensive Cancer Network. 2020. https://www.nccn.org/covid-19/default.aspx. Accessed April 9, 2021.
- 11.Society of Surgical Oncology. Resource for Management Options of Breast Cancer During COVID-19. https://www.surgonc.org/resources/covid-19-resources/. March 30, 2020. Accessed April 20, 2020.
- 12.American Society of Breast Surgeons. During the COVID-19 Pandemic: Executive Summary, Version 1.0. https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19. March 24, 2020. Accessed April 19, 2020.
- 13.American College of Surgeons. COVID-19 Guidelines for Triage of Breast Cancer Patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer. March 24, 2020. Accessed April 20, 2020.
- 14.Bartmann C, Fischer L-M, Hübner T, et al. The effects of the COVID-19 pandemic on psychological stress in breast cancer patients. BMC Cancer. 2021;21(1):1356. doi: 10.1186/s12885-021-09012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia JMX, Goh ZZS, Chua ZY, et al. Managing cancer in context of pandemic: a qualitative study to explore the emotional and behavioural responses of patients with cancer and their caregivers to COVID-19. BMJ Open. 2021;11(1):e041070. doi: 10.1136/bmjopen-2020-041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miaskowski C, Paul SM, Snowberg K, et al. Stress and symptom burden in oncology patients during the COVID-19 pandemic. J Pain Symptom Manage. 2020;60(5):e25–e34. doi: 10.1016/j.jpainsymman.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swainston J, Chapman B, Grunfeld EA, Derakshan N. COVID-19 lockdown and its adverse impact on psychological health in breast cancer. Front Psychol. 2020;11:2033. doi: 10.3389/fpsyg.2020.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes DR. Breast cancer care during a pandemic: an opportune time for cryoablation? Breast Cancer Res Treat. 2020;182(3):515–521. doi: 10.1007/s10549-020-05724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breast Cancer Surveillance Consortium (BCSC). Benchmarks for Cancers. https://www.bcsc-research.org/statistics/screening-performance-benchmarks/benchmarks-cancers. March 23, 2017. Accessed January 24, 2022.
- 20.Sud A, Jones ME, Broggio J, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanni G, Tazzioli G, Pellicciaro M, et al. Delay in breast cancer treatments during the first COVID-19 lockdown. A multicentric analysis of 432 patients. Anticancer Res. 2020;40(12):7119–7125. doi: 10.21873/anticanres.14741. [DOI] [PubMed] [Google Scholar]
- 22.Cascella M, Rajnik M, Aleem A, Dulebohn S, Napoli RD. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls. 2022. [PubMed]
- 23.Riera R, Bagattini ÂM, Pacheco RL, Pachito DV, Roitberg F, Ilbawi A. Delays and disruptions in cancer health care due to Covid-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milgrom Z, Severance T, Scanlon C, et al. An evaluation of an extension for community healthcare outcomes (ECHO) intervention in cancer prevention and survivorship care. Res Sq. 2021 doi: 10.21203/rs.3.rs-1065059/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osterman TJ, Terry M, Miller RS. Improving cancer data interoperability: the promise of the minimal common oncology data elements (mcode) initiative. JCO Clin Cancer Inform. 2020;4:993–1001. doi: 10.1200/CCI.20.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.