Abstract
Tumour cells migrate very early from primary sites to distant sites, and yet metastases often take years to manifest themselves clinically or never even surface within a patient’s lifetime. This pause in cancer progression emphasizes the existence of barriers that constrain the growth of disseminated tumour cells (DTCs) at distant sites. Although the nature of these barriers to metastasis might include DTC-intrinsic traits, recent studies have established that the local microenvironment also controls the formation of metastases. In this Perspective, I discuss how site-specific differences of the immune system might be a major selective growth restraint on DTCs, and argue that harnessing tissue immunity will be essential for the next stage in immunotherapy development that reliably prevents the establishment of metastases.
Subject terms: Immunotherapy, Immunosurveillance, Innate immunity, Cancer microenvironment
A disseminated tumour cell will grow only if it arrives at a ‘fertile’ distant site, which as Ana Luísa Correia posits is determined largely by the immune context at the site. Site-specific differences in local immune cell types, ratios and spatial locations influence whether a disseminated tumour cell establishes metastases or is kept dormant.
Introduction
Whether, and if so when, metastases will occur remains a distinct challenge in the effectiveness of treatment of patients with cancer. Metastases invariably originate from disseminated tumour cells (DTCs) that have spread beyond the primary tumour site through the circulation and have successfully colonized distant sites. But despite all spreading systemically, DTCs grow asynchronously in distant sites, and may initiate metastases within months, years or decades or not at all within a patient’s lifetime. This differential emergence of metastases stems from persistent DTCs that entered a state of dormancy1,2 upon arrival at the distant site, only to later awaken and form metastases. From a clinical perspective, this pause in cancer progression is both a threat to a long-lasting cure and a singular therapeutic window to prevent future metastases. Understanding what brings DTCs into and out of dormancy is obviously fundamental to inform the therapeutic intervention in patients at risk of currently incurable metastases.
When it comes to explaining metastatic success, a hypothesis articulated more than a century ago by Paget3 rings true even today: the ‘seed’ (that is, the DTC) will grow only if it lands on fertile ‘soil’ (that is, the microenvironment within distant tissues). Although the genetic traits of the seed are certainly important, the current lack of a systematic analysis of DTCs from patients with cancer who are asymptomatic hinders the conclusion of whether the acquisition of specific mutations is required to exit dormancy. On the contrary, the findings that the earliest transformed cells are proficient disseminators4–8 and yet are often unable to resume growth for a long time argue that the properties of the soil are rather the limiting factor of metastatic progression9. Analyses of paired primary tumours and metastases demonstrate that driver mutations precede diagnosis by many years or even decades8,10–14. Furthermore, autopsy studies have long hinted that dormant DTCs spread throughout most tissues and at much higher frequency than commonly recognized, but fail to colonize certain sites in a meaningful time frame3,15–19. This steady resistance to metastasis suggests that tissue-specific barriers to DTC outgrowth exist and contribute to the chronicity of cancer. Additional specificity of barriers to metastasis comes from even within the same tissue, as recently demonstrated in the liver, where spatially distinct sub-microenvironments allow the coexistence of breast dormant DTCs and metastases20. And, clearly, occasional disruptions of the tissue integrity can breach the barriers to metastasis and present dormant DTCs with an opportunity to awaken.
What, then, is the nature of these tissue barriers to metastasis? Being the front line defence against DTCs arriving at distant sites, the immune system is one major barrier that DTCs must overcome on the path to successful colonization. Perhaps the most striking evidence of this stems from observations that metastasis can be involuntarily transmitted by organ transplantation from apparently disease-free donors into immunosuppressed recipients21–24. Such cases support an important prediction of the classic cancer immunoediting postulate25; that is, the existence of an equilibrium phase, during which the host immune system limits the outgrowth of dormant DTCs into metastases. Clinical evidence of immune-mediated dormancy comes from the detection of an increased frequency of certain immune cells, namely natural killer (NK) cells and T cells, lining dormant breast DTCs in the bone marrow of patients with no evidence of metastases26,27. By contrast, the upsurge in metastases in animal models either depleted of those antimetastatic immune cell populations20,28–33 or enriched in other immune cell populations conducive to DTC reactivation34–41 cements a role for immunity in determining metastatic progression (Fig. 1) across several types of cancer, and raises the possibility of targeting DTCs by altering their immune microenvironment.
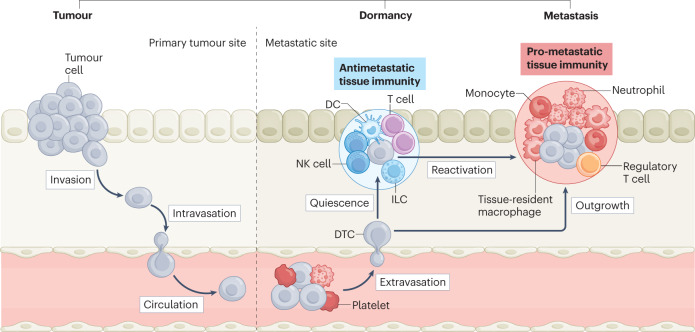
Fig. 1. Tissue immunity determines metastatic progression.
Before metastases manifest themselves clinically, tumour cells need to overcome multiple barriers throughout their journey from the primary site until they successfully colonize a distant site. First, they invade locally and intravasate the endothelium to enter the circulation, where they travel alone or in clusters with other cells, in search of a new site to extravasate and expand. The few disseminated tumour cells (DTCs) that survive the journey then face attrition from the specific immune environment within the distant site: where antimetastatic tissue-resident immune cell populations are dominant, DTCs blend into the physiological context and persist in a quiescent state for several years or even decades (dormancy stage of cancer); conversely, microenvironments depleted of antimetastatic immune cells or enriched in other immune cells conducive to DTC reactivation support metastatic outgrowth into clinically detectable metastases. Treating the specific immune microenvironment at distant sites may be a way to effectively control DTCs. DC, dendritic cell; ILC, innate lymphoid cell; NK, natural killer.
Recent breakthroughs in cancer immunotherapy42–45 have generated excitement in applying checkpoint blockade, vaccines and engineered T cells and NK cells for metastatic prevention, often searching for a one-size-fits-all approach to target DTCs. But the immune context experienced by DTCs is anything but the same in every tissue. First, each site contains a specific assortment of tissue-resident immune cells46, translated in anatomically specialized DTC–immune cell interactions. Second, tissue immunity directs the development of regional and systemic immune responses through cytokine-mediated communication47, further diversifying local antimetastatic immune responses. Third, DTC–immune cell interactions co-evolve over time48,49, and it is likely that the same immune functions that initially impede metastasis either change or provide the selective pressure that promotes outgrowth of immunoevasive DTCs. In sum, the guiding principle of antimetastatic immunity is the same one touted by real-estate experts: location, location, location.
In this Perspective, I posit that site-specific differences in the immune system are a major selective growth restraint on DTCs. I outline how anatomical organization controls local tissue immunity and discuss emerging examples of site-specific immunoregulation of metastasis. I also discuss the array of challenges to tissue integrity that may breach immune barriers to DTC outgrowth, and how normalizing tissue immunity might be explored therapeutically to prevent metastases.
Principles of site-specific immunity
Site-specific immune cell diversity, development and function
The immune system has been shaped through evolution by the need to discriminate non-self pathogens (such as viruses or bacteria) from self tissues. The first defence strategy is anatomical; that is, the physical, chemical and biological barriers to the entry of invaders in the organism. The second defence is innate immunity, which provides an immediate, less specific response that prevents invaders from functioning or multiplying50. Finally, the third defence is adaptive immunity, which provides a long-term, more specific response to the particular invader that the organism has encountered51. Achieving immunity to DTCs arriving at distant sites does not fit neatly into this self–non-self paradigm because DTCs arise from once-normal cells from the host. However, the immune system does spot and react to DTCs, presumably through either activation of innate immune pattern recognition receptors52 or adaptive strategies that involve recognition of altered-self antigens53. Equipped with a multitude of different cell types that mediate fractions of those innate and adaptive functions, at a high-level, the immune system might be viewed as a single structure that spreads throughout the body to provide host defence against insults that occur at diverse places episodically and without warning. But given the wide range of threats that beset an individual throughout life, populating all tissue sites permanently with large amounts of all the immune cell types required to mount a suitable defence response would be not only an unacceptable energetic burden54 but also incompatible with the highly organized epithelial and mesenchymal cellular sheets that ensure an organ’s function. Instead, the immune system has evolved to strategically position sentinel resident immune cells within tissues, which can locally initiate and amplify the immune response by recruiting circulating immune cells to the site of tissue damage, and readily restore tissue homeostasis.
Parked in tissues, long-lived tissue-resident immune cell populations self-maintain locally, and develop together with the site they populate55. These populations include tissue-resident macrophages56, mast cells57, NK cells58, innate lymphoid cells (ILCs)59, NKT cells60, γδ T cells61, mucosal-associated invariant T cells62 and tissue-resident memory T cells63, all of which conserve a core lineage identity and yet retain sufficient plasticity to specialize and fulfil functions specific to their tissue of residence. Taking mouse tissue-resident macrophages as an example, despite all sharing macrophage lineage markers (such as colony-stimulating factor 1 receptor (CSF1R), Fcγ receptor 1 (also known as CD64), the integrin CD11b, the adhesion G protein-coupled receptor F4/80 and the receptor tyrosine kinase MERTK), one finds that they diversify in phenotype to perform tissue-specific functions64; for example, Kupffer cells clear foreign debris and balance lipid metabolism in the liver65,66, red pulp macrophages drive senescent red blood cell removal and iron recycling in the spleen67, and alveolar macrophages clear excess lung surfactant56. Tissue-resident macrophage specification arises from selective chromatin states and expression of certain transcription factors68,69, such as Spi-C for red pulp macrophages70, ID3 for Kupffer cells71 and PPARγ for alveolar macrophages72. The past two decades have seen a revolution in the tools available for studying the diversity of immune cells in tissues. Lineage tracing and highly multiplexed immunofluorescence imaging have enabled semiquantitative analyses of the composition and spatial organization of the immune system within tissues, whereas two-photon intravital microscopy has provided insights into immune cell population dynamics, cell–cell interactions and functional responses in situ73. These advanced imaging modalities, combined with conventional flow cytometry approaches and emerging systems-level immunomics74, continue to uncover new phenotype diversity in immune cells across multiple mouse75–83 and human82,84–93 tissues. Thorough investigation of the ontogeny and function of all these new tissue-resident immune cell subsets in different physiological and disease contexts, especially during the formation of metastases, will be crucial to determining their overall importance.
Niche imprinting of site-specific immunity
If tissue compartmentalization of immunity is a given, how is it established and maintained? Resident immune cells are locally conditioned by specialized tissue microenvironments composed of several non-immune resident cell types, all embedded in extracellular matrix components, which not only provide them with a physical niche and trophic factors that confer self-maintenance but also imprint them with tissue-specific functions that are essential for the homeostasis of their tissue of residence. For example, in the skin, ILC subsets rely on hair follicle-derived factors for localization and maintenance, and tune the skin microbiota by limiting sebaceous gland growth94. In the intestine, tissue-resident macrophages respond to microbial cues to enhance regulatory T cell (Treg cell) function95 and to control gastrointestinal tract transit through the regulation of enteric neuronal function96. Immune cells are also imprinted by the microenvironment within non-barrier tissues such as the brain, where microglia rely on neuron-derived factors to ensure neuronal development and synaptic pruning97–100, or the adipose tissue, where mesenchymal stromal cell populations provide a niche for ILC2s101–103 and Treg cells104 to regulate fat metabolism. Factors such as interleukin-15 (IL-15)105–108, IL-7 (refs. 105,109) and transforming growth factor-β (TGFβ)107,110,111 are commonly required for the maintenance of most of the tissue-resident immune cells. However, given that all organs contain multiple immune cell subsets occupying very unique niches, it is likely that many other tissue-specific trophic factors and their discrete cellular sources remain to be identified.
Just as non-immune resident cells imprint immune cells with specific functions that are essential for organ homeostasis, so do they shape antimetastatic immunity. The dominant permanent resident cellular components of the metastatic niche are cancer-associated fibroblasts, endothelial cells and nerve cells, all of which have been shown to strongly influence antimetastatic immunity (Box 1). The widespread use of single-cell technologies has led to a whole new appreciation of the heterogeneity of these cell populations. As new cell subsets are revealed, functional characterization of the interactions of non-immune and immune resident cell populations under distant site-specific conditions should provide a first glimpse into how tissue microanatomy contributes to local immunity and the induction of site-specific antimetastatic immune responses.
Box 1 Non-immune tissue-resident cells: tailors of local antimetastatic immunity.
Fibroblasts
The accumulation of cancer-associated fibroblasts (CAFs)257 has become increasingly recognized as supportive of outgrowth of disseminated tumour cells (DTCs) in the lung and liver through different immuno-inhibitory mechanisms, such as the recruitment of neutrophils132,157 and inflammatory monocytes157, exclusion of cytotoxic T cells from tumour areas258 and direct suppression of natural killer (NK) cell expansion20. Recent advances in single-cell technologies have resulted in the description of heterogeneity in the CAF network across organs at the baseline, by revealing tissue-specific CAF subsets with distinct matrix-producing and immunomodulatory phenotypes259,260 and major differences in matrix composition across tissues261,262. As we start to uncover heterogeneity of CAF subsets and their immunomodulatory functions across tumour types263–268, it is anticipated that distinct CAF subsets also assist the formation of metastases.
Vascular endothelial cells
In the context of metastasis, vascular endothelium association is crucial across all organs; a normalized perivascular niche is critical for maintaining DTC dormancy, whereas endothelial cell sprouting triggers DTC outgrowth269. Endothelial cells also control DTC progression through regulation of homing and extravasation of specific immune cell subsets270,271, and direct immunosuppression through expression of programmed cell death ligand 1 (PDL1)272 or FAS ligand (FASL)273. Single-cell studies have reported remarkable differences in endothelial cell expression patterns of co-stimulatory molecules and cytokines across tissues274, but whether tissue-specific endothelial cell subsets contribute to the formation of metastases remains to be seen.
Lymphatic vasculature completes the other half of the specialized vascular system in mammals, and directly contributes to immune homeostasis and disease in a context-dependent manner275. Lymphatics contribute to metastasis by serving as intratumoural gateways to distant organs276, providing a dormancy niche for DTCs in the lung and lymph nodes277,278, and inducing peripheral tolerance279,280.
Nerve cells
Although little is known about neuronal regulation of DTCs, it has been described that sympathetic innervation reactivates breast247 and prostate247,248 dormant DTCs in the bone, and excitatory synapses with glutamatergic nerves fuel metastases in the brain281. Neuron–immune cell interactions are well-described mediators of host defence and inflammation. A notable example is the neuron–macrophage crosstalk that maintains either protective or tolerogenic environments that are vital to intestinal homeostasis: noradrenaline signalling in β2-adrenergic receptor-expressing submucosal muscularis macrophages enforces an anti-inflammatory programme, which balances the pro-inflammatory phenotype of mucosal macrophages in the lamina propria282. Akin to specialized intestinal macrophages, several other immune cells engage in a constant conversation with neurons at discrete anatomical sites to sustain tissue health283; it is, thus, tempting to speculate that disrupting neuron–immune cell interactions might result in tissue-specific disease, including the emergence of metastases. In support of this, neuroendocrine stress induces immune dysfunction and disease246, and perioperative denervation (for example, using β-adrenergic blockers) has been hypothesized to stimulate antimetastatic immunity251. Further understanding of neuron–immune cell interactions in the context of tissue-specific metastasis is among the most exciting challenges in cancer immunology for the coming years.
Site-specific immune cell density
Because immunity is tailor-made for each organ site, the cross-tissue composition of the immune system is distinct. Comprehensive studies of multiple organs from mice have illustrated the presence of distinct densities of immune cells across different sites. For example, the liver is a preferential tissue for NK cell residency58, whereas in the brain NK cells are scarce and resident myeloid-derived cells are by far the most dominant immune cell population112. The skin is largely populated by resident γδ T cells113, but these cells constitute only a small percentage of all the splenic T cells114. In the lung, tissue-resident macrophages represent more than 90% of all immune cells115, whereas in the bone, they account for only 15–20% of the immune cellular content116. In recent years, analysis of tissue samples from hundreds of healthy human organ donors has revealed that interorgan heterogeneity of immune cell density is also conserved in humans90,117,118. One important consideration is that organs consist of different regions with distinct macroanatomical and physiological traits, and these spatial topographies may account for further diversity in immune cell density even within the same organ. For instance, the way the intestine handles the challenge of tolerating food antigens and the commensal microbiota, while eliminating ingested pathogens, is by macroanatomical segregation of these reactions into functionally distinct gut-draining lymph nodes119; this anatomical parsing results in important regional differences in the distribution of immune cell populations along the length of the intestine.
Emerging studies show that site-specific immune cell density also shapes the differential emergence of metastases. Clinical data from patients with metastatic ovary120 and colorectal49 cancer show that distinct composition and distinct spatial organization of the immune cell landscape across different organs (that is, interorgan immune heterogeneity) give rise to metachronous metastases within the same individual. Additionally, spatially distinct immune microenvironments exist even within the same organ (that is, intra-organ immune heterogeneity), and disproportionally impact DTC progression. Within the liver, for instance, breast DTCs lodged in NK cell-abundant microenvironments remain dormant, whereas those experiencing a local shortage of NK cells resume growth into metastases20. An understanding of interorgan and intra-organ immune heterogeneity is, thus, necessary to determine whether there are universal immune responses that can be exploited to effectively control dormant DTCs or whether a more customized approach that considers site-specific immunity is required. In the following section, I describe the unique immune microenvironment (Table 1) within and across four frequent sites of metastasis (that is, liver, lung, bone and brain; Fig. 2) and two where metastases are rather rare (that is, spleen and thyroid; Fig. 3), in search of targets for long-lasting therapeutic prevention of metastases.
Table 1.
Types of immune cells and their contribution to disseminated tumour cell progression in four frequent sites of metastasis
| Cell type | Description | Function in DTC progression |
|---|---|---|
| Dendritic cell | Presents antigens on its surface | Suppression of bone165 and brain192 metastases |
| Group 1 innate lymphoid cell or tissue-resident natural killer cell | Generally weakly or non-cytotoxic; secretes cytokines in response to pathogens or cancer cells | IFNγ-mediated DTC dormancy in the liver20; suppression of liver metastases125 |
| Group 2 innate lymphoid cell | Responds to large extracellular parasites and allergens | Suppression of brain metastases by enhancing dendritic cell antigen presentation and T cell responses192; pro-metastatic through natural killer cell suppression in the lung152 |
| Inflammatory monocyte | Produces inflammatory molecules in response to infection and cancer | Establishment of a pre-metastatic niche in the liver132,133; support of DTC seeding and outgrowth in the lung through suppression of T cell function35,40,157 |
| Neutrophil | Releases chemicals that act against pathogens and cancer cells | Establishment of a pre-metastatic niche in the lung36; awakening of dormant DTCs in the lung34,153; colonization of the lung38,39,154, bone165 and brain189 |
| Natural killer cell | Kills pathogens and cancer cells through cytotoxic granules | DTC elimination in the lung149–151, liver150 and brain150,191; suppression of bone metastases165,170–172 |
| Natural killer T cell | Shares properties of both T cells and natural killer cells; kills pathogens and cancer cells | IFNγ-mediated antimetastatic immunity in the liver226 |
| Tissue-resident macrophage | Phagocytic cell that engulfs and destroys foreign pathogens and cancer cells | Clearance of DTCs upon liver entry130,131; establishment of a lung pre-metastatic niche through T cell suppression37 and neutrophil recruitment146; pro-metastatic in the lung147 and liver141 through acquisition of a lipid metabolism phenotype; promotion41,186,187 or suppression184,185 of brain metastases |
| Cytotoxic T cell | Processes specific immune responses to antigens; kills pathogens and cancer cells | Suppression of DTC outgrowth in the lung28,155,156,158, brain190 and bone165,171 |
| γδ T cell | Promotes inflammatory responses through cytokine production | Pro-metastatic in the lung through suppression of cytotoxic T cells39 |
| Regulatory T cell | Suppresses immune responses, to maintain peripheral tolerance, prevent autoimmunity and limit chronic inflammatory diseases | Promotion of bone165, lung148 and brain190 metastases |
DTC, disseminated tumour cell; IFNγ, interferon-γ.
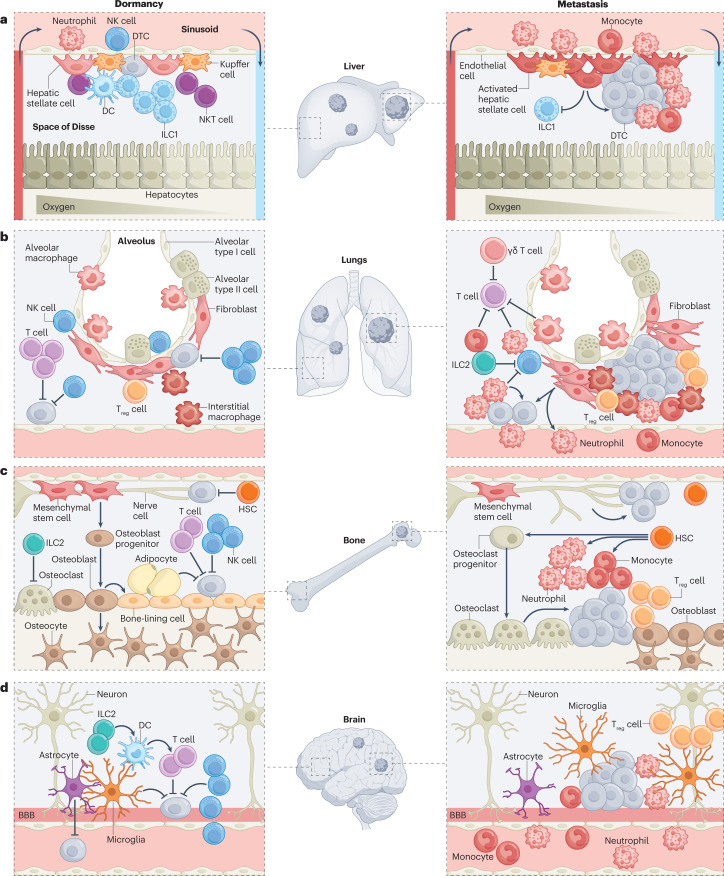
Fig. 2. Site-specific differences of the immune system contribute to differential emergence of metastases within and across common metastatic sites.
The immune system is customized by anatomical site, featuring distinct cell types that are distributed at defined ratios and spatial locations, where they engage in dynamic interactions with diverse non-immune resident cells to constantly safeguard tissue homeostasis. It is the product of these interactions that sets the immune tone for recognition of disseminated tumour cells (DTCs), and makes an ideal locale for DTCs to either be kept dormant or establish metastases within and across different sites. a, In the liver, DTCs migrate from the portal triad vessels to the sinusoid and into the subendothelial space of Disse, where they encounter immune and non-immune resident cells, all positioned in compliance with liver zonation. Liver-resident natural killer (NK) cells (or liver group 1 innate lymphoid cells (ILC1s)) are the main immune gatekeepers of DTC dormancy, whereas activation of hepatic stellate cells precipitates metastasis through suppression of liver-resident NK cell expansion or recruitment of immunosuppressive populations. b, In the lungs, NK cells and T cells are the major immune barriers to metastasis, and their function is countered by diverse immunosuppressive populations, including ILC2s, neutrophils, monocytes, regulatory T cells (Treg cells) and γδ T cells. Tissue-resident macrophages and activated fibroblasts feed the recruitment of these immunosuppressive populations by maintaining a hospitable inflammatory milieu permissive of metastasis. c, The bone is the primary niche for haematopoietic stem cells (HSCs), and is co-opted by DTCs to remain dormant. NK cells and T cells actively survey this niche, in concert with ILC2s that preserve the bone integrity through suppression of bone-resorbing osteoclasts. Conversely, an imbalance towards osteoclast differentiation supports the cycle of bone destruction that triggers dormant DTC reactivation and metastasis initiation. Other awakening factors in the bone include the accumulation of neutrophils, monocytes and Treg cells, as well as enhanced innervation. d, Access to the brain parenchyma is restricted by the blood–brain barrier (BBB), and thus DTC extravasation to the brain takes longer than in other tissues. Once in the brain, DTCs park tightly at the perivasculature, and are actively surveyed and often eliminated by NK cells and T cells, with the latter helped by ILC2-mediated enhanced antigen presentation by dendritic cells (DCs). Microglia, the main immune cell residents of the brain, act in a context-dependent manner, being either suppressive of or permissive of metastatic outgrowth. NKT cell, natural killer T cell.
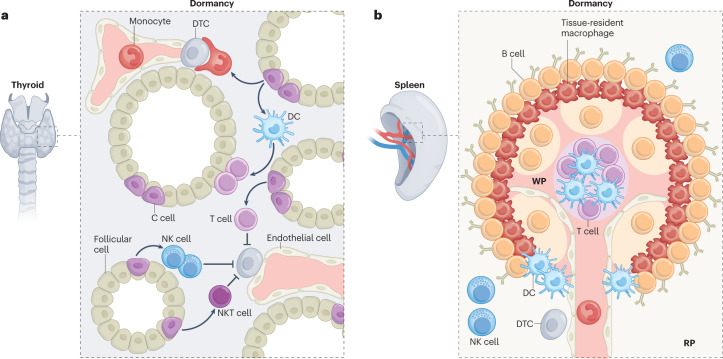
Fig. 3. Diversity and spatial distribution of tissue immunity may underlie the resistance to disseminated tumour cell outgrowth at infrequent sites of metastases.
a, In the thyroid, the continuous production and high concentration of thyroid hormones (such as tri-iodothyronine, thyroxine and calcitonin) maintains natural killer (NK) cell, T cell and NKT cell activity, stimulates the maturation and antigen presentation capacity of dendritic cells (DCs), and sparks monocyte phagocytic activity. It is plausible that this naturally hyperactive local immunosurveillance maintains dormancy of disseminated tumour cells (DTCs) and antagonizes metastatic outgrowth at this site. b, Beyond their high numbers, splenic immune cells display an intricate positioning within the three main structures of the spleen: the inner white pulp (WP), where cytokine-mediated compartmentalization separates T cell and B cell areas into distinct zones; the red pulp (RP), which harbours many immune cells with innate immune functions that selectively express distinct pattern recognition receptors, helping to tailor the nature of both the early innate immune response and the subsequent adaptive immune response; and the in-between marginal zone, harbouring concentric macrophage subsets and DCs with defined positioning to ensure efficient pathogen encounter and antigen presentation. It is possible that this sharp anatomical parsing of immunosurveillance readies the splenic antimetastatic niche by facilitating low-probability DTC–immune cell interactions. Although these hypotheses are enticing, specific mechanisms driving immune-mediated low frequency of metastases in the thyroid and spleen remain to be seen.
Setting the immune tone one site at a time
Liver
Excluding lymph nodes, the liver is the most common site of metastasis across solid tumours121. Despite having facilitated access to the liver through discontinuous sinusoids, DTCs often find resistance to immediate outgrowth and persist for a long time as dormant cells at this site122–124. This liver capacity to support dormancy is, at least in part, rooted in its exceptionally tolerogenic milieu. The particular abundance of circulating NK cells and liver-resident NK cells (also termed ‘liver ILC1s’)58 sets these innate immune cells as major effectors of hepatic immune function. In the steady state, they maintain an immature phenotype with low levels of pro-inflammatory cytokines and cytotoxic mediators, but they promptly respond to virally infected cells or DTCs by expressing tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)125 and producing interferon-γ (IFNγ)126. Recently, colleagues and I showed that IFNγ secreted by liver NK cells is essential to maintain breast DTCs dormant20. We found that the size of the NK cell pool in the liver can itself determine metastatic outgrowth, and sustaining NK cell abundance with adjuvant IL-15-based immunotherapy succeeded in preventing hepatic metastases and prolonging survival in preclinical models20. It is relevant to note that, physiologically, NK cells counter liver fibrosis by eliminating activated hepatic stellate cells (which activate from quiescent hepatic stellate cells to proliferative myofibroblasts)127,128 and by restraining the proliferation of activated hepatocytes in an IFNγ-dependent manner129; this antifibrotic role of liver NK cells already in place for normal physiology also helps to sustain DTCs in a dormant state. Similarly, the function of Kupffer cells in tissue repair and clearance of dead and senescent cells that is central to maintaining liver homeostasis also constrains the growth of DTCs upon their entry to the liver sinusoids130,131.
Although the tolerogenic milieu is intentionally kept to avoid inappropriate inflammatory responses against frequently encountered antigens, when the liver surpasses its ability to successfully clear an aggravating invader, it breaks the tolerance and triggers inflammation. It is essential that this inflammation is controlled in a timely and effective manner, otherwise the liver can initiate a cycle of chronic inflammation, fibrosis and scarring, which overall results in loss of tissue integrity and increased risk of metastasis. Activation of hepatic stellate cells, by either Kupffer cell-derived TGFβ132 or recruited inflammatory monocyte-secreted granulin133, results in a fibrotic environment that assists the pre-metastatic niche134 formation and metastasis of pancreatic cancer in the liver. Additionally, the accumulation of activated hepatic stellate cells prevents NK cell expansion and precipitates metastases20, suggesting that failure to resolve fibrosis causes incidental DTC awakening.
A particular feature of the liver is the spatial division of labour according to blood flow-generated gradients of oxygen, nutrients and hormones, together with morphogenetic fields, which run radially along the porto-central axis of the liver lobules, a phenomenon termed ‘zonation’135. Not surprisingly, resident immune cells also comply with liver zonation. Resident NKT cells and Kupffer cells concentrate around periportal regions to protect liver cell populations around the central vein from incoming blood-borne threats136. Liver ILC1s also reside primarily in the perivascular spaces surrounding the portal triad137,138, where they engage in bidirectional interplay with Kupffer cells, dendritic cells (DCs) and neutrophils, in fine coordination of an optimized immune response. Recent studies expanded on myeloid cell zonation by unravelling a population of lipid-associated macrophages that locates at the bile ducts in the healthy liver but repositions itself pericentrally to regions with steatosis (fatty liver disease)139,140 and accumulates in liver metastasis141. As more insights into the regional heterogeneity of immune cells in the context of normal liver physiology80,135,142,143 become available, how immune zonation impacts the emergence of metastases will warrant further investigation.
Lung
Pulmonary metastases are detected in 20–54% of patients with cancer and tend to occur in the basal and peripheral regions of the lung lobes, where increased vascular density raises the chances of DTC extravasation144. Because lung metastases rarely appear without other co-occurring secondary sites121, they are likely the result of default accessibility and permissiveness of this site. The lung is continuously exposed to the external environment, and it must protect the organism from inhaled pathogens and pollutants, while not mounting a disproportionate response against innocuous airborne antigens. Lung-resident immune cells are responsible for striking this delicate balance between immunity and tolerance. Among them, tissue-resident macrophages, including alveolar and interstitial macrophages145, are by far the most abundant, and there is growing appreciation of their importance also in the context of metastasis. Alveolar macrophages contribute to the pre-metastatic niche by suppressing antimetastatic cytotoxic T cell responses37 or recruiting neutrophils to the lung146. A recent study found extensive heterogeneity within macrophages isolated from metastasis-bearing lungs147, including a subset of lipid-associated macrophages enriched in transcripts associated with extracellular matrix remodelling and immunosuppression, whose functional significance awaits to be validated. Besides tissue-resident macrophages, Treg cell accumulation favours local tolerance in the lung, a feature that is fully exploited by DTCs to colonize this site148.
Lung NK cells constitute approximately 10–20% of all lung-resident lymphocytes, and are critical for antigen-independent recognition and elimination of DTCs, as demonstrated by overwhelming metastatic burden in their absence or impairment149–151. However, NK cell antimetastatic function in the lung is countered by ILC2s152 and recruited neutrophils36. Neutrophils in the lung have received particular attention, as they have been implicated in the awakening of dormant DTCs through extracellular matrix proteolysis34 and lipid metabolism153, and subsequent lung colonization38,154. Neutrophils also synergize with γδ T cells to dampen cytotoxic T cell-mediated antimetastatic immunity39, which has thus far been regarded as a main growth constraint of DTCs in the lung28,155,156. Similarly, inflammatory monocytes also promote breast DTC seeding and outgrowth by suppressing cytotoxic T cell function35,40. The recruitment of immunosuppressive neutrophils and inflammatory monocytes to the lung is orchestrated by fibroblasts, which establish a hospitable inflammatory milieu permissive of metastasis157. Even short-term inflammation, such as that induced after surgery, awakens breast dormant DTCs, the growth of which would otherwise be constrained by antimetastatic cytotoxic T cell responses158. Importantly, perioperative anti-inflammatory treatment reduces DTC outgrowth in the lung, offering an inexpensive intervention to counter inflammation and prevent the emergence of metastases particularly at this site.
Bone
After the liver and lung, the bone is the most frequent site of metastasis of all solid tumours15. Conventional bone marrow biopsies indicate that one-third of patients with early-stage breast cancer already have DTCs in their bone marrow at the time of primary tumour resection, and approximately one quarter of these patients will progress to have metastases159. Although the presence of DTCs in the bone is a common event that invariably correlates with poor outcome159–161, the inconsistently low incidence of clinically detectable bone metastases suggests that growth restraints to DTCs exist at this site. In addition, not all bones are equally susceptible to metastasis; whereas the axial skeleton (such as the spine, ribs and bony pelvis) is a preferential target for breast cancer spread, metastases rarely develop within the appendicular skeleton (such as hands and feet)162,163. Conceivably, these discrepancies might reflect differences in bone marrow composition (that is, the content of blood cell-producing red marrow and adipocyte-rich yellow marrow) within and across different bones, and also during life, which likely influence DTC outgrowth through ways still underexplored.
Despite orchestrating the vast immune cell network arising from haematopoiesis, the bone is a site of particularly dampened immunity. The small pool of cytotoxic T cells and mainly immature NK cells contrasts with a large proportion of myeloid progenitors (of both monocytic origin and granulocytic origin) and Treg cells164, creating an immunosuppressive potential ready to assist the formation of metastases. For example, heightened myeloid progenitor differentiation into immunosuppressive neutrophil and monocyte populations precedes breast DTC outgrowth in the bone165. Similarly, myeloid progenitors can differentiate into bone-resorbing osteoclasts166, feeding the vicious cycle of bone destruction that triggers breast DTCs to exit dormancy and initiate metastases167. Likewise, bone Treg cells have non-immunological activity by endowing haematopoietic stem cells with a licence to evade immunity through prevention of oxidative stress168,169, a privilege that might also be afforded to DTCs lodged in the bone.
In contrast to the default immunosuppressive microenvironment of the bone, a few antimetastatic immune responses have been described at this site. NK cell activity underpins the efficacy of adjuvant IL-2-based immunotherapy in eradicating neuroblastoma metastases to the bone170. Similarly, NK cells and cytotoxic T cells are required for interferon regulatory factor 7-mediated suppression of breast cancer bone metastases in preclinical models171. Loss of host type I interferon signalling impairs NK cell immunity and drives breast cancer metastasis in the bone172. DTC-endogenous interferon signalling also directly sustains prostate DTC dormancy in the bone, and the loss of type I interferon genes triggers DTC outgrowth into metastases173. Although the local source of interferons in the dormant DTC niche is still unclear, the fact remains that interferon abundance mediates dormancy in the bone marrow. The reports that osteal macrophages ensure ossification and maintenance of haematopoietic stem cell niches116,174 and that ILC2s suppress the generation of bone-resorbing osteoclasts175 highlight the critical function of tissue immunity in preserving bone integrity; this tissue protective mechanism may be relevant to minimize the growth of bone marrow DTCs as well.
Brain
Brain metastases are clinically observed in about 20% of all patients with cancer, even though the true incidence is estimated to be much higher and rising owing to more accurate imaging technologies and systemic therapies that extend patient survival176. For decades, the brain was considered an immune-privileged site, sealed off from peripheral immunity through the blood–brain barrier, and self-sufficient for maintenance and repair based on its own resident immune cells. This simplistic view was revisited with the discovery that bone marrow-derived immune cells enter the brain to support its function177, and immune molecules are ferried into and out of the brain through a lymphatic drainage system178. Recent studies have shown that immune cells are housed within specialized niches across spatially distinct brain regions (that is, the choroid plexus, the meninges and the cerebrospinal fluid)179. Although cellular and spatial maps of these regions during development and ageing are starting to emerge82,180, how immune phenotype diversity and spatial topographies within the brain impact the formation of metastases remains unknown and ripe for study.
Accounting for approximately 75–80% of all immune cells in the brain181, resident microglia have garnered the most acclaim in the context of brain metastasis. They build up in and around metastases182,183, and exhibit polarized phenotypes that can be either growth suppressive184,185 or permissive of metastatic colonization41,186,187. A recent study combining single-cell analyses with transgenic mouse models points to a much wider heterogeneity within the microglial compartment, by revealing seven transcriptionally different subsets of microglia that mediate brain metastatic outgrowth188. In humans, activated resident microglia seem more relevant for brain tumour development, whereas the recruitment of macrophages and neutrophils189 and the accumulation of Treg cells and exhausted cytotoxic T cells190 are dominant in brain metastases. It has been suggested that other populations of immune cells contribute as a barrier to DTC outgrowth in the brain. NK cells actively survey and eliminate DTCs that periodically re-enter proliferation and re-express NK group 2 member D (NKG2D) receptors191, whereas ILC2s limit brain metastases by enhancing DC antigen presentation and the generation of cytotoxic T cell responses192.
Thyroid
Although only 1.4–3% of malignant solid tumours clinically present as metastases in the thyroid193, autopsy studies reveal a much higher prevalence of subclinical disease (that is, small metastatic nodules and/or interspersed DTCs) in patients who die of non-thyroidal cancer194–197. This apparent discrepancy suggests that DTCs successfully reach and lodge in the thyroid, and yet they are prevented from colonizing this site in a meaningful time frame. The fast arterial blood flow, rich oxygenation and high iodine content of the thyroid gland have been long suggested to underlie its resistance to metastasis197. Another hypothesis is the naturally hyperactive immune microenvironment established by the three thyroid hormones (that is, tri-iodothyronine, thyroxine and calcitonin198), which impacts many inflammation-related processes, such as chemotaxis, phagocytosis, cytokine production and the generation of reactive oxygen species199. Both tri-iodothyronine and thyroxine stimulate IFNγ responses and NK cell activity200, and facilitate maintenance of T cells and NKT cells201. In addition, tri-iodothyronine uniquely stimulates the maturation and antigen presentation capacity of DCs202, and triggers monocyte phagocytic activity201. Thus, it is plausible that the continuous production and high concentration of thyroid hormones sustains an increased local immunosurveillance that maintains DTC dormancy and antagonizes metastatic outgrowth at this site. Although the explanation is enticing, its confirmation remains to be seen.
Spleen
Similarly to the thyroid, the spleen is also an uncommon site for metastases from solid tumours to manifest themselves clinically3,121,163,203. Many hypotheses have been offered to explain the antimetastatic nature of the spleen163,203,204, yet none seems to have been proved and all have issues. Perhaps the most obvious explanation would be the large number of immune cells within the spleen. However, an equally abundant immune cell population is present in lymph nodes, and these are not similarly protected from metastases. Beyond numbers, immune cells display an intricate positioning within the three main structures of the spleen: the red pulp, the white pulp and the in-between marginal zone205. Inside the white pulp, cytokine-mediated compartmentalization separates T cell and B cell areas into distinct zones, which synergize to perform adaptive immune functions. In contrast, the red pulp harbours many immune cells with innate immune functions, which selectively express distinct pattern recognition receptors, helping to tailor the nature of both the early innate immune response and the subsequent adaptive immune response. Just as distinct subsets of macrophages206 and correct positioning of DCs207 are critical for efficient pathogen encounter and antigen presentation, it is possible that sharp anatomical parsing of immunosurveillance readies the splenic antimetastatic niche by facilitating low-probability DTC–immune cell interactions. Assessment of the spatiotemporal dynamics of splenic immune cells during the formation of metastases will probe the validity of this hypothesis.
Hence, whether in tissues long recognized as infrequent sites of metastases (Fig. 3) or even within common metastatic sites where dormant DTCs coexist with growing metastases (Fig. 2), the diversity and spatial distribution of tissue immunity are major determinants of the differential emergence of metastases.
Systemic challenges to tissue immunity
Beyond local tissue specificities, a set of an individual’s systemic factors might influence the tissue immune microenvironment and further diversify metastasis presentation. These systemic factors include ageing, circadian rhythm, the gut microbiota, therapy and certain pathological states, all of which challenge tissue integrity, with major consequences for local tissue immunity.
Ageing
The time-dependent accumulation of cell and tissue damage during ageing poses a first major challenge to immunity. Ageing is associated with systemic, low-grade chronic inflammation leading to a gradual decline in immune function over time (or immunosenescence), which likely underlies the higher incidence of infection, autoimmunity and cancer in elderly people. Age-induced immunosenescence occurs largely in effector T cells, as illustrated by the loss of T cell function and the pronounced anti-inflammatory response that accompany the development of skin squamous cell carcinoma208. However, populations of innate immune cells, such as NK cells, macrophages and neutrophils, appear to also undergo density and phenotypic changes with ageing209. In the bone marrow, haematopoietic stem cells are skewed towards myeloid lineage specification in elderly people210,211, and increased neutrophil recruitment in aged mice accelerates breast tumour growth through suppression of cytotoxic T cell responses212. Besides a direct contribution to immunosenescence, ageing also changes the stromal compartment and indirectly favours an immunosuppressive microenvironment conducive to cancer progression. In a mouse model that mimics the aged skin microenvironment, stromal senescence establishes a tumour-permissive, chronic inflammatory milieu that allows skin squamous cell carcinoma to progress unchecked by the immune system213. Just recently, stromal changes in the aged lung were shown to induce dormant melanoma cells to exit dormancy214, strongly suggesting that age must be considered as a parameter in the design and delivery of therapies for the formation of metastases.
Circadian rhythm
The cyclic variation in behaviour and biological activity that follows every 24-h light–dark cycle is termed ‘circadian rhythm’. Virtually all organisms have developed endogenous cellular clocks that allow time sensing, so every physiological process is optimally timed to its local environment. Circadian immune circuits appear ubiquitously215, and therefore may underlie tissue integrity or the lack thereof that precedes cancer initiation and metastasis. Elegant circadian experiments have shown a clear diurnal pattern in melanoma-induced metastasis, with many lung metastases forming when the cells were injected in the morning and nearly no metastatic foci when they were injected in the evening216. Remarkably, this time-dependent difference is mitigated upon neutrophil depletion, providing a new rationale for time-controlled administration of neutrophil-targeted therapies. In another study, key circadian rhythm hormones, such as melatonin, testosterone and glucocorticoids, were shown to dictate the generation of circulating breast tumour cells in a time-dependent manner217. Although a role for tissue immunity in this context remains to be seen, evidence is growing that treatment of metastasis-prone cancers should be timed to be maximally effective.
Gut microbiota
Recent years have seen a surge of interest in the gut microbiota as a critical regulator of systemic antitumour immunity, controlling the efficacy of chemotherapies and immunotherapies218–220. However, the association with commensal bacteria extends well beyond the gut, and analyses of the microbiota from different anatomical locations in the human body have revealed site-specific commensal communities that locally shape tissue immunity221. For example, early-life microbial exposure establishes lung NKT cell tolerance to future allergens222, and commensal–DC interactions calibrate immunity in the skin223. Conversely, local dysbiosis promotes the development of lung cancer, by stimulating IL-1β and IL-23 production from myeloid cells, and downstream activation of lung-resident γδ T cells224. Altering the gut microbiota with faecal transplantation has shown promise to reverse dysbiosis and correct neutrophil-induced organ damage in models of infection225, and to restore NKT cell immunity against liver primary tumours and metastases226. In addition to studies of the gut microbiota, further analysis of the cross-tissue local microbiota and its dysbiosis may uncover novel immunoregulatory nodes in the formation of tissue-specific metastases.
Therapy
Beyond doubt, the introduction of chemotherapy and radiotherapy as the standard of care has substantially increased the survival of patients with cancer. However, growing awareness exists that they may, paradoxically, have negative consequences in some patients, largely by inducing tissue damage and twisting the immune microenvironment227. Chemotherapy has been shown to restrain antitumoural immunity228,229, elicit the release of pro-metastatic extracellular vesicles230 and nurture a microenvironment conducive to metastases through neutrophil infiltration231, T cell reprogramming232 and complement signalling233,234. Besides chemotherapy, radiotherapy has also been recently implicated in acute injury to the lung and neutrophil reprogramming, easing DTC outgrowth235. Thereby, a more thorough understanding of the effects of any therapy on tissue immunity and metastatic dynamics in patients should inform treatment strategies in the future.
Pathological states
Certain individual conditions alter the physiology of specific sites and challenge tissue immunity, thereby bringing increased risk to the sprouting of site-specific metastases. One condition with high relevance is infection, as evidenced by the high vulnerability of patients with cancer to COVID-19 (ref. 236), and the worsened clinical outcomes of patients with cancer after postoperative infection237. Even in the absence of infection, tissue injury as a consequence of surgery can itself accelerate the progression of colorectal cancer metastases in the liver through enhanced formation of neutrophil extracellular traps238. Additional studies have shown that external inflammation-inducing agents, such as bacterial lipopolysaccharides or tobacco smoke, initiate neutrophil-mediated pulmonary metastases, either by the formation of neutrophil extracellular traps34 or by enzymatic degradation of the dormancy-promoting factor thrombospondin 1 (ref. 239). Besides neutrophils, other innate immune cell populations are reshaped by airway inflammation to facilitate metastases, as shown by allergen-induced activation of an ILC2–eosinophil axis that suppresses NK cell antimetastatic immunity against breast and melanoma DTCs in the lung152.
Conditions of chronic inflammation, such as that caused by obesity, are also responsible for reprogramming tissue immunity and increasing the risk of metastasis. As adipose tissue pathologically expands, resident macrophages switch to an inflammatory phenotype and instruct the bone marrow to expand the population of myeloid progenitors and increase neutrophil density across multiple organs240. In both genetically induced obesity and diet-induced obesity animal models, increased density of peripheral neutrophils and pulmonary neutrophils facilitates breast DTC extravasation into the lung, with metastases following suit241,242. Adaptation of tumour cells to the lipotoxic obese environment also induces a metabolic tug of war between tumour cells and T cells for lipids, which results in T cell dysfunction and tumour outgrowth243. Similarly, lipid uptake by NK cells in the obesity context limits antitumour immune responses, suggesting that metabolic reprogramming may restore NK cell immunity and improve the clinical outcome in patients with obesity with cancer244. Obesity may also indirectly affect tissue immunity by altering site-specific non-immune resident cells, as for example recently illustrated by differential obesity-induced vascular endothelium dysfunction across different organs245.
Finally, chronic stress has been pointed out as an accelerator of cancer progression. Neuroendocrine factors affect the number, activity and trafficking of immune cells in a time- and tissue-specific manner246, and their influence on tissue-specific metastases is starting to emerge. For example, sympathetic activation triggers DTC outgrowth in the bone247,248, and exacerbated β-adrenergic signalling catalyses pulmonary metastases through suppression of NK cell activity249 or macrophage-mediated formation of a pre-metastatic lung niche250. Because cancer diagnosis, surgery and associated treatment is a highly stressful experience, they potentially limit antimetastatic responses and trigger awakening of dormant DTCs in distant sites; this encourages perioperative denervation as an adjuvant therapy to halt the emergence of metastases in an enduring manner251.
In summary, each individual is exposed to a unique combination of challenges throughout life that tweak tissue immunity and catalyse metastatic outgrowth. How nuanced these factors are within and across tissues is a growing area of research, and will provide additional therapeutic opportunities for preventing tissue-specific metastases.
Therapeutic implications of site-specific immunity
Clearly, immunotherapy that harnesses immunological surveillance to control DTCs has significant potential to prevent the emergence of metastases. With the growing list of approvals for immune checkpoint inhibitors, it would be tempting to consider its application to metastasis prevention in a broad manner. However, the differences in the immune microenvironment within and across distant sites discussed earlier herein argue that therapeutic interventions and the development of new immunotherapy should rather be seen in the context of site-specific immunity. From surveying different organ sites, I have identified a set of mechanisms controlling site-specific immune composition and that hold promise to either eradicate DTCs or maintain metastatic disease in a long-term dormant state.
One strategy to harness site-specific immunity directly would be to sustain pre-existing antimetastatic resident immune populations. For example, nurturing the inherent abundant pool of liver-resident NK cells with adjuvant IL-15-based immunotherapy prevents breast cancer hepatic metastases and prolongs survival in preclinical models20. Similarly, adjuvant administration of bisphosphonates halts the reactivation of breast DTCs, presumably by interfering with osteoclast biology167 and activating γδ T cells252 in the bone, thereby reducing bone metastases and extending patient survival253. The converse strategy would be to suppress pro-metastatic immune cell types in specific sites. For instance, blocking high neutrophil density and activity, either by dissolving neutrophil extracellular traps34 or by inhibiting S100A8/S100A9-mediated accumulation of oxidized lipids153, might succeed in preventing DTC reactivation in the lung.
Because antimetastatic immunity is profoundly influenced by non-immune resident cells, reversing subtle, but critical, imbalances in DTC–immune cell–non-immune cell interactions might be another way to effectively control dormant DTCs. One example would be to attempt to cause activated hepatic stellate cells to revert into quiescence as a means to reset the proliferation ability of liver-resident NK cells, and thus ensure that there are sufficient numbers to maintain DTC dormancy in the liver. Importantly, restoration of hepatic stellate cell quiescence has been achieved through use of all-trans retinoic acid254, use of vitamin D255 and exposure to nitric oxide256, encouraging the possibility that correcting one specific cellular target may indeed restore the overall tissue physiology and, thus, tissue immunity against DTCs. For these reasons, harnessing site-specific immunotherapy is likely to become an essential component of immunotherapy development that reliably prevents the establishment of metastases.
Concluding remarks
The evidence presented herein provides a renewed endorsement of Paget’s seed and soil hypothesis, yet newly contextualized to tissue immunity. Driven by anatomical organization, immune cells are indexed to tissues at defined ratios and spatial locations, where they engage in dynamic interactions with non-immune resident cells to constantly safeguard tissue homeostasis. It is the product of these interactions that sets the immune tone for DTC recognition, and makes an ideal locale for DTCs to either establish metastases or be kept dormant. Mounting an efficient antimetastatic immune response requires tissue-resident immune cells to coordinate their individual activation into global tissue-level responses, a feat that can be achieved only by long-range intercellular communication, as mediated by soluble factors (such as cytokines and growth factors). Thus, a systematic quantitative framework to monitor the different cellular and soluble architects of antimetastatic immune responses, and how they intersect with DTC intrinsic traits and individual conditions, in each tissue, in space and time, will be needed to optimize the choice of interventional approach in patients at risk of developing metastases.
As we begin to appreciate site-specific antimetastatic immunity and its therapeutic potential, a few challenges and exciting opportunities lie ahead before we leverage DTC–immune cell biology into targeted immunotherapies (Box 2). The first challenge is to improve detection of DTCs and accurately assess metastatic dynamics in patients throughout treatment, which will be greatly facilitated by increased establishment of and access to rapid autopsy programmes. The second is to continue increasing our understanding of the spatiotemporal regulation of dormant and metastatic niches, so as to predict which patients are likely to develop metastases, and how to effectively manage site-specific metastatic disease across cancer types; here, orthogonal spatial technologies have been revolutionary, and it is anticipated that their widespread implementation, combined with improved computational approaches to analyse multiple large datasets, will bring resolution of the niche full circle. The third challenge is to develop preclinical models that can translate to human immunity, and thus guide rational combination therapy strategies in future clinical trials. Finally, these trials should be large and long enough to support the complexity of precision immuno-oncology protocols, and extend clinical end points beyond the traditional progression-free survival readout that appraises only metastases to also include DTC burden. Continued progress in overcoming these challenges demands a collective interdisciplinary effort to inform the next stage in immunotherapy development that will bring long-lasting cures for all patients across all cancer types.
Box 2 Current challenges and opportunities in disseminated tumour cell–immune cell biology.
Appraising single disseminated tumour cell–immune cell interactions in patients
Given that single disseminated tumour cells (DTCs) are difficult to detect in tissues from patients who are asymptomatic and that tissue resections are not part of the treatment options in many patients with metastatic cancer, viable human tissue for researching DTC–immune cell biology is scarce. Rapid autopsies of deceased patients offer the opportunity to obtain large amounts of tissue from multiregion sampling with sufficient quality for both multi-omics analysis of DTCs within the metastatic ecosystem and the establishment of patient-derived model systems for mechanistic studies284. Increased access to and use of this admittedly tedious approach entails the chance to unravel the number of dormant DTCs in the body, and the underpinnings of DTC growth restraints in certain sites.
Expanding the understanding of site-specific antimetastatic immunity
Emerging evidence points to immune cell heterogeneity and spatial compartmentalization as having a profound influence on the propensity and timing of DTC outgrowth. The advent of high-resolution, single-cell and spatial technologies (such as co-detection by indexing (CODEX)285, cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq)286, imaging mass cytometry287, multiplexed ion beam imaging288, spatial transcriptomics289 and spatial proteomics290) has started to revolutionize the field and transcend the conventional study of individual cell types or factors in isolation to provide a global view of the rich and ever-changing ecosystem hosting DTCs. For example, multiplex imaging coupled with next-generation sequencing has revealed that immune spatial topography determines the emergence of metachronous metastases within the same individual49. Standardization of these technologies will illuminate how site-specific immunity influences DTC progression, and will help to predict which patients are likely to develop metastases and benefit from site-specific immunotherapy.
Development of preclinical models that translate to human immunity
Striking differences in immune biology between humans and animal models make the generation of an ideal preclinical model a challenging feat. Classical transplantable syngeneic mouse and human cancer lines, and genetically engineered mouse models, have provided translational utility over many years, yet with practical limitations in reflecting human disease kinetics, and often showing only modest responses to immunotherapy291. Three-dimensional heterotypic patient-derived organoid cultures better recapitulate growth and clinical treatment response292,293, but the reconstitution of single or a few selected immune cell populations may not be sufficient to capture the complexity of the metastatic microenvironment and its response to immunotherapy. In contrast, patient-derived xenografts retain the architecture of the original specimen and are being implemented as patient avatars in precision medicine programmes to identify the most effective treatment for individual patients294,295; however, because they use immunodeficient hosts, their application to studying DTC–immune cell biology awaits further development in humanized settings. Perhaps patient-derived explants296 are the closest to representing a human-specific microenvironment, at the expense of limited tissue integrity over time and suboptimal drug diffusion and uptake. Although neither is perfect on its own, combining the strengths of individual models will allow us to effectively translate immuno-oncology research to site-specific DTC targeting in human patients.
Acknowledgements
The author apologizes to those whose invaluable contributions to the field were not cited owing to space limitations. The author is grateful to J. C. Guimaraes and F. Landum for their helpful comments on the manuscript. The author’s laboratory is supported by the Champalimaud Foundation, the Beug Foundation (2021 Metastasis Research Prize) and the European Molecular Biology Organization (EMBO Installation Grant 5329).
Glossary
- Dormancy
A state of pause in cancer progression in which individual disseminated tumour cells are quiescent and reversibly arrested in G0 phase of the cell cycle.
- Disseminated tumour cells
(DTCs). Cancer cells that have left the primary tumour and survived in the circulation to land in a distant site.
- Metastases
Outgrowths of disseminated tumour cells that are histologically or radiologically detectable.
- Niche
A term borrowed from ecology that refers to a unique and optimal tissue microenvironment with defined nurturing and positional cues in a given anatomical location that allows a cell or a group of cells to survive and function.
- Pre-metastatic niche
A microenvironment within a distant site that is permissive for the survival and outgrowth of incoming disseminated tumour cells before their arrival at that site.
- Tissue-resident immune cells
Immune cells that occupy tissues for prolonged periods and undergo little or no recirculation.
- Tolerogenic milieu
An environment readily suppressive of adaptive immune responses, promoting tolerance to frequently encountered antigens, while maintaining local and systemic homeostasis.
Peer review
Peer review information
Nature Reviews Immunology thanks J. Alvarez, J. Ordovas-Montanes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein CA. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer. 2020;20:681–694. doi: 10.1038/s41568-020-00300-6. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 4.Harper KL, et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature. 2016;540:588–592. doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini H, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–558. doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husemann Y, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schardt JA, et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–239. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug. Resist. Updat. 2012;15:39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Li Z, Ma Z, Curtis C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 2020;52:701–708. doi: 10.1038/s41588-020-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brastianos PK, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Kittler O, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl Acad. Sci. USA. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shain AH, et al. The genetic evolution of metastatic uveal melanoma. Nat. Genet. 2019;51:1123–1130. doi: 10.1038/s41588-019-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoecklein NH, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell. 2008;13:441–453. doi: 10.1016/j.ccr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch. Pathol. Lab. Med. 2008;132:931–939. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 16.Hadfield G. The dormant cancer cell. Br. Med. J. 1954;2:607–610. doi: 10.1136/bmj.2.4888.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J. Surg. Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 18.Nixon IJ, et al. Surgical management of metastases to the thyroid gland. Ann. Surg. Oncol. 2011;18:800–804. doi: 10.1245/s10434-010-1408-2. [DOI] [PubMed] [Google Scholar]
- 19.Warren S, Davis A. Studies on tumor metastasis. The metastasis of carcinoma to the spleen. Am. J. Cancer. 1934;21:517–533. doi: 10.1158/ajc.1934.517. [DOI] [Google Scholar]
- 20.Correia AL, et al. Hepatic stellate cells suppress NK cell sustained breast cancer dormancy. Nature. 2021;594:566–571. doi: 10.1038/s41586-021-03614-z. [DOI] [PubMed] [Google Scholar]
- 21.Collignon FP, Holland EC, Feng S. Organ donors with malignant gliomas: an update. Am. J. Transpl. 2004;4:15–21. doi: 10.1046/j.1600-6143.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N. Engl. J. Med. 2003;348:567–568. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- 23.Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 2010;11:790–796. doi: 10.1016/S1470-2045(10)70024-3. [DOI] [PubMed] [Google Scholar]
- 24.Xiao D, et al. Donor cancer transmission in kidney transplantation: a systematic review. Am. J. Transpl. 2013;13:2645–2652. doi: 10.1111/ajt.12430. [DOI] [PubMed] [Google Scholar]
- 25.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 26.Feuerer M, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat. Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 27.Feuerer M, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer. 2001;92:96–105. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1152>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Eyles J, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 30.Rakhra K, et al. CD4+ T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 2010;18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng MW, et al. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012;72:3987–3996. doi: 10.1158/0008-5472.CAN-12-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malaise M, et al. KLRG1+ NK cells protect T-bet-deficient mice from pulmonary metastatic colorectal carcinoma. J. Immunol. 2014;192:1954–1961. doi: 10.4049/jimmunol.1300876. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl Acad. Sci. USA. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albrengues J, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitamura T, et al. Monocytes differentiate to immune suppressive precursors of metastasis-associated macrophages in mouse models of metastatic breast cancer. Front. Immunol. 2018;8:2004. doi: 10.3389/fimmu.2017.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 37.Sharma SK, et al. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J. Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 38.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffelt SB, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao S, Qian Y, Xu G, Luo Q, Zhang Z. Long-term characterization of activated microglia/macrophages facilitating the development of experimental brain metastasis through intravital microscopic imaging. J. Neuroinflammation. 2019;16:4. doi: 10.1186/s12974-018-1389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 44.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards SC, Hoevenaar WHM, Coffelt SB. Emerging immunotherapies for metastasis. Br. J. Cancer. 2021;124:37–48. doi: 10.1038/s41416-020-01160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray JI, Farber DL. Tissue-resident immune cells in humans. Annu. Rev. Immunol. 2022;40:195–220. doi: 10.1146/annurev-immunol-093019-112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altan-Bonnet G, Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019;19:205–217. doi: 10.1038/s41577-019-0131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Eynde M, et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell. 2018;34:1012–1026.e1013. doi: 10.1016/j.ccell.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Angelova M, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765.e716. doi: 10.1016/j.cell.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 51.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 52.Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat. Rev. Cancer. 2022;22:397–413. doi: 10.1038/s41568-022-00462-5. [DOI] [PubMed] [Google Scholar]
- 53.Houghton AN. Cancer antigens: immune recognition of self and altered self. J. Exp. Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;99:87–98. doi: 10.1034/j.1600-0706.2000.880110.x. [DOI] [Google Scholar]
- 55.Fan X, Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell. 2016;164:1198–1211. doi: 10.1016/j.cell.2016.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.St John AL, Rathore APS, Ginhoux F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 2023;23:55–68. doi: 10.1038/s41577-022-00731-2. [DOI] [PubMed] [Google Scholar]
- 58.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meininger I, et al. Tissue-specific features of innate lymphoid cells. Trends Immunol. 2020;41:902–917. doi: 10.1016/j.it.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 2018;18:559–574. doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribot JC, Lopes N, Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 2021;21:221–232. doi: 10.1038/s41577-020-00452-4. [DOI] [PubMed] [Google Scholar]
- 62.Toubal A, Nel I, Lotersztajn S, Lehuen A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 2019;19:643–657. doi: 10.1038/s41577-019-0191-y. [DOI] [PubMed] [Google Scholar]
- 63.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 64.Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185:4259–4279. doi: 10.1016/j.cell.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willekens FL, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 66.Bleriot C, et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101–2116.e2106. doi: 10.1016/j.immuni.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 68.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mass E, et al. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353:aaf4238. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gautier EL, et al. Systemic analysis of PPARγ in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J. Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Germain RN, et al. Understanding immunity in a tissue-centric context: combining novel imaging methods and mathematics to extract new insights into function and dysfunction. Immunol. Rev. 2022;306:8–24. doi: 10.1111/imr.13052. [DOI] [PubMed] [Google Scholar]
- 74.Bonaguro L, et al. A guide to systems-level immunomics. Nat. Immunol. 2022;23:1412–1423. doi: 10.1038/s41590-022-01309-9. [DOI] [PubMed] [Google Scholar]
- 75.McFarland AP, et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity. 2021;54:1320–1337.e1324. doi: 10.1016/j.immuni.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Hove H, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 77.Jordao MJC, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363:aat7554. doi: 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- 78.Robinette ML, et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gury-BenAri M, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166:1231–1246.e1213. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 80.Halpern KB, et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352–356. doi: 10.1038/nature21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabula Muris Consortium et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 2018;562:367–372. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masuda T, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 83.Krishnarajah S, et al. Single-cell profiling of immune system alterations in lymphoid, barrier and solid tissues in aged mice. Nat. Aging. 2022;2:74–89. doi: 10.1038/s43587-021-00148-x. [DOI] [PubMed] [Google Scholar]
- 84.Tabula Sapiens Consortium et al. The Tabula sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science. 2022;376:eabl4896. doi: 10.1126/science.abl4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dominguez Conde C, et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;375:eab15197. doi: 10.1126/science.abl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aizarani N, et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572:199–204. doi: 10.1038/s41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.James KR, et al. Distinct microbial and immune niches of the human colon. Nat. Immunol. 2020;21:343–353. doi: 10.1038/s41590-020-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park JE, et al. A cell atlas of human thymic development defines T cell repertoire formation. Science. 2020;367:eaay3224. doi: 10.1126/science.aay3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stewart BJ, et al. Spatiotemporal immune zonation of the human kidney. Science. 2019;365:1461–1466. doi: 10.1126/science.aat5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dogra P, et al. Tissue determinants of human NK cell development, function, and residence. Cell. 2020;180:749–763.e713. doi: 10.1016/j.cell.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong MT, et al. A high-dimensional atlas of human T cell diversity reveals tissue-specific trafficking and cytokine signatures. Immunity. 2016;45:442–456. doi: 10.1016/j.immuni.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Sankowski R, et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat. Neurosci. 2019;22:2098–2110. doi: 10.1038/s41593-019-0532-y. [DOI] [PubMed] [Google Scholar]
- 93.Muraro MJ, et al. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016;3:385–394.e383. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobayashi T, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–997.e916. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of Langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kana V, et al. CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J. Exp. Med. 2019;216:2265–2281. doi: 10.1084/jem.20182037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahlakoiv T, et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 2019;4:eaax0416. doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rana BMJ, et al. A stromal cell niche sustains ILC2-mediated type-2 conditioning in adipose tissue. J. Exp. Med. 2019;216:1999–2009. doi: 10.1084/jem.20190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cardoso F, et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature. 2021;597:410–414. doi: 10.1038/s41586-021-03830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spallanzani RG, et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 2019;4:eaaw3658. doi: 10.1126/sciimmunol.aaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baccala R, et al. γδ T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J. Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 106.Cui G, et al. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl Acad. Sci. USA. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mackay LK, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 108.Matsuda JL, et al. Homeostasis of Vα14i NKT cells. Nat. Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 109.Maki K, et al. Interleukin 7 receptor-deficient mice lack γδ T cells. Proc. Natl Acad. Sci. USA. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Konkel JE, et al. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat. Immunol. 2011;12:312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korin B, et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 2017;20:1300–1309. doi: 10.1038/nn.4610. [DOI] [PubMed] [Google Scholar]
- 113.Asarnow DM, et al. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 114.Sato K, Ohtsuka K, Watanabe H, Asakura H, Abo T. Detailed characterization of gamma delta T cells within the organs in mice: classification into three groups. Immunology. 1993;80:380–387. [PMC free article] [PubMed] [Google Scholar]
- 115.Haley PJ, Muggenburg BA, Weissman DN, Bice DE. Comparative morphology and morphometry of alveolar macrophages from six species. Am. J. Anat. 1991;191:401–407. doi: 10.1002/aja.1001910407. [DOI] [PubMed] [Google Scholar]
- 116.Chang MK, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 117.Granot T, et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thome JJ, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Esterhazy D, et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature. 2019;569:126–130. doi: 10.1038/s41586-019-1125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jimenez-Sanchez A, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell. 2017;170:927–938.e920. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Budczies J, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6:570–583. doi: 10.18632/oncotarget.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ikawa K, Terashima Y, Sasaki K, Tashiro S. Genetic detection of liver micrometastases that are undetectable histologically. J. Surg. Res. 2002;106:124–130. doi: 10.1006/jsre.2002.6459. [DOI] [PubMed] [Google Scholar]
- 123.Naumov GN, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- 124.Noltenius C, Noltenius H. Dormant tumor cells in liver and brain. An autopsy study on metastasizing tumors. Pathol. Res. Pract. 1985;179:504–511. doi: 10.1016/S0344-0338(85)80191-6. [DOI] [PubMed] [Google Scholar]
- 125.Takeda K, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 126.Molgora M, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Melhem A, et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J. Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 128.Radaeva S, et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterol. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 129.Shen K, et al. Activation of innate immunity (NK/IFN-γ) in rat allogeneic liver transplantation: contribution to liver injury and suppression of hepatocyte proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G1070–G1077. doi: 10.1152/ajpgi.00554.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bayon LG, et al. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology. 1996;23:1224–1231. doi: 10.1002/hep.510230542. [DOI] [PubMed] [Google Scholar]
- 131.Matsumura H, et al. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int. J. Oncol. 2014;45:2303–2310. doi: 10.3892/ijo.2014.2662. [DOI] [PubMed] [Google Scholar]
- 132.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nielsen SR, et al. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat. Cell Biol. 2016;18:549–560. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ben-Moshe S, et al. Spatial sorting enables comprehensive characterization of liver zonation. Nat. Metab. 2019;1:899–911. doi: 10.1038/s42255-019-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gola A, et al. Commensal-driven immune zonation of the liver promotes host defence. Nature. 2021;589:131–136. doi: 10.1038/s41586-020-2977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krueger PD, et al. Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells. J. Leukoc. Biol. 2017;101:329–338. doi: 10.1189/jlb.3A0516-225R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hudspeth K, et al. Human liver-resident CD56bright/CD16neg NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J. Autoimmun. 2016;66:40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guilliams M, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396.e338. doi: 10.1016/j.cell.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Remmerie A, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from Kupffer cells in the fatty liver. Immunity. 2020;53:641–657.e614. doi: 10.1016/j.immuni.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donadon M, et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J. Exp. Med. 2020;217:e20191847. doi: 10.1084/jem.20191847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hildebrandt F, et al. Spatial transcriptomics to define transcriptional patterns of zonation and structural components in the mouse liver. Nat. Comms. 2021;12:7046. doi: 10.1038/s41467-021-27354-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Droin C, et al. Space-time logic of liver gene expression at sub-lobular scale. Nat. Metab. 2021;3:43–58. doi: 10.1038/s42255-020-00323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stella GM, Kolling S, Benvenuti S, Bortolotto C. Lung-seeking metastases. Cancers. 2019;11:1010. doi: 10.3390/cancers11071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity. 2022;55:1564–1580. doi: 10.1016/j.immuni.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 147.Huggins DN, et al. Characterizing macrophage diversity in metastasis-bearing lungs reveals a lipid-associated macrophage subset. Cancer Res. 2021;81:5284–5295. doi: 10.1158/0008-5472.CAN-21-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clever D, et al. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell. 2016;166:1117–1131.e1114. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chan CJ, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat. Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 150.Malladi S, et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smyth MJ, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J. Immunol. 1999;162:6658–6662. doi: 10.4049/jimmunol.162.11.6658. [DOI] [PubMed] [Google Scholar]
- 152.Schuijs MJ, et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020;21:998–1009. doi: 10.1038/s41590-020-0745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Perego M, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci. Transl. Med. 2020;12:eabb5817. doi: 10.1126/scitranslmed.abb5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li P, et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 2020;21:1444–1455. doi: 10.1038/s41590-020-0783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Romero I, et al. T lymphocytes restrain spontaneous metastases in permanent dormancy. Cancer Res. 2014;74:1958–1968. doi: 10.1158/0008-5472.CAN-13-2084. [DOI] [PubMed] [Google Scholar]
- 156.Piranlioglu R, et al. Primary tumor-induced immunity eradicates disseminated tumor cells in syngeneic mouse model. Nat. Commun. 2019;10:1430. doi: 10.1038/s41467-019-09015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shani O, et al. Fibroblast-derived IL33 facilitates breast cancer metastasis by modifying the immune microenvironment and driving type 2 immunity. Cancer Res. 2020;80:5317–5329. doi: 10.1158/0008-5472.CAN-20-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Krall JA, et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl. Med. 2018;10:eaan3464. doi: 10.1126/scitranslmed.aan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 160.Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP. Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J. Clin. Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 161.Janni W, et al. Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse — a European pooled analysis. Clin. Cancer Res. 2011;17:2967–2976. doi: 10.1158/1078-0432.CCR-10-2515. [DOI] [PubMed] [Google Scholar]
- 162.Al-Muqbel KM. Bone marrow metastasis is an early stage of bone metastasis in breast cancer detected clinically by F18-FDG-PET/CT imaging. Biomed. Res. Int. 2017;2017:9852632. doi: 10.1155/2017/9852632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Willis, R. A. The Spread of Tumours in the Human Body (Butterworth & Co, 1952).
- 164.Zhao E, et al. Bone marrow and the control of immunity. Cell Mol. Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Monteran L, et al. Bone metastasis is associated with acquisition of mesenchymal phenotype and immune suppression in a model of spontaneous breast cancer metastasis. Sci. Rep. 2020;10:13838. doi: 10.1038/s41598-020-70788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sawant A, et al. Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Res. 2013;73:672–682. doi: 10.1158/0008-5472.CAN-12-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu X, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hirata Y, et al. CD150high bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 2018;22:445–453.e445. doi: 10.1016/j.stem.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lode HN, et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. doi: 10.1182/blood.V91.5.1706. [DOI] [PubMed] [Google Scholar]
- 171.Bidwell BN, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 2012;18:1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- 172.Rautela J, et al. Loss of host type-I IFN signaling accelerates metastasis and impairs NK-cell antitumor function in multiple models of breast cancer. Cancer Immunol. Res. 2015;3:1207–1217. doi: 10.1158/2326-6066.CIR-15-0065. [DOI] [PubMed] [Google Scholar]
- 173.Owen KL, et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 2020;21:e50162. doi: 10.15252/embr.202050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 175.Omata Y, et al. Type 2 innate lymphoid cells inhibit the differentiation of osteoclasts and protect from ovariectomy-induced bone loss. Bone. 2020;136:115335. doi: 10.1016/j.bone.2020.115335. [DOI] [PubMed] [Google Scholar]
- 176.Achrol AS, et al. Brain metastases. Nat. Rev. Dis. Prim. 2019;5:5. doi: 10.1038/s41572-018-0055-y. [DOI] [PubMed] [Google Scholar]
- 177.Shechter R, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Louveau A, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat. Immunol. 2021;22:1083–1092. doi: 10.1038/s41590-021-00994-2. [DOI] [PubMed] [Google Scholar]
- 180.Dani N, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056–3074.e3021. doi: 10.1016/j.cell.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mrdjen D, et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:380–395.e386. doi: 10.1016/j.immuni.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 182.Fitzgerald DP, et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Berghoff AS, Lassmann H, Preusser M, Hoftberger R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin. Exp. Metastasis. 2013;30:69–81. doi: 10.1007/s10585-012-9510-4. [DOI] [PubMed] [Google Scholar]
- 184.Brantley EC, et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl. Oncol. 2010;3:380–388. doi: 10.1593/tlo.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sarkar S, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat. Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]
- 186.Pukrop T, et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia. 2010;58:1477–1489. doi: 10.1002/glia.21022. [DOI] [PubMed] [Google Scholar]
- 187.Schulz M, et al. Cellular and molecular changes of brain metastases-associated myeloid cells during disease progression and therapeutic response. iScience. 2020;23:101178. doi: 10.1016/j.isci.2020.101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guldner IH, et al. CNS-native myeloid cells drive immune suppression in the brain metastatic niche through Cxcl10. Cell. 2020;183:1234–1248.e1225. doi: 10.1016/j.cell.2020.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Klemm F, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020;181:1643–1660.e1617. doi: 10.1016/j.cell.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Friebel E, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020;181:1626–1642.e1620. doi: 10.1016/j.cell.2020.04.055. [DOI] [PubMed] [Google Scholar]
- 191.Er EE, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 2018;20:966–978. doi: 10.1038/s41556-018-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Saranchova I, et al. Type 2 innate lymphocytes actuate immunity against tumours and limit cancer metastasis. Sci. Rep. 2018;8:2924. doi: 10.1038/s41598-018-20608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin JD, Weng HF, Ho YS. Clinical and pathological characteristics of secondary thyroid cancer. Thyroid. 1998;8:149–153. doi: 10.1089/thy.1998.8.149. [DOI] [PubMed] [Google Scholar]
- 194.Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland. Pathological and clinical findings. Cancer. 1962;15:557–565. doi: 10.1002/1097-0142(196205/06)15:3<557::AID-CNCR2820150315>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 195.Hull OH. Critical analysis of two hundred twenty-one thyroid glands; study of thyroid glands obtained at necropsy in Colorado. AMA Arch. Pathol. 1955;59:291–311. [PubMed] [Google Scholar]
- 196.Rice CO. Microscopic metastases in the thyroid gland. Am. J. Pathol. 1934;10:407–412.401. [PMC free article] [PubMed] [Google Scholar]
- 197.Willis RA. Metastatic tumours in the thyreoid gland. Am. J. Pathol. 1931;7:187–208.183. [PMC free article] [PubMed] [Google Scholar]
- 198.Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144:2123–2140. doi: 10.1242/dev.145615. [DOI] [PubMed] [Google Scholar]
- 199.Montesinos MDM, Pellizas CG. Thyroid hormone action on innate immunity. Front. Endocrinol. 2019;10:350. doi: 10.3389/fendo.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Provinciali M, Fabris N. Modulation of lymphoid cell sensitivity to interferon by thyroid hormones. J. Endocrinol. Invest. 1990;13:187–191. doi: 10.1007/BF03349536. [DOI] [PubMed] [Google Scholar]
- 201.Hodkinson CF, et al. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55–70 years. J. Endocrinol. 2009;202:55–63. doi: 10.1677/JOE-08-0488. [DOI] [PubMed] [Google Scholar]
- 202.Mascanfroni I, et al. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22:1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- 203.Lam KY, Tang V. Metastatic tumors to the spleen: a 25-year clinicopathologic study. Arch. Pathol. Lab. Med. 2000;124:526–530. doi: 10.5858/2000-124-0526-MTTTS. [DOI] [PubMed] [Google Scholar]
- 204.O’Reilly MS, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 205.Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci. Immunol. 2019;4:eaau6085. doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.den Haan JM, Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J. Innate Immun. 2012;4:437–445. doi: 10.1159/000335216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu E, Dang EV, McDonald JG, Cyster JG. Distinct oxysterol requirements for positioning naive and activated dendritic cells in the spleen. Sci. Immunol. 2017;2:eaal5237. doi: 10.1126/sciimmunol.aal5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Golomb L, et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death Differ. 2015;22:1764–1774. doi: 10.1038/cdd.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 210.Rossi DJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl Acad. Sci. USA. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pang WW, et al. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl Acad. Sci. USA. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grizzle WE, et al. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech. Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 213.Ruhland MK, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat. Commun. 2016;7:11762. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fane ME, et al. Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature. 2022;606:396–405. doi: 10.1038/s41586-022-04774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Palomino-Segura M, Hidalgo A. Circadian immune circuits. J. Exp. Med. 2021;218:e20200798. doi: 10.1084/jem.20200798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Casanova-Acebes M, et al. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 2018;215:2778–2795. doi: 10.1084/jem.20181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Diamantopoulou Z, et al. The metastatic spread of breast cancer accelerates during sleep. Nature. 2022;607:156–162. doi: 10.1038/s41586-022-04875-y. [DOI] [PubMed] [Google Scholar]
- 218.Viaud S, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Matson V, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gopalakrishnan V, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Olszak T, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Naik S, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jin C, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. 2019;176:998–1013.e1016. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ma C, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Karagiannis GS, Condeelis JS, Oktay MH. Chemotherapy-induced metastasis: molecular mechanisms, clinical manifestations, therapeutic interventions. Cancer Res. 2019;79:4567–4576. doi: 10.1158/0008-5472.CAN-19-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shree T, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes Dev. 2011;25:2465–2479. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bruchard M, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 230.Keklikoglou I, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019;21:190–202. doi: 10.1038/s41556-018-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bellomo G, et al. Chemotherapy-induced infiltration of neutrophils promotes pancreatic cancer metastasis via Gas6/AXL signalling axis. Gut. 2022;71:2284–2299. doi: 10.1136/gutjnl-2021-325272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Haj-Shomaly J, et al. T cells promote metastasis by regulating extracellular matrix remodeling following chemotherapy. Cancer Res. 2022;82:278–291. doi: 10.1158/0008-5472.CAN-21-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lu Y, et al. Complement signals determine opposite effects of B cells in chemotherapy-induced immunity. Cell. 2020;180:1081–1097.e1024. doi: 10.1016/j.cell.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 234.Monteran L, et al. Chemotherapy-induced complement signaling modulates immunosuppression and metastatic relapse in breast cancer. Nat. Commun. 2022;13:5797. doi: 10.1038/s41467-022-33598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nolan E, et al. Radiation exposure elicits a neutrophil-driven response in healthy lung tissue that enhances metastatic colonization. Nat. Cancer. 2022;3:173–187. doi: 10.1038/s43018-022-00336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee LYW, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Scaife CL, et al. Association between postoperative complications and clinical cancer outcomes. Ann. Surg. Oncol. 2013;20:4063–4066. doi: 10.1245/s10434-013-3267-0. [DOI] [PubMed] [Google Scholar]
- 238.Tohme S, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.El Rayes T, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl Acad. Sci. USA. 2015;112:16000–16005. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nagareddy PR, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McDowell SAC, et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat. Cancer. 2021;2:545–562. doi: 10.1038/s43018-021-00194-9. [DOI] [PubMed] [Google Scholar]
- 242.Quail DF, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell Biol. 2017;19:974–987. doi: 10.1038/ncb3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ringel AE, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–1866.e1826. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Michelet X, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018;19:1330–1340. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 245.Bondareva O, et al. Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity. Nat. Metab. 2022;4:1591–1610. doi: 10.1038/s42255-022-00674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 247.Campbell JP, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Decker AM, et al. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol. Cancer Res. 2017;15:1644–1655. doi: 10.1158/1541-7786.MCR-17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shakhar G, Ben-Eliyahu S. In vivo β-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J. Immunol. 1998;160:3251–3258. doi: 10.4049/jimmunol.160.7.3251. [DOI] [PubMed] [Google Scholar]
- 250.Chen H, et al. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J. Pathol. 2018;244:49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 251.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015;12:213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Thompson K, et al. Activation of γδ T cells by bisphosphonates. Adv. Exp. Med. Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 253.Early Breast Cancer Trialists’ Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 254.Lenk L, et al. The hepatic microenvironment essentially determines tumor cell dormancy and metastatic outgrowth of pancreatic ductal adenocarcinoma. Oncoimmunology. 2017;7:e1368603. doi: 10.1080/2162402X.2017.1368603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ding N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48:920–930. doi: 10.1002/hep.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014;2:187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 259.Buechler MB, et al. Cross-tissue organization of the fibroblast lineage. Nature. 2021;593:575–579. doi: 10.1038/s41586-021-03549-5. [DOI] [PubMed] [Google Scholar]
- 260.Krausgruber T, et al. Structural cells are key regulators of organ-specific immune responses. Nature. 2020;583:296–302. doi: 10.1038/s41586-020-2424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Beachley VZ, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat. Methods. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McCabe, M. C., Saviola, A. J. & Hansen, K. C. A mass spectrometry-based atlas of extracellular matrix proteins across 25 mouse organs. Preprint at BioRxiv10.1101/2022.03.04.482898 (2022). [DOI] [PubMed]
- 263.Bartoschek M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li H, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017;49:708–718. doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 265.Lambrechts D, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 266.Davidson S, et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep. 2020;31:107628. doi: 10.1016/j.celrep.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dominguez CX, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232–253. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 268.Kieffer Y, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020;10:1330–1351. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 269.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wieland E, et al. Endothelial Notch1 activity facilitates metastasis. Cancer Cell. 2017;31:355–367. doi: 10.1016/j.ccell.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 271.Shetty S, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 2011;186:4147–4155. doi: 10.4049/jimmunol.1002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee SS, Bindokas VP, Kron SJ. Multiplex three-dimensional optical mapping of tumor immune microenvironment. Sci. Rep. 2017;7:17031. doi: 10.1038/s41598-017-16987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Motz GT, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Amersfoort J, Eelen G, Carmeliet P. Immunomodulation by endothelial cells - partnering up with the immune system? Nat. Rev. Immunol. 2022;22:576–588. doi: 10.1038/s41577-022-00694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Steele MM, Lund AW. Afferent lymphatic transport and peripheral tissue immunity. J. Immunol. 2021;206:264–272. doi: 10.4049/jimmunol.2001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Skobe M, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 277.Demicheli R, et al. Microscopic tumor foci in axillary lymph nodes may reveal the recurrence dynamics of breast cancer. Cancer Commun. 2019;39:35. doi: 10.1186/s40880-019-0381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kim M, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 279.Tewalt EF, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–4782. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lund AW, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 281.Zeng Q, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573:526–531. doi: 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gabanyi I, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Godinho-Silva C, Cardoso F, Veiga-Fernandes H. Neuro-immune cell units: a new paradigm in physiology. Annu. Rev. Immunol. 2019;37:19–46. doi: 10.1146/annurev-immunol-042718-041812. [DOI] [PubMed] [Google Scholar]
- 284.Iacobuzio-Donahue CA, et al. Cancer biology as revealed by the research autopsy. Nat. Rev. Cancer. 2019;19:686–697. doi: 10.1038/s41568-019-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Goltsev Y, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 2018;174:968–981.e915. doi: 10.1016/j.cell.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Stoeckius M, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Giesen C, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 288.Angelo M, et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Stahl PL, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82. doi: 10.1126/science.aaf2403. [DOI] [PubMed] [Google Scholar]
- 290.Merritt CR, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020;38:586–599. doi: 10.1038/s41587-020-0472-9. [DOI] [PubMed] [Google Scholar]
- 291.Zitvogel L, Pitt JM, Daillere R, Smyth MJ, Kroemer G. Mouse models in oncoimmunology. Nat. Rev. Cancer. 2016;16:759–773. doi: 10.1038/nrc.2016.91. [DOI] [PubMed] [Google Scholar]
- 292.Weeber F, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl Acad. Sci. USA. 2015;112:13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Dijkstra KK, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174:1586–1598.e1512. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pauli C, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fior R, et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl Acad. Sci. USA. 2017;114:E8234–E8243. doi: 10.1073/pnas.1618389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Voabil P, et al. An ex vivo tumor fragment platform to dissect response to PD-1 blockade in cancer. Nat. Med. 2021;27:1250–1261. doi: 10.1038/s41591-021-01398-3. [DOI] [PubMed] [Google Scholar]