Abstract
Background and Aims
Genome size is an important plant trait, with substantial interspecies variation. The mechanisms and selective pressures underlying genome size evolution are important topics in evolutionary biology. There is considerable diversity in Allium from the Qinghai–Tibetan Plateau, where genome size variation and related evolutionary mechanisms are poorly understood.
Methods
We reconstructed the Allium phylogeny using DNA sequences from 71 species. We also estimated genome sizes of 62 species, and determined chromosome numbers in 65 species. We examined the phylogenetic signal associated with genome size variation, and tested how well the data fit different evolutionary models. Correlations between genome size variations and seed mass, altitude and 19 bioclimatic factors were determined.
Key Results
Allium genome sizes differed substantially between species and within diploids, triploids, tetraploids, hexaploids and octaploids. Size per monoploid genome (1Cx) tended to decrease with increasing ploidy levels. Allium polyploids tended to grow at a higher altitude than diploids. The phylogenetic tree was divided into three evolutionary branches. The genomes in Clade I were mostly close to the ancestral genome (18.781 pg) while those in Clades II and III tended to expand and contract, respectively. A weak phylogenetic signal was detected for Allium genome size. Furthermore, significant positive correlations were detected between genome size and seed mass, as well as between genome size and altitude. However, genome size was not correlated with 19 bioclimatic variables.
Conclusions
Allium genome size shows gradual evolution, followed by subsequent adaptive radiation. The three well-supported Allium clades are consistent with previous studies. The evolutionary patterns in different Allium clades revealed genome contraction, expansion and relative stasis. The Allium species in Clade II may follow adaptive radiation. The genome contraction in Clade III may be due to DNA loss after polyploidization. Allium genome size might be influenced by selective pressure due to the conditions on the Qinghai–Tibetan Plateau (low temperature, high UV irradiation and abundant phosphate in the soil).
Keywords: Genome size evolution, polyploidy, adaptation, ecological factors, seed mass, Allium, Qinghai–Tibetan Plateau
INTRODUCTION
Genome size is the total DNA amount in the unreplicated haploid nucleus (Greilhuber, 2005). It is an important consideration when examining plant evolution, because it represents the necessary baseline information for whole-genome sequencing, and it relationships to morphological characteristics, physiological indices and polyploidization have been reported (Beaulieu et al., 2008). Genome size differs substantially among species, varying by over 2400-fold among angiosperms (Pellicer et al., 2010). There are probably several reasons for the differences in genome size, including recombination rate, tandem repeats (Tiley and Burleigh, 2015), transposable elements (TEs) and polyploidization; however, the relative contribution of each of these factors probably varies between species (Bennetzen et al., 2005). Remarkably, genome size variations may also be linked to several other factors, including evolutionary processes (Rees et al., 1966; Laurie and Bennett, 1985; Leitch and Leitch, 2013) such as selection (Bennetzen et al., 2005), as well as abiotic factors, including elevation (Suda et al., 2009), rainfall (Qiu et al., 2019) and soil nutrient content (Guignard et al., 2016). Clearly, ecological factors contribute to genome size variations (Carta and Peruzzi, 2016).
Genome size is also related to the phenotype, karyotype and seed characteristics (Kang et al., 2014; Jordan et al., 2015). For example, there are strong correlations between genome size and cell size, guard cell length and seed mass (Knight et al., 2005). Additionally, a significant correlation between genome size and karyotype traits (e.g. total karyotype length and mean chromosome length) was observed in Echinops (Asteraceae) (Garnatje et al., 2004). Notably, a previous study demonstrated that the effects of the factors influencing genome size are greater at lower taxonomic levels than at higher taxonomic levels, implying that phylogenetic scale is an important consideration for studies on genome size evolution (Dušková et al., 2010). The genus is an ideal taxonomic level for investigating plant genome size evolution (Hawkins et al., 2008). Unfortunately, there have been relatively few studies addressing genome size variations in a genus and their relationship with the phenotype, karyotype and ecological factors (Kang et al., 2014; Jordan et al., 2015).
The Qinghai–Tibetan Plateau, which is characterized by high elevation, low pressure, low temperature and frequent precipitation (rain and snow), has long been regarded as a ‘natural laboratory’ for investigating plant evolution and environmental adaptation. However, there is limited information available regarding the genome size of plants growing there. The richness of species and high differentiation among Allium species make them ideal for studying genome size evolution on the Qinghai–Tibetan Plateau. Allium is a monophyletic taxon comprising about 971 species in the northern hemisphere, with ~111 species growing on or surrounding the Qinghai–Tibetan Plateau (Hauenschild et al., 2017). There is substantial interest in the relationship between genome size and biological characteristics. For example, previous investigations revealed significant positive correlations between genome size and karyotype characters (e.g. chromosome length and volume), as well as between genome size and seed mass in Allium (Jones and Rees, 1968; Labani and Elkington, 1987; Ohri et al., 1998). The application of model-based phylogenetic comparative methods provides a reliable means to study the mode and tempo of genome size evolution (Kang et al., 2014); however, there are only a few reports regarding Allium. Significant interspecific variations in genome size were detected on the basis of ITS phylogeny (Ricroch et al., 2005). Moreover, the mode and tempo of genome size evolution in Allium subgenus Melanocrommyum were reported to be punctuated evolution and species-specific adaptation according to the ITS phylogeny (Gurushidze et al., 2012). In the present study, we investigate Allium genome size variations and evaluate the relationships between genome size and seed mass, and as well as ecological factors in a phylogenetic framework. We also clarify the mode and tempo of Allium genome size evolution.
MATERIALS AND METHODS
Plant materials
Supplementary Data Table S1 lists 83 taxa studied, which represent 65 species from 12/15 subgenera. A total of 62 species are used for genome size analysis, 63 species used for seed mass analysis and 46 species used for ploidy analysis (Table S2). Genome size, 2C-value, seed mass, chromosome number and ploidy level data are also presented (Table S2). Combined with data downloaded from the GenBank database, the final dataset includes 71 Allium species (Table S3). Leucocoryne pauciflora, Tristagma uniflorum, Tulbaghia fragrans, Ipheion uniflorum, Nothoscordum gracile and Dichelostemma multiflorum are used as outgroups.
All Allium samples were collected from the Qinghai–Tibetan Plateau and then grown in a glasshouse at the Kunming Institute of Botany, Chinese Academy of Sciences. Voucher specimens were deposited in the Herbarium of the Kunming Institute of Botany, Chinese Academy of Sciences (KUN).
Chromosome number
Root tips were pretreated with 8-hydroxyquinoline (0.002 mol L–1) at 20–21 °C for 4–5 h, fixed in Carnoy solution (ethanol/acetic acid = 3:1) for 50 min at 4 °C, and then dissociated in a solution (1 m HCl/45 % acetic acid = 1:1) at 60 °C for 30 s. We used acetic orcein (1 %) to stain for 2–3 h and observed root tips on a glass slide (Wang et al., 2013). Three to five individuals were studied, and at least five metaphase plates from each individual were studied for counting chromosome numbers.
Genome size estimation
Genome size was measured using CyFlow Space-3000 (Partec, Germany). Triticum aestivum ‘Chinese Spring’ (2C = 35.60 pg; International Wheat Genome Sequencing Consortium, 2014) and Secale cereale (2C = 16.19 pg; Doležel et al., 1998; Li et al., 2021) were chosen as internal standards. The 1Cx-value was defined as the genome size. Leaf material was cut with a razor blade in a culture dish using WPB nuclear solution buffer (Doležel et al., 2007). After filtering cellular debris using disposable filters (30 µm), the nuclear suspension was stained for 10 min using 150 µL propidium iodide (50 μg mL–1). At least 5000–10 000 nuclei were detected for all samples. The genome size value was the average of one to three accessions, and three individuals per accession were measured. To evaluate the results, the average of coefficient of variation values (CV) <5 % was regarded as reliable. On the basis of the peak of internal standard and Allium species, Allium genome sizes were calculated (Supplementary Data Fig. S1):
Seed mass
Seed mass was calculated using the hundred-grain method. To estimate the seed mass of the investigated taxa, one to three accessions were measured, and the average value of eight repeats of 100 seeds each was recorded as the seed mass.
DNA extraction, PCR amplification and sequencing
A nuclear locus, ITS, and chloroplast loci (rps16, matK, atpB-rbcL, rpl32-trnL and trnQ-rps16) were used to reconstruct a phylogenetic tree (Friesen, 2006; Hauenschild et al., 2017). In this study, 235 of the 372 sequences were previously published and obtained from NCBI GenBank. The DNA sequences for the nuclear locus (ITS: 61 species) and five plastid loci (rps16: 44 species, rpl32-trnL: 39 species, matK: 46 species, atpB-rbcL: 18 species, and trnQ-rps16: 12 species) were downloaded (Supplementary Data Table S3). The remaining 84 sequences were newly generated by DNA extraction, polymerase chain reaction (PCR) amplification and sequencing. These sequences were available at the National Genomics Data Center (https://ngdc.cncb.ac.cn/) under BioProject number CRA005207. Genomic DNA extraction used the CTAB method (Doyle and Doyle, 1987). The volume of PCR amplification was 20 µL including 2.5 µL MgCl2 (1.5 mmol L–1), 2.5 µL dNTP mixture (0.2 mmol L–1), 1 µL primers (0.2 µmol L–1), 0.3 µL Tap polymerase, 2 µL genomic DNA as template, 2 µL PCR buffer and 9.7 µL ddH2O.
The PCR amplification conditions were as follows: ITS: 94 °C for 5 min, followed by 30 cycles of 45 s at 94 °C, 55 °C for 45 s and 72 °C for 1 min, and final extension for 7 min at 72 °C; rps16: 95 °C for 4 min, followed by 35 cycles of 30 s at 95 °C, 51.3 °C for 1 min and 72 °C for 1.5 min, with a final extension for 10 min at 72 °C; matK: 94 °C for 3 min; followed by 32 cycles of 30 s at 94 °C, 55 °C for 30 s and 72 °C for 2 min, with a final extension for 10 min at 72 °C; atpB-rbcL: 94 °C for 3 min; followed by 30 cycles of 45 s at 94 °C, 50 °C for 1 min and 72 °C for 1 min, with final extension for 10 min at 72 °C; rpl32-trnL: 80 °C for 5 min; followed by 30 cycles of 1 min at 95 °C, 50 °C for 1 min and 65 °C for 4 min, with a final extension for 5 min at 65 °C; trnQ-rps16: 94 °C for 4 min; followed by 32 cycles of 45 s at 94 °C, 53 °C for 90 s and 72 °C for 70 s, with a final extension for 10 min at 72 °C.
The polyethylene glycol (PEG) precipitation procedure was used to purify the PCR products. We used ClustalX 1.83 to align sequences (Thompson et al., 1997), and Bioedit to manually edit the sequences (Hall, 1999).
Phylogenetic analysis
We conducted a comprehensive species-level analysis of phylogenetic relationships using the DNA sequences of a nuclear locus (ITS) and chloroplast loci (rps16, rpl32-trnL, matK, atpB-rbcL and trnQ-rps16). Some unavailable sequences were treated as missing data. Bayesian inference (BI), Modeltest 3.7 and MrBayes 3.1.2 were respectively used to reconstruct phylogenetic trees, determine the optimal model of molecular evolution and implement Bayesian inference under a Markov chain Monte Carlo (MCMC) algorithm (Huelsenbeck and Ronquist, 2001; Posada and Crandall, 1998; Posada and Buckley, 2004). PAUP* 4.0 b10 was used to calculate the consensus tree (Swofford, 2003). The incongruence length difference (ILD) test was used to evaluate the congruence of the nuclear and plastid datasets (Farris et al., 1994).
Ancestral genome size
To clarify the evolutionary history of Allium genome size, we reconstructed the ancestral genome size using the square-change parsimony reconstruction method in Mesquite 2.75 (Madison and Madison, 2011). Based on the above phylogenetic tree using MrBayes 3.1.2, the file g. nex. run 1. Was used for ancestral genome calculations in Mesquite 2.75.
Models of trait evolution
To detect the mode and tempo of Allium genome size, we used the generalized least-squares method in Bayes Traits (Pagel and Meade, 2007). In the analysis, the average genome size of species was used to evaluate evolutionary models and for comparative analysis. The median values of 19 bioclimatic variables were determined for each region in which species were collected. The parameters Pagel’s lambda (λ), kappa (κ) and delta (δ) were respectively used to confirm the phylogenetic association, mode and tempo of the genome size evolution. The parameter λ represents the phylogenetic signal, which assesses the contribution of phylogeny to the interspecific covariance of a given trait. Specifically, λ = 0 suggests trait evolution independent of phylogeny, while λ = 1 indicates phylogenetic relationships can effectively predict trait variation patterns (pure Brownian motion process). Additionally, 0 < λ < 1 represents varying degrees in the phylogenetic signal. The parameter κ reflects the contributions of trait evolution to the punctuated and gradual mode in a phylogeny. Specifically, κ = 0 suggests trait evolution does not depend on branch length, which is consistent with a punctuated mode of evolution, whereas κ = 1 suggests that trait evolution depends directly on branch length. Moreover, κ < 1 reflects a greater proportion of evolution in shorter branches, while κ > 1 reflects a greater proportion of evolution in longer branches (gradual mode). The parameter δ is used to test differential evolutionary rates over time and to rescale the phylogeny according to the stability of the evolutionary rate: δ = 1 (gradual evolution). Additionally, δ < 1 suggests a temporally early trait evolution, which is a signature of adaptation radiation, whereas δ > 1 indicates longer paths contributed disproportionately to trait evolution, which represents accelerated evolution over time (species-specific adaptation). The most appropriate models were tested according to likelihood ratio tests (LRTs) (Huelsenbeck and Rannala, 1997).
Phylogenetic regression analysis
We used the phylogenetic generalized least-squares (PGLS) method to evaluate the relationship between genome size and traits. Phylogenetic regression was carried out using a phylogenetic tree, in which internal branches were all multiplied by λ and tip branches were left at their original length (Revell, 2010). Regarding the phylogenetic signal, if λ = 0, this is equal to ordinary (non-phylogenetic) least-squares regression (OLS), and OLS supposes a star phylogeny that residual variation is distinct among species; if λ = 1, PGLS and phylogenetic independent contrasts (PIC) regression are functionally equivalent (Felsenstein, 1985); if 0 < λ < 1, the OLS and the PIC methods are unsuitable, due respectively to their under- and overestimation of the impact of phylogeny (Hernández et al., 2013). In the present study, regression modelling with three types was tested, with λ equal to 0, 1 and estimated values using the R package CAPER. Furthermore, seed mass, altitude and 19 bioclimatic variables served as dependent variables, whereas genome size served as the independent variable (Orme et al., 2012). Median values for the 19 bioclimatic variables were obtained from WorldClim (http://worldclim.org). Correlation analysis involving both OLS and PGLS models were performed using the CAPER package.
RESULTS
Chromosome counts and ploidy levels
Of the 65 Allium species includes in this study (Supplementary Data Table S2), 42 are diploids, four are triploids, 15 are tetraploids, three are hexaploids and one is octaploid. Polyploids in Allium prefer to grow at higher altitude than diploids (Fig. 1). No correlation between ploidy level and latitude was observed (Fig. S2).
Fig. 1.

Boxplots illustrating the variability of diploidy and polyploidy with altitude. The outlined central box depicts the middle 50 % of the data extending from the upper to lower quartile; the horizontal bar is at the median. The ends of the vertical lines indicate the minimum and maximum data values, unless outliers are present. The P value indicates significant difference between diploid and polyploid groups at different altitude (P = 0.0064 < 0.01, t-test).
Genome size variation in Allium
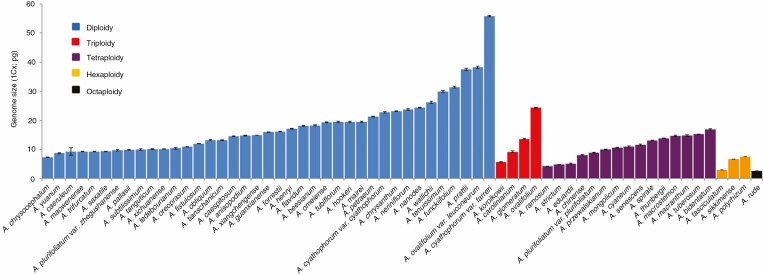
Figure 2 presents the genome size variability among 62 Allium species, including 39 diploid species, four triploid species, 15 tetraploid species, three hexaploid species and one octaploid species. The Allium genome size ranges from 2.61 pg (A. rude) to 55.78 pg (A. cyathophorum var. farreri), a 21.37-fold variablility (Supplementary Data Table S2). The somatic chromosomes are shown in Fig. S3. Of the diploid species, the estimated genome size ranges from 7.35 pg (A. chrysocephalum; 2n = 16) to 55.78 pg (A. cyathophorum var. farreri; 2n = 16) (7.59-fold), with a mean of 18.08 pg. Among the triploids, the estimated genome size ranges from 5.69 pg (A. korolkowii; 2n = 24) to 24.34 pg (A. ovalifolium; 2n = 24) (4.28-fold), with a mean of 13.21 pg. For the tetraploids, the estimated genome size ranges from 4.20 pg (A. ramosum; 2n = 28) to 16.91 pg (A. bidentatum; 2n = 32) (4.03-fold), with a mean of 9.62 pg. Among the hexaploids, the estimated genome size ranges from 2.97 pg (A. fasciculatum; 2n = 42) to 7.55 pg (A. polyrhizum; 2n = 54) (2.54-fold), with a mean of 5.74 pg. Allium rude is the only octaploid species included in this study, and it has the smallest genome (2.61 pg). The average genome size is greater for the diploids than for the triploids, tetraploids, hexaploids and octaploids. Moreover, genome size tends to decrease with increasing ploidy levels (Fig. 3).
Fig. 2.
Genome size estimates for 62 Allium species. Histogram bars represent the average of population means. Error bars represent the standard error of the population means. Species exhibiting cytotypic variation (diploidy, triploidy, tetraploidy, hexaploidy and octaploidy) are indicated.
Fig. 3.
Boxplots illustrating the variability in genome size at different ploidy levels. The outlined central box depicts the middle 50 % of the data extending from the upper to lower quartile; the horizontal bar is at the median. The ends of the vertical lines indicate the minimum and maximum data values, unless outliers are present.
Patterns of genome size evolution in Allium
The ILD test revealed significant incongruency between the ITS and chloroplast DNA (cpDNA) (rpl32-trnL, atpB-rbcL, matK, rps16 and trnQ-rps16) datasets (P = 0.01). Therefore, a combined analysis of ITS and cpDNA sequence data was not performed. Figure 4 presents the Allium phylogenetic tree with genome sizes and ploidy levels determined on the basis of ITS. The reconstructed Allium ancestral genome is estimated to be 18.781 pg (Fig. 5). The variability in the cpDNA data is insufficient for resolving the relationships within Allium (Supplementary Data Fig. S4). The following analysis is based largely on the phylogenetic reconstruction using the ITS dataset.
Fig. 4.
Molecular phyloneny of Allium used in the PGLS analysis. Genome size and ploidy levels mapped on the ITS phylogenetic tree of 71 Allium species. Leucocoryne pauciflora, Tristagma uniflorum, Tulbaghia fragrans, Ipheion uniflorum, Nothoscordum gracile and Dichelostemma multiflorum were used as outgroups.
Fig. 5.
Reconstruction of the Allium ancestral genome; different genome sizes are represented by different colours. #Genome sizes are not available.
Pagel’s λ is greater than 0 (λ = 0.0003), implying that genome size is weakly correlated with phylogeny in Allium (Table 1). The κ value (κ < 1, Table 1) and the δ value (δ < 1, Table 1) respectively indicate that the mode and tempo of the Allium genome size evolution are gradual evolution and adaptive radiation.
Table 1.
Maximum-likelihood estimates for the parameters lambda, kappa, and delta, calculated according to the generalized least square method in BayesTraits. All estimates were calculated under the assumptions of the random walk model.
| Dataset | Parameter | Estimated value | Interpretation |
|---|---|---|---|
| Genome size | Lambda (contribution of phylogeny) | 0.0003 (0 < λ < 1) | Weak phylogenetic signal |
| Kappa (mode of evolution) | –1e+200 (κ < 1) | Gradual evolution | |
| Delta (tempo of evolution) | –264.451 (δ < 1) | Adaptive radiation |
Correlation between genome size and seed mass
Maximum-likelihood tests of the continuous models suggested that genome size and seed mass best fit the Brownian motion model (Table 2). The PIC mode reveals a significant positive relationship between genome size and seed mass (R2 = 0.211, P < 0.001; Table 3), which is consistent with our linear quantile regression (Fig. 6).
Table 2.
Model selection statistics for the evolution of Allium genome size, seed mass and altitude.
| Model | Parameters | Log likelihood | k | AICc |
|---|---|---|---|---|
| Genome size | ||||
| BM | – | –262.616 | 2 | 531.589 |
| Lambda | λ = 1.089e–198 | –264.451 | 3 | 537.508 |
| Delta | δ = 8.596e–32 | –264.451 | 3 | 537.508 |
| Kappa | κ = 2.141e–83 | –1e+200 | 3 | 2e+200 |
| OU | – | –Inf | 3 | Inf |
| Seed mass | ||||
| BM | – | –307.642 | 2 | 621.642 |
| Lambda | λ = 1.424e–91 | –307.642 | 3 | 623.889 |
| Delta | δ = 1.268e–125 | –307.642 | 3 | 623.889 |
| Kappa | κ = 7.123e–194 | –e+200 | 3 | 2e+200 |
| OU | – | –Inf | 3 | Inf |
| Altitude | ||||
| BM | – | –602.006 | 2 | 1210.371 |
| Lambda | λ = 1.095e–49 | –600.848 | 3 | 1210.301 |
| Delta | δ = 5.649e–61 | –602.006 | 3 | 1212.619 |
| Kappa | κ = 2.835e–169 | –1e+200 | 3 | 2e+200 |
| OU | – | – | 3 | — |
Table 3.
Summary of Allium trait correlation as estimated by three types of linear regression models: ordinary least-squares regression (OLS: i.e. non-phylogenetic regression), phylogenetic independent contrasts (PIC) and phylogenetic generalized least-squares (PGLS). Lh: log likelihood; b: slope; Significance levels are denoted by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
| Model | Seed mass–genome size | Altitude–genome size | ||||||
|---|---|---|---|---|---|---|---|---|
| Lh | b | R 2 | P | Lh | b | R 2 | P | |
| OLS (λ = 0) | –305.465 | 0.521 | 0.0749 | 0.0209* | –598.795 | 36.553 | 0.0933 | 0.00959** |
| PIC (λ = 1) | –334.373 | 1.308 | 0.2110 | 5.639e–05*** | –615.514 | 38.382 | 0.077 | 0.0189* |
| PGLS (λ = MLE) | –305.290 | 0.465 | 0.0641 | 0.0332* | –598.215 | 36.946 | 0.010 | 0.0068** |
Fig. 6.
Scatter plot and quantile regression revealing the relationship between genome size and seed mass, and between altitude and genome size. The dashed grey lines correspond to the quantiles (0.15, 0.30, 0.45, 0.60, 0.75, 0.90), the solid blue line represents the median fit to the data, and the red line represents the least-squares estimate for the conditional mean function.
Correlation between genome size and altitude
Maximum-likelihood tests of the continuous models indicated that genome size and altitude best fit the lambda model (Table 2). The OLS mode detects a significant positive relationship between genome size and altitude (R2 = 0.093, P < 0.01; Table 3), which is consistent with our linear quantile regression (Fig. 6).
Correlation between genome size and bioclimatic variables
Under the PGLS model, the PGLS and OLS analysis revealed no correlation between genome size and 19 bioclimatic variables (Table 4).
Table 4.
Summary of the correlations between bioclimatic variables and genome size as estimated by ordinary least squares regression (OLS) and phylogenetic generalized least squares (PGLS) analyses. Significance levels are denoted by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
| R 2 (OLS) | P | R 2 (PGLS) | P | |
|---|---|---|---|---|
| BIO1 = Annual mean temperature | 0.009278 | 0.472 | 0.04109 | 0.127 |
| BIO2 = Mean diurnal range (mean of monthly (max temp − min temp)) | 0.008793 | 0.4838 | 0.004734 | 0.6078 |
| BIO3 = Isothermality (BIO2/BIO7) (*100) | 0.01453 | 0.3674 | 0.01437 | 0.3701 |
| BIO4 = Temperature seasonality (standard deviation *100) | 0.02399 | 0.2457 | 0.02169 | 0.2699 |
| BIO5 = Max temperature of warmest month | 0.01934 | 0.2979 | 0.01955 | 0.2951 |
| BIO6 = Min temperature of coldest month | 6.981e–06 | 0.9843 | 0.0002464 | 0.9069 |
| BIO7 = Temperature annual range (BIO5 − BIO6) | 0.03088 | 0.187 | 0.02712 | 0.2167 |
| BIO8 = Mean temperature of wettest quarter | 0.01755 | 0.3215 | 0.01878 | 0.305 |
| BIO9 = Mean temperature of driest quarter | 0.001019 | 0.812 | 0.002945 | 0.6858 |
| BIO10 = Mean temperature of warmest quarter | 0.01829 | 0.3115 | 0.02048 | 0.2838 |
| BIO11 = Mean temperature of coldest quarter | 5.156e–08 | 0.9987 | 0.000427 | 0.8776 |
| BIO12 = Annual precipitation | 0.008087 | 0.502 | 0.002466 | 0.7112 |
| BIO13 = Precipitation of wettest month | 0.001545 | 0.7696 | 0.0003854 | 0.8837 |
| BIO14 = Precipitation of driest month | 0.0008474 | 0.8283 | 0.001007 | 0.8131 |
| BIO15 = Precipitation seasonality (coefficient of variation) | 0.0002819 | 0.9005 | 0.0007633 | 0.8369 |
| BIO16 = Precipitation of wettest quarter | 0.001496 | 0.7731 | 0.0001141 | 0.9366 |
| BIO17 = Precipitation of driest quarter | 0.002072 | 0.7344 | 0.001209 | 0.7955 |
| BIO18 = Precipitation of warmest quarter | 0.001566 | 0.768 | 0.0001568 | 0.9257 |
| BIO19 = Precipitation of coldest quarter | 0.0006486 | 0.8495 | 0.0005248 | 0.8645 |
DISCUSSION
Polyploidy evolution of Allium on the Qinghai–Tibetan Plateau
Our results regarding the taxonomic distribution of ploidy levels in Allium confirm that of the 65 Allium species examined in this study, 42 are diploids and the rest are polyploids. Cytotypes show an interesting geographical structure, with Allium polyploids preferentially growing at higher altitude than diploids (Fig. 1); there is no correlation between ploidy level and latitude (Supplementary Data Fig. S2). This supports the hypotheses that polyploids are better adapted to extreme climates than diploids. However, some Allium diploids (e.g. A. tubiflorum, A. chrysanthum, A. prattii and A. atrosanguineum) are distributed at high altitude (Jing et al., 1999; Xue et al., 2000; Tang et al., 2005; Zhang et al., 2009; Table S1), and some Allium polyploids (e.g. A. polyrhizum, A. macrostemon, A. senescens and A. strictum) grow at low altitude (Zhu and Xu, 1999; Tang et al., 2005; Table S1). This may be because the dramatic climatic oscillations of the Quaternary caused some species on the Qinghai–Tibetan Plateau to retreat to low-altitude refugia, whereas some species survived in the on the platform (Qiu et al., 2011). Expansion of cooler and drier habitats was caused by global climate fluctuations, with several species, including Allium species, colonizing these habitats to diversify the local flora from the late Miocene to Pliocene (Xie et al., 2019; Han et al., 2020). Polyploidization events are important for the ecological radiation of Allium (Han et al., 2020). For instance, Allium przewalskianum (tetraploidy) resulted from at least eight independent autopolyploidization events that enabled it to colonize and survive in the arid conditions of the Qinghai–Tibetan Plateau (Wu et al., 2010). The small distribution ranges of some Allium species may be attributed to temporal constraints (i.e. their recent origin) (Paule et al., 2017).
Genome size variation and correlation with phylogeny
In the current study, we first comprehensively analysed the Allium genome size variation and evolution on the Qinghai–Tibetan Plateau. We present the results of our initial analysis of phylogenetic trends and the correlation between seed mass/altitude and genome size variation based on the well-resolved ITS phylogeny. Genome sizes vary substantially among the Allium diploids, triploids, tetraploids and hexaploids. A previous study showed that a large proportion of the Allium genome (44–65 %) consisted of repetitive sequences (Ranjekar et al., 1978), especially moderately repetitive sequences (Narayan, 1988). These repeating DNA segments were interspersed with lowly repetitive sequences during the evolution of various species (Narayan, 1988). Allium had one of the largest genomes among taxa in the alpine subnival belt in the Hengduan Mountains, and recent cytological analysis indicated that diploids were common, implying large genome size may be related to chromosome doubling fusion or duplicate gene copies at the chromosome level, not simply chromosome doubling (Sun, 2020). Remarkably, Allium genome size tends to decrease with increasing ploidy levels. This might reflect genome contractions after polyploidization, which is a general trend observed in angiosperms (Leitch and Bennett, 2004). Deletion of repetitive DNA sequences and TE dynamics were probably the most important factors influencing the genomic contraction (Bennetzen et al., 2005). There is cytological evidence of the loss of chromosomal segments following polyploidization. A previous investigation showed that telomeric heterochromatin of Secale cereal lost up to 100 % (Gustafson and Bennett, 1982). Therefore, repetitive sequences and chromosome doubling fusion may be the main reasons for Allium genome size variation.
Obvious differences in genome size evolution are observed among the three well-supported Allium clades (Fig. 4), which is consistent with previous studies (Hauenschild et al., 2017; Xie et al., 2020). The genomes of Clade I Allium species show minimal variations, with estimated genome sizes that were similar to the estimated size of the reconstructed ancestral genome (18.781 pg). Two noteworthy exceptions are the diploid A. wallichii (26.17 pg) and the hexaploid A. fasciculatum (2.97 pg). Accordingly, the A. wallichii genome is larger than the genomes of most diploid species. The considerable abundance of repetitive sequences in the Allium genome (44-65 %) (Ranjekar et al., 1978) suggested that the relatively large diploid A. wallichii genome may have resulted from an increase in the number of repetitive sequences. The small genome of hexaploid A. fasciculatum may be related to DNA loss during polyploidization. The genomes of most of the Clade II Allium species have experienced genomic expansion relative to the ancestral reconstructed estimates (Fig. 4). Interestingly, these species are diploids, with the exception of the triploid A. ovalifolium, and they grow at high altitude (2245–4564 m) except for A. maowenense (1500 m). A significant positive relationship between genome size and altitude was tested by the OLS model and the linear quantile regression model (Table 3; Fig. 6). The correlation between genome size and ecological factors is discussed further below. Additionally, adaptive radiation may have occurred in the species in this clade. This possibility is also addressed below. The genomes of the Allium species in Clade III have experienced genome contraction compared to the ancestral reconstructed estimate (Fig. 4). Surprisingly, A. cyathophorum var. farreri, which is a diploid, has the largest genome among these species, possibly because of an increase in the abundance of repetitive sequences. The precise mechanism underlying this change in genome size will need to be elucidated in future investigations. Clade III includes diploids, triploids, tetraploids, hexaploids and octaploids. DNA loss during polyploidization may be the main cause of the genome contraction in Clade III. The differences among the three Allium clades are interesting and confirm that the evolutionary trends in genome size variation are inconsistent with the previous result for genome size expansion (Hawkins et al., 2009).
Phylogenetic signal and evolutionary pattern
Phylogenetic signal varies among phylogenetic/taxonomic levels (Kamilar and Cooper, 2013). At higher taxonomic levels, genome size is highly dependent on phylogeny (Beaulieu et al., 2007; Knight et al., 2010; Whitney et al., 2010). Within individual taxonomic groups, a strong phylogenetic signal associated with genome size changes has been observed in a few studies, including the following genera: Orobanche (λ = 1; Weiss-Schneeweiss et al., 2006), Chenopodium (λ = 0.987; Mandák et al., 2016), Filago (λ = 0.934; Andrés-Sánchez et al., 2013), Hieracium (λ = 0.908; Chrtek et al., 2009) and Primulina (λ = 0.900; Kang et al., 2014). In the current study, the weak phylogenetic signal associated with the Allium genome sizes implies that genome size variation is minimally related to phylogenetic divergence (Table 1). A strong phylogenetic signal implies that genome size is not strongly influenced by selective pressure. Taxon-specific responses possibly obscure phylogenetic signal if genome size has been directly impacted by selective pressures (Lysak et al., 2009). This may also reflect a gradual evolution of the trait, during which selective pressures or adaptive radiation are important (Andrés-Sánchez et al., 2013).
The κ data in this study (κ < 1; Table 1) indicate that genome size evolves gradually and that this evolution is greater in the short branch. For other taxa, a gradual genome size evolution has been reported for Hieracium (Chrtek et al., 2009), Filago (Andrés-Sánchez et al., 2013) and Primulina (Kang et al., 2014), whereas punctuated evolution was reported for Orobanche (Weiss-Schneeweiss et al., 2006), Liliaceae (Leitch et al., 2007) and Allium subgen. Melanocrommyum (Gurushidze et al., 2012). Surprisingly, the results of this study are inconsistent with those of the previous examination of Allium subgen. Melanocrommyum. This discrepancy may be explained by the differences in the geographical distributions and habitats between the studies. More specifically, the Allium species in the current study are from the Qinghai–Tibetan Plateau, whereas the previously investigated Allium subgen. Melanocrommyum species were from Germany. Additionally, previous research indicated that genome size changes immediately occurred after speciation, during which punctuated evolution was more likely than a gradual evolution in genome size (Gregory, 2004). High stability in intraspecific genome size (Greilhuber, 1998; Gregory, 2004) and the rapid genome reorganization after speciation (Rieseberg et al., 1995) suggest punctuated evolution is more realistic than gradual evolution. However, this will need to be verified by analysing genome size evolution and phylogenetic relationships in more taxa.
Our δ data indicate adaptive radiation during the evolution of Allium genome size, involving a temporally early trait evolution or ‘early burst’ (δ < 1; Table 1). A rapid rate of trait change at the early evolutionary phase and a slower rate at later phases may reflect adaptive radiation ((Pagel et al., 2004)). Coincidentally, a previous study demonstrated that the Allium QTP-clade diversified most from the start of the radiation (2.34 Mya), with global cooling and climate oscillations in the Quaternary identified as the major reasons for the increased speciation rates (Hauenschild et al., 2017). Hence, Allium species on the Qinghai–Tibetan Plateau are highly diverse. Furthermore, our result is in accordance with the weak phylogenetic signal associated with Allium genome size. Thus, Allium genome size is directly affected by selective pressure, which is a feature of early adaptive radiation.
Correlated evolution of traits
Correlations among the evolution of genome size, seed mass and ecological factors were evaluated according to OLS (non-phylogenetic), PIC (phylogenetically independent contrasts) and PGLS (phylogenetic generalized least-squares) models. Our LRT results indicated the genome size and seed mass data fit the PIC model better than the OLS and PIC models. However, the genome size and altitude data fit the OLS model better than the PIC and PGLS models (Table 2).
Genome size and seed mass
Genome size variation affects numerous cellular parameters, including chromosome size, nuclear volume and cellular volume (Bennett, 1987). These are considered to be nucleotype effects (Bennett, 1972), which lead to variations in phenotypic traits (e.g. seed mass) (Beaulieu et al., 2008). Because seed mass variations are agronomically and ecologically important, clarifying the underlying molecular mechanism is critical (Beaulieu et al., 2007). Several previous studies revealed the positive correlation between genome size and seed mass among different accessions in one taxon and across species within the same genera or families (Caceres et al., 1998; Leishman, 1999; Knight and Ackerly, 2002). However, no significant correlation was detected between these two traits in 92 species in the subgenus Phillodineae of the genus Acacia (Gallagher et al., 2011).
In the present study, Allium genome size is positively correlated with seed mass according to the PIC regression and linear quantile regression (Table 3; Fig. 6), which is consistent with the findings of a previous study (Ohri et al., 1998). A general trend among species was that large genomes were present in large cells that divide relatively slowly (Knight and Beaulieu, 2008). Previous reports implied that genome size and guard cell size were strongly correlated across angiosperms (Beaulieu et al., 2008). Cells in the seeds of diploid and tetraploid species may differ dramatically in terms of size (Linkies et al., 2010). Seed mass is a key component of plant adaptation (Wang et al., 2020), and it is more highly correlated with plant size than with other factors (e.g. dispersal mode and environmental conditions) (Moles et al., 2005a, b). The correlation between plant size and seed mass may suggest there were common genetic components that determined seed size, plant size and specific plant organ size (Linkies et al., 2010). During seed development, transcription factors may play an important role in seed mass (Linkies et al., 2010). For example, in a previous study on seed phenotypes, the seed mass of the shb1-D mutant in Arabidopsis thaliana (L.) Heynh. increased largely because of coordinated endosperm enlargement as well as embryo development; additionally, compared with a wild-type control, the mutants had larger cells, accumulated more proteins and fatty acids, and showed delayed embryo development (Zhou et al., 2009). TEs were also probably important for genome size evolution (Bennetzen et al., 2005). The effects of genome size changes on the seed developmental process may be similar to the effects of loss-of-function mutations to transcription factors, and this might lead to changes in cell proliferation and developmental timing (Linkies et al., 2010).
Genome size and ecological factors
Genome size is usually regarded as an evolutionary character, suggesting that variation is not random, but generally occurs in response to external environmental fluctuations (Pellicer et al., 2018). A related issue is whether genome size varies predictably as a function of ecological factors, indicating ecological constraints on genome size. Associations between genome size and ecological factors have been explored in some plant groups at various taxonomic levels (Knight et al., 2005; Díez et al., 2013; Carta and Peruzzi, 2016; Qiu et al., 2019). In the present study, a significant positive relationship between Allium genome size and altitude was revealed by the OLS model and linear quantile regression (Table 3; Fig. 6). This is consistent with the results of a previous study that revealed the patterns in genome size variation along ecogeographical gradients (Guo et al., 2018). Our analysis of the relationship between Allium genome size and 19 bioclimatic variables did not detect any significant correlation (Table 4). Thus, ecological factors are probably relatively unimportant for determining Allium genome size, which is in accordance with the results of an earlier analysis of A. oleraceum (Duchoslav et al., 2013). Yet, we cannot exclude the possibility that selective pressure is an important factor.
The Qinghai–Tibetan Plateau is characterized by low air pressure, low temperature, high UV irradiation, and abundant precipitation in the form of rain and snow. The positive correlation between genome size and altitude may be related to the ability of Allium species to grow at low temperatures and their frost resistance. This ability to grow in cold conditions is facilitated by growth (i.e. cell division) in the previous season and by cell expansion during the early part of the season with low temperatures (Grime and Mowforth, 1982). Species that are relatively resistant to cold stress tend to have large genomes (Macgillivray and Grime, 1995; Knight and Ackerly, 2002). Large genomes lead to an increased ecological burden because of the greater need for macro-nutrients, especially nitrogen and phosphorus, to produce nucleic acids (Vitousek et al., 2010; Šmarda et al., 2013). The large genomes of alpine plants may be acceptable because soils at high altitudes are generally rich in phosphate (Körner, 1989). Nevertheless, phosphate is often a limited nutrient for DNA biosynthesis and plant growth (Raven et al., 1986). A previous study detected a correlation between large genomes and phosphate-rich soil (Hanson et al., 2001), suggesting there may be a correlation between alpine habitats and large genomes. Notably, there is also evidence of a relationship between UV-damage and genome size (Sparrow and Miksche, 1961). In the present study, we showed that plants with large genomes growing at high altitude may be affected by cell expansion early in the preceding favourable season, and then were subsequently exposed to low temperatures, phosphate-rich soil and increased UV-damage. Future studies should more precisely characterize the above-mentioned mechanisms to further clarify the evolutionary forces driving genome size variation on the Qinghai–Tibetan Plateau.
CONCLUSIONS
Genome size of Allium from the Qinghai–Tibetan Plateau varies considerably among species and within diploids, triploids, tetraploids, hexaploids and octaploids. This variability is probably influenced by repetitive sequences and chromosome doubling fusion. Genome size tends to decrease with increasing ploidy levels, probably because of DNA loss after polyploidization. The cytotypes in this study show an interesting geographical structure, with Allium polyploids preferentially distributed at higher altitude than diploids. The evolutionary pattern of Allium genome size is gradual evolution, followed by adaptive radiation. The Allium phylogenetic clades reflect genomic contraction, expansion and relative stasis. The weak phylogenetic signal for Allium genome sizes suggests that genome size variation is little associated with phylogenetic divergence. Furthermore, genome size is positively correlated with altitude. However, there is no correlation between genome size and 19 bioclimatic variables. Therefore, Allium genome size may be influenced by selection pressures on the Qinghai–Tibetan Plateau (i.e. low temperature, high UV irradiation and high soil phosphate).
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Fig. S1. Histograms of the relative flow-cytometric genome size measurements in taxa of Allium. Fig. S2. Boxplots illustrating the variability of of diploidy and polyploidy with latitude. Fig. S3. Somatic metaphase chromosome numbers in Allium species investigated in this study. Fig. S4. Phylogenetic tree of Allium based on the combined chloroplast sequences. Table S1. Voucher information of Allium in this study. Table S2. Chromosome numbers, ploidy levels, seed mass, genome size and chromosome length of Allium. Table S3. Accession numbers for DNA sequence of Allium species and the outgroup used for phylogenetic reconstruction.
ACKNOWLEDGEMENTS
We are grateful to Linlin Wang for providing Allium materials, and Chunling Zhang for her important contributions to the cytological experiments. The authors declare that they have no conflict of interest.
Contributor Information
Guangyan Wang, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
Ning Zhou, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
Qian Chen, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
Ya Yang, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
Yongping Yang, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
Yuanwen Duan, Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China; Institute of Tibetan Plateau Research at Kunming, Chinese Academy of Sciences, Kunming 650201, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (31800189), the Natural Science Foundation of Yunnan Province (202001AU070071), and the Second Tibetan Plateau Scientific Expedition and Research (STEP) programme (2019QZKK0502).
LITERATURE CITED
- Andrés-Sánchez S, Temsch EM, Rico E, Martínez-Ortega MM. 2013. Genome size in Filago L. (Asteraceae, Gnaphalieae) and related genera: phylogenetic, evolutionary and ecological implications. Plant Systematics and Evolution 299: 331–345. [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA. 2008. Genome size is a strong predictor of cell size and stomatal density in angiosperms. The New Phytologist 179: 975–986. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Moles AT, Leitch IJ, Bennett MD, Dickie JB, Knight CA. 2007. Correlated evolution of genome size and seed mass. The New Phytologist 173: 422–437. [DOI] [PubMed] [Google Scholar]
- Bennett MD. 1972. Nuclear DNA content and minimum generation time in herbaceous plants. Proceedings of the Royal Society of London. Series B, Biological Sciences 181: 109–135. [DOI] [PubMed] [Google Scholar]
- Bennett MD. 1987. Variation in genomic from in plants and its ecological implications. New Phytologist 106: 177– 200. [Google Scholar]
- Bennetzen JL, Ma J, Devos KM. 2005. Mechanisms of recent genome size variation in flowering plants. Annals of Botany 95: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres ME, de Pace C, Scarascia Mugnozza GT, Kotsonis P, Ceccarelli M, Cionini PG. 1998. Genome size variations within: correlations with chromosomal traits, environmental factors and plant phenotypic characteristics and behaviour in reproduction. Theoretical and Applied Genetics 96: 559–567. [Google Scholar]
- Carta A, Peruzzi L. 2016. Testing the large genome constraint hypothesis: plant traits, habitat and climate seasonality in Liliaceae. The New Phytologist 210: 709–716. [DOI] [PubMed] [Google Scholar]
- Chrtek J Jr, Zahradnícek J, Krak K, Fehrer J. 2009. Genome size in Hieracium subgenus Hieracium (Asteraceae) is strongly correlated with major phylogenetic groups. Annals of Botany 104: 161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Wheat Genome Sequencing Consortium . 2014. A chromosome–based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345: 1251788. [DOI] [PubMed] [Google Scholar]
- Díez CM, Gaut BS, Meca E, et al. 2013. Genome size variation in wild and cultivated maize along altitudinal gradients. The New Phytologist 199: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Lucrett S, Meister A, Lysak MA, Nard L. 1998. Plant genome size estimation by flow cytometry inter-laboratory comparison. Annals of Botany 82: 17–26. [Google Scholar]
- Doležel J, Greilhuber J, Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233–2244. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Duchoslav M, Šafářová L, Jandová M. 2013. Role of adaptive and non-adaptive mechanisms forming complex patterns of genome size variation in six cytotypes of polyploid Allium oleraceum (Amaryllidaceae) on a continental scale. Annals of Botany 111: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dušková E, Kolář F, Sklenář P, et al. 2010. Genome size correlates with growth form, habitat and phylogeny in the Andean genus Lasiocephalus (Asteraceae). Preslia 82: 127–148. [Google Scholar]
- Farris JS, Kjällersjö M, Kluge AG, Bult C. 1994. Testing significance of incongruence. Cladistics 10: 315–319. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. The American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Friesen N. 2006. Phylogeny and new intrageneric classification of Allium (Alliaceae) based on nuclear ribosomal DNA ITS sequences. Aliso 22: 372–395. [Google Scholar]
- Gallagher RV, Leishman MR, Miller JT, et al. 2011. Invasiveness in introduced Australian acacias: the role of species traits and genome size. Diversity and Distributions 17: 884–897. [Google Scholar]
- Garnatje T, Vallès J, Garcia S, et al. 2004. Genome size in Echinops L. and related genera (Asteraceae, Cardueae): karyological, ecological and phylogenetic implications. Biology of the Cell 96: 117–124. [DOI] [PubMed] [Google Scholar]
- Gregory TR. 2004. Macroevolution, hierarchy theory, and the C-value enigma. Paleobiology 30: 179– 202. [Google Scholar]
- Greilhuber J. 1998. Intraspecific variation in genome size: a critical reassessment. Annals of Botany 82: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Dolezel J, Lysák MA, Bennett MD. 2005. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany 95: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP, Mowforth MA. 1982. Variation in genome size–an ecological interpretation. Nature 299: 151–153. [Google Scholar]
- Guignard MS, Nichols RA, Knell RJ, et al. 2016. Genome size and ploidy influence angiosperm species’ biomass under nitrogen and phosphorus limitation. The New Phytologist 210: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FL, Wu JP, Chen LF, Zhou J, Yin YX, Qiu ZM. 2018. Genome size variation is correlated with altitude within Chinese species of Allium L. Pakistan Journal of Botany 50: 1517–1520. [Google Scholar]
- Gurushidze M, Fuchs J, Blattner FR. 2012. The evolution of genome size variation in Drumstick Onions (Allium subgenus Melanocrommyum). Systematic Botany 37: 96–104. [Google Scholar]
- Gustafson JP, Bennett MD. 1982. The effect of telomeric heterochromatin from Secale cereale on Triticale (x Tritico–secale). I. The influence of the loss of several blocks of telomeric heterochromatin on early endosperm development and kernel characteristics at maturity. Canadian Journal of Genetics and Cytology 24: 83–92. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Han TS, Zheng QJ, Onstein RE, et al. 2020. Polyploidy promotes species diversification of Allium through ecological shifts. The New Phytologist 225: 571–583. [DOI] [PubMed] [Google Scholar]
- Hanson L, McMahon KA, Johnson MAT, Bennett MD. 2001. First nuclear DNA C-values for another 25 angiosperm families. Annals of Botany 88: 851–858. [DOI] [PubMed] [Google Scholar]
- Hauenschild F, Favre A, Schnitzler J, Michalak I, Freiberg M, Muellner-Riehl AN. 2017. Spatio-temporal evolution of Allium L. in the Qinghai-Tibet-Plateau region: immigration and in situ radiation. Plant Diversity 39: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JS, Grover CE, Wendel JF. 2008. Repeated big bangs and the expanding universe: directionality in plant genome size evolution. Plant Science 174: 557–562. [Google Scholar]
- Hawkins JS, Proulx SR, Rapp RA, Wendel JF. 2009. Rapid DNA loss as a counterbalance to genome expansion through retrotransposon proliferation in plants. Proceedings of the National Academy of Sciences of the United States of America 106: 17811–17816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández CE, Rodríguez-Serrano E, Avaria-Llautureo J, et al. 2013. Using phylogenetic information and the comparative method to evaluate hypotheses in macroecology. Methods in Ecology and Evolution 4: 401–415. [Google Scholar]
- Huelsenbeck JP, Rannala B. 1997. Phylogenetic methods come of age: testing hypotheses in an evolutionary context. Science 276: 227–232. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jing WC, Xu JM, Yang L. 1999. A study on cytotaxonomy of sect. Anguinum of Allium. Acta Phytotaxonomica Sinica 37: 20–34. [Google Scholar]
- Jones R, Rees H. 1968. Nuclear DNA variation in Allium. Heredity 23: 591–605. [Google Scholar]
- Jordan GJ, Carpenter RJ, Koutoulis A, Price A, Brodribb TJ. 2015. Environmental adaptation in stomatal size independent of the effects of genome size. The New Phytologist 205: 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. 1989. The nutritional status of plants from high altitudes. Oecologia 81: 79–391. [DOI] [PubMed] [Google Scholar]
- Kamilar JM, Cooper N. 2013. Phylogenetic signal in primate behaviour, ecology and life history. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368: 20120341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Tao J, Wang J, et al. 2014. Adaptive and nonadaptive genome size evolution in Karst endemic flora of China. The New Phytologist 202: 1371–1381. [DOI] [PubMed] [Google Scholar]
- Knight CA, Ackerly DD. 2002. Variation in nuclear DNA content across environmental gradients: a quantile regression analysis. Ecology Letters 5: 66–76. [Google Scholar]
- Knight CA, Beaulieu JM. 2008. Genome size scaling through phenotype space. Annals of Botany 101: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CA, Clancy RB, Götzenberger L, Dann L, Beaulieu JM. 2010. On the relationship between pollen size and genome size. Journal of Botany 2010: 1–7. [Google Scholar]
- Knight CA, Molinari NA, Petrov DA. 2005. The large genome constraint hypothesis: evolution, ecology and phenotype. Annals of Botany 95: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labani RM, Elkington T. 1987. Nuclear DNA variation in the genus Allium L. Heredity 59: 119–128. [Google Scholar]
- Laurie DA, Bennett MD. 1985. Nuclear DNA content in the genera Zea and Sorghum. Intergeneric, interspecific and intraspecific variation. Heredity 55: 307–313. [Google Scholar]
- Leishman MR. 1999. How well do plant traits correlate with establishment ability? Evidence from a study of 16 calcareous grassland species. New Phytologist 141: 487–496. [Google Scholar]
- Leitch IJ, Beaulieu JM, Cheung K, Hanson L, Lysak MA, Fay MF. 2007. Punctuated genome size evolution in Liliaceae. Journal of Evolutionary Biology 20: 2296–2308. [DOI] [PubMed] [Google Scholar]
- Leitch IJ, Bennett MD. 2004. Genome downsizing in polyploid plants. Biological Journal of the Linnean Society 82: 651–663. [Google Scholar]
- Leitch IJ, Leitch AR. 2013. Genome size diversity and evolution in land plants. In: Leitch IJ, Greilhuber J, Doležel J, Wendel JF, eds. Plant genome diversity, Vol. 2. Vienna: Springer, 307–322. [Google Scholar]
- Li GW, Wang LJ, Yang JP, et al. 2021. A high-quality genome assembly highlights rye genomic characteristics and agronomically important genes. Nature Genetics 53: 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkies A, Graeber K, Knight C, Leubner-Metzger G. 2010. The evolution of seeds. The New Phytologist 186: 817–831. [DOI] [PubMed] [Google Scholar]
- Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IJ. 2009. The dynamic ups and downs of genome size evolution in Brassicaceae. Molecular Biology and Evolution 26: 85–98. [DOI] [PubMed] [Google Scholar]
- Macgillivray CW, Grime JP. 1995. Genome size predicts frost resistance in British herbaceous plants: implications for rates of vegetation response to global warming. Functional Ecology 9: 320–325. [Google Scholar]
- Madison WP, Madison DR. 2011. Mesquite: a modular system for evolutionary analysis. 2.75 ed. [WWWdocument]. http://mesquiteproject.org. (accessed 1 May 2013).
- Mandák B, Krak K, Vít P, et al. 2016. How genome size variation is linked with evolution within Chenopodium sensu lato. Perspectives in Plant Ecology, Evolution and Systematics 23: 18–32. [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, et al. 2005. Factors that shape seed mass evolution. Proceedings of the National Academy of Sciences of the United States of America 102: 10540–10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Ackerly DD, Webb CO, Tweddle JC, Dickie JB, Westoby M. 2005. A brief history of seed size. Science 307: 576–580. [DOI] [PubMed] [Google Scholar]
- Narayan RKJ. 1988. Constraints upon the organisation and evolution of chromosomes in Allium. Theoretical and Applied Genetics 75: 319–329. [Google Scholar]
- Ohri D, Fritsch RM, Hanelt P. 1998. Evolution of genome size in Allium (Alliaceae). Plant Systematics and Evolution 210: 57–86. [Google Scholar]
- Orme D, Freckleton RP, Thomas GH, et al. 2012. CAPER: comparative analyses of phylogenetics and evolution in R. Methods in Ecology and Evolution 3: 145–151. [Google Scholar]
- Pagel M, Meade A, Barker D. 2004. Bayesian estimation of ancestral character states on phylogenies. Systematic Biology53: 673–684. [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A. 2007. BayesTraits. Computer program and documentation. [WWWdocument]. Available at: http://www.evolution.rdg.ac.ukBayesTraits. (accessed 1 May 2013).
- Paule J, Wagner ND, Weising K, Zizka G. 2017. Ecological range shift in the polyploid members of the South American genus Fosterella (Bromeliaceae). Annals of Botany 120: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer J, Fay MF, Leitch IJ. 2010. The largest eukaryotic genome of them all? Botanical Journal of the Linnean Society 164: 10–15. [Google Scholar]
- Pellicer J, Hidalgo O, Dodsworth S, Leitch IJ. 2018. Genome size diversity and its impact on the evolution of land plants. Genes 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Qiu F, Baack EJ, Whitney KD, et al. 2019. Phylogenetic trends and environmental correlates of nuclear genome size variation in Helianthus sunflowers. The New Phytologist 221: 1609–1618. [DOI] [PubMed] [Google Scholar]
- Qiu YX, Fu CX, Comes HP. 2011. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Molecular Phylogenetics and Evolution 59: 225–244. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Pallotta D, Lafontaine JG. 1978. Analysis of plant genomes. V. Comparative study of molecular properties of DNAs of seven Allium species. Biochemical Genetics 16: 957–970. [DOI] [PubMed] [Google Scholar]
- Raven PH, Evert RF, Eichhorn E. 1986. Biology of plants, 4th edn. New York: Worth Publishers. [Google Scholar]
- Rees H, Cameron FM, Hazarika MH, Jones GH. 1966. Nuclear variation between diploid angiosperms. Nature 211: 828–830. [DOI] [PubMed] [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods in Ecology and Evolution 1: 319–329. [Google Scholar]
- Ricroch A, Yockteng R, Brown SC, Nadot S. 2005. Evolution of genome size across some cultivated Allium species. Genome 48: 511–520. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, van Fossen C, Desrochers AM. 1995. Hybrid speciation accompanied by genomic reorganization in wild sunflowers. Nation 375: 313– 316. [Google Scholar]
- Šmarda P, Hejcman M, Březinová A, et al. 2013. Effect of phosphorus availability on the selection of species with different ploidy levels and genome sizes in a long-term grassland fertilization experiment. The New Phytologist 200: 911–921. [DOI] [PubMed] [Google Scholar]
- Sparrow AH, Miksche JP. 1961. Correlation of nuclear volume and DNA content with higher plant tolerance to chronic radiation. Science 134: 282–283. [DOI] [PubMed] [Google Scholar]
- Suda J, Loureiro J, Trávníček P, et al. 2009. Flow cytometry and its applications in plant population biology, ecology and biosystematics: new prospects for the Cape flora. South African Journal of Botany 75: 389. [Google Scholar]
- Sun WG. 2020. Chromosome evolution and cytogeography of seed plants from the Alpine Subnival Belt in the Hengduan Mountains. PhD Thesis, Kunming Institute of Botany, Chinese Academy of Sciences. [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic analysis using parsimony (*and other methods). version 4.0b10. Sunderland: Sinauer. [Google Scholar]
- Tang H, Lihua M, Shiqimg A, Jianquaan L. 2005. Origin of the Qinghai-Tibetan Plateau endemic Milula (Liliaceae): further insights from karyological comparisons with Allium. Caryologia 58: 320–331. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiley GP, Burleigh JG, Burleigh G. 2015. The relationship of recombination rate, genome structure, and patterns of molecular evolution across angiosperms. BMC Evolutionary Biology 15: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Porder S, Houlton BZ, Chadwick OA. 2010. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecological Applications 20: 5–15. [DOI] [PubMed] [Google Scholar]
- Wang GY, Meng Y, Yang YP. 2013. Karyological analyses of 33 species of the tribe Ophiopogoneae (Liliaceae) from Southwest China. Journal of Plant Research 126: 597–604. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu Z, Upadhyaya HD, Morris GP. 2020. Genomic signatures of seed mass adaptation to global precipitation gradients in sorghum. Heredity 124: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss–Schneeweiss H, Greilhuber J, Schneeweiss GM. 2006. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. American Journal of Botany 93: 148–156. [DOI] [PubMed] [Google Scholar]
- Whitney KD, Baack EJ, Hamrick JL, et al. 2010. A role for nonadaptive processes in plant genome size evolution? Evolution 64: 2097–2109. [DOI] [PubMed] [Google Scholar]
- Wu LL, Cui XK, Milne RI, Sun YS, Liu JQ. 2010. Multiple autopolyploidizations and range expansion of Allium przewalskianum Regel. (Alliaceae) in the Qinghai-Tibetan Plateau. Molecular Ecology 19: 1691–1704. [DOI] [PubMed] [Google Scholar]
- Xie C, Xie DF, Zhong Y, et al. 2019. The effect of Hengduan Mountains Region (HMR) uplift to environmental changes in the HMR and its eastern adjacent area: tracing the evolutionary history of Allium section Sikkimensia (Amaryllidaceae). Molecular Phylogenetics and Evolution 130: 380–396. [DOI] [PubMed] [Google Scholar]
- Xie DF, Tan JB, Yu Y, et al. 2020. Insights into phylogeny, age and evolution of Allium (Amaryllidaceae) based on the whole plastome sequences. Annals of Botany 125: 1039–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue CY, Xu JM, Liu JQ. 2000. Karyotype studies of Allium prattii among 4 populations in southern Qinghai. Acta Botanica Boreali-Occidentalia Sinica 20: 288–293. [Google Scholar]
- Zhang YC, Zhou SD, Ren HY, et al. 2009. Karyotype in 20 populations belonging to 10 species of Allium from Southwest China. Journal of Wuhan Botanical Research 27: 351–360. [Google Scholar]
- Zhou Y, Zhang X, Kang X, Zhao X, Zhang X, Ni M. 2009. Short hypocotyl under BLUE1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. The Plant Cell 21: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SM, Xu JM. 1999. Karyotypic differentiation in Allium macrostemon Bunge. Acta Phytotaxonomical Sinica 37: 269–278. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.