Abstract
Introduction:
Patients with hepatic encephalopathy (HE) suffer from significant symptoms and impaired quality of life. Improved understanding on the potential benefits of first-line HE therapies may aid patient-provider discussions regarding expected benefits of HE treatments. We aimed to perform a systematic review to assess the effects of lactulose and rifaximin on patient reported outcomes (PROs).
Methods:
We searched MEDLINE, EMBASE and Cochrane Library databases for randomized trials or prospective cohort studies using lactulose and/or rifaximin for the management of HE and assessing changes in PROs using PRO instruments. Physician reviewers independently reviewed titles, abstracts and full texts and extracted data independently. We performed random effects meta-analyses to examine the effects of lactulose and rifaximin on PROs.
Results:
We identified 16 studies representing 1376 patients that met inclusion criteria. The majority of studies assessed treatment of covert hepatic encephalopathy. In patients with covert HE, lactulose significantly improved overall patient-reported HRQOL measured by the Sickness Impact Profile (SIP) with an estimated pool mean difference of 6.92 (95% CI 6.66–7.18) and showed improvements in several subscales. Conversely, rifaximin demonstrated a non-statistically significant mean difference in total SIP of 4.76 (95% CI −4.23–13.76), with strong evidence of heterogeneity between these studies. Studies examining other PRO instruments showed improvements in overall HRQOL, social functioning and sleep from both lactulose and rifaximin.
Discussion:
Patients with HE treated with lactulose or rifaximin reported improvements in important PROs. These results may inform provider-patient communication and help manage patient expectations with regards to the potential benefits of HE therapies.
Keywords: HE, quality of life, sleep, activity, communication
Graphical Abstract

Introduction
Hepatic encephalopathy (HE) is characterized by reversible cognitive dysfunction in patients with cirrhosis.(1) Patients with cirrhosis are at risk of episodic or persistent overt HE (OHE), as well as covert HE (CHE) characterized by altered executive and processing function. In addition to its significant impact on hospitalizations and death, both CHE and OHE are dominant drivers of impaired patient reported outcomes (PROs) including health-related quality of life (HRQOL), sleep and energy levels.(2)
The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend secondary prophylaxis (i.e. prevention of recurrent HE) and treatment of HE with non-absorbable disaccharides such as lactulose and add-on therapy with rifaximin.(3–6) Lactulose has several potential mechanisms of action including acidification of the gut lumen leading to conversion of ammonia to the ammonium cation, alteration of the gut microbiome, and catharsis, which reduces the time for ammonia absorption.(7) The exact mechanism of action for rifaximin is unknown but it likely is related to the modification of the small intestinal and colonic microbiome.(8) Recommendations state that lactulose should be titrated to obtain 2–3 bowel movements per day; rifaximin is generally dosed at 550 mg twice daily.(6) These recommendations are supported by evidence showing that both lactulose and rifaximin prevent recurrence of overt HE and decrease mortality in patients with overt HE.(9) Lactulose and rifaximin are the only medications approved by the US Food and Drug Administration (FDA) for the treatment of HE. While these treatments have been reported to improve overall HRQOL,(10) it remains unclear how HE treatment or prophylaxis affect individual components of HRQOL or other PROs. Knowledge regarding the impact of HE treatments on PROs may help inform provider-patient conversations surrounding expectations about which symptoms may or may not improve as a result of HE treatment.
We performed a systematic review and meta-analysis to assess how prophylaxis and treatment of HE affect PROs. We aimed to assess the possible effects of CHE treatment and prevention of OHE with US FDA-approved rifaximin or lactulose on PROs. We hypothesized that both lactulose and rifaximin would improve specific PROs including overall HRQOL, sleep and physical activity.
Methods
Study Question
We aimed to assess the following clinical question: “What effect does primary/secondary prophylaxis of hepatic encephalopathy or treatment of CHE with rifaximin and/or lactulose/lactitol have on PROs among adult patients with cirrhosis?” We included all randomized controlled trials or prospective cohort studies using PRO instruments and reporting changes in PROs. Full inclusion/exclusion criteria are in Table 1. Results were separated based on treatment indication.
Table 1.
Search Inclusion Criteria
| Inclusion/Exclusion Criteria | |
|---|---|
| Population | Adult patients with cirrhosis Primary prophylaxis in pts with no hepatic encephalopathy Secondary prophylaxis in patients with prior HE Treatment of covert hepatic encephalopathy defined as subclinical deficits in concentration, coordination, orientation, motor speed(6) |
| Intervention | rifaximin, lactulose/lactitol |
| Comparator | no treatment, placebo, active comparators |
| Outcome | PROs assessed by a PRO instrument |
| Timing of effect | any time post-treatment |
| Time of search | published any time before 11/20/2021 |
| Setting | Inpatient or outpatient setting |
| Study design | RCTs, prospective cohort |
Study Selection
We developed a conceptual framework of the drivers of HRQOL in HE (Figure 1).(11–19) Based on this model, we identified HE symptoms, side effects of treatments, psychological concerns and quality of life which are directly affected by CHE and prior OHE and indirectly affected by decreased cognitive function. This conceptual framework was used to define the list of candidate PROs for this systematic review.
Figure 1. Proposed conceptual framework of HRQOL in hepatic encephalopathy.
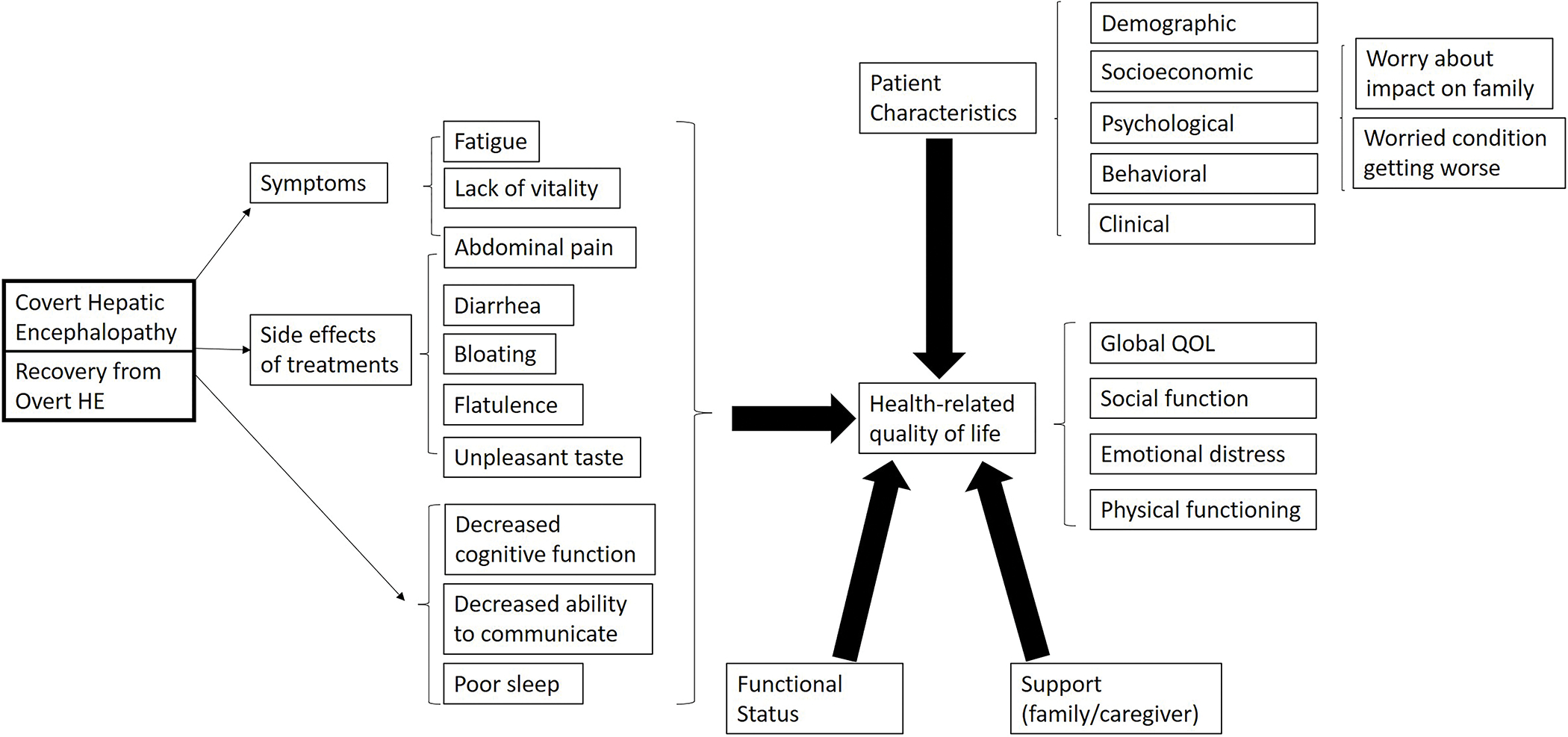
With the assistance of a health sciences librarian (JSB) and using Covidence (Melbourne, Australia), we searched the MEDLINE via PubMed, EMBASE and Cochrane Library databases for English-language articles published at any time before November 20, 2021 (full search strategy in Supplemental Table 1). Published systematic reviews were hand searched for any missing references. We contacted authors twice via email to obtain missing data not reported in manuscripts or conference abstracts.
At least two physician reviewers (AMM, HPK and/or GL) independently reviewed article titles and abstracts and applied inclusion criteria. For those identified as possibly meeting these criteria, full texts were reviewed independently. All disagreements were adjudicated via consensus.
Data Extraction
At least two reviewers (AMM, HPK and/or GL) independently extracted data from included studies using standardized forms. If multiple studies were published reporting on the same study population, the publication with the most up-to-date results was included. In concordance with the Cochrane Handbook for Systematic Reviews of Interventions, we assessed the risk of bias of included studies.(20)
Statistical Analysis
Comparison of pre/post-treatment PRO changes
We extracted pre-treatment compared to post-treatment changes in PRO measures among patients treated with lactulose, rifaximin, placebo or active comparators (e.g., LOLA). We extracted statistically significant changes (when available) for both intergroup (e.g., pre- vs post-treatment PRO scores) and intragroup (e.g., differences in pre/post-treatment PRO changes in lactulose or rifaximin vs placebo-treated patients). Changes in PRO scores were transformed so that positive numbers corresponded to improvements in the PRO measures. Additionally, for visual comparison of the change in PRO scores, we calculated the percentage change in score by taking the reported pre/post treatment change in scores and dividing them by the total range of the PRO scale or sub-scale. For example, a 12-point improvement in the Epworth Sleepiness Scale (score range 0–24) would be displayed as a 50% increase in our Figure.
Meta-analyses of PRO changes
We aimed to perform meta-analyses on the effects of lactulose and rifaximin among studies using consistent PRO instruments. Given the heterogeneity in scales and patient populations, the meta-analysis was limited to studies examining the effects of lactulose and rifaximin for the treatment of CHE on the Sickness Index Profile (SIP). We did not directly combine and compare results from different PRO scales (e.g., with standardized mean differences) given that the standardized deviations from each individual study are products of study design that lack substantive meaning and their use can distort comparisons of mean outcomes. Our meta-analysis compared post- vs. pre-treatment scores (i.e., intragroup differences) within each treatment arm. Given that there was no consistent comparator group among the studies, intergroup (i.e., lactulose vs placebo or lactulose vs rifaximin) differences were not the main focus of this meta-analysis.
We assessed for publication bias by creating a funnel plot and performing the Begg rank correlation test.(21) To account for potential heterogeneity across studies, we used a random-effects model to pool effect sizes and Knapp-Hartung adjustments(22) in calculating confidence intervals for pooled effects. The DerSimonian-Laird estimator was used to estimate the heterogeneity variance τ2; Cochran’s Q was used to evaluate statistical evidence for heterogeneity across studies.(23, 24) If the pooled effect of treatment on overall difference in SIP was found to be significant, step-down meta-analyses of the individual component SIP domains were performed using the same methodology. The Bonferroni-Holm method was applied in such step-down analyses of pooled domain-specific estimates to account for multiplicity in identifying significant pooled effects.(25) The meta package (version 4.19–0) was used in the R programming environment (version 3.6.1) to perform all meta-analyses.(26, 27) All analyses were performed at the ɑ = 0.05 significance level.
Results
Identification of Included Studies
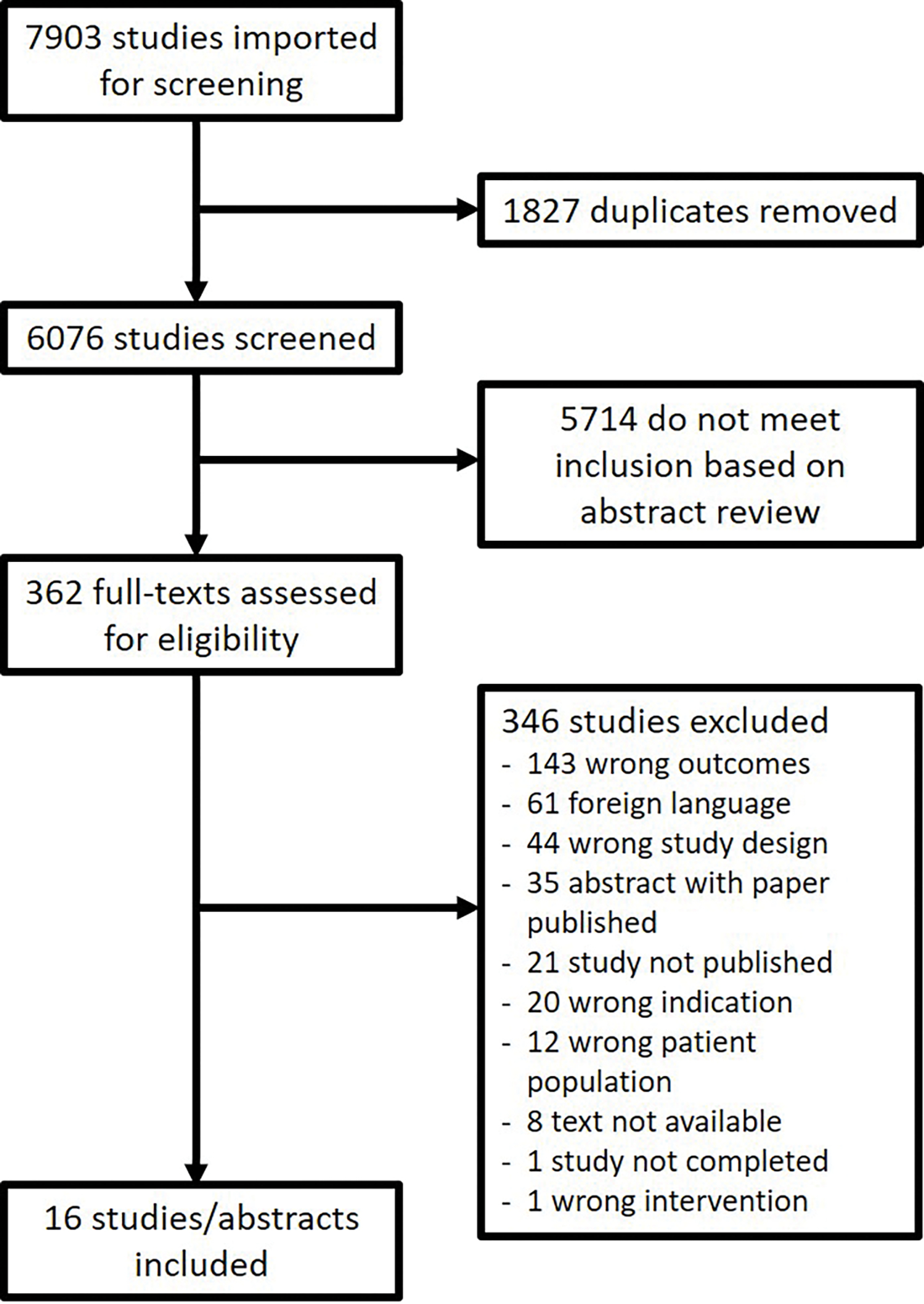
The literature search yielded 7903 results. After removing duplicates, 6076 papers were screened against eligibility criteria, of which 5714 abstracts were excluded. Of the 362 full texts reviewed for eligibility, 346 were excluded primarily due to wrong outcomes (n=143), foreign language (n=61), and wrong study design (n=44). There were an additional 21 studies listed on clinicaltrials.gov that were never published and were therefore excluded. After all exclusions, 16 studies or abstracts were included. Figure 2 illustrates the disposition of records.
Figure 2. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of studies included in systematic review.
Study Characteristics
A summary of study characteristics is included in Table 2. A total of 16 studies (14 parallel randomized controlled trials, 2 before-after studies) with a total of 1376 patients met inclusion criteria for the review.(28–43) Of these studies, 14 were full manuscripts and 2 abstracts.
Table 2.
Characteristics of included studies stratified by HE treatment indication
| Study | Location | Study Design | Indication | Duration | Intervention (n) | Comparator (n) | HRQOL Instrument(s) | Mean age, y | Male (%) | CPS A/B/C (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Poo (2006) (28) | Mexico | Parallel RCT | CHE treatment | 2 weeks | Lactulose 30–60 mL TID (n=10) | Other active comparator (LOLA) (n=10) | SF-36, Euro-QOL | 62 | 10% | Mean score 9.2 |
| Zeng (2006) (29) | China | Parallel RCT | CHE treatment | 24 weeks | Lactulose 15–90 mL daily (n=40) | Vitamin B TID (n=20) | WHOQOL-BREF | 50 | 85% | 35%/37%/28% |
| Prasad (2007) (30) | India | Parallel RCT | CHE treatment | 12 weeks | Lactulose 30–60 mL 2–3/daily (n=31) | No treatment (n=30) | SIP | 49 | 90% | 33%/54%/13% |
| Bajaj (2011) (31) | US | Parallel RCT | CHE treatment | 8 weeks | Rifaximin 550 mg BID (n=21) | Placebo one tablet BID (n=21) | SIP | 56 | N/A | 86%/14%/0% |
| Mittal (2011) (32) | India | Parallel RCT | CHE treatment | 12 weeks | Lactulose 30–60 mL 2–3/daily (n=40) | No treatment (n=40) | SIP | 43 | 78% | Median score 8.0 |
| Sidhu (2011) (34) | India | Parallel RCT | CHE treatment | 8 weeks | Rifaximin 400 mg TID (n=43) | Placebo two tablets TID (n=34) | SIP | 53 | 77% | 30%/57%/14% |
| Elnoemany (2015)* (35) | N/A | Parallel RCT | CHE treatment | N/A | Lactulose 30–60 mL BID (n=31) | Rifaximin 200 mg TID (n=32) | SIP | N/A | N/A | N/A |
| Sidhu (2016) (36) | India | Parallel RCT | CHE treatment | 12 weeks | Lactulose 30–120 mL daily (n=55) | Rifaximin 400 mg TID (n=57) | SIP | 55 | 75% | Mean score 8.0 |
| Singh (2017) (38) | India | Before-after study | CHE treatment | 24 weeks | Lactulose 30 mL (start dose) q6 hours (n=50) | N/A | SF-36 (v2), ESS, PSQI | 45 | 90% | Mean score 8.0 |
| Suzuki (2018) (40) | Japan | Parallel RCT | CHE treatment | 2 weeks | Lactitol 6–12 g TID (n=87) | Rifaximin 400 mg TID (n=84) | SF-8 | 65 | 52% | 13%/65%/22% |
| Wang (2019) (41) | China | Parallel RCT | CHE treatment | 8 weeks | Lactulose 20–60 mL BID (n=59) | No treatment (n=28) | Modified Chinese QoL questionnaire | 51 | 93% | N/A |
| Sanyal (2011) (33) | US, Canada | Parallel RCT | Secondary OHE prophylaxis | 24 weeks | Rifaximin 550 mg BID (n=101) | Placebo one tablet BID (n=118) | CLDQ | 57 | 67% | N/A |
| Bruyneel (2017) (37) | Belgium | Before-after study | Secondary OHE prophylaxis | 4 weeks | Rifaximin 550 mg BID (n=15) | N/A | SF-36, ESS, PSQI, HADS | 57 | 60% | 0%/40%/60% |
| Sanyal (2018)* (39) | US | Parallel RCT | Secondary OHE prophylaxis | 24 weeks | Rifaximin 550 mg BID (n=113) | Rifaximin 550 mg BID + lactulose (n=108) | CLDQ, CBI | 58 | 63% | 40%/56%/4% |
| Glal (2021) (42) | Egypt | Parallel RCT | Secondary OHE prophylaxis | 24 weeks | Rifaximin 550 mg BID (n=30) | Other active comparator (nitazoxanide) (n=30) | CLDQ | 55 | 60% | 0%/38%/62% |
| Patel (2022) (43) | United Kingdom | Parallel RCT | CHE treatment/secondary OHE prophylaxis | 12 weeks | Rifaximin 550 mg BID (n=19) | Placebo tablet BID (n=19) | EQ-5D | 56 | 71% | N/A |
HRQOL: health-related quality of life; CPS: Child Pugh score; RCT: randomized controlled trial; OHE: overt hepatic encephalopathy; mL: milliliters; TID: three times daily; LOLA: L-ornithine-L-aspartate; SF-36: 36 item short-form survey; SF-8: 8 item short-form survey; WHOQOL-BREF: World Health Organization Quality of Life-BREF; SIP: Sickness Impact Profile; BID: twice daily; N/A: not available; US: United States; CLDQ: Chronic Liver Disease Questionnaire; ESS: Epworth Sleepiness Scale; PSQI: Pittsburgh Sleep Quality Index; HADS: Hospital Anxiety and Depression Scale; CBI: Caregiver Burden Index; v2: version 2
abstract only
The majority of studies focused on treatment of CHE (n=11), secondary prophylaxis after OHE (n=4) and both indications (n=1). Most studies used validated definitions for MHE/CHE (Supplemental Table 2). Outcomes assessed included HRQOL, sleep, and caregiver burden using a variety of instruments (Table 2). A summary of the overall and individual component changes in PRO instruments can be found in Supplemental Tables 4–14.
A summary of bias assessments can be found in Table 3 and Table 4. The majority of RCTs (n=10) were at low risk of bias. The two non-RCTs were deemed to have low risk of bias based on the Newcastle-Ottawa Scale (NOS) (Supplemental Table 3).(44)
Table 3.
Bias assessments of included RCTs using Cochrane bias assessment tool
| Study | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Poo (2006) (28) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Zeng (2006) (29) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Prasad (2007) (30) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bajaj (2011) (31) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Mittal (2011) (32) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sanyal (2011) (33) | Low risk | Low risk | Low risk | Low risk | High risk | High risk |
| Sidhu (2011) (34) | Low risk | Low risk | Some concerns | Low risk | Low risk | Some concerns |
| Elnoemany (2015) (35) | Some concerns | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Sidhu (2016) (36) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sanyal (2018) (39) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Suzuki (2018) (40) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Wang (2019) (41) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Glal (2021) (42) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Patel (2022) (43) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Table 4:
Step-down meta-analyses for effect of lactulose (pre- vs post-treatment) for CHE treatment on SIP domains
| Domain | Pooled mean difference (pre vs post treatment) | Pooled 95% CI |
|---|---|---|
| Eating | 2.18 | (−2.51, 6.86) |
| Recreation and pastimes | 9.56 | (4.83, 14.30)* |
| Work | 9.44 | (−2.59, 21.47) |
| Communication | 2.32 | (2.00, 2.63)* |
| Ambulation | 3.36 | (−0.86, 7.59) |
| Alertness | 7.14 | (−0.71, 14.99) |
| Social interactions | 6.85 | (0.34, 13.37) |
| Mobility | 9.61 | (−11.69, 30.91) |
| Home management | 9.33 | (2.62, 16.03)* |
| Body care and movements | 3.77 | (−2.01, 9.56) |
| Emotional behavior | 8.64 | (3.21, 14.06)* |
| Sleep and rest | 9.12 | (3.06, 15.18)* |
| Total SIP (all domains) | 6.92 | (6.66, 7.18)* |
denotes statistically significant pooled effect estimates; results for each sub-domain of the SIP are corrected using the Bonferroni-Holm method
Overall HRQOL
Ten studies reported on changes in overall HRQOL. Instruments used to assess HRQOL included the SIP,(30–32, 34–36) Euro-QOL instrument,(28) Chronic Liver Disease Questionnaire (CLDQ),(33, 39, 42) and Chinese Quality of Life Questionnaire(41) (Figure 3 and 4a). Additionally, there was one study that assessed self-reported health using the EQ-5D visual analogue scale of health state score.(43)
Figure 3. Pooled mean difference in total Sickness Index Profile (SIP) scores among patients treated with lactulose.
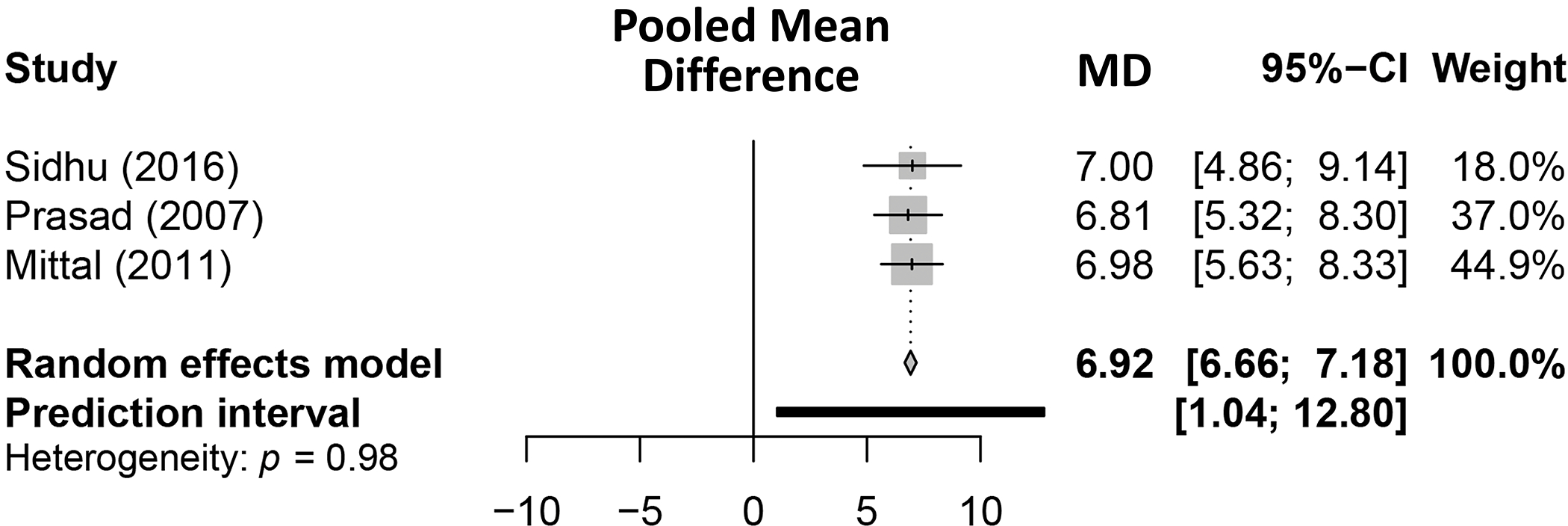
Figure 4. Percentage change in patient reported outcomes among patients with HE treated with lactulose and/or rifaximin for.
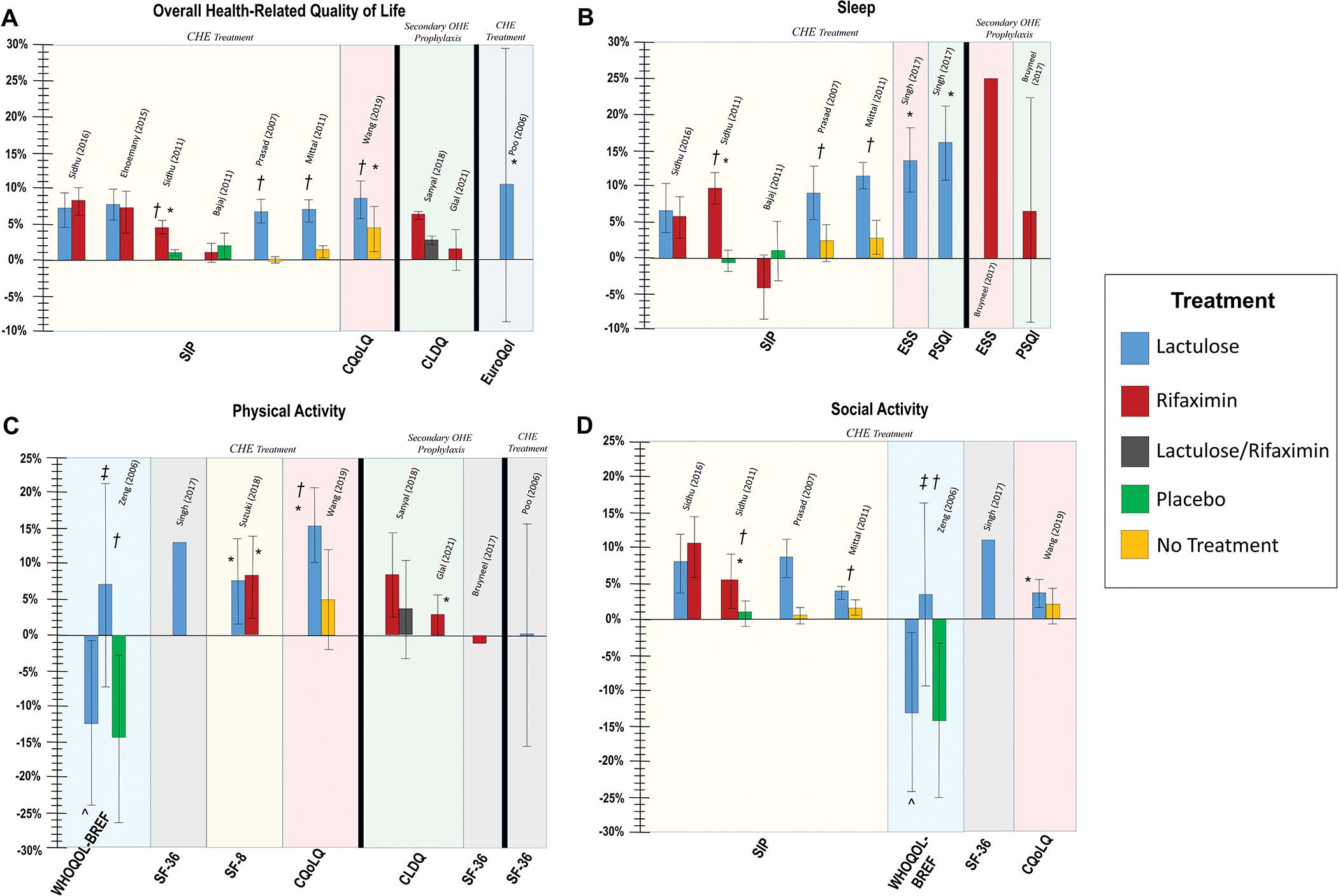
(A) overall health-related quality of life, (B) sleep, (C) physical activity, and (D) social activity
Percentage change based on total range of scores for each individual patient reported outcome instrument. Adjacent bars come from same study and positive values denote improvement in patient-reported outcomes. SIP: Sickness Index Profile; CLDQ: Chronic Liver Disease Questionnaire; CQoLQ: Modified Chinese QoL Questionnaire; ESS: Epworth Sleepiness Scale; PSQI: Pittsburgh Sleep Quality Index; WHOQOL-BREF: World Health Organization Quality of Life-BREF; SF-36: 36-Item Short Form Survey; SF-8: 8-Item Short Form Survey;
* Intragroup difference (i.e. before vs after) significant at p<0.05; † Intergroup difference (i.e. treatment vs comparator) significant at p<0.05; ^ Short-term (8 weeks) lactulose; ‡ Long-term (24 weeks) lactulose; Statistical significance not reported for CLDQ (Sanyal et al.)
For CHE treatment, there were consistent improvements in overall SIP scores among patients on lactulose (median +7.0) and these improvements were statistically significant compared to placebo or no treatment.(30, 32) Lactulose for CHE treatment was also shown to have significant pre/post improvements in Euro-QOL (+10.4)(28) and Modified Chinese QoL Questionnaire (+12.6) scores.(41)
For the meta-analysis of SIP, there was no evidence of publication bias in the studies that reported on change in SIP among patients with CHE treated with lactulose (Supplemental Figure 1) with a Begg rank correlation test p-value >0.99. The estimated pooled mean difference in SIP due to lactulose among Prasad (2007), Mittal (2011), and Sidhu (2016) was 6.92 (95% CI: 6.66–7.18) (Figure 3). We failed to find statistical evidence in favor of heterogeneity (τ2 < 0.01; Cochran’s Q = 0.03, p-value = 0.983). Step-down meta-analyses of the individual SIP domains for lactulose can be found in Table 4. Lactulose-treated patients had significant improvements in SIP sub-scores including recreation and pastimes, communication, home management, emotional behavior and sleep/rest.
Rifaximin for CHE treatment demonstrated improvements in overall SIP score in four studies (median +4.6) and this improvement was statistically significant compared to placebo in one study.(34) There were no significant differences in overall SIP improvements in the two studies that directly compared lactulose and rifaximin.
In the meta-analysis on CHE treatment, there was considerable heterogeneity between studies examining rifaximin-treated patients. While Sidhu (2011)(34) and Sidhu (2016)(36) found a statistically significant effect of rifaximin on difference in total SIP, Bajaj (2011)(31) failed to find statistically significant evidence of SIP improvement from rifaximin. Regarding the pooled effect of rifaximin among the three studies considered, there was an estimated mean difference in SIP of 4.76 (95% CI: −4.23, 13.76). Given the strong evidence suggesting heterogeneity between these three studies (τ2 = 13.11; Cochran’s Q = 46.10, p-value < 0.001) step-down meta-analyses were not performed.
Sleep
PROs for sleep were reported by seven studies, which used the SIP,(30–32, 34, 36) Epworth Sleepiness Scale (ESS)(37, 38) and Pittsburgh Sleep Quality Index (PSQI)(37, 38) (Figure 4b). Patients with CHE treated with lactulose reported improvements in sleep/rest sub-scores of SIP (median +9.0) and improvements were statistically significant compared to no treatment in two studies.(30, 32) In the step-down meta-analysis examining the effect of lactulose for patients with CHE on SIP domains (Table 5), there were statistically significant improvements in sleep and rest (mean difference 9.12, 95% CI 3.06–15.18).
Among patients with CHE, rifaximin was reported to have a 4-point improvement in sleep/rest SIP sub-scale in one study of patients with CHE(31) and had a 9.8-point improvement in another RCT,(34) which was statistically significant compared to placebo. Both lactulose and rifaximin demonstrated numerical improvements in ESS and PSQI, but these were only statistically significant for lactulose.(38)
Physical/Bodily Function/Activity
Changes in physical activity subscales were reported by seven studies, using the WHOQOL-BREF,(29) SF-36,(38) SF-8,(40) CLDQ,(39, 42) and modified Chinese QoL Questionnaire (Figure 4c).(41) There were inconsistent results regarding improvements in physical activity as measured by these various instruments.
There were improvements in lactulose and rifaximin-treated patients with CHE in ambulation and mobility, as measured by the SIP score (Supplemental Table 4). However, in the step-down meta-analyses, the improvements in SIP among patients treated with lactulose were not statistically significant for ambulation (mean difference 3.36, 95% CI −0.86–7.59) or mobility (mean difference 9.61, 95% CI −11.69–30.91). Rifaximin-treated patients reported median improvements of 3.7 points and 10.5 points in ambulation and mobility, respectively.
Social Activity/Function
Social activity or function was reported by seven studies using the SIP,(30, 32, 34, 36) WHOQOL-BREF,(29) SF-36,(38) and modified Chinese QoL Questionnaire (Figure 4d).(41) Among patients with CHE, there were numerical improvements in the social interactions sub-scale of SIP for lactulose (median +7.9) and rifaximin (median +7.8), which were improved compared to no treatment for lactulose(32) and compared to placebo for rifaximin.(34) These differences were not statistically significant in the step-down meta-analysis for lactulose. In a randomized trial comparing lactulose and rifaximin, improvements in social interactions scores were similar in the two groups.
Discussion
Hepatic encephalopathy can lead to an overwhelming impairment in HRQOL, particularly in the domains of social activity, communication and sleep.(45, 46) Recently, there has been a greater recognition of the importance of measuring and targeting PROs as important patient-centered endpoints(19, 47) and evidence suggesting that quality of life is rarely assessed among clinicians treating HE.(48) This systematic review reports on the effects of lactulose and rifaximin on PROs, demonstrating that these medications improve HRQOL, particularly when used for the treatment of covert HE. There were no apparent differences between lactulose and rifaximin in the improvement of overall HRQOL or sub-scores.
Understanding the impact of HE therapies on PROs to advise patients
This systematic review adds to previously published studies that reported that HE treatments improve overall HRQOL measures(10) by demonstrating that lactulose and rifaximin treatment are associated with improvements in specific components of HRQOL, including social functioning, communication, and sleep. These findings along with the conceptual framework in Figure 1 may be instructive for providers, who could consider assessing pre- and post-treatment PRO scores for patients suffering from social, communication, or sleep disturbances related to HE. These data may provide useful information on the potential benefits of FDA-approved HE treatments, which could guide patient-provider discussions and treatment decisions.
Impact of HE on social functioning and communication
Cognitive impairment among patients with HE likely explains patient-reported decreases in social functioning and communication. These impairments could also contribute to caregiver burden.(49) Studies included in our review report that HE treatments are associated with improvements in patient-reported social functioning and, perhaps, a decrease in caregiver burden. These are important potential benefits of HE treatment that could be targeted and measured by prescribing clinicians using validated PRO instruments such as the SIP, CLDQ, SF-36 or EQ-5D.(50, 51)
Targeting sleep disturbances in HE with lactulose and rifaximin
Lactulose and rifaximin-treated individuals reported improvements in sleep based on the generic HRQOL instrument SIP, the sleep-specific instrument PSQI and assessments of day-time sleepiness via the ESS. It is uncertain if these improvements in patient-reported sleep from HE treatments are related to a reduction in insomnia and fragmented sleep, or if this is merely a result of improved daytime sleepiness, which is interpreted by the patient as better quality sleep. Some evidence supports the latter explanation, including a lack of correlation between sleep indices and HE,(52) strong negative correlation between low levels of daytime sleepiness and subsequent HE-related hospitalizations,(53) and positive associations between serum ammonia levels and subjective sleepiness.(54) Providers may therefore consider treatment with lactulose or rifaximin for patients with excessive daytime sleepiness to improve patients’ self-reported sleep quality.
Lack of PRO instruments assessing adverse effects of medications
Our proposed conceptual framework of HRQOL in patients with HE includes some features that could be measured by PROs but were not included in studies on lactulose and rifaximin. The most notable omission among the studies included in our review were PRO instruments assessing adverse effects of these medications. Lactulose has several potential adverse effects, including unpleasant taste, bloating, and diarrhea, which may lead to therapy discontinuation in up to a third of patients.(55, 56) Furthermore, there is higher acceptance of lactulose Indian compared to American patient populations,(57) which is particularly relevant for this review since four included studies on lactulose were performed in India. The modified Chinese QoL questionnaire does include a measure of symptoms/side effects which, notably, were significantly improved in lactulose-treated patients (Supplemental Table 9). Components of instruments may capture these adverse effects, including the abdominal symptoms component of CLDQ, which was similar in combined lactulose/rifaximin compared to rifaximin alone. However, it is unclear whether these are truly valid scales for assessing adverse effects of HE treatments. Future studies assessing HE treatments should attempt to assess these side effects using standardized measures or aim to develop new validated instruments.
Limitations
While this review provides an up-to-date assessment of PRO changes among rifaximin and lactulose-treated patients, it has some potential limitations. First, due to the large heterogeneity in patient populations, study design, and PRO instruments used in this review, we were only able to perform meta-analyses examining the effects of lactulose on SIP. As a result, we were unable to provide a singular estimate of PRO improvements among lactulose and rifaximin-treated patients. We are hopeful that the development of PRO measures for cirrhosis and routine use of PROs in clinical trials of HE treatments will reduce this heterogeneity in the future. Second, given that we limited our review to PRO instruments, we may have omitted studies that included assessment of PRO concepts but did not use PRO instruments. For instance, studies may have reported visual analogue scales of sleep, psychosocial/physical functioning or medication side effects but were not included in this review given that they lack validity as clinically important outcome measures. Third, we excluded non-FDA approved treatments including some that may be available and used outside the US (e.g. L-ornithine-L-aspartate), which may limit external validity. Fourth, despite contacting each author at least twice to request missing results, our study did have some missing data on changes in PRO scores, standard deviation/variance, or statistical comparisons between treatment groups. Lastly, exclusion of non-English articles may have eliminated potentially relevant references.
Conclusion
In summary, this review demonstrates that patients with HE treated with lactulose or rifaximin report statistically significant improvements in overall HRQOL, social activity, sleep and, less consistently, physical functioning. Most evidence supporting these treatments was for the treatment of CHE. This review identifies some potential PRO domains that could be measured and targeted when prescribing medications for patients with cirrhosis and HE. Future trials assessing HE treatments should assess PROs using standardized and validated instruments that capture the important domains affected by HE.
Supplementary Material
Acknowledgements:
We would like to thank Arun Sanyal, Zeev Heimanson, Debbie Shawcross, Vishal Patel and Mark McPhail for sharing unpublished data for inclusion in this manuscript.
Grant Support:
This research was supported in part by NIH grant T32 DK007634 (AM and HK) and by an Advanced/Transplant Hepatology Grant from the AASLD Foundation (AM).
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- CBI
Caregiver Burden Inventory
- CHE
covert hepatic encephalopathy
- CLDQ
Chronic Liver Disease Questionnaire
- EASL
European Association for the Study of the Liver
- ESS
Epworth Sleepiness Scale
- FDA
Food and Drug Administration
- HADS
Hospital Anxiety and Depression Scale
- HE
hepatic encephalopathy
- HRQOL
health-related quality of life
- OHE
overt hepatic encephalopathy
- PRO
patient reported outcome
- PSQI
Pittsburgh Sleep Quality Index
- SF-36
36-item short form survey
- SF-8
8-item short form survey
- SIP
Sickness Index Profile
Footnotes
Disclosures/Conflicts of Interest: Andrew Moon consulted for TARGET RWE. A. Sidney Barritt consulted for TARGET RW and Sarpeta INC, served on the DSMB for Pfizer and did medical writing for Novo Nordisk. Elliot Tapper received grant funding to the institution from Valeant, Gilead, Madrigal, Lipocine, Ambys, Novo Nordisk, consulted for Allergan, Novartis, Takeida, Axcella, Kaleido, Novo Nordisk, and participated in advisory boards for Bausch Health and Mallinckrodt.
References
- 1.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716–21. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj JS, Cordoba J, Mullen KD, et al. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther 2011;33:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–35. [DOI] [PubMed] [Google Scholar]
- 4.American Association for the Study of Liver D, European Association for the Study of the L. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol 2014;61:642–59. [DOI] [PubMed] [Google Scholar]
- 5.Kimer N, Krag A, Moller S, et al. Systematic review with meta-analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther 2014;40:123–32. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol 2022;77:807–824. [DOI] [PubMed] [Google Scholar]
- 7.Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: A systematic review and meta-analysis. Hepatology 2016;64:908–22. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther 2016;43 Suppl 1:11–26. [DOI] [PubMed] [Google Scholar]
- 9.Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev 2016:CD003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhiman RK, Thumburu KK, Verma N, et al. Comparative Efficacy of Treatment Options for Minimal Hepatic Encephalopathy: A Systematic Review and Network Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:800–812 e25. [DOI] [PubMed] [Google Scholar]
- 11.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 1995;273:59–65. [PubMed] [Google Scholar]
- 12.Groeneweg M, Quero JC, De Bruijn I, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 1998;28:45–9. [DOI] [PubMed] [Google Scholar]
- 13.Bao ZJ, Qiu DK, Ma X, et al. Assessment of health-related quality of life in Chinese patients with minimal hepatic encephalopathy. World J Gastroenterol 2007;13:3003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003;48:1622–6. [DOI] [PubMed] [Google Scholar]
- 15.Moscucci F, Nardelli S, Pentassuglio I, et al. Previous overt hepatic encephalopathy rather than minimal hepatic encephalopathy impairs health-related quality of life in cirrhotic patients. Liver Int 2011;31:1505–10. [DOI] [PubMed] [Google Scholar]
- 16.Navasa M, Forns X, Sanchez V, et al. Quality of life, major medical complications and hospital service utilization in patients with primary biliary cirrhosis after liver transplantation. J Hepatol 1996;25:129–34. [DOI] [PubMed] [Google Scholar]
- 17.Mina A, Moran S, Ortiz-Olvera N, et al. Prevalence of minimal hepatic encephalopathy and quality of life in patients with decompensated cirrhosis. Hepatol Res 2014;44:E92–9. [DOI] [PubMed] [Google Scholar]
- 18.Nardelli S, Pentassuglio I, Pasquale C, et al. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metab Brain Dis 2013;28:239–43. [DOI] [PubMed] [Google Scholar]
- 19.Tapper EB, Kanwal F, Asrani SK, et al. Patient-reported outcomes in cirrhosis: A scoping review of the literature. Hepatology 2018;67:2375–2383. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J, Savović J, Page M, et al. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins J, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3; 2022. [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 22.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003;22:2693–710. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 24.Cochran WG. Some Methods for Strengthening the Common χ 2 Tests. Biometrics 1954;10:417. [Google Scholar]
- 25.Holm S A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 1979;6:65–70. [Google Scholar]
- 26.R Core Team. A language and environment for statistical computing. 2019. [cited 2021 September 18]; Available from: https://www.R-project.org/
- 27.Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poo JL, Gongora J, Sanchez-Avila F, et al. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol 2006;5:281–8. [PubMed] [Google Scholar]
- 29.Zeng Z, Li YY, Jia L, et al. Influence of lactulose on the cognitive level and quality of life in patients with minimal hepatic encephalopathy. Chinese Journal of Clinical Rehabilitation 2006;10:165–167. [Google Scholar]
- 30.Prasad S, Dhiman RK, Duseja A, et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007;45:549–59. [DOI] [PubMed] [Google Scholar]
- 31.Bajaj JS, Heuman DM, Wade JB, et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 2011;140:478–487 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mittal VV, Sharma BC, Sharma P, et al. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 2011;23:725–32. [DOI] [PubMed] [Google Scholar]
- 33.Sanyal A, Younossi ZM, Bass NM, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011;34:853–61. [DOI] [PubMed] [Google Scholar]
- 34.Sidhu SS, Goyal O, Mishra BP, et al. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol 2011;106:307–16. [DOI] [PubMed] [Google Scholar]
- 35.Elnoemany K, Alghoraieb A, Shebl N, et al. The effect of lactulose, rifaximin, l-ornithine laspartate, their combination on minimal hepatic encephalopathy treatment. United European Gastroenterology Journal 2015;3:A338. [Google Scholar]
- 36.Sidhu SS, Goyal O, Parker RA, et al. Rifaximin vs. lactulose in treatment of minimal hepatic encephalopathy. Liver Int 2016;36:378–85. [DOI] [PubMed] [Google Scholar]
- 37.Bruyneel M, Serste T, Libert W, et al. Improvement of sleep architecture parameters in cirrhotic patients with recurrent hepatic encephalopathy with the use of rifaximin. Eur J Gastroenterol Hepatol 2017;29:302–308. [DOI] [PubMed] [Google Scholar]
- 38.Singh J, Sharma BC, Puri V, et al. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab Brain Dis 2017;32:595–605. [DOI] [PubMed] [Google Scholar]
- 39.Sanyal AJ, Heimanson Z, Israel R, et al. Prevention of overt hepatic encephalopathy recurrence with rifaximin alone versus rifaximin plus lactulose therapy: Impact on quality of life and caregiver burden in patients with cirrhosis. In: Hospital Medicine 2018. Orlando, FL; 2018. [Google Scholar]
- 40.Suzuki K, Endo R, Takikawa Y, et al. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: A phase II/III, multicenter, randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatol Res 2018;48:411–423. [DOI] [PubMed] [Google Scholar]
- 41.Wang JY, Bajaj JS, Wang JB, et al. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J Dig Dis 2019;20:547–556. [DOI] [PubMed] [Google Scholar]
- 42.Glal KAM, Abd-Elsalam SM, Mostafa TM. Nitazoxanide versus rifaximin in preventing the recurrence of hepatic encephalopathy: A randomized double-blind controlled trial. J Hepatobiliary Pancreat Sci 2021;28:812–824. [DOI] [PubMed] [Google Scholar]
- 43.Patel VC, Lee S, McPhail MJW, et al. Rifaximin-alpha reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol 2022;76:332–342. [DOI] [PubMed] [Google Scholar]
- 44.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. [cited 2022 August 30]; Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 45.Montagnese S, Bajaj JS. Impact of Hepatic Encephalopathy in Cirrhosis on Quality-of-Life Issues. Drugs 2019;79:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabiee A, Ximenes RO, Nikayin S, et al. Factors associated with health-related quality of life in patients with cirrhosis: a systematic review. Liver Int 2021;41:6–15. [DOI] [PubMed] [Google Scholar]
- 47.Bajaj JS, Lauridsen M, Tapper EB, et al. Important Unresolved Questions in the Management of Hepatic Encephalopathy: An ISHEN Consensus. Am J Gastroenterol 2020. [DOI] [PubMed] [Google Scholar]
- 48.Halonen J, Hinde R, Amlani B. Quality of life improvement is a top-rated but rarely assessed outcome in European and Australian patients with hepatic encephalopathy: Quality of life targeting and monitoring needs to be prioritised. In: EASL International Liver Congress; 2021. [Google Scholar]
- 49.Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol 2011;106:1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nabi E, Thacker LR, Wade JB, et al. Diagnosis of covert hepatic encephalopathy without specialized tests. Clin Gastroenterol Hepatol 2014;12:1384–1389 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gluud LL, Jeyaraj R, Morgan MY. Outcomes in Clinical Trials Evaluating Interventions for the Prevention and Treatment of Hepatic Encephalopathy. J Clin Exp Hepatol 2019;9:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montagnese S, Middleton B, Skene DJ, et al. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int 2009;29:1372–82. [DOI] [PubMed] [Google Scholar]
- 53.De Rui M, Schiff S, Aprile D, et al. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis 2013;28:245–8. [DOI] [PubMed] [Google Scholar]
- 54.Bersagliere A, Raduazzo ID, Nardi M, et al. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology 2012;55:869–78. [DOI] [PubMed] [Google Scholar]
- 55.Sharma BC, Sharma P, Agrawal A, et al. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology 2009;137:885–91, 891 e1. [DOI] [PubMed] [Google Scholar]
- 56.Bajaj JS, Sanyal AJ, Bell D, et al. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther 2010;31:1012–7. [DOI] [PubMed] [Google Scholar]
- 57.Rathi S, Fagan A, Wade JB, et al. Patient Acceptance of Lactulose Varies Between Indian and American Cohorts: Implications for Comparing and Designing Global Hepatic Encephalopathy Trials. J Clin Exp Hepatol 2018;8:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


