Abstract
Background
A mismatch between tricuspid leaflet size and annular dilation is one of the morphological features tied to the development of tricuspid regurgitation (TR).
Aims
We assessed the association of the leaflet-to-annulus index (LAI) with residual TR after transcatheter edge-to-edge repair (TEER).
Methods
Consecutive patients who underwent TEER for TR were enrolled. Significant residual TR was defined as a post-procedural TR ≥3+, and patients were divided into two groups according to the amount of residual TR. The LAI was retrospectively calculated using procedural transoesophageal echocardiography and was defined as follows: (anterior leaflet length+septal leaflet length)/septolateral tricuspid annulus diameter.
Results
Of 140 patients, 43 patients had residual TR ≥3+ after TEER. The patients with residual TR ≥3+ had a lower LAI compared to those with residual TR <3+ (1.04±0.10 vs 1.13±0.09; p=0.001). In multivariable analysis, the LAI was associated with residual TR ≥3+ (odds ratio [OR] [per 0.1 increase]: 0.57; 95% confidence interval [95% CI]: 0.35-0.94; p=0.02), independent of baseline TR severity or coaptation gap size. Patients with residual TR ≥3+ had a higher incidence of the composite outcome, consisting of all-cause mortality and heart failure hospitalisation within one year after TEER (47.1% vs 26.6%, p=0.02). Residual TR ≥3+ was an independent predictor of the composite outcome within one year (hazard ratio: 2.04; 95% CI: 1.01-4.11; p=0.04).
Conclusions
The leaflet-to-annulus mismatch (i.e., LAI) is associated with residual TR ≥3+ after TEER for TR. A detailed echocardiographic analysis of the tricuspid valve will be conducive to identifing suitable subjects for TEER.
Introduction
Tricuspid regurgitation (TR) is a common valvular disease in elderly patients and has a significant impact on the functional capacity and long-term survival of patients1,2. However, surgical treatment for isolated functional TR is still controversial because of the high risk of surgery3,4. Therefore, minimally invasive catheter-based procedures are desired as a safe alternative to reduce TR with a lower procedural risk5,6. Recently, transcatheter tricuspid valve interventions (TTVI) have been reported with different technologies7,8,9,10. Among the techniques, transcatheter edge-to-edge repair (TEER) is the most prevalent technique6 and has shown encouraging results9,10. A greater reduction in TR severity is associated with improved exercise tolerance, symptoms, survival rate, and clinical outcome after TEER. Therefore, identifying anatomical parameters that can predict the reduction in TR following the procedure is a key step toward improving patient outcomes.
Tricuspid annular (TA) dilation is a common morphological feature of functional TR11,12. The TA dilation occurs secondary to right ventricular and atrial dilation, which leads to a reduction of the coaptation surface area. On the other hand, different degrees of TR can be observed in patients with the same extent of TA dilation, which may be related to differential tricuspid valve (TV) leaflet size. Theoretically, larger TV leaflets may prevent TR development when the TA dilates. That is to say, relatively smaller TV leaflets compared to the TA dilation can lead to further development of TR13. Thus, the mismatch between the tricuspid leaflet length and annulus dilation is an anatomical feature tied to TR development. Nevertheless, little is known about whether the leaflet-to-annulus mismatch is associated with procedural outcome of TEER for TR.
In the present study, we measured the leaflet-to-annulus index (LAI) for assessment of the leaflet-to-annulus mismatch and investigated the impact of the LAI on the incidence of significant residual TR after TEER.
Methods
Study population
This study was a retrospective analysis of data froma single-centre, prospective, consecutive database of patients treated at the University of Bonn. We identified consecutive symptomatic patients, who underwent TEER for TR with the MitraClip/TriClip system (Abbott Structural Heart) or PASCAL system (Edwards Lifesciences) from June 2015 to July 2020 (Supplementary Figure 1). Exclusion criteria were as follows: 1) poor quality of echocardiographic images; and 2) lack of post-procedural echocardiographic evaluation. After a standardised diagnostic workup including transoesophageal echocardiography (TOE) and computed tomography, the decision to perform the intervention, in conjunction with the device selection for TTVI, was taken by the interdisciplinary Heart Team. The registry was approved by the local ethics committee. This study was conducted in accordance with the Declaration of Helsinki and its amendments, and all patients provided written informed consent.
Procedure
Procedures were performed under general anaesthesia with 3D TOE and fluoroscopic guidance. The details of each device system and procedure have been well described previously14,15. Discretion was left to the treating physicians as to whether a second or third device needed to be used. Implantation success was defined as successful delivery and deployment of one or more clips to achieve leaflet approximation and retrieval of the delivery system. Acute procedural success was defined as implantation success with at least one grade reduction in TR severity upon discharge.
Echocardiographic parameters
Echocardiographic parameters were assessed at baseline and at discharge, according to the current guideline16. The severity of TR was graded as follows: grade 0, none; 1+, mild; 2+, moderate; 3+, severe; 4+, massive; and 5+, torrential (according to the qualitative measurements)17. Post-procedural severity of TR was assessed based on qualitative parameters, including colour flow jet, and vena contracta as a semi-quantitative parameter. Location of TR jets was evaluated in the transgastric short-axis view at 20-50°. TOE was performed at baseline and during the procedure, with a Vivid E95 ultrasound system (GE healthcare). All measurements were reviewed by two independent cardiologists dedicated to echocardiographic evaluation.
LAI measurements
We evaluated the LAI in the anteroseptal commissure, which was defined as the ratio of the sum of the anterior and septal tricuspid leaflet length in relation to the septolateral tricuspid annulus ([anterior tricuspid leaflet length+septal tricuspid leaflet length] /septolateral length of the tricuspid annulus) in the midsystole phase18 (Central illustration A). During the intraprocedural TOE evaluation, an echocardiographer identified the anterior and septal leaflets and the anteroseptal coaptation line in the biplane midoesophageal view, including the inflow-outflow view and its orthogonal four-chamber view, as previously reported19. The anterior and posterior leaflets were discriminated by the anterior papillary muscle. Then, using the midoesophageal four-chamber view, which was orthogonal to the anteroseptal coaptation line, we determined the site where the widest vena contracta of the TR jet locating in the anteroseptal coaptation line was observed. After the procedure, the leaflet length and annular dimension at the site, detected during the procedure, were retrospectively measured by an experienced cardiologist, who was blinded to procedural and outcome data.
Central illustration. Association between the leaflet-to-annulus index and residual tricuspid regurgitation after transcatheter edge-to-edge repair.
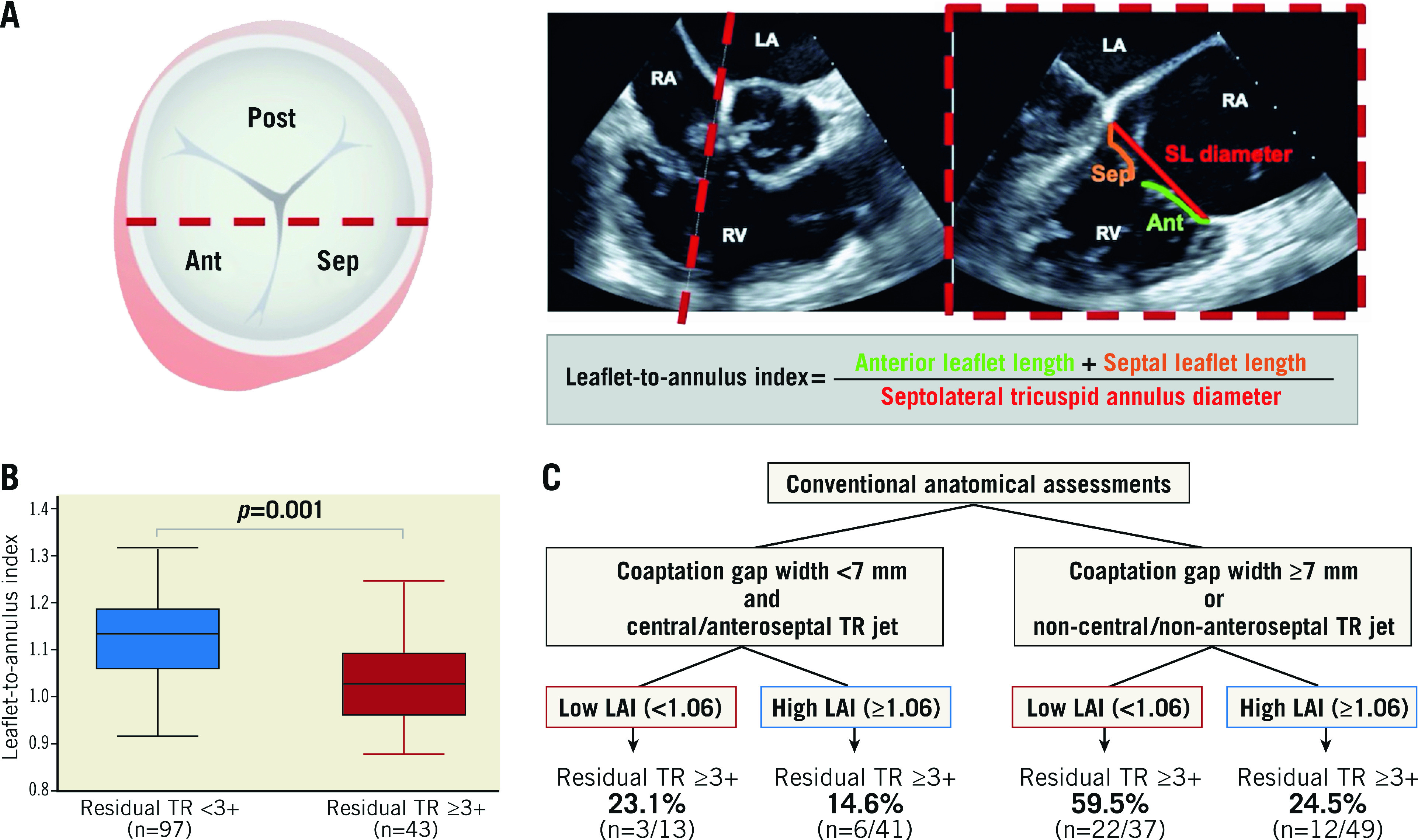
A. Assessment of the leaflet-to-annulus index. Red dashed line indicates the site in which the leaflet-to-annulus index (LAI) is evaluated. Simultaneous biplane view: the inflow–outflow view and four-chamber view. B. The LAI was lower in patients with residual tricuspid regurgitation (TR) ≥3+ compared to those without residual TR ≥3+. C. Sequential evaluation approach according to the LAI and conventional anatomical factors, including coaptation gap width and TR jet location. Ant: anterior leaflet; LA: left atrium; LAI: leaflet-to-annulus index; Post; posterior leaflet; RA: right atrium; RV: right ventricle; Sep: septal leaflet; SL diameter: septolateral tricuspid annulus diameter; TR: tricuspid regurgitation
Similarly, the LAIs in the posteroseptal and anteroposterior commissures were evaluated, described in Supplementary Appendix 1.
Outcomes and follow-up
The primary endpoint was significant residual TR, which was defined as residual TR ≥3+ upon discharge. The secondary endpoint was a composite outcome, consisting of all-cause mortality and hospitalisation due to heart failure, within one year following TEER. All suspected adverse events were independently adjudicated by the local Heart Team, according to the criteria of the Valve Academic Research Consortium 320. The occurrence of clinical events was recorded from the admission and outpatient medical records.
Statistical analysis
Continuous variables were presented as the mean±standard deviation or medians (interquartile range [IQR]) and compared using t-tests or the Mann-Whitney U-tests. Categorical variables were presented as numbers and percentages, and the differences between the groups were evaluated using the chi-square tests or Fisher's exact tests. Inter- and intra-observer variabilities for the LAI measurement were evaluated in 20 cases analysed by two examiners, and the results were analysed by means of the intraclass correlation coefficients.
First, a logistic regression analysis was conducted to assess the association of the LAI with residual TR ≥3+. The association was adjusted in the two multivariable models using geometric (model 1) and cardiac parameters (model 2). Parameters with a p-value <0.05 upon the univariate analysis were included in the models. We tested for collinearity in the multivariable models using the variance inflation factor.
Second, the receiver-operating characteristic (ROC) analysis was performed to determine the cut-off value of the LAI to predict residual TR ≥3+. The incremental effect of adding the LAI to the conventional factors, including coaptation gap width ≥7 mm, non-central/non-anteroseptal TR jet location, and TR ≥4+ at baseline, was assessed using net reclassification improvement (NRI) and integrated discrimination improvement (IDI). C-statistics were compared using DeLong’s method.
Third, event-free survival curves were constructed using the Kaplan-Meier method, and compared between patients with residual TR ≥3+ and <3+ using the log-rank test. Univariate and multivariable Cox proportional hazard models were used to explore factors associated with the composite outcome. Covariates with a p-value <0.05 upon the univariate analysis were included in the multivariable model. Statistical significance was set at p<0.05. All analyses were conducted using Stata 15.1 (StataCorp,).
Results
Clinical characteristics of the study population
Of 148 consecutive patients with TR who underwent their first TEER, a total of 140 patients were analysed (Supplementary Figure 1). The mean age was 78±7 years and 43.6% were male (Table 1). Preprocedural TR severity was graded as 3+, 4+, or 5+ in 56%, 36%, and 8% of the study participants, respectively, and the secondary aetiology of TR was observed in 94% of the patients.
Table 1. Baseline characteristics.
|
Total n=140 |
Residual TR ≥3+ n=43 |
Residual TR <3+ n=97 |
p-value | ||
|---|---|---|---|---|---|
| Male, n (%) | 61 (43.6) | 19 (44.2) | 42 (43.3) | 0.92 | |
| Age, years | 78±7 | 79±8 | 78±7 | 0.44 | |
| BMI, kg/m2 | 26.1±5.3 | 24.7±3.4 | 26.6±5.8 | 0.049 | |
| eGFR, ml/min/1.73 m2 | 46.9±16.4 | 48.1±16.2 | 46.2±16.4 | 0.53 | |
| Coronary artery disease | 83 (59.3) | 27 (62.8) | 56 (57.7) | 0.57 | |
| Previous myocardial infarction | 39 (27.9) | 14 (32.6) | 25 (25.8) | 0.41 | |
| Prior PCI, n (%) | 43 (30.7) | 15 (34.9) | 28 (28.9) | 0.48 | |
| Prior CABG, n (%) | 39 (27.9) | 16 (35.6) | 23 (24.2) | 0.16 | |
| Atrial fibrillation, n (%) | 133 (95) | 42 (97.7) | 91 (93.8) | 0.33 | |
| NYHA class III/IV, n (%) | 124 (88.6) | 38 (88.4) | 86 (88.7) | 0.64 | |
| Lead across tricuspid valve, n (%) | 49 (35.0) | 17 (39.5) | 32 (33.0) | 0.45 | |
| COPD, n (%) | 31 (22.1) | 12 (27.9) | 19 (19.6) | 0.27 | |
| Logistic EuroSCORE, % | 16.1 [8.1, 27.3] | 19.9 [9.5, 30.8] | 13.5 [7.6, 24.8] | 0.03 | |
| NT-pro BNP, pg/ml | 2,033 [1,009, 3,752] | 1,822 [888, 3,264] | 2,065 [1,202, 4563] | 0.18 | |
| Echocardiographic parameters | |||||
| Secondary TR, n (%) | 132 (94.2) | 42 (97.7) | 89 (92.7) | 0.25 | |
| TR severity, n (%) | 3+ | 78 (55.7) | 10 (23.3) | 68 (70.1) | <0.001 |
| 4+ | 50 (35.7) | 25 (58.1) | 25 (25.8) | ||
| 5+ | 12 (8.6) | 8 (18.6) | 4 (4.1) | ||
| Vena contracta, mm | 11.7 [9.0, 15.1] | 15.1 [12.5, 19.0] | 10.0 [8.1, 12.6] | <0.001 | |
| EROA, mm2 | 48.0 [36.0, 70.0] | 58.5 [37.8, 87.0] | 47.0 [35.0, 61.0] | 0.11 | |
| TR jet location | Central | 136 (97.1) | 43 (100.0) | 93 (95.9) | 0.18 |
| Anteroseptal commissure | 103 (53.6) | 35 (81.4) | 68 (70.1) | 0.16 | |
| Posteroseptal commissure | 80 (57.1) | 29 (67.4) | 51 (52.6) | 0.10 | |
| Anteroposterior commissure | 42 (30.0) | 26 (60.5) | 16 (16.5) | <0.001 | |
| LVEF, % | 55.2±10.2 | 56.0±9.4 | 54.9±10.6 | 0.55 | |
| LVEDV, ml | 69.8 [55.0, 99.1] | 62.2 [46.0, 87.9] | 72.7 [58.0, 101.6] | 0.02 | |
| Right ventricular diameter, mm | 39.9±8.8 | 40.5±8.6 | 39.4±8.6 | 0.50 | |
| Right atrial area, cm2 | 33.2 [27.0, 39.5] | 36.5 [28.7, 43.8] | 31.7 [25.4, 38.0] | 0.02 | |
| SPAP, mmHg | 36.6±12.4 | 34.3±12.3 | 37.8±12.3 | 0.14 | |
| TAPSE, mm | 17.9±5.0 | 17.9±5.3 | 17.9±4.9 | 0.99 | |
| Medication at baseline | Beta blocker, n (%) | 118 (84.3) | 40 (93.0) | 78 (80.4) | 0.06 |
| RAS inhibitor, n (%) | 90 (64.3) | 26 (60.5) | 64 (66.0) | 0.53 | |
| MRA, n (%) | 63 (45.0) | 20 (46.5) | 43 (44.3) | 0.81 | |
| Loop diuretics, n (%) | 127 (90.7) | 40 (93.0) | 87 (89.7) | 0.75 | |
| Values are n (%), mean±SD, or median [interquartile range]. BMI: body mass index; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EROA: effective regurgitant orifice area; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; MRA: mineralcorticoid receptor antagonist; NT-pro BNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; RAS: renin angiotensin system SPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation | |||||
Most cases were treated with the MitraClip/TriClip system (79.3%), followed by the PASCAL system (20.7%). The independent grasping feature was used in 21 patients (15.0%). Clip implantation failed in nine patients (6.4%), due to an insufficient view by echocardiography (n=1), insufficient TR reduction despite clip placement (n=3), or grasping failure (n=5). Single leaflet device attachment (SLDA) occurred in seven patients (5.0%) during hospitalisation, including five cases during the procedure, which did not require reintervention during hospitalisation. Acute procedural success was achieved in 123 patients (87.9%), whereas residual TR ≥3+ at discharge was observed in 43 patients (30.7%). As for demographic characteristics, the patients with residual TR ≥3+ had a lower body mass index and a higher logistic EuroSCORE compared to those with residual TR <3+ (Table 1). Other clinical characteristics were comparable between the two groups.
Echocardiographic and periprocedural findings
Patients with residual TR ≥3+ had a higher grade of TR at baseline compared to those with residual TR <3+. Additionally, patients with residual TR ≥3+ had a larger coaptation gap and coaptation depth than those with residual TR <3+.
TEER devices were mainly implanted in the anteroseptal coaptation line in the two groups (Table 2). The mean number of implanted devices was comparable between the two groups, while the occurrence of SLDA was numerically higher in patients with residual TR ≥3+ than in those with residual TR <3+ (9.3% vs 3.1%; p=0.12). No surgical conversion or periprocedural mortality occurred in either group.
Table 2. Periprocedural findings.
|
Total n=140 |
Residual TR ≥3+ n=43 |
Residual TR <3+ n=97 |
p-value | ||
|---|---|---|---|---|---|
| Device type, n (%) | MitraClip/TriClip</p> | 111 (79.3) | 30 (69.8) | 81 (83.5) | 0.06 |
| PASCAL | 29 (20.7) | 13 (30.2) | 16 (17.5) | ||
| Simultaneous TMVR, n (%) | 18 (12.9) | 3 (7.0) | 15 (15.5) | 0.17 | |
| Implant success, n (%) | 131 (93.6) | 34 (79.1) | 97 (100.0) | <0.001 | |
| Number of clips, n (%) | 0 | 9 (6.4) | 9 (20.9) | 0 (0.0) | <0.001 |
| 1 | 35 (25.0) | 7 (16.3) | 28 (28.9) | ||
| 2 | 72 (51.4) | 17 (39.5) | 55 (56.7) | ||
| 3 | 21 (15.0) | 9 (20.9) | 12 (12.4) | ||
| 4 | 3 (2.1) | 1 (2.3) | 2 (2.1) | ||
| Mean (if implanted) | 1.9±0.7 | 2.1±0.8 | 1.9±0.7 | 0.09 | |
| Implantation site of devices, n (%)* | Anteroseptal coaptation line | 202 (80.2) | 53 (73.6) | 149 (82.8) | 0.31 |
| Posteroseptal coaptation line | 47 (18.7) | 16 (22.2) | 31 (17.2) | ||
| Anteroposterior coaptation line | 3 (1.2) | 3 (4.2) | 0 (0.0) | ||
| Mean tricuspid valve gradient, mmHg | 2.6±1.5 | 2.9±1.4 | 2.4±1.5 | 0.08 | |
| Post-procedural TR grade, n (%) | 0 or 1+ | 24 (17.1) | 0 (0.0) | 24 (24.7) | <0.001 |
| 2+ | 73 (52.1) | 0 (0.0) | 73 (75.3) | ||
| 3+ | 38 (27.1) | 38 (88.4) | 0 (0.0) | ||
| 4+ | 3 (2.1) | 3 (7.0) | 0 (0.0) | ||
| 5+ | 2 (1.4) | 2 (4.7) | 0 (0.0) | ||
| Post-procedural vena contracta (mm) | 6.0 [4.1. 8.0] | 10.0 [8.1, 12.1] | 5.0 [3.9, 6.0] | <0.001 | |
| Acute procedural success, n (%) | 126 (90.0) | 29 (67.4) | 97 (100.0) | <0.001 | |
| Values are n (%) or mean±SD. *Numbers and percentages indicate number and percentage of clips implanted. TMVR: transcatheter mitral valve repair; TR: tricuspid regurgitation | |||||
The LAI analysis
The overall distribution of the LAI is shown in Supplementary Figure 2. The intraclass correlation coefficients revealed that the intra- and inter-observer reliabilities of the LAI measurement were acceptable (0.875 and 0.856, respectively). The patients with residual TR ≥3+ had a lower LAI compared to those with residual TR <3+ (1.04±0.10 vs 1.13±0.09; p=0.001) (Table 3, Central illustration B, Supplementary Figure 3). The tricuspid annulus diameter was numerically larger in patients with residual TR ≥3+ than in those with residual TR <3+. Moreover, patients with SLDA had a numerically higher LAI compared to those without SLDA (1.05±0.10 vs 1.11±0.10; p=0.14).
Table 3. Assessments of leaflet-to-annulus index.
|
Total n=140 |
Residual TR ≥3+ n=43 |
Residual TR <3+ n=97 |
p-value | |
|---|---|---|---|---|
| LAI | 1.11±0.10 | 1.04±0.10 | 1.13±0.09 | 0.001 |
| Annular diameter, mm | 42.7±6.8 | 44.4±6.4 | 42.1±6.9 | 0.07 |
| Septal leaflet length, mm | 20.0±4.7 | 20.3±5.0 | 19.8±4.6 | 0.56 |
| Anterior leaflet length, mm | 27.4±6.2 | 26.9±5.5 | 27.5±6.4 | 0.48 |
| Coaptation gap, mm | 2.9 [1.6, 4.6] | 4.7 [2.1, 7.2] | 2.2 [1.2, 3.4] | <0.001 |
| Coaptation depth, mm | 7.8±4.3 | 9.1±4.9 | 7.2±3.8 | 0.02 |
| Values are mean±SD or median [interquartile range]. LAI: leaflet-to-annulus index; TR: tricuspid regurgitation | ||||
In the logistic regression analysis, the LAI was negatively associated with the risk of residual TR ≥3+ after TEER (OR [per 0.1 increase]: 0.34; 95% CI: 0.21-0.55; p<0.001) (Table 4, Supplementary Table 1). Other covariates are listed in Supplementary Table 1. In the multivariable models, the association of the LAI remained significant after adjusting the covariates (Table 4: Model 1 and Model 2). Also, the LAI was associated with the acute procedural success (OR [per 0.1 increase]: 1.96; 95% CI: 1.10-3.50; p=0.02) in the univariate model.
Table 4. Logistic regression analysis for residual tricuspid regurgitation (≥3+).
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | Model 1 | Model 2 | |||||
| Adjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | ||||
| LAI (per 0.1 increase) | 0.34 | 0.21-0.55 | <0.001 | 0.38 | 0.22-0.66 | 0.007 | 0.41 | 0.25-0.70 | 0.008 |
| Geometric parameters | |||||||||
| Coaptation gap (mm) | 1.24 | 1.10-1.40 | <0.001 | 1.12 | 0.96-1.31 | 0.13 | |||
| Coaptation depth (mm) | 1.12 | 1.02-1.24 | 0.02 | 1.09 | 0.96-1.23 | 0.92 | |||
| Non-central/non-anteroseptal TR jet location | 2.86 | 1.27-6.43 | 0.01 | 2.01 | 0.78-5.16 | 0.15 | |||
| Annular diameter (mm) | 1.05 | 1.00-1.11 | 0.07 | ||||||
| Anterior leaflet length (mm) | 0.98 | 0.92-1.04 | 0.47 | ||||||
| Septal leaflet length (mm) | 0.96 | 0.89-1.03 | 0.27 | ||||||
| Secondary TR | 3.30 | 0.39-27.72 | 0.27 | ||||||
| Cardiac parameters | |||||||||
| TR ≥4+ | 8.13 | 3.54-18.70 | <0.001 | 4.97 | 2.02-2.25 | <0.001 | |||
| Right atrial area (cm2) | 1.03 | 1.00-1.07 | 0.04 | 1.02 | 0.98-1.06 | 0.34 | |||
| LVEF (%) | 1.01 | 0.97-1.05 | 0.55 | ||||||
| LVEDV (ml) | 0.99 | 0.98-1.00 | 0.07 | ||||||
| Right ventricular diameter (mm) | 1.02 | 0.97-1.06 | 0.49 | ||||||
| SPAP (mmHg) | 0.98 | 0.94-1.01 | 0.13 | ||||||
| TAPSE (mm) | 1.00 | 0.93-1.08 | 0.99 | ||||||
| CI: confidence interval; LAI: leaflet-to-annulus index; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; OR: odds ratio; SPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation | |||||||||
The predictive value of the LAI
The ROC analysis showed that the LAI value needed to discern residual TR ≥3+ was 1.06 (C-statistic: 0.725; p=0.001) (Supplementary Figure 4). Of the 140 patients, 50 patients (35.7%) had a lower LAI according to the cut-off value. A lower LAI was associated with an increased risk of residual TR ≥3+ (adjusted OR in model 1: 3.57; 95% CI: 1.45-8.33; p=0.006 and adjusted OR in model 2: 4.17; 95% CI: 1.69-10.00; p=0.002).
When the LAI was added to the conventional factors (i.e., coaptation gap width, non-central/non-anteroseptal TR jet location, and TR ≥4+ at baseline), the C-statistic increased from 0.780 to 0.809 (p=0.02) (Supplementary Table 2). Continuous NRI and IDI of the LAI were 0.65 (p=0.004) and 0.04 (p=0.03), respectively. By combining the LAI with the conventional anatomical factors (i.e., coaptation gap width ≥7 mm and non-central/non-anteroseptal TR jet location), patients could be stratified according to the risk of residual TR >3+ after TEER (Central illustration C).
There was no significant interaction between use of the independent grasping (binary) and the LAI (continuous) (p for interaction=0.14) (Supplementary Figure 5).
Also, the LAIs of posteroseptal and anteroposterior coaptation lines were evaluated (Supplementary Table 3). The LAIs of posteroseptal and anteroposterior coaptation lines were not associated with residual TR ≥3+ (OR [per 0.1 increase]: 1.68; 95% CI: 0.99-2.84; p=0.05, and OR [per 0.1 increase]: 0.98; 95% CI: 0.62-1.55; p=0.92, respectively).
Clinical outcome
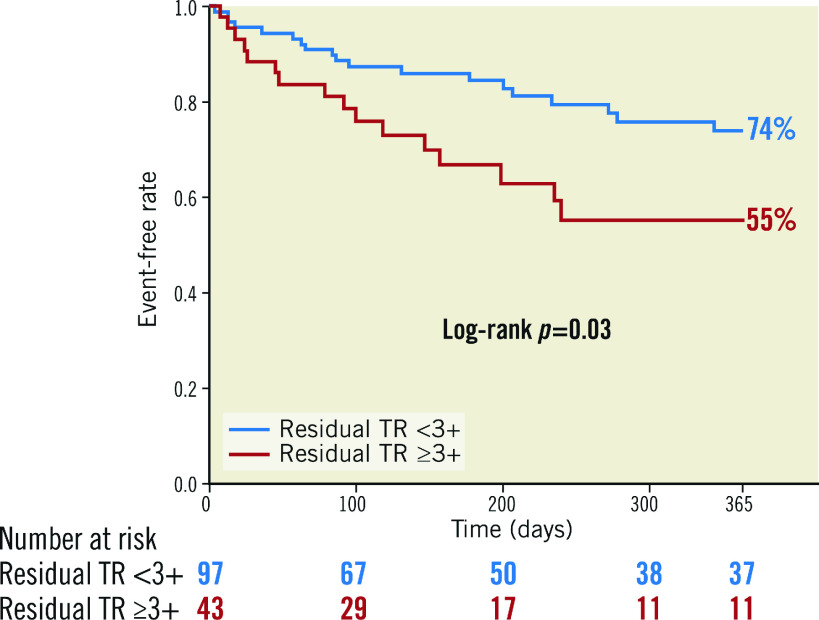
The median follow-up was 238 days (IQR: 150-365 days). Within one year following TEER, 18 patients died, of which 13 patients died due to cardiovascular causes, and 25 patients were hospitalised due to heart failure. Consequently, 35 patients experienced the composite outcome. Patients with residual TR ≥3+ had a higher incidence of the composite outcome compared to those with residual TR <3+ (45.0% vs 26.2%; p=0.03) (Figure 1).
Figure 1. Kaplan–Meier curve according to residual TR (≥3+) after transcatheter edge-to-edge repair for tricuspid regurgitation.
Event-free survival analysis of the composite outcome, consisting of all-cause mortality and heart failure hospitalisation, within one year following transcatheter edge-to-edge repair for tricuspid regurgitation.
In the Cox proportional hazard model, residual TR ≥3+ (hazard ratio [HR]: 2.08; 95% CI: 1.07-4.06; p=0.03) was significantly associated with the risk of the composite outcome within one year following TEER (Supplementary Table 4). The other significant factors were left ventricular ejection fraction (LVEF) and left ventricular end-diastolic volume (LVEDV). In the multivariable model, residual TR ≥3+ continued to be associated with the outcome (adjusted HR: 2.31; 95% CI: 1.17-4.58; p=0.02) (Table 5).
Table 5. Predictors of composite outcome within one year following transcatheter edge-to-edge repair.
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |
| Residual TR≥3+ | 2.08 | 1.07-4.06 | 0.03 | 2.31 | 1.17-4.58 | 0.02 |
| LAI (per 0.1 increase) | 0.89 | 0.63-1.24 | 0.47 | |||
| LVEF (%) | 0.97 | 0.94-0.99 | 0.04 | 0.99 | 0.95-1.02 | 0.48 |
| LVEDV (ml) | 1.01 | 1.00-1.02 | 0.03 | 1.01 | 0.99-1.02 | 0.08 |
| Significant variables (p<0.05) in the univariate analysis were included in the multivariable analysis. CI: confidence interval; HR: hazard ratio; LAI: leaflet-to-annulus index; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; TR: tricuspid regurgitation | ||||||
Discussion
This is the first study assessing the LAI and its impact on residual TR ≥3+ in patients undergoing a TEER procedure for TR. The main findings are summarised as follows:
1) a lower LAI was associated with residual TR ≥3+ after TEER, independent of the baseline TR grade and anatomical parameters;
2) the residual TR ≥3+ was a significant predictor of the composite outcome, consisting of all-cause mortality and heart failure hospitalisation, within one year following TEER in this setting.
Appropriate patient selection is essential to ensure optimal TR reduction after TEER. The impact of residual TR ≥3+ on the clinical outcome after TEER has been shown in an earlier study21 and in the present analysis. As a consequence of the increasing demand for TTVI, it is necessary to identify predictors of a greater reduction in TR following the procedure. In the present study, we revealed the association between the LAI and residual TR ≥3+ after TEER, which was still robust after accounting for known predictors (e.g., a large coaptation gap and non-central/non-anteroseptal location of the TR jet)22. Notably, the leaflet lengths themselves were not associated with an increased risk of residual TR ≥3+ after TEER, which implies that the relative leaflet length to annular dimension may be more relevant to the procedural outcome of TEER than the absolute length of the leaflets.
There are several possible explanations for the association between the LAI and residual TR. Despite the underlying mechanism of annulus dilatation, the excess of leaflet tissue (i.e., a higher LAI) can lead to the potential coaptation after edge-to-edge repair. In contrast, a short leaflet length (i.e., a lower LAI) could prevent successful clip insertion and force the clinician to place the devices farther from the main TR jet location, resulting in an ineffective reduction of TR. Alternatively, advanced TA dilation can result in an expanded TR jet area: the location of the TR expands from the centre or anteroseptal commissure to the anteroposterior or posteroseptal commissures of the TV23, which has been reported as a predictor of procedural failure22. It often requires more clips to reduce TR from multiple commissures, which can potentially lead to an elevated TV pressure gradient. In the present study, patients with residual TR ≥3+ had more clips and a higher post-procedural TV pressure gradient compared to those with residual TR <3+, which might indicate that the clinician hesitated to place further clips to address the residual TR. Furthermore, patients with SLDA had a numerically lower LAI compared to those without SLDA, which might contribute to the association between the LAI and residual TR. For these reasons, TEER, in patients with an advanced leaflet-to-annulus mismatch, can be challenging and it can be difficult to minimise TR.
The assessment of the LAI had an incremental effect of predicting residual TR after TEER on the conventional anatomical factors, such as coaptation gap width and non-central/non-anteroseptal location of the TR jet24. Edge-to-edge repair needs enough leaflet tissue to coaptate each other. The leaflet length at the site for clipping can differ between patients, regardless of the conventional factors, which may be related to the additional value of the LAI to the conventional factors. If there is enough leaflet length in relation to the enlarged annulus (i.e., a higher LAI), there will be an increased potential to achieve a greater reduction in TR with the conventional factors. By combining the LAI with the conventional anatomical factors, we could stratify the risk of residual TR ≥3+ after TEER. If patients have a low LAI in addition to a small coaptation gap width (<7 mm) and a central/anteroseptal jet location, the risk of residual TR ≥3+ was 14.6%, whereas the risk increased to 59.5% with a high LAI and the conventional anatomical factors. This risk-stratification model could assist the patient selection for TEER and potentially refine procedural results in the setting of TR.
In the present study, the LAIs of the posteroseptal and anteroposterior coaptation lines were not associated with residual TR ≥3+. The finding might reflect the lower frequency of clip implantation in the posteroseptal coaptation lines. In contrast, approximately 90% of the clip devices were implanted in the anteroseptal coaptation line, which is essential in the edge-to-edge repair technique6. From a technical perspective, the edge-to-edge devices inserted through the femoral vein are usually more accessible to the anteroseptal coaptation line than to other coaptation lines. Furthermore, an experimental study revealed that clips placed in the anteroseptal coaptation line led to a greater increase in cardiac output than those in the other coaptation lines25. Thus, the LAI of the anteroseptal coaptation line may be more relevant for predicting TR reduction by TEER compared to those of other coaptation lines.
Our observation might also imply several potential insights into the diversity of the TR development. In the present study, the LAI was negatively correlated with the TR severity at baseline (Supplementary Table 5). One plausible explanation is that patients with smaller leaflets have less reserve and develop TR when the annulus dilates. Alternatively, another possible explanation may be related to insufficient leaflet remodelling. So far, a few studies have reported leaflet remodelling on the TV side. For instance, Afilalo et al reported that patients with an enlarged TV annulus had longer tricuspid leaflets compared to the control population13. Similarly, we found a correlation between the leaflet lengths and the annulus dimension (Supplementary Figure 6), which was also true after adjusting body surface area. Further observational, longitudinal studies are needed to investigate the leaflet morphology and mechanism of TR development.
In the present study, the LAI itself was not directly associated with the composite outcome. Instead, residual TR ≥3+ was independently associated with the outcome, which may underscore the importance of TR reduction regardless of value of the LAI. Moreover, other variables, including LVEF and LVEDV, were significant outcome correlates. Given a high burden of comorbidities of patients with TR, it may be conceivable to conclude that LV systolic dysfunction and remodelling play a more important role in clinical outcomes.
For optimal TR reduction, our findings could translate into assisting with the device selection for TTVI in a clinical setting. Multiple transcatheter devices for TR have recently been developed with promising results. New generations of edge-to-edge repair devices provide the ability to independently grasp leaflets of the tricuspid valve. The ability to independently grasp leaflets might facilitate achieving an adequate clip insertion, and provide a more effective reduction in TR with a low LAI. In the present analysis, the interaction of the impact of LAI on residual TR using independent grasping was not significant. Since the number of patients in whom independent grasping was used was small, a further investigation is needed to assess the clinical relevance of independent grasping for TR with a low LAI. Furthermore, transcatheter annuloplasty may be another therapeutic option for TR with short leaflet length and severe TA dilation (i.e., a low LAI). The TRI-REPAIR study showed initial feasibility and a marked reduction in TR despite larger, wider TR jets in this population compared to the TRILUMINATE study8,9. Furthermore, the successful combination of TEER and annuloplasty for TR with severe TA dilation has been previously reported26. A preprocedural assessment of the LAI could help to determine whether leaflet-only treatment would be sufficient to reduce TR and refine therapeutic strategies in the tricuspid field.
Limitations
Several limitations to this study should be acknowledged. First, this was a single-centre, retrospective study with a relatively small number of participants. Therefore, patient selection bias might have impacted our results. Nevertheless, we conducted several multivariable models, adjusting for various clinical or anatomical covariates, which may at least partially address the issue. Second, since TV geometry can be altered by fluid volume and haemodynamics, the LAI could be different between the periprocedural and intraprocedural measurements. Third, in some cases, it may be challenging to differentiate the anterior and posterior leaflets using 2D TOE imaging, as previously reported19. Nevertheless, we used the anterior papillary muscle to identify the anteroposterior commissure27. Moreover, it might be difficult to identify the site for assessments of the LAI in patients with a triangular configuration of the central coaptation defect, which can be observed with a larger anteroposterior coaptation gap. Although our inter-observer correlation was acceptable, our preliminary data should be validated by further investigations using other imaging modalities, such as 3D echocardiography or cardiac computed tomography.
Conclusions
The LAI is independently associated with residual TR (≥3+) after TEER for TR, which is a significant predictor of one-year all-cause mortality, and hospitalisation due to heart failure. LAI evaluation could assist in making decisions about the treatment strategies for TR.
Impact on daily practice
Evaluation of the LAI, which is calculated from tricuspid leaflet length and annular dimension, is effective for predicting significant residual tricuspid regurgitation after transcatheter edge-to-edge repair for tricuspid regurgitation. LAI can help to identify suitable tricuspid valve for the edge-to-edge repair.
Supplementary data
Methods: Leaflet-to-annulus index measurements.
Univariate logistic regression analysis for residual tricuspid regurgitationtricuspid regurgitation ≥3+ after transcatheter edge-to-edge repair.
Incremental effect of adding the leaflet-to-annulus index to conventional factors for residual tricuspid regurgitation ≥3+.
Leaflet-to-annulus indexes of posteroseptal and anteroposterior coaptation lines.
Univariate Cox-proportional hazard analysis for the one-year composite outcome after transcatheter edge-to-edge repair.
Association of clinical parameters with leaflet-to-annulus index.
Study flowchart.
Distribution of the leaflet-to-annulus index.
Representative echocardiographic images of patients with a high and low leaflet-to-annulus index.
Receiver operating characteristics curve of the leaflet-to-annulus index for residual tricuspid regurgitation (≥3+) after transcatheter edge-to-edge repair.
Interaction between leaflet-to-annulus index and use of independent grasping on the risk of residual tricuspid regurgitation ≥3+.
Association between leaflet length and annulus dimension of tricuspid valve.
Acknowledgments
Acknowledgements
We would like to thank Dr. Meghan Lucas (scientific coordinator for the Heart Center Bonn, Bonn, Germany) for proofreading the manuscript.
Conflict of interest statement
M. Weber has received lecture or proctoring fees from Abbott, Boehringer-Ingelheim, Edwards Lifesciences, Janssen, Neochord, Pfizer, and Servier. G. Nickenig has received research funding from Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronic, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, the Deutsche Forschungsgemeinschaft, Edwards Lifesciences, the EU, the German Federal Ministry of Education and Research, Medtronic, Novartis, Pfizer, Sanofi, and St Jude Medical; and has received honoraria for lectures or advisory boards from Abbott, AGA Medical, AstraZeneca, Bayer, Berlin, Cardiovalve, Berlin Chemie, Biosensus, Biotronic, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St Jude Medical. T. Tanaka was financially supported in part by a Fellowship from the Japanese College of Cardiology. The other authors have no conflicts of interest to declare.
Abbreviations
- CI
confidence interval
- HR
hazard ratio
- IQR
interquartile range
- LAI
leaflet-to-annulus index
- OR
odds ratio
- ROC
receiver-operating characteristic
- TA
tricuspid annulus
- TOE
transoesophageal echocardiography
- TEER
transcatheter edge-to-edge repair
- TR
tricuspid regurgitation
- TV
tricuspid valve
- TTVI
transcatheter tricuspid valve intervention
Contributor Information
Tetsu Tanaka, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Atsushi Sugiura, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Refik Kavsur, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Johanna Vogelhuber, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Can Öztürk, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Marc Ulrich Becher, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Sebastian Zimmer, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Georg Nickenig, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
Marcel Weber, Medzinische Klinik und Poliklinik Ⅱ, Universitätsklinikum Bonn, Bonn, Germany.
References
- Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, Keren G, Banai S. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. 2020;21:157–65. doi: 10.1093/ehjci/jez216. [DOI] [PubMed] [Google Scholar]
- Topilsky Y, Maltais S, Medina Inojosa, Oguz D, Michelena H, Maalouf J, Mahoney DW, Enriquez-Sarano M. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. 2019;12:433–42. doi: 10.1016/j.jcmg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Dreyfus J, Flagiello M, Bazire B, Eggenspieler F, Viau F, Riant E, Mbaki Y, Bohbot Y, Eyharts D, Senage T, Dubrulle H, Nicol M, Doguet F, Nguyen V, Coisne A, Le Tourneau, Lavie-Badie Y, Tribouilloy C, Donal E, Tomasi J, Habib G, Selton-Suty C, Raffoul R, Iung B, Obadia JF, Messika-Zeitoun D. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. 2020;41:4304–17. doi: 10.1093/eurheartj/ehaa643. [DOI] [PubMed] [Google Scholar]
- Axtell AL, Bhambhani V, Moonsamy P, Healy EW, Picard MH, Sundt TM, Wasfy JH. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. J Am Coll Cardiol. 2019;74:715–25. doi: 10.1016/j.jacc.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramasso M, Benfari G, van der, Alessandrini H, Attinger-Toller A, Biasco L, Lurz P, Braun D, Brochet E, Connelly KA, de Bruijn, Denti P, Deuschl F, Estevez-Loureiro R, Fam N, Frerker C, Gavazzoni M, Hausleiter J, Ho E, Juliard JM, Kaple R, Besler C, Kodali S, Kreidel F, Kuck KH, Latib A, Lauten A, Monivas V, Mehr M, Muntané-Carol G, Nazif T, Nickening G, Pedrazzini G, Philippon F, Pozzoli A, Praz F, Puri R, Rodés-Cabau J, Schäfer U, Schofer J, Sievert H, Tang GHL, Thiele H, Topilsky Y, Rommel KP, Delgado V, Vahanian A, Von Bardeleben, Webb JG, Weber M, Windecker S, Winkel M, Zuber M, Leon MB, Hahn RT, Bax JJ, Enriquez-Sarano M, Maisano F. Transcatheter Versus Medical Treatment of Patients With Symptomatic Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2019;74:2998–3008. doi: 10.1016/j.jacc.2019.09.028. [DOI] [PubMed] [Google Scholar]
- Taramasso M, Hahn RT, Alessandrini H, Latib A, Attinger-Toller A, Braun D, Brochet E, Connelly KA, Denti P, Deuschl F, Englmaier A, Fam N, Frerker C, Hausleiter J, Juliard JM, Kaple R, Kreidel F, Kuck KH, Kuwata S, Ancona M, Malasa M, Nazif T, Nickenig G, Nietlispach F, Pozzoli A, Schäfer U, Schofer J, Schueler R, Tang G, Vahanian A, Webb JG, Yzeiraj E, Maisano F, Leon MB. The International Multicenter TriValve Registry: Which Patients Are Undergoing Transcatheter Tricuspid Repair? JACC Cardiovasc Interv. 2017;10:1982–90. doi: 10.1016/j.jcin.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Asmarats L, Perlman G, Praz F, Hensey M, Chrissoheris MP, Philippon F, Ofek H, Ye J, Puri R, Pibarot P, Attinger A, Moss R, Bedard E, Moschovitis A, Reineke D, Lauck S, Blanke P, Leipsic J, Spargias K, Windecker S, Webb JG, Rodés-Cabau J. Long-Term Outcomes of the FORMA Transcatheter Tricuspid Valve Repair System for the Treatment of Severe Tricuspid Regurgitation: Insights From the First-in-Human Experience. JACC Cardiovasc Interv. 2019;12:1438–47. doi: 10.1016/j.jcin.2019.04.038. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Weber M, Schuler R, Hausleiter J, Nabauer M, von Bardeleben, Sotiriou E, Schafer U, Deuschl F, Alessandrini H, Kreidel F, Juliard JM, Brochet E, Latib A, Montorfano M, Agricola E, Baldus S, Friedrichs KP, Deo SH, Gilmore SY, Feldman T, Hahn RT, Maisano F. Tricuspid valve repair with the Cardioband system: two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention. 2021;16:e1264–71. doi: 10.4244/EIJ-D-20-01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig G, Weber M, Lurz P, von Bardeleben, Sitges M, Sorajja P, Hausleiter J, Denti P, Trochu JN, Nabauer M, Dahou A, Hahn RT. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–11. doi: 10.1016/S0140-6736(19)32600-5. [DOI] [PubMed] [Google Scholar]
- Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, Gray WA, Narang A, Pislaru SV, Koulogiannis K, Grayburn P, Fowler D, Hawthorne K, Dahou A, Deo SH, Vandrangi P, Deuschl F, Mack MJ, Leon MB, Feldman T, Davidson CJ CLASP TR EFS Investigators. Feasibility Study of the Transcatheter Valve Repair System for Severe Tricuspid Regurgitation. J Am Coll Cardiol. 2021;77:345–56. doi: 10.1016/j.jacc.2020.11.047. [DOI] [PubMed] [Google Scholar]
- Taramasso M, Gavazzoni M, Pozzoli A, Dreyfus GD, Bolling SF, George I, Kapos I, Tanner FC, Zuber M, Maisano F, Hahn RT. Tricuspid Regurgitation: Predicting the Need for Intervention, Procedural Success, and Recurrence of Disease. JACC Cardiovasc Imaging. 2019;12:605–21. doi: 10.1016/j.jcmg.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Badano LP, Hahn R, Rodriguez-Zanella H, Araiza Garaygordobil, Ochoa-Jimenez RC, Muraru D. Morphological Assessment of the Tricuspid Apparatus and Grading Regurgitation Severity in Patients With Functional Tricuspid Regurgitation: Thinking Outside the Box. JACC Cardiovasc Imaging. 2019;12:652–64. doi: 10.1016/j.jcmg.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Afilalo J, Grapsa J, Nihoyannopoulos P, Beaudoin J, Gibbs JS, Channick RN, Langleben D, Rudski LG, Hua L, Handschumacher MD, Picard MH, Levine RA. Leaflet area as a determinant of tricuspid regurgitation severity in patients with pulmonary hypertension. Circ Cardiovasc Imaging. 2015;8:e002714. doi: 10.1161/CIRCIMAGING.114.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig G, Kowalski M, Hausleiter J, Braun D, Schofer J, Yzeiraj E, Rudolph V, Friedrichs K, Maisano F, Taramasso M, Fam N, Bianchi G, Bedogni F, Denti P, Alfieri O, Latib A, Colombo A, Hammerstingl C, Schueler R. Transcatheter Treatment of Severe Tricuspid Regurgitation With the Edge-to-Edge MitraClip Technique. Circulation. 2017;135:1802–14. doi: 10.1161/CIRCULATIONAHA.116.024848. [DOI] [PubMed] [Google Scholar]
- Fam NP, Braun D, von Bardeleben, Nabauer M, Ruf T, Connelly KA, Ho E, Thiele H, Lurz P, Weber M, Nickenig G, Narang A, Davidson CJ, Hausleiter J. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. 2019;12:2488–95. doi: 10.1016/j.jcin.2019.09.046. [DOI] [PubMed] [Google Scholar]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc Imaging. 2019;12:469–90. doi: 10.1016/j.jcmg.2018.07.033. [DOI] [PubMed] [Google Scholar]
- Tabata N, Weber M, Sugiura A, Öztürk C, Ishii M, Tsujita K, Nickenig G, Sinning JM. Impact of the Leaflet-to-Annulus Index on Residual Mitral Regurgitation in Patients Undergoing Edge-to-Edge Mitral Repair. JACC Cardiovasc Interv. 2019;12:2462–72. doi: 10.1016/j.jcin.2019.09.014. [DOI] [PubMed] [Google Scholar]
- Agricola E, Asmarats L, Maisano F, Cavalcante JL, Liu S, Milla F, Meduri C, Rodés-Cabau J, Vannan M, Pibarot P. Imaging for Tricuspid Valve Repair and Replacement. JACC Cardiovasc Imaging. 2021;14:61–111. doi: 10.1016/j.jcmg.2020.01.031. [DOI] [PubMed] [Google Scholar]
- VARC-3 Writing, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, Blackstone EH, Finn MT, Kapadia S, Linke A, Mack MJ, Makkar R, Mehran R, Popma JJ, Reardon M, Rodes-Cabau J, Van Mieghem, Webb JG, Cohen DJ, Leon MB. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42:1825–57. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- Mehr M, Taramasso M, Besler C, Ruf T, Connelly KA, Weber M, Yzeiraj E, Schiavi D, Mangieri A, Vaskelyte L, Alessandrini H, Deuschl F, Brugger N, Ahmad H, Biasco L, Orban M, Deseive S, Braun D, Rommel KP, Pozzoli A, Frerker C, Näbauer M, Massberg S, Pedrazzini G, Tang GHL, Windecker S, Schäfer U, Kuck KH, Sievert H, Denti P, Latib A, Schofer J, Nickenig G, Fam N, von Bardeleben, Lurz P, Maisano F, Hausleiter J. 1-Year Outcomes After Edge-to-Edge Valve Repair for Symptomatic Tricuspid Regurgitation: Results From the TriValve Registry. JACC Cardiovasc Interv. 2019;12:1451–61. doi: 10.1016/j.jcin.2019.04.019. [DOI] [PubMed] [Google Scholar]
- Besler C, Orban M, Rommel KP, Braun D, Patel M, Hagl C, Borger M, Nabauer M, Massberg S, Thiele H, Hausleiter J, Lurz P. Predictors of Procedural and Clinical Outcomes in Patients With Symptomatic Tricuspid Regurgitation Undergoing Transcatheter Edge-to-Edge Repair. JACC Cardiovasc Interv. 2018;11:1119–28. doi: 10.1016/j.jcin.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Spinner EM, Shannon P, Buice D, Jimenez JH, Veledar E, Del Nido, Adams DH, Yoganathan AP. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation. 2011;124:920–9. doi: 10.1161/CIRCULATIONAHA.110.003897. [DOI] [PubMed] [Google Scholar]
- Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, Senni M, von Bardeleben, Nickenig G, Hausleiter J, Mangieri A, Zamorano JL, Prendergast BD, Maisano F. Transcatheter treatment of the tricuspid valve. EuroIntervention. 2021;17:791–808. doi: 10.4244/EIJ-D-21-00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara R, Gelpi G, Prabhu S, Romitelli P, Troxler LG, Mangini A, Romagnoni C, Contino M, Van Hoven, Lucherini F, Jaworek M, Redaelli A, Fiore GB, Antona C. Transcatheter Edge-to-Edge Treatment of Functional Tricuspid Regurgitation in an Ex Vivo Pulsatile Heart Model. J Am Coll Cardiol. 2016;68:1024–33. doi: 10.1016/j.jacc.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Sugiura A, Weber M, Sinning JM, Werner N, Nickenig G. Staged transcatheter valve repair via MitraClip XTR after Cardioband for tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. 2019;20:118. doi: 10.1093/ehjci/jey153. [DOI] [PubMed] [Google Scholar]
- Dahou A, Levin D, Reisman M, Hahn RT. Anatomy and Physiology of the Tricuspid Valve. JACC Cardiovasc Imaging. 2019;12:458–68. doi: 10.1016/j.jcmg.2018.07.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods: Leaflet-to-annulus index measurements.
Univariate logistic regression analysis for residual tricuspid regurgitationtricuspid regurgitation ≥3+ after transcatheter edge-to-edge repair.
Incremental effect of adding the leaflet-to-annulus index to conventional factors for residual tricuspid regurgitation ≥3+.
Leaflet-to-annulus indexes of posteroseptal and anteroposterior coaptation lines.
Univariate Cox-proportional hazard analysis for the one-year composite outcome after transcatheter edge-to-edge repair.
Association of clinical parameters with leaflet-to-annulus index.
Study flowchart.
Distribution of the leaflet-to-annulus index.
Representative echocardiographic images of patients with a high and low leaflet-to-annulus index.
Receiver operating characteristics curve of the leaflet-to-annulus index for residual tricuspid regurgitation (≥3+) after transcatheter edge-to-edge repair.
Interaction between leaflet-to-annulus index and use of independent grasping on the risk of residual tricuspid regurgitation ≥3+.
Association between leaflet length and annulus dimension of tricuspid valve.