Background:
Under the hypothesis that mechanical power ratio could identify the spontaneously breathing patients with a higher risk of respiratory failure, this study assessed lung mechanics in nonintubated patients with COVID-19 pneumonia, aiming to (1) describe their characteristics; (2) compare lung mechanics between patients who received respiratory treatment escalation and those who did not; and (3) identify variables associated with the need for respiratory treatment escalation.
Methods:
Secondary analysis of prospectively enrolled cohort involving 111 consecutive spontaneously breathing adults receiving continuous positive airway pressure, enrolled from September 2020 to December 2021. Lung mechanics and other previously reported predictive indices were calculated, as well as a novel variable: the mechanical power ratio (the ratio between the actual and the expected baseline mechanical power). Patients were grouped according to the outcome: (1) no-treatment escalation (patient supported in continuous positive airway pressure until improvement) and (2) treatment escalation (escalation of the respiratory support to noninvasive or invasive mechanical ventilation), and the association between lung mechanics/predictive scores and outcome was assessed.
Results:
At day 1, patients undergoing treatment escalation had spontaneous tidal volume similar to those of patients who did not (7.1 ± 1.9 vs. 7.1 ± 1.4 ml/kgIBW; P = 0.990). In contrast, they showed higher respiratory rate (20 ± 5 vs. 18 ± 5 breaths/min; P = 0.028), minute ventilation (9.2 ± 3.0 vs. 7.9 ± 2.4 l/min; P = 0.011), tidal pleural pressure (8.1 ± 3.7 vs. 6.0 ± 3.1 cm H2O; P = 0.003), mechanical power ratio (2.4 ± 1.4 vs. 1.7 ± 1.5; P = 0.042), and lower partial pressure of alveolar oxygen/fractional inspired oxygen tension (174 ± 64 vs. 220 ± 95; P = 0.007). The mechanical power (area under the curve, 0.738; 95% CI, 0.636 to 0.839] P < 0.001), the mechanical power ratio (area under the curve, 0.734; 95% CI, 0.625 to 0.844; P < 0.001), and the pressure-rate index (area under the curve, 0.733; 95% CI, 0.631 to 0.835; P < 0.001) showed the highest areas under the curve.
Conclusions:
In this COVID-19 cohort, tidal volume was similar in patients undergoing treatment escalation and in patients who did not; mechanical power, its ratio, and pressure-rate index were the variables presenting the highest association with the clinical outcome.
Despite similar spontaneous tidal volumes, escalated patients had higher respiratory rate, minute ventilation, pleural pressure, and mechanical power ratios. Mechanical power, its ratio with the expected baseline value, and the pressure-rate index had the greatest associations with treatment escalation.
Editor’s Perspective.
What We Already Know about This Topic
Determination of the optimal timing of endotracheal intubation in patients presenting with respiratory failure from COVID-19 pneumonia based on objective physiologic parameters is controversial
The authors performed a secondary analysis of such patients in which a baseline computed tomography scan was obtained on admission and a battery of routine clinical and more complex derived respiratory parameters were quantified using esophageal manometry and transthoracic electrical impedance including mechanical power, its ratio to the expected baseline value, and the pressure-rate index (4 × driving pressure + respiratory rate)
Associations of the studied parameters with treatment escalation to intubation and mechanical ventilation was assessed using the area under the receiver operator characteristic curve
What This Article Tells Us That Is New
Despite similar spontaneous tidal volumes, escalated patients had higher respiratory rate, minute ventilation, pleural pressure, and mechanical power ratios
Mechanical power, its ratio with the expected baseline value, and the pressure-rate index had the greatest associations with treatment escalation
In patients with acute respiratory failure, the direct measurement of tidal volume, minute ventilation, and esophageal pressure, as well as their integration with other data such as gas-exchange or radiological variables, provides valuable clinical information to determine the diagnosis and to monitor the clinical response to a therapeutic intervention.1 Furthermore, such a clinical monitoring may allow for the early identification of clinical deterioration. In an era of personalized medicine, a tailored clinical approach is only possible through a careful approach to clinical monitoring, whose invasiveness should be proportional to the specific clinical severity and patients’ requirements.
The mechanical power quantifies the amount of energy transferred to the respiratory system during mechanical ventilation, and it depends on the parameters used to set the ventilator and the resulting variables in the respiratory system.2 Such energy is entirely provided by the ventilator in controlled mechanical ventilation, while it results from the combination of the energy generated by the respiratory muscles and the energy delivered by the ventilator in supported ventilation.
In this study, we aimed to (1) describe the physiologic characteristics of spontaneously breathing patients with COVID-19 pneumonia, on admission and over the course of hospitalization; (2) compare the respiratory mechanics of patients who underwent treatment escalation with those who did not; and (3) derive a diagnostic receiver operating characteristic model to assess which variables are associated with the need for respiratory treatment escalation.
Materials and Methods
Patient Population
This study is a secondary analysis of a prospective cohort, collected from September 2020 to December 2021, in which the primary objective was to assess whether an increased esophageal pressure was related to the severity of COVID-19 disease and the clinical outcome.1 All spontaneously breathing patients with COVID-19 infection, confirmed by polymerase chain reaction, and acute respiratory failure supported by continuous positive airway pressure were enrolled in the study, and the current analysis was performed a posteriori. All patients were managed in a high-dependency COVID-19 unit and were consecutively enrolled. Of 140 eligible patients, we were able to measure minute ventilation only in the last 111 individuals (see flow chart for patients’ enrollment in the supplemental material, http://links.lww.com/ALN/C988).
The clinical management of the study individuals included standard COVID-19 pharmacologic guidance (see the supplemental material for details on pharmacologic management, http://links.lww.com/ALN/C988), which was initiated on admission in all patients. Although the study individuals were undergoing sessions of awake prone positioning, during data collection, each patient was in the supine position (for at least 2 h) and supported with continuous positive airway pressure. The study was approved by the local ethical committee (Comitato Etico Milano Area I; 17263/2020-2020/ST/095), and written informed consent was obtained from each individual. The article follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Study Protocol
All patients were first assessed in the emergency department, where a computed tomography scan was performed in spontaneous breathing and without positive end-expiratory pressure (PEEP), and an arterial blood sample was collected. The patients were then transferred to the high-dependency COVID-19 unit, where continuous positive airway pressure was applied according to local guidance (Fio2 of 60% and PEEP of 5, 7.5, or 10 cm H2O, as set by the attending physician). The esophageal pressure was measured using the esophageal-balloon catheter, connected to a data acquisition system (Optivent SIDAM Srl, Modena, Italy), while minute ventilation, respiratory rate, and tidal volume were quantified by a novel impedance-based monitoring device: ExSpiron (Respiratory Motion Inc., Watertown, Massachusetts). Blood gases were sampled from an arterial catheter.
We defined two outcome groups based on whether a patient underwent the escalation of the respiratory support: (1) no treatment escalation (the individual was supported with continuous positive airway pressure from admission in the high-dependency COVID-19 unit until clinical improvement, and then transferred to the ward) and (2) treatment escalation (any additional increase of the respiratory support, either noninvasive or invasive ventilation).
The decision to escalate the ventilatory support, as well as the type of treatment escalation (to either noninvasive or invasive mechanical ventilation), were left to the discretion of the clinical care team in accordance with local institutional guidelines: tachypnea more than 28 breaths/min, esophageal pressure swing higher than 8 cm H2O, Pao2/Fio2 ratio lower than 100, delirium, increase of 2 points in the Work of Breathing score3 or 3 points in the Borg scale,4 or intolerance to continuous positive airway pressure. Similarly, the type of treatment escalation (escalation from continuous positive airway pressure to noninvasive or to invasive mechanical ventilation) was left to the decision of the treating clinical team.
Measurements
Tissue Mass and Gas Volume
After semiautomatic contour analysis of the lung computed tomography slices,5 the following variables were derived: tissue mass (g), gas volume (ml), and fractions (%) of overaerated, normally aerated, poorly aerated, and nonaerated tissue. The estimated baseline gas volume and tissue mass were calculated according to Ibanez et al.6 and Cressoni et al.7 (the equations are detailed in the supplemental material, http://links.lww.com/ALN/C988).
Tidal Volume and Minute Ventilation
Tidal volume and minute ventilation were quantified by analysis of the variation of the electrical impedance in the respiratory system,8 which was validated in acute and postacute patients9–12 (see supplemental material for specifics and details, http://links.lww.com/ALN/C988).
Esophageal Pressure
Esophageal pressure was determined using the esophageal- balloon catheter, connected to a data acquisition system (Optivent SIDAM Srl; see supplemental material for specifics and details, http://links.lww.com/ALN/C988).
The measurement of these variables allowed us to estimate the following ventilatory variables: ventilatory ratio, tidal pleural pressure, dynamic lung elastance, and tidal muscular pressure, which is the pressure exerted on the respiratory system by the respiratory muscles (see the supplemental material for the derivation of equations, http://links.lww.com/ALN/C988). In addition, we quantified the mechanical power and its normalization over the expected mechanical power at rest.
Respiratory System Mechanical Power
Respiratory system mechanical power (MPRS) was estimated as follows:
| (1) |
where 0.098 is the constant that converts l · cm H2O into J/min; RR is the respiratory rate; Vt is the tidal volume; 0.714 is a constant that accounts for the ratio between lung and total elastances (EL/Ers), that we assumed to equal 0.713; and ΔPpl is the tidal pleural pressure. See the supplemental material (http://links.lww.com/ALN/C988) for the full derivation of equation 1.
Mechanical Power Ratio
The mechanical power ratio is the ratio between the actual mechanical power of the respiratory system and the expected baseline mechanical power. The latter was estimated, analogously to the ventilatory ratio,14 as the power required to obtain a normal minute ventilation (computed as 0.1 times the ideal body weight)15 by applying an ideal transpulmonary pressure of 5 cm H2O16 at 15 breaths/min and without applied PEEP. The equations we used to quantify the expected baseline mechanical power (MPexp) and the mechanical power ratio (MP ratio) are as follows:
| (2) |
| (3) |
where 1.47, 0.006, 3.57, and 7.5 are conversion constants (see supplemental material for the full derivation, http://links.lww.com/ALN/C988), and IBW is the ideal body weight. The mechanical power ratio, in essence, measures the fold increase in mechanical power compared to normal breathing in healthy conditions.
Additional Indexes
The ratio of oxygen saturation measured by pulse oximetry/Fio2 to respiratory rate index17 was calculated as the ratio between oxygen pulse oximetry to the fraction of inspired oxygen, over the respiratory rate. The pressure-rate index18 was rearranged to account for the spontaneous breathing and for easy comparison with the mechanical power; i.e., for its computation, we used ΔPes instead of the driving pressure.
Statistical Analysis
We used a convenience sample of all eligible patients during the observation period and did not perform a sample size calculation as no previous data on the distribution of mechanical power ratio on COVID-19 patients is available. The data are reported as means ± SD or median [interquartile range], as appropriate. The comparison between the two groups was performed using the independent-samples Student’s t test or Wilcoxon’s test, according to the distribution of each variable. Chi-square or Fisher’s exact test were used to compare categorical variables. A receiver operating characteristic analysis was performed to assess the area under the curve for each variable, to estimate the association with the outcome. To evaluate the effect of multiple repeated measures in the same population during the time course, we built a model of repeated measures analysis of variance, in which the fixed effect was the need for treatment escalation and the random effect was the individual ID. No adjustment for potential confounders was performed in the statistical analysis because our aim was to assess the association between mechanical power ratio and the clinical outcome in the population as a whole. Two-tailed P values less than 0.05 were considered statistically significant. All analyses were performed with R for Statistical Computing 4.0 and SPSS version 25.
Results
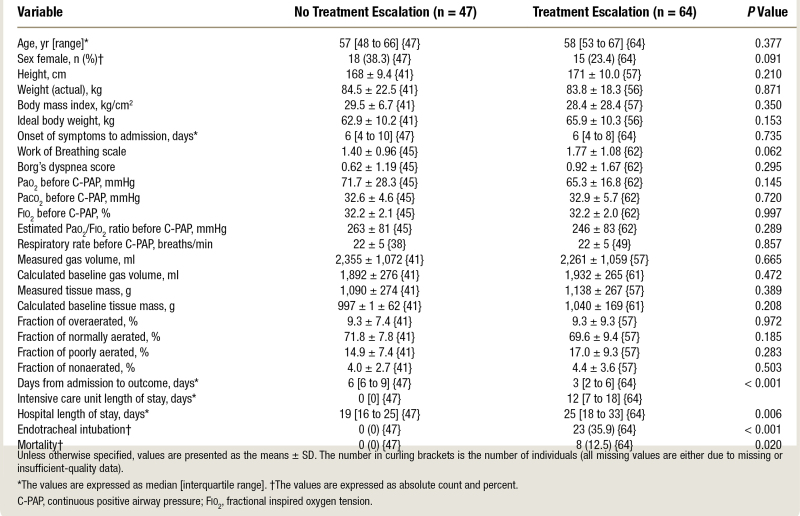
A total of 111 adults were enrolled in the study. In table 1, we present the main demographic and anatomic–physiologic characteristics measured in the emergency department before continuous positive airway pressure initiation in patients who underwent ventilatory treatment escalation (n = 64) and those who did not (n = 47). As shown, no differences were observed in terms of demographics, time from symptoms onset to the referral to the emergency department, or gas-exchange variables between the two outcome groups. In addition, no differences between groups were observed in terms of measured gas volume and tissue mass and in the fractions of overaerated, normally aerated, poorly aerated, and nonaerated tissue.
Table 1.
Baseline, Demographic, and Clinical Characteristics
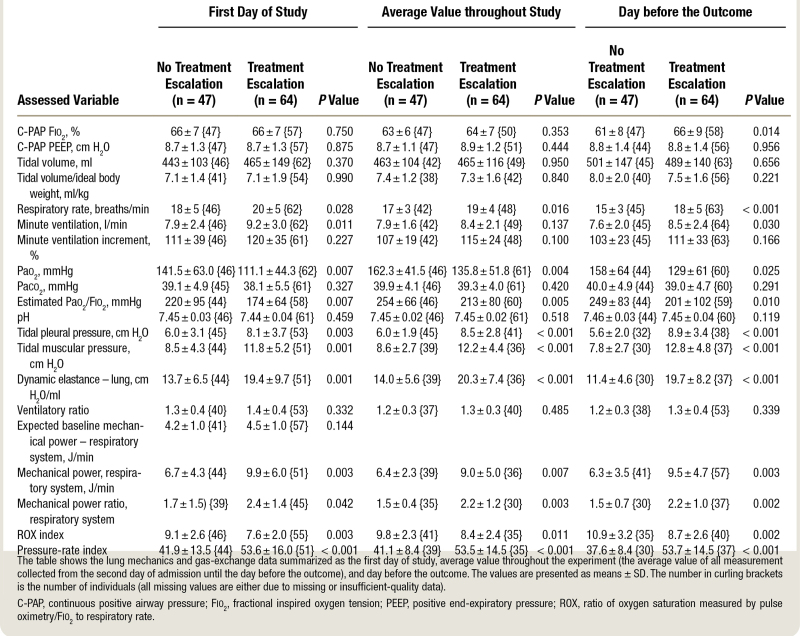
On day 1, once continuous positive airway pressure treatment was implemented, the group with no treatment escalation showed higher values of Pao2 (mean ± SD, 141.5 ± 63.0 vs. 111.1 ± 44.3 mmHg; P = 0.007) and Pao2/Fio2 (220 ± 95 vs. 174 ± 64 mmHg; P = 0.007; table 2). Paco2 was similar between groups but at the cost of higher minute ventilation (9.2 ± 3.0 vs. 7.9 ± 2.4 L/min; P = 0.011), respiratory rate (20 ± 5 vs. 18 ± 5 breaths/min; P = 0.028), tidal pleural pressure (8.1 ± 3.7 vs. 6.0 ± 3.1 cm H2O; P = 0.003), tidal muscular pressure (11.8 ± 5.2 vs. 8.5 ± 4.3 cm H2O; P = 0.001), and dynamic lung elastance (19.4 ± 9.7 vs. 13.7 ± 6.5 cm H2O/L; P = 0.001) in the treatment escalation group. Ventilatory ratio was comparable, while the absolute mechanical power of the respiratory system (9.9 ± 6.0 vs. 6.7 ± 4.3 J/min; P = 0.003) and its relative mechanical power ratio (2.4 ± 1.4 vs. 1.7 ± 1.5; P = 0.042) were higher in the treatment escalation group. The respiratory rate index and the pressure-rate indexes were also significantly different between groups (respectively, in the escalation vs. no escalation groups: 7.6 ± 2.0 vs. 9.1 ± 2.6, P = 0.003; and 53.6 ± 16.0 vs. 41.9 ± 1.4, P = 0.04).
Table 2.
Lung Mechanics and Gas-Exchange Data
The variables presenting significant differences between escalation vs. nonescalation group the day before the outcome occurred were as follows: respiratory rate (18 ± 5 vs. 15 ± 3 breaths/min; P = 0.001), Pao2 (129 ± 61 vs. 158 ± 64 mmHg; p 0.025), Pao2/Fio2 (201 ± 102 vs. 249 ± 83 mmHg; P = 0.010), tidal pleural pressure swings (8.9 ± 3.4 vs. 5.6 ± 2.0 cm H2O; P < 0.001), mechanical power (9.5 ± 4.7 vs. 6.3 ± 3.5 J/min; P = 0.003), and its ratio (2.2 ± 1.0 vs. 1.5 ± 0.7; P = 0.002). Of note, the indexed tidal volume remained similar between groups (7.5 ± 1.6 vs. 8.0 ± 2.0 ml/kgIBW; P = 0.221; table 2).
The most relevant respiratory variables from the univariate analysis on day 1 were entered in a receiver operating characteristic model to assess their association with the need for treatment escalation (table E1 in supplemental digital content, http://links.lww.com/ALN/C988). The mechanical power of the respiratory system and the mechanical power ratio showed areas under the curve of 0.738 (95% CI, 0.636 to 0.839; P < 0.001) and 0.734 (95% CI, 0.625 to 0.844; P < 0.001), while the area under the curve of tidal muscular pressure was 0.700 (95% CI, 0.595 to 0.804; P < 0.001). The area under the curve of the respiratory rate index was 0.659 (95% CI, 0.549 to 0.769; P = 0.006), while the area under the curve of pressure-rate index was 0.733 (95% CI, 0.631 to 0.835). The relative receiver operating characteristics are shown in figures E1 and E2 of the supplemental material (http://links.lww.com/ALN/C988).
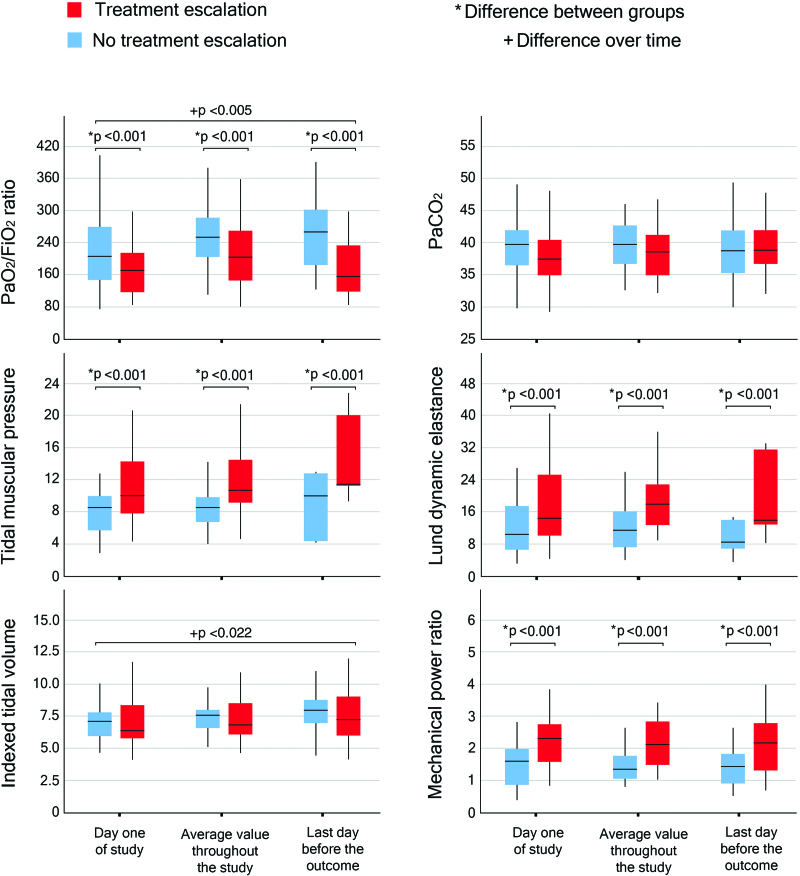
Figure 1 depicts the time evolution of Pao2/Fio2 and Paco2 (indicators of the efficiency of the gas exchanger), the tidal muscular pressure and dynamic lung elastance (indicators of the lung mechanics), and the indexed tidal volume and mechanical power ratio (indicators of the effort undertaken by the patient). The time evolution is detailed in three steps: (1) record collected on day 1; (2) average of the records collected from day 2 until the day before the outcome; and (3) record collected the day before the outcome. All tested variables but Paco2 and indexed tidal volume showed significant differences between groups when analyzed at each time point; conversely, when analyzing the evolution of such variables over time, the only variables presenting significant differences were the Pao2/Fio2 ratio and the indexed tidal volume (fig. 1).
Fig. 1.
Time course of Pao2/fractional inspired oxygen tension (Fio2) ratio and Paco2 (mmHg; top), tidal muscular pressure (cm H2O), and lung dynamic elastance (cm/l; middle), indexed tidal volume (ml/kgIBW), and mechanical power ratio (bottom). An asterisk indicates difference between groups, while a plus sign refers to difference over time.
Discussion
In a previous study based on the same cohort and data on COVID-19 patients in continuous positive airway pressure, we observed that total lung stress was the best predictive variable of disease evolution1: patients who required invasive mechanical ventilation had a lower Pao2/Fio2 ratio and greater lung stress. In this study, we were able to assess not only the stress through the esophageal pressure measurement but also tidal volume and respiratory rate, so that we could assess mechanical power, dynamic lung elastance, and muscular pressure (Pmusc). In addition, we computed two recently described predictive indexes, the respiratory rate index,17 which is designed to predict high-flow nasal cannulas and continuous positive airway pressure failure, and the pressure-rate index,18 which combined the two variables most strongly associated with the outcome (driving pressure and respiratory rate) in a mechanically ventilated population.
The novel information we observed in this current analysis is that the tidal volume, rather unexpectedly, was similar in the two outcome groups. The groups differed, however, in terms of respiratory rate, minute ventilation, and mechanical power. This behavior depicts a clear trend: for the same impairment of the lung structures, as quantified by the lung computed tomography scan, the patients in the treatment escalation group maintained normal Paco2 without increasing the tidal volume, but with a significant increase of the respiratory effort and energy requirement, as shown by the increased esophageal pressure swing, muscular pressure, mechanical power, and minute ventilation. Furthermore, we explored the association between several variables and the need for respiratory treatment escalation, and we identified the mechanical power, the mechanical power ratio, and the pressure-rate index to have the highest area under the curve, respectively: 0.738; 0.734, and 0.733.
This is an interesting finding as most current criteria evaluating the risk failure of noninvasive support are based on a threshold value of tidal volume beyond which the risk of failure is considered high: i.e., 9 to 9.5 ml/kg.19,20 On the contrary, the HACOR score (heart rate, acidosis, consciousness, oxygenation, and respiratory rate), used to determine the risk of failure of noninvasive ventilation, does not include the tidal volume but includes respiratory rate, oxygenation, and pH among the respiratory variables.21 Although these studies were performed pre-COVID-19 in patients with hypoxemic respiratory failure of mixed etiology, these criteria have been extrapolated in the management of COVID-19. Therefore, the approaches illustrated in this study may improve the detection of risk of failure specifically in COVID-19–related acute hypoxemic respiratory failure.
The escalation of the respiratory treatment from continuous positive airway pressure to noninvasive or invasive mechanical ventilation is usually consequent to a worsening in respiratory conditions. As previously reported, the decision to intubate or not a patient with COVID-19 pneumonia is cumbersome and based on several clinical assessments22; therefore, to identify all patients presenting unfavorable clinical evolution, we allocated in the poor clinical outcome group all individuals requiring either noninvasive or invasive mechanical ventilation.
In our cohort, most of the respiratory variables were significantly worse in the treatment escalation group (table 2). Unfortunately, it is not possible to discriminate the contribution of the natural course of the disease versus the potential lung damage induced by a high-stress ventilation (patient self-induced lung injury) in leading to unfavorable outcome, because the clinical presentation of these conditions is identical: progressive impairment of gas exchange and lung mechanics.23 However, although it is impossible to discriminate between the changes induced by the COVID-19 disease per se and patient self-induced lung injury, we may hypothesize that an increased respiratory effort at admission (higher stress and mechanical power in the treatment escalation group) may contribute to the further deterioration of the lung parenchyma through a vortex that we previously called the “shrinking baby lung,”24 regardless of the severity of impairment as determined by lung computed tomography. Although the two groups presented comparable patterns at computed tomography analysis, the perfusion derangements should be higher in the group undergoing treatment escalation (lower Pao2/Fio2 ratio at day 1) leading to a higher Paco2. This, in turn, should increase the respiratory effort leading to a higher stress and mechanical power, worsening the inflammation within the lungs and promoting the vortex. Of note, the discrepancy between the expected oxygenation assessed by the lung computed tomography scan (anatomical shunt) and the actual oxygenation is well known,1 and it seems to be caused by a significant alteration in perfusion, including an increased intrabronchial shunt,25 leading to an excess in venous admixture not explained by the anatomical changes in the lung parenchyma.26–29
In this study, we were able to compute the mechanical power as the patients were monitored with an esophageal balloon, and the tidal volume and minute ventilation were also collected. The total stress may be in part overestimated as it was not computed in static conditions, however, because the mechanical power estimates the energy over time; it should also include the dynamic components. For this reason, and more importantly because the respiratory rate is included in its calculation, the mechanical power may be the most comprehensive variable to quantify the lungs derangements that are actually occurring in the patient. The relevance of respiratory rate is well exemplified in the current study: whether the energetic cost per breath was only slightly different between two study cohorts (as in our cohort: similar tidal volume and different pleural pressure), the inclusion of the respiratory rate in the assessment of the energetic cost for each breath may enhance the signal effect. Of note, the measurement of esophageal pressure is a procedure that could lead to better understanding of the physiologic changes and possibly tailoring the respiratory treatment for a specific subset of patients. However, there are challenges associated with the wider adoption of this monitoring techniques, due to familiarity and invasiveness, and therefore decision-making needs to be informed by wider clinical risk–benefit ratio.
One of the unsolved problems referring to the mechanical power is its normalization; attempts were done by referring it to the respiratory system compliance,30 but the conceptual problem of the variable relationship between compliance and functional residual capacity still persists. Indeed, it is worth recalling that the mechanical power is necessary for life (i.e., it cannot be zero); therefore, more than the quantification of mechanical power in different individuals, the real issue is to quantify its relative “excess.” In this article, we tried to normalize the mechanical power by relating it to the expected normal mechanical power for a given individual, using the same approach of the ventilatory ratio. We referred to a ventilation of 0.1 l/(kg · min), airway resistances of 10 cm H2O/(ml · s), a respiratory rate of 15 breaths/min and an I:E ratio of 1:2. In this setting, a 70-kg individual at rest would require a mechanical power of 4.84 J/min, a value that is consistent with the reports in the literature.31 Using this approach, it is possible to quantify the excess and defect of the actual mechanical power. In our cohort, the mechanical power ratio of patients undergoing the escalation of the respiratory treatment was nearly 50% greater compared to patients with favorable outcome.
Limitations
The major limitation of the current study is the use of clinical criteria and the lack of a protocolized hard criteria when escalating the respiratory support. This may have led to variations in practice such that some patients received escalation later than anticipated or when other clinical criteria—not captured by respiratory parameters—occurred. However, all patients were admitted to a high-dependency COVID-19 unit, where clinical activities are focused on COVID-19 patients, and the clinical management is standardized according to the institutional protocols. Conversely, one of its strengths is the combination of tidal volume and esophageal pressure measurements in spontaneous breathing patients, allowing the calculation of mechanical power and its relative ratio. We also recognize that all measurements, taken in dynamic condition, cannot equate to the usual static assessment. This limitation, however, is mandatory during spontaneous breathing and may present the advantage of a more realistic assessment of the energy required by the patient to sustain the breathing process. Although the use of local institutional guidelines should have minimized the risk of bias, it is possible that some unrecognized bias was present during the study. Because of the design and set-up of the study, we used average values to compare different groups and time points, and this could lead to regression artifact into the analysis. Another limitation is the assumption that the ratio between lung and total elastances equals 0.7. However, because in the early stages of COVID-19, pneumonia lung mechanics are usually preserved,1 we do not expect this ratio to be significantly different from 0.7 when assessed in our population. Finally, we cannot exclude that an obese patient in a semirecumbent position may present some degree of flow limitation and, consequently, auto-PEEP.
Conclusions
In our cohort of spontaneously breathing COVID-19 patients, the patients’ respiratory pattern was comparable between groups and within the limits of lung protective strategy. Our study points out the paramount importance of respiratory rate and tidal pleural pressure, both usually ignored when lung protective strategy is applied, in modifying the outcome. Among all assessed variables, mechanical power and mechanical power ratio had the highest area under the curve when assessing the clinical outcome.
Acknowledgments
The authors thank all the nurses and physicians at Ospedale San Paolo (Milan, Italy) who helped with data collection and patient care. They are also grateful to Respiratory Motion Inc. (Watertown, Massachusetts) for providing the ExSpiron monitors.
Research Support
Supported by institutional and/or departmental sources and from Sartorius AG (Göttingen, Germany) through an unrestricted grant for lung injury-related research to the Department of Anesthesiology, University Medical Center Göttingen, Göttingen, Germany.
Competing Interests
Dr. Gattinoni reports a consultancy for General Electrics (Boston, Massachusetts) and SIDAM (Modena, Italy). He also receives lecture fees from ESTOR (Pero, Italy) and Mindray (Darmstadt, Germany). Dr. Saager has received payments from the Surgical Company Netherlands (Amersfoort, The Netherlands) for consultancy work. The other authors declare no competing interests.
Supplemental Digital Content
Supplemental Material, http://links.lww.com/ALN/C988
Footnotes
This article is featured in “This Month in Anesthesiology,” page A1.
This article is accompanied by an editorial on p. 238.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are available in both the HTML and PDF versions of this article. Links to the digital files are provided in the HTML text of this article on the Journal’s Web site (www.anesthesiology.org).
Published online first on December 26, 2022.
Contributor Information
Simone Gattarello, Email: gattarello@gmail.com.
Silvia Coppola, Email: silvia_coppola@libero.it.
Elena Chiodaroli, Email: elenachiodaroli89@gmail.com.
Tommaso Pozzi, Email: tommaso.pozzi94@gmail.com.
Luigi Camporota, Email: luigi.camporota@kcl.ac.uk.
Leif Saager, Email: leif.saager@med.uni-goettingen.de.
Davide Chiumello, Email: chiumello@libero.it.
Luciano Gattinoni, Email: gattinoniluciano@gmail.com.
References
- 1.Coppola S, Chiumello D, Busana M, Giola E, Palermo P, Pozzi T, Steinberg I, Roli S, Romitti F, Lazzari S, Gattarello S, Palumbo M, Herrmann P, Saager L, Quintel M, Meissner K, Camporota L, Marini JJ, Centanni S, Gattinoni L: Role of total lung stress on the progression of early COVID-19 pneumonia. Intensive Care Med. 2021; 47:1130–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, Protti A, Gotti M, Chiurazzi C, Carlesso E, Chiumello D, Quintel M: Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016; 42:1567–75 [DOI] [PubMed] [Google Scholar]
- 3.Apigo M, Schechtman J, Dhliwayo N, Tameemi M, Gazmuri RJ: Development of a Work of Breathing scale and monitoring need of intubation in COVID-19 pneumonia. Critical Care. 2020; 24:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg G, Ljunggren G, Ceci R: The increase of perceived exertion, aches and pain in the legs, heart rate and blood lactate during exercise on a bicycle ergometer. Eur J Appl Physiol Occup Physiol. 1985; 54:343–9 [DOI] [PubMed] [Google Scholar]
- 5.Herrmann P, Busana M, Cressoni M, Lotz J, Moerer O, Saager L, Meissner K, Quintel M, Gattinoni L: Using artificial intelligence for automatic segmentation of CT lung images in acute respiratory distress syndrome. Front Physiol. 2021; 12:676118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibanez J, Raurich JM: Normal values of functional residual capacity in the sitting and supine positions. Intensive Care Med. 1982; 8:173–7 [DOI] [PubMed] [Google Scholar]
- 7.Cressoni M, Gallazzi E, Chiurazzi C, Marino A, Brioni M, Menga F, Cigada I, Amini M, Lemos A, Lazzerini M, Carlesso E, Cadringher P, Chiumello D, Gattinoni L: Limits of normality of quantitative thoracic CT analysis. Crit Care. 2013; 17:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voscopoulos C, Brayanov J, Ladd D, Lalli M, Panasyuk A, Freeman J: Special article: Evaluation of a novel noninvasive respiration monitor providing continuous measurement of minute ventilation in ambulatory subjects in a variety of clinical scenarios. Anesth Analg. 2013; 117:91–100 [DOI] [PubMed] [Google Scholar]
- 9.Schumann R, Harvey B, Zahedi F, Bonney I: Minute ventilation assessment in the PACU is useful to predict postoperative respiratory depression following discharge to the floor: A prospective cohort study. J Clin Anesth. 2019; 52:93–8 [DOI] [PubMed] [Google Scholar]
- 10.Galvagno SM, Brayanov JB, Corneille MG, Voscopoulos CJ, Sordo S, Ladd D, Freeman JE: Non-invasive respiratory volume monitoring in patients with traumatic thoracic injuries. Trauma. 2015; 17:219–23 [Google Scholar]
- 11.Qiu C, Cheng E, Winnick SR, Nguyen VT, Hou FC, Yen SS, Custodio GD, Dang JH, LaPlace D, Morkos A, Chung EP, Desai VN: Respiratory volume monitoring in the perioperative setting across multiple centers. Respir Care. 2020; 65:482–91 [DOI] [PubMed] [Google Scholar]
- 12.Voscopoulos CJ, MacNabb CM, Brayanov J, Qin L, Freeman J, Mullen GJ, Ladd D, George E: The evaluation of a non-invasive respiratory volume monitor in surgical patients undergoing elective surgery with general anesthesia. J Clin Monit Comput. 2015; 29:223–30 [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D: Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J. 2003; 47:15s–25s [DOI] [PubMed] [Google Scholar]
- 14.Sinha P, Fauvel NJ, Singh S, Soni N: Ventilatory ratio: A simple bedside measure of ventilation. Br J Anaesth. 2009; 102:692–7 [DOI] [PubMed] [Google Scholar]
- 15.Nunn JF: Ventilation nomograms during anaesthesia. Anaesthesia. 1960; 15:65. [DOI] [PubMed] [Google Scholar]
- 16.Butler J, Caro CG, Alcala R, Dubois AB: Physiological factors affecting airway resistance in normal subjects and in patients with obstructive respiratory disease. J Clin Invest. 1960; 39:584–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernandez G, Garcia-de-Acilu M, Frat JP, Masclans JR, Ricard JD: An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019; 199:1368–76 [DOI] [PubMed] [Google Scholar]
- 18.Costa E, Slutsky A, Brochard L, Brower R, Serpa-Neto A, Cavalcanti A, Mercat A, Meade M, Morais C, Goligher E, Carvalho C, Amato M: Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021; 204:303–11 [DOI] [PubMed] [Google Scholar]
- 19.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, Schortgen F, Brochard L, Brun-Buisson C, Mekontso Dessap A: Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: Role of tidal volume. Crit Care Med. 2016; 44:282–90 [DOI] [PubMed] [Google Scholar]
- 20.Frat JP, Ragot S, Girault C, Perbet S, Prat G, Boulain T, Demoule A, Ricard JD, Coudroy R, Robert R, Mercat A, Brochard L, Thille AW; REVA network: Effect of non-invasive oxygenation strategies in immunocompromised patients with severe acute respiratory failure: A post-hoc analysis of a randomised trial. Lancet Respir Med. 2016; 4:646–52 [DOI] [PubMed] [Google Scholar]
- 21.Duan J, Han X, Bai L, Zhou L, Huang S: Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017; 43:192–9 [DOI] [PubMed] [Google Scholar]
- 22.Tobin M: The criteria used to justify endotracheal intubation of patients with COVID-19 are worrisome. Can J Anaesth. 2021; 68:258–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D: Acute respiratory failure following pharmacologically induced hyperventilation: An experimental animal study. Intensive Care Med. 1988; 15:8–14 [DOI] [PubMed] [Google Scholar]
- 24.Marini JJ, Gattinoni L: Time course of evolving ventilator-induced lung injury: the “shrinking baby lung.” Crit Care Med. 2020; 48:1203–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galambos C, Bush D, Abman SH: Intrapulmonary bronchopulmonary anastomoses in COVID-19 respiratory failure. Eur Respir J. 2021; 58:2004397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busana M, Giosa L, Cressoni M, Gasperetti A, Di Girolamo L, Martinelli A, Sonzogni A, Lorini L, Palumbo MM, Romitti F, Gattarello S, Steinberg I, Herrmann P, Meissner K, Quintel M, Gattinoni L: The impact of ventilation-perfusion inequality in COVID-19: A computational model. J Appl Physiol (1985). 2021; 130:865–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gattinoni L, Gattarello S, Steinberg I, Busana M, Palermo P, Lazzari S, Romitti F, Quintel M, Meissner K, Marini JJ, Chiumello D, Camporota L: COVID-19 pneumonia: Pathophysiology and management. Eur Respir Rev. 2021; 30:210138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrmann J, Mori V, Bates JHT, Suki B: Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat Commun. 2020; 11:4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D: Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020; 383:120–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghiani A, Paderewska J, Walcher S, Tsitouras K, Claus Neurohr C, Kneidinger N: Mechanical power normalized to lung-thorax compliance indicates weaning readiness in prolonged ventilated patients. Sci Rep. 2022; 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehder K, Marsh HM: Respiratory Mechanics during Anesthesia and Mechanical Ventilation, Handbook of Physiology. Hoboken, Wiley; pp 737–52. [Google Scholar]