Abstract
Objective
To estimate the mortality rate of patients newly diagnosed with chronic atrial fibrillation (AF) and compare it with the one in the general population. To evaluate the role of co-morbidity and other factors on the risk of dying among AF patients.
Methods
We used the General Practice Research Database in the UK to perform a retrospective cohort study. We followed a cohort of chronic AF patiens (N = 1,035) and an age and sex matched cohort of 5,000 subjects sampled from the general population. We used all deceased AF patients as cases (n = 234) and the remaining AF patients as controls to perform a nested case-control analysis. We estimated mortality risk associated with AF using Cox regression. We computed mortality relative risks using logistic regression among AF patients.
Results
During a mean follow-up of two years, 393 patients died in the general population cohort and 234 in the AF cohort. Adjusted relative risk of death in the cohort of AF was 2.5 (95%CI 2.1 – 3.0) compared to the general population. Among AF patients, mortality risk increased remarkably with advancing age. Smokers carried a relative risk of dying close to threefold. Ischaemic heart disease was the strongest clinical predictor of mortality with a RR of 3.0 (95% CI; 2.1–4.1). Current use of calcium channel blockers, warfarin and aspirin was associated with a decreased risk of mortality.
Conclusions
Chronic AF is an important determinant of increased mortality. Major risk factors for mortality in the AF cohort were age, smoking and cardiovascular co-morbidity, in particular ischaemic heart disease.
Background
Atrial fibrillation (AF) is a common cardiovascular disease among adults associated with a substantial morbidity and increased mortality [1-3]. Incidence of AF increases markedly with advancing age [4,5], with an incidence rate of less than 0.1 per 1,000 person years among subjects aged 40 to 49, rising to 8.6 among subjects aged 80–89 years. It has been recognised that AF is an important risk factor for all-cause mortality, mainly due to an increased risk of cardiovascular deaths [6,7].
Results from different studies report that patients with AF have a mortality rate about twice that of age- and sex-matched individuals without AF [8,9]. In the Framingham Heart study, the excess mortality associated with AF persisted after adjustment for the preexisting cardiovascular conditions [2]. Aging, diabetes, heart failure and myocardial infarction were found to be independent risk factors for cardiovascular mortality among patients with chronic AF [2,10]. However, the information on the impact of chronic AF on mortality in the general population is limited, and to the best of our knowledge no study with UK data has been published.
We therefore conducted a study on the mortality rate in a population-based cohort of patients newly diagnosed with permanent/chronic atrial fibrillation and compared it with the one in a general population cohort. We evaluated the role of co-morbidity, drug treatments and other factors on the risk of dying among AF patients.
Materials and Methods
We used the General Practice Research Database (GPRD) in the UK to perform a population based cohort study. The GPRD contains prospectively collected medical information on about 3 million patients. This database has been used in multiple epidemiologic studies that have confirmed the completeness and validity of the recorded data [11-13]. We identified a total of 703,730 patients in the GPRD meeting the following conditions: 40–89 years old enrolled with a general practitioner for more than two years and a computerized prescription history of at least one year before January 1996. This was done to ensure that the patients have had at least one contact with the GP in the year prior to entering the study. Patients with a code for cancer (I CD 1400–2099) before January 1996 were omitted. From this source population we identified both study cohorts.
Study cohorts
AF cohort: We identified patients aged 40–89 years with a first ever recorded diagnosis of permanent/chronic atrial fibrillation in 1996. The diagnosis was confirmed by the general practitioners (GPs) through a questionnaire, this process is describe in detailed in a previous study [4]. We requested the GPs to confirm whether the episode of chronic AF was the first ever and to provide information on diagnostic tests, procedures and aetiology of the AF (patient confidentiality was always preserved). We excluded patients classified by the GP as having paroxysmal AF, 1,035 were finally confirmed. General Population cohort: Using the same source population in which the AF patients were identified and applying the same eligibility criteria as for the AF cohort, we sampled an age and sex matched cohort of 5,000 individuals free of AF.
Analysis
The two cohorts were followed up from date of first diagnosis of chronic AF (start date) and from a random date during 1996 in the general population cohort until the earliest of death, or end of follow-up (December 1999). Survival probability was computed in both cohorts and we estimated the relative risk of dying associated with AF using Cox proportional hazard regression to control for risk factors.
A nested case-control analysis was performed in the AF cohort to assess risk factors of death. We used all deceased AF patients as cases (n = 234) and used their death date as index date. The remaining alive AF patients (n = 801) were considered controls and we sampled a random date during their follow-up period that was used as their index date. Estimates of mortality risk and 95% confidence interval (CI) were computed using logistic regression. We collected recorded information on the following risk factors: smoking status, BMI, alcohol consumption, as well as prior history of heart failure (HF), ischaemic heart disease (IHD), cerebrovascular disease (CVD), hypertension and diabetes. We used both the information in the questionnaire and computerized files to ascertain the cause of death.
Drug exposure definition
We extracted from computerized records information on drugs used to treat AF between start date and index date: diuretics, beta-blockers, ACE inhibitors, calcium channel blockers, digoxin, aspirin, warfarin, NSAIDs, and other anti-arrhythmic drugs (disopyramide, procainamide, quinidine, flecainide, propafenone and amiodarone). We defined current use when drug supply lasted until index date or ended in the previous month. We considered short-term use when duration was less than 3 months, and long-term use when duration was more than 3 months. Past use was considered when the end of the most recent prescription was more than one month before index date and non use when there was no drug use between start and index date.
Results
During a mean follow-up of close to two years, 393 patients died in the general population cohort and 234 in the AF cohort. Patients with AF had a significantly lower survival than patients from the general population without AF (Figure 1). The increased mortality was already present in the early phase of follow-up. Half of the deaths in the AF cohort were related to ischaemic heart disease (IHD) or other cardiovascular diseases (45% of all cause mortality). In the general population cohort, these two causes accounted for 29% of all deaths (table 1). The relative risk of mortality among patients with chronic atrial fibrillation was 2.5 (95% CI 2.1–3.0) compared to the general population after adjusting for co-morbidity and major clinical risk factors (table 2). This risk was 2.3 (95% CI 1.8–3.0) in men and 2.8 (95% CI 2.2–3.6) in women. When considering only cerebro- and cardio-vascular deaths (n= 306), the corresponding adjusted RR associated with chronic AF was 3.7 (95% CI 3.0–4.7).
Figure 1.
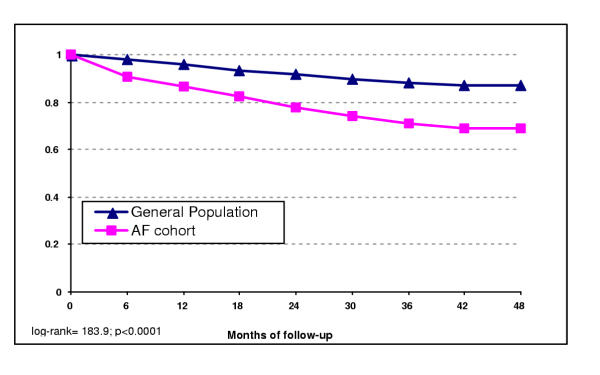
Kaplan-Meier survival curve for chronic atrial fibrillation and general population cohorts. (mean follow-up time 22 months -SD 12.9; range: 0.5–47 months).
Table 1.
Distribution of causes of death in the cohort of chronic atrial fibrillation patients and the general population
| General Population Cohort | AF Cohort | |||
| N = 393 | % | N = 234 | % | |
| Causes of death | ||||
| Ischaemic heart disease (IHD) | 70 | 17.8 | 61 | 21.1 |
| Other cardiovascular no IHD * | 42 | 10.7 | 56 | 24 |
| Cerebrovascular disease† | 44 | 11.2 | 33 | 14.1 |
| Cancer | 64 | 16.3 | 27 | 11.5 |
| Other diseases§ | 107 | 27.2 | 48 | 20.5 |
| Unknown | 66 | 16.8 | 9 | 3.8 |
* Includes heart failure, heart valvular and rhythm disorders and vascular diseases (PVD, aneurism, pulmonary embolism). †includes ischaemic and haemorrhagic cerebrovascular accidents. §Includes respiratory, gastrointestinal, liver, renal diseases, infection and trauma.
Table 2.
Incidence rate and relative risk of death in the cohort of chronic atrial fibrillation patients compared to the general population
| General Population Cohort | AF Cohort | |
| Person-years | 9261 | 1898 |
| Deaths | 393 | 234 |
| Mortality rate/100 p-years (95% Cl) | 4.2 (3.8–4.7) | 12.3 (10.8–14.0) |
| Relative Risk * (95% Cl) | 1 | 2.5 (2.1 – 3.0) |
* Relative risk estimated by Cox regression including age, sex, smoking, diabetes, heart failure, 'Ischaemic heart disease, hypertension, cerebrovascular disease
Table 3 shows the effect of several factors on the mortality risk in the cohort of chronic AF patients. The mortality risk increased remarkably with advancing age. Smokers carried a relative risk of dying close to threefold. Diabetes, heart failure and cerebrovascular disease were all associated with estimates of RR between 1.5 and 2. Ischaemic heart disease was the strongest clinical predictor of mortality with a RR of 3.0 (95% CI; 2.1–4.1). We did not find any significant association between alcohol consumption and mortality. Overweight patients (BMI of 30 or more) had a relative risk of 0.5 (95%CI: 0.3–0.9) compared to subjects with a BMI between 22 and 25 after adjusting for age, sex, smoking and preexisting conditions.
Table 3.
Risk of mortality associated with different risk factors among chronic atrial fibillation patients.
| Cases | Controls | Odds Ratio* | |||
| n = 234 | (%) | n = 801 | (%) | (95% CI) | |
| Sex | |||||
| Males | 115 | (49.1) | 372 | (53.6) | 1 |
| Females | 119 | (50.9) | 429 | (46.4) | 0.9 (0.6–1.3) |
| Age | |||||
| 40–59 years | 4 | (1.7) | 67 | (8.4) | 1 |
| 60–69 years | 19 | (8.1) | 136 | (17.0) | 1.9 (0.6–5.9) |
| 70–79 years | 93 | (39.7) | 325 | (40.6) | 4.0 (1.4 – 11.7) |
| 80–89 years | 118 | (50.4) | 273 | (34.1) | 6.0 (2.0–17.6) |
| Smoking status | |||||
| Never smoker | 108 | (46.2) | 486 | (60.7) | 1 |
| Smoker | 59 | (25.2) | 127 | (15.9) | 2.6 (1.7–4.0) |
| Ex-smoker | 28 | (12.0) | 84 | (10.5) | 1.6 (0.9–2.7) |
| Unknown | 39 | (16.7) | 104 | (13.0) | 1.7 (1.0–2.9) |
| BMI (weight /height2) | |||||
| <22 | 30 | (12.8) | 82 | (10.2) | 1.0 (0.6–1.9) |
| 22–24.9 | 49 | (20.9) | 138 | (17.2) | 1 |
| 25–30 | 60 | (25.6) | 227 | (28.3) | 0.8 (0.5–1.3) |
| +30 | 20 | (8.5) | 131 | (16.4) | 0.5 (0.3–0.9) |
| Unknown | 75 | (32.1) | 223 | (27.8) | 0.9 (0.5–1.5) |
| Prior co-morbidity | |||||
| Diabetes | 30 | (12.8) | 59 | (7.4) | 2.1 (1.3–3.6) |
| Heart Failure | 137 | (58.5) | 271 | (33.8) | 1.9 (1.4–2.7) |
| Ischemic heart disease | 127 | (54.3) | 206 | (25.7) | 3.0 (2.1 – 4.2) |
| Hypertension | 104 | (44.4) | 355 | (44.3) | 0.9 (0.7–1.3) |
| Cerebrovascular disease | 73 | (31.2) | 159 | (19.9) | 1.5 (1.1 – 2.2) |
*Estimates of risk are adjusted for all variables included in the table using logistic regression.
Current use of calcium channel blockers, aspirin and warfarin were associated with a significant reduced risk of all cause mortality (Table 4). When we separated current use into short and long term use (less than 3 months of use versus more), we observed that the reduced risk of mortality was mainly among long-term users of these drugs. Users of calcium channel blockers for more than 3 months carried a risk of 0.4 (95%CI 0.2–0.7), the respective estimates for warfarin long term users was 0.4 (95%CI 0.3–0.8) and for aspirin (RR: 0.6; 95%CI 0.4–0.9). Current use of NSAIDs was associated with an increased mortality (RR of 1.8; 95% CI 1.0–3.2).
Table 4.
Risk of mortality associated with use of cardiovascular treatment drugs in the AF cohort
| Cases | Controls | Odds Ratio* | |||
| n = 234 | (%) | n = 801 | (%) | (95% CI) | |
| Diuretics | |||||
| Non use | 46 | (19.7) | 237 | (29.6) | 1 |
| Current use | 135 | (57.7) | 419 | (52.3) | 1.1 (0.6–1.7) |
| Past use | 53 | (22.6) | 145 | (18.1) | 1.1 (0.6–1.9) |
| Beta Blockers | |||||
| Non use | 169 | (72.2) | 561 | (70.0) | 1 |
| Current use | 13 | (5.6) | 87 | (10.9) | 0.8 (0.4–1.6) |
| Past use | 52 | (22.2) | 153 | (19.1) | 1.0 (0.6–1.6) |
| ACE inhibitors | |||||
| Non use | 137 | (58.5) | 524 | (65.4) | 1 |
| Current use | 70 | (29.9) | 208 | (26.0) | 0.8 (0.5–1.3) |
| Past use | 27 | (11.5) | 69 | (8.6) | 1.0 (0.5–1.8) |
| Calcium Channel | |||||
| Blockers | |||||
| Non use | 165 | (70.5) | 560 | (69.9) | 1 |
| Current | 18 | (7.7) | 125 | (15.6) | 0.4 (0.2–0.7) |
| Past use | 51 | (21.8) | 116 | (14.5) | 1.2 (0.7–1.8) |
| Digoxin | |||||
| Non use | 51 | (21.8) | 191 | (23.8) | 1 |
| Current | 143 | (61.1) | 496 | (61.9) | 0.9 (0.6–1.4) |
| Past use | 40 | (17.1) | 114 | (14.2) | 1.0 (0.5–1.7) |
| Amiodarone † | |||||
| Non use | 212 | (90.6) | 726 | (90.6) | 1 |
| Current | 14 | (6.0) | 44 | (5.5) | 1.3 (0.6–2.8) |
| Past use | 8 | (3.4) | 31 | (3.9) | 0.8 (0.3–2.2) |
| Warfarin | |||||
| Non use | 174 | (74.4) | 503 | (62.8) | 1 |
| Current | 37 | (15.8) | 226 | (28.2) | 0.5 (0.3–0.8) |
| Past use | 23 | (9.8) | 72 | (9.0) | 1.0 (0.5–1.8) |
| Aspirin | |||||
| Non use | 109 | (46.6) | 407 | (50.8) | 1 |
| Current | 72 | (30.8) | 239 | (29.8) | 0.6 (0.4–0.9) |
| Past use | 53 | (22.6) | 155 | (19.4) | 0.8 (0.5–1.2) |
*Estimates are adjusted for age, sex, smoking, diabetes, heart failure, 'Ischaemic heart disease, hypertension, cerebrovascular disease and all the drugs groups in the table, using logistic regression. †There was no use of other antiarrhythmic drugs (disopyramide, procainamide, quinidine, flecainide and propafenone).
The estimates of mortality risk for all risk factors evaluated only changed marginally when we restricted the analysis to cardiovascular deaths that included IHD, heart failure, other cardiovascular and cerebrovascular diseases (data not shown).
Discussion
We found that permanent AF is an important independent predictor of mortality. AF patients carried an all cause mortality risk close to 3 times greater than the one in an age and sex matched general population cohort. We are not aware of other studies that have evaluated the impact of chronic AF on mortality in a UK general population setting.
Some specific characteristics of the study need to be considered. Firstly, the ascertainment of AF in general population is problematic, as some of the cases can remain undetected if these episodes are brief mild [5]. We only studied chronic AF and by using a general practice setting we tried to ensure that even less severe cases of AF could be identified. There is always room for missclassification between paroxysmal and chronic AF. We tried to minimise this potential bias by reviewing all computerized histories of AF patients and finally requesting the GP to confirm the diagnosis of chronic AF. The higher probability of detecting more severe or symptomatic cases -those that contact the GP- may lead to a slight overestimation of mortality associated with AF, and could explain the higher mortality rate found in our study compared to studies using screening methods for case ascertainment. Secondly, we could not ascertain the underlying cause of death in 12% of the subjects and this proportion was higher among the general population cohort than in than AF patients cohort. Atrial fibrillation patients have a higher consultation rate with their GP than the general population and as a result these patients tend to have more information recorded on minor morbidity and causes of death than patients from the general population. Third, we had a considerable level of missing information on BMI, mainly due to unrecorded data on height not routinely measured in elderly and other risk factors.
Estimates of mortality risk associated with AF have been reported to be around two for all cause mortality, and between two and twelve for cardiovascular mortality [14]. Our corresponding estimate for the latter end-point was close to four while our findings of all cause mortality risk associated with chronic AF were in the higher range of those from previous studies. The small difference can partly be explained by the different methods used in the identification of AF patients, the inclusion of different types of AF (chronic versus all AF) and the distinct ascertainment of causes of death [2,3,9,14]. Among patients with at least one other cardiovascular diagnosis, mortality risk was approximately 20% higher in patients with AF compared to those without AF [9]. In the Framingham Heart Study, the authors reported that the excess mortality attributed to atrial fibrillation (chronic, paroxysmal or flutter) was independent of preexisting cardiovascular conditions associated with atrial fibrillation. Their estimate of mortality associated with atrial fibrillation was 1.5 in men and 1.9 in women after adjusting for other cardiovascular co-morbidity [2]. In our study we only assessed the risk among chronic AF patients, and found higher estimates of risk for both men (RR = 2.3) and women (RR = 2.8) after taking into account preexisting diseases commonly associated with AF, such as IHD or HF. It could be argued that chronic forms of AF carry a greater mortality risk than paroxysmal AF and flutter, and this could explain the higher estimates observed in our study.
In agreement with previous studies, we confirmed that the major risk factors for mortality in AF patients are old age, smoking and cardiovascular co-morbidity [2,10,14]. We identified the same risk factors and with a similar magnitude of risk when restricting the outcome to specific cardiovascular mortality. Another study found that the increased risk for all cause mortality in AF patients was largely due to an increased mortality risk due to ventricular failure [7]. In our study, we observed that half of all deaths were due to IHD and other cardiovascular diseases in the AF cohort. A previous review by Alpert et al [15] reported that the mortality risk was age dependent and presence of HF at the time of onset of AF was an important prognostic factor. In our study, we found that coexisting heart failure carried a two fold increased risk of all cause mortality and ischaemic heart disease a three fold increase.
The use of anti-arrhythmic drugs and digoxin for atrial fibrillation among patients with coexisting cardiovascular morbidity has been controversial [16-18]. An increased risk of cardiac mortality has been reported among AF patients with a history of congestive heart failure receiving anti-arrhythmic drugs compared to patients not treated [16,19]. Other studies have concluded that anti-arrhythmic drug therapy in AF patients does not translate into an improvement in mortality and have suggested that class I anti-arrhythmic drugs should be avoided in AF patients with advanced heart failure [20]. We found no significant difference in mortality risk among those treated and not treated with anti-arrhythmic drugs such as amiodarone, beta blockers or digoxin after adjusting for cardiovascular disease and other drug treatment. Though we found some evidence for a protective effect with some drug treatments, one should be cautious in interpreting these results due to the observational design of our study and the relative statistical variability.
In our study, obesity (BMI>30) was associated with a 50 % reduced risk of all cause mortality. The Longitudinal Study of Aging reported a reduced mortality in obese elderly people after adjustment for other factors [21]. We could not find any published study that examined this association among AF patients and further studies are warranted to confirm this finding.
Competing interests
This study was supported by a research grant from AstraZeneca.
Acknowledgments
Acknowledgements
We thank the staff at GPRD, and the participating general practitioners for their collaboration. We also thank the Boston Collaborative Drug Surveillance Program (BCDSP) for providing access to the database.
Contributor Information
Ana Ruigómez, Email: aruigomez@ceife.es.
Saga Johansson, Email: Saga.Johansson@astrazeneca.com.
Mari-Ann Wallander, Email: Mari-Ann.Wallander@astrazeneca.com.
Luis Alberto García Rodríguez, Email: lagarcia@ceife.es.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. National Implications for rhythm management and stroke prevention: The anticoagulation and risk factors in atrial fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-up study. Am J Med. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- Ruigómez A, Johansson S, Wallander MA, García Rodríguez LA. The incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol. 2002;55:358–363. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risks factors for atrial fibrillation in a population-based cohort: The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- Humphries KH, Kerr CR, Connoly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: sex differences in presentation, treatment and outcome. Circulation. 2001;103:2365–70. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- Dries DL, Exner DV, Gersh BJ, Domanski MJ, Warclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- Laupacis A, Cuddy TE. Prognosis of individuals with atrial fibrillation. Can J Cardiol. 1996;12:14A–16A. [PubMed] [Google Scholar]
- Wolf PA, Mitchell JB, Baker CS, Kannel WB, Dágostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. doi: 10.1001/archinte.158.3.229. [DOI] [PubMed] [Google Scholar]
- Scardi S, Mazzone C, Pandullo C, Goldstein D, Di Lenarda A, Chersevani D. Mortality and cause of death in patients with chronic non-rheumatic atrial fibrillation after two years follow-up. G Ital Cardiol. 1999;29:637–46. [PubMed] [Google Scholar]
- Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H, Terris BZ, Derby LE, Jick SS. Further validation of information recorded on a general practitioner based computerised data resource in the United Kingdom. Pharmacoepidemiol Drug Safety. 1992;1:347–349. [Google Scholar]
- García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45:419–425. doi: 10.1046/j.1365-2125.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski MJ. The epidemiology of atrial fibrillation. Coronary Artery Disease. 1995;6:95–100. doi: 10.1097/00019501-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Alpert JS, Petersen P, Godtfrdsen J. Atrial fibrillation: Natural history, complications and management. Ann Rev Med. 1988;39:41–52. doi: 10.1146/annurev.me.39.020188.000353. [DOI] [PubMed] [Google Scholar]
- Flacker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol. 1992;20:527–32. doi: 10.1016/0735-1097(92)90003-6. [DOI] [PubMed] [Google Scholar]
- Falk RH, Leavitt JI. Digoxin for atrial fibrillation: a drug whose time has gone?. Ann Intern Med. 1991;114:573–5. doi: 10.7326/0003-4819-114-7-573. [DOI] [PubMed] [Google Scholar]
- Jung F, DiMarco JP. Treatment strategies for atrial fibrillation. Am J Med. 1998;104:272–86. doi: 10.1016/s0002-9343(97)00346-x. [DOI] [PubMed] [Google Scholar]
- Maisel WH, Kuntz KM, Reimold SC, Lee TH, Antman EM, Friedman PL, Stevenson WG. Risk of initiating antiarrhythmic drug therapy for atrial fibrillation in patients admitted to a university hospital. Ann Int Med. 1997;127:281–4. doi: 10.7326/0003-4819-127-4-199708150-00004. [DOI] [PubMed] [Google Scholar]
- Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimie A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol. 1996;28:1458–63. doi: 10.1016/s0735-1097(96)00358-0. [DOI] [PubMed] [Google Scholar]
- Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49:968–79. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]


