Abstract
Background:
Prenatal exposure to environmental chemicals may increase risk of childhood internalizing problems, but few studies have explored the potential for longer-term consequences of such exposures.
Objective:
We evaluated associations between prenatal organochlorine and metal levels and early adulthood internalizing symptoms, considering whether sociodemographic/nonchemical stressors modified these associations.
Methods:
Participants were 209 young adults, born (1993–1998) to mothers residing in or near New Bedford, Massachusetts. As part of the early-adult assessment, self-reported anxiety (7-item Generalized Anxiety Disorder scale) and depressive (8-item Patient Health Questionnaire) symptoms (: elevated symptoms) were ascertained. We previously analyzed levels of cord serum organochlorines [hexachlorobenzene, dichlorodiphenyldichloroethylene (,′-DDE), polychlorinated biphenyls (: sum of congeners 118, 138, 153, 180)] and whole blood lead shortly after participants’ birth, and levels of cord whole blood manganese from archived samples at the time of the adolescent study visit. We used modified Poisson regression models and quantile g-computation, adjusting for sociodemographics, and explored whether biological sex, race/ethnicity (proxy for unmeasured consequences of racism), prenatal social disadvantage (assessed when participants were neonates), and quality of the home environment (assessed during adolescence) modified these associations.
Results:
Participants were () y old, 76% Non-Hispanic White, and 67% female. Prenatal hexachlorobenzene, ,′-DDE, and lead exposures were moderately associated with increased risk of elevated anxiety symptoms. There were strata-specific associations for prenatal social disadvantage and quality of home environment such that adverse associations of ,′-DDE and lead and the overall mixture with anxiety and depressive symptoms were largely only evident in those with lower nonchemical stress [e.g., risk ratio and 95% confidence interval (CI) per doubling ,′-DDE for anxiety: 1.54 (95% CI: 1.20, 1.99) in high-quality home environments and 0.77 (95% CI: 0.51, 1.16) in low-quality home environments]. Associations between prenatal hexachlorobenzene and ,′-DDE and anxiety symptoms were stronger for underrepresented racial/ethnic group participants vs. Non-Hispanic Whites. We found minimal evidence for sex-specific effects, and no consistent associations with manganese or .
Discussion:
Prenatal organochlorine pesticides and lead exposure possibly increases risk of internalizing problems, particular anxiety symptoms, in young adults. Varying risk was observed by sociodemographic/nonchemical stressor strata, demonstrating the importance of considering interactions between chemical and other stressors. https://doi.org/10.1289/EHP11171
Introduction
As the prevalence of anxiety disorders and depression in young adults continues to rise,1,2 a better understanding of the contributing role of early-life stressors is critical to identifying susceptible individuals and reducing physical, mental, and societal burden caused by these internalizing disorders.3 Certain risk factors for anxiety and depression, such as female sex,3,4 lower socioeconomic status,3,4 and a poorer-quality home and family environment,4,5 have been well characterized, but there is limited research on the extent to which prenatal exposures to environmental neurotoxicant chemicals influence risk in adulthood.
Prenatal exposures to environmental chemicals, including organochlorines and metals, have been implicated as detrimental to neurobehavioral development. However, most studies evaluated these exposures in relation to externalizing (e.g., hyperactivity, inattention) rather than internalizing (e.g., anxiety, depression) symptoms and focused on outcomes in childhood prior to major core risk periods for anxiety and depression.6,7 Prenatal organochlorine and metal exposures may play a role in the development of anxiety and depression. These chemicals, which readily cross the placenta and blood–brain barrier, are hypothesized to alter the function of neurotransmitters implicated in emotion regulation, such as dopamine, gamma-aminobutyric acid (GABA), and glutamate.8–14 Insults to these neurobiological pathways may program an individual for dysregulation of emotional and behavioral function across the life span.
Previous studies of internalizing symptoms in childhood, including our prior work in the New Bedford Cohort (NBC),15 have provided limited evidence of associations (i.e., largely null results) with prenatal exposure to organochlorines, including hexachlorobenzene (HCB),16–19 dichlorodiphenyldichloroethylene (,′-DDE),16–20 and polychlorinated biphenyls (PCBs),16–21 and mixed evidence of associations (i.e., positive and null results) with prenatal exposure to lead (Pb)16,21–24 and manganese (Mn).24,25 In the NBC, we found an adverse association between prenatal Pb and self-reported anxiety-related symptoms in adolescence but not with parent-reported symptoms in mid-childhood,15 highlighting that neurobehavioral effects related to early chemical exposures may not be apparent until later in development, during or after core risk periods for onset of internalizing disorders,3,26 and when more accurate self-report of symptoms27,28 can be ascertained. Additionally, most of this literature did not explore whether coexposures to other risk factors for neurobehavioral disorders (e.g., sociodemographic, nonchemical, and other chemical stressors) modify associations.
In the current study, we examined prospective associations between prenatal organochlorine and metal levels and early-adulthood anxiety and depressive symptoms. Because sociodemographic and nonchemical risk factors, such as sex, race/ethnicity (proxy for unmeasured consequences of racism), prenatal social disadvantage, and quality of the childhood home environment, may modify the impact of chemical exposures on neurobehavior,29–31 we explored possible interactions with these stressors. We hypothesized that greater prenatal chemical levels would be associated with higher risk of elevated symptoms, with stronger adverse effects among those with sociodemographic-related or nonchemical stress risk factors (i.e., biological female sex, racial discrimination and/or structural racism, and social/material deprivation reflected in prenatal social disadvantage or a poorer-quality home environment).
Methods
Study Population: New Bedford Cohort (NBC)
The NBC is a longitudinal birth cohort of mother–child pairs who were recruited after delivery at St. Luke’s Hospital (New Bedford, Massachusetts) from 1993 to 1998.32,33 During pregnancy, NBC mothers resided in one of four towns (New Bedford, Acushnet, Fairhaven, Dartmouth) adjacent to the New Bedford Harbor Superfund site, a waste site highly contaminated with PCBs and heavy metals between 1940 and the late 1970s. Eligible women recruited into the study were y old and English- and/or Portuguese-speaking and had delivered vaginally (postsurgical narcotic analgesia restricted consent after cesarean delivery). Infants were excluded if they were not available for study-required neonatal examination. The NBC ultimately consented and enrolled 788 mother–child pairs. Child participants have undergone periodic follow-up assessments from birth through early adulthood.15,32–35
Between 2017 and 2019, a series of web-based neurobehavioral assessments were completed by NBC participants at the early-adult visit (age range: 19–25 y). Specifically, eight distinct neurobehavioral or questionnaire assessments were emailed, one at a time, typically with a few months between mailings. One of these emails included both the anxiety and depressive symptom assessments; that information was used in the present study along with data from a general questionnaire sent separately. Eligibility criteria included intact cognition (i.e., no history of central nervous system cancer or posttraumatic cognitive impairment that precluded testing), availability of at least one biomarker of prenatal chemical exposure, and trackable contact information. Among the 788 child participants enrolled at birth, 565 were eligible for young-adult follow-up and had an email address to receive study questionnaires. Of these, 325 consented to participate and completed at least one early-adult visit study questionnaire, with 240 specifically completing an anxiety and/or depressive symptom evaluation (mean age: 22.1 y). A subset of participants also completed in-person testing to validate the web-based neurobehavioral approach (89 participants did both in-person and web-based anxiety and depressive symptom assessments).
The study research protocol was approved by the human subjects committee of Brigham and Women’s Hospital (Boston, Massachusetts). Web-based written consent was obtained from all participants prior to early-adult study evaluations. Before other study visit evaluations, we obtained written consent from all participating parents and assent from all adolescent participants.
Chemical Exposure Assessment
Umbilical cord blood samples for organochlorine analyses (BD Vacutainer® red top tubes without anticoagulant) and metal analyses (BD Vacutainer® lavender top tubes with EDTA anticoagulant) were collected at birth. For organochlorines, blood samples were centrifuged, and the serum fraction was removed and transferred into solvent-rinsed glass vials and stored at until analysis. Organochlorine analyses were performed within approximately 2 to 12 months of sample collection at the Harvard T.H. Chan School of Public Health Organic Chemistry Laboratory (Boston, Massachusetts). Using high-resolution gas chromatography with electron capture detection, with primary and confirmatory capillary columns, cord serum samples were analyzed for HCB, ,′-DDE, and 51 PCB congeners (see Korrick et al.33 for complete list of congeners and their concentrations). In our analysis, we used the sum of the four most prevalent PCBs in the general population during the 1990s and early 2000s (; congeners 153, 118, 138, 180),36–38 because these were measured with the least error and are often used when exploring congener-specific effects. The limits of detection (LOD) for HCB, ,′-DDE, and most individual PCB congeners were 0.01, 0.07, and serum, respectively. For the organochlorine analyses, within-batch coefficients of variation (CV) ranged from 3% to 7% and between-batch CV from 20% to 39%.32,33,39 CVs reflected replicate analyses of cord serum quality assurance (QA)/quality control (QC) samples.
Cord whole blood samples for metal analyses were stored at 4°C and then digested prior to analysis at the Harvard T.H. Chan School of Public Health Trace Metals Laboratory (Boston, Massachusetts). We selected Pb and Mn as exposures for this analysis because a) there is animal and epidemiological evidence of associations between prenatal exposure to these metals and internalizing symptoms or other adverse neurobehavioral outcomes; and b) these metals were the only ones measured in the same biomarker as organochlorines in the NBC, and there was minimal missingness for cord blood samples in comparison with other neurotoxic metals (e.g., arsenic, cadmium, mercury) measured in maternal peripartum hair or toenails. Pb analyses were performed on average about 6 months after sample collection, and analyses of archived cord blood digests for Mn were performed as part of the 15-y assessments. Chemical analyses were conducted using isotope dilution (ID) inductively coupled plasma–mass spectrometry (ICP-MS) for assessment of concentrations of Pb (Sciex ELAN 5000; Perkin Elmer) and external calibration on a dynamic reaction cell (DRC) ICP–mass spectrometer (DRC-ICP-MS; ELAN 6100, Perkin Elmer) for assessment of concentrations of Mn. Metal concentrations were reported as the mean of five replicate measurements. The LOD for Pb and Mn was whole blood. Laboratory recovery rates for QC standards and spiked samples ranged from 90% to 110%. The relative standard deviation (SD) percentage of replicate sample analyses was .40
In the current analyses, machine concentration readings for organochlorines and metals were used to optimize statistical power and avoid biased exposure estimates due to censoring at the detection limit.41 For this analytic cohort, machine concentration readings were above zero for all included organochlorine and metals.
In addition to evaluating Pb levels in cord blood, for a subset of the NBC where comprehensive pediatric medical record review for routine Pb exposure screening data was possible (), infant and childhood blood Pb levels were abstracted. Peak early childhood Pb, an available postnatal exposure index in the NBC that has been previously linked to Pb-associated cognitive deficits,42,43 was estimated as the maximum value between ages 12 to 36 months.
Outcome Assessments
On web-based and/or in-person assessments at the early-adult visit (age range: 19–25 y), participants completed the 7-item Generalized Anxiety Disorder scale (GAD-7)44 and 8-item Patient Health Questionnaire (PHQ-8).45 The GAD-7 and PHQ-8 are questionnaires that assess severity of anxiety and depressive symptoms, respectively, in the prior 2 wk, using a 4-point Likert scale (0–3). These questionnaires are used as diagnostic screening tools for generalized anxiety disorder and depression, dichotomized at a score of to indicate moderate levels of symptoms and a clinical cut point for further evaluation.44,45
In analyses, we included GAD-7 and PHQ-8 scores that were fully complete or only lacked a response to one question. In a few cases where one response was not answered [ (1%) for GAD-7 and PHQ-8, each], we prorated the score and rounded it to the nearest whole number (e.g., multiplying an incomplete GAD-7 score by seven-sixths). Web-based questionnaire scores were used in cases where participants also completed an in-person study visit, but in some instances participants only filled out these assessments in-person, and in those cases, we used the available scores from the in-person assessment ( for GAD-7, for PHQ-8; 13%). Although web-based and in-person visits were typically completed 4 months apart [median (range): 4.0 (0.8–19) months], retest scores were positively and highly correlated, with Spearman’s rank correlations () of 0.71 for GAD-7 and 0.81 for PHQ-8 scores. Reliability among retest scores remained high regardless of timing between questionnaires (e.g., for GAD-7 was 0.70 when retested in a time span of months, and 0.71 for months). Of the 240 participants who completed either questionnaire at least once, 239 participants had available GAD-7 and PHQ-8 scores, because two individuals completed only one of the assessments.
On an early-adult visit general questionnaire, participants reported whether, during the past 10 y, they had ever had a diagnosis of an anxiety disorder or depression by a psychologist, therapist, school professional, or other doctor. At this time, study participants also reported medications taken on a regular basis, including psychotropic drugs frequently used to treat anxiety or mood disorders (see Table S1).
Covariate Assessment
Just after the child’s birth, a trained study nurse abstracted information on the medical course of each NBC mother’s pregnancy and delivery, and on infant’s race/ethnicity, biological sex, and birth outcomes from hospital records. As previously described,34 an obstetrical risk score was derived to summarize adverse conditions prior to or during pregnancy, labor and delivery, and the neonatal period.46
During home visits occurring approximately 2 wk after birth, information on parental and household characteristics, including maternal age, marital status, diet, smoking during pregnancy, maternal and paternal education, and annual household income were ascertained via questionnaires completed by study mothers. Maternal marital status and smoking status, parental education, and annual household income questions were asked of study mothers at the 6-month, 8-y, and/or 15-y assessments to update information. Generally, child race/ethnicity was ascertained from infant medical record review data, but where those data were not available, race/ethnicity information collected from study mothers at 6-month or 8-y assessments was used. The child’s race/ethnicity was determined using questionnaire categories that included White; Hispanic; Black or African American; Asian American; Native American; and Other, with a free-text option for specifying. Because a significant portion of “Other” responses included “Cape Verdean,” it was added as its own category.
Modifying methods used in prior studies that examined childhood social disadvantage,47,48 we constructed a measure of prenatal social disadvantage specific to the NBC, based on five sociodemographic factors at birth. This index summarized data on maternal age (, y); maternal and paternal education ( graduate, post–high school education); maternal marital status (not married, married); and annual household income (, ), scored as 1 or 0, for a total possible range of 0–5. In cases where data on one of these covariates were missing in our analytic cohort (; 1%), we weighted the composite score to five responses and rounded to the nearest whole number (i.e., multiplied by five-fourths); if covariate was missing data, then the prenatal social disadvantage index (PNSDI) score was considered missing. For analyses, we derived a dichotomized variable in which was considered high disadvantage,15,40,49,50 because this cut point was sensitive to greater risk of exposure to other prenatal and childhood environmental and social stressors in the NBC.
The 8-y and 15-y study assessments included a home visit in which the Home Observation for Measurement of the Environment (HOME) was used to assess and summarize the quality of the home environment and parenting skills. Consisting of direct questions as well as observed items, the HOME is a validated tool that measures the physical environment, as well as the amount and quality of support and stimulation available to a child at home, with a higher score indicating a more enriching environment.51,52 We prioritized HOME scores (total score: 0–60) from the 15-y assessment, using the previous (8-y) scores if available in cases (; 8%) when 15-y scores were not. For analyses, we derived a dichotomized variable in which less than the sample median HOME score value of 45 was considered a low score that indicated a poorer-quality home environment.
Statistical Analysis
For all analyses, we biomarkers of chemical exposure levels. Using prior literature and with consideration of avoiding overfitting regression models,53 we used directed acyclic graphs (Figure S1) to select a priori potential confounders, predictors of internalizing symptoms, and effect modifiers in regression models.54 The covariates included in all models were parental and household characteristics [PNSDI score (, ), maternal smoking during pregnancy (no, yes), HOME score ( of 45, )] and participant characteristics [biological sex (female, male), age at early-adult assessment (continuous; years), race/ethnicity (Non-Hispanic White, underrepresented racial/ethnic group)]. Due to limited sample size in racial/ethnic identities besides Non-Hispanic White, we categorized Hispanic, Black or African American, Asian American, Native American, Cape Verdean, and Other racial/ethnic groups together for analysis, because we hypothesize these groups experience a greater, although varying, degree of discrimination and racism.
Because elevated GAD-7 and PHQ-8 scores were not a rare outcome and because we were interested in relative risk, we used modified Poisson regression with a robust error variance approach55 to estimate associations between prenatal chemical concentrations and risk of elevated symptoms. We first examined potential nonlinear associations between prenatal biomarker concentrations and the log risk ratio (RR) of outcomes using covariate-adjusted generalized additive models and, in most cases, found linear relationships. Some of the modeled splines indicated possible quadratic associations; consequently, we compared goodness of fit, using the quasi-likelihood under the independence model information criterion (), between models including linear terms vs. linear and quadratic terms for exposures. In these cases, values were better for the linear term model or not appreciably different (within 1 point of each other) between the two models (Figure S2); thus, for the sake of parsimony, all exposures were modeled as linear terms, with effect estimates representing risk per doubling cord blood biomarker concentration. We evaluated the presence of associations based on both magnitude and precision of effect estimates.
Based on prior literature, we identified sociodemographic and nonchemical stressors (hereafter referred to as other stressors), including participant biological sex, race/ethnicity (surrogate for experience with racial discrimination and/or structural racism), PNSDI, and adolescent HOME score; we explored whether these other stressors modified the association of prenatal chemical concentrations with internalizing symptoms. We did so by a) including product interaction terms between chemical concentrations and the other stressors in models (our primary approach) and b) running main effects models in data sets stratified by each dichotomized other stressor. These two approaches were used due to possible concerns of varying confounder relationships in exposure–outcome relationships by strata, in which the use of an interaction term would be inappropriate and bias results.
In main analyses, we fit single-exposure covariate-adjusted models for the overall analytic cohort and then models that additionally included product interaction terms between the exposure and one of the other stressors. Thus, for each exposure and outcome, we ran five separate models (i.e., main effects model; ones additionally including an exposure–sex, exposure–race/ethnicity, exposure–PNSDI, or exposure–HOME product interaction term). With respect to an explicit statistical significance level, we used , but we also highlighted findings that were suggestive but did not meet this threshold. For possible heterogeneity in strata-specific effects, we assessed the magnitude and precision of associations within strata and interaction term -values.
Additionally, to evaluate the effect of the five-chemical mixture on risk of elevated internalizing symptoms, we used quantile-based g-computation.56 Briefly, this approach specifies an index formed as a weighted combination of quantile scores for each pollutant in the model and applies g-computation to estimate the joint association of a quantile increase of the total mixture. Based on our assessment of generalized additive models, we assumed linearity of associations. We fit logistic regression models with no bootstraps to obtain weights for each exposure (i.e., strength/direction of the association between each exposure and outcome). Then, running 10,000 bootstrap samples, we estimated RRs (95% “pointwise” bounds) for the overall mixture effect. We ran quantile g-computation in the overall analytic cohort and in data sets stratified by the other stressors to obtain effect estimates. Then, in the overall analytic cohort, we fit models with a product term between the chemical mixture and each of the other stressors to test statistical interactions.
We investigated concordance between elevated GAD-7 and PHQ-8 symptoms in the overall analytic cohort. Furthermore, in the subset of participants who completed medical history questionnaires at the early-adult assessment (), we first determined concordance between a) elevated GAD-7 symptoms and self-report of clinical diagnosis of an anxiety disorder and b) elevated PHQ-8 symptoms and self-report of a clinical diagnosis of depression. Then, we performed secondary single-chemical analyses considering self-report of an anxiety disorder and of depression in the prior 10 y as outcomes of interest. As with the GAD-7 and PHQ-8, we explored overall and strata-specific associations.
We performed sensitivity analyses in which we examined potential selection bias, residual confounding, or influence of outliers for the single-chemical main analysis of GAD-7 and PHQ-8 outcomes. First, we used inverse probability of censoring weighting (IPCW) to quantify potential selection bias due to cohort attrition and exclusion of participants without complete case information.57,58 We explored predictors of dropout (i.e., missing exposure, outcome, and/or covariate data) and then calculated stabilized truncated weights to include in the analysis. To evaluate possible coexposure confounding, we a) mutually adjusted for all chemical exposures in one model (i.e., by including the five separate chemical variables) and b) further adjusted models of organochlorine exposures for maternal seafood (continuous; servings per day) and local produce consumption (yes/no) during pregnancy, because these dietary exposures may act as negative confounders (i.e., consumption is associated with higher organochlorine exposures, and with positive neurobehavioral effects due to coexposure to essential nutrients).59 We also fit models that excluded participants on regular psychotropic medications, because these individuals’ symptoms may be altered by medication use. When considering cord blood Pb as the exposure of interest, we included peak early childhood Pb as a covariate to investigate the effect of Pb in these two separate exposure windows. Last, in generalized additive models, we observed that the HCB minimum value was an influential outlier, and so we ran all single-chemical HCB models excluding that observation.
Among 240 participants who completed the GAD-7 and/or PHQ-8 assessments in early adulthood, 234 had cord serum organochlorine measures, and of those, 204 had complete covariate data. For analyses of prenatal Pb and Mn, 228 and 220 participants had cord blood biomarker measures, respectively. Of those with these prenatal metal biomarkers, 198 and 193 participants had measures of cord blood Pb and Mn, respectively, as well as complete data on covariates. Data from 188 participants were used in five-chemical Poisson regression and quantile g-computation analyses. A total of 209 participants were included in at least one complete case analysis (see flow chart: Figure S3).
Results
Study Population Characteristics
Of those 209 participants included in the analytic data set, a large proportion of their parents [ () of mothers and 63% () of fathers] had an education level of high school graduate or lower at the time of their child’s birth. One-quarter of mothers reported smoking during pregnancy (, 25%), and 33% () were categorized with a high PNSDI (more disadvantaged). At the time of GAD-7 and PHQ-8 assessment, the age of study participants was y. Approximately two-thirds of early-adult study participants were female (, 67%), and most identified as Non-Hispanic White (, 76%) (Table 1). Of the 51 participants from underrepresented groups, most participants identified as Cape Verdean (, 10%) or Hispanic (, 9%); the others identified as Black or African American, Native American, Asian American, or another race/ethnicity. Among those categorized as belonging to a higher risk sociodemographic or psychosocial stressor group, concordances between high PNSDI, low HOME score, and the underrepresented racial/ethnic group were moderate to strong, with the greatest overlap between those with a high PNSDI also having a low HOME score (Table S2).
Table 1.
Characteristics of 209 young adult New Bedford Cohort participants included in at least one complete case analysis and their associated RRs (95% CI) [RR (95% CI)] for elevated anxiety symptoms on the GAD-7 scale and elevated depressive symptoms on the PHQ-8.
| or (%) | RR (95% CI) of elevated GAD-7 anxiety symptoms | RR (95% CI) of elevated PHQ-8 depressive symptoms | |
|---|---|---|---|
| Parental and household characteristics | |||
| PNSDI score (0–5 scale) | |||
| High () | 68 (33) | 2.33 (1.34, 4.02) | 2.33 (1.46, 3.72) |
| Low () | 141 (67) | Ref | Ref |
| Maternal age at child’s birthb | |||
| yb | 24 (11) | 2.01 (1.06, 3.82) | 1.65 (0.92, 2.95) |
| y | 185 (89) | Ref | Ref |
| Maternal educationa,b | |||
| High school graduate or lessb | 101 (49) | 2.82 (1.49, 5.34) | 1.78 (1.08, 2.94) |
| Junior college, college, and/or postgraduate | 107 (51) | Ref | Ref |
| Paternal educationa,b | |||
| High school graduate or lessb | 131 (63) | 1.97 (0.99, 3.91) | 1.65 (0.94, 2.91) |
| Junior college, college, and/or postgraduate | 76 (37) | Ref | Ref |
| Annual household income at birthb (USD) | |||
| b | 64 (31) | 1.88 (1.09, 3.26) | 2.01 (1.27, 3.20) |
| 145 (69) | Ref | Ref | |
| Maternal marital status at birthb | |||
| Not marriedb | 68 (33) | 2.33 (1.34, 4.02) | 2.16 (1.35, 3.44) |
| Married | 141 (67) | Ref | Ref |
| Maternal prenatal smoking | |||
| Yes | 53 (25) | 1.57 (0.89, 2.78) | 1.47 (0.90, 2.41) |
| No | 156 (75) | Ref | Ref |
| HOME score (0–60 scale)c | |||
| Low [ (45)] | 93 (44) | 1.89 (1.07, 3.35) | 2.29 (1.38, 3.79) |
| High [ (45)] | 116 (56) | Ref | Ref |
| Participant characteristics | |||
| Age at GAD-7 and PHQ-8 assessment (per year) | 1.04 (0.87, 1.24) | 1.11 (0.95, 1.29) | |
| Sex | |||
| Female | 139 (67) | 1.52 (0.79, 2.93) | 1.47 (0.84, 2.58) |
| Male | 70 (33) | Ref | Ref |
| Race/ethnicity | |||
| Underrepresented groupd | 51 (24) | 1.90 (1.09, 3.30) | 1.55 (0.95, 2.53) |
| Non-Hispanic White | 158 (76) | Ref | Ref |
| Early adult anxiety and depressive symptom characteristics | |||
| GAD-7 anxiety symptoms score (0–21 scale)a | — | — | |
| PHQ-8 depressive symptoms score (0–24 scale) | — | — | |
| GAD-7 symptomsa | |||
| Elevated () | 40 (19) | — | — |
| Not elevated () | 168 (81) | — | — |
| PHQ-8 symptoms | |||
| Elevated () | 51 (24) | — | — |
| Not elevated () | 158 (76) | — | — |
| Psychotropic medication usea | |||
| Yes | 15 (9) | 3.29 (1.81, 5.98) | 2.88 (1.72, 4.84) |
| No | 149 (91) | Ref | Ref |
| Diagnosis of an anxiety disordera | |||
| Yes | 43 (25) | 4.58 (2.50, 8.40) | 2.73 (1.66, 4.49) |
| No | 129 (75) | Ref | Ref |
| Diagnosis of depressiona | |||
| Yes | 36 (21) | 5.77 (3.19, 10.4) | 3.78 (2.34, 6.11) |
| No | 136 (79) | Ref | Ref |
Note: —, no data; CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HOME: Home Observation for Measurement of the Environment; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index; Ref, reference; SD, standard deviation.
For participants included in any of the complete case analyses, the following covariates have missing data (n missing): 1 with maternal education level, 2 for paternal education level; 1 with GAD-7 score; 45 with psychotropic medication use; 37 for diagnosis of an anxiety disorder/depression.
These parental and household characteristics contribute toward the total score for the PNSDI.
For 16 participants who were missing the HOME score at the 15-y assessment, the HOME score from the 8-y assessment was used.
The underrepresented group includes those categorized as Cape Verdean, Hispanic, Black or African American, Native American, Asian American, or another race/ethnicity.
Participants who completed the GAD-7 and PHQ-8 assessments at the early-adult visit were more likely to be female and Non-Hispanic White and have low PNSDI and high HOME scores than those initially enrolled in the cohort who did not participate in these assessments (Table S3).
Organochlorine and Metal Exposures
The median biomarker levels of organochlorines in cord serum were 0.02, 0.33, and for HCB, ,′-DDE, and , respectively. Median peak early childhood Pb concentration was higher than cord blood Pb (5.8 vs. ), and median cord blood Mn was (Table 2). The cord serum organochlorines were positively and moderately correlated, with of 0.36 for HCB and , 0.38 HCB and ,′-DDE, and 0.61 ,′-DDE and . The cord blood metals, Pb and Mn, were positively correlated (). Across the two chemical classes, concentrations were not strongly correlated (: to 0.14). Peak early childhood Pb was positively and moderately correlated with cord blood Pb () (Figure S4).
Table 2.
Distributions of cord blood biomarkers of organochlorine and metal exposures among 209 New Bedford Cohort participants included in at least one complete case analysis.
| Minimum | Percentiles | Maximum | |||||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| Organochlorines (ng/g)a,b,c | |||||||
| HCB | 0.001 | 0.02 | 0.02 | 0.03 | 0.13 | ||
| ,′-DDE | 0.03 | 0.21 | 0.33 | 0.46 | 9.74 | ||
| d | 0.01 | 0.12 | 0.20 | 0.31 | 4.41 | ||
| Metals ()a,b,c | |||||||
| Pb | |||||||
| Cord blood | 0.07 | 0.77 | 1.14 | 1.66 | 9.39 | ||
| Peak childhoode | 1.00 | 4.00 | 5.82 | 8.66 | 22.8 | ||
| Mn | 1.92 | 3.19 | 4.01 | 4.91 | 14.6 | ||
Note: GM, geometric mean; GSD, geometric standard deviation; HCB, hexachlorobenzene; LOD, limit of detection; Mn, manganese; Pb, lead; PCB, polychlorinated biphenyl; ,′-DDE, dichlorodiphenyldichloroethylene; SD, standard deviation; , sum of four polychlorinated biphenyls congeners.
Organochlorines were measured in cord serum; prenatal Pb and Mn were measured in cord whole blood; peak childhood Pb was measured in whole blood.
For participants included in any of the complete case analyses, the organochlorine and metal exposures have missing data (n missing): 5 with HCB, ,′-DDE, and ; 11 with cord blood Pb; 16 with peak early childhood Pb and Mn.
The LOD for the cord blood biomarkers were: for HCB, for ,′-DDE, for individual PCB congeners, and for Pb and Mn. In cases where concentrations were but , we used machine-read values in analyses.
Sum of four PCB congeners (118, 138, 153, 180).
Peak early childhood blood lead levels from ages 12 to 36 months.
Average concentrations of chemical biomarker levels were similar across those included and not included in any complete case analysis, except for cord blood and peak early childhood Pb, which were lower among those in complete case analyses (Table S3).
Symptoms and Diagnoses of Anxiety and Depression
In the analytic cohort, 19% () of participants had elevated GAD-7 anxiety symptoms, and 24% () had elevated PHQ-8 depressive symptoms (Table 1; Table S4). The strongest risk factors for elevated symptoms included high PNSDI, low HOME score, underrepresented racial/ethnic group, psychotropic medication use, and prior diagnosis of an anxiety disorder and depression (Table 1). Elevated anxiety and depressive symptoms were often comorbid, although there were more cases of elevated depressive symptoms in the absence of elevated anxiety symptoms in comparison with to anxiety without depressive symptoms (Table S5). Among participants who completed the GAD-7 and/or PHQ-8 and self-reported on medical diagnoses in the past 10 y, there was moderate concordance between elevated recent symptoms and prior clinical diagnosis. For example, 61% () of participants with elevated recent anxiety symptoms also self-reported a diagnosis of an anxiety disorder, and 50% () of participants with elevated recent depressive symptoms also self-reported a diagnosis of depression (Table S5).
Single-Chemical Associations with Elevated Anxiety and Depressive Symptoms
Overall (i.e., main effects models), a doubling of prenatal Pb level was associated with 1.41 (95% CI: 1.03, 1.94) times the risk of elevated anxiety symptoms. There was also a positive association between Pb and elevated depressive symptoms, but the RR was weaker than for anxiety, and CIs included the null [1.18 (95% CI: 0.91, 1.53)] (Figure 1; Table S6). Although CIs included the null, prenatal HCB and ,′-DDE levels were also associated with higher risk of elevated anxiety, but not depressive, symptoms (Figure 2; Table S6). Prenatal and Mn levels were not associated with risk of elevated symptoms (Figures 1 and 2; Table S6). In comparison with effect estimates in unadjusted models (Table S7), covariate-adjusted RRs were typically attenuated for prenatal Pb and Mn biomarkers and slightly strengthened for HCB, ,′-DDE, and biomarkers (Table S6).
Figure 1.
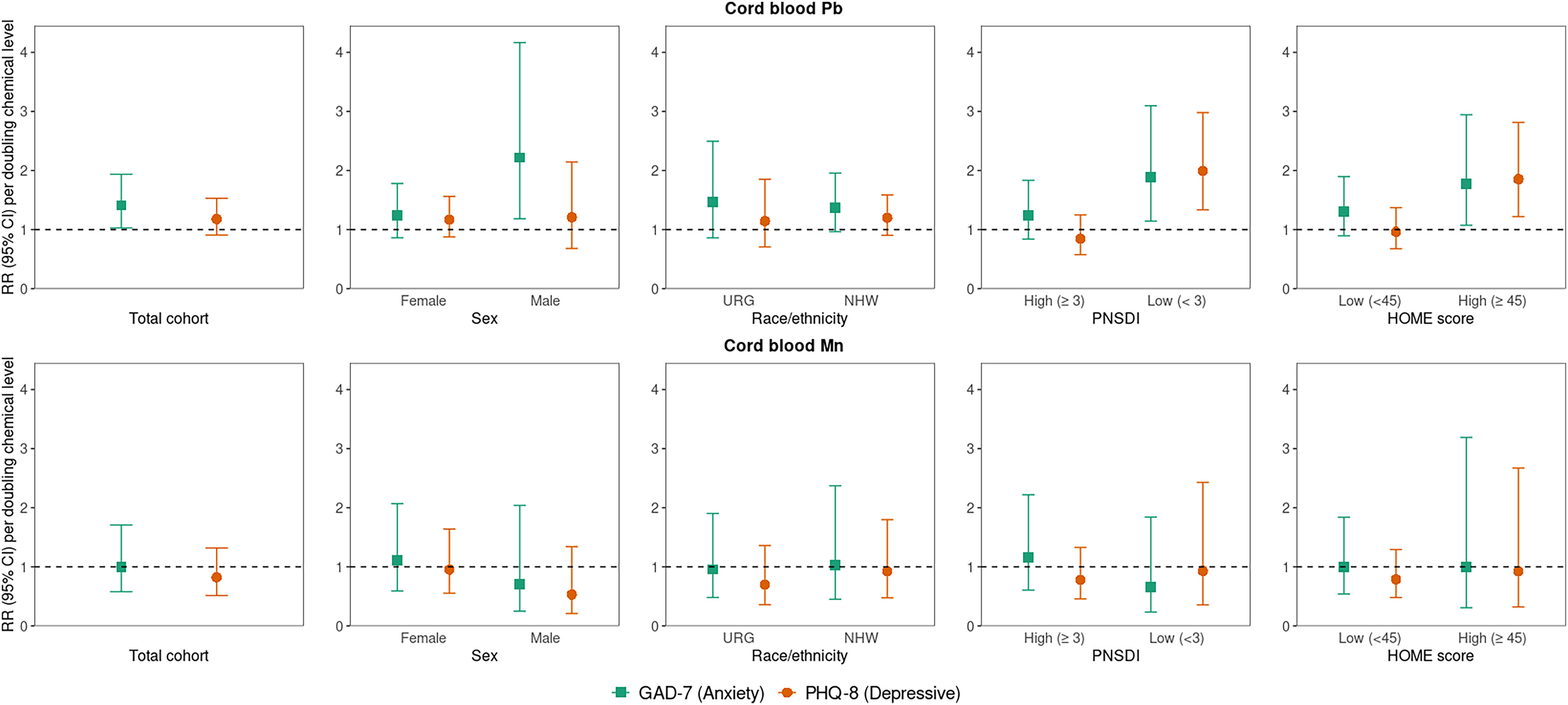
Overall and sociodemographic/nonchemical stressor strata-specific RRs (95% CI) [RR (95% CI)] for early adulthood elevated anxiety (GAD-7) and depressive symptoms (PHQ-8) associated with a doubling of cord whole blood Pb and Mn (micrograms per deciliter). Note: Single-exposure modified Poisson regression models adjusted for parental and household characteristics (PNSDI score, maternal smoking during pregnancy) and participant characteristics [age at assessment, race/ethnicity (URG, NHW), sex, HOME score]. For each outcome and chemical, RRs were estimated from five models: one with no interaction term and four including a product interaction term between the chemical and each of the four specified other stressors. See Table S6 for corresponding numeric data. CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HOME, Home Observation for Measurement of the Environment; Mn, manganese; NHW, Non-Hispanic White; Pb, lead; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index score; RR, risk ratio; URG, underrepresented group.
Figure 2.
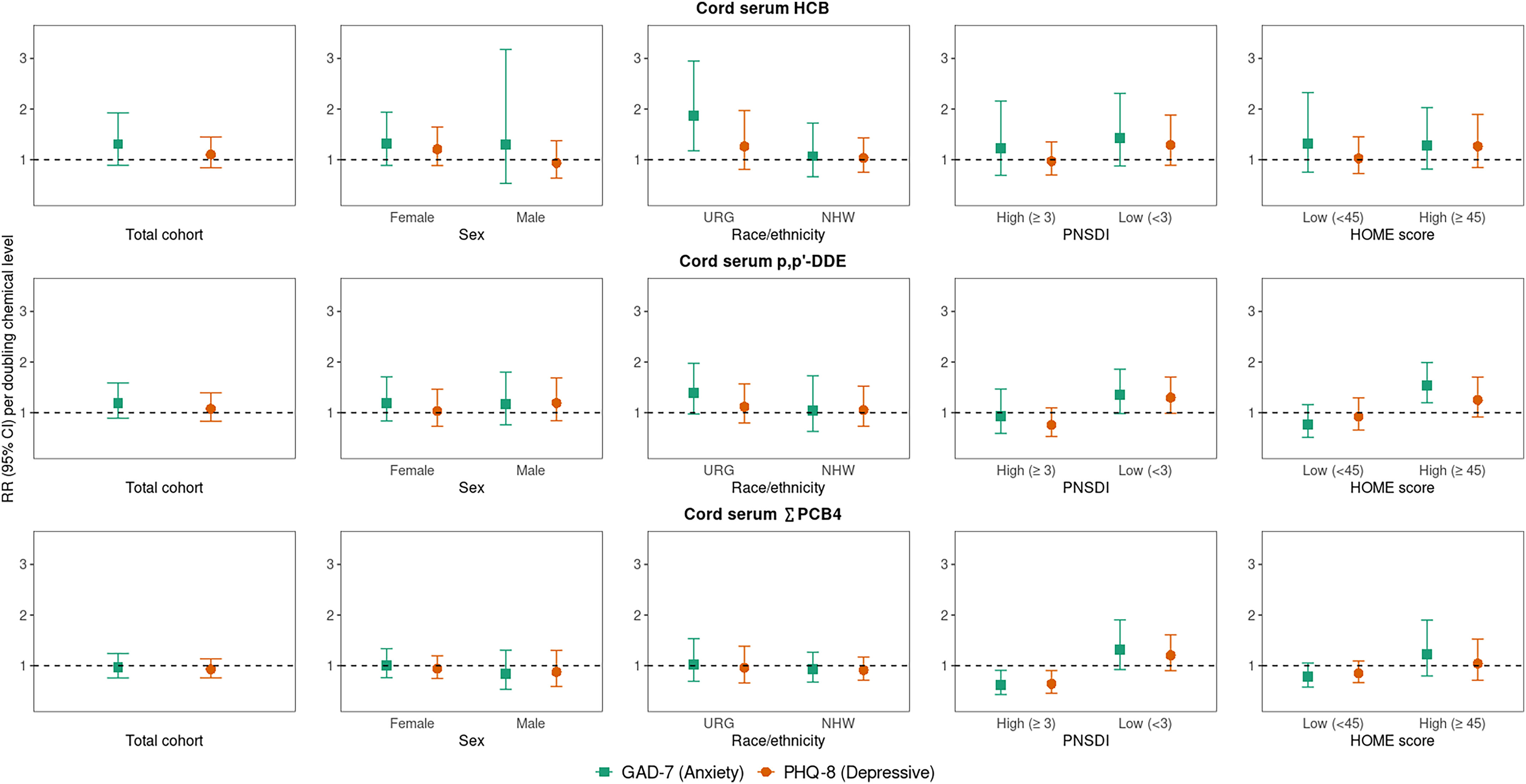
Overall and sociodemographic/nonchemical stressor strata-specific RRs (95% CI) [RR (95% CI)] for early adulthood elevated anxiety (GAD-7) and depressive symptoms (PHQ-8) associated with a doubling of cord serum HCB, ,′-DDE, and (congeners: 118, 138, 153, 180) (nanograms per gram). Note: Single-exposure modified Poisson regression models adjusted for parental and household characteristics (PNSDI score, maternal smoking during pregnancy) and participant characteristics [age at assessment, race/ethnicity (URG; NHW), sex, HOME score]. For each outcome and chemical, RRs were estimated from five models: one with no interaction term and four including a product interaction term between the chemical and each of the four specified other stressors; See Table S6 for corresponding numeric data. CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HCB, hexachlorobenzene; HOME, Home Observation for Measurement of the Environment; NHW, Non-Hispanic White; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index; ,′-DDE, dichlorobiphenyldichloroethylene; RR, risk ratio; , sum of four polychlorinated biphenyls congeners; URG, underrepresented group.
In analyses exploring whether other stressors modified associations between prenatal chemical exposures and risk of elevated symptoms, we observed statistically significant interactions () for PNSDI and HOME score, but not sex or race/ethnicity, with at least one chemical (Figures 1 and 2; see Table S6 for interaction -values). For prenatal Pb, ,′-DDE, and , a doubling in chemical concentration was associated with higher risk of elevated depressive symptoms for those with a low PNSDI but associated with no or lower risk of symptoms for those with a high PNSDI (more disadvantaged) [e.g., RR per doubling Pb: 1.99 (95% CI: 1.34, 2.98) for low vs. 0.85 (95% CI: 0.58, 1.25) for high PNSDI]. Although CIs included the null, a doubling of prenatal levels of ,′-DDE and were also associated with higher risk of elevated anxiety symptoms only for those with a low PNSDI [ (95% CI: 0.98, 1.86) for ,′-DDE, (95% CI: 0.92, 1.91) for ]. Similarly, for those with a high HOME score (better home environment), a doubling of prenatal ,′-DDE was associated with higher risk of elevated anxiety symptoms [ (95% CI: 1.20, 1.99)], and prenatal Pb was associated with higher risk of depressive symptoms [ (95% CI: 1.22, 2.81)]. The pattern of adverse risk of depressive symptoms only for those with a low PSNDI score or high HOME score (lower nonchemical stress groups) was also observed for prenatal HCB exposure, but CIs included the null and interactions were not statistically significant at the level [e.g., RR per doubling prenatal HCB: 1.29 (95% CI: 0.89, 1.88) in low PNSDI strata] (Figures 1 and 2; Table S6).
Additionally, prenatal HCB and ,′-DDE levels were associated with higher risk of elevated anxiety symptoms among underrepresented racial/ethnic group participants, and associations were null for Non-Hispanic White participants [e.g., (95% CI: 1.18, 2.95) for the underrepresented racial/ethnic group vs. 1.07 (95% CI: 0.66, 1.72) for Non-Hispanic White per doubling HCB] (Figure 2; Table S6). Although there was no statistically significant evidence of sex-specific associations with any chemical exposure, the adverse association between prenatal Pb and anxiety symptoms was stronger for males [RR per doubling Pb: 2.22 (95% CI: 1.18, 4.16) vs. 1.24 (95% CI: 0.86, 1.78) for females] (Figure 1; Table S6). Magnitude and directionality of effect estimates did not appreciably change when estimating strata-specific effects using interaction terms vs. stratifying the data set by the modifier (Table S8).
Mixture Associations with Anxiety and Depressive Symptoms
In the overall analytic cohort, quantile g-computation analysis showed that a quartile increase in the chemical mixture was moderately, albeit imprecisely with CIs that included the null, associated with an increased risk of elevated anxiety symptoms [ (95% CI: 0.71, 2.44)]. The overall mixture was not associated with risk of elevated depressive symptoms [ (95% CI: 0.67, 1.69)] (Table 3). The chemicals did not have coefficients in the same direction, with HCB, ,′-DDE, and Pb having positive scaled effect weights (i.e., contributing to higher risk of elevated symptoms), and and Mn having negative scaled effect weights (i.e., lower risk of elevated symptoms) (Figure S5). In models stratified by the four other stressors, RRs between the overall mixture and elevated symptoms somewhat varied by strata, but most estimates were imprecise, and CIs overlapped for strata-specific estimates. The strongest example of a stressor modifying the association between the mixture and an outcome was in a model stratified by PNSDI score. A quartile increase in all chemicals was associated with increased risk of depressive symptoms in the low PNSDI group (lower nonchemical stress) and with lower risk of depressive symptoms in the high PNSDI group (higher nonchemical stress); specifically, with 2.04 (95% CI: 1.01, 4.12) times the risk of elevated depressive symptoms for those with a low PNSDI and 0.64 (95% CI: 0.32, 1.26) times the risk for those with a high PNSDI. This was the only stratified quantile g-computation model in which the interaction between the mixture and another stressor was statistically significant at the level (Table 3).
Table 3.
Adjusted overall and sociodemographic/nonchemical stressor strata-specific RRs (95% CI) [RR (95% CI)] for early adulthood elevated anxiety (GAD-7) and depressive symptoms (PHQ-8) associated with a quartile increase in all five chemicals [cord blood HCB, ,′-DDE, (congeners: 118, 138, 153, 180), lead, and manganese] in quantile g-computation models.
| Anxiety (GAD-7) | Depressive (PHQ-8) | |||||
|---|---|---|---|---|---|---|
| RR (95% CI)a,b | c | RR (95% CI)a,b | c | |||
| Overall | 187 | 1.32 (0.71, 2.44) | — | 188 | 1.07 (0.67, 1.69) | — |
| Sex-stratified | — | — | 1.00 | — | — | 0.85 |
| Female | 125 | 1.39 (0.68, 2.83) | — | 126 | 1.27 (0.73, 2.22) | — |
| Male | 62 | 1.46 (0.10, 20.6) | — | 62 | 0.86 (0.25, 2.98) | — |
| Race/ethnicity-stratified | — | — | 0.36 | — | — | 0.72 |
| Underrepresented group | 47 | 1.68 (0.72, 3.94) | — | 48 | 0.87 (0.39, 1.95) | — |
| Non-Hispanic White | 140 | 0.99 (0.36, 2.72) | — | 140 | 1.05 (0.55, 1.99) | — |
| PNSDI-stratified | — | — | 0.73 | — | — | 0.04 |
| High () | 64 | 1.19 (0.39, 3.59) | — | 65 | 0.64 (0.32, 1.26) | — |
| Low () | 123 | 1.66 (0.45, 6.06) | — | 123 | 2.04 (1.01, 4.12) | — |
| HOME-stratified | — | — | 0.78 | — | — | 0.30 |
| Low () | 85 | 1.22 (0.49, 3.02) | — | 86 | 0.85 (0.49, 1.50) | — |
| High () | 102 | 1.71 (0.35, 8.35) | — | 102 | 1.86 (0.72, 4.76) | — |
Note: —, no data; CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HCB, hexachlorobenzene; HOME, Home Observation for Measurement of the Environment; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index; ,′-DDE, dichlorodiphenyldichloroethylene; RR, risk ratio; , sum of four polychlorinated biphenyls congeners.
Adjusted for parental and household characteristics (PNSDI, maternal smoking during pregnancy, HOME score) and participant characteristics (sex, race/ethnicity, age at assessment).
Effect estimates obtained from quantile g-computation models run in data sets stratified by the four other stressors.
Interaction term -values obtained from quantile g-computation models run in overall analytic data set and fit with a product term between the mixture and each of the four other stressors.
Single-Chemical Associations with Physician Diagnosis of Anxiety Disorders or Depression
Prenatal ,′-DDE and Pb levels were moderately associated with higher risk of diagnosis of an anxiety disorder or depression in the prior ten years, but CIs included the null [e.g., (95% CI: 0.90, 1.61)] for diagnosis of an anxiety disorder per doubling Pb]. Strata-specific associations by HOME score were observed with a doubling of prenatal ,′-DDE and , with higher risk of diagnoses for those with a high HOME score and lower risk for those with a low HOME score (poorer-quality home environment) [e.g., RR for depression diagnosis: 1.51 (95% CI: 1.08, 2.11) for high HOME score vs. 0.67 (95% CI: 0.45, 1.00) for low HOME score]. A similar strata-specific pattern was present for and PNSDI with diagnosis of depression [ (95% CI: 0.89, 1.77) for low PNSDI vs. 0.70 (95% CI: 0.46, 1.05) for high PNSDI (more disadvantaged) per doubling ]. Additionally, a doubling of prenatal ,′-DDE was associated with higher risk of anxiety diagnosis among the underrepresented racial/ethnic group participants [ (95% CI: 1.01, 1.60)], but not for Non-Hispanic White participants [ (95% CI: 0.64, 1.53)]. Prenatal HCB and Mn levels were not associated with self-report of physician-diagnosed anxiety or depression (Table S9).
Sensitivity Analyses
Findings were largely unchanged when using IPCW vs. complete case analysis, although the association between doubling of prenatal HCB and elevated anxiety symptoms in underrepresented racial/ethnic group participants was strengthened with IPCW [ (95% CI: 1.49, 3.18)] (Figure 3; Table S10). When excluding the minimum HCB outlier, the association with depressive symptoms did not change, but prenatal HCB was more strongly associated with elevated anxiety symptoms [i.e., overall (95% CI: 1.09, 2.07) per doubling HCB] (Table S11). We saw no meaningful changes to the magnitude of associations when further adjusting for coexposure by other chemicals (Figure 3; Table S12) or when additionally adjusting for potential dietary confounders (Figure 3; Table S13). Effect estimates also did not appreciably differ when comparing results from the subset that had information available on medication use (Table S14) to the further restricted subset of those not taking regular psychotropic medications (Table S15). However, the overall RR of prenatal Pb and elevated anxiety symptoms was attenuated in the 155 participants with data available on medication use (Table S14) and with exclusion of those on medications (Table S15) vs. the 197 participants in the complete case subset (Table S6).
Figure 3.
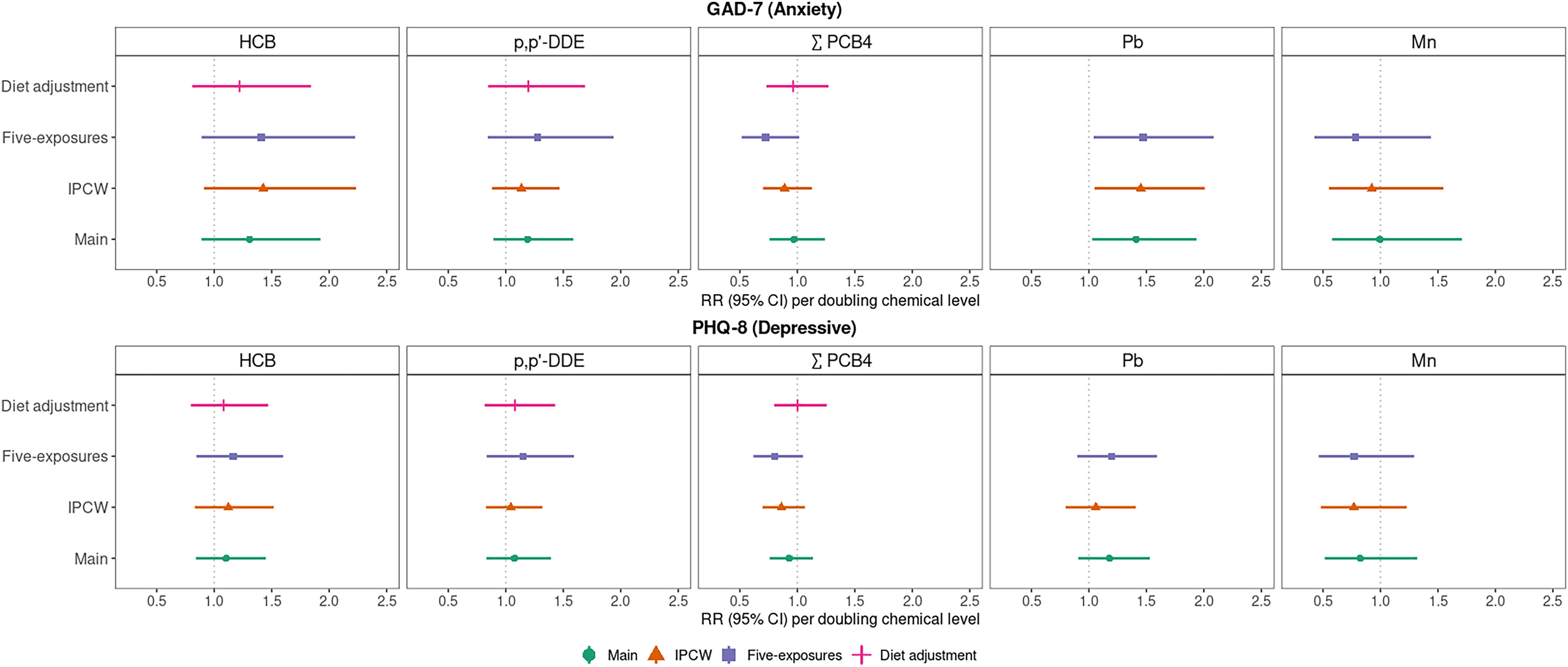
Overall RRs (95% CI) [RR (95% CI)] for early adulthood elevated anxiety (GAD-7) and depressive symptoms (PHQ-8) associated with a doubling of cord serum HCB, p,p’-DDE, (congeners: 118, 138, 153, 180) (nanograms per gram), and cord blood Pb and Mn (micrograms per deciliter), in the main models vs. sensitivity models using a) IPCW, b) adjusting for all five chemical coexposures, and c) adjusting for dietary factors associated with organochlorine exposure. Note: Modified Poisson regression models adjusted for parental and household characteristics (PNSDI score, maternal smoking during pregnancy) and participant characteristics (age at assessment, race/ethnicity, sex, HOME score). See Table S6 (main), Table S9 (IPCW), Table S11 (five exposures), and Table S12 (diet adjustment) for corresponding numeric data of overall and stratified models. CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HCB, hexachlorobenzene; HOME, Home Observation for Measurement of the Environment; IPCW, inverse probability of censoring weighted; Mn, manganese; Pb, lead; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index; ,′-DDE, dichlorobiphenyldichloroethylene; RR risk ratio; , sum of four polychlorinated biphenyls congeners.
In regression models including both prenatal and peak early childhood blood Pb, cord blood Pb was associated with higher risk of elevated anxiety and depressive symptoms, whereas peak childhood Pb was not associated with elevated symptoms. In analyses assessing strata-specific estimates, associations with peak childhood Pb remained largely null, except for associations with higher risk of elevated anxiety symptoms among the underrepresented racial/ethnic group participants and with lower risk of depressive symptoms for male, Non-Hispanic White, low PNSDI, and high HOME score participants (Figure 4; Table S16).
Figure 4.
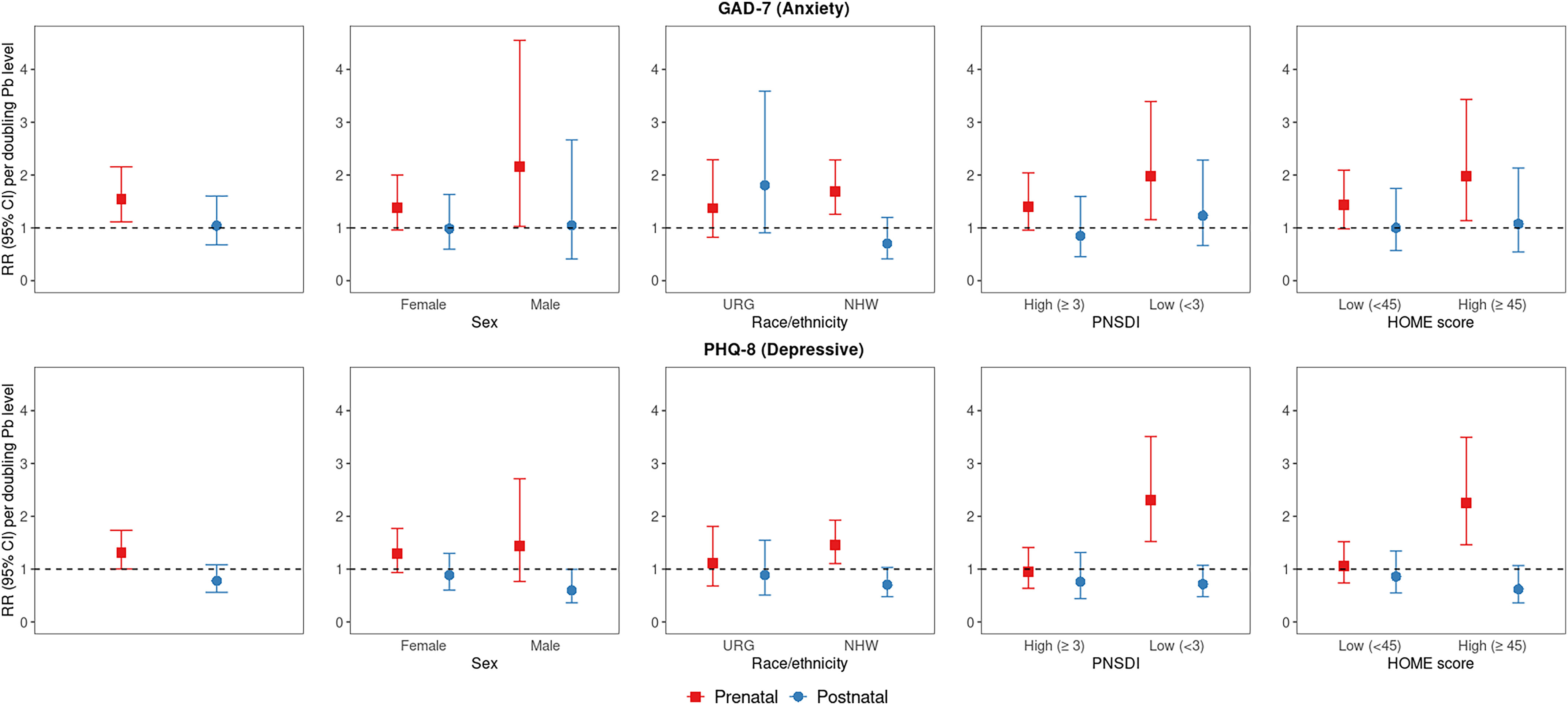
Overall and sociodemographic/nonchemical stressor strata-specific RRs (95% CI) [RR (95% CI)] for early adulthood moderately elevated anxiety (GAD-7) and depressive symptoms (PHQ-8) associated with a doubling of prenatal and postnatal (i.e., peak early childhood) Pb (micrograms per deciliter) ( for GAD-7; for PHQ-8). Note: Modified Poisson regression models, including both prenatal and postnatal lead, were adjusted for parental and household characteristics (PNSDI score, maternal smoking during pregnancy) and participant characteristics [age at assessment, race/ethnicity (URG, NHW), sex, HOME score]. See Table S16 for corresponding numeric data. CI, confidence interval; GAD-7, Generalized Anxiety Disorder scale; HOME, Home Observation for Measurement of the Environment; Mn, manganese; NHW, Non-Hispanic White; Pb, lead; PHQ-8, Patient Health Questionnaire; PNSDI, prenatal social disadvantage index; RR, risk ratio; URG, underrepresented group.
Discussion
In our study of NBC participants followed from birth through early adulthood, we found evidence of associations between greater prenatal exposure to HCB, ,′-DDE, and Pb and increased risk of elevated anxiety symptoms at approximately age 22 y. These adverse associations were strongest with prenatal Pb exposure, and more modest with the organochlorine exposures. In addition, higher prenatal organochlorines and Pb levels, as well as the overall effect of the mixture, were associated with increased risk of elevated depressive symptoms for those categorized as exposed to lower levels of nonchemical stress (i.e., low PNSDI, high HOME score). These findings suggest a possible “signal-to-noise ratio” scenario60 in which strong psychosocial risk factors for internalizing disorders obscure modest additional risk from prenatal chemical exposures. The strata-specific associations among those with a high PNSDI (more disadvantaged) or low HOME score (poorer-quality home environment) were mostly null, but in some analyses, prenatal ,′-DDE and appeared to be paradoxically associated with lower risk of elevated symptoms in these other stressor groups. In the total analytic cohort, the overall mixture was not associated with risk of depressive symptoms, but there was evidence of a relatively strong in magnitude, but imprecise, association with anxiety symptoms. We observed an interaction between the overall mixture and PNSDI score, in which a quartile increase in the mixture was associated with greater risk of depressive symptoms for the low PNSDI group (lower nonchemical stress group). Other than that, the overall effect of the mixture appeared to slightly vary in the presence other stressors, but strata-specific CIs were wide and overlapped. In general, prenatal and Mn were not adversely associated with risk of early adulthood anxiety and depressive symptoms. We did not observe strong evidence of sex-specific effects of these prenatal chemicals on risk for these symptoms in early adulthood.
Consistent with associations of prenatal Pb and self-report anxiety symptoms in adolescence in the NBC,15 higher prenatal Pb levels were associated with increased risk of elevated anxiety symptoms in early adulthood. Prior studies of prenatal Pb exposure and internalizing symptoms have mainly assessed outcomes in early or mid-childhood,16,21–24 prior to core risk periods for the onset of anxiety and depressive disorders,3,26 and largely have not observed associations. Our study findings suggested that in utero exposure to Pb may be one factor that increases anxiety vulnerability, possibly via the hypothesized mechanisms of disruption of dopaminergic and GABAergic systems9; notably, these effects may not manifest until later in development during or after critical risk periods for anxiety. Similar findings have been reported in rodent models, with low-level exposure to Pb during pregnancy and lactation shown to have little impact on weaned rats but to be associated with heightened anxiety behaviors in adult rats.14
Research on postnatal Pb exposure, either prospectively examining childhood exposure61 or cross-sectionally assessing Pb levels in adulthood,62 has demonstrated increased risk for internalizing symptoms in early adulthood. Despite the facts that, in the NBC, average peak early childhood blood Pb levels were a) higher than those measured in cord blood and b) higher than the CDC’s recently updated (in 2021) and previous blood reference levels of 3.5 and , respectively,63 we observed that prenatal Pb was more consistently associated with risk of elevated symptoms than early-childhood blood Pb. Although the literature on early Pb exposure and child neurodevelopment supports the postnatal period as a critical time,64 our study indicated that prenatal exposure may also influence risk for developing internalizing problems, particularly anxiety symptoms.
Although most prior literature, including in the NBC, has reported no associations between prenatal organochlorine levels and subsequent internalizing symptoms,15–21 we found evidence of prenatal HCB and ,′-DDE as risk factors for elevated internalizing symptoms in early adulthood, in most cases with stronger associations in certain levels of the sociodemographic or nonchemical stressors. The adverse associations of prenatal HCB and ,′-DDE levels and risk of elevated anxiety symptoms was stronger for young adult participants who identified as underrepresented racial/ethnic group participants. This finding aligns with the hypothesis that individuals experiencing stress due to racial discrimination and/or structural racism may have increased susceptibility to the effects of environmental exposures.65,66 It is important to note that our study did not have the power to look at more distinct categories of race/ethnicity. Experiences with racial discrimination and structural racism may differ vastly for those who identify as Cape Verdean, Hispanic, Black or African American, Native American, Asian American, or in other underrepresented racial/ethnic groups. Our study used a composite definition of race/ethnicity and thus was unable to parse out possible strata-specific effects by more meaningful constructs. Thus, to better understand possible synergistic effects between the stressors of chemicals and racism on subsequent mental health, future studies should be conducted in racially diverse cohorts that measure participants’ personal experiences with discrimination and other manifestations of structural racism using validated questionnaires (e.g., Everyday Discrimination Scale67).
Aside from potential differential susceptibility to ,′-DDE-associated anxiety by race/ethnicity, associations of prenatal ,′-DDE exposure with higher risk of internalizing symptoms were otherwise only apparent for those in lower stressor groups as indicated by PNSDI and HOME score ratings. This pattern of findings suggests a possible signal-to-noise ratio problem60 in which the modest effects of ,′-DDE are only detectable among those experiencing fewer psychosocial stressors. Strata-specific patterns may be more similar for PNSDI and HOME score strata in comparison with race/ethnicity strata, because a large portion of participants with a low HOME score (poorer-quality home environment) were also categorized with high PNSDI (more disadvantaged).
In comparison with most other studies of prenatal organochlorine exposures and internalizing symptoms, our study is unique in that we evaluated anxiety and depressive symptoms in early adulthood. In the NBC, a study in which prenatal concentrations of organochlorines were relatively low in comparison with other high-risk exposure cohorts,33,68 prenatal HCB, ,′-DDE, and were not associated with mid-childhood and adolescent internalizing symptoms, even when considering sex-specific effects.15 Similarly, other studies exploring prenatal HCB16,18,19 and ,′-DDE16,18–20 exposures and symptoms in childhood observed no adverse associations. One study with follow-up through early adulthood found no associations of these organochlorines with diagnosis of depression.17 When we considered self-report of physician diagnosed anxiety or depression as outcomes, we also observed no associations with prenatal HCB and mainly strata-specific adverse associations with ,′-DDE and . Our findings a) highlight the importance of considering coexposures to chemical and other stressors because adverse associations with HCB and ,′-DDE appeared to be strata-specific and b) suggest that modest effects of prenatal chemical exposures may be more likely to be observed with self-report of symptoms rather than physician diagnoses. As is the case for metals such as Pb, organochlorines are also hypothesized to dysregulate dopamine-mediated, glutamate, and GABAergic functions8,10 and thereby could contribute to later susceptibility to anxiety or depressive symptoms, which may not become apparent until adulthood.
With prenatal HCB, ,′-DDE, and Pb exposures, we found more consistent evidence of associations with anxiety in comparison with depressive symptoms. Although anxiety and depressive symptoms are often comorbid,69 as was the case in our study, and several of the hypothesized biological pathways by which prenatal exposures may alter neurobiological functions would be expected to increase vulnerability to both types of symptoms, it is plausible that neural pathways associated specifically with an anxiety phenotype are more susceptible to early-life chemical insults. For example, anxiety and depression phenotypes have been proposed to align with different constructs within the systems of negative valence (anxiety with “acute threat” or “potential threat” and depression with “loss”), and these constructs are associated with differing neural and physiological pathways.70,71 Thus, certain chemical exposures may dysregulate pathways more relevant to anxiety vulnerability (e.g., bed nucleus of the stria terminalis). Research is needed to explicate the potential neural mechanisms by which specific chemical exposures exert their effects.
We did not observe consistent associations between prenatal exposure and internalizing symptoms in early adulthood. In fact, in some cases, cord blood levels of , as well as ,′-DDE, were associated with lower risk of elevated symptoms for those categorized as having a high PNSDI and low HOME score (i.e., hypothesized higher nonchemical stressor groups). These apparent protective associations, in the opposite direction than hypothesized, could be due to chance findings in an analytic cohort with a moderate sample size or differential sources/levels of confounding in nonchemical stressor strata. In addition to fitting models with interaction terms, we stratified the data set by PNSDI and HOME score groups to explore whether this strata-specific pattern was due to differing associations of confounders with exposure–outcome relationships for high vs. low groups. However, this did not appear to be the case, because RRs remained similar when estimating effect estimates both ways. Alternatively, a small study of mother–child pairs found that greater maternal stress and adversity during pregnancy was associated with decreased cord blood DNA methylation of the oxytocin receptor gene.72 This decrease may lead to increased expression and easier activation of the oxytocin receptor gene in childhood, in turn mitigating the adverse impacts of stress and exerting a protective effect toward chemical exposures. Further exploration into this potential and other mechanisms is needed to better understand whether higher nonchemical maternal stress could induce certain protective adaptations.
Prenatal Mn levels were not associated with internalizing symptoms here, in contrast to adverse associations observed for girls in mid-childhood and adolescence in the NBC.15 When analogous outcomes were evaluated in the NBC at earlier ages, symptoms were mainly assessed using continuous measures (i.e., Conners’ Rating Scale,73 Behavior Assessment System for Children, Second Edition74) whereas the GAD-7 and PHQ-8 are binary screening tools. Thus, this attenuation of the sex-specific association by early adulthood could be due to reduced power to detect moderate differences with a dichotomized outcome and smaller sample size. It is also possible that prenatal Mn exposure does not influence anxiety and depressive symptoms evident in early adulthood. In other cohorts with follow-up through mid-childhood,24,25 there is limited, mixed evidence of associations between prenatal Mn and subsequent internalizing symptoms. Because Mn is an essential metal, prenatal exposure via diet is fundamental for growth and neurodevelopment, unlike prenatal exposure to Pb. However, deficient or excess levels Mn can cause neurotoxicity.75 Thus, if NBC participants were largely exposed to nutritionally optimal Mn levels while in utero, it is plausible that Mn would have no impact on anxiety and depressive symptoms in early adulthood.
Although this study is novel by virtue of its prospective longitudinal assessment of the effects of prenatal exposures on internalizing symptom risk in early adulthood, our findings may have been influenced by selection bias due to loss to follow-up of 20 y or longer, residual and unmeasured confounding, a limited sample size, and type II error. We aimed to address possible selection bias due to cohort attrition with use of IPCW and residual confounding bias by adjusting for prenatal seafood consumption, local produce consumption, and chemical coexposures. Although these sensitivity analyses showed that results were robust, IPCW model misspecification or dietary measurement error still may result in remaining bias. Our study also had a modest overall sample size, with relatively small numbers of participants in some of the other stressor subgroups. Consequently, we were only able to evaluate participants from underrepresented racial/ethnic groups in a composite group, and strata-specific findings may not be generalizable. Moreover, our analyses may not have been sufficiently powered to detect modest effect sizes or interactive effects. External validity of our results may have also been impacted by the timing of biomarker collection (1993–1998) and changes in exposure distributions of organochlorines and metals over time. Additionally, we did not correct for multiple comparisons in our models. Therefore, some of the observed associations, including both those that supported and contradicted our a priori hypotheses, may be the result of type II error (i.e., false positives). We elected to prioritize risk of false positives over false negatives; thus, replication of our findings, particularly those examining interactive effects between prenatal chemical exposures and other stressors, is needed in other cohort studies. Furthermore, missing data on medical history at the early-adult visit (i.e., different analytic cohorts for sensitivity analyses) limited our ability to determine whether treatment for a psychiatric illness contributed to outcome measurement error. Last, we were unable to assess other critical windows of exposure vulnerability, because all chemicals, except peak early childhood Pb, were only measured in cord blood. Although this limits our ability to understand specific exposure windows when the developing brain may be most susceptible to chemical impacts, postnatal exposure levels would not confound the observed associations, unless they were highly correlated with prenatal ones; thus, their absence does not detract from the validity of these findings.
That said, elevated anxiety and depressive symptoms were prevalent among the young adult NBC participants, and we observed associations between HCB, ,′-DDE, and Pb and internalizing symptoms and differing associations in strata of other stressor groups. In addition, our study had several strengths and addressed gaps in the extant literature. In limited prior studies, there has been evaluation of coexposures to prenatal organochlorines or metals with mid-childhood internalizing symptoms,18,19,24 single-chemical associations with early adulthood outcomes,17 or sex-specific effects.25 However, to our knowledge, the present study is distinct as a singular study that examined prenatal organochlorines and metal exposures as individual components and as a mixture, assessed internalizing symptoms in early adulthood using both self-report questionnaires and physician-diagnosed disorders, and considered interactions between chemical exposures and several sociodemographic and nonchemical stressors. We used quantile g-computation, in addition to standard parametric regression models, to better understand how simultaneous exposure to these two classes of chemicals may influence risk of elevated symptoms. Additionally, we examined the outcome in early adulthood, a core risk period for anxiety and depressive disorders. By including assessments a) not dependent on access and use of medical care and b) not subject to variability in clinical practice (i.e., standardized questionnaires), we minimized outcome misclassification that can occur when only considering physician diagnosis of internalizing disorders. The use of self-report questionnaires also better captures subclinical disease and thus may have greater sensitivity for detecting more modest associations. In fact, we observed stronger magnitudes of associations for GAD-7 anxiety symptoms than for diagnosis of an anxiety disorder with HCB, ,′-DDE, and Pb. Finally, the NBC is socioeconomically diverse, which not only improves the generalizability of the findings, but also allowed us to consider a number of other stressors as potential modifiers of the associations between chemical exposures and internalizing symptoms.
In summary, our findings indicated adverse associations of prenatal HCB, ,′-DDE, and Pb exposure with risk of early adulthood internalizing symptoms, particularly anxiety symptoms. Prevalence of anxiety in young adults has been rapidly increasing in recent years1; thus, understanding how early-life chemical exposures, in addition to more well-studied psychosocial stressors, influence susceptibility to internalizing disorders is critical. Because anxiety is a risk factor for poor work and social and psychiatric outcomes over the life course,76 it is important to consider that reductions to chemical exposures during pregnancy, even if associations are modest, may improve the overall population’s well-being.
Supplementary Material
Acknowledgments
Support for this research was provided by grants P42ES005947, R01ES014864, P30ES000002, and R21ES024513 from the National Institute of Environmental Health Sciences (NIEHS/NIH). L.B.R. was additionally supported by the CDC/NIOSH Harvard Education and Research Center (T42OH008416).
References
- 1.Goodwin RD, Weinberger AH, Kim JH, Wu M, Galea S. 2020. Trends in anxiety among adults in the United States, 2008–2018: rapid increases among young adults. J Psychiatr Res 130:441–446, PMID: , 10.1016/j.jpsychires.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojtabai R, Olfson M, Han B. 2016. National Trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 138(6):e20161878, PMID: , 10.1542/peds.2016-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenen KC, Rudenstine S, Susser E, Galea S. 2013. A Life Course Approach to Mental Disorders. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Blanco C, Rubio J, Wall M, Wang S, Jiu CJ, Kendler KS. 2014. Risk factors for anxiety disorders: common and specific effects in a national sample. Depress Anxiety 31(9):756–764, PMID: , 10.1002/da.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sander JB, McCarty CA. 2005. Youth depression in the family context: familial risk factors and models of treatment. Clin Child Fam Psychol Rev 8(3):203–219, PMID: , 10.1007/s10567-005-6666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellinger DC. 2013. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf Health Work 4(1):1–11, PMID: , 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korrick SA, Sagiv SK. 2008. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr 20(2):198–204, PMID: , 10.1097/MOP.0b013e3282f6a4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell MR. 2014. Endocrine-disrupting actions of PCBs on brain development and social and reproductive behaviors. Curr Opin Pharmacol 19:134–144, PMID: , 10.1016/j.coph.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason LH, Harp JP, Han DY. 2014. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int 2014:1–8, PMID: , 10.1155/2014/840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addae C, Cheng H, Martinez-Ceballos E. 2013. Effect of the environmental pollutant hexachlorobenzene (HCB) on the neuronal differentiation of mouse embryonic stem cells. Int J Environ Res Public Health 10(10):5244–5256, PMID: , 10.3390/ijerph10105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betharia S, Maher TJ. 2012. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology 33(5):1117–1127, PMID: , 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 12.van den Bosch M, Meyer-Lindenberg A. 2019. Environmental exposures and depression: biological mechanisms and epidemiological evidence. Annu Rev Public Health 40:239–259, PMID: , 10.1146/annurev-publhealth-040218-044106. [DOI] [PubMed] [Google Scholar]
- 13.de Souza Lisboa SF, Gonçalves G, Komatsu F, Queiroz CA, Almeida AA, Moreira EG. 2005. Developmental lead exposure induces depressive-like behavior in female rats. Drug Chem Toxicol 28(1):67–77, PMID: , 10.1081/dct-39696. [DOI] [PubMed] [Google Scholar]
- 14.Moreira EG, Vassilieff I, Vassilieff VS. 2001. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23(5):489–495, PMID: , 10.1016/S0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 15.Rokoff LB, Shoaff JR, Coull BA, Enlow MB, Bellinger DC, Korrick SA. 2022. Prenatal exposure to a mixture of organochlorines and metals and internalizing symptoms in childhood and adolescence. Environ Res 208:112701, 10.1016/j.envres.2022.112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, et al. 2013. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8 years. Environ Int 59:225–231, PMID: , 10.1016/j.envint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, et al. 2014. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes–a prospective study with long-term follow-up. Environ Int 68:41–48, PMID: , 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Jedynak P, Maitre L, Guxens M, Gützkow KB, Julvez J, López-Vicente M, et al. 2021. Prenatal exposure to a wide range of environmental chemicals and child behaviour between 3 and 7 years of age – an exposome-based approach in 5 European cohorts. Sci Total Environ 763:144115, PMID: , 10.1016/j.scitotenv.2020.144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maitre L, Julvez J, López-Vicente M, Warembourg C, Tamayo-Uria I, Philippat C, et al. 2021. Early-life environmental exposure determinants of child behavior in Europe: a longitudinal, population-based study. Environ Int 153:106523, PMID: , 10.1016/j.envint.2021.106523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenquist AH, Høyer BB, Julvez J, Sunyer J, Pedersen HS, Lenters V, et al. 2017. Prenatal and postnatal PCB-153 and p,p’-DDE exposures and behavior scores at 5–9 years of age among children in Greenland and Ukraine. Environ Health Perspect 125(10):107002, PMID: , 10.1289/EHP553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plusquellec P, Muckle G, Dewailly E, Ayotte P, Bégin G, Desrosiers C, et al. 2010. The relation of environmental contaminants exposure to behavioral indicators in Inuit preschoolers in arctic Quebec. Neurotoxicology 31(1):17–25, PMID: , 10.1016/j.neuro.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Bellinger D, Leviton A, Allred E, Rabinowitz M. 1994. Pre- and postnatal lead exposure and behavior problems in school-aged children. Environ Res 66(1):12–30, PMID: , 10.1006/enrs.1994.1041. [DOI] [PubMed] [Google Scholar]
- 23.Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J. 2016. Bisphenol A, phthalates and lead and learning and behavioral problems in Canadian children 6–11 years of age: CHMS 2007–2009. Neurotoxicology 54:89–98, PMID: , 10.1016/j.neuro.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. 2018. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int 121(pt 1):148–158, PMID: , 10.1016/j.envint.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernández-Bonilla D, et al. 2015. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int 84:39–54, PMID: , 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62(6):593–602, PMID: , 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 27.Sourander A, Helstelä L, Helenius H. 1999. Parent-adolescent agreement on emotional and behavioral problems. Soc Psychiatry Psychiatr Epidemiol 34(12):657–663, PMID: , 10.1007/s001270050189. [DOI] [PubMed] [Google Scholar]
- 28.van der Ende J, Verhulst FC, Tiemeier H. 2012. Agreement of informants on emotional and behavioral problems from childhood to adulthood. Psychol Assess 24(2):293–300, PMID: , 10.1037/a0025500. [DOI] [PubMed] [Google Scholar]
- 29.Appleton AA, Holdsworth EA, Kubzansky LD. 2016. A Systematic review of the interplay between social determinants and environmental exposures for early-life outcomes. Curr Environ Health Rep 3(3):287–301, PMID: , 10.1007/s40572-016-0099-7. [DOI] [PubMed] [Google Scholar]
- 30.Barrett ES, Padula AM. 2019. Joint impact of synthetic chemical and non-chemical stressors on children’s health. Curr Environ Health Rep 6(4):225–235, PMID: , 10.1007/s40572-019-00252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowell WJ, Wright RJ. 2017. Sex-specific effects of combined exposure to chemical and non-chemical stressors on neuroendocrine development: a review of recent findings and putative mechanisms. Curr Envir Health Rpt 4(4):415–425, 10.1007/s40572-017-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi AL, Levy JI, Dockery DW, Ryan LM, Tolbert PE, Altshul LM, et al. 2006. Does living near a superfund site contribute to higher polychlorinated biphenyl (PCB) exposure? Environ Health Perspect 114(7):1092–1098, PMID: , 10.1289/ehp.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korrick SA, Altshul LM, Tolbert PE, Burse VW, Needham LL, Monson RR. 2000. Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J Expo Anal Environ Epidemiol 10(6 pt 2):743–754, PMID: , 10.1038/sj.jea.7500120. [DOI] [PubMed] [Google Scholar]
- 34.Sagiv SK, Nugent JK, Brazelton TB, Choi AL, Tolbert PE, Altshul LM, et al. 2008. Prenatal organochlorine exposure and measures of behavior in infancy using the Neonatal Behavioral Assessment Scale (NBAS). Environ Health Perspect 116(5):666–673, PMID: , 10.1289/ehp.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA. 2012. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect 120(6):904–909, PMID: , 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen LG. 1998. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect 106(suppl 1):171–189, PMID: , 10.1289/ehp.98106s1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schantz SL, Widholm JJ, Rice DC. 2003. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect 111(3):357–576, PMID: , 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Environmental Protection Agency. 2013. America’s Children and the Environment. 3rd ed. https://www.epa.gov/system/files/documents/2022-10/ace3_2013.pdf [accessed 16 November 2022].
- 39.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. 2010. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol 171(5):593–601, PMID: , 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oppenheimer AV, Bellinger DC, Coull BA, Weisskopf MG, Zemplenyi M, Korrick SA. 2021. Prenatal exposure to chemical mixtures and inhibition among adolescents. Toxics 9(11):311, PMID: , 10.3390/toxics9110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. 1995. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol 40(9):1475–1485, PMID: , 10.1088/0031-9155/40/9/007. [DOI] [PubMed] [Google Scholar]
- 42.Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. 2003. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N Engl J Med 348(16):1517–1526, PMID: , 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. 2005. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899, PMID: , 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spitzer RL, Kroenke K, Williams JB, Löwe B. 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10):1092–1097, PMID: , 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 45.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. 2009. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114(1–3):163–173, PMID: , 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Hobel CJ, Hyvarinen MA, Okada DM, Oh W. 1973. Prenatal and intrapartum high-risk screening. I. Prediction of the high-risk neonate. Am J Obstet Gynecol 117(1):1–9, PMID: , 10.1016/0002-9378(73)90720-5. [DOI] [PubMed] [Google Scholar]
- 47.Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, et al. 2014. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. Am J Epidemiol 180(3):263–271, PMID: , 10.1093/aje/kwu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, et al. 2017. Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc Natl Acad Sci USA 114(26):6728–6733, PMID: , 10.1073/pnas.1617698114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oppenheimer AV, Bellinger DC, Coull BA, Weisskopf MG, Korrick SA. 2022. Prenatal exposure to chemical mixtures and working memory among adolescents. Environ Res 205:112436, PMID: , 10.1016/j.envres.2021.112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oppenheimer AV, Bellinger DC, Coull BA, Weisskopf MG, Korrick SA. 2021. Prenatal exposure to chemical mixtures and cognitive flexibility among adolescents. Toxics 9(12):329, PMID: , 10.3390/toxics9120329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caldwell BM, Bradley RH. 2003. HOME Inventory Administration Manual: Comprehensive Edition. Little Rock, AR. University of Arkansas for Medical Sciences and University of Arkansas at Little Rock. [Google Scholar]
- 52.Bradley RH, Corwyn RF, Caldwell BM, Whiteside-Mansell L, Wasserman GA, Mink IT. 2000. Measuring the home environments of children in early adolescence. J Res Adolesc 10(3):247–288, 10.1207/SJRA1003_1. [DOI] [Google Scholar]
- 53.Hosmer DW, Lemeshow S, Sturdivant RX. 2013. Applied Logistic Regression. 3rd ed. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 54.Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: . [PubMed] [Google Scholar]
- 55.Zou G. 2004. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706, PMID: , 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 56.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A Quantile-Based g-Computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004, PMID: , 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole SR, Hernán MA. 2008. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168(6):656–664, PMID: , 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hernán MA, Hernández-Díaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15(5):615–625, PMID: , 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 59.Choi AL, Cordier S, Weihe P, Grandjean P. 2008. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol 38(10):877–893, PMID: , 10.1080/10408440802273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vieira VM, Levy JI, Fabian MP, Korrick S. 2021. Assessing the relation of chemical and non-chemical stressors with risk-taking related behavior and adaptive individual attributes among adolescents living near the New Bedford Harbor Superfund site. Environ Int 146:106199, PMID: , 10.1016/j.envint.2020.106199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuben A, Schaefer JD, Moffitt TE, Broadbent J, Harrington H, Houts RM, et al. 2019. Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry 76(4):418–425, PMID: , 10.1001/jamapsychiatry.2018.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. 2009. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry 66(12):1313–1319, PMID: , 10.1001/archgenpsychiatry.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.U.S. Centers for Disease Control and Prevention. 2022. Childhood Lead Poisoning Prevention: Blood Lead Reference Value. https://www.cdc.gov/nceh/lead/data/blood-lead-reference-value.htm [accessed 18 November 2022].
- 64.Bellinger DC. 2008. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr 20(2):172–177, PMID: , 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- 65.Hicken MT, Gee GC, Connell C, Snow RC, Morenoff J, Hu H. 2013. Black-white blood pressure disparities: depressive symptoms and differential vulnerability to blood lead. Environ Health Perspect 121(2):205–209, PMID: , 10.1289/ehp.1104517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yitshak-Sade M, Lane KJ, Fabian MP, Kloog I, Hart JE, Davis B, et al. 2020. Race or racial segregation? Modification of the PM2.5 and cardiovascular mortality association. PLoS One 15(7):e0236479, PMID: , 10.1371/journal.pone.0236479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams DR, Yan Y, Jackson JS, Anderson NB. 1997. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 2(3):335–351, PMID: , 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 68.Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, et al. 2003. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect 111(1):65–70, PMID: , 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollack MH. 2005. Comorbid anxiety and depression. J Clin Psychiatry 66(suppl 8):22–29, PMID: . [PubMed] [Google Scholar]
- 70.Ahmed AT, Frye MA, Rush AJ, Biernacka JM, Craighead WE, McDonald WM, et al. 2018. Mapping depression rating scale phenotypes onto research domain criteria (RDoC) to inform biological research in mood disorders. J Affect Disord 238:1–7, PMID: , 10.1016/j.jad.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.National Institute of Mental Health. Definitions of the RDoC Domains and Constructs. https://www.nimh.nih.gov/research/research-funded-by-nimh/rdoc/definitions-of-the-rdoc-domains-and-constructs [accessed 29 August 2021].
- 72.Unternaehrer E, Bolten M, Nast I, Staehli S, Meyer AH, Dempster E, et al. 2016. Maternal adversities during pregnancy and cord blood oxytocin receptor (OXTR) DNA methylation. Soc Cogn Affect Neurosci 11(9):1460–1470, PMID: , 10.1093/scan/nsw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conners CK. 1997. Conners’ Rating Scales-Revised Technical Manual. Multi-Health Systems. North Tonawanda, NY. [Google Scholar]
- 74.Reynolds CR, Kamphaus RW. 2004. Behavior Assessment System for Children (BASC-2). 2nd ed. Circle Pines, MN: American Guidance Service. [Google Scholar]
- 75.Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M, et al. 2020. Brain manganese and the balance between essential roles and neurotoxicity. J Biol Chem 295(19):6312–6329, PMID: , 10.1074/jbc.REV119.009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wittchen HU. 2002. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety 16(4):162–171, PMID: , 10.1002/da.10065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


