Abstract
Background
Recent clinical data suggest that treatment with terlipressin (TP) may be more advantageous for septic shock than catecholamines. However, it is unknown whether TP would be effective for acute respiratory distress syndrome (ARDS) patients with shock.
Objectives
The aim of this study was to compare the impact of TP vs. dopamine on hemodynamic variables and vascular endothelial growth factor (VEGF) in ARDS patients with shock.
Methods
We studied 32 ARDS patients with shock despite fluid loading, who were randomized to receive TP (16 patients) or dopamine (16 patients). TP was administered as a continuous intravenous dose of 1.3 μg/kg/h and dopamine was administered in doses up to 20 μg/kg/min to maintain a mean arterial pressure of 70 ± 5 mm Hg for 48 h. Hemodynamic changes, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2), and VEGF were recorded prospectively.
Results
There was a significant correlation between the plasma VEGF level and the lung injury score at baseline (r = 0.387, p < 0.01). VEGF concentrations significantly decreased from baseline levels in the TP group (p < 0.05) at 48 h; there was no difference in the dopamine group (p > 0.05) at 48 h vs. baseline. There was no significant difference in the tumor necrosis factor-α concentration between the groups.
Conclusions
TP treatment has the potential to inhibit VEGF and improve oxygenation in patients with shock in the early stage of ARDS.
Keywords: acute respiratory distress syndrome, vascular endothelial growth factor, oxygenation, terlipressin, dopamine
Introduction
Sepsis is an important risk factor for acute respiratory distress syndrome (ARDS). ARDS is characterized by increased pulmonary permeability edema, the cause of which is still incompletely understood. Shock and ARDS frequently co-exist, and the mortality rate of patients with ARDS and shock is very high.
Vascular endothelial growth factor (VEGF) has been identified as a vascular permeability factor (1). VEGF regulates endothelial cell differentiation, angiogenesis, and the maintenance of existing vessels. The role of VEGF in the pathogenesis of ARDS is controversial (1). Several authors have suggested a pathologic role for VEGF in the lung, whereas others claim a protective role for VEGF. Dopamine is generally used to treat shock 2, 3. Terlipressin (TP) is a synthetic long-acting analogue of vasopressin with comparable pharmacodynamic, but different pharmacokinetic, properties. Terlipressin is recommended to treat hypotension in patients with catecholamine-resistant septic shock 4, 5. Recently, several studies have demonstrated that TP can improve oxygenation. Kalambokis et al. found that TP significantly reduced the magnitude of the shunt in normoxemic cirrhotic patients (6). Asfar et al. confirmed that titrated continuous infusion of TP not only reversed the hypotensive hyperdynamic circulation in porcine endotoxemia, but also decreased global oxygen consumption without compromising splanchnic metabolism and organ functions (7). However, little information about the effect of TP and dopamine on VEGF is available.
Materials and Methods
Patients
After approval by the local Institutional Ethics Committee, the study was conducted in an 18-bed multidisciplinary intensive care unit (ICU). Informed consent was obtained from each participant or an immediate family member at the time of admission. Enrollment of patients commenced in January 2007 and ended in December 2009. We enrolled patients who fulfilled the criteria of having ARDS and septic shock. ARDS was defined as the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FiO2) <200, the presence of bilateral pulmonary infiltrates on a chest radiograph, and no clinical evidence of left atrial hypertension (8). Septic shock was defined as sepsis-induced hypotension persisting despite adequate fluid resuscitation (2). Patients were studied within 48 h of admission to the ICU. Exclusion criteria were age <18 years, catecholamine therapy before randomization, severe cardiovascular disorder, chronic renal failure, severe liver dysfunction (Child-Pugh grade C), aneurysm or arteriovenous malformation, pregnancy, and present or suspected acute mesenteric ischemia. All patients were sedated with midazolam and received mechanical ventilation using a volume-controlled mode. A lung-protective strategy for mechanical ventilation was utilized during this study. The target tidal volume was to 6–8 mL/kg predicted body weight. Fraction of inspired oxygen (FiO2), positive end expiratory pressure, and respiratory rate were set to achieve an oxygen saturation of 88–92%.
Data Collection and Measurements
Disease severity was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II, Simplified Acute Physiology Score (SAPS) II, and the Murray Lung Injury Score (LIS), a composite variable that includes components of oxygenation, compliance, positive end-expiratory pressure, and the appearance of the chest radiograph 9, 10.
Blood pressure was measured via a radial artery catheter. Central venous pressure (CVP) was determined via an internal carotid vein catheter. Heart rate was analyzed from a continuous recording of the electrocardiogram with monitoring of the ST segments. Arterial blood samples were drawn for blood gas analysis. VEGF and tumor necrosis factor (TNF)-α concentration were determined using a human VEGF and TNF-α immunoassay kit (R&D Systems, Minneapolis, MN). According to the data sheet, the minimum detectable concentration of VEGF and TNF-α was <5.0 pg/mL. Outcomes measures included 28-day mortality, length of hospital and ICU stay, pressor-free days, and days of mechanical ventilation until day 28.
Study Design
Patients were randomized to one of two study groups using a computer-generated random number table. Patients assigned to the TP group received a continuous TP infusion of 1.3 μg/kg/h. The dopamine group received a dopamine infusion up to 20 μg/kg/min. Doses of dopamine could be increased or decreased by 2 μg per kilogram per minute, as needed. In each group, open-label dobutamine in doses up to 20 μg/min/kg was additionally infused, if the goal mean arterial pressure (MAP) of 70 ± 5 mm Hg was not achieved with study drug infusion alone. Fluid challenge was performed to maintain central venous pressure at 8–12 mm Hg during the 48-h intervention period. Packed red blood cells were transfused when hemoglobin concentrations decreased to <8 g/dL. Hemodynamic measurements, laboratory variables, and blood gases were determined at baseline and 48 h after randomization. Plasma cytokine concentrations were measured at baseline and after 48 h. In patients surviving the 48-h intervention period, the study drug infusion was terminated, and open-label dopamine was titrated to maintain the MAP at 70 ± 5 mm Hg.
Statistical Analysis
Data are expressed as the mean ± SD. SPSS 13.0 software (SPSS Inc., Chicago, IL) was used for statistical analysis. Differences within and among groups were analyzed using a two-way analysis of variance (ANOVA) for repeated measurements with group and time as factors. Time-independent variables were compared with one-way ANOVA. In the case of significant group differences over time, appropriate post hoc comparisons (Student-Newman-Keuls) were performed. The relationship between the VEGF levels and lung injury scores were analyzed using the Spearman rank-order correlation test. Statistical significance was defined as a p < 0.05.
Results
Patients
Of the 72 patients with ARDS and shock who met the inclusion criteria of the study, 40 were excluded due to prior catecholamine therapy (n = 27), severe cardiovascular disorders (n = 6), and severe liver or renal dysfunction (n = 7). Thirty-two consecutive patients were enrolled in the study and randomized equally to one of the study groups (n = 16 per group). None of the enrolled patients died during the study period.
Demographic Data
Baseline characteristics including age, gender, body weight, origin of septic shock, APACHE II, LIS, SAPS II, and hemodynamic variables are presented in Table 1 . There were no significant differences in the baseline characteristics between the groups.
Table 1.
Baseline Characteristics of the Study Patients
| TP Group (n = 16) | Dopamine Group (n = 16) | |
|---|---|---|
| Mean age (years) | 56.6 ± 16.4 | 52.2 ± 14.0 |
| Gender (M/F) | 10/6 | 8/8 |
| Body weight (kg) | 62.4 ± 2.1 | 58.6 ± 3.6 |
| Etiology | Pneumonia (n = 8) | Pneumonia (n = 9) |
| Pancreatitis (n = 4) | Pancreatitis (n = 3) | |
| Severe trauma (n = 2) | Severe trauma (n = 1) | |
| Aspiration of gastric contents or irritant (n = 2) | Aspiration of gastric contents or irritant (n = 1) | |
| Major surgery (n = 2) | ||
| Blood culture positive | 5 | 4 |
| APACHE II | 19.3 ± 9.6 | 17.6 ± 5.3 |
| LIS | 2.7 ± 0.4 | 3.0 ± 0.5 |
| SAPS | 42.3 ± 16.8 | 48.73 ± 17.2 |
TP = terlipressin; APACHE = Acute Physiology and Chronic Health Evaluation; LIS = Lung Injury Score; SAPS = Simplified Acute Physiology Score.
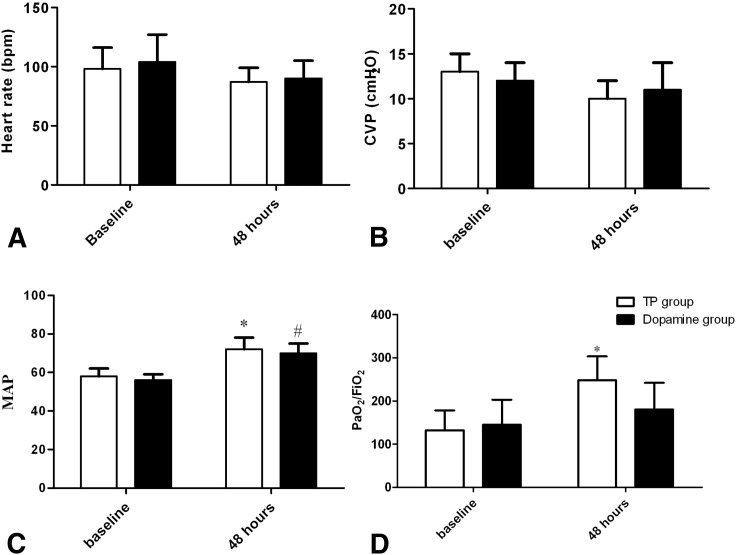
Hemodynamic and Oxygenation Variables
Hemodynamic and oxygenation variables are summarized in Figure 1 . There was no significant overall group difference in heart rate or CVP. Both TP and dopamine treatment elevated the MAP from baseline in ARDS patients with shock (p < 0.05). The PaO2/FiO2 significantly increased from baseline in the TP group at 48 h (p < 0.05). Although the PaO2/FiO2 at 48 h was higher in the TP group than that in the dopamine group, the difference did not reach statistical significance (p > 0.05).
Figure 1.
Hemodynamic and oxygenation variables in the terlipressin (TP) group and dopamine group. (A) Heart rate (HR) variable; (B) central venous pressure (CVP) variable; (C) mean arterial pressure (MAP) variable; (D) ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) variable; *Compared with baseline of TP group, p < 0.05; #Compared with baseline of dopamine group, p < 0.05.
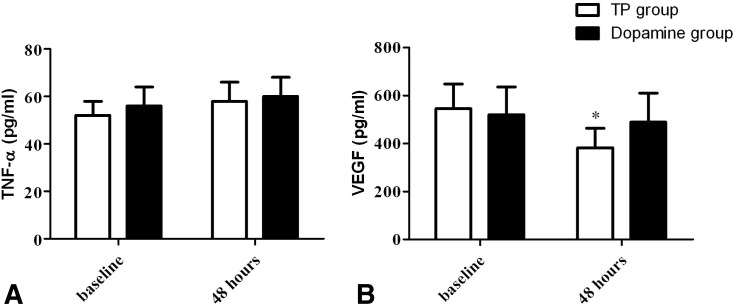
VEGF and TNF-α Value
The mean VEGF level in all groups was 486.6 ± 107.2 pg/mL. There was a significant correlation between the plasma VEGF level and the LIS at baseline (p = 0.387, p < 0.01). The VEGF levels in plasma from the TP group were significantly lower than baseline (p < 0.05), and compared to the dopamine group (p < 0.05). However, there was no difference from baseline in the dopamine group at 48 h (p > 0.05), and there was no significant difference in the TNF-α concentration between the groups (Figure 2 ).
Figure 2.
Levels of vascular endothelial growth factor (VEGF) in plasma from the terlipressin (TP) group and dopamine group. (A) Plasma level of VEGF; (B) plasma level of tumor necrosis factor (TNF)-α; *Compared with baseline of dopamine group, p < 0.05.
Outcome Measures
There were no significant differences between the two groups in the length of ICU stay (p = 0.7), the length of hospital stay (p = 0.74), the number of pressor-free days until day 28 (p = 0.29), the number of ventilation days until day 28 (p = 0.65), or the mortality until day 28 (p = 0.72; Table 2 ).
Table 2.
Comparison of Outcomes between Terlipressin Group and Dopamine Group
| Terlipressin | Dopamine | p Value | |
|---|---|---|---|
| Pressor-free days until day 28 | 13.0 ± 3.4 | 10.7 ± 3.8 | 0.29 |
| Length of ICU stay, days | 5.5 ± 3.5 | 6.4 ± 3.7 | 0.70 |
| Length of hospital stay, days | 18.1 ± 6.4 | 16.3 ± 8.5 | 0.74 |
| Days of ventilation | 4.3 ± 2.5 | 5.3 ± 3.6 | 0.65 |
| Mortality until day 28 | 44% | 50% | 0.72 |
ICU = intensive care unit.
Discussion
Terlipressin is involved in the regulation of vascular tone by inducing vascular smooth muscle cell contraction, and is used to treat hypotension in patients with catecholamine-resistant septic shock 2, 11. Although the main catecholamine therapy for septic shock is norepinephrine alone or in combination, there is increasing evidence that continuous infusion of low-dose TP is effective as a first-line vasopressor agent in reversing sepsis-induced arterial hypotension, is safe, and has similar survival outcomes 5, 12, 13, 14. Almost all studies involving TP have focused on the cardiovascular actions of TP. Whether or not TP can also influence vascular permeability, in particular in VEGF, is unknown.
Despite the known role of VEGF in a variety of conditions characterized by vascular leakage, the effect of VEGF in patients with ARDS is controversial. Thickett et al. reported increased levels of plasma VEGF in patients with ARDS compared to those at risk, suggesting a potential role for VEGF in the development of pulmonary edema (15). However, several studies have suggested a protective role for VEGF. Corne et al. showed that interleukin 13 protects the mouse lung from hyperoxic lung injury through VEGF up-regulation (16). Thickett et al. showed that increasing intrapulmonary VEGF levels are associated with resolution of lung injury in ARDS patients in a manner opposite to that of plasma (1). To date, most studies support the notion that VEGF may contribute to the development of pulmonary edema in the early stages of ARDS, and may subsequently participate in the recovery process of the damaged endothelial–epithelial barrier 1, 15, 17, 18.
In the present study, VEGF levels in plasma were correlated with lung injury score. These results suggest that down-regulation of VEGF in the plasma of patients with lung injury and ARDS may be associated with a more favorable outcome (19). The current study has demonstrated that VEGF levels at 48 h after TP treatment are significantly lower than in the dopamine group. The PaO2/FiO2 was higher in ARDS patients with shock after TP treatment than in those after dopamine treatment. There was no significant difference between the TP group and dopamine group in TNF-α. We suggest that TP treatment with down-regulation of VEGF may reduce the extensive local and general tissue edema and improve oxygenation.
However, it is important to note that TP has the potential to reduce the cardiac index and excessively increase vascular resistance in a dose-dependent manner 19, 20. The adverse effects resulting from intermittent high-dose TP bolus injections are probably linked to overshooting increases in vascular resistance, and thus reduced cardiac output and global oxygen supply (21). To overcome the adverse effects associated with TP bolus infusion, the drug may be administered as a low-dose continuous infusion 19, 22. In this study, we administered TP at 1.3 ug/kg/h, similar to Morelli et al. (5). The TP effect on pulmonary function has not been thoroughly described in the literature. Matok et al. recently reported that TP caused a statistically insignificant decrease in the oxygenation index in septic shock patients who survived (23). They suggested that improvement in respiratory index values may be attributed to the vasodilatory effect of TP on pulmonary blood vessels via V1 receptors. In the current study, results showed that a low-dose continuous TP infusion increased the MAP and PaO2/FiO2 ratio. Clearly, additional trials are needed to determine whether or not an early low-dose continuous TP infusion improves the overall outcome of patients with ARDS and shock.
Limitations
This study may have looked at an atypical group of people and situations, and only some of the possible variables. Thus, it is unknown whether the findings can be generalized to other people or situations.
Conclusion
In conclusion, we demonstrated that the VEGF concentration in plasma was significantly lower in ARDS patients with shock who were treated with TP than in those treated with dopamine. Although the underlying mechanism remains to be determined, our findings suggest that continuous TP infusion has the potential to decrease VEGF release and improve oxygenation in patients with ARDS and shock.
Article Summary
1. Why is this topic important?
Recent clinical data suggest that treatment with terlipressin (TP) may be more effective for septic shock than catecholamines. However, it has not been determined whether TP is effective for acute respiratory distress syndrome (ARDS) patients with shock.
2. What does this study attempt to show?
The aim of the present study was to compare the impact of TP and of dopamine on hemodynamic variables and vascular endothelial growth factor (VEGF) in ARDS patients with shock.
3. What are the key findings?
TP treatment has the potential to inhibit VEGF and improve oxygenation in patients with ARDS and shock.
4. How is patient care impacted?
Based on the combined data from all trials, the benefits of TP treatment for acute respiratory distress syndrome outweigh dopamine. Discussing TP usage with patients in the Emergency Department will enable Emergency Physicians to better evaluate possible drug effects, educate and learn from our patients, and refer them when appropriate.
Acknowledgments
We thank Dr. Bin Wang for help in collecting and analyzing the patient samples.
References
- 1.Thickett D.R., Armstrong L., Millar A.B. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care. 2002;166:1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger R.P., Levy M.M., Carlet J.M., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2008;2008(34):17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Backer D., Biston P., Devriendt J., et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 4.Delmas A., Leone M., Rousseau S., Albanese J., Martin C. Clinical review: vasopressin and terlipressin in septic shock patients. Crit Care. 2005;9:212–222. doi: 10.1186/cc2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morelli A., Ertmer C., Rehberg S., et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): a randomized, controlled pilot study. Crit Care. 2009;13:R130. doi: 10.1186/cc7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalambokis G., Baltayiannis G., Tsiouris S., et al. Scintigraphic evaluation of intrapulmonary shunt in normoxemic cirrhotic patients and effects of terlipressin. Hepatol Res. 2010;40:1015–1021. doi: 10.1111/j.1872-034X.2010.00715.x. [DOI] [PubMed] [Google Scholar]
- 7.Asfar P., Hauser B., Ivanyi Z., et al. Low-dose terlipressin during long-term hyperdynamic porcine endotoxemia: effects on hepatosplanchnic perfusion, oxygen exchange, and metabolism. Crit Care Med. 2005;33:373–380. doi: 10.1097/01.ccm.0000152253.45901.fb. [DOI] [PubMed] [Google Scholar]
- 8.Bernard G.R., Artigas A., Brigham K.L., et al. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 9.Perkins G.D., McAuley D.F., Thickett D.R., Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 10.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 11.Ryckwaert F., Virsolvy A., Fort A., et al. Terlipressin, a provasopressin drug exhibits direct vasoconstrictor properties: consequences on heart perfusion and performance. Crit Care Med. 2009;37:876–881. doi: 10.1097/CCM.0b013e31819b8199. [DOI] [PubMed] [Google Scholar]
- 12.Morelli A., Ertmer C., Westphal M. “Terlipressin in the treatment of septic shock: the earlier the better”? Best Pract Res Clin Anaesthesiol. 2008;22:317–321. doi: 10.1016/j.bpa.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Rehberg S., Ertmer C., Kohler G., et al. Role of arginine vasopressin and terlipressin as first-line vasopressor agents in fulminant ovine septic shock. Intensive Care Med. 2009;35:1286–1296. doi: 10.1007/s00134-009-1470-z. [DOI] [PubMed] [Google Scholar]
- 14.Morelli A., Ertmer C., Pietropaoli P., Westphal M. Terlipressin: a promising vasoactive agent in hemodynamic support of septic shock. Expert Opin Pharmacother. 2009;10:2569–2575. doi: 10.1517/14656560903257808. [DOI] [PubMed] [Google Scholar]
- 15.Thickett D.R., Armstrong L., Christie S.J., Millar A.B. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 16.Corne J., Chupp G., Lee C.G., et al. IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest. 2000;106:783–791. doi: 10.1172/JCI9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosmidou I., Karmpaliotis D., Kirtane A.J., Barron H.V., Gibson C.M. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis. 2008;25:259–264. doi: 10.1007/s11239-007-0062-4. [DOI] [PubMed] [Google Scholar]
- 18.Maitre B., Boussat S., Jean D., et al. Vascular endothelial growth factor synthesis in the acute phase of experimental and clinical lung injury. Eur Respir J. 2001;18:100–106. doi: 10.1183/09031936.01.00074701. [DOI] [PubMed] [Google Scholar]
- 19.Westphal M., Stubbe H., Sielenkamper A.W., et al. Terlipressin dose response in healthy and endotoxemic sheep: impact on cardiopulmonary performance and global oxygen transport. Intensive Care Med. 2003;29:301–308. doi: 10.1007/s00134-002-1546-5. [DOI] [PubMed] [Google Scholar]
- 20.Morelli A., Ertmer C., Lange M., et al. Effects of short-term simultaneous infusion of dobutamine and terlipressin in patients with septic shock: the DOBUPRESS study. Br J Anaesth. 2008;100:494–503. doi: 10.1093/bja/aen017. [DOI] [PubMed] [Google Scholar]
- 21.Albanese J., Leone M., Delmas A., Martin C. Terlipressin or norepinephrine in hyperdynamic septic shock: a prospective, randomized study. Crit Care Med. 2005;33:1897–1902. doi: 10.1097/01.ccm.0000178182.37639.d6. [DOI] [PubMed] [Google Scholar]
- 22.Lange M., Morelli A., Ertmer C., et al. Continuous versus bolus infusion of terlipressin in ovine endotoxemia. Shock. 2007;28:623–629. doi: 10.1097/shk.0b013e318050c78d. [DOI] [PubMed] [Google Scholar]
- 23.Matok I., Vard A., Efrati O., et al. Terlipressin as rescue therapy for intractable hypotension due to septic shock in children. Shock. 2005;23:305–310. doi: 10.1097/01.shk.0000158115.69704.11. [DOI] [PubMed] [Google Scholar]