Summary
Facial sutures contribute significantly to postnatal facial development, but their potential role in craniofacial pathology is understudied. Since interest in their development and physiology peaked in the mid-20th century, facial sutures have not garnered nearly the same clinical research interest as calvarial sutures or cranial base endochondral articulations. In addition to reinforcing the complex structure of the facial skeleton, facial sutures absorb mechanical stress and generally remain patent into adolescence as they mediate growth and refine the shape of facial bones. However, premature closure of these sites of postnatal osteogenesis leads to disrupted growth vectors and consequent dysmorphologies. While pathology in individual sutures results in isolated facial deformities, we posit that generalized pathology across multiple may be involved in complex craniofacial conditions such as syndromic craniosynostosis. In this work, we comprehensively review 27 key facial sutures, including physiologic maturation and closure, contributions to postnatal facial development, and clinical consequences of premature closure.
Introduction
Facial sutures are overshadowed by their calvarial counterparts in the modern craniofacial literature. As craniosynostosis is the principal context in which sutures are discussed, it is unsurprising that those of the calvarium have dominated the clinical and research spheres. Facial sutures, however, were studied on par with those of the calvarium during the rise of craniofacial biology in the 1950s-60s. Scott, Enlow, Sarnat, and others were deeply interested in both their physiology and the craniofacial defects secondary to their disruption (1-3).
Craniosynostosis can occur in isolation or as part of a syndrome involving a spectrum of physical manifestations, including profound facial anomalies (4-6). While the etiology of these facial deformities is debated, the current leading theory suggests they are sequelae of aberrant cranial base development (5,7). However, for several decades craniofacial experts and basic scientists have endorsed the alternative hypothesis that premature facial suture synostosis plays a primary role in the development of these deformities (8-13).
To further explore this hypothesis, we sought to comprehensively review the existing literature on facial sutures. This review reveals that beyond their general developmental and physiologic roles, individual human facial sutures have not been rigorously or systematically studied since the mid-20th century. Our current understanding of the maturation course of individual sutures is derived from a select few forensic anthropology and human cadaveric studies, with limited contribution from clinical imaging studies. We herein synthesize all knowledge to-date on the physiology and pathophysiology of 27 prominent facial sutures. Particular attention is paid to their posited contributions to facial deformity in both craniosynostotic and non-craniosynostotic contexts.
Development
The neurocranium (bones encasing the brain) is derived from a combination of head mesoderm and neural crest, while the viscerocranium (bones forming the face) is derived exclusively from neural crest mesenchyme (14,15).
Most craniofacial bony interfaces are true sutures, fibrous joints consisting mainly of type I collagen (16). There are notable histologic distinctions between facial and calvarial sutures early in development (17). Several cranial base articulations are synchondroses (cartilaginous joints with predominantly type II collagen) but often referenced inaccurately as sutures.
Facial sutures are patent at birth and progressively ossify, at variable rates and to variable extents. Generally, the ossification of facial sutures differs from that of calvarial sutures. While calvarial sutures achieve significant closure – involving dense interdigitations with a remnant visible suture line – by age 20-30, most of their facial counterparts remain patent through late adulthood (18-20). Some facial sutures do not form appreciable interdigitations until the 7th to 8th decades of life (20).
Physiologic Roles
Throughout life, facial sutures provide structural support and mechanical stress absorption for deformational forces induced during motion such as in mastication (21). While patent, facial sutures also adjoin the periostea of adjacent bones and serve as centers for osteogenesis (20). Their dual functionality as sites of intramembranous osteogenesis and absorbers of mechanical stress allows them to play an essential transducing role in directing facial development (20).
Recognizing the potential impact of suture pathology on facial development requires understanding sutural growth dynamics, a topic heavily studied in the 1950s-60s. Scott documented the permissive growth theory in 1954, hypothesizing that facial bones grow at sutures because underlying organs and cartilage – namely, the eyes and nasal septum – increase in size and physically separate adjacent bones, allowing new bone to be deposited at the junction (1). He posited that sutural growth ceases and closure commences when the underlying structures stop growing. Enlow also endorsed this theory, suggesting that in the same way dura mater and brain tissue provide stimuli for new bone formation at expanding calvarial sutures, facial cartilage such as the nasal septum provides signals for bone deposition at their overlying facial sutures (3).
These experts also investigated how postnatal suture growth relates to macroscopic development of the facial skeleton. Scott described three “suture systems”, each mediating facial growth in a particular direction (22). Sarnat characterized how concerted growth at the frontomaxillary, zygomaticomaxillary, and pterygomaxillary sutures translocates the maxilla outward (2). Enlow found that inferolateral displacement of the zygoma requires growth at the frontozygomatic and zygomaticomaxillary sutures (3). Similarly, Björk demonstrated the necessity of frontozygomatic and intermaxillary suture growth in achieving proper maxillary expansion (23,24).
By 1963, sutural growth was widely considered a main catalyst of postnatal facial growth, along with endochondral cranial base ossification, appositional growth at facial bone surfaces, and nasal septal growth (2). In contrast to reactive, intramembranous sutural growth, endochondral osteogenesis at the cranial base synchondroses is active and prolific through early adolescence (25, 26).
Although facial sutures remain patent for a considerable duration, bony growth does not continue indefinitely. Certain aspects of facial growth do persist throughout life, as evidenced by the continuous increase in measurements like the nasion to anterior nasal spine distance and bizygomatic width; however, midfacial growth is typically complete by the mid-teen years, when endochondral growth momentum decelerates with the ossification of cranial base synchondroses (27,28). While additional research is required to quantify the correlation between facial suture patency with sutural growth velocity, it can be extrapolated from calvarial craniosynostosis that early gross ossification appreciated on imaging signifies the restriction of subsequent growth at that suture site.
Anatomy and Terminology
The neurocranium, totaling eight bones, encases the brain and is further divided into the calvarium and cranial base. The viscerocranium, or facial skeleton, totals 14 bones.
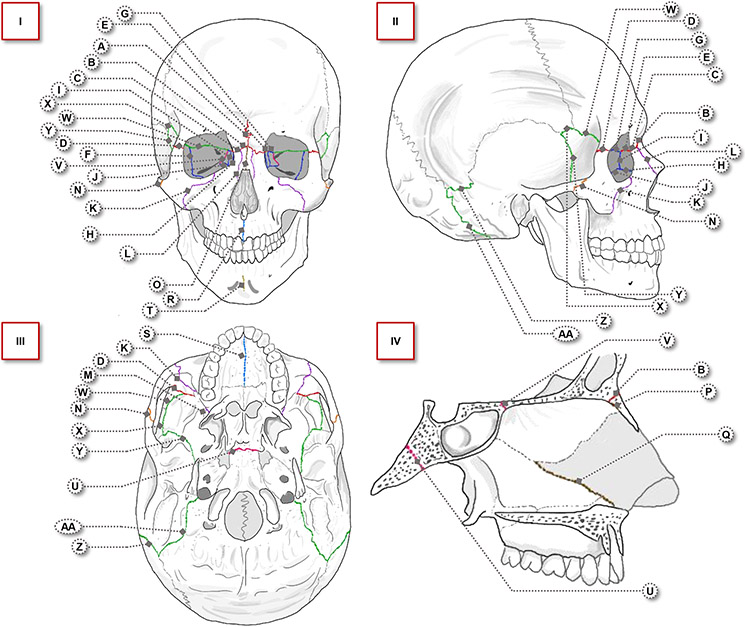
The “face” is a colloquial term lacking defined anatomic borders. “Facial sutures” are similarly vaguely defined, referring to various articulations between craniofacial bones. Given these intricacies, we take an anatomically oriented approach to systematically cover the bony articulations supporting the face. We delineate nine facial regions as our framework to discuss 27 articulations (Figure 1, Table 1). For conciseness, only sutures with the most significant clinical findings are discussed in the body of this review (all others can be found in Table 1).
Figure 1.
Four views of the craniofacial skeleton, with 27 labeled facial sutures. Suture labels correspond to those in Table 1. Sutures are color-coded by facial region (frontal: red, orbital: dark blue, maxillary: purple, lateral: orange, nasal: brown, palatal: light blue, mandibular: gold, basal: magenta, circummeatal: green). I) Anterior view, II) lateral view, III) inferior view, IV) midline sagittal view, with focus on the basal and nasal regions.
Table 1.
Detailed review of 27 facial sutures, by region. Abbreviations of the articulations correspond to the labels in Figure 1. Includes timing of closure, notable growth functions, and all documented cases of premature closure in the literature. Abbreviations are as follows: M = metopic, FN = frontonasal, FM = frontomaxillary, FZ = frontozygomatic, FL = frontolacrimal, SZ = sphenozygomatic, FE = frontoethmoidal, LM = lacrimomaxillary, EL = ethmoidolacrimal, EM = ethmoidomaxillary, ZM = zygomaticomaxillary, NM = nasomaxillary, PMx = pterygomaxillary, ZT = zygomaticotemporal, IN = internasal, NE = nasoethmoidal, SV = septovomerine, IMx = intermaxillary, MP = midpalatal, IM = intermandibular, SO = sphenooccipital, SE = sphenoethmoidal, FS = frontosphenoidal, SP = sphenoparietal, SS = sphenosquamosal, PM = parietomastoid, OM = occipitomastoid, CT = computed tomography.
| Clinical Consequences of Premature Closure in Humans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of articulation | Timing of closure | In humans | |||||||||
| Region | Articulation | True Suture |
Synchondrosis | Symphysis | In humans | In Animal (if limited human studies) |
Notable functions of suture growth |
Cases of isolated synostosis |
Involvement in non-syndromic craniosynostosis |
Involvement in syndromic craniosynostosis |
Animal models of isolated synostosis |
| Frontal | M |
|
CT: Starts at 3 months, completes by 9 months | Transverse frontal bone growth, across supraorbital ridge | Non-syndromic trigonocephaly (raised midline ridge, hypotelorism, possible neuropsychologic disturbances) | ||||||
| FN |
|
Cadaveric: Starts in 40s, completes in 50s | Protrusion of the nasal complex |
|
|
||||||
| FM |
|
Cadaveric: Does not start until 7th-8th decade | Protrusion of the maxilla |
|
|||||||
| FZ |
|
Cadaveric: Starts in 8th decade, completes in 10th | Inferolateral displacement of the zygoma | Currarino: Frontal flattening, small/round orbit, broadened/shortened lateral orbital rim |
|
||||||
| FL |
|
Prolonged patency | Ateles monkeys: Closes late in adult life | ||||||||
| Orbital | SZ |
|
Cadaveric: Starts in 5th decade, completes in 6th | Currarino: One case of synostosis concomitant with frontozygomatic synostosis. Contributes to lateral orbital wall broadening/shortening |
|
|
|||||
| FE |
|
Cadaveric: Starts in 40s, completes by early 50s |
|
|
|||||||
| LM |
|
Prolonged patency |
|
||||||||
| EL |
|
Cadaveric: Starts in mid 30s, completes by age 50 | |||||||||
| EM |
|
Cadaveric: Starts and completes in late 40s to 50s | |||||||||
| Maxillary | ZM |
|
|
|
Calandrelli et al.: Synostosedin 26% of syndromic infants (4 Crouzon, 1 Pfeiffer). Severe maxillary retrusion and reduced SNA angle. Involvement indicates higher severity facial skeletal deficit, exacerbates maxillary retrusion | Thimaporn et al.: Nasomaxillary complex asymmetry, constrained downward/ forward displacement of the maxilla/ zygoma (guinea pigs) | |||||
| NM |
|
Cadaveric: Does not begin until 60s-70s | Miri et al.: Synostosed in 62% of unilateral coronal synostosis subjects. Contributes to facial twist | ||||||||
| PMx |
|
CT: Starts in mid teens | Protrusion of maxilla | ||||||||
| Lateral | ZT |
|
Cadaveric: Significant closure does not start until 7th decade |
|
Kreiborg & Bjork: Synostosed in postmortem Crouzon skull | ||||||
| Nasal | IN |
|
Cadaveric: Starts in 20s, completes after age 30 |
|
|||||||
| NE |
|
Patent through mid-teens at least (based on critical growth roles through adolescence) |
|
||||||||
| SV |
|
Haug & Foss: Deep facial trauma can cause premature ossification of the septovomerine and consequent midface growth disruption. Also asymmetric nasal deformity, septal deviation, obstructed airway | |||||||||
| Palatal | IMx |
|
Cadaveric: Mostly closed by age 18, variable patency in 20s | Transverse maxillary growth | Transverse maxillary hypoplasia, possible severe malocclusion | ||||||
| MP |
|
CT and cadaveric: Starts in teens, completion varies considerably | Kreiborg & Bjork: Synostosed in postmortem Crouzon skull. Hypoplastic maxilla in vertical and transverse dimensions | ||||||||
| Mandibular | IM |
|
Cadaveric: Ossifies by 7-8 months, completely closed by 12 months | Wang et al.: Narrowed mandible with missing mandibular teeth (mice exposed to all-trans retinoic acid) | |||||||
| Basal | SO |
|
|
Midfacial growth, cranial base lengthening |
|
Rosenberg et al.: Severe midface retrusion (rabbit) | |||||
| SE |
|
CT: Starts at age 2, completes by age 15 | Burdi et al.: Isolated fusion in warfarin-exposed fetus. Severe exorbitism | Rogers et al.: Involved in one case of unilateral frontosphenoidal synostosis. Frontal flattening, orbital rim depression/recession |
|
||||||
| Circummeatal | FS |
|
CT: Starts at age 5, completes by age 15 | Anteroposterior lengthening of cranial base from birth to age 7 |
|
Showalter et al.: If synostosis occurs in unilateral coronal synostosis, it blunts the harlequin orbital deformity and can delay diagnosis |
|
||||
| SP |
|
CT: Starts before age 7, completes during teen | Lambert & Wineski: Can be involved in scaphocephaly with sagittal synostosis |
|
|||||||
| Circummeatal | SS |
|
CT: Starts at age 2, completes by age 6 | Sakamoto et al.: Suture growth is arrested in unilateral coronal synostosis. Shortened sphenoid greater wing and temporal bone |
|
||||||
| PM |
|
CT: Starts in 40s-50s, completes after age 70 | Jimenez et al.: Unilateral posterior plagiocephaly without lambdoid synostosis, but with parietomastoid and occipitomastoid synostosis. Anterior displacement of ear. Compensatory frontal bossing, malar protrusion |
|
|||||||
| OM |
|
CT: Partially patent through late teens |
|
||||||||
Frontal Region
The metopic suture, often considered a calvarial suture, courses down the frontal bone midline (M in Figure 1, Table 1) (29). Closure on CT imaging is detectable as early as 3 months and frequently completes before 1 year (30,31). Synostosis generates the trigonocephalic phenotype, characterized by a triangular forehead with a raised bony midline ridge, a shortened anterior cranial fossa, and hypotelorism. Severe cases have been associated with ethmoidal hypoplasia, neuropsychologic sequelae, and minor cranial base suture involvement (32, 33). While most patients with metopic craniosynostosis are non-syndromic, patients with syndromic craniosynostoses have also been reported to have metopic synostosis (34, 35).
The frontonasal sutures run transversely at the nasion (FN in Figure 1, Table 1). Closure occurs in the 5th through 6th decades in cadaveric studies (36,37). A synostotic mouse model found consequent hypertelorism, snout shortening, and midface hypoplasia, suggesting patency is required for normal early midfacial development (38). Similarly, a lagomorph model of stunted frontonasal growth demonstrated midface shortening and secondary growth restrictions at the coronal and internasal sutures (39). Clinically, frontonasal synostosis has been reported in both metopic and coronal craniosynostosis patients. Calandrelli et al. found that most of their 59 pediatric subjects with metopic synostosis actually had synostotic extension into the frontonasal suture (40). In their cohort of 7 patients with the same condition, Udayakumaran et al. reported frontonasal synostosis in all and hypothesized that it contributes to metopic suture angulation (41). Asymmetric frontonasal closure has also been associated with unilateral coronal synostosis (UCS), implicating facial suture synostosis in the characteristic facial twist of UCS patients (42).
The frontomaxillary sutures connect the frontal process of the maxilla to the inferior margin of the frontal bone (FM in Figure 1, Table 1). This suture does not start closing until the 7th decade in a cadaveric study (19). Similar to nasofrontal sutures, asymmetric synostosis of the frontomaxillary sutures is increased on the synostotic side in UCS patients (42). A CT study found a 70% prevalence of concomitant frontomaxillary synostosis in non-syndromic metopic synostosis patients and established a significant positive association between frontomaxillary synostosis and severity of the trigonocephalic phenotype (40).
The frontozygomatic is the sole suture of the lateral orbital rim (FZ in Figure 1, Table 1). Cadaveric study suggests closure occurs between the 8th and 10th decades (43). Patency enables growth and contributes to increasing bizygomatic width until age 60. Isolated synostosis mimics the fronto-orbital dysplasia of UCS with frontal flattening, small orbit, and shortened lateral orbital rim (44). Synostosis has also been found in UCS and metopic synostosis patients, and contributes to posterior malpositioning of the zygomatic bone frequently seen in the latter (45,46).
Orbital Region
The sphenozygomatic sutures contribute to both the lateral orbital wall and the anterior wall of the temporal fossa (SZ in Figure 1, Table 1) (47). Closure occurs between the 5th and 6th decades in a cadaveric study (37). Despite a lack of isolated cases, sphenozygomatic synostosis occurs concomitantly with other synostoses. Rogers et al. reported synostotic extension into the sphenozygomatic in a frontal plagiocephalic patient with unilateral frontosphenoidal synostosis (48). They thus consider frontal plagiocephaly a phenotypic spectrum with multiple synostotic etiologies, including coronal ring fusions that may extend into adjacent sutures. Genitori et al. found 7% of their metopic synostosis patients had concurrent sphenozygomatic synostosis (46). Within the radiologic literature, simultaneous sphenozygomatic and frontozygomatic synostoses were found in an infant with the phenotypic appearance of UCS in the absence of coronal suture involvement (44).
The frontoethmoidal sutures are found in the medial orbital walls (FE in Figure 1, Table 1). Closure occurs between the 5th and 6th decades (37). As basilar continuations of the coronal ring, the frontoethmoidal and frontosphenoidal are frequently closed prematurely in coronal synostosis (49). When basilar synostoses are involved, the classic forehead flattening of UCS is accompanied by a more severely shortened anterior fossa, thickened pterion, and shallowed orbits. Because of cranial base tethering, lateral canthal advancement enhances the treatment of coronal ring synostosis. Artificial coronal and cranial base sutures are created, enabling complete mobilization of the frontal bone. A study of syndromic and non-syndromic trigonocephalic subjects found a 7% prevalence of concomitant frontoethmoidal synostosis (46). In the case of severe trigonocephaly, metopic suture synostosis may extend down the face to affect the frontoethmoidal, causing hypotelorism (50). Frontoethmoidal synostosis has also been observed on CT imaging of Crouzon, Pfeiffer, and Apert subjects (34,35).
Maxillary Region
The zygomaticomaxillary suture is the longest and thickest maxillary suture (ZM in Figure 1, Table 1) (51). It is completely patent on CT until age 10-15 and remains incompletely interdigitated through the 7th decade in cadavers (19,52,53). Induced unilateral synostosis in newborn guinea pigs resulted in asymmetry of the nasomaxillary complex due to constrained anteroinferior displacement of the maxilla and zygoma (54). A CT study demonstrated that suture patency enabled greater orthodontic maxillary protraction in malocclusion patients (51). These data suggest that synostosis causes tethering of the maxilla that limits its outward growth potential. The seminal CT study of Calandrelli et al. found that while all other viscerocranial sutures were patent in their syndromic subjects, the zygomaticomaxillary was prematurely closed bilaterally in 5 (26%) of the infants (4 Crouzon and 1 Pfeiffer) (35). These infants demonstrated significant maxillary retrusion, leading the authors to interpret zygomaticomaxillary synostosis as a marker of severe facial deficit and a potential cause of maxillary retrusion.
The pterygomaxillary sutures are located where the maxilla and sphenoid are most closely approximated (PMx in Figure 1, Table 1) (55). It appears considerably interdigitated on CT by age 12, and orthodontic maxillary protraction becomes more difficult after this age (56). Synostosis during development is rare, given the neurovascular intricacy of the region. Suture disruption is performed to mobilize the maxilla during midface advancement, a mainstay procedure for correcting syndromic facial deformities (57,58). However, surgical manipulation may prematurely obliterate the suture and consequently disrupt transverse maxillary growth (58). Growth at this suture also contributes to anterior maxillary growth, which is thought to be limited in cleft patients due to the iatrogenic effects of reconstructive surgeries and therapies compounded on an intrinsic growth disturbance, resulting in higher incidences of maxillary hypoplasia (59,60).
Lateral Region
The zygomaticotemporal suture is the most lateral suture of the face (ZT in Figure 1, Table 1). It is one of the last sutures to close, with interdigitation starting in the 7th decade in cadavers (52). An isolated synostosis case presented as “progressive midfacial and orbital asymmetry, angulation of the cranial base, and nasal deviation” (61). The patient also displayed maxillary retrusion and asymmetric calvarial shortening. CT noted a thickened lateral orbital wall and zygomatic body, suggesting compensatory growth. Another report described unilateral midfacial and frontal retrusion and nose and chin deviation (62). The surgeons performed a segmental zygomaticotemporal suturectomy, hypothesizing that release would facilitate normal growth. The effectiveness of this procedure is to be determined.
Nasal Region
The internasal suture runs in the midline of the anterior face (IN in Figure 1, Table 1). Closure begins in the 20s and completes in the 30s in cadavers (63). Udayakumaran et al. found all their non-syndromic metopic synostosis subjects had concomitant synostosis of the internasal and frontonasal sutures, hypothesizing that premature nasion sutural complex synostosis leads to severe metopic angulation (41). The internasal suture was surgically released in 3 infants, with improved angulation and hypotelorism.
The nasoethmoidal and septovomerine sutures are deep nasal structures (NE and SV in Figure 1, Table 1). The timing of closure for these sutures are not well studied, but bony growth at these sutures contributes significantly to nose and airway development through the mid-teens (64,65). Disruption due to trauma or surgery can cause premature ossification, leading to downstream growth arrest, asymmetric nasal deformities, septal deviation, or airway obstruction (65,66). The effect of the deep nasal sutures on nasal growth is the rationale for delaying surgical manipulation of the septum until skeletal maturity (64,67-69).
Palatal Region
The intermaxillary and midpalatal sutures are contiguous (IMx and MP in Figure 1, Table 1). The former is mostly closed by age 18 but variably patent through the late 20s (70). Closure of the latter begins in the teens, with variable progression among individuals influenced by masticatory forces, genetic heterogeneity, and hormonal parameters (71-74). Transverse growth at the intermaxillary suture through the late teens may be the paramount determinant of adult maxillary width (20). Restricted growth across prematurely closed palatal sutures results in transverse maxillary hypoplasia, which can be effectively managed with non-surgical palatal expansion before the teenage years (75). However, the sutures are considerably interdigitated by the late teens, thus requiring surgical release for expansion (76,77).
Basal Region
We briefly mention the sphenooccipital (SOS) and sphenoethmoidal (SES) synchondroses here, as they are well studied with extensive literature on their closure timing and synostotic involvement in syndromic craniosynostosis (SO and SE in Figure 1, Table 1) (34,35,78-81). SOS ossification starts and completes in the teens, occurring earlier in females (82-90). SES closure occurs between age 2 and 15 (91). Both are major contributors to midfacial growth through adolescence, mediating anteroposterior lengthening of the cranial base and protrusion of the midface (7,46,82,92,93). Premature ossification, reported in Apert, Crouzon, Pfeiffer, and Saethre-Chotzen patients, is strongly associated with midface retrusion (34,35,78-80,93,94).
Circummeatal Region
The frontosphenoidal sutures (FSS) are the inferomedial extensions of the coronal sutures and joined at the midline by the SES (FS in Figure 1, Table 1). The coronal sutures, FSS, and midline SES form the coronal ring. Closure of the FSS on CT occurs between age 5 and 15 (91). With the SES, it facilitates anteroposterior cranial base expansion from birth to age 7 (46). Since Francel et al. first documented frontal plagiocephaly caused by an isolated FSS synostosis, there have been over 20 published cases (95-105). Puente-Espel et al. characterized the phenotype of isolated FSS synostosis as ipsilateral frontal flattening and retrusion, and inferolateral positioning of the ipsilateral orbit with compensatory medial elongation (97). Variation in orbital deformity is the key differentiator between isolated coronal and FSS synostoses (106). FSS synostosis occurs more often with UCS than as an isolated synostosis. There is also a high likelihood of FSS synostosis in syndromic craniosynostosis. Early studies identified extension of coronal synostosis to the FSS as a likely contributor to brachycephaly in Apert and Crouzon patients (107-109). Recent CT studies have documented synostosis in Pfeiffer and Saethre-Chotzen patients as well (34,35).
The sphenoparietal sutures are short sutures on the lateral skull at the pterion (SP in Figure 1, Table 1). On CT, closure starts before age 7 and is complete by the teens (110). In addition to sagittal synostosis, premature closures of the sphenoparietal and sphenosquamosal may occur in scaphocephaly (111). The sphenoparietal may also be synostosed in Apert because of the frontal bone’s shortened orbital plate (112). Synostosis results in retrusion and elevation of the supraorbital wings, a fronto-orbital defect with cutaneous manifestations like disrupted eyebrows and excessive forehead skin wrinkling. Synostosis is also observed in Crouzon, Pfeiffer, and Saethre-Chotzen patients (34,35).
Discussion
Facial sutures are an essential yet presently under-studied element of craniofacial anatomy. Aside from maintaining structural integrity and absorbing mechanical stress, they mediate fine-tuned postnatal growth for the facial skeleton (1-3,17,22,23). Early studies demonstrated that multiple growth processes required to achieve a normal adult midface necessitate precise facial sutural growth (2,3,23,24).
The focus on neurocranial articulations in the craniosynostosis literature has contributed to the current paucity of systematic studies on facial sutures. We hereby present the first comprehensive review of the numerous facial sutures of the human craniofacial skeleton with discussion of the maturation, physiology, and pathophysiology of each respective suture.
Despite the notoriety of syndromic craniosynostosis facial deformities, the pathogenesis thereof has yet to be solidly understood. The current majority opinion considers premature ossification of cranial base growth centers the primary culprit (7,78-81,113). Although cranial base tethering may play an overarching role, the severity and multifocal nature of syndromic facial deformities suggest multiple factors are at play.
Sarnat elucidated endochondral, appositional, and sutural growth as the key modalities of postnatal facial development (2). Because suture growth is inherently permissive and reactive to stimuli, it is finely controlled along defined vectors rather than rapidly expansionary (20). Therefore, while endochondral pathology could stunt cranial base lengthening and thereby explain maxillary retrusion, it may be unable to completely explain the characteristic morphologic and dimensional aberrations of individual facial bones in syndromic craniosynostosis (92). Rather, the tightly regulated nature of sutural growth may make suture synostosis a more compatible explanation for these granular abnormalities with significant macroscopic consequences. The last decade has seen an increase in reports of facial suture synostosis, both in isolated cases and in the context of craniosynostosis (35,42,103).
Our synthesis of the literature demonstrates multifaceted ways in which facial suture synostosis is detrimental to craniofacial development. Discrete patterns of suture pathology can exacerbate facial deformities in non-syndromic craniosynostosis, generate focal abnormalities in isolated synostosis cases, and disrupt downstream growth vectors. Asymmetric frontonasal and frontomaxillary synostoses augment the facial twist phenotype in UCS (42). Frontonasal, internasal, and frontoethmoidal synostoses intensify the metopic angulation and hypotelorism seen in severe, non-syndromic trigonocephaly (40,41,46). Premature closures of the midpalatal and intermaxillary sutures result in transverse maxillary deficiency by limiting lateral growth (20,75). Furthermore, frontonasal synostosis restricts growth at the coronal and internasal sutures, zygomaticomaxillary synostosis tethers the maxilla posteriorly and prevents its outward growth, and traumatic premature ossification of the nasoethmoidal and septovomerine sutures predisposes to septal deviation and airway obstruction by stunting nasal complex growth (39,51,54,65,66).
Despite these exciting insights, exploration of facial suture involvement in human syndromic craniosynostosis is still in its nascency. The recent imaging studies of Runyan et al. and Calandrelli et al. have begun to elucidate patterns of orbital and circummeatal region suture synostoses in infants with various craniosynostosis syndromes (34,35). The latter study – as the only thus far to evaluate sutures located primarily within the viscerocranium – also reported zygomaticomaxillary involvement in a small subset of patients, preliminarily suggesting a potential contributory role to midface retrusion and airway hypoplasia (35). While these studies are novel, their limitations and dearth of related research leave further questions yet to be answered. Aside from small sample sizes, Runyan et al. focused on minor calvarial and cranial base sutures with a peri-facial location and did not assess sutures located primarily within the viscerocranium, and Calandrelli et al. evaluated young infants at a single timepoint likely too early for the effects of facial suture synostosis to manifest.
The gap in the literature may be addressed with multi-institutional, in vivo imaging studies that comprehensively and longitudinally evaluate facial osteology in patients with various craniosynostosis syndromes, from birth through adolescence. While ambitious, such a study would answer key questions regarding the magnitude of facial suture synostosis as a pathology in syndromic craniosynostosis and the mechanisms by which suture pathology contributes to the development of syndromic facial deformities. By quantifying the extents of both cranial base ossification and facial suture synostosis over time and correlating these findings with the developing craniofacial phenotype, this study could test our hypothesis that endochondral and sutural pathology produce disparate aspects of facial deformity in syndromic patients. Ultimately, we hope that further research in this realm generates a more complete understanding of syndromic facial deformities and their pathoetiology, with the objective of optimizing outcomes.
Acknowledgments
This work was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award numbers R01 DE028098 and DE029234 (JCL), the Jean Perkins Foundation (JCL), and Bernard G. Sarnat Endowment for Craniofacial Biology (RRR and JCL).
Footnotes
Disclosures
All authors have no financial interests including products, devices, or drugs associated with this manuscript. There are no commercial associations that might pose or create a conflict of interest with information presented in this submitted manuscript such as consultancies, stock ownership, or patent licensing arrangements. All sources of funds supporting the completion of this manuscript are under the auspices of the University of California Los Angeles.
References
- 1.Scott JH. The growth of the human face. Proc R Soc Med. 1954;47:91–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Sarnat B. Postnatal growth of the upper face: some experimental considerations. Angle Orthod. 1963;33(3):139–161. [Google Scholar]
- 3.Enlow DH, Hunter WS. A differential analysis of sutural and remodeling growth in the human face. Am J Orthod. 1966;52:823–830. [DOI] [PubMed] [Google Scholar]
- 4.Ciurea AV, Toader C. Genetics of craniosynostosis: review of the literature. J Med Life. 2009;2:5–17. [PMC free article] [PubMed] [Google Scholar]
- 5.Katzen JT, McCarthy JG. Syndromes involving craniosynostosis and midface hypoplasia. Otolaryngol Clin North Am. 2000;33:1257–1284. [DOI] [PubMed] [Google Scholar]
- 6.Derderian C, Seaward J. Syndromic craniosynostosis. Semin Plast Surg. 2012;26:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath J, Gerety PA, Derderian CA, et al. Differential closure of the spheno-occipital synchondrosis in syndromic craniosynostosis. Plast Reconstr Surg. 2012;130:681e–689e. [DOI] [PubMed] [Google Scholar]
- 8.Purushothaman R, Cox TC, Maga AM, Cunningham ML. Facial suture synostosis of newborn Fgfr1(P250R/+) and Fgfr2(S252W/+) mouse models of Pfeiffer and Apert syndromes. Birt Defects Res A Clin Mol Teratol. 2011;91:603–609. [DOI] [PubMed] [Google Scholar]
- 9.Motch Perrine SM, Cole TM, Martínez-Abadías N, Aldridge K, Jabs EW, Richtsmeier JT. Craniofacial divergence by distinct prenatal growth patterns in Fgfr2 mutant mice. BMC Dev Biol. 2014;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Abadías N, Percival C, Aldridge K, et al. Beyond the closed suture in apert syndrome mouse models: evidence of primary effects of FGFR2 signaling on facial shape at birth. Dev Dyn Off Publ Am Assoc Anat. 2010; 239:3058–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreiborg S, Björk A. Description of a dry skull with Crouzon syndrome. Scand J Plast Reconstr Surg. 1982;16:245–253. [DOI] [PubMed] [Google Scholar]
- 12.Kreiborg S Craniofacial growth in plagiocephaly and Crouzon syndrome. Scand J Plast Reconstr Surg. 1981;15:187–197. [DOI] [PubMed] [Google Scholar]
- 13.David D, Poswillo D, Simpson D. The Craniosynostoses: Causes, Natural History, and Management. 1st ed. Springer-Verlag; 1982. [Google Scholar]
- 14.Jin S-W, Sim K-B, Kim S-D. Development and Growth of the Normal Cranial Vault : An Embryologic Review. J Korean Neurosurg Soc. 2016;59:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuratani S, Matsuo I, Aizawa S. Developmental patterning and evolution of the mammalian viscerocranium: genetic insights into comparative morphology. Dev Dyn Off Publ Am Assoc Anat. 1997;209:139–155. [DOI] [PubMed] [Google Scholar]
- 16.Katsianou MA, Adamopoulos C, Vastardis H, Basdra EK. Signaling mechanisms implicated in cranial sutures pathophysiology: Craniosynostosis. BBA Clin. 2016;6:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard JJ, Scott JH, Girgis FG. The structure and development of cranial and facial sutures. J Anat. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- 18.Sim SY, Yoon SH, Kim SY. Quantitative Analysis of Developmental Process of Cranial Suture in Korean Infants. J Korean Neurosurg Soc. 2012;51:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Maza C, Rosas A, Nieto-Díaz M. Postnatal changes in the growth dynamics of the human face revealed from bone modelling patterns. J Anat. 2013;223:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice DP. Developmental anatomy of craniofacial sutures. Front Oral Biol. 2008;12:1–21. [DOI] [PubMed] [Google Scholar]
- 21.Persson M. The role of sutures in normal and abnormal craniofacial growth. Acta Odontol Scand. 1995;53:152–161. [DOI] [PubMed] [Google Scholar]
- 22.Scott J. Growth at facial sutures. Am J Orthod Dentofacial Orthop. 1956;42:381–387. [Google Scholar]
- 23.Björk A. Sutural growth of the upper face studied by the implant method. Acta Odontol Scand. 1966;24:109–127. [DOI] [PubMed] [Google Scholar]
- 24.Björk A, Skieller V. Growth of the maxilla in three dimensions as revealed radiographically by the implant method. Br J Orthod. 1977;4:53–64. [DOI] [PubMed] [Google Scholar]
- 25.Cendekiawan T, Wong R, Rabie AB. Relationships Between Cranial Base Synchondroses and Craniofacial Development: A Review. Open Anat J. 2010;2:67–75. [Google Scholar]
- 26.Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn Off Publ Am Assoc Anat. 2000;219:472–485. [DOI] [PubMed] [Google Scholar]
- 27.Gruber DP, Brockmeyer D. Pediatric skull base surgery. 1. Embryology and developmental anatomy. Pediatr Neurosurg. 2003;38:2–8. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg. 1992;90:592–600. [DOI] [PubMed] [Google Scholar]
- 29.Erşahin Y. Endoscope-assisted repair of metopic synostosis. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2013;29:2195–2199. [DOI] [PubMed] [Google Scholar]
- 30.Anantheswar YN, Venkataramana NK. Pediatric craniofacial surgery for craniosynostosis: Our experience and current concepts: Part −1. J Pediatr Neurosci. 2009;4:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vu HL, Panchal J, Parker EE, Levine NS, Francel P. The timing of physiologic closure of the metopic suture: a review of 159 patients using reconstructed 3D CT scans of the craniofacial region. J Craniofac Surg. 2001;12:527–532. [DOI] [PubMed] [Google Scholar]
- 32.van der Meulen J. Metopic synostosis. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2012;28:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzaferro DM, Naran S, Wes AM, et al. Incidence of Cranial Base Suture Fusion in Infants with Craniosynostosis. Plast Reconstr Surg. 2018;141:559e–570e. [DOI] [PubMed] [Google Scholar]
- 34.Runyan CM, Xu W, Alperovich M, et al. Minor Suture Fusion in Syndromic Craniosynostosis. Plast Reconstr Surg. 2017;140:434e–445e. [DOI] [PubMed] [Google Scholar]
- 35.Calandrelli R, Pilato F, Massimi L, et al. Quantitative evaluation of facial hypoplasia and airway obstruction in infants with syndromic craniosynostosis: relationship with skull base and splanchnocranium sutural pattern. Neuroradiology. 2018;60:517–528. [DOI] [PubMed] [Google Scholar]
- 36.Alesbury HS, Ubelaker DH, Bernstein R. Utility of the frontonasal suture for estimating age at death in human skeletal remains. J Forensic Sci. 2013;58:104–108. [DOI] [PubMed] [Google Scholar]
- 37.Sakaue K. A Bayesian approach to age estimation from cranial suture closure in Japanese people. Bull Natl Mus Nat Sci. 2015;41:1–11. [Google Scholar]
- 38.Nelson DK, Williams T. Frontonasal process-specific disruption of AP-2alpha results in postnatal midfacial hypoplasia, vascular anomalies, and nasal cavity defects. Dev Biol. 2004;267:72–92. [DOI] [PubMed] [Google Scholar]
- 39.Babler WJ, Persing JA, Nagorsky MJ, Jane JA. Restricted growth at the frontonasal suture: alterations in craniofacial growth in rabbits. Am J Anat. 1987;178:90–98. [DOI] [PubMed] [Google Scholar]
- 40.Calandrelli R, Pilato F, Massimi L, et al. Craniofacial Sutural Pattern and Surgical Management in Patients With Different Degrees of Trigonocephaly Severity. J Comput Assist Tomogr. 2020. [DOI] [PubMed] [Google Scholar]
- 41.Udayakumaran S, Krishnadas A, Subhash P. Why do metopic sutural synostoses angulate? The concept of nasion sutural complex and its implication on the management of hypotelorism-early results and proof of concept. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2019;35:907–912. [DOI] [PubMed] [Google Scholar]
- 42.Miri S, Mittermiller P, Buchanan EP, Khosla RK. Facial twist (asymmetry) in isolated unilateral coronal synostosis: does premature facial suture fusion play a role? J Craniofac Surg. 2015;26:655–657. [DOI] [PubMed] [Google Scholar]
- 43.Kokich VG. Age changes in the human frontozygomatic suture from 20 to 95 years. Am J Orthod. 1976;69:411–430. [DOI] [PubMed] [Google Scholar]
- 44.Currarino G. Premature closure of the frontozygomatic suture: unusual frontoorbital dysplasia mimicking unilateral coronal synostosis. AJNR Am J Neuroradiol. 1985;6:643–646. [PMC free article] [PubMed] [Google Scholar]
- 45.Barrabé A, Weber E, Czorny A, Godfrin G, Meyer C, Louvrier A. A scanographic study of asymmetry of the frontal process of zygoma in unilateral coronal synostosis. J Cranio-Maxillo-fac Surg. 2018;46:1504–1510. [DOI] [PubMed] [Google Scholar]
- 46.Genitori L, Cavalheiro S, Lena G, Dollo C, Choux M. Skull base in trigonocephaly. Pediatr Neurosurg. 1991;17:175–181. [DOI] [PubMed] [Google Scholar]
- 47.Standring S, Borley N, Gray H. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 40th ed. Churchill Linvgstone/ Elsevier; 2008. [Google Scholar]
- 48.Rogers GF, Proctor MR, Mulliken JB. Unilateral fusion of the frontosphenoidal suture: a rare cause of synostotic frontal plagiocephaly. Plast Reconstr Surg. 2002;110:1011–1021. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman HJ, Mohr G. Lateral canthal advancement of the supraorbital margin. A new corrective technique in the treatment of coronal synostosis. J Neurosurg. 1976;45:376–381. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy JG, Bradley JP, Longaker MT. Step expansion of the frontal bar: correction of trigonocephaly. J Craniofac Surg. 1996;7:333–335. [DOI] [PubMed] [Google Scholar]
- 51.Angelieri F, Ruellas AC, Yatabe MS, et al. Zygomaticomaxillary suture maturation: Part II-The influence of sutural maturation on the response to maxillary protraction. Orthod Craniofac Res. 2017;20:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latham RA, Burston WR. The postnatal pattern of growth at the sutures of the human skull. An histological survey. Dent Pract Dent Rec. 1966;17(2):61–67. [PubMed] [Google Scholar]
- 53.Angelieri F, Franchi L, Cevidanes LHS, Hino CT, Nguyen T, McNamara JA. Zygomaticomaxillary suture maturation: A predictor of maxillary protraction? Part I - A classification method. Orthod Craniofac Res. 2017;20:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thimaporn J, Goldberg JS, Enlow DH. Effects of unilateral premature fusion of the zygomaxillary suture on the growth of nasomaxillary complex. J Oral Maxillofac Surg. 1990;48:835–841. [DOI] [PubMed] [Google Scholar]
- 55.Icen M, Orhan K, Oz U, Horasan S, Avsever H. Relationship between pterygomaxillary fissure morphology and maxillary/mandibular position : A cone beam computed tomography assessment. J Orofac Orthop Fortschritte Kieferorthopadie OrganOfficial J Dtsch Ges Kieferorthopadie. 2020. [DOI] [PubMed] [Google Scholar]
- 56.Ghoneima A, Abdel-Fattah E, Hartsfield J, El-Bedwehi A, Kamel A, Kula K. Effects of rapid maxillary expansion on the cranial and circummaxillary sutures. Am J Orthod Dentofac Orthop. 2011;140:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watzek G, Grundschober F, Plenk H, Eschberger J. Experimental investigations into the clinical significance of bone growth at viscerocranial sutures. J Maxillofac Surg. 1982;10:61–79. [DOI] [PubMed] [Google Scholar]
- 58.Bachmayer DI, Ross RB, Munro IR. Maxillary growth following LeFort III advancement surgery in Crouzon, Apert, and Pfeiffer syndromes. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 1986;90:420–430. [DOI] [PubMed] [Google Scholar]
- 59.Ross RB. The clinical implications of facial growth in cleft lip and palate. Cleft Palate J. 1970;7:37–47. [PubMed] [Google Scholar]
- 60.Liao Y-F, Mars M Long-term effects of palate repair on craniofacial morphology in patients with unilateral cleft lip and palate. Cleft Palate-Craniofacial J. 2005;42:594–600. [DOI] [PubMed] [Google Scholar]
- 61.Rogers GF, Greene AK, Oh AK, Robson C, Mulliken JB. Zygomaticotemporal synostosis: a rare cause of progressive facial asymmetry. Cleft Palate-Craniofacial J. 2007;44:106–111. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan SR, Greene AK, Mulliken JB, Rogers GF. Zygomaticotemporal suture synostosis causes progressive facial deformity and asymmetry. Plast Reconstr Surg. 2009;123:146e–147e. [DOI] [PubMed] [Google Scholar]
- 63.Sivakumaran R. Critical analysis of the factors affecting the “cranial suture aging method” using the Hamann Todd Collection (Master’s dissertation). Published online 2014. [Google Scholar]
- 64.Farkas LG, Posnick JC, Hreczko TM, Pron GE. Growth patterns of the nasolabial region: a morphometric study. Cleft Palate-Craniofacial J. 1992;29:318–324. [DOI] [PubMed] [Google Scholar]
- 65.Cole P, Kaufman Y, Hollier LH. Managing the pediatric facial fracture. Craniomaxillofacial Trauma Reconstr. 2009;2:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haug RH, Foss J. Maxillofacial injuries in the pediatric patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:126–134. [DOI] [PubMed] [Google Scholar]
- 67.Stucker FJ, Bryarly RC, Shockley WW. Management of nasal trauma in children. Arch Otolaryngol Chic Ill 1960. 1984;110:190–192. [DOI] [PubMed] [Google Scholar]
- 68.Baskaran M, Packiaraj I, Arularasan SG, Divakar TK. Cleft rhinoplasty. J Pharm Bioallied Sci. 2015;7:S691–S694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anastassov GE, Joos U, Zöllner B. Evaluation of the results of delayed rhinoplasty in cleft lip and palate patients. Functional and aesthetic implications and factors that affect successful nasal repair. Br J Oral Maxillofac Surg. 1998;36:416–424. [DOI] [PubMed] [Google Scholar]
- 70.Wagemans PA, van de Velde JP, Kuijpers-Jagtman AM. Sutures and forces: a review. Am J Orthod Dentofac Orthop Off Publ Am Assoc Orthod Its Const Soc Am Board Orthod. 1988;94:129–141. [DOI] [PubMed] [Google Scholar]
- 71.Persson M, Thilander B. Palatal suture closure in man from 15 to 35 years of age. Am J Orthod. 1977;72:42–52. [DOI] [PubMed] [Google Scholar]
- 72.Angelieri F, Cevidanes LHS, Franchi L, Gonçalves JR, Benavides E, McNamara JA. Midpalatal suture maturation: classification method for individual assessment before rapid maxillary expansion. Am J Orthod Dentofac Orthop. 2013;144:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bell RA. A review of maxillary expansion in relation to rate of expansion and patient’s age. Am J Orthod. 1982;81:32–37. [DOI] [PubMed] [Google Scholar]
- 74.Petrick S, Hothan T, Hietschold V, Schneider M, Harzer W, Tausche E. Bone density of the midpalatal suture 7 months after surgically assisted rapid palatal expansion in adults. Am J Orthod Dentofac Orthop. 2011;139:S109–116. [DOI] [PubMed] [Google Scholar]
- 75.Stuart DA, Wiltshire WA. Rapid palatal expansion in the young adult: time for a paradigm shift? J Can Dent Assoc. 2003;69:374–377. [PubMed] [Google Scholar]
- 76.Sumer AP, Ozer M, Sumer M, Danaci M, Tokalak F, Telcioglu NT. Ultrasonography in the evaluation of midpalatal suture in surgically assisted rapid maxillary expansion. J Craniofac Surg. 2012;23:1375–1377. [DOI] [PubMed] [Google Scholar]
- 77.Profitt W. The biological basis of orthondontic therapy. In: Contemporary Orthodontics. 3rd ed. Mosby, Inc.; 2000:296–325. [Google Scholar]
- 78.Goldstein JA, Paliga JT, Wink JD, Bartlett SP, Nah H-D, Taylor JA. Earlier evidence of spheno-occipital synchondrosis fusion correlates with severity of midface hypoplasia in patients with syndromic craniosynostosis. Plast Reconstr Surg. 2014;134:504–510. [DOI] [PubMed] [Google Scholar]
- 79.Tahiri Y, Paliga JT, Vossough A, Bartlett SP, Taylor JA. The spheno-occipital synchondrosis fuses prematurely in patients with Crouzon syndrome and midface hypoplasia compared with age- and gender-matched controls. J Oral Maxillofac Surg. 2014;72:1173–1179. [DOI] [PubMed] [Google Scholar]
- 80.Paliga JT, Goldstein JA, Vossough A, Bartlett SP, Taylor JA. Premature closure of the spheno-occipital synchondrosis in Pfeiffer syndrome: a link to midface hypoplasia. J Craniofac Surg. 2014;25:202–205. [DOI] [PubMed] [Google Scholar]
- 81.Driessen C, Rijken BF, Doerga PN, Dremmen MH, Joosten KF, Mathijssen IM. The effect of early fusion of the spheno-occipital synchondrosis on midface hypoplasia and obstructive sleep apnea in patients with Crouzon syndrome. J Cranio-Maxillo-fac Surg. 2017;45:1069–1073. [DOI] [PubMed] [Google Scholar]
- 82.Alhazmi A, Vargas E, Palomo JM, Hans M, Latimer B, Simpson S. Timing and rate of spheno-occipital synchondrosis closure and its relationship to puberty. PloS One. 2017;12:e0183305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirley NR, Jantz RL. Spheno-occipital synchondrosis fusion in modern Americans. J Forensic Sci. 2011;56:580–585. [DOI] [PubMed] [Google Scholar]
- 84.Hisham S, Flavel A, Abdullah N, Noor MHM, Franklin D. Quantification of spheno-occipital synchondrosis fusion in a contemporary Malaysian population. Forensic Sci Int. 2018;284:78–84. [DOI] [PubMed] [Google Scholar]
- 85.Mahon T-J, Friedling LJ, Gordon GM. Spheno-occipital synchondrosis: Examining the degree of fusion in a South African Black skeletal sample. Forensic Sci Int. 2017;278:408.e1–408.e5. [DOI] [PubMed] [Google Scholar]
- 86.Lottering N, MacGregor DM, Alston CL, Gregory LS. Ontogeny of the spheno-occipital synchondrosis in a modern Queensland, Australian population using computed tomography. Am J Phys Anthropol. 2015;157:42–57. [DOI] [PubMed] [Google Scholar]
- 87.Franklin D, Flavel A. Brief communication: timing of spheno-occipital closure in modern Western Australians. Am J Phys Anthropol. 2014;153:132–138. [DOI] [PubMed] [Google Scholar]
- 88.Akhlaghi M, Taghaddosinejad F, Sheikhazadi A, Valizadeh B, Shojaei SMR. Age-at-death estimation based on the macroscopic examination of spheno-occipital sutures. J Forensic Leg Med. 2010;17:304–308. [DOI] [PubMed] [Google Scholar]
- 89.Can IO, Ekizoglu O, Hocaoglu E, Inci E, Sayin I, Kaya KH. Forensic age estimation by spheno-occipital synchondrosis fusion degree: computed tomography analysis. J Craniofac Surg. 2014;25:1212–1216. [DOI] [PubMed] [Google Scholar]
- 90.Sinanoglu A, Kocasarac HD, Noujeim M. Age estimation by an analysis of spheno-occipital synchondrosis using cone-beam computed tomography. Leg Med Tokyo Jpn. 2016;18:13–19. [DOI] [PubMed] [Google Scholar]
- 91.Madeline LA, Elster AD. Suture closure in the human chondrocranium: CT assessment. Radiology. 1995;196:747–756. [DOI] [PubMed] [Google Scholar]
- 92.Forte AJ, Alonso N, Persing JA, Pfaff MJ, Brooks ED, Steinbacher DM. Analysis of midface retrusion in Crouzon and Apert syndromes. Plast Reconstr Surg. 2014;134:285–293. [DOI] [PubMed] [Google Scholar]
- 93.Burdi AR, Kusnetz AB, Venes JL, Gebarski SS. The natural history and pathogenesis of the cranial coronal ring articulations: implications in understanding the pathogenesis of the Crouzon craniostenotic defects. Cleft Palate J. 1986;23:28–39. [PubMed] [Google Scholar]
- 94.Rosenberg P, Arlis HR, Haworth RD, Heier L, Hoffman L, LaTrenta G. The role of the cranial base in facial growth: experimental craniofacial synostosis in the rabbit. Plast Reconstr Surg. 1997;99:1396–1407. [DOI] [PubMed] [Google Scholar]
- 95.Francel PC, Park TS, Marsh JL, Kaufman BA. Frontal plagiocephaly secondary to synostosis of the frontosphenoidal suture. Case report. J Neurosurg. 1995;83:733–736. [DOI] [PubMed] [Google Scholar]
- 96.Dundulis JA, Becker DB, Govier DP, Marsh JL, Kane AA. Coronal ring involvement in patients treated for unilateral coronal craniosynostosis. Plast Reconstr Surg. 2004;114:1695–1703. [DOI] [PubMed] [Google Scholar]
- 97.Puente-Espel J, Kozusko SD, Konofaos P, Boop FA, Wallace RD. Isolated Frontosphenoidal Suture Craniosynostosis: Treatment Approaches and Literature Review for a Unique Condition. J Craniofac Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 98.Ben Nsir A, Darmoul M, Zemmali M, Slimane A, Hattab N. Plagiocephaly due to Frontosphenoidal Suture Synostosis: Report of 2 Cases and Literature Review. Pediatr Neurosurg. 2016;51:204–209. [DOI] [PubMed] [Google Scholar]
- 99.Plooij JM, Verhamme Y, Bergé SJ, van Lindert EJ, Borstlap-Engels VMF, Borstlap WA. Unilateral craniosynostosis of the frontosphenoidal suture: a case report and a review of literature. J Cranio-Maxillo-fac Surg. 2009;37:162–166. [DOI] [PubMed] [Google Scholar]
- 100.Mathijssen IMJ, van der Meulen JJNM, van Adrichem LNA, et al. The frontosphenoidal suture: fetal development and phenotype of its synostosis. Pediatr Radiol. 2008;38:431–437. [DOI] [PubMed] [Google Scholar]
- 101.de Ribaupierre S, Czorny A, Pittet B, Jacques B, Rilliet B. Frontosphenoidal synostosis: a rare cause of unilateral anterior plagiocephaly. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2007;23:1431–1438. [DOI] [PubMed] [Google Scholar]
- 102.Yasonov SA, Lopatin AV, Kugushev AY. Craniosynostosis of the Sphenofrontal Suture: Definition of the Main Signs of Craniofacial Deformity. Ann Maxillofac Surg. 2017;7:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sauerhammer TM, Oh AK, Boyajian M, et al. Isolated frontosphenoidal synostosis: a rare cause of synostotic frontal plagiocephaly. J Neurosurg Pediatr. 2014;13:553–558. [DOI] [PubMed] [Google Scholar]
- 104.Pickrell BB, Lam SK, Monson LA. Isolated Unilateral Frontosphenoidal Craniosynostosis: A Rare Cause of Anterior Plagiocephaly. J Craniofac Surg. 2015;26:1944–1946. [DOI] [PubMed] [Google Scholar]
- 105.Marucci DD, Jones BM, Dunaway DJ, Hayward RD. Unilateral isolated frontosphenoidal craniosynostosis causing frontal plagiocephaly. J Plast Reconstr Aesthetic Surg. 2009;62:e255–258. [DOI] [PubMed] [Google Scholar]
- 106.Mittermiller PA, Yeom KW, Menard RM. Isolated Intraorbital Frontosphenoidal Synostosis. J Craniofac Surg. 2018;29:82–87. [DOI] [PubMed] [Google Scholar]
- 107.Kreiborg S, Prydsoe U, Dahl E, Fogh-Anderson P. Clinical conference I. Calvarium and cranial base in Apert’s syndrome: an autopsy report. Cleft Palate J. 1976;13:296–303. [PubMed] [Google Scholar]
- 108.Seeger JF, Gabrielsen TO. Premature closure of the frontosphenoidal suture in synostosis of the coronal suture. Radiology. 1971;101(3):631–635. [DOI] [PubMed] [Google Scholar]
- 109.Kreiborg S, Cohen MM. Characteristics of the infant Apert skull and its subsequent development. J Craniofac Genet Dev Biol. 1990;10:399–410. [PubMed] [Google Scholar]
- 110.Wilkinson CC, Stence NV, Serrano CA, et al. Fusion patterns of major calvarial sutures on volume-rendered CT reconstructions. J Neurosurg Pediatr. 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 111.Lambert HW, Wineski LE. Lippincott’s Illustrated Q&A Review of Anatomy and Embryology. Lippincott Williams & Wilkins/Wolters Kluwer Health; 2010. [Google Scholar]
- 112.Cohen MM, Kreiborg S. Cutaneous manifestations of Apert syndrome. Am J Med Genet. 1995;58:94–96. [DOI] [PubMed] [Google Scholar]
- 113.Krishan K, Kanchan T. Evaluation of spheno-occipital synchondrosis: A review of literature and considerations from forensic anthropologic point of view. J Forensic Dent Sci. 2013;5:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chopra S. The cranial suture closure in monkeys. 1957;128:67–112. Proc Zool Soc Lond. 1957;128:67-112. [Google Scholar]
- 115.Becker MJ. Mandibular symphysis (medial suture) closure in modern Homo sapiens: preliminary evidence from archaeological populations. Am J Phys Anthropol. 1986;69:499–501. [DOI] [PubMed] [Google Scholar]
- 116.Khandare S, Bhise S, Shinde A. Age estimation from cranial sutures: CT scan study. Indian J Basic Appl Med Res. 2014;3:203–211. [Google Scholar]
- 117.Singh P, Oberoi S, Gorea R. Age estimation in old individuals by CT scan of skull. J Indian Acad Forensic Med. 2004;26:10–13. [Google Scholar]
- 118.Wang W, Jian Y, Cai B, Wang M, Chen M, Huang H. All-Trans Retinoic Acid-Induced Craniofacial Malformation Model: A Prenatal and Postnatal Morphological Analysis. Cleft Palate-Craniofacial J. 2017;54:391–399. [DOI] [PubMed] [Google Scholar]
- 119.Showalter BM, David LR, Argenta LC, Thompson JT. Influence of frontosphenoidal suture synostosis on skull dysmorphology in unicoronal suture synostosis. J Craniofac Surg. 2012;23:1709–1712. [DOI] [PubMed] [Google Scholar]
- 120.Sakamoto Y, Nakajima H, Tamada I, Miyamoto J, Kishi K. Involvement of the sphenosquamosal suture for unilateral coronal synostosis. J Craniofac Surg. 2012;23:1267–1269. [DOI] [PubMed] [Google Scholar]
- 121.Jimenez DF, Barone CM, Argamaso RV, Goodrich JT, Shprintzen RJ. Asterion region synostosis. Cleft Palate-Craniofacial J. 1994;31:136–141. [DOI] [PubMed] [Google Scholar]
- 122.Coll G, Sakka L, Botella C, et al. Pattern of Closure of Skull Base Synchondroses in Crouzon Syndrome. World Neurosurg. 2018;109:e460–e467. [DOI] [PubMed] [Google Scholar]