IMPORTANCE:
Provider staffing models for ICUs are generally based on pragmatic necessities and historical norms at individual institutions. A better understanding of the role that provider staffing models play in determining patient outcomes and optimizing use of ICU resources is needed.
OBJECTIVES:
To explore the impact of transitioning from a low- to high-intensity intensivist staffing model on patient outcomes and unit composition.
DESIGN, SETTING, AND PARTICIPANTS:
This was a prospective observational before-and-after study of adult ICU patients admitted to a single community hospital ICU before (October 2016–May 2017) and after (June 2017–November 2017) the transition to a high-intensity ICU staffing model.
MAIN OUTCOMES AND MEASURES:
The primary outcome was 30-day all-cause mortality. Secondary outcomes included in-hospital mortality, ICU length of stay (LOS), and unit composition characteristics including type (e.g., medical, surgical) and purpose (ICU-specific intervention vs close monitoring only) of admission.
RESULTS:
For the primary outcome, 1,219 subjects were included (779 low-intensity, 440 high-intensity). In multivariable analysis, the transition to a high-intensity staffing model was not associated with a decrease in 30-day (odds ratio [OR], 0.90; 95% CI, 0.61–1.34; p = 0.62) or in-hospital (OR, 0.89; 95% CI, 0.57–1.38; p = 0.60) mortality, nor ICU LOS. However, the proportion of patients admitted to the ICU without an ICU-specific need did decrease under the high-intensity staffing model (27.2% low-intensity to 17.5% high-intensity; p < 0.001).
CONCLUSIONS AND RELEVANCE:
Multivariable analysis showed no association between transition to a high-intensity ICU staffing model and mortality or LOS outcomes; however, the proportion of patients admitted without an ICU-specific need decreased under the high-intensity model. Further research is needed to determine whether a high-intensity staffing model may lead to more efficient ICU bed usage.
Keywords: hospital mortality, intensive care unit, intensive care unit triage, intensivist staffing, length of stay
KEY POINTS
Question: We hypothesized that transition to a high-intensity intensivist staffing model would reduce all-cause 30-day and in-hospital mortality.
Findings: We performed a prospective observational study examining patient outcomes before and after the transition to a high-intensity staffing model in a single community hospital ICU. Multivariable modeling did not show any association between the staffing model change and mortality outcomes.
Meaning: Transition to a high-intensity intensivist-driven staffing model did not reduce in-hospital or 30-day mortality in this community hospital ICU.
Although the majority of ICUs in the United States are based in community hospitals (1), very little research has been done to assess the impact of ICU staffing models on patient outcomes in the community setting. Instead, staffing generally reflects local historical precedent and practical necessity rather than optimization based on formal assessment (2, 3). Existing studies have been largely based in academic centers and observed effects on patient outcomes have been heterogeneous. Implementation of 24-hour in-house intensivist care has generally shown no association with mortality outcomes (4, 5) but may reduce mortality in ICUs with inconsistent daytime intensivist coverage (5). Analyses of patient-to-provider ratios have shown mixed results with some demonstrating a deleterious mortality effect of higher ratios (6, 7) but others suggesting no association (8). Mandatory involvement of trained intensivists in ICU patient care, often referred to as a “closed” ICU model, has been associated with improved patient outcomes in multiple studies at single academic medical centers (9–15); however, large cohort studies of multiple academic and community-based ICUs have not been able to replicate this finding (16, 17). One factor contributing to this inconsistency could be differences of effects between academic and community sites.
To better understand the effects of ICU staffing models on patient outcomes in community settings, we evaluated the impact of an administratively planned transition from a low-intensity to high-intensity ICU provider staffing model on unit composition, ICU turnover, and clinical outcomes of patients admitted to a community-based ICU. We hypothesized that the introduction of a high-intensity staffing model and consistent intensivist involvement would be associated with improvements in patient mortality outcomes (30-d and in-hospital mortality), reduction in ICU length of stay (LOS), and transition of unit composition to more patients with active critical care needs, rather than simply observational needs.
MATERIALS AND METHODS
Complete details on the Materials and Methods used can be found in the Supplemental Methods (http://links.lww.com/CCX/B136).
Study Design and Setting
A prospective observational before-and-after study was conducted at a 24-bed, combined-specialty, community-based, open ICU under two staffing paradigms to evaluate their effect on patient outcomes as well as unit composition and turnover.
Study Population and Timeframe
Data were collected from October 2016 to November 2017 on all adults admitted to the ICU over the study period (Fig. 1). This timeframe encompassed an 8-month period before and 6-month period after a planned transition in ICU staffing from a low-intensity to high-intensity model (June 2017).
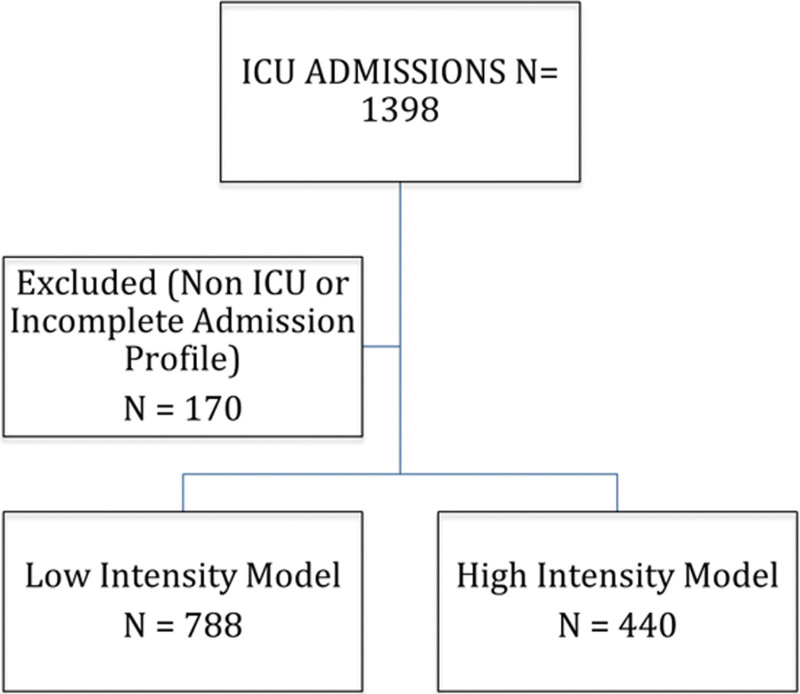
Figure 1.
Flowchart of ICU patient admissions and subject enrollment. Patients who were non-ICU status (boarding) or had incomplete admission profiles were excluded.
Low-Intensity and High-Intensity Staffing Models
Similar to prior literature descriptions (2, 18), the low-intensity staffing model (November 2016–May 2017) employed an open admission policy with elective intensivist consultation at the discretion of the admitting provider, although primary intensivist management of patients requiring mechanical ventilation or invasive hemodynamic support (e.g., vasopressors, or mechanical cardiac support devices) was mandated. The ICU was covered by two in-house board-certified intensivists (anesthesiology, internal medicine, or surgical critical care trained physician) from 7:00 am to 9:00 pm (provider 1, 7:00 am–2:00 pm; provider 2, 10:00 am–9:00 pm resulting in 4 hr of overlap between providers). Overnight coverage was provided by an off-site tele-ICU physician with access via the electronic medical record and high-resolution cameras, and a second on-call physician available to return to the hospital if needed for bedside management at the discretion of the tele-ICU provider.
The high-intensity model (June 2017–November 2017) implemented three major staffing changes. First, a third daytime provider was added to the staffing complement in the form of a critical care advanced practice provider (APP) working from 7 am to 5 pm, in addition to the two existing critical care physicians. The APP responsibilities included managing a share of admissions and consults and assisting with cross cover. Physician oversight was provided but most APPs functioned largely independently. Second, unlike the low-intensity model in which intensivist consultation was optional except in the case of invasive mechanical ventilation and vasopressor use, all patients admitted to the ICU under the high-intensity model were either primarily admitted and managed by the intensivist team or co-managed by the nonintensivist admitting team and the intensivist team via mandatory consultation. Third, overnight coverage was changed from remote to in-house coverage by one intensivist who was responsible for ICU admissions, cross covering on all ICU patients, and covering tele-ICU responsibilities for four other community hospital ICUs in the area.
Data Collection
Patient-to-provider ratios were calculated at the time of admission for each patient by dividing the ICU census at time of admission by the number of scheduled daytime providers that day, regardless of provider overlap. Select patient and census-specific data were collected by the unit clerk using a data collection tool and additional variables were extracted from the electronic medical record by research coordinators and medical students trained to abstract data according to study protocol. Additional census data were obtained from the hospital administration. Full details of collected data can be found in the Supplemental Methods (http://links.lww.com/CCX/B136). The primary outcome of this study was 30-day all-cause mortality. Secondary outcomes included in-hospital mortality, ICU LOS, and unit composition, defined as the proportion of patients admitted to the ICU for a specific intervention versus observation.
Statistical Analysis
Patient characteristics and ICU staffing, census, and turnover were summarized by staffing model using standard descriptive statistics. Normally distributed variables were compared using Student t test with unequal variances, skewed variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using Fisher exact test. Thirty-day and in-hospital all-cause mortality were compared between the staffing models using Fisher exact test. ICU LOS was compared between staffing models while treating in-hospital mortality as a competing risk using Aalen-Johansen cumulative incidence plots and Gray test (19), the competing risks analog to the log-rank test. To visually assess the presence of seasonal variations or other cyclical effects, 30-day mortality, in-hospital mortality, and ICU LOS by month of hospital admission were plotted. The associations between staffing model and mortality outcomes were reevaluated using multiple logistic regression models accounting for additional patient and unit variables. The association between staffing model and ICU LOS was similarly reevaluated using a Fine-Gray competing risk regression model (20) accounting for patient and unit factors. The adjusted models were then refit using an interaction term between staffing model and Sequential Organ Failure Assessment (SOFA) score to determine whether the association between each patient outcome and staffing model differed by patient acuity. All analyses were conducted using R Version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and two-sided p values of less than 0.05 were considered statistically significant. This study was approved by the University of Minnesota Institutional Review Board (Study No. 1606M89741, October 28, 2016). Research procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975.
RESULTS
Patient and Admission Characteristics
Among the 1,398 ICU patients admitted between October 2016 and November 2017, 170 had incomplete data or were deemed to have non-ICU status (e.g., boarding in the ICU) and were excluded from all analyses. Of the remaining 1,228 patients, 788 were admitted during the low-intensity model timeframe (October 2016–May 2017) and 440 during the high-intensity model timeframe (June 2017–November 2017) (Fig. 1).
Patient demographics and information regarding ICU staffing, census, and turnover are provided in Table 1 for the overall study population as well as by staffing model. Although most observed characteristics were similar between groups, patients admitted during the low-intensity staffing model had, on average, a higher SOFA score than those admitted during the high-intensity staffing model (4.5 [sd: 3.4] vs 4.1 [sd: 4.1]; p = 0.05), however, both of these scores correlate clinically with a similar predicted mortality rate of ~20% (21).
TABLE 1.
Baseline Characteristics of the Study Sample in Addition to ICU Census, Staffing, and Acuity Data by Staffing Model
| Characteristic | Overall (n = 1,228) | Low-Intensity Staffing Model (n = 788) | High-Intensity Staffing Model (n = 440) | p |
|---|---|---|---|---|
| Demographics and admission circumstance | ||||
| Female sex assigned at birth, n (%) | 677 (55.2) | 426 (54.1) | 251 (57.2) | 0.31 |
| Non-Hispanic White, n (%) | 1,009 (82.2) | 656 (83.2) | 353 (80.2) | 0.19 |
| Age, mean (sd) | 64.5 (16.5) | 64.7 (16.5) | 64.0 (16.3) | 0.43 |
| Night admission (7 pm–7 am), n (%) | 366 (29.8) | 238 (30.2) | 128 (29.1) | 0.70 |
| Weekday admission, n (%) | 988 (80.5) | 622 (78.9) | 366 (83.2) | 0.07 |
| Reason for admission, n (%) | ||||
| Intervention | 912 (74.3) | 561 (71.2) | 351 (79.8) | < 0.001 |
| Monitoring | 291 (23.7) | 214 (27.2) | 77 (17.5) | |
| Overflow | 25 (2.0) | 13 (1.6) | 12 (2.7) | |
| Type of ICU admission, n (%) | ||||
| Cardiac/cardiovascular surgery | 282 (23.0) | 155 (19.7) | 127 (28.9) | 0.003 |
| Medical | 594 (48.4) | 406 (51.5) | 188 (42.7) | |
| Neurologic | 262 (21.3) | 168 (21.3) | 94 (21.4) | |
| Surgical (postoperative) | 86 (7.0) | 57 (7.2) | 29 (6.6) | |
| Other | 4 (0.3) | 2 (0.3) | 2 (0.5) | |
| Charlson Comorbidity Index, mean (sd) | 4.7 (2.9) | 4.7 (2.9) | 4.6 (2.9) | 0.35 |
| Sequential Organ Failure Assessment score, mean (sd) | 4.4 (3.3) | 4.5 (3.4) | 4.1 (3.2) | 0.05 |
| Census and turnover | ||||
| Midnight average ICU census (sd)a | 13.9 (1.4) | 14.1 (1.3) | 13.6 (1.6) | 0.55 |
| Total ICU census at time of admission for each subject, mean (sd) | 14.5 (3.2) | 14.8 (3.1) | 13.8 (3.4) | < 0.001 |
| ICU turnover (number of pts transferred/discharged from ICU on day of admission), mean (sd) | 6.2 (2.6) | 6.5 (2.6) | 5.6 (2.4) | < 0.001 |
| Distinct patient averagea | 19.2 (1.9) | 19.7 (1.8) | 18.6 (1.9) | 0.31 |
| Requiring renal replacement therapy at admission, n (%) | 3 (0.24) | 1 (0.13) | 2 (0.45) | 0.29 |
| Staffing and ICU acuity | ||||
| Patient to attending ratio, mean (sd) | 5.8 (1.7) | 5.8 (1.7) | 5.8 (1.7) | 0.91 |
| Number of patients on unit with 1:1 nurse:patient ratio at time of subject admission, mean (sd) | 1.6 (1.4) | 1.7 (1.4) | 1.5 (1.3) | 0.02 |
| Number of patients on unit with 1:2 nurse:patient ratio at time of subject admission, mean (sd) | 12.8 (3.5) | 13.0 (3.5) | 12.3 (3.6) | 0.001 |
| Nurse:patient ratio at time of subject admission, n (%) | ||||
| 1 RN: 1 Pt | 380 (31.0) | 238 (30.2) | 142 (32.3) | 0.09 |
| 1 RN: 2 Pt | 826 (67.3) | 532 (67.5) | 294 (67.0) | |
| 2 RN: 1 Pt | 21 (1.7) | 18 (2.3) | 3 (0.7) | |
| Number of mechanically ventilated patients on unit at time of admission, mean (sd) | 6.1 (2.8) | 6.2 (2.6) | 5.9 (3.0) | 0.10 |
| Number of patients on pressors on unit at time of admission, mean (sd) | 2.3 (1.8) | 2.4 (1.8) | 2.1 (1.7) | 0.002 |
| Case mix index, averagea | 3.6 (0.3) | 3.6 (0.3) | 3.5 (0.3) | 0.61 |
RN = registered nurse, Pt = patient, pts = patients.
Derived retrospectively from monthly administrative reports.
Purpose for ICU Admission and Unit Composition
The transition to a high-intensity staffing model was associated with significant differences in the patient composition of the ICU (Table 1). In the high-intensity model, the proportion of patients admitted for direct therapeutic interventions significantly increased relative to low-intensity (561 [71.2%] low-intensity compared with 351 [79.8%] high-intensity; p = 0.001), and the proportion admitted for observation decreased (214 [27.2%] low-intensity compared with 77 [17.5%] high-intensity; p = 0.001). During the high-intensity period, an increase in the proportion of patients admitted for cardiac and cardiovascular surgery indications (19.7% low-intensity vs 28.9% high-intensity; p = 0.003) and a decrease in the proportion of medical patients (51.5% low-intensity vs 42.7% high-intensity; p = 0.003) were also observed.
ICU Census and Turnover
The mean ICU census at the time of patient admission was significantly higher under the low-intensity model than the high-intensity model (14.8 [sd: 3.1] low-intensity vs 13.8 [sd: 3.4] high-intensity; p < 0.001). This finding differed from the administrative reports for which no significant difference was observed between staffing models in the monthly average midnight ICU census (14.1 [sd: 1.26] low-intensity vs 13.6 [sd: 1.59] high-intensity; p = 0.55), nor the distinct patient monthly average (19.7 [sd: 1.8] low-intensity vs 18.6 high-intensity [sd: 1.9]; p = 0.31). This difference in findings may reflect the decreased time resolution afforded by daily rather than continuous census sampling, with the former having more susceptibility to undercounting during periods of high turnover than the latter. ICU turnover significantly decreased between the low- and high-intensity models, with an average of 6.5 (sd: 2.6) patients being transferred or discharged on the day of a subject’s ICU admission during the low-intensity period versus 5.6 (sd: 2.4) during the high-intensity period (p < 0.001).
Staffing Ratios and ICU Acuity
As gauges of ICU acuity, nursing ratios, numbers of patients on mechanical ventilation, and numbers of patients requiring pressors was compared between staffing models. The nurse-to-patient ratio (RN: Pt ratio) at the time of admission for each individual subject was not significantly different between staffing models (p = 0.09). However, the average number of total patients on the unit with a 1:1 or 1:2 RN: Pt ratio at the time of each subject’s admission did significantly differ. Whereas the mean number of patients on unit with a 1:1 or 1:2 RN: Pt ratio at the time of subject admission was 1.7 (sd: 1.4) and 13.0 (sd: 2.5) for the low-intensity model, these numbers decreased slightly to 1.5 (sd: 1.3) and 12.3 (sd: 3.6) for the high-intensity model (p = 0.02 and 0.001). The average number of mechanically ventilated patients was similar between staffing models (6.2 [sd: 2.6] low-intensity vs 5.9 [sd: 3.0] high-intensity; p = 0.10). Although the average number of patients on vasopressors was also similar, a small but statistically significant difference was found (low-intensity, 2.4 [sd: 1.8]; high-intensity, 2.1 [sd: 1.7]; p = 0.002).
Mortality Outcomes
A multivariable model was used to explore associations between staffing model and mortality, while adjusting for patient acuity and demographics, type and timing of admission, ICU acuity, and nurse-to-patient ratio at time of admission as potential confounding variables (Tables 2 and 3). Adjusting for potential confounders, no significant difference was observed in the odds of in-hospital mortality between the high-intensity and low-intensity staffing models (odds ratio [OR], 0.89; 95% CI, 0.57–1.38; p = 0.60; n = 1,220). Similar results were observed in the OR for 30-day mortality (OR, 0.90; 95% CI, 0.61–1.34; p = 0.62; n = 1,219). In contrast, significant associations with both in-hospital and 30-day mortality were found for patient age (OR for both mortality outcomes, 1.04; 95% CI, 1.03–1.06; p < 0.001), SOFA score (in-hospital OR, 1.42; 95% CI, 1.32–1.52; p < 0.001 and 30-d OR, 1.35; 95% CI, 1.27–1.43; p < 0.001), admission for a cardiac or cardiovascular surgery indication compared with medical indication (in-hospital OR, 0.18; 95% CI, 0.08–0.37; p < 0.001 and 30-d OR, 0.21; 95% CI, 0.11–0.41; p < 0.001), and admission for a surgical (postoperative) indication rather than a medical indication (in-hospital OR, 0.23; 95% CI, 0.08–0.58; p < 0.001 and 30-d OR, 0.38; 95% CI, 0.16–0.81; p = 0.02).
TABLE 2.
Multivariable Model for In-Hospital Mortality
| Covariate | OR (95% CI) | p |
|---|---|---|
| Intercepta | –6.70 (–8.22 to –5.30) | < 0.001 |
| High-intensity staffing model | 0.89 (0.57–1.38) | 0.60 |
| Subject nurse:patient ratio at time of admissionb | ||
| 1 RN: 1 Pt | 1.12 (0.63–1.97) | 0.71 |
| 2 RN: 1 Pt | 0.32 (0.02–2.10) | 0.32 |
| Age | 1.04 (1.03–1.06) | < 0.001 |
| Female sex assigned at birth | 0.78 (0.51–1.17) | 0.23 |
| Non-Hispanic White | 1.43 (0.80–2.69) | 0.24 |
| Charlson Comorbidity Index | 1.06 (0.97–1.16) | 0.18 |
| Sequential Organ Failure Assessment score | 1.42 (1.32–1.52) | < 0.001 |
| Night admission (7 pm–7 am) | 0.98 (0.63–1.51) | 0.92 |
| Weekend admission | 0.73 (0.44–1.20) | 0.23 |
| Number of mechanically ventilated patients on unit at time of admission | 0.95 (0.88–1.03) | 0.26 |
| Number of patients on pressors on unit at time of admission | 1.06 (0.93–1.20) | 0.37 |
| Type of ICU admissionc | ||
| Cardiac/cardiovascular surgery | 0.18 (0.08–0.37) | < 0.001 |
| Neurologic | 0.80 (0.43–1.48) | 0.50 |
| Surgical (postoperative) | 0.23 (0.08–0.58) | < 0.001 |
OR = odds ratio, RN = registered nurse, pt = patient.
Presented on the log(OR) scale.
OR relative to nurse:patient ratio 1 RN: 2 Pt.
OR relative to medical admission. OR for admission type “Other” was inestimable due to quasi-complete separation.
TABLE 3.
Multivariable Model for 30-Day Mortality
| Covariate | OR (95% CI) | p |
|---|---|---|
| Intercepta | –5.99 (–7.29 to –4.77) | < 0.001 |
| High-intensity staffing model | 0.90 (0.61–1.34) | 0.62 |
| Subject nurse:patient ratio at time of admissionb | ||
| 1 RN: 1 Pt | 0.95 (0.57–1.60) | 0.86 |
| 2 RN: 1 Pt | 0.21 (0.01–1.32) | 0.17 |
| Age | 1.04 (1.03–1.06) | < 0.001 |
| Female sex assigned at birth | 0.78 (0.54–1.12) | 0.18 |
| Non-Hispanic White | 1.13 (0.68–1.92) | 0.64 |
| Charlson Comorbidity Index | 1.09 (1.01–1.19) | 0.03 |
| Sequential Organ Failure Assessment score | 1.35 (1.27–1.43) | < 0.001 |
| Night admission (7 pm–7 am) | 0.97 (0.65–1.42) | 0.87 |
| Weekend admission | 0.71 (0.45–1.12) | 0.15 |
| Number of mechanically ventilated patients on unit at time of admission | 0.97 (0.90–1.04) | 0.38 |
| Number of patients on pressors on unit at time of admission | 1.02 (0.91–1.14) | 0.75 |
| Type of ICU admissionc | ||
| Cardiac/cardiovascular surgery | 0.21 (0.11–0.41) | < 0.001 |
| Neurologic | 0.90 (0.53–1.52) | 0.71 |
| Surgical (postoperative) | 0.38 (0.16–0.81) | 0.02 |
OR = odds ratio, RN = registered nurse, pt = patient.
Presented on the log(OR) scale.
OR relative to nurse:patient ratio 1 RN: 2 Pt.
OR relative to medical admission. OR for admission type “Other” was inestimable due to quasi-complete separation.
The incorporation of an interaction term between staffing model and SOFA score had little impact on the estimated coefficients in the multiple logistic regression models for in-hospital and 30-day mortality (eTABLES 1 and 2, http://links.lww.com/CCX/B136). There was no statistically significant interaction between staffing model and SOFA score (in-hospital mortality p = 0.83; 30-d mortality p = 0.92).
Because one of the major interventions associated with the higher intensity staffing model was mandatory intensivist consultation for all ICU patients not admitted by the intensivist service, subgroup analyses were performed separately comparing mortality outcomes across staffing models for patients admitted by a nonintensivist service (low-intensity group: 603/788 subjects [77.5%]; high-intensity group: 314/440 subjects [71.3%]), and for patients admitted by intensivists. For intensivist admissions, the odds of in-hospital and 30-day mortality were essentially the same in the lower versus higher intensity groups (in-hospital OR, 0.98; 95% CI, 0.48–1.99; p = 0.96 and 30-d OR, 1.03; 95% CI, 0.53–2.01; p = 0.93; eTABLES 3 and 4, http://links.lww.com/CCX/B136). For nonintensivist admissions who had newly mandated intensivist consults under the high-intensity service, the estimated odds of mortality were lower during the higher intensity staffing period, but the changes were not statistically significant (in-hospital OR, 0.73; 95% CI, 0.38–1.34; p = 0.32 and 30-d OR, 0.79; 95% CI, 0.47–1.31; p = 0.37; eTABLES 5 and 6, http://links.lww.com/CCX/B136).
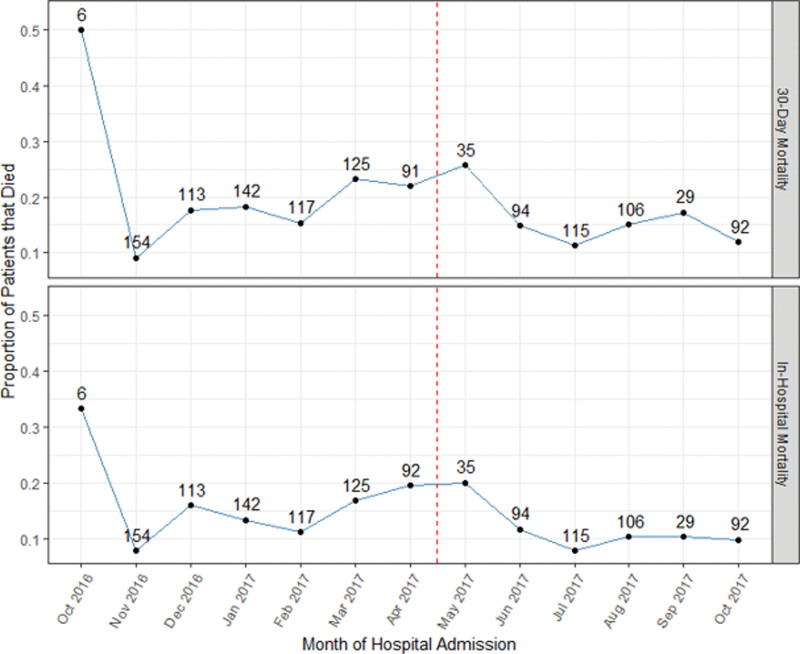
To assess whether other underlying temporal trends could be affecting mortality, these outcomes were plotted by month of hospital admission (Fig. 2). Notably, there is no sustained trend in mortality spanning both the low-intensity and high-intensity model periods. An unadjusted analysis of mortality outcomes can be found in eTABLE 7 (http://links.lww.com/CCX/B136).
Figure 2.
The proportion of patients that died in-hospital or within 30 d after admission by month of hospital admission. The dashed red line denotes the time at which the transition from the low-intensity to high-intensity staffing model occurred. The numbers above each point reflect the number of patients admitted to the hospital for whom mortality data were available.
Length of ICU Stay
The subdistribution hazard ratios (HRs) of each covariate for ICU LOS, along with corresponding 95% CIs and p values, are presented in eTABLE 8 (http://links.lww.com/CCX/B136). Treating in-hospital mortality as a competing risk, the subdistribution HR of discharge from the ICU for the high-intensity versus low-intensity staffing models was 0.99 (95% CI, 0.89–1.09; p = 0.80), indicating that staffing model was not associated with the rate of being discharged. In contrast, age (p < 0.001), SOFA score (p < 0.001), being admitted for a cardiac/cardiovascular surgery versus a medical reason (p < 0.001), being admitted for a surgical reason versus a medical reason (p = 0.01), and having a nursing ratio of two RN: one Pt versus one RN: one Pt (p = 0.03) were significantly associated with the incidence of being discharged. There was not a statistically significant interaction between staffing model and SOFA score (p = 0.73; eTABLE 9, http://links.lww.com/CCX/B136).
The cumulative incidence curve for ICU discharge did not significantly differ between staffing models (p = 0.14; eFIG. 1, http://links.lww.com/CCX/B136). The median ICU LOS for the entire study sample never exceeded 2.5 days for a given hospital admission month (eFIG. 2, http://links.lww.com/CCX/B136).
DISCUSSION
We performed a prospective before-and-after study at a single community hospital ICU to explore the impact on patient outcomes of transitioning from a low-intensity to a high-intensity intensivist staffing model. Analysis using a multivariable logistic regression model did not demonstrate a significant association between staffing model and in-hospital or 30-day mortality, nor was there an association between staffing model and ICU LOS. We did note a shift in the usage of ICU resources between staffing models with an increased proportion of patients under the high-intensity staffing model being admitted for active ICU-based interventions (e.g., vasoactive medications, mechanical ventilation) and a higher proportion of patients under the low-intensity staffing model being admitted to the ICU without a specific ICU need (e.g., close observation).
Transition to a high-intensity staffing model was not associated with changes in mortality or ICU LOS.
Although overall mortality was lower during the higher intensity staffing period, multivariable models suggest that these changes were largely driven not by the staffing model change but rather by changes between the two time periods in the proportion of patients admitted for cardiac surgery versus medical indications, as well as changes in patient age and acuity. Admission for cardiac or cardiac surgery indications was associated with an 82% decrease in odds of in-hospital mortality and a 79% decrease for 30-day mortality relative to admission for a medical indication. Likely, this is related to the significantly lower in-hospital mortality rates reported with cardiac surgery procedures (2–4% [22–24]) relative to those reported for common medical ICU indications such as septic shock (40–50% [25, 26]) and acute respiratory distress syndrome (35–50% [27]). During the high-intensity staffing model time period, the proportion of admissions for cardiac or cardiac surgery indications increased from 19.7% to 28.9%, while admissions for medical indications decreased from 51.5% to 42.7%. Patient age and acuity also slightly decreased during the high-intensity staffing model time frame, and both of these changes were also associated with decreased odds of mortality. ICU LOS did not significantly change between staffing models in either unadjusted or adjusted analysis.
Our findings differ somewhat from those previously reported (12–14), in which LOS reductions were observed for at least a subset of patients in all studies, and mortality improvements were seen in some, but not all, studies (12, 14). One potential source of discrepancy may relate to differences in study setting. Our setting was a community hospital multidisciplinary ICU, whereas most prior reports have examined only the medical ICU at academic centers. Observed effects may not directly translate between these sites. Also, a statistically significant increase in the proportion of cardiac and cardiovascular surgery patients occurred between study periods, and patients of this type had significantly lower mortality rates and longer lengths of stay in our multivariable analyses. Sensitivity to such changes in disciplinary demographic may limit our ability to detect other underlying changes in ways not experienced by studies of single-discipline ICUs.
Transition to a High-Intensity Staffing Model Reduced Admissions Without a Specific ICU Indication
We observed a statistically significant shift toward ICU admissions for ICU-specific interventions (e.g., vasopressors, mechanical ventilation) and away from “soft” indications (e.g., close monitoring) associated with the transition to the higher intensity staffing model. We believe that this may be due at least in part to increased intensivist participation in the triaging process, leading to more judicious use of ICU beds. This economization of space was further reflected by a statistically significant decrease in ICU census at the time of each patient’s admission under the high-intensity staffing model.
The need for strategies to ensure optimal use of ICU space has been underscored by a number of studies showing associations between mortality and ICU operation at or near maximum capacity (28–30). The use of decision support tools to assist with ICU triage can reduce inappropriate (“too well”) ICU admissions in some settings (31), but in others has been shown to exclude ICU admission for patients who might benefit from it (32). Consequently, the ICU triaging guidelines of the Society of Critical Care Medicine (33) as well as the World Federation of Societies of Intensive and Critical Care Medicine (34) both recommend direct involvement of intensivists in triage decisions. The observed reduction in patients admitted to the ICU without a direct ICU need under the high-intensity staffing model would seem to support these guidelines.
ICU Staffing Model and COVID-19 Surge Management
The recent COVID-19 pandemic strained ICU resources throughout the world with adverse effects to patient outcomes (35, 36). At the hospital examined in this study, two findings associated with the higher intensity model were particularly helpful during local surges. First, the association between increased intensivist involvement and tighter triage helped to minimize the ICU load, and this association was extrapolated to justify the creation of a system-wide triage intensivist position. Second, staffing has been identified as a chief driver of capacity limitations in analyses of ICU operations during the pandemic (37, 38). Because the study hospital had recently increased its standing ICU staff complement of the hospitals in our system, this one required the fewest ad hoc staffing additions to manage the increased capacity.
Limitations
The limitations of this study are several-fold. First, it is limited to a single center. Second, the observational nature of the study prevents assignment of causality. Third, the before-and-after design may introduce temporal biases such as those related to seasonal changes in disease (e.g., respiratory viral season) or simultaneous but unrecognized temporal shifts in other factors related to ICU care (e.g., turnover of nursing staff). Finally, the overall census numbers for the center were relatively low under both the low- and high-intensity models compared with similar studies of the same nature, which may limit generalizability.
CONCLUSIONS
In this before-and-after study examining the transition to a high-intensity ICU staffing model, multivariable analysis showed no association of this transition with all-cause in-hospital and 30-day mortality. However, this change was associated with a statistically significant decrease in the proportion of patients admitted to the ICU without the need for an ICU-specific intervention. The observational nature of our study precludes determination of causality; thus, further research will be needed to determine whether this triaging effect is indeed due to increased intensivist involvement.
Supplementary Material
Footnotes
Dr. Proper and Dr. Wacker contributed equally to this work.
This work was funded by the University of Minnesota Critical Care Program (grant to Dr. Reilkoff). Support was also provided by the University of Minnesota Clinical and Translational Science Institute (Grant Number UL1TR002494 from the National Institutes of Health’s National Center for Advancing Translational Sciences) in the form of access to Research Electronic Data Capture database software.
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Angus DC, Shorr AF, White A, et al. : Critical care delivery in the United States: Distribution of services and compliance with Leapfrog recommendations. Crit Care Med 2006; 34:1016–1024 [DOI] [PubMed] [Google Scholar]
- 2.Garland A, Gershengorn HB: Staffing in ICUs: Physicians and alternative staffing models. Chest 2013; 143:214–221 [DOI] [PubMed] [Google Scholar]
- 3.Holdorf JD, Lilly CM: Intensivist staffing: Evolving challenges and solutions. Semin Respir Crit Care Med 2015; 36:842–850 [DOI] [PubMed] [Google Scholar]
- 4.Wilcox ME, Harrison DA, Short A, et al. : Comparing mortality among adult, general intensive care units in England with varying intensivist cover patterns: A retrospective cohort study. Crit Care 2014; 18:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace DJ, Angus DC, Barnato AE, et al. : Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med 2012; 366:2093–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershengorn HB, Harrison DA, Garland A, et al. : Association of intensive care unit patient-to-intensivist ratios with hospital mortality. JAMA Intern Med 2017; 177:388–396 [DOI] [PubMed] [Google Scholar]
- 7.Tarnow-Mordi WO, Hau C, Warden A, et al. : Hospital mortality in relation to staff workload: A 4-year study in an adult intensive-care unit. Lancet 2000; 356:185–189 [DOI] [PubMed] [Google Scholar]
- 8.Gershengorn HB, Pilcher DV, Litton E, et al. : Association of patient-to-intensivist ratio with hospital mortality in Australia and New Zealand. Intensive Care Med 2022; 48:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JJ, Sullivan G: Effect on ICU mortality of a full-time critical care specialist. Chest 1989; 96:127–129 [DOI] [PubMed] [Google Scholar]
- 10.Pronovost PJ, Jenckes MW, Dorman T, et al. : Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA 1999; 281:1310–1317 [DOI] [PubMed] [Google Scholar]
- 11.Treggiari MM, Martin DP, Yanez ND, et al. : Effect of intensive care unit organizational model and structure on outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2007; 176:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawari FI, Al Najjar TI, Zaru L, et al. : The effect of implementing high-intensity intensive care unit staffing model on outcome of critically ill oncology patients. Crit Care Med 2009; 37:1967–1971 [DOI] [PubMed] [Google Scholar]
- 13.Wise KR, Akopov VA, Williams BR, Jr, et al. : Hospitalists and intensivists in the medical ICU: A prospective observational study comparing mortality and length of stay between two staffing models. J Hosp Med 2012; 7:183–189 [DOI] [PubMed] [Google Scholar]
- 14.Tanios MA, Teres D, Park H, et al. : The impact of implementing an intensivist model with nighttime in-hospital nocturnist and effect on ICU outcomes. J Intensive Care Med 2020; 35:461–467 [DOI] [PubMed] [Google Scholar]
- 15.Miller PE, Chouairi F, Thomas A, et al. : Transition from an open to closed staffing model in the cardiac intensive care unit improves clinical outcomes. J Am Heart Assoc 2021; 10:e018182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy MM, Rapoport J, Lemeshow S, et al. : Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa DK, Wallace DJ, Kahn JM: The association between daytime intensivist physician staffing and mortality in the context of other ICU organizational practices: A multicenter cohort study. Crit Care Med 2015; 43:2275–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pronovost PJ, Angus DC, Dorman T, et al. : Physician staffing patterns and clinical outcomes in critically ill patients: A systematic review. JAMA 2002; 288:2151–2162 [DOI] [PubMed] [Google Scholar]
- 19.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16:1141–1154 [Google Scholar]
- 20.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509 [Google Scholar]
- 21.Ferreira FL, Bota DP, Bross A, et al. : Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286:1754–1758 [DOI] [PubMed] [Google Scholar]
- 22.Mazzeffi M, Zivot J, Buchman T, et al. : In-hospital mortality after cardiac surgery: Patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg 2014; 97:1220–1225 [DOI] [PubMed] [Google Scholar]
- 23.Manlhiot C, Rao V, Rubin B, et al. : Comparison of cardiac surgery mortality reports using administrative and clinical data sources: A prospective cohort study. CMAJ Open 2018; 6:E316–E321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan PG, Seese L, Aranda-Michel E, et al. : Operative mortality in adult cardiac surgery: Is the currently utilized definition justified? J Thorac Dis 2021; 13:5582–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar-Hari M, Phillips GS, Levy ML, et al. : Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellani G, Laffey JG, Pham T, et al. : Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 28.Wilcox ME, Harrison DA, Patel A, et al. : Higher ICU capacity strain is associated with increased acute mortality in closed ICUs. Crit Care Med 2020; 48:709–716 [DOI] [PubMed] [Google Scholar]
- 29.Anesi GL, Gabler NB, Allorto NL, et al. : Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: A 2-hospital study in South Africa. J Intensive Care Med 2020; 35:1104–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bihari S, McElduff P, Pearse J, et al. : Intensive care unit strain and mortality risk in patients admitted from the ward in Australia and New Zealand. J Crit Care 2022; 68:136–140 [DOI] [PubMed] [Google Scholar]
- 31.Ramos JGR, Ranzani OT, Perondi B, et al. : A decision-aid tool for ICU admission triage is associated with a reduction in potentially inappropriate intensive care unit admissions. J Crit Care 2019; 51:77–83 [DOI] [PubMed] [Google Scholar]
- 32.Guest T, Tantam G, Donlin N, et al. : An observational cohort study of triage for critical care provision during pandemic influenza: “Clipboard physicians” or “evidenced based medicine?”. Anaesthesia 2009; 64:1199–1206 [DOI] [PubMed] [Google Scholar]
- 33.Nates JL, Nunnally M, Kleinpell R, et al. : ICU admission, discharge, and triage guidelines: A framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med 2016; 44:1553–1602 [DOI] [PubMed] [Google Scholar]
- 34.Blanch L, Abillama FF, Amin P, et al. : Triage decisions for ICU admission: Report from the task force of the World Federation of Societies of Intensive and Critical Care Medicine. J Crit Care 2016; 36:301–305 [DOI] [PubMed] [Google Scholar]
- 35.Demoule A, Fartoukh M, Louis G, et al. : ICU strain and outcome in COVID-19 patients-a multicenter retrospective observational study. PLoS One 2022; 17:e0271358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcox ME, Rowan KM, Harrison DA, et al. : Does unprecedented ICU capacity strain, as experienced during the COVID-19 pandemic, impact patient outcome? Crit Care Med 2022; 50:e548–e556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vranas KC, Golden SE, Mathews KS, et al. : The influence of the COVID-19 pandemic on ICU organization, care processes, and frontline clinician experiences: A qualitative study. Chest 2021; 160:1714–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litton E, Huckson S, Chavan S, et al. : Increasing ICU capacity to accommodate higher demand during the COVID-19 pandemic. Med J Aust 2021; 215:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]