Abstract
Background Polycythemia vera (PV) is a myeloproliferative disease with overproduction of erythrocytes, leukocytes, and platelets causing an increased risk of both thrombosis and hemorrhage. There are limited reports and no established guidelines for managing such patients undergoing reconstructive surgery.
Methods We present four patients with PV and head and neck cancer who required reconstruction after resection and provide a review of the current literature.
Results Preoperatively, patients on cytoreductive therapy continued with their treatment throughout their hospital course and had hematologic parameters normalized with phlebotomy or transfusions if needed. Two patients who underwent free flap surgery (cases 1 and 2) had postoperative courses complicated by hematoma formation and persistent anemia, requiring multiple transfusions. Cases 3 and 4 (JAK2+ PV and JAK2− PV, respectively) underwent locoregional flap without postoperative complications.
Conclusion Concomitant presentation of PV and head and neck cancer is uncommon and presents unique challenges for the reconstructive surgeon. Overall, we recommend that patients should have hematologic parameters optimized prior to surgery, continue ruxolitinib or hydroxyurea, and hold antiplatelet/anticoagulation per established department protocols. It is essential to engage a multidisciplinary team involving hematology, head and neck and reconstructive surgery, anesthesia, and critical care to develop a standardized approach for managing this unique subset of patients.
Keywords: microvascular free flap, plastic surgery, reconstruction, polycythemia vera, reconstructive surgery, hydroxyurea, ruxolitinib, head and neck cancer, microvascular surgery, head and neck surgery
Introduction
Polycythemia vera (PV) is a myeloproliferative disease with overproduction of erythrocytes, leukocytes, and platelets (PLTs) causing an increased risk of both thrombosis and hemorrhage. There are two types of PV: primary PV, which is due to intrinsic mutations within hematopoietic cell lines, most commonly via JAK2 mutation, and secondary PV, which is due to chronic hypoxic conditions or mutations that cause the overproduction of erythropoietin, which is not seen in primary PV. 1 2 Thromboses, both arterial and venous, are the most lethal complications of PV. Up to 39% of patients present with major thrombotic events at the time of diagnosis. 2
There are limited studies regarding surgical management among these patients and even fewer regarding reconstructive surgery and the challenges in perioperative management. We report our experience from a single academic institution for patients ( n = 4) with a diagnosis of PV ( n = 3 with JAK2+ PV; n = 1 with JAK2− PV) who underwent head and neck reconstructive surgery. We also present a review of the literature regarding surgical management in PV. Additionally, we provide perioperative recommendations for patients with PV undergoing reconstructive surgery.
Methods
This study was approved by the Institutional Review Board within our institution. We performed a retrospective review of PV patients who required defect reconstruction for the head and neck with either a locoregional flap and/or microvascular free flap. Data were abstracted from medical charts at a single institution over a 1-year period from 2019 to 2020. Patients were identified by Current Procedural Terminology and International Classification of Diseases (ICD) codes (ICD-9: 238.4, ICD-10: D45) ( Table 1 ).
Table 1. CPT codes used to study the database.
| 15732 | Muscle, myocutaneous, or fasciocutaneous flap; head and neck or trunk |
| 15756 | Free muscle or myocutaneous flap with microvascular anastomosis |
| 15757 | Free skin flap with microvascular anastomosis |
| 15758 | Free fascial flap with microvascular anastomosis |
| 20955 | Bone graft with microvascular anastomosis; fibula |
| 20956 | Bone graft with microvascular anastomosis; iliac crest |
| 20962 | Bone graft with microvascular anastomosis; other than fibula, iliac crest, or metatarsal |
Abbreviation: CPT, Current Procedural Terminology.
We have a standard head and neck reconstructive surgery protocol within our institution. For preoperative management, patients are optimized for surgery with regard to chronic conditions. If patients are on anticoagulation (e.g., apixaban), these are typically withheld for a few days before surgery. Intraoperatively, 150 mg of aspirin is given at the end of the surgery, and 81 mg thereafter for 30 days, starting on postoperative day (POD) 1. Perioperative antibiotic prophylaxis with cefazolin and metronidazole are given for 24 hours. Venous thromboembolism (VTE) prophylaxis is started on POD1 with subcutaneous enoxaparin, 30 mg BID. Flap monitoring is done using a combination of physical examination and Doppler ultrasound probes, both implantable and/or handheld. Flaps checks for implantable Doppler signals, color, turgor, capillary refill, and overall appearance occur every hour (Q1h) for the first 24 hours, Q2h on POD2 to 3, Q4h on POD4, and Q8h from POD5 until discharge.
A systematic review was performed following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses ( Fig. 1 ). Articles in English using the PubMed database for the keywords “reconstructive surgery, head and neck surgery, microvascular surgery, and PV” were selected for full article review after screening for relevance based on title and abstract by two authors (S.D. and R.T.).
Fig. 1.
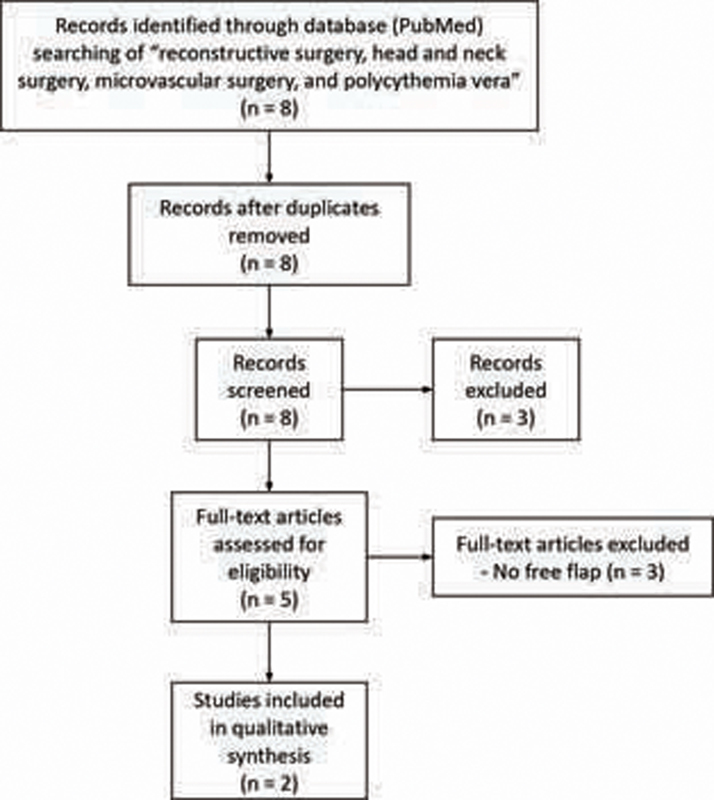
Flow diagram of systematic literature review.
Case Presentations
Case 1
A 66-year-old man with JAK2+ PV on chronic ruxolitinib, chronic obstructive pulmonary disease, alcohol and tobacco use presented with squamous cell carcinoma (SCC) of the mandible (pT4aN3b) and bilateral cervical metastases. He underwent an anterior composite mandible resection, bilateral neck dissection, and two free flap reconstructions—right fibula and right anterolateral thigh. His preoperative laboratories are presented in Table 2 . Aspirin was held and the patient was transfused with 1 unit (U) of PLTs due to thrombocytopenia. His operative course was complicated by blood loss requiring multiple blood transfusions.
Table 2. Clinical components of previously published head and neck free flap transfer cases and cases reported in this article.
| Previously published reports | Time | Preoperative laboratories and medications | Intraoperative course/complications | Postoperative laboratories | Postoperative complications/course | ||
|---|---|---|---|---|---|---|---|
| Lin and Yu 4 | Resection of mandible |
Hgb—18.5 (15.9
a
)
Hct—52.7% |
No blood transfusions or anticoagulation EBL = 400 mL |
Hgb—12 Hct—38% |
No thrombotic events and flap survived. Received daily aspirin 81 mg and no other anticoagulation | ||
| Ghazali et al 5 | Resection of mandible | Hgb—14.8 Hct—37.6% |
Aspirin Hydroxyurea |
No blood transfusions or anticoagulation was used | Hgb—9.7 Hct—30.6% |
Transient sinus bradycardia, otherwise uneventful. Continued aspirin and hydroxyurea. Received daily subcutaneous LMWH | |
| Current series | |||||||
| Case 1 | Resection of mandible Flaps: 1. Right fibula flap (9 cm × 6 cm) to mandible 2. Right ALT flap (15 cm × 8 cm) to submental/central neck 3. Left pectoralis major (8 cm × 4 cm) to oral cavity and neck 4. STSG from left thigh (4 cm × 3 cm) to right lower extremity |
34 d | Hgb—10.0 Hct—31% PLT—55,000 b PT—16.5 PTT—34.4 |
Aspirin Ruxolitinib |
Received a total of 4 U of pRBCs, 6 L of crystalloid solution, 1.2 L of colloid solution EBL = 550 mL |
Hgb—6.4 Hct—18.4% PLT—76,000 PT—19.7 PTT—57.9 |
Persistent anemia requiring five separate blood transfusions, right thigh hematoma at the ALT free flap donor site, poor wound healing leading to neck flap dehiscence requiring partial reconstruction and repair with pectoralis flap. Prolonged antibiotic therapy. No thrombosis or hemorrhage |
| Case 2 | Resection of temporalis AVM Flap: Left anteromedial thigh flap (15 cm × 7 cm) to temple |
56 d | Hgb—10.1 Hct—30.9% PLT—320,000 PT—15.1 PTT—57.8 |
Ruxolitinib Apixaban |
Received 2.3 L of crystalloid, 250 mL of colloid solution, and 25 mg of ephedrine EBL = 250 mL |
Hgb—9.3 Hct—27.9% PLT—292,000 PT—15.1 PTT—40.1 |
Left scalp and thigh hematomas requiring 1 U of pRBC transfusion, washout, and flap exploration. Aspirin 325 mg once after AVM embolization. Aspirin 81 mg on POD2 of flap |
| Case 3 | Resection of mandible Flap: Supraclavicular locoregional flap (8 cm × 25 cm) to mandible |
23 d | Hgb—9.4 Hct—29% PLT—1,185,000 c |
Aspirin Hydroxyurea |
Received 1.6 L of lactated ringers, 1 L of NS and 2 U of pRBCs EBL = 50 mL |
Hgb—8.1 Hct—22.9% PLT—217,000 |
Received an additional 1 U of pRBC on POD2 due to a Hgb drop (6.9, from 8.1). Restarted enoxaparin 30 mg on POD1, aspirin 81 mg on POD2 |
| Case 4 | Resection of temporal bone, auricle, and parotid Flap: SCM, temporalis locoregional flap (5 cm × 6 cm) to temporal bone defect |
92 d | Hgb—13.9 Hct—43% PLT—not reported |
Aspirin Clopidogrel Enoxaparin |
Received 1 L of albumin, 1 L of lactated ringers, 2 L of NS and 2 L of crystalloid solution | Hgb—12.6 Hct—36.4% PLT—218,000 |
81 mg starting on POD3. Apixaban was resumed on POD6 |
Abbreviations: ALT, anterolateral thigh; AVM, arteriovenous malformation; EBL, estimated blood loss; Hct, hematocrit; Hgb, hemoglobin (g/dL); LMWH, low-molecular-weight heparin; NS, normal saline; PLT, platelet (per μL); POD, postoperative day; pRBC, packed red blood cell; PT, prothombin time (second); PTT, partial thromboplastin time (second); SCM, sternocleidomastoid; STSG, split-thickness skin graft; U, unit.
Note: “Time” describes time from presentation to surgery.
Postphlebotomy.
Platelet transfusion required.
Most recent available laboratory value obtained more than 2 weeks prior to surgery.
On POD1, he was started on VTE prophylactic dosing of enoxaparin at 30 mg twice daily but stopped on POD8 due to bleeding complications. Aspirin was not restarted due to persistent postoperative thrombocytopenia. Ruxolitinib was continued for the entirety of hospitalization. Donor-site hematoma was developed on POD5 and was evacuated; prothrombin time (PT) and partial thromboplastin time (PTT) were within normal limits at the time. Poor wound healing and neck flap dehiscence were noted on POD11 which required return to the operating room (OR) for washout and partial reconstruction with a pectoralis flap on POD12. He was discharged on POD23 with no further complications on follow-up a week later ( Fig. 2 ).
Fig. 2.

Hematocrit trends throughout hospital courses. Case 1: 66-year-old man JAK2+ PV on ruxolitinib. Case 2: 71-year-old man JAK2+ PV on ruxolitinib. Case 3: 82-year-old woman JAK2+ PV on hydroxyurea. Case 4: 64-year-old man JAK2 PV with serial phlebotomies. PV, polycythemia vera.
Case 2
A 71-year-old man with JAK2+ PV on ruxolitinib and history of pulmonary embolism (PE) on apixaban presented with a large, painful arteriovenous malformation (AVM, pT1) of the left temple and cheek. AVM embolization was performed 2 days before resection and anteromedial thigh free flap reconstruction ( Table 2 ; Figs. 3 and 4 ). He received 325 mg of aspirin after AVM embolization. Apixaban was held 2 days prior to surgery. His preoperative and postoperative PT/PTT were elevated. He remained on ruxolitinib throughout his hospitalization. After surgery, he received VTE prophylaxis with enoxaparin 30 mg twice daily and was started on aspirin 81 mg on POD2. However, on POD5, he developed left scalp and thigh hematomas, causing acute anemia from a hemoglobin (Hgb) of 7.2 to 6.6 g/dL, and PT/PTT was 15.4/41.8 seconds. He required 1 U of packed red blood cell (pRBC) transfusion and returned to the OR for a washout and flap exploration. Anticoagulation was held for 3 days following his return from the OR and he was started on a heparin drip 4 days later due to a history of PE. Heparin was bridged to apixaban on the day before discharge (POD12). On follow-up 10 days after discharge, the patient was doing well without complications ( Fig. 5 ).
Fig. 3.
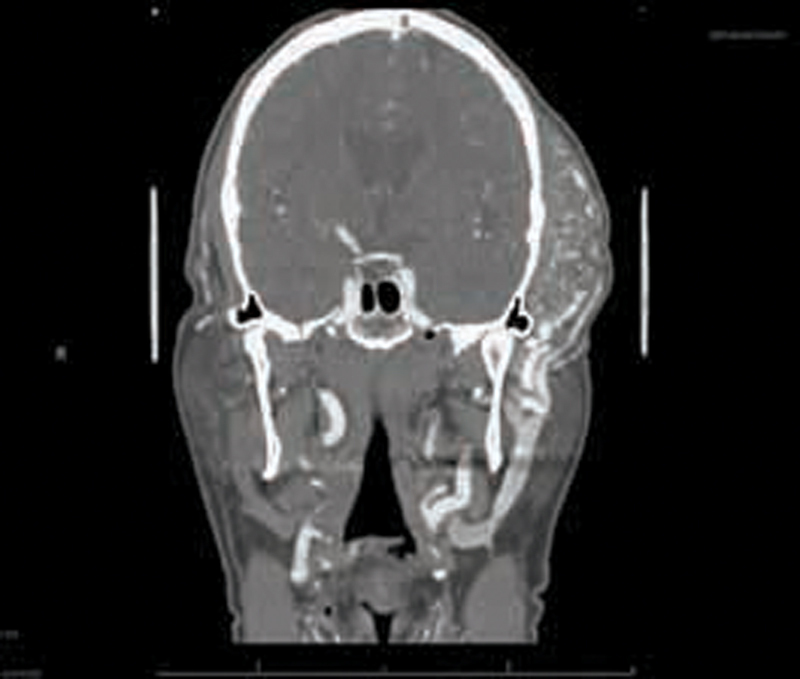
AVM seen on computed tomography angiogram of the head. Case 2: 71-year-old man JAK2+ PV on ruxolitinib who presented with AVM of temporal region requiring microvascular free flap reconstruction with anteromedial thigh free flap. AVM, arteriovenous malformation; PV, polycythemia vera.
Fig. 4.
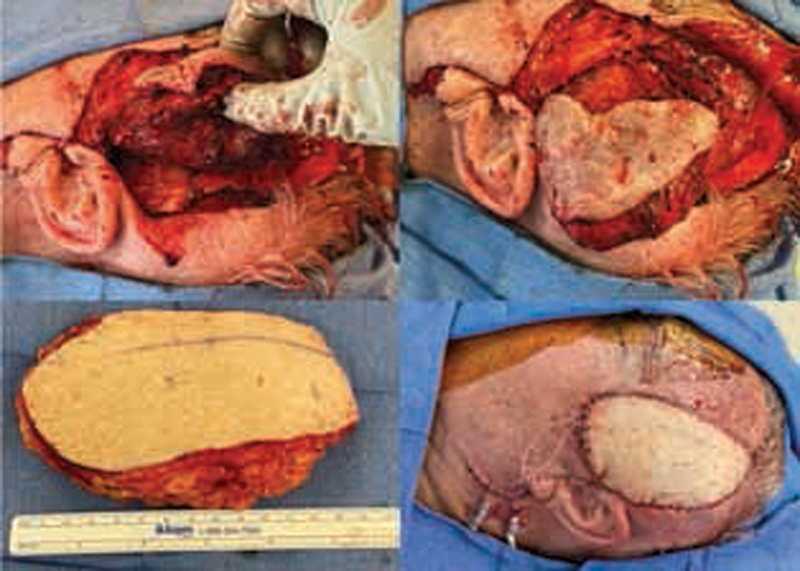
AVM resection with anteromedial thigh free flap reconstruction. Case 2: 71-year-old man JAK2+ PV on ruxolitinib who presented with AVM of temporal region requiring microvascular free flap reconstruction with anteromedial thigh free flap. AVM, arteriovenous malformation; PV, polycythemia vera.
Fig. 5.
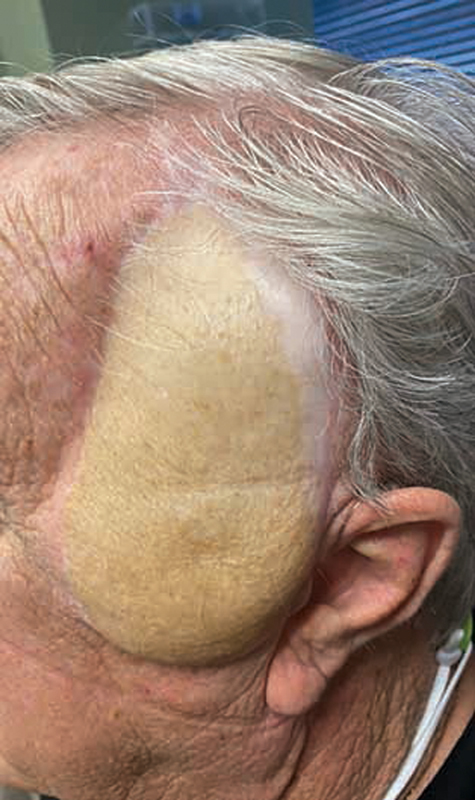
Case 2 approximately 1 year after free flap reconstruction.
Case 3
An 82-year-old woman with JAK2+ PV and essential thrombocythemia on hydroxyurea presented with a large SCC originating from the left floor of the mouth. Surgery was performed 23 days after the initial presentation. She underwent a segmental mandibulectomy with a left supraclavicular island flap ( Table 2 , Fig. 6 ). Preoperatively, she had moderate anemia and thrombocytosis. She remained on hydroxyurea and aspirin throughout the perioperative period. EBL was 50 mL. Postoperatively, her anemia worsened but did not require immediate transfusion and her PLT count normalized. She was started on VTE prophylaxis with enoxaparin at 30 mg daily on POD1 and aspirin 81 mg on POD2. She received 1 U of pRBC on POD2 due to a Hgb drop from 8.1 to 6.9 g/dL without signs of bleeding. Her postoperative course was otherwise unremarkable, and she was discharged home on POD6. On follow-up 8 days after discharge, she was doing well without complications.
Fig. 6.
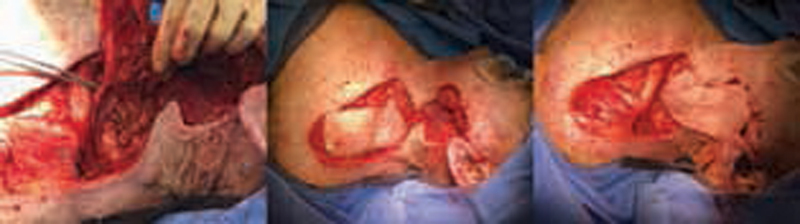
Case 3: 82-year-old woman with JAK2+ PV and essential thrombocythemia on hydroxyurea and aspirin who underwent a locoregional flap.
Case 4
A 64-year-old man with JAK2− PV managed by serial phlebotomies, atrial fibrillation on apixaban, and coronary artery disease on dual antiplatelet therapy (aspirin, clopidogrel) presented with SCC (pT3N0) of the left auricle and cartilaginous ear canal. Surgery was delayed from initial presentation due to need for cardiac catheterization and dual antiplatelet therapy for at least 30 days. He underwent left temporal bone resection with partial auriculectomy, parotidectomy, and left neck dissection and locoregional reconstruction with left sternocleidomastoid and left temporalis muscle and split-thickness skin graft ( Table 2 ). He required preoperative phlebotomies for polycythemia. Aspirin, clopidogrel, and apixaban were held the morning of surgery. EBL was 100 mL. POD1 laboratories revealed normal RBC and PLT counts. On POD3, he restarted daily 81 mg aspirin. Due to hematuria, apixaban was not restarted until POD6. His postoperative course was uncomplicated, and he was discharged home on POD7. However, he returned to the emergency department 2 days later due to bleeding around his neck drain which resolved with suture reinforcement. He recovered without complications.
Discussion
PV is a myeloproliferative disease with overproduction of erythrocytes, leukocytes, and PLTs causing an increased risk of both thrombosis and hemorrhage. Up to 39% of patients present with major thrombotic events at the time of diagnosis. 2 As a result, PV presents a unique challenge for head and neck reconstruction as the increased risk of bleeding and/or thrombosis may lead to flap failure and wound complications. In patients with PV, malignancy and thrombotic complications are the leading causes of death, and median survival is 19 years after diagnosis. 3 There are limited studies on surgical management in this population and few that have specifically focused on head and neck reconstructive surgery and associated challenges in perioperative management. We present four cases of head and neck reconstruction after tumor resection highlighting the risk of postoperative complications in PV patients. Moreover, we discuss the lessons learned in medical management before and after major head and neck surgery.
To the best of our knowledge, there are only two previously reported cases of patients with PV who have undergone successful microvascular free flap surgery, summarized in Table 2 . One reported a mandibulectomy followed by free flap transfer for oral SCC. 4 The other reported a microvascular free flap with bisphosphonate-induced mandibular osteonecrosis. 5 In our case series, two patients underwent microvascular reconstruction with one patient requiring two free flaps. With no evidence of intraoperative or postoperative microvascular thrombosis in our patients nor in previously published reports, we would recommend microvascular reconstruction as a safe option in patients with PV. The two patients with regional flap reconstruction did well postoperatively without wound dehiscence or partial flap necrosis. In case 3, the patient was offered both free tissue reconstruction and regional flap reconstruction with the patient ultimately deciding to pursue regional reconstruction. It is our recommendation that all surgical reconstructive options should be available to patients with a final reconstructive plan made from joint decision-making between the surgeon and patient. All patients with PV required extensive discussions with hematology teams before undergoing head and neck reconstruction.
Cytoreductive therapy remains the mainstay for treatment of high-risk PV with goals of hematocrit (Hct) less than 45% in men and less than 42% in women. 6 7 In untreated patients, risk of arterial thrombosis (hazard ratio [HR]: 2.0) and venous thrombosis (HR: 4.7) is elevated. 6 7 Low-risk patients (age < 60 years without a history of thrombosis) can be treated with phlebotomy alone, while high-risk patients (age ≥ 60 years with or without a history of thrombosis) are also treated with cytoreductive agents. 6 7 The recommended first-line cytoreductive agent is hydroxyurea. Patients refractory to hydroxyurea are often treated with ruxolitinib, a tyrosine kinase inhibitor. Although several studies suggest that cytoreductive therapy improves thrombosis-related outcomes, these patients remain at an increased risk for adverse events despite controlled disease and anticoagulation.
The National Comprehensive Cancer Network (NCCN) recommends that cytoreductive therapy should be continued throughout the perioperative period, but that dose adjustments may be required before and after surgery. 8 We recommend goal Hct levels of less than 42% (women) and 45% (men) based on cytoreductive therapy goals and NCCN recommendations. 8 These levels are associated with lower risks of cardiovascular death 9 and thrombotic events. 8 The NCCN also recommends control of Hct for 3 months before elective surgery; however, this time is not always available based on the patient's disease. 8 A previous study on PLT thresholds in microvascular surgeries showed that patients who experienced venous thrombosis had higher preoperative PLT values as compared with those with arterial thrombosis. 10 Moreover, PLT levels of 200,000/μL or less were associated with higher salvage success rates. 10 Cho et al reported an increased risk of flap thrombosis in trauma patients with PLT levels more than 361,000/μL. 11 Similarly, Tarabishy et al reported an increased risk for blood transfusion in patients with preoperative thrombocytosis (> 450,000/μL). 12 Therefore, based on available evidence and a lack of literature on the current population, we recommend PLT levels to be maintained above the lower limit of normal and below 361,000/μL with a goal of approximately 200,000/μL to optimize postoperative outcomes.
Aspirin should be held 7 days before surgery when feasible and restarted 24 hours after surgery when bleeding risk is acceptable. 8 Careful discussions should be had with the hematology team regarding dose adjustments of cytoreductive agents especially with ruxolitinib. Surgery should be postponed to achieve optimal cell count levels but should not significantly interfere with timely oncologic care in head and neck cancer patients.
Our two patients requiring microvascular reconstruction were both on ruxolitinib, a scenario not previously reported upon in head and neck reconstruction. Though neither patient had microvascular compromise nor free flap failure, they both developed hematomas requiring surgical intervention and blood transfusions. Review of these cases leads us to conclude that, though cytoreductive agents are vital to treatment of PV, aggressive treatment can also lead to anemia, thrombocytopenia, and risk of bleeding. Patient 1 presented to surgery with adequate Hct levels but also had significant thrombocytopenia due to ruxolitinib. Patient 2 presented with a low Hct of 35 and developed a hematoma requiring blood transfusions. Both cases exemplify the need to create better thresholds for hematologic parameters preoperatively so that a careful balance can be maintained between reducing thrombotic risk and bleeding risk.
When patients present with a high risk of VTE, the surgical and hematology teams must decide the appropriate timing for cessation and initiation of anticoagulation. Patients may be already at risk of bleeding complications due to thrombocytopenia combined with the risk of major head and neck surgery. In case 2, the patient had a history of PE and apixaban was held prior to surgery. Afterward, he developed a hematoma after starting prophylactic anticoagulation. Ultimately, after surgical control of bleeding, the patient was placed on a heparin drip and bridged to apixaban. The patient in case 4 had a history of atrial fibrillation and his apixaban was also held before surgery. His anticoagulation was restarted when his bleeding risk was acceptable, on POD 3, and he did not have any bleeding events. In those patients undergoing free flap reconstruction, when bleeding risk is high, we recommend consideration of heparin bridging therapy before restarting preoperative anticoagulation regimen. Otherwise, prophylactic or therapeutic anticoagulation should be restarted as soon as the surgeon feels it is safe.
Several other factors should be considered with head and neck reconstruction in PV patients. Chronic hydroxyurea therapy can cause nonhealing ulcers, typically perimalleolar. 13 14 15 16 The mechanism is unclear but appears to be related to cutaneous atrophy and poor wound healing from megaloblastic erythrocytes poorly perfusing small capillary beds. 17 Ulcers typically resolve within weeks after discontinuing hydroxyurea. 15 17 Ruxolitinib also has immunosuppressive effects and increases risk for both bacterial and viral infections; therefore, patients should be monitored closely for postoperative infection. 7 18 In our case series, we have found that routine postsurgical antibiotic prophylaxis to be adequate. Poor wound healing and infection were noted in our patient in case 1 who was on ruxolitinib, but he eventually was able to heal with a secondary regional flap. Finally, it should be noted that PV naturally predisposes an increased risk toward splenomegaly, 3 which is a marker of response to therapy, and should be assessed prior to placement of a gastronomy tube (G-tube). We have provided a summary of our recommendations based on the discussed literature and experiences within our institution Fig. 7 .
Fig. 7.
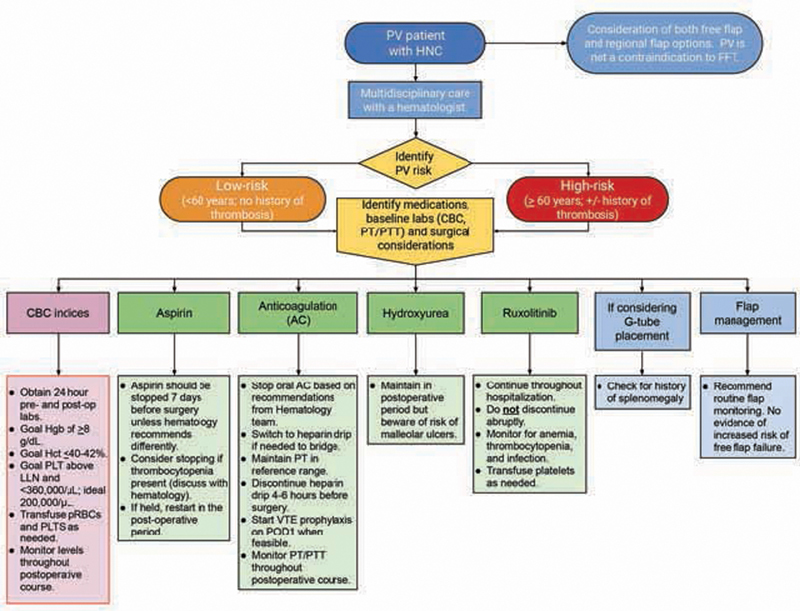
Flow diagram of recommendations for preoperative and postoperative management of the PV patient with HNC undergoing reconstruction surgery. CBC, complete blood count; FFT, free flap transfer; G-tube, gastrostomy tube; Hct, hematocrit; Hgb, hemoglobin; HNC, head and neck cancer; LLN, lower limit of normal; PLT, platelet; POD, postoperative day; pRBC, packed red blood cell; PT, prothrombin time; PTT, partial thromboplastin time; PV, polycythemia vera; VTE, venous thromboembolism.
Conclusion
Concomitant presentation of PV and head and neck cancer is uncommon and presents unique challenges for the reconstructive surgeon. These patients have complex hematologic issues with increased thrombotic and bleeding complications which are both part of the disease and treatment. We report four patients with PV who successfully underwent head and neck reconstruction. Though these patients did not experience flap failure, complications included persistent preoperative and postoperative cytopenias requiring transfusions, hematoma formation, and wound dehiscence. Quality improvement initiatives for the care of these patients should include multidisciplinary collaboration with the patients' hematologists as soon as the decision to pursue surgery is made. Per NCCN recommendations, cell counts should be optimized 3 months prior to surgery; however, this may not be possible due to the aggressive nature of some cancers. We recommend maintaining preoperative Hct less than 42% (women) and less than 45% (men) and PLT counts around 200,000/μL (with high risk for thrombosis above 360,000/μL). Careful examination for splenomegaly should occur before G-tube placement. Patients with medication-induced cytopenias before surgery may need dosing adjustments. Hematology inpatient consultation is recommended to guide the use of cytoreductive agents, antiplatelet therapies, and anticoagulation. The microvascular surgeon should also be aware of cytoreductive therapy side effects including wound healing, infection, and cytopenias in these patients. Based on our experiences, the presence of PV does not preclude successful flap reconstruction.
Footnotes
Conflict of Interest None declared.
References
- 1.Briere P J.Secondary polycythemiaIn: Orphanet Ency-clopedia. Last accessed in December 2020 at:https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=98428
- 2.Cuthbert D, Stein B L. Polycythemia vera-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. J Blood Med. 2019;10:359–371. doi: 10.2147/JBM.S189922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A, Rumi E, Finazzi G. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(09):1874–1881. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S J, Yu P. Free tissue transfer in a head and neck cancer patient with polycythemia vera. Plast Reconstr Surg. 2009;124(01):169e–170e. doi: 10.1097/PRS.0b013e3181a83a4a. [DOI] [PubMed] [Google Scholar]
- 5.Ghazali N, Collyer J C, Tighe J V. Hemimandibulectomy and vascularized fibula flap in bisphosphonate-induced mandibular osteonecrosis with polycythaemia rubra vera. Int J Oral Maxillofac Surg. 2013;42(01):120–123. doi: 10.1016/j.ijom.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Griesshammer M, Kiladjian J-J, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019;98(05):1071–1082. doi: 10.1007/s00277-019-03625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbui T, Tefferi A, Vannucchi A M. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(05):1057–1069. doi: 10.1038/s41375-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesa R A, Jamieson C, Bhatia R. NCCN Guidelines Insights: Myeloproliferative Neoplasms, Version 2.2018. J Natl Compr Canc Netw. 2017;15(10):1193–1207. doi: 10.6004/jnccn.2017.0157. [DOI] [PubMed] [Google Scholar]
- 9.Marchioli R, Finazzi G, Specchia G. CYTO-PV Collaborative Group. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(01):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 10.Mirzabeigi M N, Wang T, Kovach S J, Taylor J A, Serletti J M, Wu L C. Free flap take-back following postoperative microvascular compromise: predicting salvage versus failure. Plast Reconstr Surg. 2012;130(03):579–589. doi: 10.1097/PRS.0b013e31825dbfb7. [DOI] [PubMed] [Google Scholar]
- 11.Cho E H, Bauder A R, Centkowski S. Preoperative platelet count predicts lower extremity free flap thrombosis: a multi-institutional experience. Plast Reconstr Surg. 2017;139(01):220–230. doi: 10.1097/PRS.0000000000002893. [DOI] [PubMed] [Google Scholar]
- 12.Tarabishy S P, Inglesby D, Tapp M, Corral G D, Herrera F A. Thrombocytosis is associated with complications after microvascular surgery: an NSQIP data analysis. Microsurgery. 2020;40(03):288–297. doi: 10.1002/micr.30530. [DOI] [PubMed] [Google Scholar]
- 13.Badawi M, Almazrooa S, Azher F, Alsayes F. Hydroxyurea-induced oral ulceration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(06):e232–e234. doi: 10.1016/j.oooo.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Stone T, Berger A, Blumberg S. A multidisciplinary team approach to hydroxyurea-associated chronic wound with squamous cell carcinoma. Int Wound J. 2012;9(03):324–329. doi: 10.1111/j.1742-481X.2011.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saravu K, Velappan P, Lakshmi N, Shastry B A, Thomas J. Hydroxyurea induced perimalleolar ulcers. J Korean Med Sci. 2006;21(01):177–179. doi: 10.3346/jkms.2006.21.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bader U, Banyai M, Böni R, Burg G, Hafner J. Leg ulcers in patients with myeloproliferative disorders: disease- or treatment-related? Dermatology. 2000;200(01):45–48. doi: 10.1159/000018315. [DOI] [PubMed] [Google Scholar]
- 17.Sirieix M-E, Debure C, Baudot N. Leg ulcers and hydroxyurea: forty-one cases. Arch Dermatol. 1999;135(07):818–820. doi: 10.1001/archderm.135.7.818. [DOI] [PubMed] [Google Scholar]
- 18.Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(03):339–347. doi: 10.1002/ajh.24976. [DOI] [PubMed] [Google Scholar]


