Abstract
The MerR family transcription factors (TFs) are a large group of bacterial proteins responding to cellular metal ions and multiple antibiotics by binding within central RNA-polymerase-binding regions of a promoter. While most TFs alter transcription through protein-protein interactions, MerR TFs are capable of reshaping promoter DNA. To address the question of which mechanism prevails, we determined two cryo-EM structures of transcription activation complexes (TAC) comprising E. coli CueR (a prototype MerR TF), RNAP holoenzyme and promoter DNA. The structures reveal that this TF promotes productive promoter-polymerase association without canonical protein-protein contacts seen between other activator proteins and RNAP. Instead, CueR realigns the key promoter elements in the TAC by clamp-like protein-DNA interactions: these induce four distinct kinks that ultimately position the −10 element for formation of the transcription bubble. These structural and biochemical results provide strong support for the DNA-distortion paradigm of allosteric transcriptional control by MerR TFs.
Introduction
Bacterial transcription factors typically activate gene expression by acting as a physical linkage between RNAP and promoter DNA in a sequence specific or non-specific manner 1, 2. In bacterial transcription initiation complexes comprising canonical class I or class II activators, the transcription activators recognize and bind their cognate cis elements, which locate at either upstream of central promoter elements (in the case of class I activators) or the region partially overlaps with the −35 element (in the case of class II activators), and make extensive interactions with RNAP-α subunit and/or the transcription initiation σ factor3, 4. In the bacterial transcription initiation complexes comprising non-canonical activators, such as M. tuberculosis RpbA and CarD, the activators anchor distinct surfaces of RNAP holoenzyme and make interaction with promoter DNA regardless of promoter sequences5,6.
The MerR family TFs—including the metalloregulatory protein subcategory of metal-dependent factors—are highly discriminative and provide an ultrasensitive physiological response to specific metal ions (such as CueR, ZntR, PbrR, CadR, and CoaR). Other MerR regulators are larger proteins capable of binding multiple antibiotic drugs (such as BmrR, BltR, and Mta). Proposed mechanisms of trans-activation include protein-protein contacts with RNAP or distortion of promoter DNA upon ligand binding 7–15. Strong support for TF-induced DNA distortion mechanism is found in both biophysical and solid state structures 11–15; however the structure of active transcription complexes containing MerR proteins have not been reported. Given that the majority of TFs employ obligatory RNAP-protein contacts and given the overlapping binding sites of MerR-family members and RNAP σ70 subunit within the core promoter elements, the mechanism of transcription activation at an atomic level has been difficult to resolve.
To understand the structural basis underlying transcription activation of MerR-family TFs, we examined the structure of Escherichia coli CueR transcription activation complex (CueR-TAC) comprising CueR, a metal responsive MerR family TF, by footprinting and cryo-EM methods. The structure sheds light on how the metal-bound form of CueR activates transcription and addresses possible polymerase-activator protein contacts. Unlike the canonical TF mechanisms, these results support a model in which CueR lowers the energetic barriers for formation of the TAC by switching the topology of the DNA in a manner that makes the promoter an optimal substrate for polymerase.
Results
CueR and RNAP induce cooperative structural change of promoter
The CueR-TAC was reconstituted in vitro with either CuI or AgI-bound CueR, σ70-RNAP holoenzyme and DNA based on the copA promoter (PcopA) and examined by footprinting methods that reveal sites of DNA distortion (Fig. 1 and Supplementary Fig. 1). Like MerR and ZntR 13, 16–18, the activator form of CueR decreases hypersensitivity to DNase I cleavage at bending sites but increases Cu-5-phenyl-OP hypersensitivity at the central protein induced kink. Permanganate (KMnO4) footprinting methods reveal more profound DNA distortion consistent with a single strand region from −12 to +2 corresponding to a TAC with a canonical transcription bubble (Figs. 1a, 1b, and Supplementary Figs. 1a and 1b). Telltale CueR contact sites with DNA are preserved within the region protected by the RNAP (Fig. 1). These data establish that the TF and RNAP induce cooperative changes in structure of the core promoter; however, they do not reveal the stereochemistry of these changes, nor do they establish whether the activator-mediated DNA distortions are sufficient to promote formation of the TAC. To examine these issues and determine whether explicit activator-polymerase contacts are present in the stable TAC complex, we turn to single particle cryo-EM methods.
Figure 1. Summary of footprints for CueR and polymerase complexes with PcopA DNA.
The DNase footprints (Fig. S1) of metal-free CueR and CuI-CueR are essentially the same (A), differing in only the strength of the hypersensitivity to DNaseI at the indicated sites. (B) Hypersensitive sites between −12 and +3 of PcopA in KMnO4 and 5-phenyl-1,10-phenanthroline-Cu footprinting datasets reveal clear formation of the transcription bubble upon addition of inducing silver or copper ions. The addition of ApG and NTPs results in the appearance of a KMnO4 sensitive site at +7 of the NT strand indicating formation of the TAC.
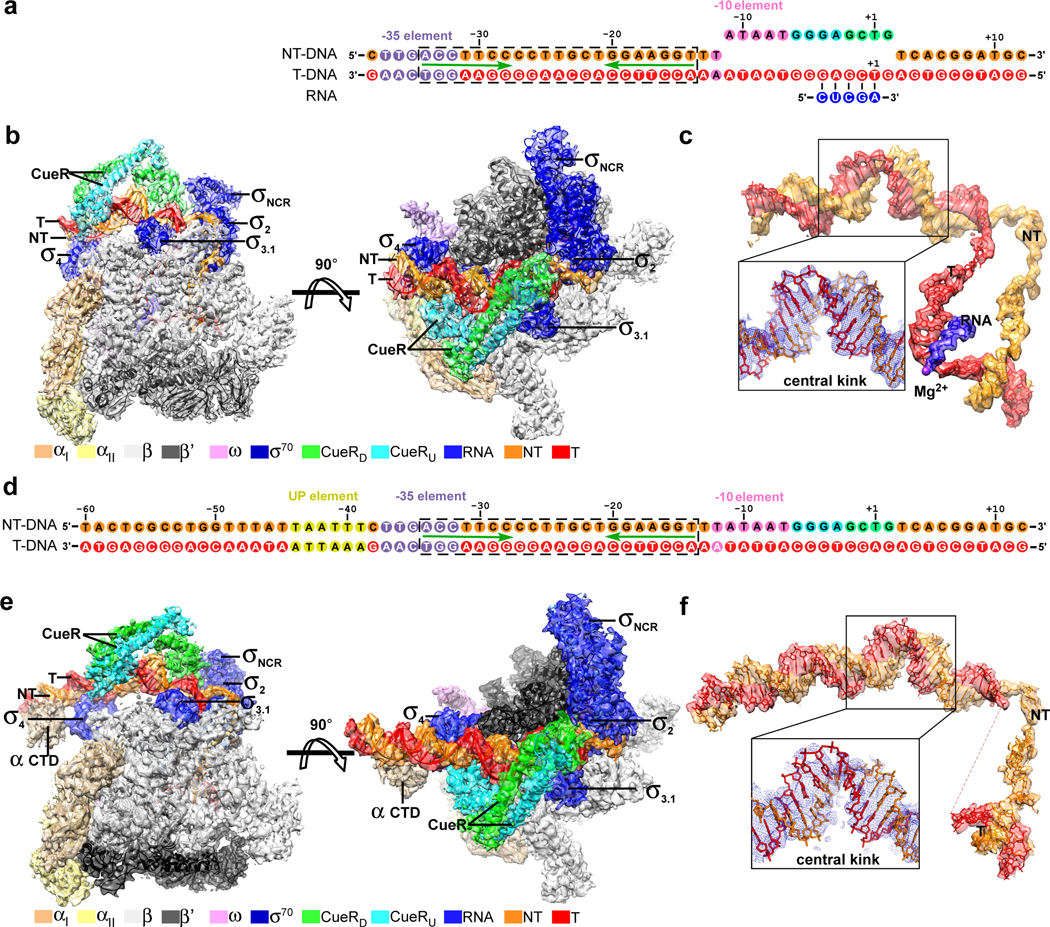
The cryo-EM structure of E. coli CueR-TAC
Two Cryo-EM samples employed either a pre-melted nucleic-acid scaffold comprising a double-stranded promoter DNA (−38 to +12 respective to the transcription start site) derived from E. coli PcopA with a palindromic CueR-binding box (−34 to −14), an unwound transcription bubble (non-complimentary sequences from −11 to +2 with consensus non-template single-stranded DNA), and a 5-mer RNA primer (CueR-TAC-1; Fig. 2a and Supplementary Fig. 2a–e), or a fully duplex promoter DNA (−60 to +12 respective to the transcription start site) derived from E. coli PcopA with a wild-type palindromic CueR-binding box (−34 to −14), and consensus sequence for promoter elements in the transcription bubble (CueR-TAC-2; Fig. 2d and Supplementary Fig. 2f). The structures were obtained through a cryo-EM single-particle method at 3.7 Å for CueR-TAC-1 and 4.2 Å for CueR-TAC-2 (Supplementary Table 2; Supplementary Figs. 3 and 4). The single-particle reconstruction of E. coli CueR-TAC-1 map shows clear features for domains σ1.2, σ2, σNCR, σ3, and σ4 of E. coli σ70, all nucleotides of promoter DNA and RNA, and a CueR dimer bound to its operator at the −35/−10 spacer of promoter DNA (Figs. 2b and 2c, and Supplementary Fig. 3e). The single-particle reconstruction of E. coli CueR-TAC-2 map shows clear features for σ1.2, σ2, σNCR, σ3, and σ4 of E. coli σ70, nucleotides of −46 to +12 of promoter DNA, a CueR dimer bound to its operator at the −35/−10 spacer of promoter DNA, and one αCTD bound to the UP element of promoter DNA (Figs. 2e and 2f, and Supplementary Fig. 4e). The two structures exhibit essentially the same closed-clamp conformation of RNAP (RMSD values of 3.0 Å of all RNAP Cα atoms), dimerization of CueR (RMSD values of 2.1 Å of all CueR Cα atoms), and promoter DNA with exception that the CueR-TAC-2 shows ordered “UP” element and disordered template ssDNA (Supplementary Fig. 5). Both structures also exhibit essentially the same interactions among RNAP, promoter DNA, and CueR; however the additional one helical turn of upstream DNA in CueR-TAC-2 reveals additional physiologically significant interactions of polymerase with the distal region of the promotor: the σR4 and RNAP-αCTD that bind the −35 and UP elements, respectively, which are clear in footprint characterization of the TAC in solution (Fig. 1 and Supplementary Fig. 6). The two structures of the two TACs are otherwise quite similar. Given the higher resolution and better map quality of CueR-TAC-1 (referred to as CueR-TAC hereafter), our discussion focuses on this structure.
Figure 2. The cryo-EM structures of E. coli CueR-TAC-1 and E. coli CueR-TAC-2.
(a) The nucleic-acid scaffold used for determination of E. coli CueR-TAC-1. The entire CueR operator DNA is highlighted in a dashed box and the palindromic sequence is underline by green arrows. The nontemplate ssDNA, orange; the template ssDNA, red; the −35 element, light blue; the −10 element, violet; the discriminator element, cyan; the CRE, green; RNA, blue. (b) Front and top view orientations of the cryo-EM map of E. coli CueR-TAC-1. The RNAP subunits and CueR protomers are colored as in the color scheme. (c) The cryo-EM map for the promoter DNA of the E. coli CueR-TAC-1. The cryo-EM map is shown as orange, red, and blue surfaces for nontemplate DNA, template promoter DNA, and RNA, respectively. The blue mesh in the scaled-up box represents cryo-EM map for the central DNA kink at the center of CueR-binding region. Purple sphere, RNAP active-center Mg2+. (d) The sequence of the fully duplex PcopA derivative promoter DNA. The PcopA derivative comprises wild-type sequence of PcopA (−60 to −11) and consensus sequences for the −10, discriminator, and core-recognition elements (gray-shaded) Colors are as in (a). (e) Front and top view orientations of the cryo-EM map of E. coli CueR-TAC-2. The RNAP subunits and CueR protomers are colored as in the color scheme. (f) The cryo-EM map for the promoter DNA of the structure of E. coli CueR-TAC-2.
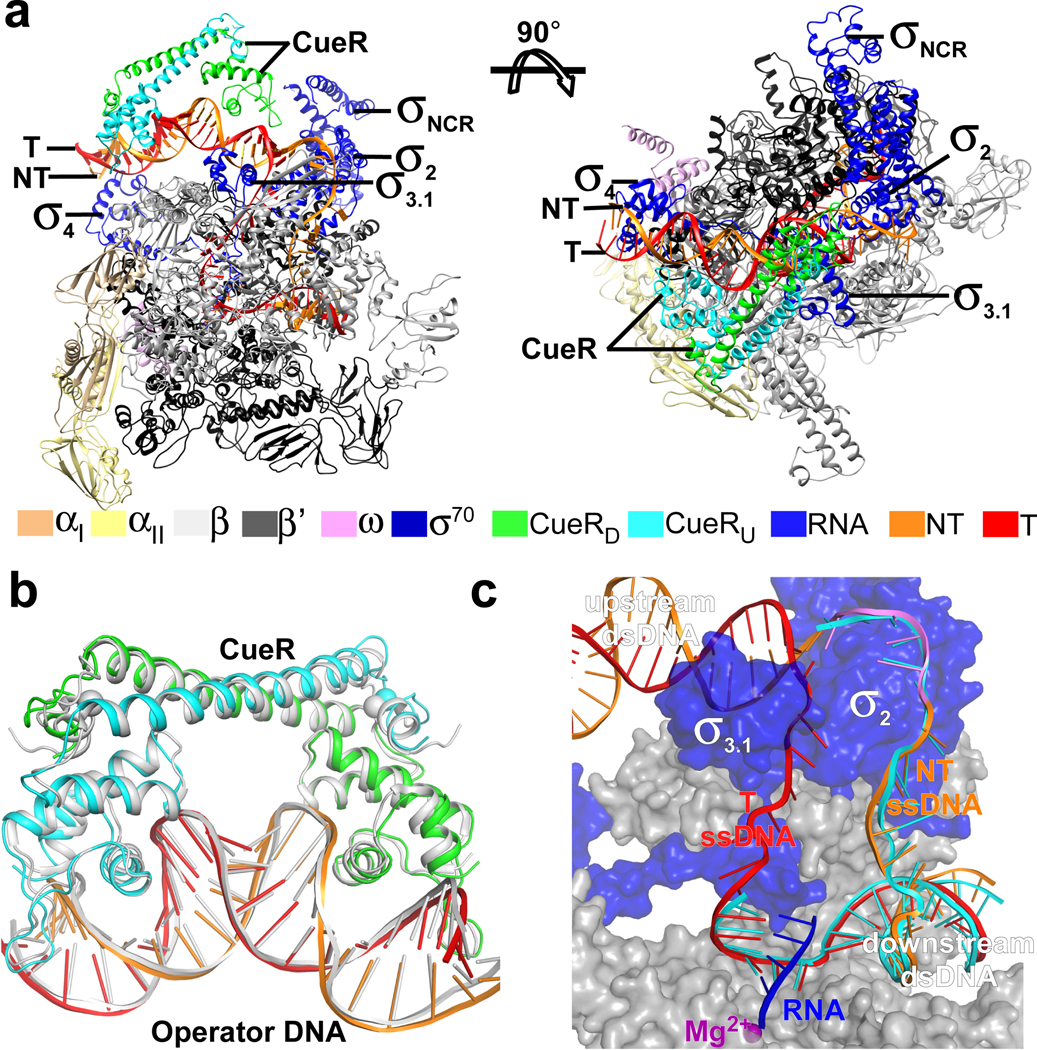
In the structure of E. coli CueR-TAC, σ4 anchors the −35 element of the upstream dsDNA, σ2 and RNAP core enzyme embrace the transcription bubble and downstream dsDNA; and the CueR dimer distorts and kinks the dsDNA structure at several sites between the −35 and −10 elements of promotor (Fig. 3a). The metal coordination, dimer orientation of CueR, and CueR/DNA interactions are similar to those reported for the binary crystal structure of AgI-CueR/DNA (Fig. 3b) 19, indicating that the AgI-bound CueR pre-organizes its operator DNA to a conformation ready for engagement with σ70-RNAP holoenzyme. The overall conformation and the interaction with RNAP of the single-stranded DNA of transcription bubble, the template DNA:RNA hybrid, as well as the downstream dsDNA correlate well with structures of bacterial RNA polymerase-promoter DNA open complex (RPo) and transcription activation complexes (Fig. 3c) 3, 4, 20. The entire upstream dsDNA (−38 to −12) is protected by contacts with σ70 and CueR (Fig. 4a), and domains σ4 and σ3.1 locate at the same side of the upstream promoter DNA and interact with the −35 region and extended −10 region, respectively (Fig. 4a), in agreement with the footprinting results (Fig. 1).
Figure 3. The overall structure of E. coli CueR-TAC.
(a) The front and top view orientations of the E. coli CueR-TAC-1. (b) The superimposition of CueR/DNA in our structure of E. coli CueR-TAC-1 colored as above and the crystal structure of AgI-CueR/DNA binary complex (PDB: 4WLW; colored in white). The AgI ion is represented as in spheres (The AgI in CueRU is not modeled due to poor electron density). (c) The comparison of the transcription bubble and downstream dsDNA in E. coli CueR-TAC-1 colored as above and T. thermophilus RPo (PDB: 4G7H; colored in cyan).
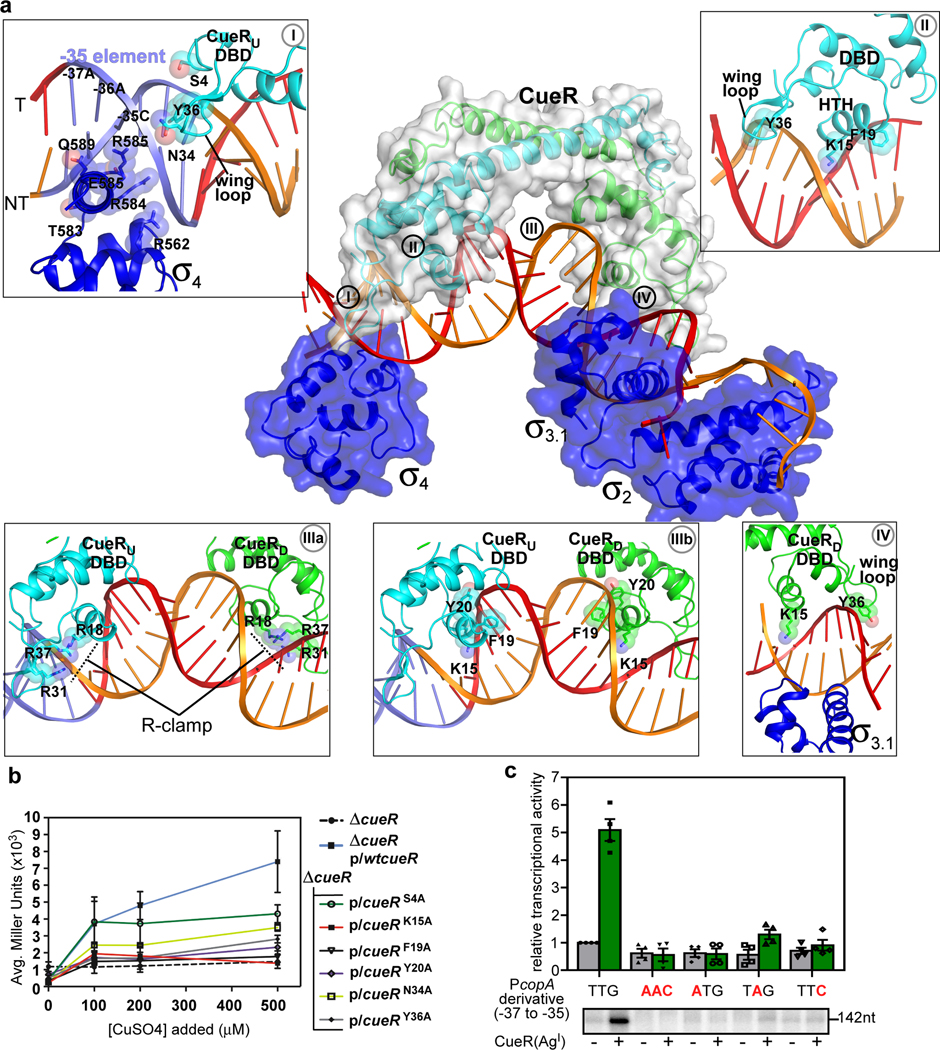
Figure 4. A cooperative binding of promoter DNA by CueR and E. coli σ70.
(a) The upstream dsDNA is shielded by both CueR and s70 from opposite surfaces. The CueR protomers (green and cyan) and the σ702, σ703.1, and σ704 (blue) are represented as cartoon and surface. Box I, σ704 and CueR create a “sandwich clamp” around the −35 element; Box II, the interaction between CueR DNA binding domain (DBD) and the operator DNA; Box IIIa, the “R-clamp” (R18, R31, and R37) of two CueR-protomers interacts with the phosphate backbone of the CueR operator; Box IIIb, base-specific (K15 and F19) and phosphate backbone (Y20) CueR-DNA interactions that envelope the third kink in the TAC additional; Box IV, the cooperative binding of the extended - 10 region by CueR and σ703.1. The side chains of nucleic acid-contacting residues from σ70 and CueR are shown as spheres and sticks. (b) β-galactosidase activity for various CueR DNA-binding residue variants. The Cu binding to CueR activates transcription through DNA distortion induced by conformational change of CueR and mutation of DNA-interacting residues decreased the activation activity. Data are presented as mean ± SD. n= 6 biologically independent samples except for the alanine substitution of CueR variant (n=3). (c) Mutating any of the first three nucleotides of the −35 element substantially impairs the transcription activation activity of CueR. Data are presented as mean ± SEM, n=4 biologically independent experiments. The lower panel shows the representative gel image of the 4 biologically independent experiments.
CueR and σ70 promote localized DNA distortions
A mechanistically important feature of the TAC are a series of short clamp-like interactions of the TF and polymerase proteins associated with DNA distortions that progress from the −35 element through the TF binding site up to the transcription bubble. CueR dimer forms clamp-like interactions through a winged helix-turn-helix fold and recognizes its long operator DNA spanning 21 bp (−34 to −14) (Fig. 4a). The helix-turn-helix module and the wing loop of each CueR protomer inserts into the major groove and minor groove of CueR palindromic box, respectively (Box II in Fig. 4a). Residues K15 and F19 of the helix-turn-helix module and residue Y36 of the wing loop reach the bottom of the DNA grooves and make base-specific interactions; while several other residues (S4, R18, Y20, R31, N34, R37, R54, L60) interact with backbone phosphates of CueR operator DNA (Box II in Fig. 4a and Supplementary Figs. 7a–c).
In the upstream region of the promoter, the TAC structure reveals that CueR residues S4, Y36 and N34, along with RNAP σ4 residues R562, T583, R584, E585, R588, Q589 create a “sandwich clamp” around −35 (Box I in Fig. 4a and Supplementary Figs. 7b–d). This leads to one of four distortion sites in dsDNA structure (defined here as kinks; see SI), and is the most upstream of four kink sites in the TAC. To test which of these CueR interactions with the −35 element are mechanistically important, transcription activity of each mutant was examined in vivo. Mutations in N34 and Y36, and to a lesser extent in S4, reduce transcription activation (Fig. 4b), supporting the importance of these three CueR residues in the sandwich clamping interaction with the −35 element. Directly downstream of the −35 element, we observe a subset of CueR interactions with the phosphate backbone, i.e. the “R-clamp” (R18, R31 and R37) residues (Box IIIa in Fig 4a and Supplementary Fig. 7c). Our previous work has shown that the “R-clamp” residues play essential roles in CueR-mediated transcription repression and activation12. At the center of the promoter, we observe base-specific (K15 and F19) and phosphate backbone (Y20) CueR-DNA interactions that envelope the third kink in the TAC (Box IIIb in Fig 4a and Supplementary Fig. 7a and c). Like the R-clamp, this arises exclusively from interactions with CueR. To test whether these interactions are also critical to activation, mutant forms of CueR (K15A, F19A, and Y20A) were examined in vivo and found to severely affect transcription activation, relative to wild type CueR (Fig. 4b). Importantly, each CueR variant maintains full transcription repression activity (Fig. 4b; compare to wt-CueR and uncontrolled promoter ΔcueR), indicating that these mutations do not significantly alter CueR-promoter recognition per se.
The unusual long operator DNA (spanning from −34 to −14) required for DNA recognition and distortion by CueR leaves only the first three base pairs of the −35 element available for sequence-specific recognition by σ70 (Fig. 4a). In our structure, σ4 inserts its recognition helix into the major groove and makes the clamp-like contacts with the first three nucleotides (TTG; −37 to −35) of the −35 element as does the σ4 in T. aquaticus and E. coli RPo 21, 22 (Box I in Fig. 4a and Supplementary 7d and 7e), confirming a sequence-specific recognition. A survey of promoters regulated by CueR and other MerR family TFs indeed shows a high sequence identity (“TTG”) at the first three positions of the −35 element 23, 24 (Supplementary Fig. 8), and base substitution at any of the three positions almost completely abolished the effect of transcription activation by CueR (Fig. 4c).
Due to the palindromic nature of the promoter, modification of the specific CueR residues mentioned above, inevitably affect the downstream half of the promoter as well. The downstream half of CueR operator (CueR-OD; −20 to −14) completely overlaps with the extended −10 region of promoter DNA, suggesting that the extended −10 region has been expropriated by CueR for sequence-specific recognition. Intriguingly, σ70 still retains interactions with backbone phosphates and bases of nucleotides at extended −10 region from the other side of CueR (Box IV in Fig. 4a and Supplementary Fig. 7f). These observations, along with the results from the in vivo CueR variant assays (Fig. 4b), further support the idea that CueR and RNAP work cooperatively across several domains (CueR DNA-binding domain, RNAP σ4 and σ3.1) to reinforce kinks in the promoter that work together with RNAP contacts at the −35 and −10 to stabilize the TAC.
CueR realigns promoter stereochemistry
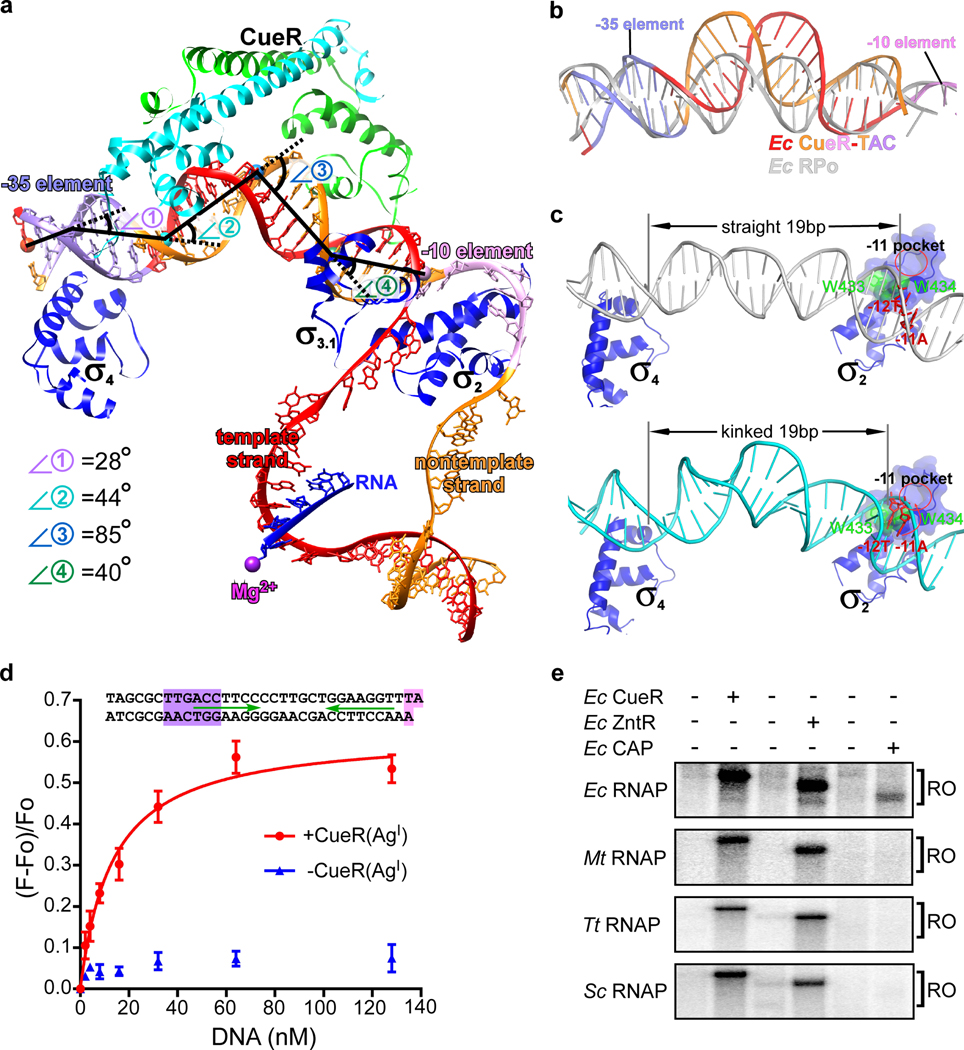
The most striking features in the structure of E. coli CueR-TAC are four kinks which stand in stark contrast with the near straight DNA conformation of that in bacterial RPo (Fig. 5a and 5b) 21, 22. Kink 1, imposed by CueR and RNAP σ4, as described above, corresponds to a 28° change in helical axis at the center of the −35 element 21, 24. CueR alone induces kinks 2, 3 and 4 in the promoter DNA (Fig. 5a). Kink 2 (44°) occurs at position −30—the middle point of the upstream half of the promoter DNA (CueR-OU). Kink 3 (85°) is the largest distortion and occurs at the central base pair (T:A; position −24). Kink 4 (40°) occurs at around position −18—the middle point of the downstream half of the promoter (CueR-OD) (Fig. 5a). The A-DNA-like conformation of the large central kink 3 is imposed by CueR and not RNAP, as the roll angles over the two central base pair steps (T:A – T:A – G:C), the width of major and minor grooves in the E. coli CueR-TAC structure are nearly identical to that of the previously reported binary structure of AgI-CueR/DNA, but not in the apo-CueR/DNA complex12 (Fig. 3b and Supplementary Figs. 9a–d). This indicates that the central kink (Kink 3) is formed prior to formation of the TAC. As previously predicted, the central kink formed by CueR shortens the distance of the suboptimal 19-bp spacer between the −35 and −10 elements (Supplementary Fig. 9e) 12. It also underwinds the promoter DNA to realign the −35 and −10 on the same phase plane for simultaneous engagement by σ4 and σ2, respectively, and for subsequent unwinding of the −10 region (Fig. 5c). In this manner the AgI-CueR and RNAP induced kinks change the overall stereochemistry of the promoter in manner that puts the key residues of the −10 element in direct contact with σ2 residues that are important for formation of the transcription bubble. These results support a model in which the combined DNA clamp activities of the TF and the polymerase stabilize the series of four kinks that remodel the suboptimal spacer region into a better substrate for RNAP.
Figure 5. CueR realigns the non-optimal −35/−10 spacer of promoter DNA.
(a) The kinks of the upstream promoter DNA at positions −35 (⦟1, 28°), −30 (⦟2, 44°), −24 (⦟3, 85°), and −18 (⦟4, 40°). Kink 1 at −35 is induced by CueR and σ704, and kinks 2, 3 and 4 are induced by the CueR dimer. (b) The superimposition of upstream promoter dsDNA in E. coli CueR-TAC complex (orange and red) and RPo (white). (c) The kink realigns the −35 and −10 elements to a proper space and phase for simultaneous engagement by σ4 and σ2 according to structure models of RNAP-promoter DNA closed complexes (RPc) comprising straight or kinked promoter dsDNA (see SI for details of structure modeling). Green spheres and sticks show the tryptophan dyad critical for promoter unwinding. The red oval indicates the protein pocket for securing the first unwound nucleotide (−11A). (d) A RNAP molecular beacon assays shows that CueR dramatically increases affinity of promoter dsDNA to E. coli RNAP holoenzyme. The scaffold used in the beacon assay is shown in top. The Kd value (13 ± 2 nM; red curve) was obtained from the three independent repeats. The data were presented as mean ± S.E.M. (e) The transcription activation of CueR, ZntR, or CAP on four different bacterial RNAP species, including Escherichia coli, Mycobacterium tuberculosis, Thermus thermophilus, and Streptomyces coelicolor. The representative gel image was from one biologically independent experiment.
The structure of E. coli CueR-TAC suggests that CueR activates transcription by altering the shape of the DNA in a manner that facilitates interaction with σ70-RNAP holoenzyme of promoter dsDNA. To further test this hypothesis, we employed the RNAP molecular beacon assay to explore interactions between RNAP and an upstream fork promoter DNA derived from PcopA 25, 26. The results in Fig. 5d clearly show that, in the absence of AgI-CueR, PcopA DNA with a non-optimal −35/−10 spacer barely binds RNAP holoenzyme, consistent with its very weak transcription activity (Figs. 4b and 4c). Notably, AgI-CueR dramatically improves the binding affinity between PcopA fork promoter and RNAP (Fig. 5d), promotes RPo formation on a full-length PcopA promoter derivative (Supplementary Figs. 2g and 2h), and increases transcription activity (Figs. 4b and 4c and Supplementary Fig. 2e), providing support for this model. This mechanism of transcription activation stands in sharp contrast to that of the canonical class I and class II transcription activators (E. coli CRP as an example) as well as non-canonical activators such as mycobacterial rbpA, CarD, and Caulobacter crescentus GcrA. Each of these stimulate transcription by creating a physical linkage between RNAP and promoter DNA 3–6, 27–30.
CueR activates transcription independent of RNAP contact
The promoter regions contacted by CueR and σ70 overlap substantially (Figs. 1, 3a, 4a and Supplementary Figs. 6 and 7); however, neither of the two CueR protomers make contacts with conserved domains of RNAP core enzyme or σ70 (Figs. 2b, 2e, 4a, and 5a). The closest apparent approach is between residue E33 of the CueRD protomer and non-conserved region of σ70 (Supplementary Fig. 10a and 10b). Mutation of this residue does not alter CueR activity, indicating it is dispensable for CueR-mediated transcription activation (Supplementary Figs. 10c). Although Multidrug MerR TFs contain a larger C-terminal domain for multidrug binding 11 compared with CueR, structure modeling of a B. subtilis BmrR-TAC suggests that its extra multidrug-binding domain extrudes out and makes no contact with RNAP either (Supplementary Fig. 11). These arguments suggest the broader MerR family of factors stimulate polymerase action at the promotor in RNAP contact-independent manner. To further test this model, we evaluated the activity of a series of MerR TFs from E. coli in transcription complexes from other bacterial species. The assumption is that the evolutionarily distant RNAPs would still recognize the E. coli promoter motifs in the test promoters, but that the potential TF-RNAP interactions would not be conserved in the more distant species. The results from the in vitro transcription assay show that E. coli CueR and ZntR are capable of activating transcription with all tested RNAP of E. coli, M. tuberculosis, T. thermophilus, and S. coelicolor from the CueR-dependent or ZntR-dependent promoters of E. coli, respectively (Fig. 5e). In contrast, E. coli catabolic activating protein (CAP; an RNAP-contact dependent transcription activator) only activates E. coli RNAP-initiated transcription from the CAP-dependent promoter of E. coli (Fig. 5e) 3. Although the footprinting data of CueR, MerR, and ZntR implicate a large overlap on promoter DNA of the MerR-family TFs with RNAP (Fig. 1) 13, 16–18, our structure and biochemical evidence show how these TFs activate transcription in a manner that involves little, if any TF interaction with the polymerase.
Discussion
Any mechanism for MerR family TFs must account for the ability of polymerase and TF to work in a cooperative manner to rapidly switch transcriptional activity from a tightly repressed state to one that can be as much as a thousand fold more active in response to a small change in ligand concentration 31. Our results in physiological buffers at room temperature and frozen on substrates strongly support a model in which MerR family TFs work by manipulating the gross stereochemistry core promoter elements and reinforce torsional stiffness through a series of kinks in dsDNA structure, induced by protein-DNA clamping interactions, upstream of the site of transcription bubble formation. We propose that these torsional restraints facilitate formation of the TAC by RNAP and ultimately favor unidirectional progression of the RNAP once NTPs are added to the TAC, further increasing transcriptional efficiency. Given that MerR-regulated promoters are otherwise poor substrates for RNAP in the absence of a TF, their ability to impose ligand-responsive, protein-induced torsional stress in the DNA likely plays a key role in lowering the barrier to transcriptional initiation. Our previous work and this study show that CueR functions as a metal ion sensor that represses transcription by stably associating with and stabilizing a nonpermissive conformation of promoter DNA in the absence of copper 12, but efficiently switches to a transcription activator by inducing distortion of promoter DNA. MerR family TFs act as transistor-like switching elements 32 that respond to small changes in the gate voltage (i.e. metal concentration) by inducing a major change in drain current (i.e. the rate of transcription) and thus could have a number of applications in synthetic biology. We anticipate that this type of ‘square wave’ transcription switching mechanism poses a threat for horizontal transfer of multidrug resistance operons comprising MerR family TFs.
Online Methods
Plasmid construction
The plasmid information is summarized in Table S1. The pTolo-EX5/EcCueR and pTolo-EX5/EcZntR were constructed by incorporating into pTolo-EX5 (Tolo Biotech.) the DNA fragments of E. coli cueR and E. coli zntR (obtained from genomic DNA of E. coli cells with primers listed in Table S3), respectively, using a homogenous recombination method (NovoRec Plus One step PCR Cloning kit; Novoprotein). The pET28a/EcCAP and pET28a/MtbσA were constructed by incorporating into pET28a the DNA fragments of E. coli crp, M. tuberculosis sigA, and Streptomyces coelicolor hrdB (amplified from respective genomic DNA with primers listed in Table S3), respectively, as described above. The pET28a/ScσA was constructed by ligation of a NcoI-EcoRI DNA fragment, amplified from S. coelicolor genomic DNA with primers listed in Table S3, into pET28a using T4 ligase (New England Biolabs, Inc).
For footprinting experiments, the pET24a/cueR and pUC19/copA plasmids (Table S1) were used 33. For β-galactosidase assays, the pACYC184/cueR plasmid (Table S1) was used 33. CueR variants (pACYC184/cueR) were made by site-directed mutagenesis as in 12 with the primers in Table S3.
The pEASY/P-copA, and pEASY/P-zntA were constructed by ligating promoter regions (−50 to +50) of respective genes, obtained by PCR from E. coli genomic DNA, with pEASY-blunt vector (Transgen biotech). The pEASY/P-CC (41.5) was constructed by ligating pEASY-blunt vector and a DNA fragment derived from melR promoter [a 22-bp region centered at the position −41.5 of the wild-type melR promoter was replaced by a 22-bp consensus CAP-cis element 34; the melR promoter derivative (−66 to +80) was obtained by PCR amplification from E. coli genomic DNA with primers listed in Table S3]. The pEASY/P-copA derivatives were made by site-directed mutagenesis (Transgene Biotech) with primers listed in Table S3.
E. coli CueR, ZntR, and CAP
The expression of EcCueR, EcZntR, and EcCAP were induced for 14h by addition of 0.5 mM IPTG at 18 °C to LB cultures of E. coli BL21(DE3) cells carrying pTolo-EX5/EcCueR, pTolo-EX5/EcZntR, or pET28a/EcCAP, respectively, at OD600 of 0.6 to 0.8. Cell pellets were resuspended using lysis buffer (50 mM Tris-HCl pH 8.0, 0.3 M NaCl, 5% (v/v) glycerol, 5 mM β-mercaptoethanol, protease inhibitor cocktail (Bimake.cn Inc.)) and lysed using an Avestin EmulsiFlex-C3 cell disrupter (Avestin, Inc.). The lysate was centrifuged at 16,000 g for 45 min at 4 °C and the supernatant was loaded on to a Ni-NTA column (Smart-lifesciences, Inc., China). The column was washed with lysis buffer supplemented with 20 mM imidazole and eluted with lysis buffer supplemented with 300 mM imidazole. The Sumo fusion tag was cleaved with TEV protease. The protein samples were further purified by a Heparin column (HiTrap Heparin HP 5ml column, GE healthcare Life Sciences) using a salt gradient of buffer A (50 mM Tris-HCl pH 8.0, 0.05 M NaCl, 5% (v/v) glycerol, 1 mM DTT) and buffer B (50 mM Tris-HCl pH 8.0, 1 M NaCl, 5% (v/v) glycerol, 1 mM DTT). The elute fractions containing target proteins were concentrated to 2 mg/mL and stored at −80 °C.
E. coli RNAP and σ70
The E. coli RNAP core enzyme was overexpressed in E. coli BL21(DE3) cells carrying pEcABC and pCDF/EcrpoZ and purified as described 27. The E. coli σ70 was overexpressed in E. coli BL21(DE3) cells carrying pET28c/Ecσ70 as described in 35. The E. coli σ70-211C were prepared as in 25.
M. tuberculosis RNAP and σA
The M. tuberculosis RNAP core enzyme was overexpressed in E. coli BL21(DE3) cells carrying pETDuet/Mtb-rpoA-rpoZ and pACYCDuet/Mtb-rpoB-rpoC and purified as described in 36. M. tuberculosis σA was overexpressed in E. coli BL21(DE3) cells carrying pET28a-MtbσA and purified by a similar procedure as E. coli CAP.
T. thermophilus RNAP core enzyme and T. thermophilus sA
T. thermophilus RNAP core enzyme was isolated from T. thermophilus strain HB-8 as described in 20. The T. thermophilus σA was over-expressed in E. coli BL21(DE3) cells carrying pET28a/TthσA and purified as described in 20.
S. coelicolor RNAP core enzyme and σA
S. coelicolor RNAP core enzyme was isolated from S. coelicolor strain M145. The bacteria cells were cultured at 37 °C in LB medium for 2 days. Cell pellet was re-suspended in lysis buffer (40 mM Tris-HCl, pH 7.7, 200 mM NaCl, 5% glycerol, 2 mM EDTA, 2 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail (Biomake.cn. Inc.)) and lysed with an EmulsiFlex-C3 cell disrupter (Avestin, Inc.). The supernatant was precipitated with polyethylenimine (PEI; final concentration 0.7%) at 4°C for 30 min. The pellet was washed twice with 10 mM Tris-HCl, pH 7.7, 5% glycerol, 300 mM NaCl, 1 mM DTT, and 2mM EDTA. The RNAP in the pellet was released into supernatant by addition of 10 mM Tris-HCl, pH 7.7, 5% glycerol, 1 M NaCl, 1 mM DTT, and 2 mM EDTA and precipitated again by addition of ammonium sulfate (final concentration; 30 g/ml). The pellet was dissolved with TGED buffer (10 mM Tris-HCl, pH 7.7, 5% glycerol, 1 mM DTT, and 2mM EDTA). The RNAP sample was sequentially loaded on to a Heparin column (HiTrap Heparin HP 5ml column, GE healthcare Life Sciences; buffer HPA: 50 mM Tris-HCl pH 8.0, 0.05 M NaCl, 5% (v/v) glycerol, 1 mM DTT; buffer HPB: 50 mM Tris-HCl pH 8.0, 1 M NaCl, 5% (v/v) glycerol, 1 mM DTT) and a Mono Q column (Mono Q 10/100 GL, GE healthcare Life Sciences; buffer QA: 10 mM Tris-HCl pH 7.7, 50 mM NaCl, 5% (v/v) glycerol, 1 mM DTT, 0.1mM EDTA; buffer QB: 10 mM Tris-HCl pH 7.7, 1 M NaCl, 5% (v/v) glycerol, 1 mM DTT, 0.1mM EDTA). The fractions containing RNAP were concentrated to 5 mg/mL and stored at −80 °C. The S. coelicolor σA was overexpressed in E. coli BL21(DE3) cells carrying pET28a-ScσA and purified by a similar procedure as E. coli CAP.
Nucleic-acid scaffolds.
Nucleic-acid scaffold for E. coli CueR-TAC-1 was prepared as follows: nontemplate-strand DNA (5’- CTTGACCTTCCCCTTGCTGGAAGGTTTATAATGGGAGCTGTCACGGATGC-3’; 0.5 mM final; Sangon Biotech), template-strand DNA (5’- GCATCCGTGAGTCGAGGGTAATA AAACCTTCCAGCAAGGGGAAGGTCAAG-3’; 0.6 mM final; Sangon Biotech) and RNA (5’- CUCGA-3’; 0.75 mM final; GenScript Biotech Corp.) in annealing buffer (5 mM Tris-HCl, pH 8.0, 200 mM NaCl, and 10 mM MgCl2) were heated for 5 min at 95°C, cooled to 22 °C in 2 °C steps with 30 s per step using a thermal cycler.
Fully duplex promoter PcopA derivative DNA for E. coli CueR-TAC-2 reconstitution, gel-shift assay, and molecular-beacon assay, was prepared as follows: nontemplate-strand DNA (5’- TACTCGCCTGGTTTATTAATTTCTTGACCTTCCCCTTGCTGGAAGGTTTATAATG GGAGCTGTCACGGATGC-3’; 0.2 mM final; Sangon Biotech), and template-strand DNA (5’- GCATCCGTGACAGCTCCCATTATAAACCTTCCAGCAAGGGGAAGGTCAAGAAATTAA TAAACCAGGCGAGTA-3’; 0.22 mM final; Sangon Biotech) in annealing buffer (5 mM Tris-HCl, pH 8.0, 200 mM NaCl, and 10 mM MgCl2) were heated for 5 min at 95°C, cooled to 22 °C in 2 °C steps with 30 s per step using a thermal cycler.
Fork PcopA promoter for molecular beacon assay was prepared as above using nontemplate-strand DNA (5’-TAGCGCTTGACCTTCCCCTTGCTGGAAGGTTTA-3’) and template-strand DNA (5’-AAACCTTCCAGCAAGGGGAAGGTCAAGCGCTA-3’).
Gel shift assay
Reaction mixtures contained (7 μl): 10 μM E. coli RNAP holoenzyme, 7 μM PcopA derivative promoter DNA, 0 or 28 μM CueR in 10 mM Hepes pH 7.5, 50 mM KCl, 5 mM MgCl2, 3 mM DTT, and 100 μM AgNO3. Reaction mixtures were incubated for 1h on ice and followed by heparin challenge (100 μg/ml; final concentration) when indicated. The complexes were separated by 5% TBE gel in the TBE buffer (90 mM Tris-borate, pH 8.0, and 2 mM EDTA), stained using SYBR-Gold, and analyzed by Tanon-2500 (Tanon Science & Technology Co., Ltd).
E. coli CueR-TAC cryo-EM structure determination
The E. coli CueR-TAC complex was reconstituted by mixing 600 μL 12.8 μM E. coli RNAP core enzyme [in 10 mM Tris-HCl pH 7.7,420 mM NaCl, 5% (v/v) glycerol, 1 mM DTT, 0.1 mM EDTA; 9 μM final], 140 μL 110 μM σ70 [in 50 mM Tris-HCl pH 7.7, 500 mM NaCl, 5% (v/v) glycerol, 1 mM DTT; 18 μM final], 120 μl 260 μM CueR (in 50 mM Tris-HCl pH 7.7, 420 mM NaCl, 5% (v/v) glycerol, 1 mM DTT; 36 μM final), and 20 μL 500 μM nucleic-acid scaffold (in 5 mM Tris-HCl, pH 8.0, 200 mM NaCl, and 10 mM MgCl2; 11 μM final) in a ~1:2:4:1.2 molar ratio. The mixture was supplemented with AgNO3 (10 μM final) and incubated at 4 °C overnight. The CueR-TAC was further purified by a Superose 6 10/300 GL column (GE Healthcare) in S6 buffer (10 mM Hepes pH 7.5, 50 mM KCl, 5 mM MgCl2, 3 mM DTT). Fractions containing E. coli CueR-TAC were concentrated to ~15 mg/mL and supplemented with AgNO3 (final concentration 5 μM).
The freshly purified E. coli CueR-TAC at 15 mg/mL was incubated with 3-([3-cholamidopropyl] dimethylammonio)-2-hydroxy-1-propanesulfonate (CHAPSO, 8 mM final; Hampton Research Inc.) prior to grid preparation. 3 μL complex was applied on a glow-discharged C-flat CF-1.2/1.3 400 mesh holey carbon grids (Protochips, Inc.), blotted with Vitrobot Mark IV (FEI), and plunge-frozen into liquid ethane with 95% chamber humidity at 10 °C.
The micrographs were collected using SerialEM on a 300 keV Titan Krios (FEI) equipped with a Gatan K2 Summit direct electron detector (1.307 Å/pixel; 8 electrons/pixel/s). A total 2,278 images were recorded with the Serial EM using counting mode (12 s exposures in 48 subframes resulting in a total dose of 56 electrons/Å2; defocus range of 1.5–2.5 μm. Frames of individual movies were aligned using MotionCor2 37, and Contrast-transfer-function estimations were performed using CTFFIND4 38. About 1,000 particles were manually picked and subjected to 2D classification in RELION3.0 39. Protein aggregates and ice spots were removed, and the resulting particles were subjected to 2D classification in RELION3.0 again. Particles in poorly populated classes were removed, resulting in a dataset of 617,318 particles. We used a 40 Å low-pass-fitmicroscopy data bank with accession codes lered cryo-EM map of E coli σE-RPo as the starting reference model for 3D classification40. A total of 184,524 particles were used to calculate the final map. The steps of 3D auto-refinement, CTF-refinement, Bayesian polishing, and post-processing were performed in RELION 3.0 and the Gold-standard Fourier-shell-correlation analysis indicated a mean map resolution of 3.7 Å 41.
The cryo-EM structure of E. coli CAP-TAC (PDB: 6B6H) 4 and crystal structure of E. coli CueR-DNA (PDB:4WLW) 12 were fit into the cryo-EM density map in Coot42, followed by adjustment of each single residue in Coot, and real-space refined using Phenix 43. The final coordinate and cryo-EM map were deposited into the protein data bank and the electron microscopy data bank with accession codes of 6LDI and EMD-0874, respectively.
E. coli CueR-TAC-2 cryo-EM structure determination
The E. coli CueR-TAC-2 complex was reconstituted by mixing 100 μL 18.7 μM E. coli RNAP holo enzyme, 60 μl 130μM CueR, and 9.5 μL 200 μM fully duplex prmoter DNA, in cryo-EM buffer (10 mM Hepes pH 7.5, 50 mM KCl, 5 mM MgCl2, 5 mM DTT), in a ~1:4:1 molar ratio. The mixture was supplemented with AgNO3 (100 μM final) and incubated on ice 1.5h. The mixture was concentrated to ~13 mg/mL.
The grid preparation, image collection, and cryo-EM map calculation of CueR-TAC-2 were performed as for CueR-TAC-1. The final maps calculated from 23,109 particles were obtained through 3D auto-refinement, CTF-refinement, Bayesian polishing, and post-processing in RELION 3.0. Gold-standard Fourier-shell-correlation analysis indicated a mean map resolution of 4.2 Å 41.
The RNAP-αCTD from cryo-EM structure of E. coli CAP-TAC (PDB: 6B6H) 4 and CueR-TAC from cryo-EM structure of E. coli CueR-TAC-1 were fit into the cryo-EM density map in Phenix 43 and Coot42, followed by adjustment of main-chain and side-chain conformations in Coot, and real-space refined using Phenix 43. The final coordinate and cryo-EM map were deposited into the protein data bank and the electron microscopy data bank with accession codes of 7C17 and EMD-30268, respectively.
DNA structure analysis
The groove widths were measured with Curves+ program 44, which calculated the groove widths for each half base pair step. The data were extracted from the Curves+ calculations and graphed using Microsoft Office Excel (Microsoft Corp., Redmond, WA). The DNA roll angles were measured and represented with x3DNA v2.3 (http://x3dna.org) using the recipes from 45. The DNA distortion angles (kinks) were measured with UCSF Chimera 46. The centroid positions were created at sites −38, −35, −30, −24, −18, and −13, and the angle between the centroid positions were measured using the Angles/Torsion function.
Structure modeling of RPc
The RNAP clamp has to open to allow entry of promoter dsDNA during formation of RNAP promoter DNA close complex (RPc)47–49. As Lipiramycin has been demonstrated to lock the RNAP clamp in the open conformation by a collection of biochemical and structural evidence 50–52, we modeled a structure of E. coli RNAP-σ70 holoenzyme with an open clamp (E. coli RNAP-σ70-OC) by rotating E. coli σ702 and RNAP-clamp domains of our E. coli CueR-TAC to a position where the corresponding domains of M. tuberculosis RNAP locate in the cryo-EM structure of M. tuberculosis RNAP-Lipiarmycin (PDB 6FBV)51. Subsequently, we superimposed the E. coli class I CAP-TAC (PDB 6B6H)4 and our E. coli CueR-TAC onto the model of E. coli RNAP-σ70-OC. Finally, the E. coli RPc comprising a straight promoter dsDNA with 19-bp spacer was modeled by copying the upstream straight promoter dsDNA (−36 to −17) from E. coli class I CAP-TAC (PDB 6B6H) and by extending to position −6 as a B-form dsDNA. The E. coli RPc comprising a kinked promoter dsDNA with 19-bp spacer was modeled by copying the upstream kinked promoter dsDNA (−38 to −17) from our E. coli CueR-TAC and by extending to position −6 as a B-form dsDNA.
Radiochemical in vitro transcription assay
To measure the transcription activation by E. coli CueR, reaction mixtures (20 μl) containing RNAP core enzyme (50 nM E. coli, M. tuberculosis, T. thermophilus, or S. coelicolor RNAP core enzyme; final), σ factor (250 nM E. coli σ70, M. tuberculosis σA, T. thermophilus σA, or S. coelicolor σA; final), 40 mM Tris-HCl, pH 7.9, 75 mM KCl, 5 mM MgCl2, 2.5 mM DTT, and 12.5% glycerol were incubated 10 min at 37 °C, and then supplemented with 1 μl wild-type or derivatives of copA promoter DNA (50 nM final; prepared by PCR using pEASY/P-copA as template and M13 primers listed in Table S3). CueR-AgNO3 (400 nM CueR and 5 μM AgNO3; final) was added when indicated. The mixtures were further incubated for 10 min at 37 °C. The reactions were initiated by adding 0.7 μL NTP mixture (100 nM [α−32P] UTP (0.04 Bq/fmol), 100 nM ATP, 100 nM GTP, and 100 nM CTP; final) and were allowed to proceed for 15 min at 37 °C. The reactions were terminated by addition of 5 μl loading buffer (8 M urea, 20 mM EDTA, 0.025 % xylene cyanol, and 0.025 % bromophenol blue). The samples were boiled for 2 min, and immediately cooled down in ice for 5 min. The RNA transcripts were separated using 15% (19:1 acrylamide/bisacrylamide) urea-polyacrylamide slab gels in 90 mM Tris-borate (pH 8.0) and 0.2 mM EDTA. The radiograph was obtained by storage-phosphor scanning (Typhoon; GE Healthcare, Inc.) and the RNA intensity was quantified by FUJIFILM Multi Gauge.
The transcription activation by E. coli ZntR was measured by essentially the same procedure as above except that zntR promoter DNA (prepared by PCR using pEASY/P-zntR as template and M13 primers listed in Table S3) was used and ZnSO4 (100 μM final) was supplemented when indicated. The transcription activation by E. coli CAP was measured by essentially the same procedure as above except that CC (41.5) promoter (prepared by PCR using pEASY/P- CC (41.5) as template and M13 primers listed in Table S3) and cAMP (0.7 mM final) were supplemented when indicated.
Fluorescence-detected in vitro transcription assay
The transcription activity was measured by taking advantage of the drastically increased fluorescence of TO1–3PEG-Biotin (Applied Biological Materials Inc.) upon association of the Mango riboswitch53. To measure the transcription activation by CueR from the pcopA derivative (Supplemental fig. 2i), reaction mixtures (20 μL) containing E. coli, RNAP-σ70 holoenzyme (50 nM), 50 mM Tris-HCl, pH 7.9, 100 mM KCl, 10 mM MgCl2, 1 mM DTT, 5% glycerol, and 0.01% Tween-20, the wild-type or derivative of copA promoter DNA (50 nM final; prepared by PCR using pEASY/P-copA and pEASY/P-copA-chimeric as template and F: 5’-CAGGAAACAGCTATGAC-3’, R:5’-GGCACGTACGAATATACCACATACCAATCCTTCC TTCGTACGTGCCGTCGATAATTTGTGACATAAAACACTCC-3’ primers; the sequence of Mango riboswitch is underscored) were incubated at 37 °C for 10 min. CueR-AgNO3 (400 nM CueR and 100 μM AgNO3; final) were added when indicated. The mixtures were then challenged with 0.25 μL 4 mg/ml heparin (50 μg/ml heparin; final concentration). The reactions were started by addition of 0.5 μL NTP mix (0.1 mM; final concentration) and 0.2 μL 50 μM TOl-3PEG-Biotin (0.5 μM; final concentration) and incubated for 30 min. The fluorescence signals were measured using a plate reader (SPARK, TECAN Inc) at an excitation wavelength of 510 nm and an emission wavelength of 550 nm. The data were plotted in SigmaPlot14.0 (Systat software, Inc)
RNAP molecular beacon assay
The affinity of E. coli RNAP holoenzyme for upstream fork DNA (sequences in Fig. 5d) was measured by a RNAP beacon assay 25, 26. Reaction mixtures contained (100 μl): 1 nM E. coli RNAP holoenzyme containing σ70 labelled at position 211 with 5-tetramethylrhodamine and 0, 2, 4, 8, 16, 32, 64, or 128 nM nucleic-acid scaffold, 10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM DTT, 0.025% Tween-20, and 1% glycerol. Reaction mixtures were incubated for 15 min at room temperature. Fluorescence emission intensities were recorded using a microplate reader (TECAN, Inc; Ex, 550/10 nM; Em, 580/20 nM). The equilibrium dissociation constants (Kd) were estimated by non-linear regression using the equation:
where F is the fluorescence signal at a given concentration of the scaffold, F0 is the fluorescence signal in the absence of the scaffold, [S] is the concentration of scaffold, and B is an unconstrained constant.
The RPo formation detected by the molecular beacon assay were performed using essentially the same procedures as above by using the full-length PcopA derivative promoter DNA (sequences in Fig. 2d). Reaction mixtures contained (100 μl): 2 nM E. coli RNAP holoenzyme containing σ70 labelled at position 211 with 5-tetramethylrhodamine and 0, 2, 4, 8, 16, 32, 64, or 128 nM PcopA derivative, 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM DTT, 0.05% Tween-20, and 1% glycerol. Reaction mixtures were incubated for 15 min at room temperature and challenged with heparin (100 μg/ml; final concentration) for 10 min. Fluorescence emission intensities were measured using a microplate reader (TECAN, Inc; excitation wavelength = 550/10 nM; emission wavelength = 580/20 nM). The equilibrium dissociation constants (Kd) were estimated by nonlinear regression using the equation:
where F is the fluorescence signal at a given concentration of the scaffold, F0 is the fluorescence signal in the absence of the scaffold, [S] is the concentration of scaffold, and B is an unconstrained constant.
Footprinting
Purified CueR protein was obtained as in 33. Labeling of the PcopA non-template and template strands with [α−32P]dATP was carried out as in 16. DNaseI, KMnO4 and 5-phenyl-1,10-phenanthroline-Cu footprinting of labeled PcopA were carried out in footprinting buffer, with all components (except for TPEN) treated with Chelex® 100 chelating resin (Bio-Rad Laboratories, Inc., Hercules, CA, USA), as in 16, with the following changes: for DNaseI footprinting, NaCN, instead of TPEN, was added to generate Cu-free CueR; for KMnO4 and 5-phenyl-1,10-phenanthroline-Cu footprinting, the dinucleotide initiator ApG and nucleotides ATP, GTP and CTP were added where indicated; for DNaseI and KMnO4 footprinting, Cu+ was added as Cu+(CH3N)4PF6 and Ag+ was added as AgNO3. See Figure S1 legend for component concentrations.
β-galactosidase assay
In vivo β-galactosidase reporter assays were essentially carried out as in ref 33. Briefly, wild-type or variant cueR (see table S3 for oligonucleotides), cloned into the low-copy plasmid pACYC184 and under the control of its own promoter, were transformed into the cueR knock-out strain (BW25113 derivative WOII260A) 33, which carries a single genomic copy of the lacZ gene controlled by the copA promoter. Transformants, in distinct colonies, were maintained on LB/Chloramphenicol (Cam, 35 μg/ml) agar plates. Three colonies were chosen and grown to saturation in LB/Cam. Liquid cultures were grown at 37°C, with 250 rpm shaking, in 14 ml Polypropylene round-bottom culture tubes (BD Falcon) slanted at a 45° angle. An aliquot of saturated culture was pelleted by centrifugation, washed once with CDM, resuspended 1:50 in CDM/Cam and split into 4 cultures (one culture for each added CuSO4 concentration). Cultures were grown, as above, to exponential phase and exposed to varying CuSO4 concentrations (see Fig. 4b and Supplementary Fig. 10c) for 60 minutes. The optical densities (at 600 nm) of the cultures were recorded. The following assays were performed in triplicates for each culture. 50 μL culture aliquots were added to 80 μL of permeabilization solution (0.8 mg/mL hexadecyltrimethylammonium bromide, 0.4 mg/mL sodium deoxycholate, 100 mM Na2HPO4, 20 mM KCl, 2 mM MgSO4, 5.4 μL/mL 2-mercaptoethanol) and incubated at 30°C for at least 60 minutes. 600 μL of substrate solution (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mg/mL 2-Nitrophenyl β-D-galactopyranoside, 2.7 μL/mL 2-mercaptoethanol) was added to the permeabilized cells. The assays were timed and allowed to proceed until yellow color was observed (if any), after which 700 μL of stop solution (1 M Na2CO3) was added. The stopped assay samples were then micro-centrifuged at top speed for 2 minutes to remove cellular debris. 100 μL of each assay solution supernatant were added to wells of a Costar® black with clear flatbottom 96-well assay plate (Corning Inc., Corning, NY) and the absorbances at 420 nm were measured, using 100 μL of a mixture of substrate and stop solutions (50:50) as a blank. Miller units were calculated essentially as in 54 (centrifugation of the stopped assay samples allowed for the omission of the 550 nm measurement). Miller unit and standard deviation calculations were performed in Microsoft Office Excel (Microsoft Corp., Redmond, WA). Calculation results were analyzed by Two-way ANOVA using the Tukey post-hoc test with alpha=0.05 (95% confidence interval), performed using Prism version 8.1.2 for macOS (GraphPad Software, La Jolla California USA, www.graphpad.com). The graphs in Fig. 4b and Supplementary Fig. 10c were created in Prism 8.1.2. Transcription repression and activation are defined as follows: average Miller units fall below and above, respectively, of the uncontrolled promoter (ΔcueR, dashed black lines in Fig. 4b and Supplementary Fig. 10c).
Supplementary Material
Acknowledgements
The work was supported by the National Key Research and Development Program of China (2018YFA0900701 to Y.Z. and 2018YFA0507800 to Y.F.), the Strategic Priority Research Program of the CAS to Y.Z. (XDB29020000), the National Natural Science Foundation of China (31822001 to Y.Z. and 31970040 to Y.F.), the Leading Science Key Research Program of CAS to Y.Z. (QYZDB-SSW-SMC005), and the National Institutes of Health of the United States to T.V.O. (GM038784-29 and CA193419). We thank Dr. Shenghai Chang at the cryo-EM center of Zhejiang University, Liangliang Kong and Fangfang Wang at the cryo-EM center of the National Center for Protein Science Shanghai (NCPSS) for assistance with data collection, Dr. Chengyuan Wang at Rutgers University for assistance with figure preparation, and Alfonso Mondragón at Northwestern University for helpful discussions.
Footnotes
Competing Financial Interests Statement
The authors declare no conflict of interest with the contents of this article.
Data Availability Statement
The PDB accession code for the CueR-TAC-1 is 6LDI and the EMDB accession code is EMD-0874. The PDB accession code for the CueR-TAC-2 is 7C17 and the EMDB accession code is EMD-30268.
References
- 1.Browning DF, Butala M. & Busby SJW Bacterial Transcription Factors: Regulation by Pick “N” Mix. J Mol Biol 431, 4067–4077 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Danson AE, Jovanovic M, Buck M. & Zhang X. Mechanisms of sigma(54)-Dependent Transcription Initiation and Regulation. J Mol Biol 431, 3960–3974 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Zhang Y. & Ebright RH Structural basis of transcription activation. Science 352, 1330–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Hong C, Huang RK, Yu Z. & Steitz TA Structural basis of bacterial transcription activation. Science 358, 947–951 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Hubin EA et al. Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. eLife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae B. et al. CarD uses a minor groove wedge mechanism to stabilize the RNA polymerase open promoter complex. eLife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown NL, Stoyanov JV, Kidd SP & Hobman JL The MerR family of transcriptional regulators. FEMS Microbiol Rev 27, 145–163 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Lopez R, Ruiz R, de la Cruz F. & Moncalian G. Transcription factor-based biosensors enlightened by the analyte. Front Microbiol 6, 648 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni RD & Summers AO MerR cross-links to the alpha, beta, and sigma 70 subunits of RNA polymerase in the preinitiation complex at the merTPCAD promoter. Biochemistry 38, 3362–3368 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Ishihama A. Promoter selectivity control of RNA polymerase, in Nucleic acids and molecular biology, Vol. 11. (eds. E. FD) 53–70 (Heidelberg: Springer-Verlag, 1997). [Google Scholar]
- 11.Heldwein EE & Brennan RG Crystal structure of the transcription activator BmrR bound to DNA and a drug. Nature 409, 378–382 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Philips SJ et al. Allosteric transcriptional regulation via changes in the overall topology of the core promoter. Science 349, 877–881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansari AZ, Bradner JE & O’Halloran TV DNA-bend modulation in a repressor-to-activator switching mechanism. Nature 374, 371–375 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Ansari AZ, Chael ML & O’Halloran TV Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature 355, 87–89 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Watanabe S, Kita A, Kobayashi K. & Miki K. Crystal structure of the [2Fe-2S] oxidative-stress sensor SoxR bound to DNA. Proc Natl Acad Sci U S A 105, 4121–4126 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Outten CE, Outten FW & O’Halloran TV DNA distortion mechanism for transcriptional activation by ZntR, a Zn(II)-responsive MerR homologue in Escherichia coli. J Biol Chem 274, 37517–37524 (1999). [DOI] [PubMed] [Google Scholar]
- 17.O’Halloran TV, Frantz B, Shin MK, Ralston DM & Wright JG The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell 56, 119–129 (1989). [DOI] [PubMed] [Google Scholar]
- 18.Frantz B. & O’Halloran TV DNA distortion accompanies transcriptional activation by the metal-responsive gene-regulatory protein MerR. Biochemistry 29, 4747–4751 (1990). [DOI] [PubMed] [Google Scholar]
- 19.Martell DJ et al. Metalloregulator CueR biases RNA polymerase’s kinetic sampling of dead-end or open complex to repress or activate transcription. Proc Natl Acad Sci U S A 112, 13467–13472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y. et al. Structural basis of transcription initiation. Science 338, 1076–1080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae B, Feklistov A, Lass-Napiorkowska A, Landick R. & Darst SA Structure of a bacterial RNA polymerase holoenzyme open promoter complex. eLife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayanan A. et al. Cryo-EM structure of Escherichia coli sigma(70) RNA polymerase and promoter DNA complex revealed a role of sigma non-conserved region during the open complex formation. J Biol Chem 293, 7367–7375 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultzaberger RK, Chen Z, Lewis KA & Schneider TD Anatomy of Escherichia coli sigma70 promoters. Nucleic Acids Res 35, 771–788 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell EA et al. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell 9, 527–539 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Mekler V, Pavlova O. & Severinov K. Interaction of Escherichia coli RNA polymerase sigma70 subunit with promoter elements in the context of free sigma70, RNA polymerase holoenzyme, and the beta’-sigma70 complex. J Biol Chem 286, 270–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mekler V. & Severinov K. RNA polymerase molecular beacon as tool for studies of RNA polymerase-promoter interactions. Methods 86, 19–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson BP et al. Three-dimensional EM structure of an intact activator-dependent transcription initiation complex. Proceedings of the National Academy of Sciences of the United States of America 106, 19830–19835 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rammohan J, Ruiz Manzano A, Garner AL, Stallings CL & Galburt EA CarD stabilizes mycobacterial open complexes via a two-tiered kinetic mechanism. Nucleic Acids Res 43, 3272–3285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rammohan J. et al. Cooperative stabilization of Mycobacterium tuberculosis rrnAP3 promoter open complexes by RbpA and CarD. Nucleic Acids Res 44, 7304–7313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen D, Manzano AR, Rammohan J, Stallings CL & Galburt EA CarD and RbpA modify the kinetics of initial transcription and slow promoter escape of the Mycobacterium tuberculosis RNA polymerase. Nucleic Acids Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralston DM & O’Halloran TV Ultrasensitivity and heavy-metal selectivity of the allosterically modulated MerR transcription complex. Proc Natl Acad Sci U S A 87, 3846–3850 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Halloran TV Transition metals in control of gene expression. Science 261, 715–725 (1993). [DOI] [PubMed] [Google Scholar]
Methods-only References
- 33.Outten FW, Outten CE, Hale J. & O’Halloran TV Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J Biol Chem 275, 31024–31029 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Gaston K, Bell A, Kolb A, Buc H. & Busby S. Stringent spacing requirements for transcription activation by CRP. Cell 62, 733–743 (1990). [DOI] [PubMed] [Google Scholar]
- 35.Fang C. et al. Structures and mechanism of transcription initiation by bacterial ECF factors. Nucleic Acids Res 47, 7094–7104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Fang C, Zhuang N, Wang T. & Zhang Y. Structural basis for transcription initiation by bacterial ECF sigma factors. Nat Commun 10, 1153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng SQ et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang K. Gctf: Real-time CTF determination and correction. J Struct Biol 193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Leiro R. & Scheres SHW A pipeline approach to single-particle processing in RELION. Acta Crystallogr D Struct Biol 73, 496–502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degen D. et al. Transcription inhibition by the depsipeptide antibiotic salinamide A. eLife 3, e02451 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson R. et al. Outcome of the first electron microscopy validation task force meeting. Structure 20, 205–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emsley P. & Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Adams PD et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavery R, Moakher M, Maddocks JH, Petkeviciute D. & Zakrzewska K. Conformational analysis of nucleic acids revisited: Curves+. Nucleic Acids Res 37, 5917–5929 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu XJ & Olson WK 3DNA: a versatile, integrated software system for the analysis, rebuilding and visualization of three-dimensional nucleic-acid structures. Nat Protoc 3, 1213–1227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pettersen EF et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Murakami KS, Masuda S. & Darst SA Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science 296, 1280–1284 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Boyaci H, Chen J, Jansen R, Darst SA & Campbell EA Structures of an RNA polymerase promoter melting intermediate elucidate DNA unwinding. Nature 565, 382–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feklistov A. et al. RNA polymerase motions during promoter melting. Science 356, 863–866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyaci H. et al. Fidaxomicin jams Mycobacterium tuberculosis RNA polymerase motions needed for initiation via RbpA contacts. Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin W. et al. Structural Basis of Transcription Inhibition by Fidaxomicin (Lipiarmycin A3). Mol Cell 70, 60–71 e15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakraborty A. et al. Opening and closing of the bacterial RNA polymerase clamp. Science 337, 591–595 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trachman RJ 3rd et al. Structural basis for high-affinity fluorophore binding and activation by RNA Mango. Nat Chem Biol 13, 807–813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller JH Experiments in molecular genetics. (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.; 1972). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PDB accession code for the CueR-TAC-1 is 6LDI and the EMDB accession code is EMD-0874. The PDB accession code for the CueR-TAC-2 is 7C17 and the EMDB accession code is EMD-30268.