Abstract
Background:
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a rare and challenging demyelinating disorder. It is necessary to increase our understanding of potential connections between imaging, electromyography, and clinical characteristics.
Objective:
The aim of this study was to evaluate the relationships between multisequence magnetic resonance neurography (MRN) findings, electrophysiological parameters, and clinical characteristics in CIDP patients.
Design:
A cross-sectional study.
Methods:
Overall, 51 CIDP patients underwent MRN of the brachial and lumbosacral plexus, and nerve conduction studies. The inflammatory Rasch-built overall disability scale (I-RODS) questionnaire, CIDP disease activity status (CADS) scale, and muscle strength scores were evaluated by two neurologists. Electrophysiological parameters, clinical information, and multiparameter-MRN were analyzed for correlations. Multiparameter-MRN includes diameter, nerve-to-muscle T2 signal intensity ratio (nT2), contrast-enhanced ratio (CR), fractional anisotropy (FA), and apparent diffusion coefficient (ADC) of bilateral plexus nerve roots.
Results:
Electrophysiological parameters that were not elicited were significantly higher in the lower extremities than in the upper extremities, and those were higher in sensory nerve conduction than in motor. There were moderate correlations between motor nerve conduction velocity and distal motor latency in nerve diameter, nT2, FA, and ADC, respectively (|r|, 0.45–0.64, p < 0.05). The correlations between CR and sensory nerve conduction velocity and peak latency were moderate, and ADC had a positive correlation with compound motor action potential amplitude (|r|, 0.45–0.63). FA correlated negatively with the course (r = −0.62) and cerebrospinal fluid (CSF) protein (r = −0.41), whereas ADC had correlated positively with CSF protein (r = 0.34). Only CR had a moderately negative correlation with CADS (r’s = −0.57). Muscle strength in all extremities was positively correlated with FA (r’s range, 0.41–0.49). There was no significant correlation between I-RODS scores and multiparameter-MR.
Conclusion:
MRN-derived multiparameter [nerve size, nT2, and diffusion tensor imaging (DTI) parameters] could serve as quantitative biomarkers of myelin sheath integrity in CIDP. DTI parameters and CR correlated with clinical characteristics better than morphological parameters-MR for CIDP patients.
Keywords: chronic inflammatory demyelinating, diffusion tensor imaging, electrophysiology, magnetic resonance imaging, polyradiculoneuropathy
Introduction
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an immune-mediated inflammatory polyneuropathy characterized by sensorimotor or autonomic nerve involvement and muscular weakness.1,2 Demyelination and remyelination are pathological characteristics of CIDP.3 The CIDP is typically diagnosed based on usual clinical signs combined with electrophysiological evidence of demyelinating disease.4 Other auxiliary methods [e.g. lumbar puncture, inflammatory Rasch-built overall disability scale (I-RODS), CIDP disease activity status (CADS), and muscle strength assessments] for the diagnosis and evaluation of the disease progression in this domain are also available.5,6 However, the onset of CIDP is insidious, and diagnosis is unreliable in the early stages of the disease and in cases with clinically atypical presentation. Currently, clinical, serological, and electrophysiological CIDP characteristics are insufficiently sensitive to provide accurate information on disease progression and treatment response.3,7 In addition, electromyography is less helpful for disease monitoring in CIDP patients with severe nerve injury because waveforms are frequently not evoked, and clinical scales have much more subjectivity in common.8 Therefore, objective and serially applicable non-invasive biomarkers are urgently needed.
Magnetic resonance imaging (MRI) and ultrasound are both non-invasive neuropathy evaluation technologies that have been recognized as supporting diagnostic criteria in the European Federation of Neurological Societies / Peripheral Nerve Society guidelines.4 Ultrasound is an easy and inexpensive method to detect morphological abnormalities in the brachial plexus nerve. However, some limitations of ultrasound include user dependence and the inability to assess nerves at all sites, especially the proximal nerve roots of the deep plexus that are often involved.9 Furthermore, magnetic resonance neurography (MRN) not only allows imaging to clearly identify deep nerves conspicuously and accurately locate lesions but it also provides measurement of microstructure and function of nerve tissue. Diffusion tensor imaging (DTI), for example, as nerve fiber biomarkers, improves the diagnostic value of conventional MRI in peripheral neuropathy.9–11 Before these neuroimaging techniques can be used in clinical setting, it is crucial to be compared to the ‘gold standard’ in terms of imaging potential and association with clinical and electrophysiological parameters. However, previous studies have primarily focused on morphological indicators, and the correlation between multiparameter MRN, electrophysiological parameters, and clinical scales has not been investigated systematically.
Therefore, this study aims to evaluate the relationships between multisequence MRN findings, electrophysiology, and clinical characteristic, such as the period between symptom onset and study participation, cerebrospinal fluid (CSF) protein content, muscle strength, CIDP scales of activity and overall impairment, and electrophysiological features in CIDP patients.
Materials and methods
This study was approved by the ethical review committee (No. IORG0003571) and was registered on ClinicalTrials.gov (ChiCTR1800016450). All patients voluntarily signed a written informed consent prior to inclusion in the study. The study was conducted from March 2017 to November 2021.
Subjects
A total of 51 patients with CIDP were consecutively recruited from the Neuromuscular Center of our hospital. Their conditions were confirmed to meet the European Federation of Neurological Societies / Peripheral Nerve Society diagnostic criteria by a neurologist.4 Exclusion criteria included (1) any contraindication to MRI and the presence of severe renal insufficiency (eGFR < 30 ml/min/1.73 m2) and (2) patients who were unable to undergo routine MRI examinations or had poor images with severe artifacts.
Clinical characteristics
Clinical data from electronic medical records were collected, including age, sex, disease course, and CSF protein. The I-RODS questionnaire scores,5 CADS scale,7 and the UK Medical Research Council (MRC) muscle strength scores12 were documented before MRI scans individually by two neurologists. I-RODS questionnaire scores have proven to be an outcome measurement for assessing activity restrictions and disability with 24-item levels ranging from easy to difficult (e.g. ‘Reading’ a newspaper/book is an easy event; ‘Running’ is a difficult event). The CADS scale was proposed by the Guillain Barré syndrome (GBS)/CIDP Foundation International Medical Advisory Board to assess CIDP disease activity, which graded from 1A to 5C (a total of 10 grades). MRC muscle strength score was quantified from grade 0 to grade 5 (a total of 13 grades). Any assessment disagreements were settled by consensus to reach a final conclusion.
Nerve conduction studies
Nerve conduction examinations on CIDP patients were performed by a board-certified neurologist using a Keypoint 4 electromyograph (Medtronic, Denmark), with skin temperature of extremities maintained at 32°C. The motor nerves (median, ulnar, tibial, and common peroneal nerves) and sensory nerves (median, ulnar, sural, and superficial peroneal nerves) were studied in detail. The electrophysiological parameters include the motor nerve conduction velocity (MCV), amplitude of compound motor action potential (CMAP), distal motor latency (DML), sensory nerve conduction velocity (SCV), amplitude of sensory nerve action potential (SNAP), and peak latency (PL). If the response waveform in all the discovered nerves can be elicited, the patient was assigned to group A; otherwise, the patient with one or more nerves failed to be elicited was in group B.
Multisequence MRN
MRI protocol
A 3-T whole-body MR system (MAGNETOM Trio, Siemens Healthcare) was used for the MRN. The MRN protocol of the brachial and lumbosacral (LS) plexus is based on previously published literature,10 which includes volumetric interpolated breath-hold examination, turbo inversion recovery magnitude, sampling perfection with application-optimized contrasts using different flip angle evolution sequences on the coronal plane, and echo-planar-imaging-based DTI sequence on the axial plane. A macrocyclic contrast agent (Gadovist, Bayer Healthcare) was injected intravenously at a dose of 0.1 ml/kg with a flow rate of 1.5 ml/s. Finally, following gadolinium injection, contrast-enhanced (ce)-volumetric interpolated breath-hold examination and sampling perfection with application-optimized contrasts using different flip angle evolution sequences were applied. Detailed parameters are provided in Supplementary table 1.
Image postprocessing and analysis
Postprocessing software of MRI data was provided by the MR system manufacturer (Syngo MR Workspace, Siemens Healthcare) and references to previously published literature.10 Qualitative assessment of abnormal findings (hypertrophy/ hypersignal/contrast enhancement) of the brachial and LS plexus was evaluated by two experienced radiologists. The final conclusion was reached through consensus for any disagreements. The brachial (bilateral C5–C8) and LS plexus (bilateral L4–S1) nerve roots were subjected to a multiparameter quantitative analysis that included nerve diameter, nerve-to-muscle T2 signal intensity ratio (nT2), contrast-enhanced ratio (CR), and DTI parameters. Using a different flip angle evolution sequence, the diameter of nerve roots was measured perpendicular to the long axis of the maximum intensity projection image of the sampling perfection with application-optimized contrasts. The nT2 was obtained by placing the region of interest (ROI) nerve roots and calculated in relation to the adjacent muscles on the turbo inversion recovery magnitude sequence. The CR of nerve roots was determined at the same location by coping the ROI between the ce and non-ce volumetric interpolated breath-hold examination sequences. The apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values were calculated on the DTI by hand-operated circular ROI at nerve roots. The final averaged values were obtained by three repeated measurements in each parameter. For diffusion tensor tractography, a flip angle of 30° and an FA threshold of 0.10 were employed. The schematic diagrams of the multiparameter measurement were depicted in Figures 1 and 2.
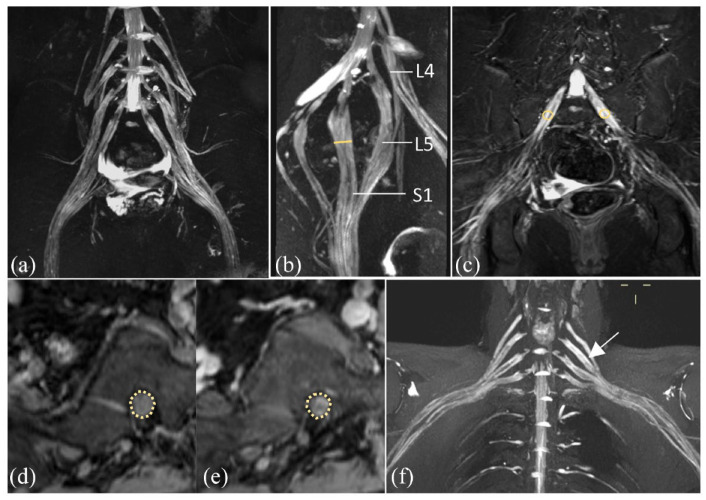
Figure 1.
MRN showed that diffuse hypertrophy of the LS plexus (a, b) and brachial plexus (f) nerve roots, bundles, and branches with increased T2 signal intensity, accompanied by mild enhancement (d, e) in CIDP patient. Schematic diagram showed the measurement of the diameter in the left S1 nerve root (yellow line in b), signal intensity of bilateral S1 nerve roots on TIRM sequence (circles in c), and on non-ce VIBE and ce VIBE sequences, separately (circles in d, e).
Figure 2.
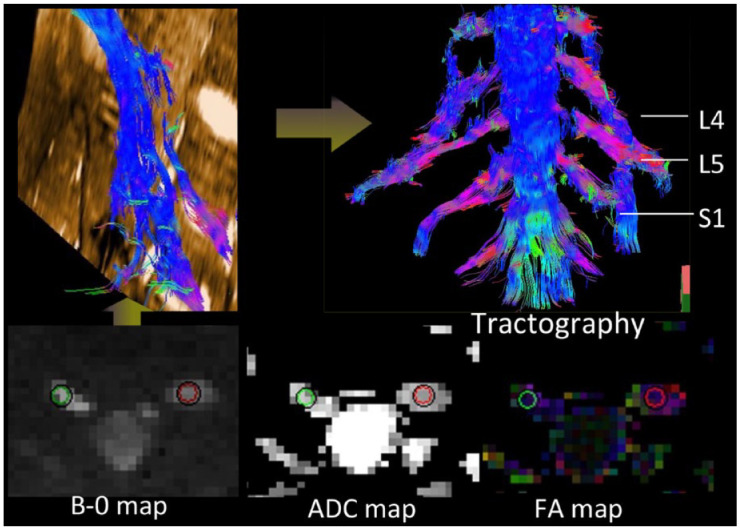
Schematic diagram showed the measurement of the DTI parameters, including FA and ADC values (circles in bilateral S1 nerve roots). Diffusion tensor tractography showed thickened and distorted discontinuous tracts in the LS plexus.
Statistical analysis
Statistical analysis was performed by IBM SPSS statistical software version 25 (IBM corp., Armonk, NY, USA) and GraphPad prism 7.0 (San Diego, USA). Kolmogorov–Smirnov and homogeneity variance tests were performed for the quantitative data. Categorical variables were summarized as frequencies and proportions. The quantitative data of normal distribution were expressed by mean ± standard deviation (X ± S). Median (M) and quartile (q1, q3) were expressed in discontinuous variance or non-normal distributed data. The Bonferroni correction was used to address multiple hypothesis testing. The difference of rates was calculated by adjusted chi-square test between related two samples. Mann–Whitney U test was used to assess the differences between two independent samples. Paired-sample t-test (normal distributed data) or Wilcoxon signed-rank test was used to assess the differences between two related samples. Pearson’s (normal distributed data) and Spearman’s correlation were performed. Two-tailed p < 0.05 was considered statistically significant.
Results
Clinical features
A total of 51 CIDP patients were enrolled, with 14 females and 37 males, and a body mass index of 23.5 ± 0.4 kg/m2. Their average age was 49.3 ± 11.8 years (ranged from 32 to 67 years). The mean age at the onset was 44.5 ± 11.9 years, and the median course of disease at the time was 24 (8, 48) months. A total of 39 CIDP patients had lumbar puncture, with the mean value of CSF protein was 0.998 ± 0.71 g/liter. Their median score of the I-RODS questionnaires was 35.5 (27, 41), and the CADS scale was 8 (5, 9). The median scores of MRC muscle strength were 11 (9, 12) for right lower limb, 10 (8, 12) for left lower limb, 12 (10, 12) for right upper limb, and 12 (10, 12) for left upper limb, respectively.
Nerve conduction studies
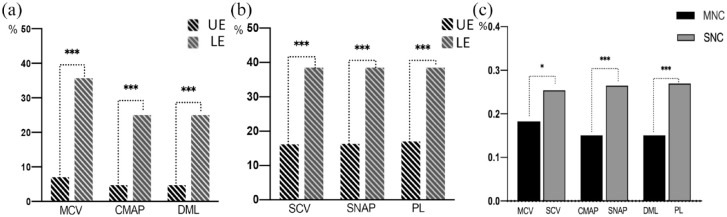
A total of 51 patients were examined with 331 motor and 354 sensory nerves. Non-elicited rates of motor nerves ranged 15.1–35.7% and sensory nerves ranged 26.4–26.9%. Non-elicited rates of each parameter, both in motor and sensory nerves conduction, were significantly higher in the lower extremities than in the upper extremities [p < 0.001, Figure 3(a) and (b)]. Non-elicited rates of the conduction velocity, latency and amplitude of action potential in sensory nerve conductions were significantly higher than those of motor nerve conductions [p < 0.05, Figure 3(c)].
Figure 3.
Non-elicited rate of the motor and sensory nerve conduction in lower extremities was significantly higher than those of upper extremities (a, b). Non-elicited rate of the conduction velocity, latency, and amplitude of action potential in sensory nerve conduction was significantly higher than those of motor nerve conduction (c).
CMAP, compound muscle action potential; DML, distal motor latency; LE, lower extremities; MCV, motor conduction velocity; MNC, motor nerve conduction; PL, peak latency; SCV, sensory nerve conduction velocity; SNAP, sensory nerve action potential; SNC, sensory nerve conduction; UE, upper extremities.
*p < 0.05.
***p < 0.001.
In motor nerve conduction, the amplitude of CMAP and MCV of all nerves in group B was significantly lower than those in group A (p range, 0.002–0.041). The DML of the ulnar nerve was significantly longer in group B than in group A (p = 0.002). See Supplementary table 2. In sensory nerve conduction, the SCV of the sural and ulnar nerves and amplitude SNAP of sural nerve in group B were significantly lower than in group A (p < 0.01), while the PL in group B was longer (p = 0.008). See Supplementary table 3.
Image characteristics and analysis
A total of 47 CIDP patients underwent MR examination. Two cases were excluded due to MR contraindications and another MR images of two cases were discarded because of severe motion artifacts. The abnormal rate of the LS plexus (76.6%, 36/47) was significantly higher than brachial plexus (61.7%, 29/47) in CIDP patients (κ = 4.0, p < 0.05). Representative hypertrophy with increased signal intensity and enhancement were illustrated as seen in the LS and brachial plexus of the CIDP patients (Figures 1 and 2 and S. Fig.1). The FA values of nerve roots in the LS plexus were significantly lower than those in the brachial plexus, while CR values in LS plexus were higher (p < 0.05, Table 1). There were no significant differences between the LS and brachial plexus never roots in the nT2 and ADC values (p > 0.05, Table 1).
Table 1.
Multiparameter for brachial and LS plexus.
| Brachial plexus | LS plexus | p value | |
|---|---|---|---|
| Diameter (mm) | 5.11 (4.21, 5.94) | 7.50 (6.33, 9.35) | <0.000**** |
| nT2 | 4.275 ± 1.81 | 3.970 ± 1.54 | 0.053 |
| CR | 1.196 ± 0.21 | 1.331 ± 0.23 | <0.000**** |
| FA | 0.295 ± 0.08 | 0.279 ± 0.07 | 0.028* |
| ADC (10−3 mm2) | 1.586 ± 0.27 | 1.628 ± 0.29 | 0.107 |
ADC, apparent diffusion coefficient; CR, contrast-enhanced ratio; FA, fractional anisotropy; LS, lumbosacral; nT2, nerve-to muscle T2 signal intensity ratio.
The numbers in parentheses indicate the quartiles (q1, q3).
p < 0.05.
p < 0.0001.
Correlations of electrophysiological and MRN parameters
The MCV and DML of motor nerve conduction have significantly moderate correlations with the nerve diameter, nT2, FA, and ADC values, respectively (all ps < 0.05,|r| range, 0.45–0.64, Figure 4). The CR value had significantly moderate correlations with the SCV and PL of sensory nerve conduction, and the ADC had moderate correlations with the amplitude SNAP, respectively (p < 0.05,|r| range, 0.45–0.63, Figure 4).
Figure 4.
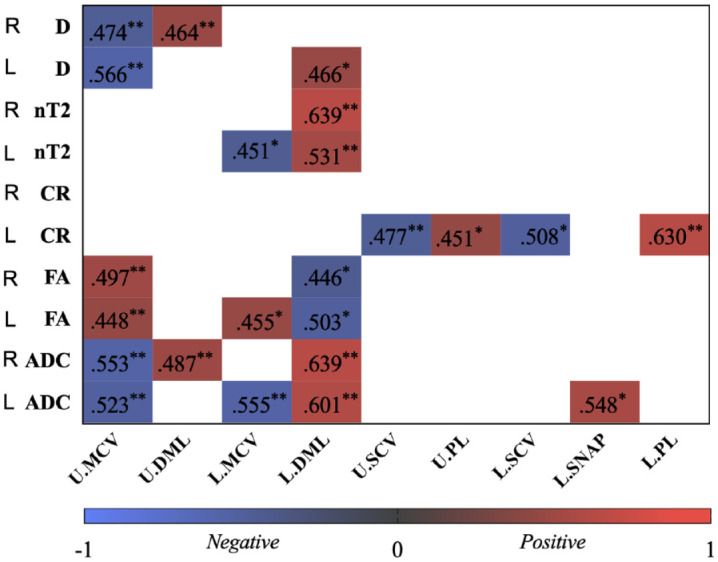
The heatmap shows significant correlation coefficients (r) between quantitative magnetic resonance multiparameters and electrophysiological parameters.
ADC, apparent diffusion coefficient; CR, contrast-enhanced ratio; D, diameter; DML, distal motor latency; FA, fractional anisotropy; L., lower extremities; MCV, motor conduction velocity; nT2, nerve-to muscle T2 signal intensity ratio; PL, peak latency; SCV, sensory nerve conduction velocity; SNAP, sensory nerve action potential; U., upper extremities.
*p < 0.05.
**p < 0.01.
Correlations of clinical characteristics and MRN parameters
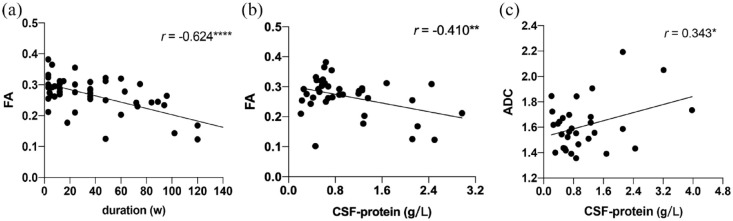
The FA value had significantly moderate negative correlations between course duration (r = −0.624) and CSF protein (r = −0.410) in patients with CIDP, respectively [p < 0.05, Table 2 and Figure 5(a) and (b)].
Table 2.
Correlations of clinical characteristic and MRN parameters.
| Course duration | I-RODS | CSF protein | CADS | |||||
|---|---|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | r’s | p value | |
| D | 0.154 | 0.336 | 0.083 | 0.620 | 0.150 | 0.396 | −0.162 | 0.410 |
| nT2 | −0.096 | 0.558 | 0.216 | 0.200 | 0.246 | 0.167 | 0.055 | 0.786 |
| CR | −0.16 | 0.36 | −0.294 | 0.097 | −0.269 | 0.158 | 0.570 | 0.003** |
| FA | −0.624 | <0.000**** | −0.107 | 0.542 | −0.410 | 0.001** | 0.025 | 0.908 |
| ADC | 0.106 | 0.531 | −0.120 | 0.481 | 0.343 | 0.041* | 0.092 | 0.664 |
ADC, apparent diffusion coefficient; CADs, CIDP disease activity status; CR, contrast-enhanced ratio; CSF, cerebrospinal fluid; D, diameter; FA, fractional anisotropy; I-RODS, inflammatory Rasch-built overall disability scale; nT2, nerve-to muscle T2 signal intensity ratio; r, Pearson correlation coefficient; r’s, Spearman correlation coefficient.
p < 0.05.
p < 0.01
p < 0.0001.
Figure 5.
Pearson correlation shows that FA value has significantly moderate negative correlations between (a) disease course and (b) CSF protein, respectively (p < 0.05). There was a slight significantly positive correlation between (c) ADC value and CSF protein.
ADC, apparent diffusion coefficient; CSF, cerebrospinal fluid; FA, fractional anisotropy.
*p < 0.05.
**p < 0.01.
****p < 0.0001.
There was a significantly mild positive correlation between ADC value and CSF protein [r = 0.343, p < 0.05, Table 2 and Figure 5(c)]. Only the CR value had a significantly moderate negative correlation with CADS scores (r’s = 0.570, p < 0.01, Table 2). No significant correlation was found between I-RODS questionnaire scores and multiparameter MRN (Table 2). MRC muscle strength scores of all extremities had significantly moderate positive correlations with corresponding FA values, respectively, but no correlation with rest MR parameters (r’s range, 0.410–0.493, all ps < 0.05, Table 3 and S. Table 4).
Table 3.
Correlations of MRC strength scores and MRN parameters.
| Brachial plexus | LS plexus | ||||
|---|---|---|---|---|---|
| r’s | p value | r’s | p value | ||
| Left UE | 0.493 | 0.017* | Left LE | 0.440 | 0.041* |
| Right UE | 0.410 | 0.021* | Right LE | 0.470 | 0.038* |
LE, lower extremity; r’s, Spearman correlation coefficient; UE, upper extremity.
p < 0.05.
Discussion
CIDP is mostly characterized by chronic progressive and relapsing courses.3 Clinical, electrophysiological, and serological assessments may not be sensitive enough to monitor disease activity at the moment.2,13 The multiparametric MRN paradigm is non-invasiveness and repeatable approach, allowing for measurements of architectural configuration and microstructural properties in deep plexus and nerve trunks in extremities. Only histological examinations provide reliable evidence of disease activity and parameter abnormalities, while sural nerve biopsy is both difficult and invasive, and it is not feasible in deep nerve trunks.14,15 Considering fibers from the plexus nerve root contributes to the creation of peripheral nerves extremities, and alterations of the plexus nerve roots may indicate an impairment in nerve conduction function in both motor and sensory nerves of the extremities.16 In this study, electrophysiology suggests that nerve injuries in the lower extremities were more severe than upper extremities, which is consistent with MRN findings. The degree of nerve injury was greater in patients without elicited waveform, making monitoring and prognosis difficult in those patients. Furthermore, there were significant correlations among morphological and microstructural indices of plexus nerve roots in multiparameter MRN, electrophysiological parameters, and clinical characteristics (disease duration, CSF protein, MRC muscle strength, and CADS scale scores) except I-RODS questionnaire scores.
Latency and conduction velocity are well known electrophysiological parameters in demyelinating neuropathy. Nerve size, such as diameter or cross-sectional area, is now the most commonly used metric in MRI or ultrasound in correlation studies, while a few studies have been reported correlations between the nT2 value and electrophysiologic parameters.17–19 We found that larger diameter and nT2 value were correlated with lower MCV and longer DML but not with amplitude of CMAP, implying that nerve size and nT2 value were correlated with more demyelination rather than axonal injury. That results could have been corroborated, and the part of nerve size was consistent with the findings from nerve ultrasound studies.19 Hypertrophy with increased signal of peripheral nerves was mainly attributed by the proliferation of Schwann cell and the edema in the endoneurium, which resulted in repeated demyelination and regeneration of myelin sheath in CIDP.20 Moreover, the increased T2 signal in nerve tissues was caused by pathologic processes with the interstitial edema, increased myelin membranes or macromolecules by remyelination, rather than an increase in free water.21,22 However, regarding clinical characteristics (course, CSF protein, MRC muscle strength, I-RODS, and CADS scale scores), we did not find any significant correlation with diameters or nT2 values, which was in line with previous studies.17,19,22 Moreover, nerve size and nT2 value have been reported to be insensitive to disease prognosis in previous literature23 that may be down to the fact that it serves as morphological indices of nerves, probably not changing markedly over time or in the early stages of disease.
As mentioned before, DTI is a non-invasive functional MR for peripheral nerves that portrays the microstructural integrity of nervous tissue.24 FA is a biomarker of nerve fiber integrity and is the most commonly used DTI parameter. The ADC value reflects the diffusion degree of molecules in each voxel and can be used as an indirect indicator to assess the size of diffusion barriers, such as cell membranes or myelin sheaths. Nerves inflammation, edema, or injury can result in decreased FA and increased ADC values.25,26 However, the association of DTI with electrophysiological parameters is a contentious issue. A study reported a lack of a significant correlation,9 while others reported significant correlations between DTI parameters and electrophysiological parameters.27–29 In this study, we found that microstructural parameters derived by DTI correlated with electrophysiological parameters of demyelinating neuropathy. That is, FA was positively associated with MCV, while negatively associated with DML, and the ADC tended to be in the opposite direction as the FA, as predicted by theory. Furthermore, DTI parameters were not found to be associated with CMAP amplitude, as an electrophysiological marker of axonal neuropathy, which was consistent with previous studies in peripheral neuropathy.27,29 However, it also has been reported that FA was positively correlated with CMAP amplitude in a study with small sample size.28 The possibility for this discrepancy is that the severity of affiliated patients varies, or that a bias develops in a limited sample.
Gadolinium enhancement of spinal roots and cauda equina on MRI is hypothesized to be caused by increased permeability or destruction of blood nerve barrier, may work by signaling the presence of an inflammatory process, as seen in CIDP.30,31 CR was used as an MR biomarker for detecting the permeability of blood nerve barrier, and greater CR associated with more severely impaired blood nerve barrier integrity.10,32 Present studies focusing on correlation between the permeability of blood nerve barrier and electrophysiological parameters are rare. Our study provides, for the first time, evidence of correlations between the CR value and demyelinating parameters of sensory nerve conduction that is SCV and PL rather than motor conduction in our cohort. However, there was a lack of understanding of molecular basis that needs to be addressed further. The one possibility is that the CR represents specific pathophysiological changes that would serve as a complementary MRI parameter to DTI and morphological index. Given that CIDP is primarily a demyelinating disease,3 these findings supported the current hypothesis that multiparameter-MRN could serve as specific biomarkers of demyelinating neuropathy.
In this study, DTI parameters and CR correlated well with clinical characteristics, but not with morphological features, such as nerve diameter and nT2. Therefore, DTI and CR seem to be more robust biomarkers, which might be attributed to their greater sensitivity to neural function and microstructure. We found that patients with lower FA and higher ADC values of the nerve roots had a moderate trend toward a higher CSF protein and a longer disease duration. CSF protein has been reported to be positively associated with the severity of CIDP;33,34 it might be proposed for FA representing the severity of CIDP over time since lumbar puncture is an invasive procedure that does not allow for frequent examination. FA of the plexus nerve roots was found to be positively related to MRC muscular strength of the ipsilateral extremities. However, we did not find a link between changes in multiparameter-MRN and I-RODS scores in CIDP patients. Previous studies have reported a moderate negative correlation between FA and the inflammatory neuropathy cause and treatment scale and the neuropathy impairment score.27,35 However, this score was not implemented in our study because the I-RODS scale has been proved to be more appropriate for inflammatory neuropathy with more sensitive to clinical changes.36 In addition, CADS, as a measure of assessing CIDP activity, relates the determination of disease activity to treatment status.6 To the best of our knowledge, we are the first to discover that CR has a positive association with CADS, indicating that it might be used as a biomarker to monitor disease activity, although further studies with larger sample size are required.
According to the results of this study, FA and CR values could be used as potential markers in the management of prognosis for the patients of CIDP, which would contribute to further studies into disease progression and developed technologies for monitoring the efficacy of new therapies. It will most likely be useful in severe cases of neuropathy and in the patients who are unable to perform electromyography (EMG), such as those with bleeding tendencies and fainting needles.
Limitations
Our study has several limitations. First, given the disease’s low incidence, the sample size was inadequate. Second, our patient population was not completely representative as it mainly comprised moderate and severe affected patients, with no variant CIDP included. The third issue is a lack of histology confirmation, which is difficult to execute biopsies on all patients with neuritis. Finally, we did not examine other neuropathies with mimics of CIDP, so that lack of disease controls that documented morphological and microstructure alterations of peripheral nerves cannot be declared as specific to CIDP. Future study will further expand our patient cohort and disease types. Also, longitudinal studies are still needed to include any of these parameters or other indicators, such as radial and axial diffusivity, and their correlations change over time in the future.
Conclusion
In CIDP, MRI-derived multiparameter validated quantitative biomarkers of myelin sheath integrity. DTI parameters and CR had a stronger correlation with clinical characteristics than morphological parameters (nerve diameter and nT2). Therefore, we believe that estimating these quantitative parameters will be the future function of MRN in treatment monitoring or prediction of CIDP patients. Multisequence MRN may be especially useful in situations with confusing electrophysiological findings or in severe cases of neuropathy, where the waveform may not be retrieved, making stratification and monitoring problematic in those patients.
Multisequence MRN could be an objective and useful tool for quantifying and monitoring subclinical or severe damage of peripheral nerves in patients with CIDP. It may be more helpful to doctors in non-specialized hospitals due to not sensitive to an uncommon disease.
All subjects signed written informed consent prior to enrollment.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221150540 for Multisequence magnetic resonance neurography of brachial and lumbosacral plexus in chronic inflammatory demyelinating polyneuropathy: correlations with electrophysiological parameters and clinical features by Xiaoyun Su, Xiangquan Kong, Xiangchuang Kong, Qing Zhu, Zuneng Lu and Chuansheng Zheng in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iD: Xiaoyun Su  https://orcid.org/0000-0003-2375-9231
https://orcid.org/0000-0003-2375-9231
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaoyun Su, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan 430022, China.
Xiangquan Kong, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Xiangchuang Kong, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Qing Zhu, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Zuneng Lu, Department of Neurology, Renming Hospital of Wuhan University, Wuhan 430060, China.
Chuansheng Zheng, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan 430022, China.
Declarations
Ethics approval and consent to participate: This prospective study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No. IORG0003571). All included patients gave their verbal and written informed consent.
Consent for publication: Not applicable.
Author contributions: Xiaoyun Su: Conceptualization; Data curation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Xiangquan Kong: Formal analysis; Investigation; Software.
Xiangchuang Kong: Investigation; Methodology; Validation.
Qing Zhu: Data curation; Formal analysis; Investigation; Methodology; Validation.
Zuneng Lu: Conceptualization; Project administration; Resources; Supervision; Writing – review & editing.
Chuansheng Zheng: Conceptualization; Funding acquisition; Project administration; Supervision; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant Nos. 81470076 and 81701653).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Latov N. Diagnosis and treatment of chronic acquired demyelinating polyneuropathies. Nat Rev Neurol 2014; 10: 435–446. [DOI] [PubMed] [Google Scholar]
- 2. Rajabally YA, Stettner M, Kieseier BC, et al. CIDP and other inflammatory neuropathies in diabetes – diagnosis and management. Nat Rev Neurol 2017; 13: 599–611. [DOI] [PubMed] [Google Scholar]
- 3. Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol 2010; 9: 402–412. [DOI] [PubMed] [Google Scholar]
- 4. Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force-Second revision. Eur J Neurol 2021; 28: 3556–3583. [DOI] [PubMed] [Google Scholar]
- 5. van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built overall disability scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 2011; 76: 337–345. [DOI] [PubMed] [Google Scholar]
- 6. Gorson KC, van Schaik IN, Merkies IS, et al. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst 2010; 15: 326–333. [DOI] [PubMed] [Google Scholar]
- 7. Alabdali M, Abraham A, Alsulaiman A, et al. Clinical characteristics, and impairment and disability scale scores for different CIDP disease activity status classes. J Neurol Sci 2017; 372: 223–227. [DOI] [PubMed] [Google Scholar]
- 8. Kuwabara S, Isose S, Mori M, et al. Different electrophysiological profiles and treatment response in ‘typical’ and ‘atypical’ chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 2015; 86: 1054–1059. [DOI] [PubMed] [Google Scholar]
- 9. Lichtenstein T, Sprenger A, Weiss K, et al. MRI biomarkers of proximal nerve injury in CIDP. Ann Clin Transl Neurol 2018; 5: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su X, Kong X, Alwalid O, et al. Multisequence quantitative magnetic resonance neurography of brachial and lumbosacral plexus in chronic inflammatory demyelinating polyneuropathy. Front Neurosci 2021; 15: 649071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breckwoldt MO, Stock C, Xia A, et al. Diffusion tensor imaging adds diagnostic accuracy in magnetic resonance neurography. Invest Radiol 2015; 50: 498–504. [DOI] [PubMed] [Google Scholar]
- 12. Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med 2008; 40: 665–671. [DOI] [PubMed] [Google Scholar]
- 13. Albulaihe H, Alabdali M, Alsulaiman A, et al. Disease activity in chronic inflammatory demyelinating polyneuropathy. J Neurol Sci 2016; 369: 204–209. [DOI] [PubMed] [Google Scholar]
- 14. Vallat JM, Tabaraud F, Magy L, et al. Diagnostic value of nerve biopsy for atypical chronic inflammatory demyelinating polyneuropathy: evaluation of eight cases. Muscle Nerve 2003; 27: 478–485. [DOI] [PubMed] [Google Scholar]
- 15. Kulkarni GB, Mahadevan A, Taly AB, et al. Sural nerve biopsy in chronic inflammatory demyelinating polyneuropathy: are supportive pathologic criteria useful in diagnosis. Neurol India 2010; 58: 542–548. [DOI] [PubMed] [Google Scholar]
- 16. Tazawa K, Matsuda M, Yoshida T, et al. Spinal nerve root hypertrophy on MRI: clinical significance in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy. Intern Med 2008; 47: 2019–2024. [DOI] [PubMed] [Google Scholar]
- 17. Wu F, Wang W, Zhao Y, et al. MR neurography of lumbosacral nerve roots: diagnostic value in chronic inflammatory demyelinating polyradiculoneuropathy and correlation with electrophysiological parameters. Eur J Radiol 2020; 124: 108816. [DOI] [PubMed] [Google Scholar]
- 18. Feng Y, Zhang Y, Su X, et al. The comparison of MRN, electrophysiology and progression among typical CIDP and atypical CIDP subtypes. Sci Rep 2020; 10: 16697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitarokoili K, Kronlage M, Bäumer P, et al. High-resolution nerve ultrasound and magnetic resonance neurography as complementary neuroimaging tools for chronic inflammatory demyelinating polyneuropathy. Ther Adv Neurol Disord 2018; 11: 1756286418759974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin 2004; 22: 643–682, vi–vii. [DOI] [PubMed] [Google Scholar]
- 21. Kronlage M, Bäumer P, Pitarokoili K, et al. Large coverage MR neurography in CIDP: diagnostic accuracy and electrophysiological correlation. J Neurol 2017; 264: 1434–1443. [DOI] [PubMed] [Google Scholar]
- 22. Tanaka K, Mori N, Yokota Y, et al. MRI of the cervical nerve roots in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy: a single-institution, retrospective case-control study. BMJ Open 2013; 3: e003443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jongbloed BA, Bos JW, Rutgers D, et al. Brachial plexus magnetic resonance imaging differentiates between inflammatory neuropathies and does not predict disease course. Brain Behav 2017; 7: e00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kronlage M, Pitarokoili K, Schwarz D, et al. Diffusion tensor imaging in chronic inflammatory demyelinating polyneuropathy: diagnostic accuracy and correlation with electrophysiology. Invest Radiol 2017; 52: 701–707. [DOI] [PubMed] [Google Scholar]
- 25. Morrow JM, Reilly MM. Early detection of nerve injury in transthyretin-related familial amyloid polyneuropathy. Brain 2015; 138(Pt. 3): 507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 2006; 51: 527–539. [DOI] [PubMed] [Google Scholar]
- 27. Mathys C, Aissa J, Meyer Zu, Hörste G, et al. Peripheral neuropathy: assessment of proximal nerve integrity by diffusion tensor imaging. Muscle Nerve 2013; 48: 889–896. [DOI] [PubMed] [Google Scholar]
- 28. Kakuda T, Fukuda H, Tanitame K, et al. Diffusion tensor imaging of peripheral nerve in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a feasibility study. Neuroradiology 2011; 53: 955–960. [DOI] [PubMed] [Google Scholar]
- 29. Wu C, Wang G, Zhao Y, et al. Assessment of tibial and common peroneal nerves in diabetic peripheral neuropathy by diffusion tensor imaging: a case control study. Eur Radiol 2017; 27: 3523–3531. [DOI] [PubMed] [Google Scholar]
- 30. Duggins AJ, McLeod JG, Pollard JD, et al. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain 1999; 122: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 31. Kuwabara S, Nakajima M, Matsuda S, et al. Magnetic resonance imaging at the demyelinative foci in chronic inflammatory demyelinating polyneuropathy. Neurology 1997; 48: 874–877. [DOI] [PubMed] [Google Scholar]
- 32. Su X, Kong X, Liu D, et al. Multimodal magnetic resonance imaging of peripheral nerves: establishment and validation of brachial and lumbosacral plexi measurements in 163 healthy subjects. Eur J Radiol 2019; 117: 41–48. [DOI] [PubMed] [Google Scholar]
- 33. Lucke IM, Peric S, van Lieverloo GGA, et al. Elevated leukocyte count in cerebrospinal fluid of patients with chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst 2018; 23: 49–54. [DOI] [PubMed] [Google Scholar]
- 34. Matsumoto H, Hanajima R, Terao Y, et al. A significant correlation between cauda equina conduction time and cerebrospinal fluid protein in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci 2018; 384: 7–9. [DOI] [PubMed] [Google Scholar]
- 35. Markvardsen LH, Vaeggemose M, Ringgaard S, et al. Diffusion tensor imaging can be used to detect lesions in peripheral nerves in patients with chronic inflammatory demyelinating polyneuropathy treated with subcutaneous immunoglobulin. Neuroradiology 2016; 58: 745–752. [DOI] [PubMed] [Google Scholar]
- 36. Draak TH, Vanhoutte EK, van Nes SI, et al. Changing outcome in inflammatory neuropathies: Rasch-comparative responsiveness. Neurology 2014; 83: 2124–2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221150540 for Multisequence magnetic resonance neurography of brachial and lumbosacral plexus in chronic inflammatory demyelinating polyneuropathy: correlations with electrophysiological parameters and clinical features by Xiaoyun Su, Xiangquan Kong, Xiangchuang Kong, Qing Zhu, Zuneng Lu and Chuansheng Zheng in Therapeutic Advances in Neurological Disorders