Abstract
Papillary breast carcinomas comprise <1% of all breast cancers. They are notorious among surgical pathologists for posing diagnostic difficulty, especially with small sample sizes, such as a core-needle biopsy and carry potential for overtreatment. Solid-papillary carcinoma is a subtype of papillary breast carcinomas that affects elderly females and generally has a favorable diagnosis in its in-situ form. This report focuses on the unique and clinically aggressive presentation and treatment of invasive solid-papillary carcinoma that was discovered along the axillary chest wall after an ipsilateral mastectomy for multifocal ductal carcinoma in situ.
Keywords: Papillary breast cancer, PALOMA 2, Recurrent breast cancer
Introduction
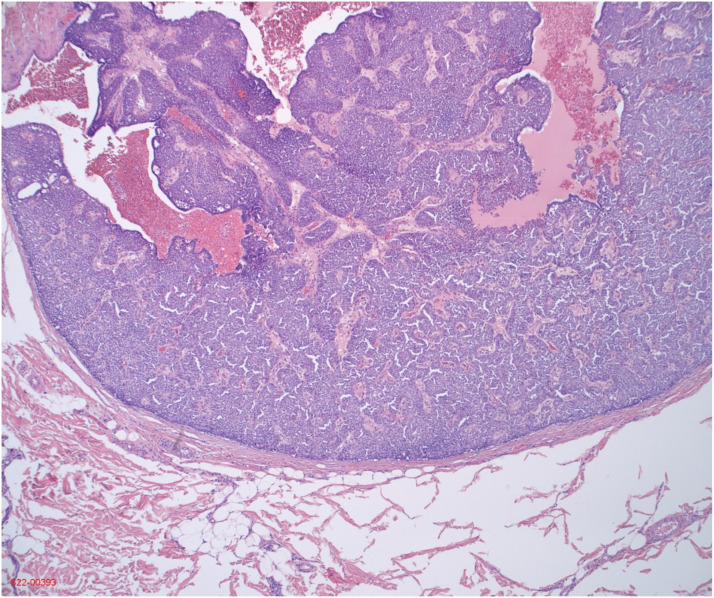
Papillary breast carcinoma is a rare form of breast cancer, comprising of <1% of all breast cancers. Lesions have a distinct histological appearance containing finger-like projections, commonly described as fronds, with central fibrovascular cores and overlying epithelium with or without myoepithelial cells (Figure 1).1,2 Papillary lesions are further characterized by their epithelium to delineate if they are benign, atypical, or carcinomatous.2 The presence of myoepithelial cells and how they are distributed allows for better classification of papillary lesions but require additional staining techniques beyond hematoxylin and eosin. An accurate neoplastic diagnosis is frequently difficult to ascertain for many pathologists, especially with small samples such as core-needle biopsies, which can carry significant implications for management.1
Figure 1.
Solid papillary carcinoma histopathology from final excisional biopsy.
According to the 2019 WHO classification, five subtypes of papillary breast neoplasms are defined: Intraductal papilloma, Papillary ductal carcinoma in situ (DCIS), Encapsulated papillary carcinoma, Solid-papillary carcinoma (in situ and invasive), and Invasive papillary carcinoma.3 Here, we explore an unusual case presentation of a clinically aggressive invasive solid-papillary carcinoma (SPC) that was found in the axillary chest wall 2 years post mastectomy for multifocal ductal carcinoma in situ (DCIS).
Case report
Our patient is a 62-year-old post-menopausal female with past medical history of psoriatic arthritis and left breast DCIS. She is status post left lumpectomy followed by left skin-sparing mastectomy with sentinel node biopsy and reconstruction for positive margins due to multifocal low-grade DCIS, ER/PR+, HER2-. Post mastectomy margins and lymph nodes were negative for disease, however of note, the patient never started adjuvant anti-endocrine therapy.
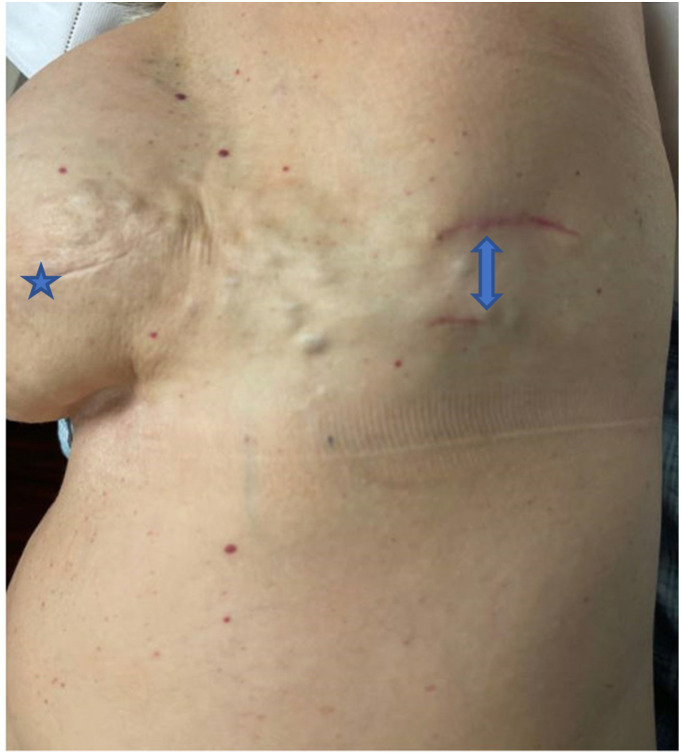
Two years later, she presented to clinic with a chief complaint of several small nodular masses along the left mastectomy scar extending from the reconstructed nipple to the lateral axillary chest wall. Ultrasound of the palpable masses showed diffuse thickening with skin edema. Over a week-long period, the nodules progressed in number and size (20–25 in total – Figure 2) prompting histologic evaluation. A core-needle biopsy was performed but was read as fibroadipose tissue and discordant to our findings on physical exam. We then proceeded with an excisional biopsy, which showed benign fatty tissue; again, discordant with our physical exam.
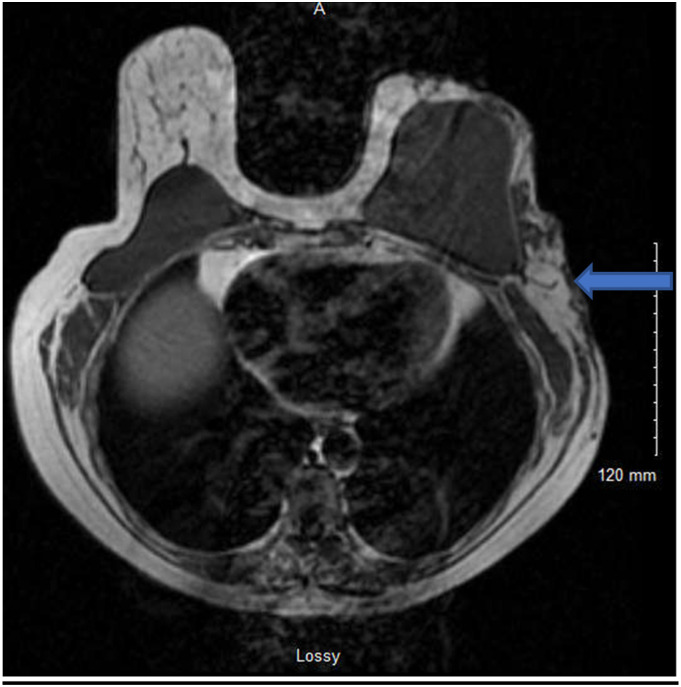
Figure 3.
T2 MR: Arrow denotes subcutaneous nodular enhancement with skin thickening.
Figure 2.
Clinical photo. Star denotes left breast mastectomy incision. Arrows point to left axillary chest wall with two healing excisional biopsy sites.
MRI with and without contrast was performed (Figure 3) showing multiple areas of nodular enhancement with skin thickening and no evidence of lymphadenopathy. A second excisional biopsy was performed, removing more tissue from the lateral chest wall. Immunohistochemical stains of this sample showed: nodules with proliferation of solid and papillary plasmacytoid cells with amphophilic cytoplasm and focal pink cytoplasmic granules. The lesional cells were CK-5 - and diffusely ER/PR+, HER 2- (Ki67 18%) with absence of myoepithelial cells by p63 and smooth muscle myosin. Findings were most compatible with invasive solid-papillary carcinoma, grade 1. The case was submitted for expert consultation to Johns Hopkins Laboratories. In their test center, the lesional cells were found to be diffusely GATA-3 + with absence of myoepithelial cells by p63/SMMHC (multiplex stain). No lymphovascular invasion was identified via D2-40 and ERG immunostains.
After presentation at multidisciplinary tumor board (MTB), she was determined to have unresectable disease due to the extent of axillary and chest wall skin involved. PET/CT showed no evidence of distant disease. The consensus recommendation was to proceed with anti-endocrine therapy with an aromatase inhibitor (AI) plus cyclin-dependent kinase (CDK 4/6) inhibitor based off the phase three PALOMA-2 trial. PALOMA-2 has shown significant benefit in the progression free survival of hormone receptor positive, HER2 negative invasive breast cancers.4 She was initiated on letrozole 2.5 mg daily and palbociclib 125 mg daily.
Our patient is currently still undergoing neoadjuvant anti-endocrine and immunotherapy. Thus far, she has been tolerating treatment well without complication. After 6 months, she has experienced a complete clinical resolution of her nodules and an almost complete resolution of disease on restaging MRI. After discussions with out multi-disciplinary tumor board, we plan to continue current therapy for a total of 2 years with surveillance imaging. Pending adequate response to neoadjuvant therapy, we plan to proceed with definitive surgery and adjuvant radiation therapy. The extent of her disease was tattooed shortly after initiating neoadjuvant therapy in preparation for future radiation therapy.
Discussion
Papillary breast carcinoma is an exceedingly rare form of breast cancer and prognosis is dependent on subtype characteristics. Accurately diagnosing papillary subtypes as invasive or in situ remains difficult for many surgical pathologists, although this has improved some with newer staining techniques on core-needle biopsy samples.1
Core-needle biopsies can displace epithelium during sampling and mislead pathologists to a diagnosis of invasive carcinoma.1 Additionally, many benign papillary lesions harbor foci of DCIS, malignancy, or atypical features. Upgrade rates after excisional biopsy from core-needle biopsy have been reported as high as 28–38%.5,6 Thus, it is universal practice to perform an excisional biopsy for all papillary lesions noted on core-needle biopsy. Several immunostains have been identified to aid in differentiating invasive versus in situ by assessing myoepithelial markers such as cytokeratin 5/6, smooth muscle myosin heavy chain, calponin, and p63.1,7,8
Solid-papillary carcinom is histologically described as a discrete papillary structure that has evidence of an underlying fibrovascular stromal network. It is viewed as an expansile nodule that is well-circumscribed with crowded epithelial cells. Cells often have mild to moderate atypia and present as round to oval, columnar, or spindle morphology. Neuroendocrine features have been described (ex. Chromogranin A) along with mucin production. It is strongly ER+ and CK5/6- with intermittent positivity for synaptophysin. Unlike other papillary subtypes, myoepithelial cells are often lacking and rarely aid in diagnosis of SPC.9 Concordant tissue diagnosis proved to be a challenge in this case. An excisional biopsy was required, twice, but the decision to perform an excisional biopsy was made based off discordant findings, not the inability to obtain an accurate histological diagnosis.
Solid-papillary carcinom is more commonly found in post-menopausal women, however cases have been reported in men.10–12 Dysplastic cells originate from the ductal epithelium with 50% of cases presenting as retroareolar or subareolar masses with bloody nipple discharge. 90% of lesions present as localized tumors with 8% presenting with locoregional disease and 0.4% distant metastasis.3,13 About 95% of the tumors are unilateral. Our patient’s disease was especially unusual due to the number of nodules and their location. The overall recurrence rate of breast cancer in mastectomy flaps can occur 4–11% of cases.14 Most of our patient’s lesions appeared to be in the skin, not the subcutaneous tissue, and far lateral to any tissue that would normally be excised with a mastectomy. It is possible that she had an incomplete mastectomy leaving some residual breast tissue behind, however most of her disease was lateral to normal breast margins and of a completely different pathology. It is also possible that the patient had a small focus of SPC within her mastectomy that was not sampled within histologic evaluation. Upon extensive literature review, we found no evidence of a similar presentation.
There are currently no set guidelines for treatment of SPC.12,13,15 Surgical excision is recommended when atypia or invasive features are confirmed. The role of sentinel node biopsy, hormone therapy and radiotherapy are still controversial.13 Additionally, there is a dearth of information regarding chemotherapy’s utility in the treatment of invasive SPC. Our patient had no evidence of distant metastasis as seen on her PET/CT, although her disease presentation of rapidly growing nodules suggests a more aggressive locoregional tone. Thus, we elected to base our treatment off the phase three PALOMA-2 trial using immunotherapy with a CDK 4/6 inhibitor plus AI, which has shown to have significantly increased progression free survival for hormone receptor positive, HER2 negative breast cancers.4,16 To our knowledge, this is the first known case of SPC treated as such.
Our patient presented with an unusual case of multi-nodular SPC along her axilla extending from a mastectomy incision for multifocal DCIS treated 2 years prior. She is currently undergoing neoadjuvant immunotherapy (palbociclib) with anti-hormone therapy (letrozole) and has already experienced a significant clinical improvement of her nodules. Restaging MRI was preformed after 6 months of therapy which showed an excellent partial response. Clinical exam at 6 months also showed no evidence of disease. After discussion with our multi-disciplinary tumor board, we plan to continue therapy for a total of 2 years with interval imaging. Pending adequate response, we plan to proceed with definitive surgery (+/− sentinel node biopsy) and adjuvant radiation therapy.
Conclusion
The solid-papillary carcinoma subtype of papillary breast carcinoma is an extremely rare form of breast cancer with minimal data on its management. Our patient presented with rapidly growing chest wall nodules extending from a mastectomy incision for multifocal DCIS preformed 2 years prior. Immunotherapy with anti-endocrine therapy was initiated based off prior success seen in the PALOMA-2 trial against other hormone receptor positive, HER2 negative breast cancers. This is the first known case report of SPC presenting in such a manner in addition to being treated with immunotherapy. While our patient is still amidst neoadjuvant treatment, we have already appreciated a complete clinical resolution of her nodules along with significant improvement on imaging. Depending on final outcomes, a multimodal approach using anti-endocrine and immunotherapy may prove to be beneficial for future cases of invasive SPC.
Acknowledgements
I would like to thank Dr. Priscilla Strom for her assistance and guidance in this research.
Footnotes
Author contributions: Harper Niver researched literature, conceived the study, obtained consent, and wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical IRB approval was waived for this case study by BRENAU UNIVERSITY.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
Data availability: The original data for this study is available. Please contact harper.niver@nghs.com to request access to the data.
ORCID iD
Harper E. Niver https://orcid.org/0000-0002-3869-1818
References
- 1.Collins LC, Schnitt SJ. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology 2007; 52(1): 20–29. DOI: 10.1111/j.1365-2559.2007.02898.x [DOI] [PubMed] [Google Scholar]
- 2.Brogi E, Krystel-Whittemore M. Papillary neoplasms of the breast including upgrade rates and management of intraductal papilloma without atypia diagnosed at core needle biopsy. Modern Pathology 2021; 34(S1): 78–93. DOI: 10.1038/s41379-020-00706-5 [DOI] [PubMed] [Google Scholar]
- 3.Breast Tumours, WHO Classification of Tumours, ed. 5. WHO Classification Editorial Board, 2019. [Google Scholar]
- 4.Finn R, Martin M, Rugo H, et al. Palbociclib and letrozole in advanced breast cancer. New England Journal of Medicine 2016; 375(375): 1925–1936. DOI: 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 5.Pal SK, Lau SK, Kruper L, et al. Papillary carcinoma of the breast: an overview. Breast Cancer Research and Treatment 2010; 122(3): 637–645. DOI: 10.1007/s10549-010-0961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo M, Linebarger J, Lowe M, et al. Management of papillary breast lesions diagnosed on core-needle biopsy: clinical pathologic and radiologic analysis of 276 cases with surgical follow-up. J Am Coll Surg 2012; 214(3): 280–287. DOI: 10.1016/j.jamcollsurg.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 7.Tse G, Tan P, Moriya T. The role of immunohistochemistry in the differential diagnosis of papillary lesions of the breast. J Clin Pathol 2009; 62(5): 407–413. DOI: 10.1136/jcp.2008.063016 [DOI] [PubMed] [Google Scholar]
- 8.Shah V, Flowers C, Douglas-Jones A, et al. Immunohistochemistry increases the accuracy of diagnosis of benign papillary lesions in breast core needle biopsy specimens. Histopathology 2006; 48(6): 683–691. DOI: 10.1111/j.1365-2559.2006.02404 [DOI] [PubMed] [Google Scholar]
- 9.Morgan S, Dodington D, Wu JM, et al. Solid papillary carcinoma and encapsulated papillary carcinoma of the breast: clinical-pathologic features and basement membrane studies of 50 cases. Pathobiology 2021; 88(5): 359–373. DOI: 10.1159/000517189 [DOI] [PubMed] [Google Scholar]
- 10.Burga AM, Fadare O, Lininger RA, et al. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Archiv 2006; 449(5): 507–512. DOI: 10.1007/s00428-006-0305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong E, Cheng E, Goldfischer M, et al. Papillary lesions of the male breast: a study of 117 cases and brief review of the literature demonstrate a broad clinicopathologic spectrum. Am J Surg Pathol 2020; 44(1): 68–76. DOI: 10.1097/PAS.0000000000001340 [DOI] [PubMed] [Google Scholar]
- 12.Guo S, Wang Y, Rohr J, et al. Solid papillary carcinoma of the breast: a special entity needs to be distinguished from conventional invasive carcinoma avoiding over-treatment. Breast 2016; 26(26): 67–72. [DOI] [PubMed] [Google Scholar]
- 13.Nunez D, Gonzalez F, Ibarguengoitia M, et al. Papillary lesions of the breast: a review. Breast Cancer Management 2020; 9(4). [Google Scholar]
- 14.Chung S, Shin S, Chen X, et al. Recurrent breast carcinoma arising in a transverse rectus abdominis myocutaneous flap. Arch Pathol Lab Med 2004; 128(10): 1157–1160. DOI: 10.1043/1543-2165 [DOI] [PubMed] [Google Scholar]
- 15.Clement Z, Jones M. Solid papillary carcinoma of the breast: a review. Int J Surg Med 2017; 3(1). [Google Scholar]
- 16.Shah M, Nunes M, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor–positive advanced breast cancer? Oncol (Williston Park) 2018; 32(5): 216–222. [PMC free article] [PubMed] [Google Scholar]