Abstract
Background/Objectives
Although it has been reported that thyroid-stimulating immunoglobulin (TSI) is associated with the clinical characteristics of thyroid eye disease (TED), there is a paucity of literature regarding the role of TSI in diagnosing active TED. This study investigated the relationship between the level of TSI and the activity of TED and assessed the cut-off value of TSI discriminating active TED from inactive TED.
Methods
This cross-sectional study included 101 patients with TED. TSI was quantitatively measured with a cell-based bioassay using a chimeric TSH receptor and a cyclic adenosine monophosphate response element-dependent luciferase. The association between TSI and a variety of demographic and clinical features of TED was analysed. Multivariate regression analysis was performed to determine possible independent factors affecting the level of TSI.
Results
TSI level was higher in males than in females (p = 0.023) and smokers than in nonsmokers (p = 0.004). TSI level was inversely correlated with the duration of ocular symptoms (r = −0.295, p = 0.003). The level of TSI was also significantly different when compared to the thyroid function (p = 0.003), TED activity (p < 0.001), and TED severity (p = 0.001). Multivariate regression analysis revealed a significant relationship between TED activity and thyroid function jointly and the TSI level. The cut-off level of TSI for predicting active TED was a specimen-to-reference ratio of 406.7 (p < 0.001, area under the curve = 0.847, sensitivity 77.4%, specificity 81.3%).
Conclusions
TSI was a functional biomarker strongly associated with TED activity even after being adjusted by other clinical characteristics. Serum TSI level may help identify patients with active TED in clinics.
Subject terms: Predictive markers, Thyroid diseases
Introduction
Thyroid eye disease (TED) is one of the most common orbital inflammatory diseases. Approximately 20–25% of patients with Grave’s disease (GD) have orbitopathy [1]. TED causes not only cosmetic disruptions but also significant functional problems like permanent visual disturbance and diplopia. Patients with TED have a much-reduced quality of life, similar to individuals with diabetes or cancer [2].
The pathogenesis of TED is not yet fully understood, but autoantibodies to the thyroid-stimulating hormone (TSH) receptor (TSH-R) are thought to play a key role [3]. TSH-R is the GD’s primary autoantigen: the breaking of self-tolerance to TSH-R leads to TSH-R antibodies inducing hyperthyroidism [4, 5]. TSH-R, which is expressed in the thyroid follicular cells, also expressed in orbital tissue, including orbital fibroblasts, adipocytes, and lymphocytes [6, 7]. Traditionally, TSH-R autoantibodies were measured by thyrotropin-binding inhibiting immunoglobulin (TBII) assays which quantified receptor binding assay that measured their inhibiting ability for TSH-R binding. Although it has fairly good sensitivity and specificity for diagnosing GD, it has been criticized because it cannot distinguish stimulating and blocking antibodies [8, 9].
The thyroid-stimulating immunoglobulin (TSI) bioassay is the most recently developed technique to detect TSH-R autoantibodies. It measures cyclic adenosine monophosphate that is produced after binding of TSH-R and autoantibodies. TSI bioassay represents the functional components of TSH-R autoantibody [10]. In 2011, Ponto et al. [11] reported that TSI was associated with various clinical manifestations of TED, including activity and severity. Afterward, TSI has been reported to be correlated with clinical manifestations of TED in several studies and popularly measured in clinics [12–18]. However, it is ambiguous to interpret the meaning of TSI in reality because there are numerous factors that affect its level.
We aimed to investigate the usefulness of TSI as a potential biomarker of the activity of TED. This study investigated the association between TSI and demographic and clinical factors in patients with TED. We subsequently performed multivariate regression analysis to determine the factors significantly associated with TSI. Then, we assessed the discriminative ability of the TSI to diagnose active TED.
Methods
This study adhered to the tenets of the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board at Hallym University Sacred Heart Hospital (2020-09-006-001). The Institutional Review Board at Hallym University Sacred Heart Hospital waived the need for informed consent because it was a retrospective study based on an electronic medical record review. All clinical records were anonymized and de-identified before analysis. This study included 101 patients who had clinically confirmed TED and TSI levels recorded at the time of diagnosis. The TED diagnosis was made based on comprehensive ophthalmic and orbital examinations and thyroid function tests [19]. All subjects were newly diagnosed TED patients. Patients who had been treated with oral or intravenous steroid or radiation therapy were excluded.
A total of 101 consecutive patients with TED were reviewed. We collected data from the electronic medical records which included age, gender, smoking history, thyroid function, duration of ocular symptom, and thyroid disease treatment modality. The thyroid function was classified into hyperthyroid, subclinical hyperthyroid, and euthyroid [20]. The hyperthyroid TED was defined when the patient showed elevated serum fT4 and T3 or had a history of antithyroidal treatment including antithyroidal drug, surgery or radioiodine. The subclinical hyperthyroid TED was diagnosed when the serum levels of fT4 and T3 were normal but the level of TSH was decreased. The diagnosis of euthyroid TED was established when the patient showed normal serum levels of fT4, T3, and TSH without a clinical history of thyroid disease.
The activity and severity of TED were assessed by one oculoplasty specialist (MJL), unaware of the laboratory data. The clinical activity score (CAS) consisted of 7 items representing classic inflammatory symptoms and signs: (1) spontaneous pain behind the globe, (2) pain on attempted gazes, (3) swelling of the eyelids, (4) redness of the eyelids, (5) redness of the conjunctiva, (6) chemosis, and (7) swollen caruncle [21]. Thus, the CAS ranged between 0 and 7, and a CAS ≥3 was considered active TED. The severity of TED was evaluated and classified into mild, moderate to severe, and sight-threatening TED by the European Group on Graves’ Orbitopathy classification [22]. Mild TED was defined when the patient had minor lid retraction (<2 mm), mild soft tissue involvement, exophthalmos <3 mm, above normal, transient or no diplopia, and corneal exposure responsive to lubricants. Moderate TED was assessed when the lid retraction ≥2 mm, moderate or severe soft tissue involvement, exophthalmos ≥3 mm above normal, and inconstant or constant diplopia. Patients with dysthyroid optic neuropathy and/or corneal breakdown were classified into sight-threatening TED.
TSI level was measured using a commercially available bioassay kit (ThyretainTM, Diagnostic Hybrids Inc., OH, USA), according to the manufacturer’s instructions. Briefly, a frozen vial of CHO-Mc4 cells was seeded and grown to a confluent cell monolayer in 96-well plates for 15–18 h at 37 °C and 5% carbon dioxide (CO2). Serum samples and positive, reference, and normal controls were diluted 1:11 in a reaction buffer, added to the cell monolayers, and each plate was incubated for 3 h at 37 °C, 5% CO2. Subsequently, the CHO-Mc4 cells were lysed, and the Relative Light Unit (RLU) was quantified in the luminometer. The TSI level was considered positive when the specimen-to-reference ratio percentage (SRR%) measured ≥140%.
The descriptive statistic was performed by calculation of the mean, standard deviation, and range. Unpaired t-tests or Kruskal Wallis tests tested differences in continuous variables between groups. Differences between categorical variables within groups were tested with the chi-square test or Fisher’s exact test, as appropriate. Correlation analyses were performed using Spearman’s rank correlation coefficient. All variables that showed a significant effect on TSI levels on univariate analyses were included in multivariate regression analysis. Receiver operating characteristics (ROC) plot analysis was performed to obtain the area under the curve (AUC) using the TSI level and TED activity. The sensitivity and specificity were examined at an optimal cut-off point in the ROC curve regarding the discrimination ability between active and inactive TED groups. The statistical analysis was computed using SPSS (IBM SPSS® Statistics, Version 26, IBM Inc., Armonk, NY, USA) and GraphPad Prism (GraphPad Prism, Version 8, GraphPad Inc., San Diego, CA, USA). A statistical significance was defined at a p-value < 0.05.
Results
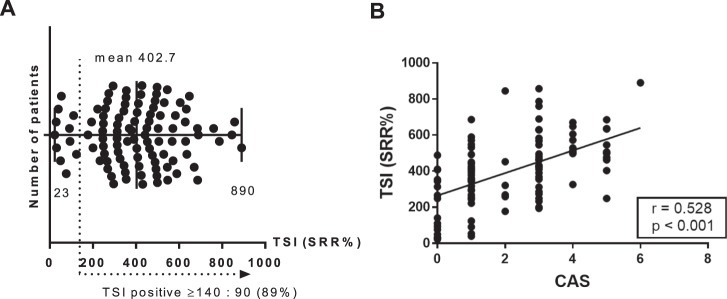
The demographic, clinical, and serologic data are summarized in Supplemental Table 1. The mean age of the study population was 46 years and 71 patients were female. The mean TSI level was 402.7 SRR% (range 23–890 SRR%), and the prevalence of TSI-positive patients with TED was 89% (Fig. 1). The mean duration of ocular symptom was 8 months (range 1–60 months). The average CAS was 2.21, and active TED was assessed in 51 patients. TED severity was mild in 52 (51.5%) patients and moderate to severe in 41 (40.6%) patients. Eight patients (7.9%) showed evidence of dysthyroid optic neuropathy.
Fig. 1. The distribution of thyroid-stimulating immunoglobulin (TSI) level in patients with thyroid eye disease (n = 101).
A The mean TSI level was 402.7(SRR%), and the prevalence of TSI-positive patients with TED was 89%. B There was a significant positive correlation between TSI and clinical activity score (CAS) (r = 0.528, p < 0.001 by Pearson correlation analysis).
The TSI levels were higher in smokers (492 ± 207 SRR%) than nonsmokers (370 ± 178 SRR%, p = 0.004, Table 1). The levels of TSI were also significantly different according to the thyroid function (p = 0.003). Hyperthyroid TED patients showed higher TSI (439 ± 181 SRR%) than subclinical hyperthyroid TED (397.8 ± 96.1 SRR%) or euthyroid TED patients (251 ± 204 SRR%). The duration of ocular symptoms inversely correlated with TSI levels (r = −0.295, p = 0.003, Table 2).
Table 1.
Comparison of thyroid-stimulating immunoglobulin levels according to the clinical features of thyroid eye disease.
| Clinical variables | n | TSI (mean ± SD) | p-value | |
|---|---|---|---|---|
| Sex | Male | 30 | 469.3 ± 204.8 | 0.023a |
| Female | 71 | 374.6 ± 181.8 | ||
| Smoking | No | 74 | 369.9 ± 177.8 | 0.004a |
| Yes | 27 | 492.4 ± 207.1 | ||
| Thyroid function | Hyperthyroid TED | 75 | 439.5 ± 181.3 | 0.003b |
| Subclinical hyperthyroid TED | 8 | 397.8 ± 96.1 | ||
| Euthyroid TED | 18 | 251.3 ± 204.1 | ||
| Diplopia | No | 70 | 383.5 ± 191.9 | 0.134a |
| Yes | 31 | 446.0 ± 190.9 | ||
| TED activity | Inactive | 50 | 315.4 ± 181.1 | <0.001a |
| Active | 51 | 488.3 ± 164.8 | ||
| TED severity | Mild | 52 | 342.9 ± 183.6 | 0.001a |
| Moderate to severe | 41 | 446.6 ± 177.9 | ||
| Dysthyroid optic neuropathy | 8 | 566.6 ± 190.1 | ||
TSI thyroid-stimulating immunoglobulin, SD standard deviation, TED thyroid eye disease.
aStudent T-test.
bKruskal Wallies test.
Table 2.
Correlation analysis between thyroid-stimulating immunoglobulin levels and age or ocular symptom duration.
| Clinical variables | n | Mean ± SD | Pearson Corr | r2 | p-value |
|---|---|---|---|---|---|
| Age (year) | 101 | 46.28 ± 13.36 | 0.125 | 0.016 | 0.214 |
| Ocular symptom duration (month) | 101 | 8.01 ± 11.97 | −0.295 | 0.087 | 0.003 |
SD standard deviation, Pearson Corr pearson correlation coefficient.
Active TED patients showed a significantly higher TSI level than inactive TED patients (488 ± 164 vs. 315 ± 191 SRR%, p < 0.001). All active TED patients showed TSI positivity with a cut-off of 140 SRR%, whereas 22% (11/50) of inactive TED patients showed TSI negativity. In addition, CAS showed positive correlation with TSI levels (r = 0.528, p < 0.001, Fig. 1). TSI was also different among the severity of TED: mild (342 ± 183 SRR%), moderate to severe (446 ± 177 SRR%), and dysthyroid optic neuropathy (566% ± 190 SRR%) TED groups. TSI was compared among four groups, considering activity and severity (mild-active, mild-inactive, moderate to severe-active, moderate to severe-inactive) and showed a significant difference (p < 0.001). Thereafter, the TSI level was significantly higher in active subgroups than inactive subgroups regardless of the severity, suggesting TSI was more influenced by activity rather than severity (Supplemental Fig. 1).
Before multivariate regression analysis, multicollinearity was tested, and the variance inflation factor was less than 10 in all independent variables, which meant that the variables did not correlate with each other. Multivariate regression analysis showed that TED activity and thyroid function were significant factors associated with the TSI level (p = 0.010, p = 0.026, respectively, Table 3).
Table 3.
Multivariate regression analyses of thyroid-stimulating immunoglobulin levels and clinical variables of thyroid eye disease.
| Clinical variables | ß | p-value |
|---|---|---|
| Gender | 0.027 | 0.832 |
| Age | 0.067 | 0.461 |
| Smoking | 0.184 | 0.150 |
| Ocular symptom duration | −0.174 | 0.050 |
| Thyroid function | −0.205 | 0.026 |
| TED activity | 0.272 | 0.010 |
| TED severity | 0.093 | 0.364 |
TED thyroid eye disease.
The cut-off TSI level that can discriminate between active and inactive TED groups was determined by comparing clinical variables between active and inactive TED groups. To determine this cut-off, we performed multiple logistic regression analysis and, subsequently, ROC analysis (Supplemental Tables 2 and 3). The ROC curve showed an AUC of 0.847 (p < 0.001), indicating good discriminatory performance. A cut-off of 406.7 SRR% showed the best discriminatory performance with a 77.4% sensitivity and 81.3% specificity (Fig. 2).
Fig. 2. Receiver operating characteristics (ROC)-curve.
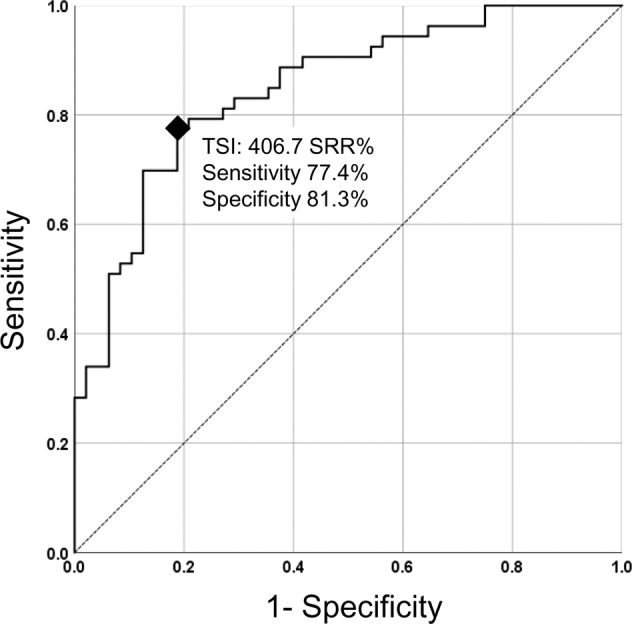
The ROC curve of cut-off 406.7 SRR% thyroid-stimulating immunoglobulin (TSI) level represented good performance in discriminating active thyroid eye disease (TED) and inactive TED (area under the curve = 0.847, p < 0.001).
Discussion
This study demonstrated that TSI was correlated with various demographic and clinical factors in patients with TED, including smoking, thyroid function status, the duration of ocular symptoms, TED activity, and the severity of TED. Since several factors were associated with TSI, multivariate regression analysis was subsequently performed to identify the key factor. Multivariate regression analysis revealed that TED activity and thyroid function status were the most significantly correlated factors with TSI level. The ROC curve showed that TSI had a good discriminatory performance classifying active and inactive TED patients.
Several previous studies reported the correlation between TSI and the activity/severity of TED. Lytton SD et al. [23] reported that TSI was strongly correlated with both activity and severity of TED. Jang et al. [12] investigated the association between the severity and activity of TED and three kinds of TSH-R antibody assays: 1st generation TBII, 3rd generation TBII, and TSI. All these TSH-R antibody assays showed a significant correlation with both activity and severity of TED. The authors emphasized the positive correlation between TSI and the severity indices. Recently, Lytton et al. [24] published a review paper embracing the results of meta-analysis about the clinical performance of TSI and reported the significant correlation of TSI with CAS, proptosis, soft tissue involvement, muscle involvement, and total eye score.
Our study results also showed the both the activity and severity were associated with TSI. However, multivariate regression analysis revealed that TSI was much more significantly correlated with the activity of TED than the severity of TED. We also conducted a subgroup analysis comparing the TSI levels among four groups: mild-active, mild-inactive, moderate to severe-active, moderate to severe-inactive groups. All patients with dysthyroid optic neuropathy had active TED (n = 8) excluded from this analysis. The results revealed that the TSI level was significantly higher in active subgroups than inactive subgroups in mild and moderate to severe groups.
The accurate assessment of TED activity is essential to determine treatment plans in patients with TED. Immunomodulating agents, including corticosteroids, rituximab, and tocilizumab, are effective and administered only in the active phase [1, 22, 25]. Surgical rehabilitation is still the main treatment for inactive TED. CAS is a summed score of inflammatory signs around the eye and orbit. It is a well-established clinical scoring system and has been regarded as a gold standard for assessing TED activity so far. However, CAS is subjective and has a limited power to discriminate against those who do not respond to immunomodulating treatment. CAS uses a binary scale, and each clinical item has an equal weight [26]. The results of this study suggested TSI can be used as an additional biomarker for TED activity. TSI was significantly correlated with TED activity even after multivariate regression analysis, suggesting TSI independently reflected TED activity.
In clinics, the diagnosis of TED is not difficult in most cases because the patients usually have a history of GD and TED itself has discriminating ophthalmic characteristics such as eyelid retraction and lid lag. However, it is sometimes difficult to be diagnosed if the eyelid signs are not evident or thyroid function tests are normal. There have been several reports on the value of TSI as a diagnostic marker for TED. TSI positivity is more evident in patients with TED than GD without ophthalmic manifestations. Lytton et al. [23] reported a higher positive rate and fold change of TSI in patients with TED than in those with GD. TSI level was also higher in Hashimoto’s thyroiditis patients with TED than without [27]. In this cohort, TSI was positive in 90% of patients. All patients with active TED showed positive TSI. Eleven patients showed negative TSI: 3 patients with GD with chronic inactive TED and eight euthyroid TED patients who had only eyelid retraction and low CAS. Woo et al. [28] previously reported 70% of TSI positivity in patients with chronic and inactive TED patients. Suzuki et al. [29] also reported 70.5% TSI positivity in Euthyroid TED patients. Although TSI is relatively sensitive diagnostic marker of TED, clinicians should keep in mind that negative TSI may not exclude the possibility of TED.
We suggested a cut-off TSI level that might be able to discriminate between active and inactive TED. Since most TED patients had positive TSI with a diagnostic standard of 140 SRR% and TED activity was significantly associated with TSI level, we tried to develop a diagnostic TSI level for TED activity. We analysed a ROC curve and calculated a discriminatory cut-off level. The ROC curve showed an acceptable discriminatory performance (AUC = 0.841, p < 0.001) with a cut-off point of 406.7 SRR%. A few previous articles suggested a new TSI standard level predicting specific clinical features of GD or TED. Takakura et al. [15] measured TSI level in newly diagnosed GD patients, compared the TSI level according to orbitopathy’s development, and reported that high TSI, greater than 400, at the time of GD diagnosis is a significant risk factor for the development of TED. Ponto et al. [14] suggested a cut-off of 377 SRR% for predicting recent onset dysthyroid optic neuropathy. Jang et al. [30] reported the cut-off level of 555.10 SRR% to predict severe TED development. To confirm the clinical utility of cut-off level presented in this study, subsequent further research would be needed using independent external subjects for validation.
This report has several limitations. This study is a retrospective study from a single center, and our number of cases was limited, especially patients with dysthyroid optic neuropathy. The results of this study were further limited by the retrospective and cross-sectional study design. Heterogeneous demographic and clinical factors were associated with the TSI level. They may act as confounding factors for analysing the relationship between TED activity and TSI level, although we performed multivariate regression analysis. We, therefore, plan to do further research using longitudinal TSI data. That study design may verify the change of TSI according to the Rundle’s curve of an individual patient, reflecting TED activity’s change more accurately. We used a commercially available TSI bioassay kit in this study and the ROC curve and the cut-off value are applicable for this specific type of test. The TSI has its own assay variability and further studies would be needed to validate the feasibility of the routine use of TSI to assess TED in clinics.
In conclusion, we investigated the relationships between various clinical characteristics of TED patients and the TSI level. We demonstrated that TED activity and initial endocrinologic status were independently associated with the TSI levels. We believe that this is the first report to provide the discriminatory cut-off level of TSI for TED activity. Serum TSI levels seem to be a useful functional biomarker for diagnosing the active phase of TED. Therefore, it may help decide the treatment strategy when the inflammatory signs are unclear or the CAS is borderline.
Summary
What was known before
Thyroid-stimulating immunoglobulin (TSI) bioassay is a cell-based assay that can measure functional activity and potency. TSI has been reported to be associated with clinical features of thyroid eye disease (TED).
What this study adds
Multivariate regression analysis revealed TED activity and thyroid function were significant factors associated with the level of TSI. Our findings suggested TSI had discriminative power between active and inactive TED groups.
Supplementary information
Acknowledgements
The statistical analysis used in this manuscript was supported by Division of Biostatistics, Hallym Institute for Clinical Medicine of Hallym University Medical Center.
Author contributions
All authors contributed to the conception of the study, data analysis, revision of the manuscript and approval of the final version.
Funding
This research was supported by the National Research Foundation of Korea (NRF): 2019R1G1A1100257.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-022-01981-z.
References
- 1.Li Z, Cestari DM, Fortin E. Thyroid eye disease: what is new to know? Curr Opin Ophthalmol. 2018;29:528–34. doi: 10.1097/ICU.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 2.Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, Wiersinga WM. Quality of life in patients with Graves’ ophthalmopathy is markedly decreased: measurement by the medical outcomes study instrument. Thyroid. 1997;7:885–9. doi: 10.1089/thy.1997.7.885. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Graves’ ophthalmopathy. N. Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khong JJ, McNab AA, Ebeling PR, Craig JE, Selva D. Pathogenesis of thyroid eye disease: review and update on molecular mechanisms. Br J Ophthalmol. 2016;100:142–50. doi: 10.1136/bjophthalmol-2015-307399. [DOI] [PubMed] [Google Scholar]
- 5.McLachlan SM, Rapoport B. Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev. 2014;35:59–105. doi: 10.1210/er.2013-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–8. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahn RS, Dutton CM, Joba W, Heufelder AE. Thyrotropin receptor expression in cultured Graves’ orbital preadipocyte fibroblasts is stimulated by thyrotropin. Thyroid. 1998;8:193–6. doi: 10.1089/thy.1998.8.193. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, 3rd, Hay ID, Nelson RE, Jiang NS. Clinical utility of thyrotropin-receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clin Proc. 1988;63:707–17. doi: 10.1016/S0025-6196(12)65533-5. [DOI] [PubMed] [Google Scholar]
- 9.Gupta MK. Thyrotropin receptor antibodies: advances and importance of detection techniques in thyroid diseases. Clin Biochem. 1992;25:193–9. doi: 10.1016/0009-9120(92)90302-9. [DOI] [PubMed] [Google Scholar]
- 10.Lytton SD, Li Y, Olivo PD, Kohn LD, Kahaly GJ. Novel chimeric thyroid-stimulating hormone-receptor bioassay for thyroid-stimulating immunoglobulins. Clin Exp Immunol. 2010;162:438–46. doi: 10.1111/j.1365-2249.2010.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ. Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology. 2011;118:2279–85. doi: 10.1016/j.ophtha.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Jang SY, Shin DY, Lee EJ, Choi YJ, Lee SY, Yoon JS. Correlation between TSH receptor antibody assays and clinical manifestations of Graves’ orbitopathy. Yonsei Med J. 2013;54:1033–9. doi: 10.3349/ymj.2013.54.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampmann E, Diana T, Kanitz M, Hoppe D, Kahaly GJ. Thyroid Stimulating but Not Blocking Autoantibodies Are Highly Prevalent in Severe and Active Thyroid-Associated Orbitopathy: A Prospective Study. Int J Endocrinol. 2015;2015:678194. doi: 10.1155/2015/678194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponto KA, Diana T, Binder H, Matheis N, Pitz S, Pfeiffer N, et al. Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J endocrinological Investig. 2015;38:769–77. doi: 10.1007/s40618-015-0254-2. [DOI] [PubMed] [Google Scholar]
- 15.Takakura A, Kirkeby K, Earle K, Silkiss RZ. Predicting the Development of Orbitopathy in Graves Thyroidopathy Patients: The Potential Role of TSI Testing. Ophthalmic Plast Reconstructive Surg. 2015;31:369–72. doi: 10.1097/IOP.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 16.Diana T, Kahaly GJ. Thyroid Stimulating Hormone Receptor Antibodies in Thyroid Eye Disease-Methodology and Clinical Applications. Ophthalmic Plast Reconstructive Surg. 2018;34:S13–s19. doi: 10.1097/IOP.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 17.Seo S, Sánchez Robledo M. Usefulness of TSH receptor antibodies as biomarkers for Graves’ ophthalmopathy: a systematic review. J Endocrinol Investig. 2018;41:1457–68. doi: 10.1007/s40618-018-0945-6. [DOI] [PubMed] [Google Scholar]
- 18.Roos JCP, Paulpandian V, Murthy R. Serial TSH-receptor antibody levels to guide the management of thyroid eye disease: the impact of smoking, immunosuppression, radio-iodine, and thyroidectomy. Eye. 2019;33:212–7. doi: 10.1038/s41433-018-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–5. doi: 10.1016/S0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 20.Jang SY, Lee SY, Lee EJ, Yoon JS. Clinical features of thyroid-associated ophthalmopathy in clinically euthyroid Korean patients. Eye. 2012;26:1263–9. doi: 10.1038/eye.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol. 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 22.Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158:273–85. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 23.Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–31. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 24.Lytton SD, Schluter A, Banga PJ. Functional diagnostics for thyrotropin hormone receptor autoantibodies: bioassays prevail over binding assays. Front Biosci. 2018;23:2028–43. doi: 10.2741/4687. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez, et al. Efficacy of Tocilizumab in Patients With Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am J Ophthalmol. 2018;195:181–90. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Dolman PJ. Grading Severity and Activity in Thyroid Eye Disease. Ophthalmic Plast Reconstructive Surg. 2018;34:S34–s40. doi: 10.1097/IOP.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 27.Kahaly GJ, Diana T, Glang J, Kanitz M, Pitz S, König J. Thyroid Stimulating Antibodies Are Highly Prevalent in Hashimoto’s Thyroiditis and Associated Orbitopathy. J Clin Endocrinol Metab. 2016;101:1998–2004. doi: 10.1210/jc.2016-1220. [DOI] [PubMed] [Google Scholar]
- 28.Woo YJ, Jang SY, Lim TH, Yoon JS. Clinical Association of Thyroid Stimulating Hormone Receptor Antibody Levels with Disease Severity in the Chronic Inactive Stage of Graves’ Orbitopathy. Korean J Ophthalmol. 2015;29:213–9. doi: 10.3341/kjo.2015.29.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki N, Noh JY, Kameda T, Yoshihara A, Ohye H, Suzuki M, et al. Clinical course of thyroid function and thyroid associated-ophthalmopathy in patients with euthyroid Graves’ disease. Clin Ophthalmol. 2018;12:739–46. doi: 10.2147/OPTH.S158967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang SY, Shin DY, Lee EJ, Lee SY, Yoon JS. Relevance of TSH-receptor antibody levels in predicting disease course in Graves’ orbitopathy: comparison of the third-generation TBII assay and Mc4-TSI bioassay. Eye. 2013;27:964–71. doi: 10.1038/eye.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.