Abstract
Protein arginine methyltransferase 7 (PRMT7) pathogenetic variants have been associated with the human disorder of Short Stature, Brachydactyly, Intellectual Developmental Disability and Seizures syndrome (SBIDDS). Only 15 cases have been described in the literature. Here we report two female dizygotic twins with novel compound heterozygous deleterious variants of PRMT7 and describe the associated endocrine manifestations and short-term response to recombinant growth hormone (rGH) treatment. They were born at 36 + 3 weeks from a dichorionic diamniotic twin pregnancy. Twin A was appropriate for gestational age while Twin B was small for gestational age. Whole exome sequencing analyses showed the same novel compound heterozygous genetic defects in the PRMT7 gene (c.1220 G > A of maternal origin; c.1323 + 2 T > G of paternal origin, Fig. 1). Due to severe short stature and growth impairment, at six years of age, endocrine investigations were performed to rule out growth hormone (GH) deficiency, and revealed GH deficiency (GHD) in Twin A and an appropriate GH response in Twin B. Therefore, both started rGH, albeit at different dosages according to the underlying diagnosis. Both showed a satisfactory short-term response to treatment with height gain (∆HT) of +0.52 SDS (Twin A) and +0.88 SDS (Twin B) during the first year. In conclusion, our findings expand the knowledge about the endocrine manifestations associated with PRMT7 pathogenetic variants, including GH deficiency and rGH response. Further studies are needed to investigate long-term outcomes and establish whether PRMT7 genetic defects can be included among syndromic short stature treatable with rGH.
Subject terms: Growth disorders, Genetics research
Introduction
Homozygous/compound heterozygous mutations in Protein Arginine Methyltransferase 7 (PRMT7, OMIM*610087) gene have been linked to a human disorder known as Short Stature, Brachydactyly, Intellectual Developmental Disability and Seizures syndrome (SBIDDS) (OMIM #617157) [1–6]. PRMT7 encodes for an arginine methyltransferase that methylates arginine residues on various protein substrates and is involved in several biological processes, such as DNA transcription and repairing, RNA splicing, cell differentiation, and metastasis.
So far, only 15 patients with pathogenetic variants in PRMT7 have been described. Akawi et al. initially identified six individuals belonging to three families with compound heterozygous pathogenic variants in PRMT7 Fig. 1. These patients showed a syndromic condition characterized by mild intellectual disability (ID), facial dysmorphisms, microcephaly, short stature, and brachydactyly. Their clinical phenotype was considered a phenocopy of Albright hereditary osteodystrophy/Pseudohypoparathyroidism AHO/PHP (OMIM#103580; Orphanet 457059) [1]. In 2017, Kernohan et al. described a 6-year-old male with similar clinical findings along with cryptorchidism and seizures who was found to have a homozygous deletion in the transcription start site of the PRMT7 gene [2]. In 2017, Agolini et al. reported three additional patients with severe/moderate ID, short stature, brachydactyly, mild dysmorphic features and possible genotype/ phenotype correlation [3]. More recently, in 2019, Valenzuela et al. described a 2-year-old girl with short stature, psychomotor delay, hearing loss and brachydactyly due to two alterations in PRMT7 for whom parental segregation studies detected biallelic mutation inheritance [4]. Birnbaum et al. reported two male siblings with a novel PRMT7 homozygous deleterious variants and brain calcifications, delayed myelination and congenital orbital tumor [5]. Finally, a very recent report by Poquérusse et al. described a novel homozygous substitution in PRMT7 in two brothers from a consanguineous Iraqi family with associated compound heterozygous defects in the dysplasia-associated perlecan-encoding HSPG2 gene (OMIM*142461) [6]. No endocrine evaluation was performed in all these patients.
Fig. 1.
Whole exome sequencing analysis showing the compound heterozygous genetic defects in the PRMT7 gene, of maternal and paternal origin.
Over the past few decades, PRMT7 has been the subject of extensive research. In murine models, PRMT7-null mice showed reduced body size and shortened metatarsal bones. These mice had high mortality rates shortly after birth, and the few mice that survived developed increased fat mass as adults. The phenotypes of PRMT7 alterations in humans are mainly represented as SBIDDS syndrome. The similarities in phenotype between PRMT7-null mice and humans with PRMT7 pathogenetic variants demonstrate the crucial role of PRMT7 in the development of skeletal muscle, neurons, bone and adipose tissues. Even though short stature can be considered as a distinctive feature of the syndrome, its underlying pathogenetic aspects are yet to be established. Indeed, postnatal growth restriction of prenatal onset, mild intellectual disability (ID), facial dysmorphisms and brachydactyly resemble phenotypically conditions associated with methylation disturbances.
Here we report the case of two dizygotic twins diagnosed by whole exome sequencing (WES) analysis of DNA from whole peripheral blood. Of note, the parents also have one unaffected son (born before the two sisters).
Patients and methods
In accordance with hospital ethics committee standards, parents signed written informed consent for DNA analysis and for publication of the present article with accompanying images. No ethical approval was required for this clinical case series as patients were treated in accordance with latest guidelines and local regulations for recombinant growth hormone (rGH) therapy.
Clinical characteristics
The study included two prepubertal twins born after in vitro fertilization at 36 + 3 weeks from a dichorionic diamniotic pregnancy complicated by threatened abortion at 13 weeks of gestational age. Amniocentesis performed at 25 weeks of gestation showed in both a normal female karyotype, confirmed after birth at chromosomal microarray analysis. Twin A was born appropriate for gestational age (AGA, birth weight 1990 gr, −1.73 SDS according to Bertino charts, [7]), whereas Twin B had postnatal growth restriction of prenatal onset, was born small for gestational age (SGA, birth weight 1425 gr, −2.8 SDS according to Bertino charts [7]), and needed non-invasive ventilation support for respiratory distress syndrome. Postnatally, they both showed global developmental delay and progressive decline in growth. No episode of seizure has been reported. Twin A also had bilateral epicanthus, convergent strabismus and bilateral hypermetropic astigmatism, while Twin B broad forehead, sparse eyebrows, hypertelorism, depressed nasal bridge and thin lips.
Genetic evaluation and testing
At 1.7 years of age, both children underwent genetic evaluation. Poor growth, global developmental delay and shared mild dysmorphic features suggested a possible genetic condition. The karyotype and array-based comparative genomic hybridization (aCGH) were unremarkable. At four years, both twins and their parents were examined by whole exome sequencing (WES) through next generation sequencing (NGS). WES identified the same compound heterozygous genetic defects in the PRMT7 gene in both twins: the missense variant NM_019023.5:c.1220 G > A p.(Cys407Tyr) of maternal origin and the splicing variant NM_019023.5:c.1323 + 2 T > G of paternal origin.
Given the still poorly defined role of the methyltransferase PRTMT7 in humans and the presence of phenotypic features resembling those found in some imprinting disorders, Methylation-Sensitive Multiplex Ligand-dependent Probe Amplification (MS-MLPA, using the assay SALSA MS-MLPA Probemix ME034) analysis was performed to investigate the methylation and copy number status of known imprinted loci associated with imprinting disorders and/or Multilocus imprinting disorders (MLID). The probe panel included genes involved in Transient Neonatal Diabetes Mellitus 1 (TNDM1), Silver Russel syndrome (SRS), Beckwith-Wiedemann syndrome (BWS), Prader–Willi syndrome (PWS), Angelman syndrome (AS), Kagami–Ogata syndrome, Temple syndrome and Pseudohypoparathyroidism type B (PHP1B, recently named as inactivation PTH/PTHrp signaling disorder 3, iPPSD3, according to the novel proposed classification of conditions related to the impairment of the PTH/PTHrp signaling pathway). No epigenetic alterations at imprinted loci were found in either patient.
Auxological evaluation and hormonal testing
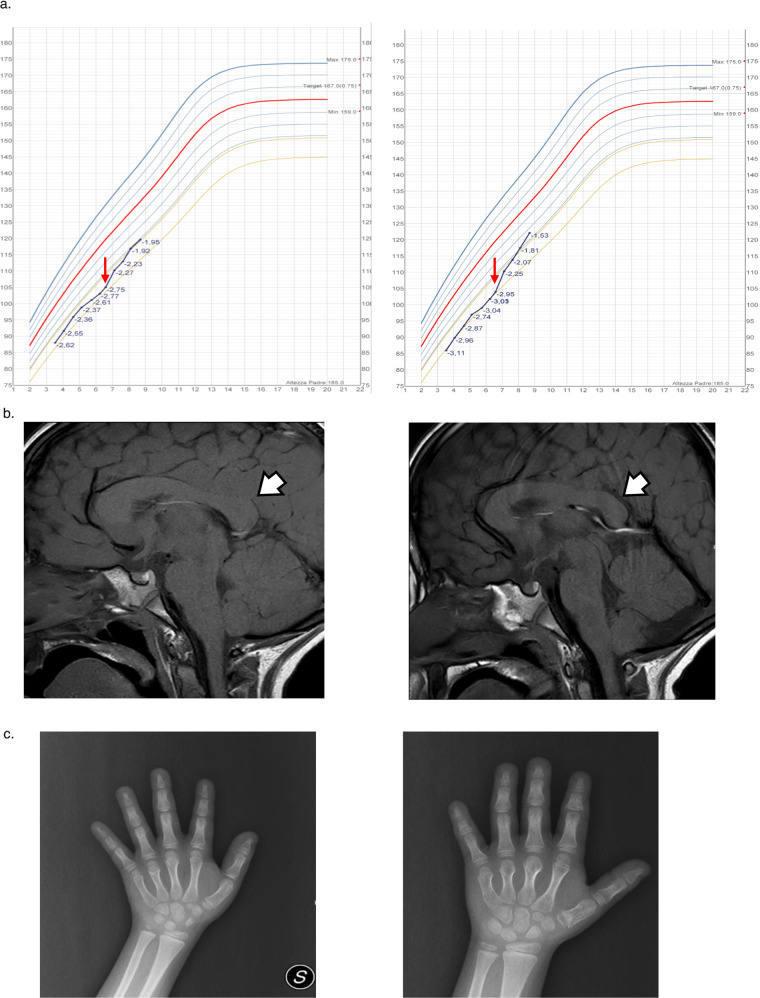
The twins came to our attention at 3.5 years of age. At 6 years of age, they both showed severe short stature and progressive growth impairment (Fig. 2a [8]). For this reason, endocrine assessment was planned to rule out growth hormone (GH) deficiency. GH deficiency and reduced IGF-I levels were found in Twin A (peak GH at arginine test: 4.61 µg/L; peak GH at glucagon test: 5.14 µg/L, IGF-I 53 ng/mL, −2.02 SDS) whereas an appropriate GH response, even in the presence of reduced IGF-I concentrations, was observed in Twin B (peak GH at arginine test: 11.8 µg/L, IGF-I 52 ng/mL, −2.05 SDS). Other blood tests were normal. In both, skeletal maturation, evaluated according to the standards of Tanner-Whitehouse [9], was consistent with chronological age.
Fig. 2. Growth Curves according to Cacciari Growth Charts.
Red arrow: rGH start (a), Brain MRI. White arrow: dysmorphic features of corpus callosum (b) and X-ray of Hand and Wrist for Bone Age Assessment (c) of Twin A (left panel) and B (right panel).
An MRI of whole brain and pituitary region was performed showing normal hypothalamic-pituitary region with corpus callosum thickening in Twin A (Fig. 2b) and dysmorphic features of corpus callosum and cerebellar vermis with a pars intermedia cyst in Twin B (Fig. 2c).
Laboratory methods/hormone assays
Growth hormone values were assessed with a chemiluminescence method (Immulite 2000, Siemens Medical Solutions Diagnostics, Los Angeles, CA) with a detection limit of 0.01 μg/L and calibrated to the WHO International Standard IS 98/574.
Insulin-like growth factor 1 (IGF-I) levels were measured by a chemiluminescent immunometric assay (Immulite 2000 IGF-I; Siemens Medical Solutions Diagnostics, Los Angeles, CA), with an intra- and interassay coefficient of variation of 2.9 and 7.4%, respectively and calibrated according to IS 02/254 standard. IGF-I concentrations were compared with those from an appropriate age- and sex-adjusted range and expressed as SDS. All the other biochemical parameters were measured by standard procedures.
Results
Growth hormone therapy and response
Twin A was treated with rGH at the replacement dosage of 0.025 mg/kg/day, whereas Twin B with 0.035 mg/kg/day, as indicated in SGA [9]. After two-year therapy, Twin A and B showed a good response to treatment with a total ∆HT of +0.82 SDS and +1.42 SDS, respectively (Table 1) [8]. Growth hormone therapy was increased at each visit according to body weight changes. No sign of pubertal development was recorded and bone age showed a regular progression during the first two years of treatment.
Table 1.
Auxological Data at baseline and after 1 and 2 years of rGH therapy.
| CAa, years | BAb, years | HTc, cm (SDS) | GVd, cm/year (SDS) | ΔHTa-HTabaseline, cm (SDS) | ΔTHe-HTa (SDS) | rGHf dose (mcg/kg/day) | |
|---|---|---|---|---|---|---|---|
| Twin A (GHDg) | |||||||
| Baseline | 6.6 | 6.3 | 105 (−2.75) | 3.8 (−2.5) | – | 3.42 | 0.025 |
| 1 year | 7.6 | 6.7 | 112.3 (−2.23) | 7.5 (2.1) | 7.3 (0.52) | 2.90 | 0.022 |
| 2 years | 8.7 | 7.2 | 119.6 (−1.95) | 6.2 (0.9) | 14.6 (0.82) | 2.62 | 0.022 |
| Twin B (SGAh) | |||||||
| Baseline | 6.6 | 6.7 | 104 (−2.95) | 4.3 (−2.0) | – | 3.62 | 0.035 |
| 1 year | 7.6 | 7.9 | 113.8 (−2.07) | 9.5 (4.1) | 9.4 (0.88) | 2.74 | 0.032 |
| 2 years | 8.7 | 8.7 | 122.1 (−1.53) | 7.9 (2.9) | 18.1 (1.42) | 2.20 | 0.033 |
aChronological Age.
bBone Age.
cHeight.
dGrowth Velocity.
eTarget Height.
fRecombinant Growth Hormone.
gGrowth Hormone Deficiency.
hSmall for Gestational Age.
Growth Curves are displayed in Fig. 2a. In Twin B hand and wrist X-Ray assessment ruled out the presence of brachydactyly with cone-shaped epiphysis of the first metacarpal and intermediate phalanx of the second finger (Fig. 2c).
Safety and adverse events
Neither twin experienced adverse effects over 2 years of therapy. Parameters of glucose metabolism remained unchanged. IGF-I levels were always within the reference range for age and sex.
Hormonal and metabolic parameters are shown in Table 2.
Table 2.
Hormonal and biochemical at baseline and after 1 and 2 years of rGH therapy.
| IGF-Ia, ng/mL (nv 65–225) | IGF-Ia, SDS | Glycemia, mg/dL (nv 80–100) | Insulin, IU/L (nv 1.8–24) | TSHb, mIU/L (nv 0.26–4.2) | Free T4c, pg/mL (nv 8–17) | |
|---|---|---|---|---|---|---|
| Twin A (GHDd) | ||||||
| Baseline | 53 | −2.02 | 83 | 8.0 | 1.76 | 10.6 |
| 1 year | 172 | 0.66 | 90 | 10.4 | – | – |
| 2 years | 164 | 0.49 | 91 | 11.2 | 1.5 | 10.3 |
| Twin B (SGAe) | ||||||
| Baseline | 52 | −2.05 | 84 | 7.3 | 1.83 | 12.7 |
| 1 year | 149 | 0.1 | 89 | 11.8 | – | – |
| 2 years | 170 | 0.61 | 92 | 8.4 | 1.37 | 11.8 |
1Insulin-like Growth Factor-I.
bThyroid Stimulating Hormone.
cThyroxine.
dGrowth Hormone Deficiency.
eSmall for Gestational Age.
Discussion
To the best of our knowledge, this represents the first report of short-term good response to rGH treatment in SBIDDS Syndrome, either with or without underlying GH deficiency. We also investigated for the first time the methylation effects of PRMT7 in the context of SBIDDS by MS-MLPA analysis for MLID and didn’t find any epigenetic alteration at known imprinted loci associated with imprinting syndromes in either twin.
In SBIDDS Syndrome, growth delay, though not always present at birth [3], seems to be constantly reported later in life, usually occurring after 6 years of age [2, 4, 6] with an adult height usually falling under the 3rd centile [3]. Despite being a distinctive feature of the syndrome usually found in all the patients described to date (Table 3), the underlying pathogenetic aspects of short stature are far to be clear. Given the role of PRMT7 as methyltransferase, to investigate its contribution to growth impairment and phenotypic overlap with AHO/PHP, genetic analysis through MS-MLPA involving genes related to imprinting disorders was performed. AHO/PHP, resulting from genetic or epigenetic alterations in the GNAS locus [10], can sometimes be attributed to imprinting-associated altered methylation patterns [11, 12]. In our case series, molecular investigations through MS-MLPA targeting imprinted genes related to imprinting disorders was normal.
Table. 3.
Summary of previously reported auxological data.
| Akawi et al. [1–6] | Kernohan et al. [7] | Agolini et al. [8–10] | Valenzuelaa et al. [11] | Birnbaum et al. [12, 13] | Poquérusse et al. [14, 15] | |
|---|---|---|---|---|---|---|
| Short stature | X | X | X | X | X | X |
| Length at birth | NA | 46 cm, −2.06 SD |
Case 1: 47 cm, −2.05 SD Case 2: 49 cm, −0.98 SD Case 3: 48 cm, −1.31 SD |
45 cm, −2.3 SD | NA | NA |
| Height at last follow-up |
Case 1: 151.5 cm, −1.98 SD (adult height) Case 2: NA Case 3: 146.6 cm, −2.8 SD (adult height) Case 4: 150 cm, −1.4 SD (14 years) Case 5: 132 cm, −2.0 SD (9 years 7 months) Case 6: 102 cm, −2.9 SD (6 years 2 months) |
110 cm, −1.93 SD (8 years) |
Case 1: 90.3 cm, −2.37 SD (3 years and 7 months) Case 2: 160 cm, −1.92 SD (adult height) Case 3: 145.5 cm, −2.77 SD (adult height) |
66 cm, −2 SD (10 years) |
Case 1: NA Case 2: length<4 SD (21 months) |
Case 1: 3rd centile (6 years) Case 2: 0.4th centile (5 years) |
Indeed, the striking height gain observed in our patients over the two-year rGH treatment is highly suggestive of a possible role of GH/IGF-I axis alterations as contributors of growth impairment though, to date, only one study performed an unspecified endocrinological evaluation for short stature, which was negative [4]. In Twin A we found an insufficient response to GH stimulation tests, confirming the presence of GHD. This endocrine manifestation has never been described before in the spectrum of SBIDDS Syndrome, even though PRMT7-null murine models showed growth impairment and increased fat mass, as frequently encountered in human GHD. On the other hand, Twin B was found to be GH sufficient at stimulation test but, being born SGA from IUGR, was treated with rGH as indicated in Europe since 2003 in SGA children over 4 years of age in the absence of an appropriate catch-up growth [13].
Studies on rGH effects have clearly shown that responsiveness to therapy is highly related to the entity of the GH/IGF-I axis defect. However, there are few standardised definitions of “good” and “poor” short-term auxological response in the literature. Nonetheless, it is of paramount importance to optimize first year growth during rGH treatment, as it represents the strongest indicator of future adult height outcomes [14, 15]. In 2010, Ranke et al. defined a first-year “poor response” as a height gain less than 0.4 SDS in severe GHD (GH peak after stimulation test < 5 ng/dL) and less than 0.3 SDS in mild GHD or SGA (GH peak > 5 ng/dL) [16]. Second-year “poor response” was defined as a height gain less than 0.15 SDS and less than 0.12 SDS in severe GHD and mild GHD or SGA, respectively [16]. Instead, Bang et al. in 2011 defined a first-year “poor response” as a height gain below 0.5 SDS [17]. In this context, our patients showed a good response after 1- and 2-year therapy according to both the above reported criteria. Moreover, bone age showed regular progression during treatment.
These findings suggest the potential role of rGH treatment in the management of growth disorders associated with PRMT7 pathogenetic variants, as observed in other syndromic short stature namely Noonan Syndrome [18] or aggrecan (ACAN) deficiency [19]. The diagnostic process as well as the subsequent clinical response may indicate the presence of an underlying growth and developmental disorder of multifactorial origin, with endocrine involvement (with variable GH/IGF-I axis associated defect) being only one aspect of a more complex impairment in which several signalling pathways can be implied [1]. Indeed, GH/IGF-I axis defects represent a continuum ranging between severe GHD to complete GH resistance [20], with the entity of idiopathic short stature (ISS) situated midway, probably including some children with mild GHD and some with partial GH resistance. In this context, both twins had low concentrations of IGF-I, thus supporting a possible underlying role of GH/IGF-I axis impairment of variable entity.
Moreover, Twin B, who was treated with higher doses in the absence of GHD, showed the best clinical response to treatment. This seems to reflect a dose-related response with greater efficacy from higher (pharmacological) rGH doses than physiological replacement, as frequently encountered in other syndromic short stature, namely Noonan Syndrome [18, 21], Turner Syndrome [22], or in children born SGA [23]. Furthermore, studies conducted in patients with ISS treated with rGH showed good response in the first year of treatment, though followed by a relatively reduced growth in the ensuing years [24].
In the literature, studies on SGA children treated with rGH have shown that most patients reached a normal height after the first years of rGH treatment and remained in the normal range until adult height. Nonetheless, at the end of GH treatment, a slight decrease in height SDS was found because of early onset of puberty and/or acceleration of bone maturation. This would lead to attainment of adult height at a relatively young age compared with peers and/or a reduced pubertal height gain [15]. Only long-term follow-up will probably show whether this good response reflects a faster bone age progression or a sustained height gain with an appropriate target height retrieval.
Another aspect to take into consideration is the safety profile, especially in the presence of underlying genetic abnormalities potentially associated with risk of malignancies. The long-term safety of rGH therapy in disease categories at higher risk of developing cancer per se should be considered in treatment decision-making. Long-term follow-up is ongoing in these patients to provide data on adult height and safety profile. As far as short-term-safety is concerned, in both children parameters of glucose metabolism remained normal and IGF-I levels were in the normal range for sex and age over the whole treatment period.
In conclusion, this report expands the knowledge in the field of PRMT7-associated manifestations, with particular focus on short stature. The diagnostic evaluation as well as the subsequent response to treatment may indicate the presence of an underlying growth impairment of multifactorial origin, with endocrine involvement (with variable GH/IGF-I axis associated defect) being only an aspect of a more complex impairment in which several signalling pathways can be involved. This study demonstrates for the first time the effectiveness of rGH in two children with PRMT7 deleterious variants: the striking and sustained good response leads to wonder if SBIDDS Syndrome should be probably counted among syndromic short stature treatable with growth hormone. Given the rarity of the disease as well as the shortage of data in the literature, it is difficult to determine which proportion of affected children could benefit from rGH treatment. However, every patient with SBIDDS Syndrome presenting with growth failure should undergo through endocrine assessment and be treated with rGH according to the current guidelines [25]. Further studies are needed to identify the causal mechanisms and potential benefits of rGH in an attempt to have a more individualized treatment approach for these patients and to confirm rGH long-term efficacy and safety.
Supplementary information
Acknowledgements
The Authors would like to thank the patients and their parents for their kind cooperation. This work has been generated within the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (ERN-ITHACA) (EU Framework Partnership Agreement ID: 3HP-HP-FPA ERN-01-2016/739516).
Author contributions
RG wrote the first draft of the manuscript; MG, AM and GC have revised the text; EF, BMF and GC have critically reviewed the text and provided substantial scientific contributions. All authors approved the final version of the manuscript.
Funding
The research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01220-9.
References
- 1.Akawi N, McRae J, Ansari M, Balasubramanian M, Blyth M, Brady AF, et al. Discovery of four recessive developmental disorders using probabilistic genotype and phenotype matching among 4,125 families. Nat Genet. 2015;47:1363–9. doi: 10.1038/ng.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kernohan KD, McBride A, Xi Y, Martin N, Schwartzentruber J, Dyment DA, et al. Loss of the arginine methyltranserase PRMT7 causes syndromic intellectual disability with microcephaly and brachydactyly. Clin Genet. 2017;91:708–16. doi: 10.1111/cge.12884. [DOI] [PubMed] [Google Scholar]
- 3.Agolini E, Dentici ML, Bellacchio E, Alesi V, Radio FC, Torella A, et al. Expanding the clinical and molecular spectrum of PRMT7 mutations: Three additional patients and review. Clin Genet. 2018;93:675–81. doi: 10.1111/cge.13137. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela I, Segura-Puimedon M, Rodríguez-Santiago B, Fernández-Alvarez P, Vendrell T, Armengol L, et al. Further delineation of the phenotype caused by loss of function mutations in PRMT7. Eur J Med Genet. 2019;62:182–5. doi: 10.1016/j.ejmg.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum R, Yosha-Orpaz N, Yanoov-Sharav M, Kidron D, Gur H, Yosovich K, et al. Prenatal and postnatal presentation of PRMT7 related syndrome: Expanding the phenotypic manifestations. Am J Med Genet A. 2019;179:78–84. doi: 10.1002/ajmg.a.6. [DOI] [PubMed] [Google Scholar]
- 6.Poquérusse J, Whitford W, Taylor J, Alburaiky S, Snell RG, Lehnert K, et al. Novel PRMT7 mutation in a rare case of dysmorphism and intellectual disability. J Hum Genet. 2022;67:19–26. doi: 10.1038/s10038-021-00955-5. [DOI] [PubMed] [Google Scholar]
- 7.Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal Anthropometric Charts: The Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. 2010;51:353–61. doi: 10.1097/MPG.0b013e3181da213e. [DOI] [PubMed] [Google Scholar]
- 8.Growth Calculator 4. [Online] http://www.weboriented.it/gh4/.
- 9.Tanner JM. Assessment of Skeletal Maturity and Prediction of Adult Height (TW2 Method) Academic Press; 1983. [Google Scholar]
- 10.Linglart A, Levine MA, Jüppner H. Pseudohypoparathyroidism. Endocrinol Metab Clin North Am. 2018;47:865–88. doi: 10.1016/j.ecl.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani G, de Sanctis L, Barbieri AM, Elli FM, Bollati V, Vaira V, et al. Pseudohypoparathyroidism and GNAS epigenetic defects: clinical evaluation of albright hereditary osteodystrophy and molecular analysis in 40 patients. J Clin Endocrinol Metab. 2010;95:651–8. doi: 10.1210/jc.2009-0176. [DOI] [PubMed] [Google Scholar]
- 12.Linglart A, Maupetit-Méhouas S, Silve C. GNAS -Related Loss-of-Function Disorders and the Role of Imprinting. Horm Res Paediatr. 2013;79:119–29. doi: 10.1159/000348516. [DOI] [PubMed] [Google Scholar]
- 13.Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92:804–10. doi: 10.1210/jc.2006-2017. [DOI] [PubMed] [Google Scholar]
- 14.De Ridder MA, Stijnen T, Hokken-Koelega AC. Prediction of adult height in growth-hormone-treated children with growth hormone deficiency. J Clin Endocrinol Metab. 2007;92:925–31. doi: 10.1210/jc.2006-1259. [DOI] [PubMed] [Google Scholar]
- 15.Van Pareren Y, Mulder P, Houdijk M, Jansen M, Reeser M, Hokken-Koelega A. Adult height after long-term, continuous growth hormone (GH) treatment in short children born small for gestational age: results of a randomized, double-blind, dose-response GH trial. J Clin Endocrinol Metab. 2003;88:3584–90. doi: 10.1210/jc.2002-021172. [DOI] [PubMed] [Google Scholar]
- 16.Ranke MB, Lindberg A. KIGS International Board. Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95:1229–37. doi: 10.1210/jc.2009-1471. [DOI] [PubMed] [Google Scholar]
- 17.Bang P, Ahmed SF, Argente J, Backeljauw P, Bettendorf M, Bona G, et al. Identification and management of poor response to growth-promoting therapy in children with short stature. Clin Endocrinol (Oxf) 2012;77:169–81. doi: 10.1111/j.1365-2265.2012.04420.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelnar CJ. Growth hormone therapy in noonan syndrome. Horm Res. 2000;53:77–81. doi: 10.1159/000053209. [DOI] [PubMed] [Google Scholar]
- 19.Muthuvel G, Dauber A, Alexandrou E, Tyzinski L, Andrew M, Hwa V, et al. Treatment of short stature in aggrecan deficient patients with recombinant human growth hormone: one-year response. J Clin Endocrinol Metab. 2021;107:e2103–9. doi: 10.1210/clinem/dgab904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage MO, Burren CP, Rosenfeld RG. The continuum of growth hormone–IGF-I axis defects causing shortstature: diagnostic and therapeutic challenges. Clin Endocrinol (Oxf) 2010;72:721–8. doi: 10.1111/j.1365-2265.2009.03775.x. [DOI] [PubMed] [Google Scholar]
- 21.Ozono K, Ogata T, Horikawa R, Matsubara Y, Ogawa Y, Nishijima K. Efficacy and safety of two doses of Norditropin((R)) (somatropin) in short stature due to Noonan syndrome: a 2-year randomized, double-blind, multicenter trial in Japanese patients. Endocr J. 2018;65:159–74. doi: 10.1507/endocrj.EJ17-0313. [DOI] [PubMed] [Google Scholar]
- 22.Dahlgren J, Albertsson-Wikland K. GH responsiveness in children with noonan syndrome compared to turner syndrome. Front Endocrinol (Lausanne) 2021;12:737893. doi: 10.3389/fendo.2021.737893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatelain P, Colle M, Nako JP, Le Luyer B, Wagner K, Berlier P, et al. Optimization of growth hormone dosing in children born small for gestational age: an open-label, randomized study of children during the fourth year of therapy. Horm Res Paediatr. 2012;77:156–63. doi: 10.1159/000337216. [DOI] [PubMed] [Google Scholar]
- 24.Deodati A, Cianfarani S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: systematic review. BMJ. 2011;342:c7157. doi: 10.1136/bmj.c7157. [DOI] [PubMed] [Google Scholar]
- 25.Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, Biller BMK, Boguszewski MCS, et al. Diagnosis, genetics, and therapy of short stature in children: a growth hormone research society international perspective. Horm Res Paediatr. 2019;92:1–14. doi: 10.1159/000502231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.