Abstract
Elevated blood pressure and hypertension have been associated with increased risk of atrial fibrillation in a number of epidemiological studies, however, the strength of the association has differed between studies. We conducted a systematic review and meta-analysis of the association between blood pressure and hypertension and atrial fibrillation. PubMed and Embase databases were searched for studies of hypertension and blood pressure and atrial fibrillation up to June 6th 2022. Cohort studies reporting adjusted relative risk (RR) estimates and 95% confidence intervals (CIs) of atrial fibrillation associated with hypertension or blood pressure were included. A random effects model was used to estimate summary RRs. Sixty eight cohort studies were included in the meta-analysis. The summary RR was 1.50 (95% CI: 1.42–1.58, I2 = 98.1%, n = 56 studies) for people with hypertension compared to those without hypertension (1,080,611 cases, 30,539,230 participants), 1.18 (95% CI: 1.16–1.21, I2 = 65.9%, n = 37 studies) per 20 mmHg increase in systolic blood pressure (346,471 cases, 14,569,396 participants), and 1.07 (95% CI: 1.03–1.11, I2 = 91.5%, n = 22 studies) per 10 mmHg increase in diastolic blood pressure (332,867 cases, 14,354,980 participants). There was evidence of a nonlinear association between diastolic blood pressure and atrial fibrillation with a steeper increase in risk at lower levels of diastolic blood pressure, but for systolic blood pressure the association appeared to be linear. For both systolic and diastolic blood pressure, the risk increased even within the normal range of blood pressure and persons at the high end of systolic and diastolic blood pressure around 180/110 mmHg had a 1.8–2.3 fold higher risk of atrial fibrillation compared to those with a blood pressure of 90/60 mmHg. These results suggest that elevated blood pressure and hypertension increases the risk of atrial fibrillation and there is some increase in risk even within the normal range of systolic and diastolic blood pressure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10654-022-00914-0.
Keywords: Blood pressure, Hypertension, Atrial fibrillation, Systematic review, Meta-analysis, Cohort studies
Introduction
Atrial fibrillation presents a considerable public health burden and is the most common type of arrhythmia affecting around 1–2% of the general population, increasing to around 10% of persons by 80 years of age [1]. Five million incident cases were diagnosed worldwide in 2010 [2] and the prevalence of atrial fibrillation has been estimated at 33 million in 2015 [3]. In the USA the prevalence of atrial fibrillation has been projected to increase from 2.3 million in 1996–1997 to 5.6 million by 2050 [4]. Patients with atrial fibrillation are at increased risk of a number of complications, most notably stroke, heart failure, dementia and all-cause mortality [5, 6]. Several risk factors for atrial fibrillation have been established including age, sex, diabetes, coronary heart disease, heart failure, smoking, alcohol, obesity, low physical activity and possibly high intensity physical activity [7–15].
Elevated blood pressure is the leading cause of death and disability-adjusted life-years (DALYs) globally with 10.4 million deaths and 218 million DALYs attributable to elevated systolic blood pressure in 2017 according to the Global Burden of Disease Study [16]. Although elevated blood pressure is an established risk factor for several cardiovascular diseases, data regarding blood pressure and risk of atrial fibrillation have to our knowledge not been summarized in a meta-analysis. A large number of cohort studies have investigated the association between hypertension and the risk of atrial fibrillation [7–9, 17–65], and most of these found an increased risk [7, 8, 17–22, 24–31, 33, 34, 36–49, 51–62, 64, 65], with few studies reporting no association [9, 23, 32, 35, 50, 63], however, the strength of the association has differed considerably between studies with reported relative risks reported ranging from 0.93 to 2.85 [7–9, 17–59]. In addition, several studies have investigated the association between systolic [8, 18, 20, 29, 33, 34, 39, 41, 42, 44, 46, 51, 55, 63, 65–80] or diastolic [8, 29, 33, 39, 41, 44, 46, 51, 55, 67–70, 72–74, 79, 81] blood pressure and risk of atrial fibrillation with most studies reporting increased risk for increasing systolic blood pressure [18, 20, 29, 34, 39, 41, 44, 46, 51, 55, 65–72, 74–80], while results have been more mixed for diastolic blood pressure with some showing an increased risk [39, 44, 51, 55, 67–69, 72, 79, 82] but other studies showing no association [8, 29, 33, 46, 70, 73, 74, 81], or even reduced risk [41, 77] with higher diastolic blood pressure.
Establishing whether hypertension and elevated blood pressure increases the risk of atrial fibrillation would be important from a preventive point of view as it is a risk factor that could be modified by diet, physical activity, weight control and pharmaceutical drugs [83]. In addition it would be useful to better characterize the strength and shape of the dose–response relationship between blood pressure and atrial fibrillation to clarify whether the association is dose-dependent or if there are threshold effects. We conducted a systematic review and meta-analysis of cohort studies on hypertension and blood pressure in relation to the risk of atrial fibrillation to clarify the strength and shape of the dose–response relationship, and to identify potential sources of heterogeneity in the results.
Material and methods
Search strategy and inclusion criteria
We searched Pubmed, and Embase databases up to June 9th 2022 for eligible studies. The search strategy is provided in the Supplementary Text. We followed standard criteria for conducting and reporting meta-analyses [84]. In addition, we searched the reference lists of the identified publications for further studies.
Study selection
We included published retrospective and prospective cohort studies and nested case–control studies within cohorts that investigated the association between blood pressure or hypertension and the risk of atrial fibrillation (any type). Retrospective case–control studies were excluded because of their potential for recall bias and selection bias and cross-sectional studies were excluded because of difficulties in establishing cause and effect relationships. Estimates of the relative risk adjusted for at least one confounding factor had to be available with the 95% confidence intervals (CIs) in the publication. Conference abstracts, grey literature and non-English publications were not included. When multiple publications were available from the same study, the study with the largest number of cases was used in general. However, overlapping publications were used in specific subgroup analyses by sex or ethnicity, when the article used for the main analysis did not report such stratified analyses. Overlapping publications that reported risk estimates in three categories or more were also used for the nonlinear dose–response analyses (as the nonlinear analysis requires categorical data) if the article included in the main analysis only reported risk estimates on a continuous scale. A list of the excluded studies can be found in Supplementary Table 1. DA, YMS, EK and TF did the study selection in duplicate and any disagreements were resolved by discussion.
Data extraction
The following data were extracted from each study: The first author’s last name, publication year, country where the study was conducted, study period, sample size, number of cases and participants, exposure (hypertension, systolic blood pressure, or diastolic blood pressure), subgroup (e.g. sex, race), relative risks (RRs) and 95% CIs for hypertension versus no hypertension or for increments in systolic or diastolic blood pressure and variables adjusted for in the analysis. DA did the data extraction and it was checked for accuracy by YMS.
Statistical methods
We calculated summary RRs (95% CIs) of atrial fibrillation for participants with hypertension compared with participants without hypertension and for systolic and diastolic blood pressure using the random-effects model by DerSimonian and Laird [85] which takes into account both within and between study variation (heterogeneity). The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted by the inverse of its variance. Linear dose–response analyses were conducted per 20 mmHg for systolic blood pressure and per 10 mmHg for diastolic blood pressure (consistent with previous studies [86–88]) using the method of Greenland and Longnecker [89]. For studies that reported blood pressure by ranges we estimated the midpoint for each category by calculating the average of the upper and lower cut-off points. For open-ended categories we used the width of the adjacent interval to estimate an upper or lower cut-off value for the extreme category. Fractional polynomial models were used to investigate a potential nonlinear association between systolic and diastolic blood pressure and risk of atrial fibrillation [90]. A log-likelihood test was used to test for nonlinearity [91].
Heterogeneity between studies was evaluated using Q and I2 statistics [92]. I2 is an estimate of how much of the heterogeneity that is due to between study variation rather than chance. I2-values of 25%, 50% and 75% indicates low, moderate and high heterogeneity respectively. We conducted main analyses (all studies combined) and stratified by study characteristics such as sample size, number of cases, whether prevalent cases were excluded or not, geographic location, study quality and by adjustment for confounding factors to investigate potential sources of heterogeneity and we used meta-regression analyses to test for differences in summary estimates between subgroups. Study quality was assessed using the Newcastle Ottawa scale which rates studies according to selection, comparability and outcome assessment with a score range from 0 to 9 [93].
Publication bias was assessed using Egger’s test [94] and by inspection of funnel plots. The statistical analyses were conducted using the software package Stata, version 13.1 software (StataCorp, Texas, US).
Results
From a total of 32,876 records that were identified by the search we included a total of 69 publications [7–9, 17–82] with data from 68 cohort studies (two of these were nested case–control studies within cohort studies [21, 66]) in the systematic review and meta-analysis of hypertension and blood pressure and atrial fibrillation (Fig. 1). Five of these publications were identified from separate searches on other risk factors for atrial fibrillation [9, 21, 32, 34, 42]. Each of two publications reported results from two studies combined [73, 81], and another publication reported results from six studies combined [65]. Two publications [42, 75] reported results from two separate studies each and one publication reported results from five separate studies [29], two of which were included in the main analysis (the other three were surpassed by more recent publications, but results of two of these duplicate studies were included in subgroup analyses by ethnicity). Twenty six studies (23 publications) [18, 20, 21, 25, 27–30, 33, 36, 42, 43, 48, 54, 64, 65, 69, 71–73, 75, 76, 78] were from Europe, twenty studies (25 publications) [7, 17, 19, 22, 24, 26, 29, 31, 34, 37, 40, 41, 45–47, 52, 53, 56, 59, 67, 68, 70, 74, 77, 81] were from North America, nineteen studies (19 publications) [23, 32, 35, 38, 39, 44, 49–51, 55, 57, 60–63, 66, 79, 80, 82] were from Asia, and three studies (3 publications) [8, 9, 58] were from Australia.
Fig. 1.

Flow-chart of study selection
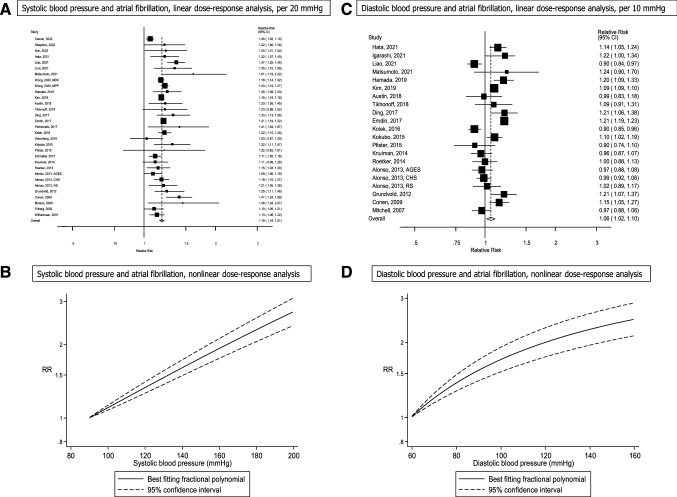
Fifty six cohort studies (52 publications, 52 risk estimates) [7–9, 17–65] were included in the analysis of hypertension and atrial fibrillation risk including 1,080,611 cases and 30,539,230 participants (Fig. 1, Table 1). Data on hypertension and atrial fibrillation by ethnicity [29, 59] and sex [25] from three studies (ARIC, REGARDS, and Malmö Diet and Cancer Study) were only included in the respective subgroup analyses as the publications overlapped with more recent publications from the same studies which were used for the main analysis [27, 45, 56]. The summary relative risk for persons with hypertension compared to persons without hypertension was 1.50 (95% CI: 1.42–1.58, I2 = 98.1%, pheterogeneity < 0.0001) (Fig. 2). There was no evidence of publication bias neither with Egger’s test (p = 0.74) or by inspection of the funnel plot (Supplementary Fig. 1). The summary RR ranged from 1.47 (95% CI: 1.42–1.52) when excluding the study by Zoller et al. [30] to 1.51 (95% CI: 1.43–1.59) when excluding the study by Sano et al. [53] (Supplementary Fig. 2).
Table 1.
Cohort studies of hypertension and atrial fibrillation
| First author, publication year, country | Study name or description | Study period | Number of participants, number of cases | Type of hypertension, subgroup | Comparison | Relative risk (95% confidence interval) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|
| Krahn AD et al., 1995, Canada | Manitoba Follow-Up Study | 1948–1992, 44 years follow-up | 3983 men, age 18–62 years: 299 atrial fibrillation cases | Hypertension | Yes vs. no | 1.42 (1.10–1.84) | Age |
| Wilhelmsen L et al., 2001, Sweden | Multifactor Primary Prevention Study | 1970–1973–1996, 25.2 years follow-up | 7495 men, age 47–55 years: 754 atrial fibrillation cases | Treatment for hypertension | Yes vs. no | 1.73 (1.31–2.29) | Age |
| Tsang TSM et al., 2001, USA | Olmsted County–ECG | 1990–1998–NA, 3.97 years follow-up | 1655 men and women, age 65–105 years: 189 atrial fibrillation cases | Hypertension | Yes vs. no | 1.44 (1.01–2.05) | Age, sex, history of valvular disease, congestive heart failure, myocardial infarction, diabetes mellitus |
| Ruigomez A et al., 2002, United Kingdom | UK General Practice Research Database | 1996–1996, 1 year follow-up |
1035 atrial fibrillation cases 5000 controls (nested case–control study) Men and women, age 40-89 years |
Hypertension | Yes vs. no | 1.80 (1.50–2.10) | Age, sex, cardiovascular morbidity |
| Friberg J et al., 2003, Denmark | Copenhagen City Heart Study |
1981–1983 - 1991–1994–1994, 7 years follow-up |
18,167 men and women, age 40–79 years: 379 atrial fibrillation cases | Arterial hypertension | Yes vs. no | 1.3 (1.0–1.6) | Age, sex, prior myocardial infarction, diabetes mellitus, systolic blood pressure, ECG-LVH voltage, ECG-LVH ST/T, height, weight, BMI, FEV1, smoking status, alcohol, period |
| Gami AS et al., 2007, USA | Olmsted County Study | 1987–2003, 4.7 years follow-up | 3542 men and women, mean age 49 years: 133 atrial fibrillation cases | Hypertension | Yes vs. no | 2.85 (2.02–4.02) | Age, sex, coronary artery disease, lowest nocturnal oxygen saturation |
| Kim HJ et al., 2007, Korea | Health Promotion Center, Samsung Medical Center | 2001–2006, 3.7 years follow-up | 16,568 men and women, median age 49 years: 61 atrial fibrillation cases | Hypertension medication use | Yes vs. no | 1.63 (0.71–3.75) | Age, sex, history of coronary artery disease, BMI, fibrinogen, left atrial enlargement on ECG |
| Nichols GA et al., 2009, USA | Kaiser Permanente Northwest | 1999–2008, 7.2 years follow-up |
16,057 diabetes patients and 16,471 persons without diabetes, mean age 58.4 years: 1835 atrial fibrillation cases |
Hypertension, all | Yes vs. no | 1.32 (1.20–1.46) | Age, sex, race, smoking, BMI, systolic blood pressure, ischemic heart disease, valvular disease, hypertension, heart failure |
| Hypertension, men | Yes vs. no | 1.29 (1.13–1.46) | |||||
| Hypertension, women | Yes vs. no | 1.34 (1.15–1.55) | |||||
| Smith JG et al., 2010, Sweden | Malmo Diet and Cancer study | 1991–1996–2005, 11.2 years follow-up | 30,447 men and women, age 44–73 years: 1430 atrial fibrillation cases | Hypertension, men | Yes vs. no | 1.78 (1.48–2.14) | Age |
| Hypertension, women | Yes vs. no | 1.74 (1.42–2.13) | |||||
| Lipworth L et al., 2012, USA | Southern Community Cohort Study | 1999–2008, 5.7 years follow-up | 8836 men and women, age ≥ 65 years: 1062 atrial fibrillation cases | Hypertension, all | Yes vs. no | 1.29 (1.07–1.55) | Length of Medicare follow-up |
| Hypertension, blacks | Yes vs. no | 1.37 (1.05–1.80) | |||||
| Hypertension, whites | Yes vs. no | 1.19 (0.92–1.54) | |||||
| Hypertension, men | Yes vs. no | 1.18 (0.90–1.55) | |||||
| Hypertension, women | Yes vs. no | 1.38 (1.07–1.78) | |||||
| Smith JG et al., 2013, Sweden | Malmo Diet and Cancer study | 1991–1996–2009, ~ 15.5 years follow-up | 30,447 men and women, age 44–73 years: 2339 atrial fibrillation cases | Hypertension | Yes vs. no | 1.54 (1.37–1.72) | Age, sex, BMI, history of myocardial infarction, diabetes, current smoking |
| Alonso A et al., 2013, USA | Cardiovascular Health Study | 1989–1990, 1992–1993–2000, NA years of follow-up | 5043 men and women, age 65 years: 624 atrial fibrillation cases | Hypertension, whites | Yes vs. no | 1.63 (1.38–1.92) | Age, sex |
| Hypertension, African Americans | Yes vs. no | 1.31 (0.77–2.22) | |||||
| Alonso A et al., 2013, USA | Atherosclerosis Risk in Communities Study | 1987–1989–1996–1998, -2005, NA years of follow-up | 10,675 men and women, age 45–64 years: 419 atrial fibrillation cases | Hypertension, whites | Yes vs. no | 2.02 (1.63.2.51) | Age, sex |
| Hypertension, African Americans | Yes vs. no | 2.31 (1.34–3.98) | |||||
| Alonso A et al., 2013, Iceland | Age, Gene/ Environment Susceptibility Reykjavik Study | 2002–2011, NA years of follow-up | 4469 men and women, mean age 76 years: 408 atrial fibrillation cases | Hypertension | Yes vs. no | 1.59 (1.28–1.97) | Age, sex |
| Alonso A et al., 2013, Netherlands | Rotterdam Study | 1997–2005, NA years of follow-up | 3203 men and women, mean age 72 years: 177 atrial fibrillation cases | Hypertension | Yes vs. no | 1.62 (1.21–2.19) | Age, sex |
| Conen D et al., 2013, USA | Women's Health Study | 1992–1995–2011, 16.4 years follow-up | 34,713 women, age ≥ 45 years: 796 atrial fibrillation cases | Hypertension, atrial fibrillation without LA enlargement | Yes vs. no | 1.55 (1.25–1.92) | Age, height, body weight, diabetes, race, education, alcohol, smoking, exercise |
| Hypertension, atrial fibrillation with LA enlargement | Yes vs. no | 1.99 (1.49–2.65) | |||||
| Nyrnes A et al., 2013, Norway | Tromsø Study | 1994–1995–2007, 11.1 years follow-up | 22,815 men and women, age 25–96 years: 461/361 atrial fibrillation cases | Hypertension, men | Yes vs. no | 1.98 (1.46–2.69) | Age, height, BMI, total cholesterol, HDL cholesterol, diabetes, palpitations, coronary artery disease |
| Hypertension, women | Yes vs. no | 1.40 (1.13–1.74) | |||||
| Zoller B et al., 2013, Sweden | Sweden Nationwide study | 2000–2008, ~ 8 years follow-up | 4,266,289 men and women, age ≥ 25 years: 101,985 atrial fibrillation cases | Hypertension, men | Yes vs. no | 2.03 (1.99–2.07) | Age, neighborhood deprivation, marital status, family income, education, country of origin, urban/rural status, mobility, hospitalized for chronic lower respiratory disease, type 2 diabetes, alcohol, obesity, heart failure, coronary heart disease, hyperthyroidism |
| Hypertension, women | Yes vs. no | 2.32 (2.27–2.38) | |||||
| Perez MV et al., 2013, USA | Women's Health Initative | 1994–1998–2007, 9.8 years follow-up | 81,892 women, age 50–79 years: 8252 atrial fibrillation cases | Hypertension | Yes vs. no | 1.43 (1.36–1.50) | Age, race/ethnicity, peripheral arterial disease, diabetes, hyperlipidemia, heart failure, coronary heart disease, BMI, postmenopausal hormone use, smoking status, alcohol, education |
| Knuiman M et al., 2014, Australia | Busselton Health Study | 1994–1995–NA, 15 years follow-up | 4267 men and women, age 25–84 years: 343 atrial fibrillation cases | Hypertension treatment | Yes vs. no | 1.70 (1.35–2.13) | Age, sex, height |
| Sano F et al., 2014, Japan | Circulatory Risk in Communities Study | 1991–1995–2000, 6.4 years follow-up | 8602 men and women, age 30–80 years: 296 atrial fibrillation cases | Hypertension, all | Yes vs. no | 0.93 (0.73–1.19) | Age, sex, alcohol, smoking status, BMI, hyperglycemia, hyperlipidemia, major ST-T abnormality, previous MI, heart failure |
| Hypertension, men | Yes vs. no | 1.02 (0.69–1.50) | |||||
| Hypertension, women | Yes vs. no | 0.89 (0.66–1.22) | |||||
| Pfister R et al., 2015, United Kingdom | EPIC-Norfolk | 1993–1997–2009, 5 years follow-up (analysis restricted to first 5 years) | 24,020 men and women, age 39–79 years: 236 atrial fibrillation cases | Hypertension treatment | Yes vs. no | 2.01 (1.51–2.66) | Age, height, weight, current smoking, systolic blood pressure, diastolic blood pressure, diabetes mellitus, heart failure, myocardial infarction |
| Schnabel RB et al., 2015, USA | Framingham Cohort Study | 1958–2007, 50 years follow-up | 9511 men and women, age 50–89 years: 1544 atrial fibrillation cases | Hypertension, 1958–1967 | Yes vs. no | 1.71 (0.96–3.06) | Age, sex |
| Hypertension, 1968–1977 | Yes vs. no | 1.63 (1.18–2.27) | |||||
| Hypertension, 1978–1987 | Yes vs. no | 1.35 (1.06–1.71) | |||||
| Hypertension, 1988–1997 | Yes vs. no | 1.68 (1.37–2.06) | |||||
| Hypertension, 1998–2007 | Yes vs. no | 1.32 (1.08–1.60) | |||||
| Suzuki H et al., 2015, Japan | Fukushima Health Management Survey | 2008–2010–2013, 1.4 years follow-up | 12,410 men and women, age 40–90 years: 79 atrial fibrillation cases | Hypertension | Yes vs. no | 1.08 (0.66–1.77) | Age, sex, obesity, excess ethanol intake, current smoking, hypertension |
| Nystrom PK et al., 2015, Sweden | Stockholm—60 year old men and women | 1997–1999–2012, 13.6 years follow-up | 4021 men and women, age 60 years: 285 atrial fibrillation cases | Hypertension, normal waist | Yes vs. no | 2.54 (1.54–4.21) | Sex, birth country, smoking status, alcohol, moderate-intensity exercise, history of myocardial infarction |
| Hypertension, medium waist | Yes vs. no | 1.92 (1.10–3.33) | |||||
| Hypertension, elevated waist | Yes vs. no | 1.18 (0.77–1.79) | |||||
| Qureshi WT et al., 2015, USA | Henry Ford Exercise Testing (FIT) Project | 1991–2009, 5.4 years follow-up | 64,561 men and women, mean age 54.5 years: 4616 atrial fibrillation cases | Hypertension | Yes vs. no | 1.23 (1.12–1.34) | Age, sex, race/ethnicity, hypertension, sedentary, obesity, family history of coronary artery disease, smoking, history of hyperlipidemia, known coronary artery disease, hypertensive response, thyroid medication use, digoxin use, lung medication use, beta blocker use, ACE inhibitor use, ARB inhibitor, calcium channel blocker use, lipid lowering medication use, METs |
| Guo Y et al., 2015, China | Yunnan Medical Insurance Database | 2001–2012, 11 years follow-up | 471,446 men and women, age ≥ 20 years: 866 atrial fibrillation cases | Hypertension | Yes vs. no | 1.72 (1.48–2.01) | Age, sex, rheumatic heart disease, dilated cardiomyopathy, heart failure, hyperthyroidism, coronary artery disease, chronic obstructive pulmonary disease, diabetes, renal dysfunction, hyperlipidemia, peripheral vascular disease |
| Kokubo Y et al., 2015, Japan | Suita Study | 1989–2006–2015, 12.8 years follow-up | 6906 men and women, age 30–79 years: 253 atrial fibrillation cases | Hypertension | < 120/ < 80 mmHg | 1.00 | Age, sex, BMI, hypercholesterolemia, diabetes, smoking status, drinking status, cohort groups, chronic kidney disease, stroke, coronary heart disease, chronic heart failure, premature contractions |
| 120–139/80–89 | 1.20 (0.83–1.73) | ||||||
| ≥ 140/ ≥ 90 | 1.53 (1.07–2.19) | ||||||
| Chyou JY et al., 2015, USA | Truven Health MarketScan Commercial and Medicare Supplemental Databases | 2007–2010, 3 years follow-up | 3,007,874 men and women, all ages: 165,741 atrial fibrillation cases | Hypertension | Yes vs. no | 1.31 (p < 0.0001) | Age, sex, heart failure, diabetes, coronary artery disease, chronic kidney disease, sleep apnea, chest pain, dizziness, palpitations, tachycardia unspecified, shortness of breath, respiratory other, respiratory—unspecified, geographic region, medications, use of internal/ external electrocardiographic recording device |
| Diouf I et al., 2016, Australia | Australian Diabetes, Obesity and Lifestyle study cohort | 1999/2000–2004/2005, 5 years follow-up | 5389 men and women, age ≥ 35 years: 53 atrial fibrillation cases | Hypertension | Yes vs. no | 1.20 (0.60–2.40) | Age, sex, smoking status, usual number of alcoholic drinks, physical activity, level of education, BMI |
| Kolek MJ et al., 2016, USA | Vanderbilt University Medical Center Electronic Medical Records | 2005–2010, 5 years follow-up | 33,494 men and women, age ≥ 40 years: 2455 atrial fibrillation cases | Hypertension treatment | Yes vs. no | 1.41 (1.30–1.54) | Age, race, height, weight, smoking status, systolic blood pressure, diastolic blood pressure, diabetes, heart failure, myocardial infarction |
| Svennberg E et al., 2016, Sweden | Uppsala Longitudinal Study of Adult Men (ULSAM) | 1970—NA, 12.6 years follow-up | 883 men, age 50 years: 113 atrial fibrillation cases | Antihypertensive treatment | Yes vs. no | 2.32 (1.55–3.46) | Age, sex, smoking, BMI, systolic blood pressure, total cholesterol, HDL cholesterol, lipid-lowering treatment, type 2 diabetes, heart failure |
| Svennberg E et al., 2016, Sweden | Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) | 2001—NA, 10 years follow-up | 978 men and women, age 70 years: 148 atrial fibrillation cases | Antihypertensive treatment | Yes vs. no | 1.52 (1.05–2.21) | Age, sex, smoking, BMI, systolic blood pressure, total cholesterol, HDL cholesterol, lipid-lowering treatment, type 2 diabetes |
| O'Neal WT et al., 2017, USA | Reasons for Geographic And Racial Differences in Stroke (REGARDS) Study | 2003–2007—NA, 9.4 years follow-up | 13,688 men and women, mean age 63 years: 997 atrial fibrillation cases | Hypertension, whites | Yes vs. no | 1.28 (1.11–1.48) | Age, sex, ethnicity, income, smoking status, diabetes, obesity, exercise, dyslipidemia, left ventricular hypertrophy, cardiovascular disease |
| Hypertension, blacks | Yes vs. no | 1.24 (0.90–1.71) | |||||
| Ding L et al., 2017, China | Shandong Multi-Center Health Check-up Longitudinal Study | 2004–2014, 2.6 years follow-up | 33,186 men and women, age 45–85 years: 134 atrial fibrillation cases | Hypertension | Yes vs. no | 1.64 (1.12–2.40) | Age, sex |
| Hobbelt AH et al., 2017, Netherlands | PREVEND Study | 1997—NA, NA | 8042 men and women, mean age 48.5 years: 319 atrial fibrillation cases | Hypertension (anti-hypertensive treatment), atrial fibrillation without 2-year recurrence | Yes vs. no | 1.50 (0.88–2.56) | Age, sex, BMI, heart rate, lipid-lowering treatment, MR-proANP, eGFR |
| Hypertension (anti-hypertensive treatment), self-terminating atrial fibrillation | Yes vs. no | 2.52 (1.19–5.33) | |||||
| Hypertension (anti-hypertensive treatment), non-self-terminating atrial fibrillation | Yes vs. no | 1.33 (0.85–2.08) | |||||
| Ogunmoroti O et al., 2018, USA | Multi-Ethnic Study of Atherosclerosis | 2000–2002—NA, 11.2 years follow-up | 6506 men and women, age 45–84 years: 709 atrial fibrillation cases | Hypertension/blood pressure, all | < 120/ < 80 mmHg | 0.71 (0.58–0.87) | Age, sex, race/ethnicity, education, income, health insurance |
| 120–139/80–89 | 0.86 (0.72–1.03) | ||||||
| ≥ 140/ ≥ 90 | 1.00 | ||||||
| Hypertension/blood pressure, whites |
< 120/ < 80 mmHg 120–139/80–89 ≥ 140/ ≥ 90 |
0.70 (0.53–0.93) 0.86 (0.67–1.11) 1.00 |
|||||
| Hypertension/blood pressure, Chinese Americans |
< 120/ < 80 mmHg 120–139/80–89 ≥ 140/ ≥ 90 |
0.73 (0.42–1.29) 1.17 (0.72–1.91) 1.00 |
|||||
| Hypertension/blood pressure, African Americans |
< 120/ < 80 mmHg 120–139/80–89 ≥ 140/ ≥ 90 |
0.76 (0.46–1.25) 0.89 (0.60–1.31) 1.00 |
|||||
| Hypertension/blood pressure, Hispanic |
< 120/ < 80 mmHg 120–139/80–89 ≥ 140/ ≥ 90 |
0.64 (0.40–1.02) 0.72 (0.47–1.09) 1.00 |
|||||
| Austin TR et al., 2018, USA | Jackson Heart Study | 2005–2008–2012, 8.5 years follow-up | 5240 men and women, mean age 55 years: 242 atrial fibrillation cases | Hypertension (antihypertensive medication) | Yes vs. no | 1.52 (1.08–2.15) | Age, sex, height, weight, glycemic status, history of myocardial infarction, FEV1, eGFR, ECG PR interval, current smoking, insured, high school graduate |
| Aronson D et al., 2018, USA | Maccabi Health Services | 2005–2015, 9.7 years follow-up | 96,778 men and women, age ≥ 50 years: 5660 atrial fibrillation cases | Hypertension | Yes vs. no | 1.48 (1.39–1.57) | Age, sex, BMI, systolic blood pressure, history of myocardial infarction, peripheral arterial disease, history of heart failure, chronic obstructive pulmonary disease, inflammatory disease, age at prevalent heart failure, female with inflammatory disease |
| Khurshid S et al., 2018, United Kingdom | UK Biobank | 2006–2010–2015–2016, 7 years follow-up | 489,194 men and women, age 40–69 years: 10,619 atrial fibrillation cases | Hypertension | Yes vs. no | 1.49 (1.38–1.62) | Age, sex, race, BMI, smoking status, alcohol, hyperlipidemia, diabetes, chronic kidney disease, sleep apnea, asthma, chronic obstructive pulmonary disease, hyperthyroidism, hypothyroidism, depression, venous thromboembolism, peripheral arterial disease, stroke, coronary artery disease, heart failure |
| Kim YG et al., 2018, Korea | Ulsan University Hospital | 2003–2008–2016, 8.7 years follow-up | 21,813 men, mean age 45.9 years: 168 atrial fibrillation/ flutter cases | Hypertension | Yes vs. no | 1.35 (0.99–1.86) | Age, smoking status, regular exercise, alcohol drinking, chronic kidney disease, central obesity, raised fasting glucose, raised triglycerides, reduced HDL-cholesterol |
| Kodani E et al., 2019, Japan | TAMA MED Project-AF | 2008–2015, 6.9 years follow-up | 10,430 men and women, age 40–74 years: 133 atrial fibrillation cases | Hypertension | Yes vs. no | 1.44 (0.96–2.15) | Age, sex, cardiac disease, diabetes, LDL-cholesterol, triglycerides, GGTP, smoking status |
| Hamada R et al., 2019, Japan | Seirei Center for Health Promotion and Preventive Medicine, Hamamatsu City | 2008–2014–2015, 7 years follow-up | 65,984 men and women, age 40–79 years: 349 atrial fibrillation cases | Hypertension (antihypertensive agent use) | Yes vs. no | 1.40 (1.09–1.79) | Age, sex |
| Bose A et al., 2019, USA | Reasons for Geographic and Racial Differences in Stroke [REGARDS] Study | 2003–2007—NA, 9.3 years follow-up | 11,806 men and women, mean age 63 years: 1016 atrial fibrillation cases | Hypertension (use of blood pressure medication), men | Yes vs. no | 1.46 (1.22–1.76) | Age, race, height, weight, cardiovascular disease |
| Hypertension (use of blood pressure medication), women | Yes vs. no | 1.25 (1.00–1.56) | |||||
| Hulme OL et al., 2019, USA | Partners HealthCare System Research Patient Data Registry | 2000–2014, NA | 206,042 men and women, age 45–95 years: 7216 atrial fibrillation cases | Hypertension | Yes vs. no | 1.11 (1.05–1.18) | Age, age squared, sex, ethnicity/race, smoking, height, height squared, weight, weight squared, diastolic blood pressure, hyperlipidemia, heart failure, coronary heart disease, valvular disease, previous stroke/TIA, peripheral artery disease, chronic kidney disease, hypothyroidism |
| Feng T et al., 2019, Norway | HUNT | 2006–2008–2015, 8.1 years follow-up | 47,870 men and women, age ≥ 20 years: 1758 atrial fibrillation cases | Hypertension | No, BMI < 25.0 | 1.0 | Age, sex, height, smoking status, time since last meal, type of work, marital status, physical activity, alcohol, C-reactive protein, sex, blood glucose, triglycerides, HDL-cholesterol, abdominal obesity |
| Yes, BMI ≥ 25.0 | 1.6 (1.2–2.1) | ||||||
| No, BMI 25.0–29.9 | 1.3 (1.0–1.7) | ||||||
| Yes, BMI 25.0–29.9 | 1.7 (1.4–2.2) | ||||||
| No, BMI < 30 | 1.3 (0.9–1.9) | ||||||
| Yes, BMI ≥ 30 | 2.1 (1.6–2.8) | ||||||
| Kim YG et al., 2019, Korea | Korea National Health Insurance Database | NA-NA, 8.2 years follow-up | 9,797,418 men and women, mean age 47 years: 196,136 atrial fibrillation cases | Hypertension, age 20- < 30 | Non-hypertension | 1.00 | Age, sex, smoking status, alcohol, regular physical activity, low income, diabetes mellitus, dyslipidemia |
| Pre-hypertension | 1.14 (1.07–1.22) | ||||||
| Hypertension with med., <5 years | 1.37 (1.19–1.58) | ||||||
| Hypertension with med., <5 years | 3.67 (2.83–4.76) | ||||||
| Hypertension with med., ≥ 5 years | 6.22 (3.74–10.36) | ||||||
| Hypertension, age 30- < 40 | Non-hypertension | 1.00 | |||||
| Pre-hypertension | 1.15 (1.09–1.20) | ||||||
| Hypertension without medication | 1.42 (1.32–1.53) | ||||||
| Hypertension with medication, < 5 years | 2.72 (2.48–3.00) | ||||||
| Hypertension with medication, ≥ 5 years | 3.40 (2.88–4.02) | ||||||
| Hypertension, age 40- < 50 | Non-hypertension | 1.00 | |||||
| Pre-hypertension | 1.10 (1.07–1.13) | ||||||
| Hypertension without medication | 1.27 (1.21–1.32) | ||||||
| Hypertension with medication, < 5 years | 1.84 (1.76–1.93) | ||||||
| Hypertension with medication, ≥ 5 years | 2.13 (2.01–2.25) | ||||||
| Hypertension, age 50- < 60 | Non-hypertension | 1.00 | |||||
| Pre-hypertension | 1.05 (1.02–1.08) | ||||||
| Hypertension without medication | 1.15 (1.10–1.19) | ||||||
| Hypertension with medication, < 5 years | 1.53 (1.49–1.58) | ||||||
| Hypertension with medication, ≥ 5 years | 1.76 (1.70–1.81) | ||||||
| Hypertension, age 60- < 70 | Non-hypertension | 1.00 | |||||
| Pre-hypertension | 1.01 (0.99–1.04) | ||||||
| Hypertension without medication | 1.11 (1.07–1.15) | ||||||
| Hypertension with medication, < 5 years | 1.38 (1.34–1.43) | ||||||
| Hypertension with medication, ≥ 5 years | 1.66 (1.61–1.70) | ||||||
| Hypertension, age ≥ 70 | Non-hypertension | 1.00 | |||||
| Pre-hypertension | 0.99 (0.96–1.02) | ||||||
| Hypertension without medication | 1.06 (1.02–1.10) | ||||||
| Hypertension with medication, < 5 years | 1.30 (1.26–1.35) | ||||||
| Hypertension with medication, ≥ 5 years | 1.54 (1.49–1.58) | ||||||
| Rattani A et al., 2019, USA | Atherosclerosis Risk in Communities Study | 1987–2017, 21.4 years follow-up | 14,915 men and women, age 45–64 years: 2891 atrial fibrillation cases | Hypertension categories (JNC 7) | Normal | 1.00 | Age, sex, race, height, education, field center, BMI, smoking, drinking status, diabetes, heart failure, coronary heart disease, stroke |
| Prehypertension | 1.24 (1.12–1.36) | ||||||
| Hypertension | 1.58 (1.44–1.74) | ||||||
| Hypertension (2017 ACC/AHA) | Yes vs. no | 1.37 (1.26–1.48) | |||||
| Hypertension categories (2017 ACC/AHA) | Normal | 1.00 | |||||
| Elevated | 1.26 (1.11–1.43) | ||||||
| Stage 1 hypertension | 1.21 (1.07–1.37) | ||||||
| Stage 2 hypertension | 1.58 (1.44–1.74) | ||||||
| Abbas SS et al., 2020, Australia | Australian Longitudinal Study on Women's Health | 2000–2015, NA | 6671 women, mean age 77.79 years: 1827 atrial fibrillation cases | Hypertension | Yes vs. no | 1.24 (1.09–1.42) | Exercise, heart attack, angina, arthritis |
| Koshiyama M et al., 2021, Japan | Iwate Prefecture | 2010–2013, 3 years follow-up | 130,396 men and women, age 45- ≥ 85 years: 824 atrial fibrillation cases | Hypertension, men | Yes vs. no | 1.20 (1.01–1.43) | Age, diabetes mellitus, dyslipidemia, overweight, coronary artery disease, stroke, smoking, drinking, regular exercise |
| Hypertension, women | Yes vs. no | 1.70 (1.29–2.45) | |||||
| Ninomiya Y et al., 2021, Japan | JA Kagoshima Kouseiren Medical Health Care Center | 2008–2016, 5 years follow-up | 67,379 men and women, mean age 54 years: 280 atrial fibrillation cases | Hypertension, all | Yes vs. no | 1.75 (1.35–2.27) | Age, waist circumference, diabetes, dyslipidemia, drinking, chronic kidney disease |
| Hypertension, men | Yes vs. no | 1.46 (1.09–1.95) | |||||
| Hypertension, women | Yes vs. no | 2.69 (1.52–4.77) | |||||
| Chao TF et al., 2021, Taiwan | Taiwan Health Insurance Database | 2000–2016, 16 years follow-up | 7,220,654 men and women, age 40 years: 438,930 atrial fibrillation cases | Hypertension | Yes vs. no | 1.41 (1.40–1.42) | Age, sex, diabetes mellitus, heart failure, stroke, coronary artery disease with or without myocardial infarction, peripheral vascular diseases, COPD, autoimmune diseases, liver cirrhosis, hyperthyroidism, chronic kidney disease with or without end-stage renal disease, gout, alcoholism |
| Matsuoka S et al., 2022, Japan | JMDC Claims Database | 2005–2020, 3.3 years follow-up | 2,597,441 men and women, age 20–75 years: 12,773 atrial fibrillation cases | Hypertension, age 20–49 years | Yes vs. no | 1.68 (1.45–1.82) | Age, sex, obesity, high waist circumference, diabetes, dyslipidemia, cigarette smoking, alcohol, physical inactivity |
| Hypertension, age 50–59 years | Yes vs. no | 1.60 (1.50–1.69) | |||||
| Hypertension, 60–75 years | Yes vs. no | 1.45 (1.35–1.55) | |||||
| Shapkina M et al., 2022, Russia | HAPIEE study | 2003–2005–2017, 12.85 years follow-up | 3871 men and women, age 45–69 years: 122 atrial fibrillation cases | Hypertension | Yes vs. no | 1.13 (0.66–1.93) | Age, BMI, total cholesterol, triglycerides, smoking, alcohol, education, marital status |
| Schnabel RB et al., 2022, Germany | InGef research database | 2013–2016, | 1,476,391 men and women, age ≥ 45 years: 98,958 atrial fibrillation cases | Hypertension, treated | Yes vs. no | 1.76 (1.72–1.79) | Age, sex, treated heart failure, valvular heart disease, chronic kidney disease, stroke—not specified as hemorrhage or infarction, hemiplegia, other pulmonary heart diseases, paroxysmal tachycardia, other cardiac arrhythmias, ulcer of lower limb not elsewhere classified, personal history of medical treatment |
| Camen S et al., 2022, Europe |
6 cohort studies: DAN-MONICA study FINRISK study Moli-sani study Northern Sweden MONICA study Scottish Heart Health Extended Cohort (SHHEC) The Tromsø Study |
1982–2010, max 10 years follow-up | 108,363 men and women, median age 46.0 years: 2413 atrial fibrillation cases | Antihypertensive treatment | Yes vs. no | 1.35 (1.04–1.66) | Age, sex, cohort, BMI, total serum cholesterol, diabetes, daily smoking, systolic blood pressure |
ARB = angiotensin receptor blocker, ACE = angiotensin converting enzyme, BMI = body mass index, ECG = electrocardiography, eGFR = estimated glomerular filtration rate, FEV1 = forced expiratory volume per 1 s, GGTP = gamma glutamyl transpeptidase, HDL = high-density lipoprotein, LVH = left ventricular hypertrophy, MET = metabolic equivalent task, MR-proANP = mid-regional pro atrial natriuretic peptide, NA = not available, PR = pulse rate, ST/T = ST and T wave ratio, TIA = transient ischemic attack
Fig. 2.
Hypertension and atrial fibrillation
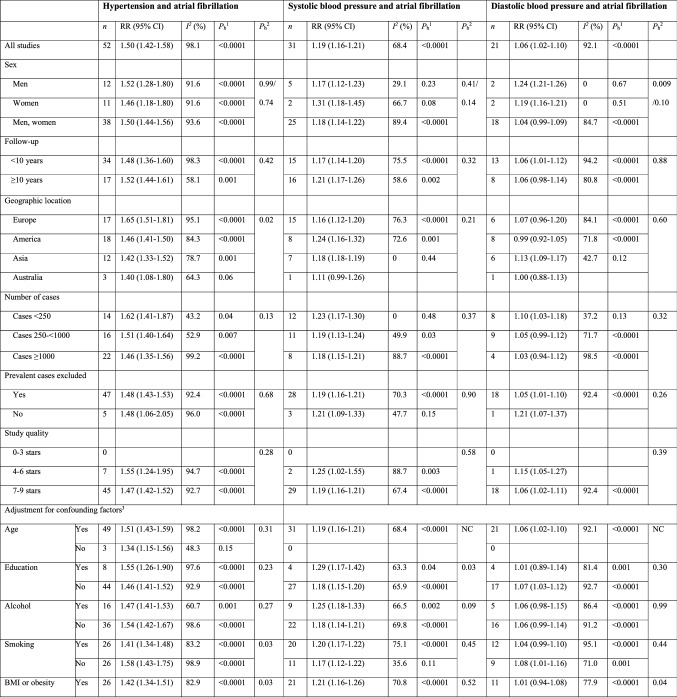
Thirty seven cohort studies (28 publications, 31 risk estimates) [8, 18, 20, 29, 33, 34, 39, 41, 42, 44, 46, 51, 55, 63, 65–67, 69–75, 77–80] (347,813 cases, 14,565,763 participants) were included in the analysis of systolic blood pressure and atrial fibrillation (Table 2). The summary RR was 1.19 (95% CI: 1.16–1.21, I2 = 68.4%, pheterogeneity < 0.0001) per 20 mmHg increment (Fig. 3a). There was no evidence of publication bias with Egger's test (p = 0.46), but some indication of asymmetry in the funnel plot (Supplementary Fig. 3a). When using the trim and fill method, eight studies were added, but the results were similar, summary RR = 1.17 (95% CI: 1.14–1.19) (Supplementary Fig. 4). The summary RR ranged from 1.18 (95% CI: 1.16–1.20) when the Atherosclerosis Risk in Communities Study [77] was excluded to 1.20 (95% CI: 1.18–1.22) when a pooled analysis [65] was excluded (Supplementary Fig. 5). Ten cohort studies [18, 34, 39, 55, 67–69, 76, 79, 80] were included in the nonlinear dose–response analysis. Although the test for nonlinearity was significant, pnonlinearity < 0.0001, the association was approximately linear, and there was a dose-dependent increase in risk with increasing systolic blood pressure from a systolic blood pressure level of 90 mmHg and above (Fig. 3b).
Table 2.
Cohort studies of blood pressure and atrial fibrillation
| First author, publication year, country | Study name or description | Study period | Number of participants, number of cases | Exposure, subgroup | Comparison | Relative risk (95% confidence interval) | Adjustment for confounders |
|---|---|---|---|---|---|---|---|
| Wilhelmsen L et al., 2001, Sweden | The Multifactor Primary Prevention Study | 1970–1973–1996, 25.2 years follow-up | 7495 men, age 47–55 years: 754 atrial fibrillation cases | SBP |
≤ 145 mmHg 146–175 ≥ 176 |
1.00 1.30 (1.11–1.54) 1.37 (1.09–1.74) |
Age |
| Friberg J et al., 2003, Denmark | Copenhagen City Heart Study |
1981–1983 - 1991–1994–1994, 7 years follow-up |
18,167 men and women, age 40–79 years: 379 atrial fibrillation cases | SBP | Per 10 mmHg | 1.0 (1.0–1.1) | Age, sex, prior myocardial infarction, diabetes mellitus, arterial hypertension, ECG-LVH voltage, ECG-LVH ST/T, height, weight, BMI, FEV1, smoking status, alcohol, period |
| Mitchell GF et al., 2007, USA | Framingham Heart Study and Framingham Offspring Study | 1979–1982/ 1983–2004, 16 years follow-up | 5331 men and women, age ≥ 35 years: 698 atrial fibrillation cases | DBP | Per 10 mmHg | 0.97 (0.88–1.06) | Age, sex, BMI, smoking, valvular disease, myocardial infarction, heart failure, diabetes, ECG—left ventricular hypertrophy, hypertension treatment |
| Conen D et al., 2009, USA | Women's Health Study | 1993–2006, 12.4 years follow-up | 34,221 women, age ≥ 45 years: 644 atrial fibrillation cases | SBP |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 1.00 (0.78–1.28) 1.28 (1.00–1.63) 1.56 (1.22–2.01) 2.74 (1.77–4.22) |
Age, BMI, diabetes, smoking, hypercholesterolemia, exercise, alcohol, education, randomized treatment assignment + mutual adjustment between systolic and diastolic blood pressure |
| DBP |
< 65 mmHg 65–74 75–84 85–89 90–94 ≥ 95 |
1.00 1.17 (0.81–1.69) 1.18 (0.84–1.65) 1.53 (1.05–2.23) 1.35 (0.82–2.22) 2.15 (1.21–3.84) |
|||||
| SBP |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 1.01 (0.77–1.33) 1.30 (0.97–1.74) 1.58 (1.16–2.16) 2.69 (1.63–4.46) |
|||||
| DBP |
< 65 mmHg 65–74 75–84 85–89 90–94 ≥ 95 |
1.00 1.14 (0.78–1.66) 1.02 (0.70–1.49) 1.11 (0.72–1.71) 0.91 (0.53–1.58) 1.22 (0.64–2.33) |
|||||
| Minami M et al., 2009, Japan | Ishikawa Prefecture | 1998–2006, NA |
69 atrial fibrillation cases (men) 138 controls (nested case–control study) |
SBP | Per 1 mmHg | 1.019 (1.002–1.037) | Age, time period, BMI, total cholesterol, GGTP, uric acid, fasting plasma glucose, hemoglobin, cardiomegaly, Brinkman index, drinking habits |
| Chamberlain AM et al., 2011, USA | Atherosclerosis Risk in Communities Study | 1987–1989–1998, 10 years follow-up | 14,546 men and women, age 45–64 years: 515 atrial fibrillation cases | SBP |
< 100 mmHg 100- < 120 120- < 140 140- < 160 ≥ 160 |
0.82 (0.53–1.28) 1.00 1.42 (1.15–1.76) 2.16 (1.67–2.79) 2.63 (1.83–3.78) |
Age, sex, race |
| DBP |
< 70 mmHg 70- < 80 80- < 90 90 < 100 ≥ 100 |
0.93 (0.75–1.15) 1.00 1.24 (0.98–1.57) 1.53 (1.06–2.22) 2.02 (1.20–3.41) |
|||||
| Grundvold I et al., 2012, Norway | Oslo 1972–1975 | 1972–1975–2007, 30 years follow-up | 2014 men, age 40–59 years: 270 atrial fibrillation cases | SBP |
88–116 mmHg 118–126 128–138 140–220 Per 18 mmHg |
1.00 1.26 (0.74–2.14) 1.98 (1.22–3.27) 1.84 (1.07–3.19) 1.22 (1.10–1.42) |
Age, BMI, left ventricular hypertrophy, smoking, total cholesterol, physical fitness, resting heart rate |
| DBP |
54–78 mmHg 80–86 88–92 94–130 Per 10 mmHg |
1.00 1.67 (1.00–2.85) 1.76 (1.01–3.11) 2.36 (1.38–4.15) 1.25 (1.11–1.43) |
|||||
| Alonso A et al., 2013, USA | Cardiovascular Health Study | 1989–1990, 1992–1993–2000, NA years of follow-up | 5043 men and women, age 65 years: 624 atrial fibrillation cases | SBP, whites | Per 20 mmHg | 1.19 (1.10–1.28) | Age, sex |
| SBP, African Americans | Per 20 mmHg | 1.15 (0.92–1.42) | |||||
| DBP, whites | Per 10 mmHg | 1.00 (0.93–1.08) | |||||
| DBP, African Americans | Per 10 mmHg | 0.90 (0.72–1.13) | |||||
| Alonso A et al., 2013, USA | Atherosclerosis Risk in Communities Cohort | 1987–1989–1996–1998, -2005, NA years of follow-up | 10,675 men and women, age 45–64 years: 419 atrial fibrillation cases | SBP, whites | Per 20 mmHg | 1.14 (1.02–1.28) | Age, sex |
| SBP, African Americans | Per 20 mmHg | 0.98 (0.78–1.24) | |||||
| DBP, whites | Per 10 mmHg | 0.89 (0.80–0.99) | |||||
| DBP, African Americans | Per 10 mmHg | 1.04 (0.84–1.29) | |||||
| Alonso A et al., 2013, Iceland | Age, Gene/ Environment Susceptibility Reykjavik Study | 2002–2011, NA years of follow-up | 4469 men and women, mean age 76 years: 408 atrial fibrillation cases | SBP | Per 20 mmHg | 1.09 (1.00–1.19) | Age, sex |
| DBP | Per 10 mmHg | 0.97 (0.88–1.08) | |||||
| Alonso A et al., 2013, Netherlands | Rotterdam Study | 1997–2005, NA years of follow-up | 3203 men and women, mean age 72 years: 177 atrial fibrillation cases | SBP | Per 20 mmHg | 1.21 (1.05–1.38) | Age, sex |
| DBP | Per 10 mmHg | 1.02 (0.89–1.17) | |||||
| Roetker NS et al., 2014, USA | Multi-Ethnic Study of Atherosclerosis | 2000–2002–2009, 7.8 years follow-up | 6630 men and women, age 45–84 years: 307 atrial fibrillation cases | SBP | Per 21.5 mmHg | 1.16 (1.03–1.31) | Age, sex, race/ethnicity, site, education, height, BMI, smoking status, antihypertensive medication use, diabetes, ECG-based LVH, P-R interval, resting heart rate |
| DBP | Per 10.3 mmHg | 1.00 (0.88–1.13) | |||||
| Knuiman M et al., 2014, Australia | Busselton Health Study | 1994–1995–2010, 15 years follow-up | 4267 men and women, age 25–84 years: 343 atrial fibrillation cases | SBP | Per 17.8 mmHg | 1.10 (0.99–1.23) |
Age, sex, height + hypertension treatment, BMI |
| DBP | Per 10 mmHg | 1.04 (0.93–1.15) | |||||
| SBP | Per 17.8 mmHg | 1.02 (0.91–1.14) | |||||
| DBP | Per 10 mmHg | 0.96 (0.87–1.07) | |||||
| Schnabel RB et al., 2015, USA | Framingham Cohort Study | 1958–2007, 50 years follow-up | 9511 men and women, age 50–89 years: 1544 atrial fibrillation cases | SBP, 1958–1967 |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 1.76 (0.59–5.25) 1.29 (0.42–3.95) 1.95 (0.73–5.21) 2.63 (1.00–6.93) |
Age, sex |
| SBP, 1968–1977 |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 0.53 (0.26–1.07) 0.79 (0.43–1.45) 1.39 (0.83–2.34) 1.36 (0.79–2.32) |
|||||
| SBP, 1978–1987 |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 0.75 (0.46–1.21) 0.97 (0.63–1.49) 1.16 (0.79–1.72) 1.21 (0.79–1.85) |
|||||
| SBP, 1988–1997 |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 1.11 (0.76–1.61) 1.27 (0.89–1.81) 1.47 (1.06–2.03) 1.28 (0.89–1.83) |
|||||
| SBP, 1998–2007 |
< 120 mmHg 120–129 130–139 140–159 ≥ 160 |
1.00 0.94 (0.69–1.29) 1.19 (0.89–1.60) 0.89 (0.67–1.19) 1.15 (0.84–1.58) |
|||||
| Pfister R et al., 2015, United Kingdom | EPIC-Norfolk | 1993–1997–2009, 5 years follow-up (analysis restricted to first 5 years) | 24,020 men and women, age 39–79 years: 236 atrial fibrillation cases | SBP | Per 1 mmHg | 1.01 (0.99–1.03) | Age, height, weight, current smoking, hypertension treatment, diabetes mellitus, heart failure, myocardial infarction |
| DBP | Per 1 mmHg | 0.99 (0.97–1.01) | |||||
| Kokubo Y et al., 2015, Japan | Suita Study | 1989–2006–2015, 12.8 years follow-up | 6906 men and women, age 30–79 years: 253 atrial fibrillation cases | SBP |
< 120 mmHg 120–139 ≥ 140 |
1.00 1.29 (0.91–1.85) 1.74 (1.22–2.49) |
Age, sex, BMI, hypercholesterolemia, diabetes, smoking status, drinking status, cohort groups, chronic kidney disease, stroke, coronary heart disease, chronic heart failure, premature contractions + mutual adjustment between systolic and diastolic blood pressure |
| DBP |
< 80 mmHg 80–89 ≥ 90 |
1.00 1.16 (0.84–1.61) 1.47 (1.08–1.99) |
|||||
| SBP |
< 120 mmHg 120–139 ≥ 140 |
1.00 1.29 (0.88–1.90) 1.74 (1.12–2.69) |
|||||
| DBP |
< 80 mmHg 80–89 ≥ 90 |
1.00 1.03 (0.73–1.46) 1.14 (0.77–1.69) |
|||||
| Svennberg E et al., 2016, Sweden | Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) | 2001—NA, 10 years follow-up | 978 men and women, age 70 years: 148 atrial fibrillation cases | SBP | Per 22.5 mmHg | 1.03 (0.86–1.23) | Age, sex, smoking, BMI, antihypertensive treatment, total cholesterol, HDL cholesterol, lipid-lowering treatment, type 2 diabetes |
| Kolek MJ et al., 2016, USA | Vanderbilt University Medical Center Electronic Medical Records | 2005–2010, 5 years follow-up | 33,494 men and women, age ≥ 40 years: 2455 atrial fibrillation cases | SBP | Per 20 mmHg | 1.22 (1.15–1.30) | Age, race, height, weight, smoking status, systolic blood pressure, diastolic blood pressure, diabetes, heart failure, myocardial infarction |
| DBP | Per 10 mmHg | 0.90 (0.85–0.96) | |||||
| Emdin CA et al., 2017, United Kingdom | UK General Practice Research Database | 1990–2013—NA, 6.9 years follow-up | 4,301,349 men and women, age 30–90 years: 128,468 atrial fibrillation cases | SBP, all | Per 20 mmHg | 1.21 (1.19–1.22) | Age, sex, smoking status, diabetes |
| SBP, men | Per 20 mmHg | 1.16 (1.14–1.18) | |||||
| SBP, women | Per 20 mmHg | 1.26 (1.24–1.28) | |||||
| DBP, all | Per 10 mmHg | 1.21 (1.19–1.23) | |||||
| DBP, men | Per 10 mmHg | 1.24 (1.21–1.26) | |||||
| DBP, women | Per 10 mmHg | 1.19 (1.16–1.21) | |||||
| Ding L et al., 2017, China | Shandong Multi-Center Health Check-up Longitudinal Study | 2004–2014, 2.6 years follow-up | 33,186 men and women, age 45–85 years: 134 atrial fibrillation cases |
SBP Diastolic blood pressure |
Per 20.75 mmHg Per 12.82 mmHg |
1.34 (1.14–1.59) 1.28 (1.08–1.51) |
Age, sex |
| Perkiomaki JS et al., 2017, Finland | Oulu Project Elucidating Risk of Atherosclerosis study | 1990–1993–2009, 16.4 years follow-up | 903 men and women, age 40–59 years: 91 atrial fibrillation cases | Mean ambulatory SBP | Per 5 mmHg | 1.09 (1.01–1.17) | Age, sex, height, BMI, ALAT, uric acid, smoking—pack-years |
| Austin TR et al., 2018, USA | Jackson Heart Study | 2005–2008–2012, 8.5 years follow-up | 5240 men and women, mean age 55 years: 242 atrial fibrillation cases | SBP | Per 20 mmHg | 1.23 (1.05–1.45) | Age, sex, height, weight, glycemic status, history of myocardial infarction, FEV1, eGFR, ECG PR interval, current smoking, insured, high school graduate |
| DBP | Per 10 mmHg | 0.99 (0.83–1.18) | |||||
| Tikhonoff V et al., 2018, Belgium, Italy, Poland, Russia, Czech Republic | FLEMENGHO and EPOGH | 1985—NA, 14 years follow-up | 3956 men and women, mean age 43.1 years: 143 atrial fibrillation cases | SBP | Per 17.1 mmHg | 1.19 (0.99–1.43) | Age, sex, BMI, serum cholesterol, tobacco, alcohol, history of cardiovascular disease, diabetes mellitus, antihypertensive treatment |
| DBP | Per 10.9 mmHg | 1.10 (0.90–1.34) | |||||
| Hamada R et al., 2019, Japan | Seirei Center for Health Promotion and Preventive Medicine, Hamamatsu City | 2008–2014–2015, NA | 65,984 men and women, age 40–79 years: 349 atrial fibrillation cases | SBP | Per 15.4 mmHg | 1.19 (1.07–1.32) | Age, sex |
| DBP | Per 10.8 mmHg | 1.22 (1.10–1.36) | |||||
| Kim YG et al., 2019, Korea | Korea National Health Insurance Database | NA-NA, 8.2 years follow-up | 9,797,418 men and women, mean age 47 years: 196,136 atrial fibrillation cases | SBP |
< 120 mmHg 120–139 140–159 ≥ 160 Per 5 mmHg |
1.00 1.17 (1.16–1.19) 1.42 (1.40–1.44) 1.64 (1.61–1.68) 1.043 (1.042–1.045) |
Age, sex, smoking status, alcohol, regular physical activity, low income, diabetes mellitus, dyslipidemia |
| DBP |
< 80 mmHg 80–89 90–99 ≥ 100 Per 5 mmHg |
1.00 1.14 (1.13–1.15) 1.26 (1.24–1.28) 1.36 (1.33–1.39) 1.046 (1.044–1.048) |
|||||
| Wong JA et al., 2020, Sweden | Malmo Preventive Project | 1974–1992–2013, 27.6 years follow-up |
32,625 men and women, mean age 45.7 years: 3277 atrial fibrillation cases (no heart failure) 1153 atrial fibrillation cases (with heart failure) |
SBP, atrial fibrillation (no heart failure) | Per 10 mmHg | 1.08 (1.06–1.10) | Age, sex, height, BMI, current smoking, prevalent coronary event, prevalent diabetes |
| SBP, atrial fibrillation (with heart failure) | Per 10 mmHg | 1.20 (1.16–1.24) | |||||
| Wong JA et al., 2020, Sweden | Malmo Diet and Cancer Study | 1991–1996–2014, 17.7 years follow-up |
27,695 men and women, mean age 58.2 years: 3167 atrial fibrillation cases (no heart failure) 890 atrial fibrillation cases (with heart failure) |
SBP, atrial fibrillation (no heart failure) | Per 10 mmHg | 1.07 (1.05–1.09) | Age, sex, height, BMI, current smoking, prevalent coronary event, prevalent diabetes |
| SBP, atrial fibrillation (with heart failure) | Per 10 mmHg | 1.13 (1.09–1.16) | |||||
| Igarashi Y et al., 2021, Japan | Morinomiyako Occupational Health Center, Miyagi Prefecture | 2013–2016—NA, 3 years follow-up | 37,562 men and women, age ≥ 40 years: 135 atrial fibrillation cases | DBP | Per 1 mmHg | 1.02 (1.00–1.03) | Age, sex, waist circumference, diabetes, logγ-GTP, Minnesota codes |
| Espnes H et al., 2021, Norway | The Tromsø Study | 1994–1995–2016, 17.6 years follow-up | 24,804 men and women, age 25–97 years: 914 atrial fibrillation cases | SBP, paroxysmal/persistent atrial fibrillation, men |
100 mmHg 110 120 130 140 150 160 170 180 |
0.81 (0.73–0.88) 0.90 (0.86–0.94) 1.00 1.11 (1.07–1.16) 1.24 (1.13–1.35) 1.38 (1.21–1.57) 1.53 (1.29–1.82) 1.70 (1.37–2.12) 1.90 (1.46–2.46) |
Age, BMI, total cholesterol, current smoking, leisure-time physical activity, history of myocardial infarction, angina pectoris, stroke, diabetes mellitus |
| SBP, paroxysmal/persistent atrial fibrillation, women |
100 mmHg 110 120 130 140 150 160 170 180 |
0.56 (0.45–0.69) 0.78 (0.71–0.85) 1.00 1.22 (1.13–1.31) 1.43 (1.25–1.62) 1.62 (1.36–1.93) 1.79 (1.45–2.22) 1.95 (1.53–2.50) 2.10 (1.60–2.76) |
|||||
| SBP, permanent atrial fibrillation, men |
100 mmHg 110 120 130 140 150 160 170 180 |
0.92 (0.83–1.01) 0.96 (0.91–1.00) 1.00 1.04 (1.00–1.10) 1.09 (0.99–1.20) 1.14 (0.99–1.31) 1.19 (0.99–1.44) 1.25 0.98–1.58) 1.30 (0.98–1.73) |
|||||
| SBP, permanent atrial fibrillation, women |
100 mmHg 110 120 130 140 150 160 170 180 |
0.63 (0.49–0.80) 0.82 (0.74–0.91) 1.00 1.17 (1.08–1.27) 1.32 (1.15–1.53) 1.46 (1.20–1.78) 1.59 (1.25–2.02) 1.70 (1.29–2.24) 1.80 (1.33–2.44) |
|||||
| Matsumoto K et al., 2021, USA | Cardiovascular Abnormalities and Brain Lesions (CABL) Study (derived from Northern Manhattan Study) | 1993–2001—NA, 9.5 years follow-up | 769 men and women, mean age 70.5 years: 83 atrial fibrillation cases | Office SBP | Per 10 mmHg | 0.96 (0.82–1.11) | Age, sex, race, hypertension status at baseline, number of antihypertensive drugs |
| Office DBP | Per 10 mmHg | 1.02 (0.80–1.29) | |||||
| Central SBP | Per 10 mmHg | 1.11 (1.00–1.25) | |||||
| Central DBP | Per 10 mmHg | 1.10 (0.85–1.43) | |||||
| 24-h SBP | Per 10 mmHg | 1.27 (1.09–1.49) | |||||
| 24-h diastolic blood pressure | Per 10 mmHg | 1.24 (0.90–1.70) | |||||
| Daytime SBP | Per 10 mmHg | 1.24 (1.06–1.45) | |||||
| Daytime DBP | Per 10 mmHg | 1.18 (0.87–1.59) | |||||
| Night-time SBP | Per 10 mmHg | 1.24 (1.08–1.43) | |||||
| Night-time DBP | Per 10 mmHg | 1.24 (0.94–1.65) | |||||
| Lind L et al., 2021, Sweden | Uppsala Longitudinal Study of Adult Men (ULSAM) | 1970–1974–2014, NA | 2322 men, age 50 years: 556 atrial fibrillation cases | SBP | Per 10 mmHg | 1.16 (1.05–1.26) | Triglycerides, HDL cholesterol, LDL cholesterol, BMI, diabetes, smoking |
| Liao LZ et al., 2021, USA | Atherosclerosis Risk in Communities Study | 1987–1989–2011–2013, 24.1 years follow-up | 9474 men and women, age: 1414 atrial fibrillation cases | SBP | Per 10 mmHg | 1.17 (1.12–1.22) | Age, sex, race, BMI, smoking, drinking, education, sport, heart failure, coronary heart disease, diabetes, creatine, HDL-cholesterol, LDL-cholesterol, triglycerides, glucose, statin use, aspirin, anticoagulants |
| DBP | Per 10 mmHg | 0.90 (0.84–0.97) | |||||
| Hata J et al., 2021, Japan | The Hisayama Study | 1988–2012, 24 years follow-up | 2442 men and women, age 40 years: 230 atrial fibrillation cases | SBP |
< 120 mmHg 120–129 130–139 140–159 160–179 ≥ 180 |
1.00 1.21 (0.77–1.90 1.43 (0.91–2.24) 1.49 (0.96–2.30) 1.89 (1.10–3.23) 2.12 (1.07–4.17) |
Age, sex, fasting plasma glucose, serum HDL cholesterol, serum non-HDL cholesterol, serum triglycerides, BMI, waist circumference, eGFR, coronary artery disease, abnormal cardiac murmur, high R-wave amplitude on ECG, arrhythmia other than atrial fibrillation, smoking status and cigarettes per day, alcohol intake, regular exercise Age, sex |
| DBP |
< 80 mmHg 80–84 85–89 90–99 100–109 ≥ 110 |
1.00 1.07 (0.75–1.54) 2.00 (1.35–2.96) 1.14 (0.74–1.75) 2.67 (1.38–5.18) 1.98 (0.49–8.05) |
|||||
| Shapkina M et al., 2022, Russia | HAPIEE study | 2003–2005–2017, 12.85 years follow-up | 3871 men and women, age 45–69 years: 122 atrial fibrillation cases | SBP | Per 1 mmHg | 1.01 (1.00–1.02) | Age, BMI, total cholesterol, triglycerides, smoking, alcohol |
| Son MK et al., 2022, Korea | Korean Genome and Epidemiology Study | 2001–2002–2018, 13.1 years follow-up | 9049 men and women, age 40–69 years: 182 atrial fibrillation cases | SBP |
< 120 mmHg 120–139 ≥ 140 |
1.00 1.22 (0.87–1.69) 1.54 (1.01–2.35) |
Age, sex, area, obesity and central obesity, leisure-time physical activity, chronic kidney disease, cardiovascular disease, HbA1c, total cholesterol |
| Camen S et al., 2022, Europe |
6 cohort studies: DAN-MONICA study FINRISK study Moli-sani study Northern Sweden MONICA study Scottish Heart Health Extended Cohort (SHHEC) The Tromsø Study |
1982–2010, max 10 years follow-up | 108,363 men and women, median age 46.0 years: 2413 atrial fibrillation cases | SBP | Per 10 mmHg | 1.03 (1.01–1.05) | Age, sex, cohort, BMI, total serum cholesterol, diabetes, daily smoking, antihypertensive treatment, stroke |
ALAT = alanin aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, ECG = electrocardiography, eGFR = estimated glomerular filtration rate, FEV1 = forced expiratory volume per 1 s, GGTP = gamma glutamyl transpeptidase, HDL = high-density lipoprotein, LVH = left ventricular hypertrophy, NA = not available, PR = pulse rate, SBP = systolic blood pressure, ST/T = ST and T wave ratio
Fig. 3.
Systolic and diastolic blood pressure and atrial fibrillation, linear and nonlinear dose–response analysis
Twenty three cohort studies (19 publications, 21 risk estimates) [8, 29, 33, 39, 41, 44, 46, 51, 55, 67, 69, 70, 72–74, 77, 79, 81, 82] (333,901 cases, 14,387,470 participants) were included in the analysis of diastolic blood pressure and atrial fibrillation. The summary RR was 1.06 (95% CI: 1.02–1.10, I2 = 92.1%, pheterogeneity < 0.0001) per 10 mmHg increment (Fig. 3c). There was no evidence of publication bias with Egger's test (p = 0.55) or by inspection of the funnel plot (Supplementary Fig. 6). The summary RR ranged from 1.05 (95% CI: 1.00–1.10) when excluding the UK GPRD study [72] to 1.07 (95% CI: 1.03–1.11) when excluding a study at Vanderbilt University [41] (Supplementary Fig. 7). Six cohort studies [39, 55, 67–69, 79] were included in the nonlinear dose–response analysis of diastolic blood pressure and atrial fibrillation. There was evidence of nonlinearity (pnonlinearity < 0.0001) with a slightly steeper increase in risk at lower levels of diastolic blood pressure than at higher levels, however, there was an increased risk from a diastolic blood pressure level of around 60 mmHg (Fig. 3d).
Subgroup and sensitivity analyses
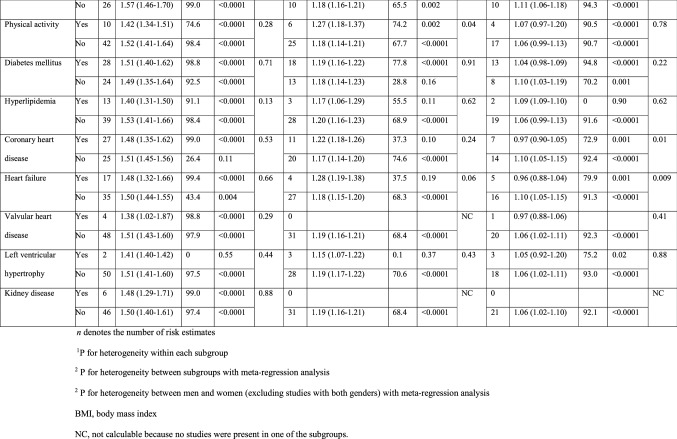
There were positive associations between hypertension and risk of atrial fibrillation across all subgroup analyses defined by sex, duration of follow-up, geographic location, number of cases, whether prevalent cases were excluded or not, study quality and adjustment for confounding (and in some cases potentially mediating) factors (including age, education, alcohol, smoking, BMI, physical activity, diabetes, hyperlipidemia, coronary heart disease, heart failure, valvular heart disease, left ventricular hypertrophy, and kidney disease), although the number of studies was small in some subgroups (Table 3). With meta-regression analyses there was some indication of heterogeneity between some subgroups for hypertension, with a stronger association among European studies than studies from the other geographic locations (p = 0.02), and a weaker association among studies with adjustment for smoking (p = 0.03) when compared to those without such adjustment. Further subgroup analyses by ethnicity showed summary RRs of 1.53 (95% CI: 1.29–1.80, I2 = 70.4%, n = 5) for Caucasians [26, 29, 45, 59] and 1.35 (95% CI: 1.16–1.59, I2 = 7.5%, n = 6) for African Americans [26, 29, 45, 46, 59] with no significant heterogeneity between subgroups (p = 0.77) (Supplementary Fig. 8).
Table 3.
Subgroup analyses of hypertension, systolic and diastolic blood pressure and atrial fibrillation
There was evidence of heterogeneity in the subgroup analysis of systolic blood pressure and atrial fibrillation when stratified by adjustment for education (p = 0.03) and physical activity (p = 0.04) with stronger associations among studies with compared to without such adjustments, however, relatively few studies made such adjustments. For diastolic blood pressure there was heterogeneity in analyses stratified by sex (p = 0.009) and by adjustment for BMI or obesity (p = 0.04), coronary heart disease (p = 0.01), and heart failure (p = 0.009). However, the association was weaker in studies of both sexes combined than in studies among either men or women, and there was no heterogeneity when comparing men with women (and excluding studies in both sexes combined) (Table 3). There was no association in studies that adjusted for BMI or obesity, coronary heart disease, or heart failure, but a positive association in studies that did not make such adjustments (Table 3).
Mean (median) study quality scores were 7.7 (8.0) for the analysis of hypertension, 7.8 (8.0) for systolic blood pressure, and 7.8 (8.0) for diastolic blood pressure.
Discussion
This meta-analysis of cohort studies suggests that persons with hypertension have a 50% increase in the relative risk of developing atrial fibrillation compared to persons without hypertension. There was a 19% increase in the relative risk of atrial fibrillation per 20 mmHg increase in systolic blood pressure and 6% increase in relative risk per 10 mmHg of diastolic blood pressure. Although the test for nonlinearity was significant both for systolic and diastolic blood pressure in relation to atrial fibrillation, the association with systolic blood pressure appeared to be approximately linear, while the association for diastolic blood pressure was nonlinear with a slightly steeper increase in risk at lower levels than at higher levels of diastolic blood pressure. However, there was an increased risk even within what is considered the normal blood pressure range and the lowest risk was observed at a systolic and diastolic blood pressure of 90/60 mmHg, respectively, while there was a 1.8–2.3 fold increase in risk at the high end of systolic and diastolic blood pressure around 180/110 mmHg. Positive associations were observed both in men and women, and among European, American, Asian and Australian studies, however, data from other regions are lacking. In the few studies that reported results stratified by ethnicity, there was a positive association between hypertension and atrial fibrillation among both Caucasians and African Americans. Our findings of an increased risk of atrial fibrillation with higher systolic and diastolic blood pressure are partly consistent with several recent Mendelian Randomization (MR) studies [95–97], as well as a randomized open-label trial which found a 54% reduction in risk of new-onset atrial fibrillation among participants allocated to tight vs usual blood systolic blood pressure control (target of < 130 mmHg and < 140 mmHg, respectively) [98], suggesting a possible causal relation between elevated blood pressure and atrial fibrillation. The MR studies reported stronger associations between blood pressure and atrial fibrillation when compared to the current analysis with 17–19% vs. 9% increases in risk of atrial fibrillation per 10 mmHg increase in systolic blood pressure and 25–29% vs. 6% increases in risk of atrial fibrillation per 10 mmHg increase in diastolic blood pressure, respectively. The stronger associations observed in the MR studies could be due to a stronger impact of lifelong elevated blood pressure that may be better captured in the MR studies, and potential overadjustment for intermediate risk factors in some of the observational studies. We did not observe significant differences in the association between hypertension or blood pressure and atrial fibrillation by sex, in contrast to what has been previously observed for cardiovascular disease incidence [99], but consistent with that observed for stroke [100] and cardiovascular disease mortality [99]. This suggests that for the prevention of atrial fibrillation, blood pressure lowering may be equally important among men and women.
Several biological pathways could explain an increased risk of atrial fibrillation in patients with hypertension. Elevated blood pressure increases the risk of coronary heart disease and heart failure [101], conditions that predisposes to atrial fibrillation [29, 34, 47, 48]. Hypertension induces structural remodelling of the left atrium with excessive fibroblast proliferation, and fibroblasts can switch and proliferate to myofibroblasts which have a higher profibrotic potential and also contribute to collagen accumulation [102]. Epidemiological studies have shown that elevated blood pressure predisposes to left ventricular hypertrophy [103–106], which again increases the risk of atrial fibrillation [17, 20, 29, 34, 41, 51]. It also stimulates apoptosis and inflammation of the cardiomyocytes, leading to fibrosis and left ventricular hypertrophy. Activation of the renin–angiotensin–aldosterone system and autonomic dysregulation are major factors behind these changes. Long-term hypertension can through ventricular thickening, left ventricular hypertrophy and impaired left ventricular systolic-diastolic function increase atrial pressure, ultimately leading to atrial stretch, enlargement and deterioration of atrial contraction [102]. Dysregulation of the autonomic nervous system may also contribute to the development of atrial fibrillation and it has been shown that both sympathetic and parasympathetic overactivation may trigger atrial fibrillation [102].
The present systematic review and meta-analysis has some limitations that need to be discussed. Persons with hypertension often have less healthy lifestyles than persons without hypertension, including higher BMI, less physical activity and they may be more likely to smoke. Several of the included studies adjusted for the most important confounding factors and the results persisted across most subgroup analyses, and we found little evidence of heterogeneity between these subgroups. However, we cannot exclude the possibility that residual confounding could partly explain the results. There was very high heterogeneity in the analyses of hypertension and diastolic blood pressure and moderately high heterogeneity in the analysis of systolic blood pressure and this persisted in many of the subgroup analyses, but there was lower heterogeneity in studies with a longer duration of follow-up. The heterogeneity observed appeared to be more driven by differences in the effect sizes rather than differences in the direction of the association, as all except one study found positive associations between hypertension or systolic blood pressure and atrial fibrillation. Since the studies only had one baseline assessment of hypertension status or blood pressure, regression dilution bias could have attenuated the association between hypertension or blood pressure and risk of atrial fibrillation. Some participants with high blood pressure at baseline may have undergone subsequent treatment for high blood pressure with pharmaceutical medications or lifestyle changes, which would have lowered their blood pressure, but any such effects would most likely have led to conservative estimates of the associations between hypertension and blood pressure and risk of atrial fibrillation. Some of the included studies may also have over-adjusted by including hypertension status and blood pressure in the same models, adjusting rather than stratifying for blood pressure treatment, and/or by adjusting for potentially intermediary conditions such as coronary heart disease, heart failure, valvular heart disease, and left ventricular hypertrophy in the multivariable models. Any further studies might want to adjust for potential confounders and mediators separately to evaluate the impact of both on the observed associations.
Strengths of the present meta-analysis include (1) the cohort design of the included studies (which avoids recall bias and reduces the potential for selection bias), (2) the detailed subgroup and sensitivity analyses, (3) the very large sample size with 14.3–30.5 million participants and 333,000 to 1,080,000 cases providing a more robust estimate of the association between blood pressure and hypertension and risk of atrial fibrillation than most individual studies, and (4) the detailed dose–response analyses. Our findings have important clinical and public health implications as the number of people with hypertension worldwide increased from 594 million in 1975 to 1.13 billion in 2015, mainly due to population growth and ageing, but also due to lifestyle factors [107]. This increase in the number of people with hypertension may at least have partly contributed to increased rates of atrial fibrillation. Routine screening for hypertension and lifestyle interventions to reduce blood pressure that emphasize healthy diets, physical activity, weight control and proper pharmaceutical treatment of hypertension may therefore also reduce the risk of atrial fibrillation as well as other cardiovascular complications.
Conclusion
In conclusion, this meta-analysis suggests that people with hypertension have a 50% increase in the relative risk of developing atrial fibrillation compared to those without hypertension. Increasing systolic and diastolic blood pressure even within the normal range was associated with increased risk and at the high end was associated with a twofold increase in risk. These results strongly support a role of elevated blood pressure in the development of atrial fibrillation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
DA designed the research, conducted the literature search and analyses and wrote the first draft of the manuscript. DA, YMS, EK, and TF did the literature screening. All authors interpreted the data, revised the subsequent drafts for important intellectual content, read and approved the final manuscript. This work has been supported by funding from the South-East Regional Health Authority of Norway (DA), Norwegian Heart and Lung Association (TF), and Liaison Committee for Education, Research and Innovation in Central Norway (TF). The authors declare that there is no duality of interest associated with this manuscript. D. Aune takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;6(354):i4482. doi: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 6.Liu DS, Chen J, Jian WM, Zhang GR, Liu ZR. The association of atrial fibrillation and dementia incidence: a meta-analysis of prospective cohort studies. J Geriatr Cardiol. 2019;16(3):298–306. doi: 10.11909/j.issn.1671-5411.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conen D, Glynn RJ, Sandhu RK, Tedrow UB, Albert CM. Risk factors for incident atrial fibrillation with and without left atrial enlargement in women. Int J Cardiol. 2013;168(3):1894–1899. doi: 10.1016/j.ijcard.2012.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knuiman M, Briffa T, Divitini M, Chew D, Eikelboom J, McQuillan B, Hung J. A cohort study examination of established and emerging risk factors for atrial fibrillation: the Busselton Health Study. Eur J Epidemiol. 2014;29(3):181–190. doi: 10.1007/s10654-013-9875-y. [DOI] [PubMed] [Google Scholar]
- 9.Diouf I, Magliano DJ, Carrington MJ, Stewart S, Shaw JE. Prevalence, incidence, risk factors and treatment of atrial fibrillation in Australia: the Australian Diabetes, Obesity and Lifestyle (AusDiab) longitudinal, population cohort study. Int J Cardiol. 2016;15(205):127–132. doi: 10.1016/j.ijcard.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Aune D, Feng T, Schlesinger S, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complicat. 2018;32(5):501–511. doi: 10.1016/j.jdiacomp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol. 2018;25(13):1437–1451. doi: 10.1177/2047487318780435. [DOI] [PubMed] [Google Scholar]
- 12.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64(3):281–289. doi: 10.1016/j.jacc.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Sen A, Schlesinger S, Norat T, Janszky I, Romundstad P, Tonstad S, Riboli E, Vatten LJ. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol 2017. [DOI] [PMC free article] [PubMed]
- 14.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK biobank study. Circulation. 2018;137(24):2583–2591. doi: 10.1161/CIRCULATIONAHA.117.032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thelle DS, Selmer R, Gjesdal K, Sakshaug S, Jugessur A, Graff-Iversen S, Tverdal A, Nystad W. Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309,540 men and women. Heart. 2013;99(23):1755–1760. doi: 10.1136/heartjnl-2013-303825. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250(5):382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 20.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14(6):666–672. doi: 10.1097/01.ede.0000091649.26364.c0. [DOI] [PubMed] [Google Scholar]
- 21.Ruigomez A, Johansson S, Wallander MA, Rodriguez LA. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. J Clin Epidemiol. 2002;55(4):358–363. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 22.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Young KO, Sung J, Kim JH, Song YB, Lee W-S, Choi J-O, Shin D-H, Cho S-W, Choi JH, Hahn J-Y, Kim JS. Risk factors for predicting new-onset atrial fibrillation in persons who received health screening tests. Korean Circulat J. 2007;37(12):609–615. [Google Scholar]
- 24.Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32(10):1851–1856. doi: 10.2337/dc09-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25(2):95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 26.Lipworth L, Okafor H, Mumma MT, Edwards TL, Roden DM, Blot WJ, Darbar D. Race-specific impact of atrial fibrillation risk factors in blacks and whites in the southern community cohort study. Am J Cardiol. 2012;110(11):1637–1642. doi: 10.1016/j.amjcard.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JG, Melander O, Sjogren M, Hedblad B, Engstrom G, Newton-Cheh C, Platonov PG. Genetic polymorphisms confer risk of atrial fibrillation in patients with heart failure: a population-based study. Eur J Heart Fail. 2013;15(3):250–257. doi: 10.1093/eurjhf/hfs176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyrnes A, Mathiesen EB, Njolstad I, Wilsgaard T, Lochen ML. Palpitations are predictive of future atrial fibrillation. An 11-year follow-up of 22,815 men and women: the Tromso Study. Eur J Prev Cardiol. 2013;20(5):729–36. doi: 10.1177/2047487312446562. [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoller B, Li X, Sundquist J, Sundquist K. Neighbourhood deprivation and hospitalization for atrial fibrillation in Sweden. Europace. 2013;15(8):1119–1127. doi: 10.1093/europace/eut019. [DOI] [PubMed] [Google Scholar]
- 31.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative Observational Study. Heart. 2013;99(16):1173–1178. doi: 10.1136/heartjnl-2013-303798. [DOI] [PubMed] [Google Scholar]
- 32.Sano F, Ohira T, Kitamura A, Imano H, Cui R, Kiyama M, Okada T, Yamagishi K, Sankai T, Tanigawa T, Kario K, Iso H. Heavy alcohol consumption and risk of atrial fibrillation. The Circulatory Risk in Communities Study (CIRCS) Circ J. 2014;78(4):955–61. doi: 10.1253/circj.cj-13-1387. [DOI] [PubMed] [Google Scholar]
- 33.Pfister R, Bragelmann J, Michels G, Wareham NJ, Luben R, Khaw KT. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015;22(7):932–939. doi: 10.1177/2047487314544045. [DOI] [PubMed] [Google Scholar]
- 34.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki H, Ohira T, Takeishi Y, Hosoya M, Yasumura S, Satoh H, Kawasaki Y, Takahashi A, Sakai A, Ohtsuru A, Kobashi G, Ozasa K, et al. Increased prevalence of atrial fibrillation after the Great East Japan Earthquake: results from the Fukushima health management survey. Int J Cardiol. 2015;1(198):102–105. doi: 10.1016/j.ijcard.2015.06.151. [DOI] [PubMed] [Google Scholar]
- 36.Nystrom PK, Carlsson AC, Leander K, De FU, Hellenius ML, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a Swedish, prospective cohort study. PLoS One. 2015;10(5):e0127111. doi: 10.1371/journal.pone.0127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al-Mallah MH. Cardiorespiratory fitness and risk of incident atrial fibrillation: results from the henry ford exercise testing (FIT) project. Circulation. 2015;131(21):1827–1834. doi: 10.1161/CIRCULATIONAHA.114.014833. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y, Tian Y, Wang H, Si Q, Wang Y, Lip GYH. Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest. 2015;147(1):109–119. doi: 10.1378/chest.14-0321. [DOI] [PubMed] [Google Scholar]
- 39.Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kobayashi T, Watanabe T, Okamura T, Okayama A, Miyamoto Y. Interaction of blood pressure and body mass index with risk of incident atrial fibrillation in a Japanese Urban Cohort: the suita study. Am J Hypertens. 2015;28(11):1355–1361. doi: 10.1093/ajh/hpv038. [DOI] [PubMed] [Google Scholar]
- 40.Chyou JY, Hunter TD, Mollenkopf SA, Turakhia MP, Reynolds MR. Individual and combined risk factors for incident atrial fibrillation and incident stroke: an analysis of 3 million at-risk US patients. J Am Heart Assoc. 2015;4(7):e001723. doi: 10.1161/JAHA.114.001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolek MJ, Graves AJ, Xu M, Bian A, Teixeira PL, Shoemaker MB, Parvez B, Xu H, Heckbert SR, Ellinor PT, Benjamin EJ, Alonso A, et al. Evaluation of a prediction model for the development of atrial fibrillation in a repository of electronic medical records. JAMA Cardiol. 2016;1(9):1007–1013. doi: 10.1001/jamacardio.2016.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svennberg E, Lindahl B, Berglund L, Eggers KM, Venge P, Zethelius B, Rosenqvist M, Lind L, Hijazi Z. NT-proBNP is a powerful predictor for incident atrial fibrillation—Validation of a multimarker approach. Int J Cardiol. 2016;15(223):74–81. doi: 10.1016/j.ijcard.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Hobbelt AH, Siland JE, Geelhoed B, Van der HP, Hillege HL, Van GI, Rienstra M. Clinical, biomarker, and genetic predictors of specific types of atrial fibrillation in a community-based cohort: data of the PREVEND study. Europace. 2017;19(2):226–32. doi: 10.1093/europace/euw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding L, Li J, Wang C, Li X, Su Q, Zhang G, Xue F. Incidence of atrial fibrillation and its risk prediction model based on a prospective urban Han Chinese cohort. J Hum Hypertens. 2017;31(9):574–579. doi: 10.1038/jhh.2017.23. [DOI] [PubMed] [Google Scholar]
- 45.Ogunmoroti O, Michos ED, Aronis KN, Salami JA, Blankstein R, Virani SS, Spatz ES, Allen NB, Rana JS, Blumenthal RS, Veledar E, Szklo M, et al. Life's simple 7 and the risk of atrial fibrillation: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2018;275:174–181. doi: 10.1016/j.atherosclerosis.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 46.Austin TR, Wiggins KL, Blackshear C, Yang Y, Benjamin EJ, Curtis LH, Sotoodehnia N, Correa A, Heckbert SR. Atrial fibrillation in an African-American cohort: the Jackson heart study. Clin Cardiol. 2018;41(8):1049–1054. doi: 10.1002/clc.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aronson D, Shalev V, Katz R, Chodick G, Mutlak D. Risk score for prediction of 10-year atrial fibrillation: a community-based study. Thromb Haemost. 2018;118(9):1556–1563. doi: 10.1055/s-0038-1668522. [DOI] [PubMed] [Google Scholar]
- 48.Khurshid S, Choi SH, Weng LC, Wang EY, Trinquart L, Benjamin EJ, Ellinor PT, Lubitz SA. Frequency of cardiac rhythm abnormalities in a half million adults. Circ Arrhythm Electrophysiol. 2018;11(7):e006273. doi: 10.1161/CIRCEP.118.006273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YG, Choi KJ, Han S, Hwang KW, Kwon CH, Park GM, Won KB, Ann SH, Kim J, Kim SJ, Lee SG, Nam GB, et al. Metabolic syndrome and the risk of new-onset atrial fibrillation in middle-aged East Asian men. Circ J. 2018;82(7):1763–1769. doi: 10.1253/circj.CJ-18-0113. [DOI] [PubMed] [Google Scholar]
- 50.Kodani E, Kaneko T, Fujii H, Nakamura H, Sasabe H, Tamura Y, Shimizu W. Prevalence and incidence of atrial fibrillation in the general population based on national health insurance special health checkups- TAMA MED project-AF. Circ J. 2019;83(3):524–531. doi: 10.1253/circj.CJ-18-1038. [DOI] [PubMed] [Google Scholar]
- 51.Hamada R, Muto S. Simple risk model and score for predicting of incident atrial fibrillation in Japanese. J Cardiol. 2019;73(1):65–72. doi: 10.1016/j.jjcc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Bose A, O'Neal WT, Wu C, McClure LA, Judd SE, Howard VJ, Howard G, Soliman EZ. Sex differences in risk factors for incident atrial fibrillation (from the reasons for geographic and racial differences in stroke [REGARDS] study) Am J Cardiol. 2019;123(9):1453–1457. doi: 10.1016/j.amjcard.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 53.Hulme OL, Khurshid S, Weng LC, Anderson CD, Wang EY, Ashburner JM, Ko D, McManus DD, Benjamin EJ, Ellinor PT, Trinquart L, Lubitz SA. Development and validation of a prediction model for atrial fibrillation using electronic health records. JACC Clin Electrophysiol. 2019;5(11):1331–1341. doi: 10.1016/j.jacep.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng T, Vegard M, Strand LB, Laugsand LE, Morkedal B, Aune D, Vatten L, Ellekjaer H, Loennechen JP, Mukamal K, Janszky I. Metabolically healthy obesity and risk for atrial fibrillation: the HUNT study. Obesity (Silver Spring) 2019;27(2):332–338. doi: 10.1002/oby.22377. [DOI] [PubMed] [Google Scholar]
- 55.Kim YG, Han KD, Choi JI, Yung BK, Kim DY, Oh SK, Lee KN, Shim J, Kim JS, Kim YH. Impact of the duration and degree of hypertension and body weight on new-onset atrial fibrillation: a nationwide population-based study. Hypertension. 2019;74(5):e45–e51. doi: 10.1161/HYPERTENSIONAHA.119.13672. [DOI] [PubMed] [Google Scholar]
- 56.Rattani A, Claxton JS, Ali MK, Chen LY, Soliman EZ, Alvaro A. Association and impact of hypertension defined using the 2017 AHA/ACC guidelines on the risk of atrial fibrillation in The Atherosclerosis Risk in Communities study. BMC Cardiovasc Disord. 2019;19(1):262. doi: 10.1186/s12872-019-1259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koshiyama M, Tamaki K, Ohsawa M. Age-specific incidence rates of atrial fibrillation and risk factors for the future development of atrial fibrillation in the Japanese general population. J Cardiol 2020. [DOI] [PubMed]
- 58.Abbas SS, Majeed T, Nair BR, Forder P, Weaver N, Byles J. Burden of atrial fibrillation and stroke risk among octagenarian and nonagenarian women in Australia. Ann Epidemiol. 2020;44:31–37. doi: 10.1016/j.annepidem.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 59.O'Neal WT, Judd SE, Limdi NA, McIntyre WF, Kleindorfer DO, Cushman M, Howard VJ, Howard G, Soliman EZ. Differential Impact of Risk Factors in Blacks and Whites in the Development of Atrial Fibrillation: the Reasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Racial Ethn Health Disparities 2016. [DOI] [PMC free article] [PubMed]
- 60.Chao TF, Chiang CE, Chen TJ, Liao JN, Tuan TC, Chen SA. Clinical risk score for the prediction of incident atrial fibrillation: derivation in 7220654 Taiwan patients With 438930 Incident atrial fibrillations during a 16-year follow-up. J Am Heart Assoc. 2021;10(17):e020194. doi: 10.1161/JAHA.120.020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ninomiya Y, Kawasoe S, Kubozono T, Tokushige A, Ichiki H, Miyahara H, Tokushige K, Ohishi M. Sex-specific relationship between abdominal obesity and new-onset atrial fibrillation in the general Japanese population. Heart Vessels. 2021;36(12):1879–1884. doi: 10.1007/s00380-021-01880-5. [DOI] [PubMed] [Google Scholar]
- 62.Matsuoka S, Kaneko H, Okada A, Morita K, Itoh H, Michihata N, Jo T, Takeda N, Morita H, Fujiu K, Nakamura S, Node K, et al. Age modified relationship between modifiable risk factors and the risk of atrial fibrillation. Circ Arrhythm Electrophysiol. 2022;15(1):e010409. doi: 10.1161/CIRCEP.121.010409. [DOI] [PubMed] [Google Scholar]
- 63.Shapkina M, Ryabikov A, Mazdorova E, Titarenko A, Avdeeva E, Mazurenko E, Shcherbakova L, Pikhart H, Bobak M, Malyutina S. The determinants of the 13-year risk of incident atrial fibrillation in a Russian population cohort of middle and elderly age. J Pers Med. 2022;12(1):122. doi: 10.3390/jpm12010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnabel RB, Witt H, Walker J, Ludwig M, Geelhoed B, Kossack N, Schild M, Miller R, Kirchhof P. Machine learning-based identification of risk-factor signatures for undiagnosed atrial fibrillation in primary prevention and post-stroke in clinical practice. Eur Heart J Qual Care Clin Outcomes 2022. [DOI] [PMC free article] [PubMed]
- 65.Camen S, Csengeri D, Geelhoed B, Niiranen T, Gianfagna F, Vishram-Nielsen JK, Costanzo S, Søderberg S, Vartiainen E, Borschel CS, Donati MB, Lochen ML, et al. Risk factors, subsequent disease onset, and prognostic impact of myocardial infarction and atrial fibrillation. J Am Heart Assoc. 2022;11(7):e024299. doi: 10.1161/JAHA.121.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minami M, Kobayashi Y, Toyokawa S, Inoue K, Takeshita Y. Risk factors for new-onset atrial fibrillation during routine medical checkups of Japanese male workers. Int Heart J. 2009;50(4):457–464. doi: 10.1536/ihj.50.457. [DOI] [PubMed] [Google Scholar]
- 67.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation. 2009;119(16):2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107(1):85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grundvold I, Skretteberg PT, Liestol K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension. 2012;59(2):198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713. [DOI] [PubMed] [Google Scholar]
- 70.Roetker NS, Chen LY, Heckbert SR, Nazarian S, Soliman EZ, Bluemke DA, Lima JA, Alonso A. Relation of systolic, diastolic, and pulse pressures and aortic distensibility with atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;114(4):587–592. doi: 10.1016/j.amjcard.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perkiomaki JS, Nortamo S, Ylitalo A, Kesaniemi A, Ukkola O, Huikuri HV. Ambulatory blood pressure characteristics and long-term risk for atrial fibrillation. Am J Hypertens. 2017;30(3):264–270. doi: 10.1093/ajh/hpw149. [DOI] [PubMed] [Google Scholar]
- 72.Emdin CA, Anderson SG, Salimi-Khorshidi G, Woodward M, MacMahon S, Dwyer T, Rahimi K. Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int J Epidemiol. 2017;46(1):162–72. doi: 10.1093/ije/dyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tikhonoff V, Kuznetsova T, Thijs L, Cauwenberghs N, Stolarz-Skrzypek K, Seidlerova J, Malyutina S, Gilis-Malinowska N, Swierblewska E, Kawecka-Jaszcz K, Filipovsky J, Narkiewicz K, et al. Ambulatory blood pressure and long-term risk for atrial fibrillation. Heart. 2018;104(15):1263–1270. doi: 10.1136/heartjnl-2017-312488. [DOI] [PubMed] [Google Scholar]
- 74.Matsumoto K, Jin Z, Homma S, Elkind MSV, Schwartz JE, Rundek T, Mannina C, Ito K, Sacco RL, Di Tullio MR. Office, central and ambulatory blood pressure for predicting incident atrial fibrillation in older adults. J Hypertens. 2021;39(1):46–52. doi: 10.1097/HJH.0000000000002613. [DOI] [PubMed] [Google Scholar]
- 75.Wong JA, Conen D, Healey JS, Johnson LSB. Modifiable risk factors predict incident atrial fibrillation and heart failure. Open Heart. 2020;7(1):e001092. doi: 10.1136/openhrt-2019-001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Espnes H, Ball J, Løchen ML, Wilsgaard T, Njølstad I, Mathiesen EB, Gerdts E, Sharashova E. Sex-Specific associations between blood pressure and risk of atrial fibrillation subtypes in the Tromsø study. J Clin Med. 2021;10(7):1514. doi: 10.3390/jcm10071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao LZ, Wen XY, Zhang SZ, Li WD, Zhuang XD. Hypertension and atrial fibrillation: a study on epidemiology and mendelian randomization causality. Front Cardiovasc Med. 2021;8:644405. doi: 10.3389/fcvm.2021.644405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lind L, Ingelsson M, Sundstrom J, Arnlov J. Impact of risk factors for major cardiovascular diseases: a comparison of life-time observational and Mendelian randomisation findings. Open Heart. 2021;8(2):e001735. doi: 10.1136/openhrt-2021-001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hata J, Nagata T, Sakata S, Oishi E, Furuta Y, Hirakawa Y, Honda T, Yoshida D, Kitazono T, Ninomiya T. Risk prediction model for incident atrial fibrillation in a general Japanese population - The Hisayama study. Circ J. 2021;85(8):1373–1382. doi: 10.1253/circj.CJ-20-0794. [DOI] [PubMed] [Google Scholar]
- 80.Son MK, Song DS, Lee K, Park HY. Lower number of modifiable risk factors was associated with reduced atrial fibrillation incidence in an 18-year prospective cohort study. Sci Rep. 2022;12(1):9207. doi: 10.1038/s41598-022-13434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297(7):709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 82.Igarashi Y, Nochioka K, Sakata Y, Tamai T, Ohkouchi S, Irokawa T, Ogawa H, Hayashi H, Fujihashi T, Yamanaka S, Shiroto T, Miyata S, et al. Risk prediction for new-onset atrial fibrillation using the Minnesota code electrocardiography classification system. Int J Cardiol Heart Vasc. 2021;34:100762. doi: 10.1016/j.ijcha.2021.100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302(4):401–411. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 85.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 86.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 87.Kobeissi E, Hibino M, Pan H, Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2019;34(6):547–555. doi: 10.1007/s10654-019-00510-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan H, Hibino M, Kobeissi E, Aune D. Blood pressure, hypertension and the risk of sudden cardiac death: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2020;35(5):443–454. doi: 10.1007/s10654-019-00593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 90.Royston P. A strategy for modelling the effect of a continuous covariate in medicine and epidemiology. Stat Med. 2000;19(14):1831–1847. doi: 10.1002/1097-0258(20000730)19:14<1831::aid-sim502>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 91.Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159(11):1077–1086. doi: 10.1093/aje/kwh142. [DOI] [PubMed] [Google Scholar]
- 92.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 93.Wells G, Shea B, O'Connell D., Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp, Accessed 20 May 2022.
- 94.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nazarzadeh M, Pinho-Gomes AC, Bidel Z, Canoy D, Dehghan A, Smith BK, Bennett DA, Smith GD, Rahimi K. Genetic susceptibility, elevated blood pressure, and risk of atrial fibrillation: a Mendelian randomization study. Genome Med. 2021;13(1):38. doi: 10.1186/s13073-021-00849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Georgiopoulos G, Ntritsos G, Stamatelopoulos K, Tsioufis C, Aimo A, Masi S, Evangelou E. The relationship between blood pressure and risk of atrial fibrillation: a Mendelian randomization study. Eur J Prev Cardiol 2021. [DOI] [PubMed]
- 97.Hyman MC, Levin MG, Gill D, Walker VM, Georgakis MK, Davies NM, Marchlinski FE, Damrauer SM. Genetically predicted blood pressure and risk of atrial fibrillation. Hypertension. 2021;77(2):376–382. doi: 10.1161/HYPERTENSIONAHA.120.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verdecchia P, Staessen JA, Angeli F, De SG, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374(9689):525–33. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 99.Wei YC, George NI, Chang CW, Hicks KA. Assessing sex differences in the risk of cardiovascular disease and mortality per increment in systolic blood pressure: a systematic review and meta-analysis of follow-up studies in the United States. PLoS ONE. 2017;12(1):e0170218. doi: 10.1371/journal.pone.0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peters SA, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013;44(9):2394–401. doi: 10.1161/STROKEAHA.113.001624. [DOI] [PubMed] [Google Scholar]
- 101.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gumprecht J, Domek M, Lip GYH, Shantsila A. Invited review: hypertension and atrial fibrillation: epidemiology, pathophysiology, and implications for management. J Hum Hypertens. 2019;33(12):824–836. doi: 10.1038/s41371-019-0279-7. [DOI] [PubMed] [Google Scholar]
- 103.McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ. Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National high blood pressure education program working group staging criteria. Hypertension. 2007;50(2):392–395. doi: 10.1161/HYPERTENSIONAHA.107.092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soliman EZ, Byington RP, Bigger JT, Evans G, Okin PM, Goff DC, Jr, Chen H. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: action to control cardiovascular risk in diabetes blood pressure trial. Hypertension. 2015;66(6):1123–1129. doi: 10.1161/HYPERTENSIONAHA.115.06236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mosterd A, D'Agostino RB, Silbershatz H, Sytkowski PA, Kannel WB, Grobbee DE, Levy D. Trends in the prevalence of hypertension, antihypertensive therapy, and left ventricular hypertrophy from 1950 to 1989. N Engl J Med. 1999;340(16):1221–1227. doi: 10.1056/NEJM199904223401601. [DOI] [PubMed] [Google Scholar]
- 106.Cao X, Broughton ST, Waits GS, Nguyen T, Li Y, Soliman EZ. Interrelations between hypertension and electrocardiographic left ventricular hypertrophy and their associations with cardiovascular mortality. Am J Cardiol. 2019;123(2):274–283. doi: 10.1016/j.amjcard.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 107.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017; 389(10064):37–55. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.