Abstract
Objective
To compare opioid use in patients with obesity treated with bariatric surgery versus adults with obesity who underwent intensive lifestyle modification.
Summary background data
Previous studies of opioid use after bariatric surgery have been limited by small sample sizes, short follow-up, and lack of control groups.
Methods
Nationwide matched cohort study including individuals from the Scandinavian Obesity Surgery Registry (SOReg) and the Itrim health database with individuals undergoing structured intensive lifestyle modification, between August 1, 2007, and September 30, 2015. Participants were matched on BMI, age, sex, education, previous opioid use, diabetes, cardiovascular disease, and psychiatric status (n=30,359:21,356). Dispensed opioids were retrieved from the Swedish Prescribed Drug Register from 2 years before to up to 8 years after intervention.
Results
During the 2-year period before treatment, prevalence of individuals receiving ≥1 opioid prescription was identical in the surgery and lifestyle group. At 3 years, the prevalence of opioid prescriptions was 14.7% versus 8.9% in the surgery and lifestyle groups (mean difference 5.9%, 95%CI 5.3–6.4) and at 8 years 16.9% versus 9.0% (7.9%, 6.8–9.0). The difference in mean daily dose also increased over time and was 3.55mg in the surgery group versus 1.17mg in the lifestyle group at 8 years (mean difference [adjusted for baseline dose] 2.30mg, 95%CI 1.61–2.98).
Conclusions
Bariatric surgery was associated with a higher proportion of opioid users and larger total opioid dose, compared to actively treated obese individuals. These trends were especially evident in patients who received additional surgery during follow-up.
MINI ABSTRACT
We use Swedish registers to describe trends in opioid use after bariatric surgery versus controls undergoing intensive lifestyle modification. After surgery, opioid use rises relative to controls, especially among patients with additional surgery, and surgery patients reach a dose roughly three times as large as controls 8 years after treatment.
INTRODUCTION
The US opioid epidemic was declared a public health emergency in 2017 and opioid-related deaths are still increasing. On average, 130 Americans die every day due to opioids and more than 35% of these deaths are due to prescription opioids.1 In Sweden, annual opioid-related deaths more than doubled between 2011 and 2016 from 24 to 55 deaths per million inhabitants.2
Long-term pain is more frequently reported in the morbidly obese3–5 than in the general population and these patients more often using analgesics, including opioids. As bariatric surgery is the treatment demonstrating best long-term weight loss and improvement of obesity-related comorbidities,6 it is important to investigate its association with the use of opioid analgesics.
Opioid analgesics should not be used for long-term non-cancer pain due to development of tolerance and risk of side effects.7 Concerns have been raised regarding substance misuse after bariatric surgery,8,9 for example due to anatomical changes that lead to faster absorption and higher maximum concentration of alcohol and liquid formulation morphine.10
Bariatric surgery has been associated with reduced musculoskeletal11,12 and bodily pain, and improved physical function13,14, effects that could potentially reduce the use of opioid analgesics. However, in 3 large uncontrolled studies, with a before/after design, the majority of patients with a high/chronic use of opioids before surgery continued with or increased their use of opioids during the first year after surgery.15–17
Existing studies regarding long term opioid use after bariatric surgery are conflicting. US data show an increase in self-reported opioid use from 15% at baseline to 20% after 7 years.15 Norwegian data show a higher incidence of opioid use after bariatric surgery compared to medical controls, but no increase in the prevalence of opioid use after surgery (36% at baseline and approximately 33% at 7 years after surgery).18
The primary aim of this study was to compare opioid use from 2 years before to 8 years after intervention in patients with obesity treated with bariatric surgery versus intensive lifestyle modification. Secondary aims were to compare opioid use after gastric bypass versus after sleeve gastrectomy, and opioid-related mortality.
METHODS
Study design
This nationwide matched cohort study with prospectively registered data includes individuals from the Scandinavian Obesity Surgery Registry (SOReg) covering >97% of all bariatric surgery patients in Sweden,19 and the Itrim health database with individuals undergoing a 1-year structured intensive lifestyle modification program.20–22 All analyses were conducted on de-identified data, and the study was approved by the regional ethics review board in Stockholm, Sweden.
Inclusion and exclusion criteria
We restricted the study population to individuals, at least 18 years old with a BMI between 30 and <50 kg/m2 at start of treatment (eFigure 1). Bariatric surgery patients with a BMI above 50 were excluded since there were few suitable intensive lifestyle comparators for the matching procedure. Only primary (not revisional) surgeries were considered. As we required prescription data from 2 years prior to at least 3 years after initiation of treatment, we restricted the study population to individuals who initiated treatment between August 1, 2007, and September 30, 2015. During the study period, no major changes in opioid prescription guidelines were implemented in Sweden, and between 2006 and 2015, the annual prevalence of opioid prescriptions was stable and the daily-defined-dose (DDD) per treated individual decreased.23
Interventions
Bariatric surgery:
Patients in the surgery cohort underwent bariatric surgery (92.6% gastric bypass, 6.7% sleeve gastrectomy, 0.7% other). The majority of patients underwent a short low-calorie diet as a preparation for surgery.
Intensive lifestyle intervention: Individuals underwent a 3-month dietary weight loss phase with low- or very-low-calorie diets (LCD/VLCD) followed by a 9-month weight maintenance phase.20 The choice of LCD or VLCD was based on baseline BMI, personal preference, and contraindication status. After the weight-loss phase, patients entered a 9-month weight maintenance program including exercise (circuit training at a centre 2–3 times per week for 30–45 min, and pedometer use to encourage walking) and dietary advice. Behavioral changes were facilitated by a structured program including 20 group sessions lasting 1h and face-to-face counselling sessions.
Intensive lifestyle participants were selected as comparators since they were also obese, intended to lose weight, and their treatment leads to more weight loss22 than most other control treatments.20,24
General population comparators: Up to 10 general population comparators were matched on age, sex and place of residence to each surgery patient to provide a reference level of opioid use in Sweden.
Outcome and follow-up
The main outcome was opioid use, measured as the semi-annual percentage of patients with a filled opioid prescription and the semi-annual dose of opioids in oral milligram morphine equivalents. Data on filled prescriptions were retrieved from the nationwide Swedish Prescribed Drug Register. Conversion to oral morphine equivalents has been described previously17 and information about drugs, conversion factors, and calculations are available in eTable 2. Longitudinal data were presented from 2 years before to 8 years after the index date. Individuals were followed from treatment initiation until death, emigration or end of follow-up.
In Sweden, bariatric surgery patients are often prescribed analgesics shortly before treatment so they do not have to visit a pharmacy directly after surgery. In order to attribute these early prescriptions intended for use after surgery to the bariatric surgery treatment, we moved forward all dates for opioid prescriptions by one month for all individuals in the study population (surgery patients, individuals receiving intensive lifestyle modification, and general population comparators).
As an additional outcome, we examined the incidence of opioid-related deaths in the bariatric surgery group compared with the intensive lifestyle group and the general population comparators (ICD-10 codes X42, X62, Y12, Y450, T400-T404).
Covariates
Baseline weight and height were used to calculate BMI (kg/m2). Data on age, sex, education, marital status, and emigrations were collected from the Longitudinal Integration Database for Health Insurance and Labor Market studies25 and the Total Population Register26 from Statistics Sweden.
Data on hospital visits for substance use disorder, other psychiatric causes, and cardiovascular disease were obtained from the National Patient Register (nationwide inpatient data from 1987; hospital-based outpatient data from 2001). The date of death was obtained from the Causes of Death Register, and filled prescriptions for antidepressants, anxiolytics and diabetes drugs before inclusion from the Prescribed Drug Register (ICD and ATC codes used are provided in eTable 3).
Statistical analysis
To make the surgery and the intensive lifestyle groups comparable, coarsened exact matching was used.27 Participants were matched by baseline opioid use (none, intermittent and chronic), BMI (<35, 35-<40, 40-<45, 45-<50kg/m2), age (18-<30, 30-<40, 40-<50, 50-<60, ≥60y), sex, education level (<10, 10–12, >12y, missing data), diabetes status, cardiovascular disease, history of psychiatric care, substance use disorder, antidepressant use, and anxiolytics use. Intermittent opioid use was defined as having ≥1 prescription during the 2 years before treatment but not in all 6-month periods. Chronic opioid use was defined as having dispensed prescriptions in every 6-month period during the 2 years before treatment.
To minimize loss of information, we allowed matching strata to include different numbers of surgery and intensive lifestyle participants. To account for the differential strata sizes, analyses were weighted. All surgery patients were given the weight one. If, for example, there were two surgery participants and four lifestyle participants in a stratum, each surgery participant was given the weight 1 and each lifestyle participant the weight 0.5.
In the matched cohort, we compared the percentage of individuals with filled opioid prescriptions after treatment in the surgery and intensive lifestyle groups using risk differences. To compare total opioid dose between the groups, we used linear regression to estimate the mean difference in dose measured as morphine equivalents, adjusting for dose before treatment.
Subgroup analyses
Baseline opioid use: Subgroup analyses were performed by baseline opioid use with patients divided into 3 groups based on opioid use during the 2 years before treatment (none/intermittent/chronic).
Surgical procedure: Gastric bypass and sleeve gastrectomy patients operated between January 1st 2013 and September 30th 2015 were compared using the same matching procedure as for surgery versus intensive lifestyle treatment, with the addition of matching for surgery year.
Weight loss: To investigate if weight loss is associated with long term opioid use, we divided the bariatric surgery group into 3 equally large groups based on percentage 1-year total weight loss (%TWL): low (<28.7%), medium (28.7–35.2%), and high (>35.2%).
Additional surgery: To investigate the association between additional surgery after treatment and changes in opioid use, we divided the bariatric surgery patients into one group with hip surgery (procedure code: NFB), knee surgery (NGB), surgery in the gastrointestinal tract (J), or surgery on the skin of the main body (QB) during follow-up, and a second group with no additional surgery of this kind.
Initial post-surgery opioid use: To investigate if early use of opioids after bariatric surgery was associated with a subsequent increase in opioid use, we compared the prevalence and dose of opioid use among bariatric surgery patients with versus without opioid use during the first 6 months after surgery. We required that patients in these subgroups did not receive opioid prescriptions during 2 years before surgery.
Sensitivity analysis
To make our results comparable with previous studies on chronic opioid use, we examined the prevalence of ≥5 opioid prescriptions per 6-month period among surgery patients and comparators.
Analyses were performed using SAS version 9.4 and STATA version 14. Statistical significance was defined as a two-sided P-value of <0.05.
RESULTS
Participant characteristics
After matching and weighting, the surgery and the intensive lifestyle group were balanced regarding sex, age, obesity status, educational level, previous opioid use, as well as diabetes, cardiovascular disease, history of psychiatric care, substance use disorder, use of antidepressants, and use of anxiolytics (Table 1).
Table 1.
Participant characteristics of matched sample1
| Bariatric surgery (n=30,359) | Intensive lifestyle (n=21,356) | Matched general population (n=303,507) | |
|---|---|---|---|
| Women, n (%) | 24,116 (79.4) | 16,965 (79.4) | 241,103 (79.4) |
| Age (years), mean (SD) | 40 (10.8) | 41 (11.3) | 41 (10.8) |
| BMI at screening (kg/m2), mean (SD) | 40.5 (4.0) | 40.0 (4.2) | Not available |
| BMI 30 to <35, n (%) | 2094 (6.9) | 1473 (6.9) | Not available |
| BMI 35 to <40, n (%) | 12,316 (40.6) | 8664 (40.6) | Not available |
| BMI 40 to <45, n (%) | 11,773 (38.8) | 8282 (38.8) | Not available |
| BMI 45 to <50, n (%) | 4176 (13.8) | 2938 (13.8) | Not available |
| Married, n (%) | 13,370 (44.0) | 8614 (40.3) | 130,413 (43.0) |
| Education level, n (%) | |||
| Primary school | 3983 (13.1) | 2802 (13.1) | 38,053 (12.5) |
| High school | 19,200 (63.2) | 13,506 (63.2) | 143,428 (47.3) |
| University | 7119 (23.4) | 5008 (23.4) | 115,340 (38.0) |
| Missing data | 57 (0.2) | 40 (0.2) | 6686 (2.2) |
| Previous opioid use, n (%) | |||
| None | 23,839 (78.5) | 16,770 (78.5) | 266,302 (87.7) |
| Intermittent use2 | 6015 (19.8) | 4231 (19.8) | 32,472 (10.7) |
| Chronic use3 | 505 (1.7) | 355 (1.7) | 4733 (1.6) |
| Comorbidity and drug use, n (%) | |||
| Diabetes | 2476 (8.2) | 1742 (8.2) | 7399 (2.4) |
| Cardiovascular disease | 5712 (18.8) | 4018 (18.8) | 32,909 (10.8) |
| Psychiatric care | 5149 (17.0) | 3622 (17.0) | 43,610 (14.4) |
| Substance use disorder | 590 (1.9) | 415 (1.9) | 10,320 (3.4) |
| Antidepressants | 9240 (30.4) | 6500 (30.4) | 58,248 (19.2) |
| Anxiolytics | 5750 (18.9) | 4045 (18.9) | 43,220 (14.2) |
Using coarsened exact matching, bariatric surgery and intensive lifestyle participants were matched on sex, age, BMI, education, history of opioid use, diabetes, cardiovascular disease, psychiatric care, substance use disorder, antidepressant use, and anxiolytics use; general population comparators were matched on age, sex and place of residence
≥1 prescription in the 2 years before intervention but not all 6-month periods
Dispensed prescriptions in every 6-month period in the 2 years before intervention
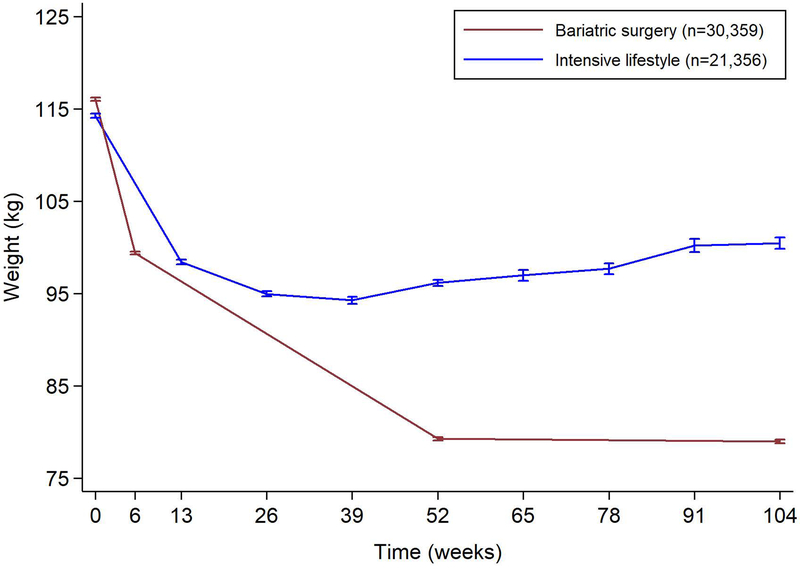
Over the first 2 years after treatment, bariatric surgery patients lost 37.0kg compared to 15.1kg in the matched intensive lifestyle group (mean difference 21.9kg, 95%CI 21.4–22.3; P<0.001; Figure 1).
Figure 1.
Weight change after bariatric surgery and intensive lifestyle intervention over 2 years of follow-up. Error bars indicate 95% confidence intervals.
Prevalence of opioid use
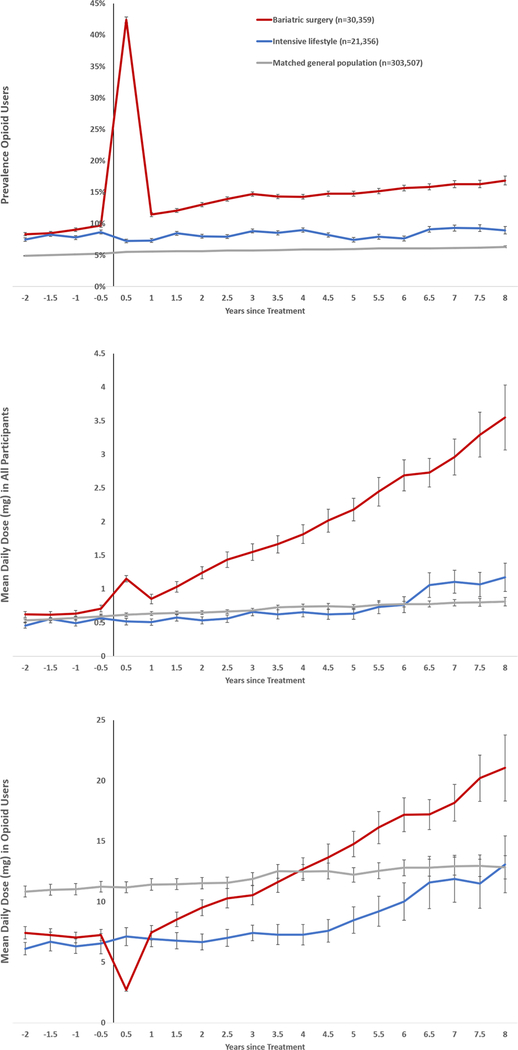
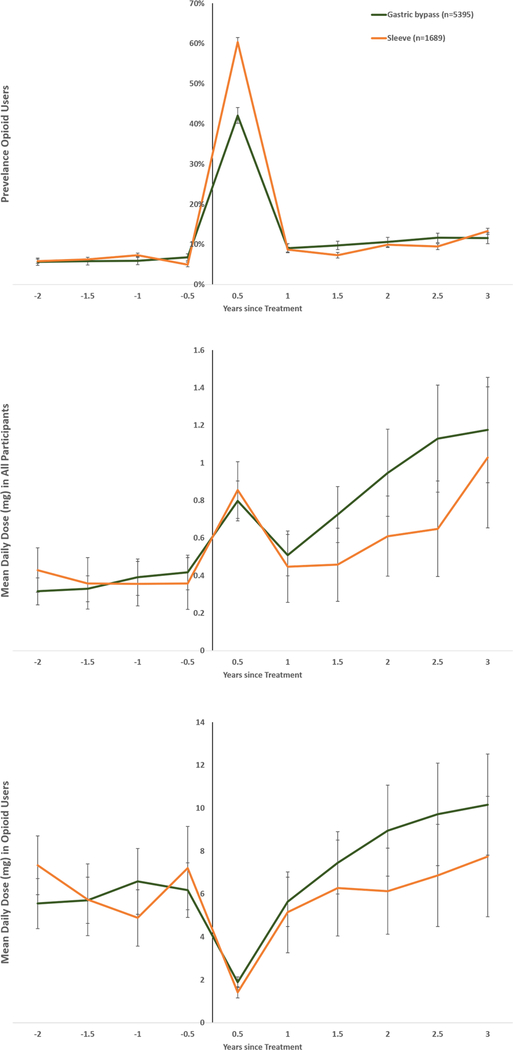
During the 2-year period before treatment, the fraction of individuals receiving at least one opioid prescription was similar in the surgery and lifestyle group at 21.5% (Table 1). The prevalence in the surgery group increased to 42% during the 6 months after treatment (Figure 2), as patients were prescribed opioids in conjunction with surgery. At 1 year after surgery, the prevalence had dropped to 11.5%, but then increased steadily reaching 16.9% at 8 years after surgery.
Figure 2.
Prevalence of opioid use (upper panel), mean daily dose in all participants (middle panel) and in opioid users (lower panel).
Opioid use was defined as ≥1 dispensing of opioid analgesics per 6-month period.
Using coarsened exact matching, bariatric surgery and intensive lifestyle participants were matched on sex, age, BMI, education, history of opioid use, diabetes, cardiovascular disease, psychiatric care, substance use disorder, antidepressant use, and anxiolytics use; general population comparators were matched on age, sex and place of residence.
The prevalence of opioid use in the intensive lifestyle group remained below 10% during the study period and was 9.0% at 8 years after treatment (Figure 2). In the age-sex-matched general population, the prevalence was lower than in both the surgery and the intensive lifestyle group (Figure 2).
Total opioid dose
The mean daily dose of opioids (in morphine equivalent units) 1 year before treatment was 0.64mg in the surgery and 0.49mg in the intensive lifestyle group (mean difference 0.14mg, 95%CI 0.07–0.22; Figure 2). The mean daily dose of opioids increased to 1.15mg in the surgery group during the 6 months after the operation and then fell to 0.85mg at 1 year. Thereafter the daily dose increased steadily and reached 3.55mg at 8 years after surgery.
The mean daily dose in the intensive lifestyle group also rose over time but at a lower rate, reaching 1.17mg at 8 years. The mean differences, adjusted for baseline dose, between the bariatric surgery and intensive lifestyle groups were 0.79mg at 3 years (95%CI 0.62–0.97) and 2.30mg at 8 years (95%CI 1.61–2.98) after treatment.
In the age-sex-matched general population, the mean daily dose of opioids was similar to the level in the intensive lifestyle group.
Dose among opioid users
The mean daily dose of opioids in a 6-month period among those individuals who received at least 1 opioid prescription in that period (dose among opioid users) was similar before treatment across surgery patients and the intensive lifestyle group. After the first 6 months post intervention, the dose among opioid users rose more quickly in the surgery group. Opioid users in the general population had an initially higher mean dose which remained stable during follow-up.
As the prevalence of opioid use after the index date was below 50% for all groups, the median dose was 0 over the whole study period. The 95th percentile of the opioid dose showed a similar pattern as the mean (eFigure 2), with increasing levels in the bariatric surgery group but largely stable levels in the intensive lifestyle group and the general population.
Opioid-related deaths
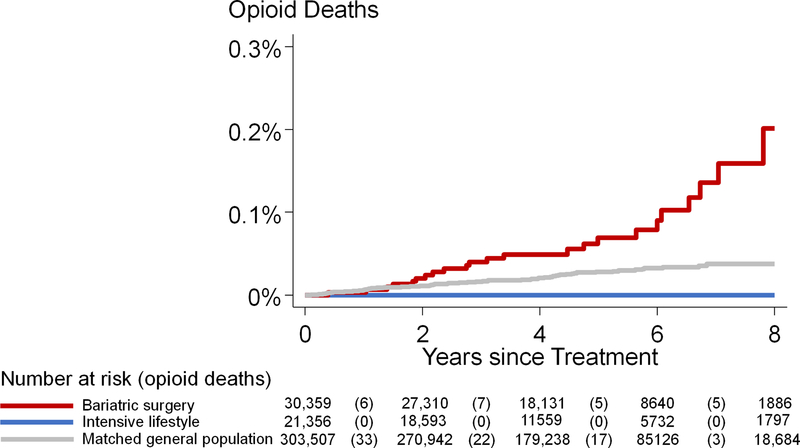
The cumulative incidence of opioid-related deaths over 8 years was 0.2% in the surgery and 0% in the intensive lifestyle group (P=0.047 in log-rank test) (Figure 3).
Figure 3.
Cumulative incidence of opioid-related deaths after bariatric surgery, after intensive lifestyle intervention, and in the matched general population.
Subgroup analyses
Baseline opioid use:
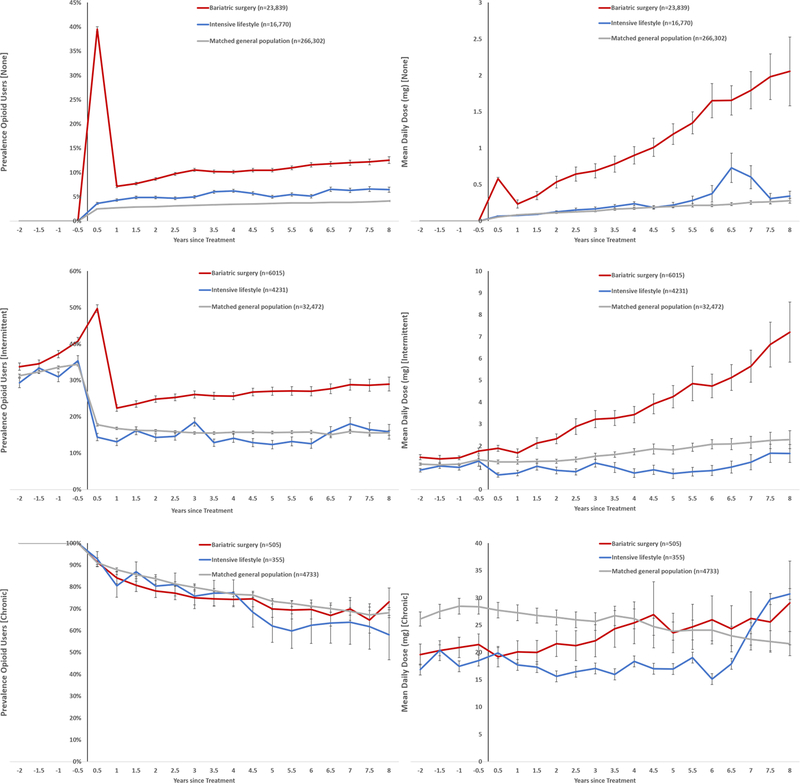
Among individuals with no opioid use before intervention (78.5%) or some previous use (19.8%), both the prevalence and the mean daily dose increased more in the surgery than in the intensive lifestyle group (Figure 4). In the small group with chronic opioid use before treatment (1.7%), estimates were associated with much uncertainty.
Figure 4.
Subgroup analysis by previous opioid use: none (top), intermittent (middle), and chronic (bottom). Left column shows prevalence of opioid use and right column shows mean daily dose (mg).
Opioid use was defined as ≥1 dispensing of opioid analgesics per 6-month period.
Intermittent previous use: ≥1 prescription in the 2 years before intervention but not all 6-month periods.
Chronic previous use: Dispensed prescriptions in every 6-month period in the 2 years before intervention.
Using coarsened exact matching, bariatric surgery and intensive lifestyle participants were matched on sex, age, BMI, education, history of opioid use, diabetes, cardiovascular disease, psychiatric care, substance use disorder, antidepressant use, and anxiolytics use; general population comparators were matched on age, sex and place of residence.
Surgical procedure:
Baseline characteristics were similar in patients receiving gastric bypass and sleeve gastrectomy (eTable 4). The prevalence of opioid use 1 year before treatment was lower in the gastric bypass than the sleeve gastrectomy group (6.0% versus 7.3%; P=0.047). The difference was more pronounced during the 6-month period after surgery (42.1% versus 60.4%; P<0.001), but at 3 years after surgery, there was no statistically significant difference in prevalence between the two groups (11.6% versus 13.3%; P=0.058; Figure 5).
Figure 5.
Subgroup analysis of gastric bypass versus sleeve gastrectomy: prevalence of opioid use (upper panel), mean daily dose in all participants (middle panel), and in opioid users (lower panel).
Opioid use was defined as ≥1 dispensing of opioid analgesics per 6-month period.
Using coarsened exact matching, gastric bypass and sleeve gastrectomy participants were matched on sex, age, BMI, education, history of opioid use, diabetes, cardiovascular disease, psychiatric care, substance use disorder, antidepressant use, and anxiolytics use.
The mean daily dose of opioids was similar among gastric bypass and sleeve gastrectomy patients 1 year before surgery (0.39mg versus 0.36mg; mean difference 0.035mg, 95%CI −0.19 to 0.26). After the initial 6-month period post-surgery, mean doses started diverging but 3 years after surgery the mean daily dose did not differ between gastric bypass and sleeve gastrectomy patients (1.18mg versus 1.03mg; mean difference 0.12mg, 95%CI −0.51 to 0.75; P=0.70).
Weight loss:
Surgery patients with high 1-year weight loss used higher doses of opioids during follow up, but there were no between-group-differences in opioid use prevalence (eFigure 3).
Additional surgery:
Of the bariatric surgery patients, 33.7% received additional surgery during follow-up. The prevalence and dose of opioid use were higher in this subgroup of surgery patients already before treatment. After surgery, the increase in prevalence and dose was largest in the subgroup of surgery patients receiving additional surgery (eFigure 4). Bariatric surgery patients who did not receive additional surgery also had a higher prevalence of opioid use after surgery and used a higher total dose relative to individuals undergoing intensive lifestyle modification (eFigure5).
Initial post-surgery opioid use:
The use and dose of opioids increased after surgery in patients with no prescription during the first 6 months after surgery, but the increase was greater among patients with an initial opioid prescription (eFigure 6).
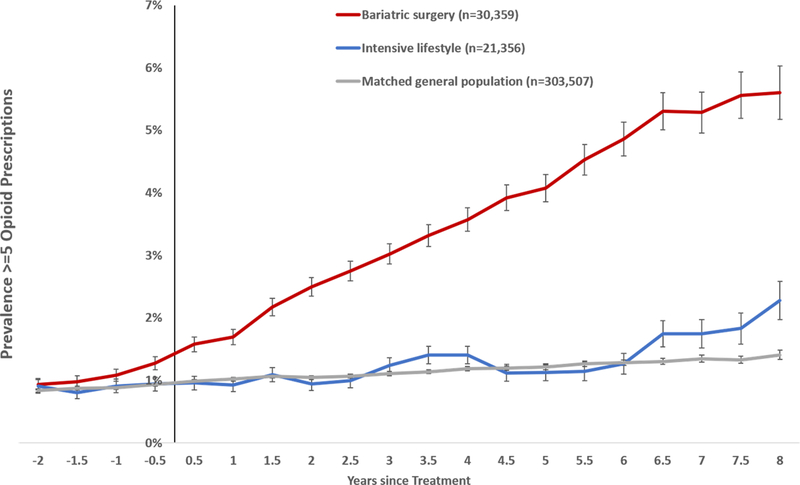
Sensitivity analysis
The prevalence of ≥5 opioid prescriptions per 6-month period increased among surgery patients and was more than 5 times higher at 8 years after surgery relative to before treatment (Figure 6).
Figure 6.
Prevalence of ≥5 opioid prescriptions after bariatric surgery, after intensive lifestyle intervention, and in the matched general population
DISCUSSION
Main findings
In this nationwide matched cohort study of obese adults undergoing bariatric surgery or intensive lifestyle modification, we found a continuous increase in opioid use (proportion users and total dose) over 8 years after surgery. Actively treated obese comparators displayed only small changes during follow-up. The prevalence of patients dispensed ≥5 opioid prescriptions per 6-month period increased steadily over time and was >5 times higher at the end of follow-up relative to before surgery. Surgery patients were also found to be at an increased risk of dying from opioid-related causes.
Previous research
To our knowledge, there are 4 studies investigating opioid use as primary end-point after bariatric surgery without comparators,15–17,28 one controlled study with opioid use as a secondary end-point,18 and one controlled study with long-term follow-up that also compared surgery methods (gastric bypass versus sleeve gastrectomy) in a predominantly elderly, male military veteran cohort.29 The studies by Raebel et al16,28 and Wallén et al17 used oral morphine equivalents of dispensed opioids to compare daily doses before and after surgery, but lacked control groups and had only 1 and 2 years post-surgery follow-up, respectively. In another study without comparators, King et al15 found increasing self-reported opioid use over 7 years after bariatric surgery in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) cohort. Participants were classified as opioid users if they reported daily, weekly or “on demand” use of a prescribed opioid during the preceding 90 days at the annual follow-up, but there were no dose data. In a recent Norwegian study, Jakobsen et al18 reported more new onset opioid use after bariatric surgery compared to medically treated controls, but the proportion of opioid users trended below baseline after 6 years of follow-up.
Recently, Maciejewski et al29 reported increased risk of chronic prescription opioid use after bariatric surgery compared to non-surgically treated controls in military veterans. No differences were found in hazard ratios for opioid use incidence between surgical procedures (open gastric bypass, laparoscopic gastric bypass, and laparoscopic sleeve gastrectomy). We also did not find any statistically significant difference in opioid use by surgical procedure.
Potential mechanisms
After bariatric surgery there is a risk for painful complications not present among the comparators, but few of those complications would lead to long-term pain. More than 30% of bariatric surgery patients received additional surgery during the 8-year follow-up. Some gastrointestinal surgical (e.g. cholecystectomy) and body contouring procedures are consequences of bariatric surgery, which complicates interpretation. Other procedures are performed to reduce pain, e.g. hip and knee arthroplasties. The fact that opioid use was highest already before baseline in patients receiving additional surgery, and that opioid use increased more among these patients, suggests that a combination of selection of patients and a direct effect is responsible for the association between opioid use and additional surgery. Further research is needed to discern if continued opioid use after procedures such as hip and knee arthroplasties is associated with prior exposure to bariatric surgery.
Gastric bypass and sleeve gastrectomy have been shown to increase the uptake of alcohol30,31 and the risk of alcohol use disorder.32–35 Gastric bypass also affects the pharmacokinetic properties of liquid morphine formulations10 (shorter time to and higher maximum concentration) but not extended release formulations.36 Bioavailability of methadone has also been described to increase after sleeve gastrectomy.37 However, liquid formulation opioids are not commonly used in Sweden.
Abdominal pain is not uncommon after gastric bypass and could be one explanation for the increasing prevalence and escalating mean dose compared to the intensive lifestyle group. Previous research has estimated a risk difference versus medically treated controls of 12.6% for abdominal pain and 15.8% for any gastrointestinal surgery. 18 Other uncontrolled studies investigating abdominal pain after gastric bypass surgery have reported that 34% of patients lived with chronic abdominal pain after 5 years,38 and 40% had abdominal pain resulting in computer tomography scans.39
Another explanation is that bariatric surgery and other types of surgery40 leads to exposure to opioids. In our study, 39.5% of surgery patients without previous opioid use filled an opioid prescription during the 6 months following surgery. Compared with surgery patients that did not fill an opioid prescription during the first 6 months, this group had a higher prevalence and use during the subsequent 8 years (eFigure 6).
Strengths and limitations
Strengths of this study include its nationwide design and prospectively collected outcome data with complete information about filled prescriptions from 2 years before to 8 years after intervention. Most previous studies lack control groups,15–17,28 while we used actively treated obese comparators, matched on multiple physical, psychiatric and social variables. In addition to comparing the prevalence of opioid users, we also compared the development of total dose with a previously used and validated method for calculating and comparing oral morphine equivalents.16,17,28,41
A limitation is the nonrandomized design, and we cannot exclude the possibility of unknown and residual confounding. It is possible that we have underestimated opioid use in the surgery group through exclusion of patients with BMI>50. Further, while we had information on filled prescriptions, we do not know if participants used all dispensed opioids. We lack information about opioids given in inpatient care, which results in an underestimation of the total dose used in the surgery compared to the intensive lifestyle group, as surgery patients are more likely to be hospitalized after intervention.42 Finally, we only had 3 years of data for the comparison between gastric bypass and sleeve gastrectomy, as the number of sleeve operations with longer follow-up was limited.
Summary
We observed greater increases in the prevalence of opioid use and higher mean opioid dosing over 8 years after bariatric surgery compared to intensively treated obese comparators. There were no indications of a plateau in mean dose, which continued to increase. No difference in opioid use between surgical procedure types was found after 3 years of follow-up.
Our study illustrates that even though bariatric surgery patients lose more weight than individuals undergoing intensive lifestyle modification, they also have a higher risk of long-term opioid use. It is thus important to develop strategies to mitigate the risk of elevated opioid use after bariatric surgery, for example protocols for opioid-sparing postoperative pain management.43
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Näslund reports personal fees from Baricol Bariatrics AB, Sweden, personal fees from Ethicon, Johnson & Johnson, personal fees from AstraZeneca A/S Denmark, during the conduct of the study; Dr. Ottosson reports personal fees from Vifor Pharma member of advisory board, personal fees from Johnson & Johnson member of advisory board, outside the submitted work; Drs. Sundström, Neovius and Marcus report advisory board participation at Itrim. Dr. Neovius also reports personal fees for advisory board participation for Ethicon, Johnson & Johnson.
Funding Grants Office at Region Örebro County, US National Institutes of Health, Swedish Research Council, and Swedish Research Council for Health, Working Life and Welfare
REFERENCES
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.OECD. Addressing Problematic Opioid Use in OECD Countries. 2019.
- 3.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8(5):430–436. [DOI] [PubMed] [Google Scholar]
- 4.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity (Silver Spring). 2012;20(7):1491–1495. [DOI] [PubMed] [Google Scholar]
- 6.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014(8):CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. [DOI] [PubMed] [Google Scholar]
- 8.Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Subst Use Misuse. 2014;49(4):405–417. [DOI] [PubMed] [Google Scholar]
- 9.Saules KK, Wiedemann A, Ivezaj V, Hopper JA, Foster-Hartsfield J, Schwarz D. Bariatric surgery history among substance abuse treatment patients: prevalence and associated features. Surg Obes Relat Dis. 2010;6(6):615–621. [DOI] [PubMed] [Google Scholar]
- 10.Lloret-Linares C, Hirt D, Bardin C, et al. Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53(10):919–930. [DOI] [PubMed] [Google Scholar]
- 11.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes (Lond). 2007;31(1):114–120. [DOI] [PubMed] [Google Scholar]
- 12.Ryder JR, Edwards NM, Gupta R, et al. Changes in Functional Mobility and Musculoskeletal Pain After Bariatric Surgery in Teens With Severe Obesity: Teen-Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Pediatr. 2016;170(9):871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King WC, Chen JY, Belle SH, et al. Change in Pain and Physical Function Following Bariatric Surgery for Severe Obesity. JAMA. 2016;315(13):1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raoof M, Näslund I, Rask E, et al. Health-Related Quality-of-Life (HRQoL) on an Average of 12 Years After Gastric Bypass Surgery. Obesity Surgery. 2015;25(7):1119–1127. [DOI] [PubMed] [Google Scholar]
- 15.King WC, Chen JY, Belle SH, et al. Use of prescribed opioids before and after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. Jama. 2013;310(13):1369–1376. [DOI] [PubMed] [Google Scholar]
- 17.Wallen S, Szabo E, Palmetun-Ekback M, Naslund I. Use of Opioid Analgesics Before and After Gastric Bypass Surgery in Sweden: a Population-Based Study. Obes Surg. 2018;28(11):3518–3523. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen GS, Smastuen MC, Sandbu R, et al. Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA. 2018;319(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundbom M, Näslund I, Näslund E, Ottosson J. High acquisition rate and internal validity in the Scandinavian Obesity Surgery Registry. Surg Obes Relat Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 20.Hemmingsson E, Johansson K, Eriksson J, Sundstrom J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr. 2012;96(5):953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson K, Sundstrom J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond). 2014;38(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight Loss and Heart Failure: A Nationwide Study of Gastric Bypass Surgery Versus Intensive Lifestyle Treatment. Circulation. 2017;135(17):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bäckryd E, Heilig M, Hoffmann M. Dynamiken i förskrivningen av opioider i Sverige 2000–2015. Läkartidningen. 2017;114:1–6. [PubMed] [Google Scholar]
- 24.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludvigsson JF, Svedberg P, Olen O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. [DOI] [PubMed] [Google Scholar]
- 27.Blackwell M, Iacus S, King G, Porro G. cem: Coarsened exact matching in Stata. Stata Journal. 2009;9(4):524–546. [Google Scholar]
- 28.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014;23(12):1247–1257. [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski ML, Smith VA, Berkowitz TSZ, et al. Long-term opioid use after bariatric surgery. Surg Obes Relat Dis. 2020;16(8):1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK, Pepino MY. Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surg Obes Relat Dis. 2018;14(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klockhoff H, Naslund I, Jones AW. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol. 2002;54(6):587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg. 2013;148(2):145–150. [DOI] [PubMed] [Google Scholar]
- 33.King WC, Chen JY, Courcoulas AP, et al. Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13(8):1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. Jama. 2012;307(23):2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostlund MP, Backman O, Marsk R, et al. Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surg. 2013;148(4):374–377. [DOI] [PubMed] [Google Scholar]
- 36.Hachon L, Reis R, Labat L, et al. Morphine and metabolites plasma levels after administration of sustained release morphine in Roux-en-Y gastric bypass subjects versus matched control subjects. Surg Obes Relat Dis. 2017;13(11):1869–1874. [DOI] [PubMed] [Google Scholar]
- 37.Strommen M, Helland A, Kulseng B, Spigset O. Bioavailability of Methadone After Sleeve Gastrectomy: A Planned Case Observation. Clin Ther. 2016;38(6):1532–1536. [DOI] [PubMed] [Google Scholar]
- 38.Hogestol IK, Chahal-Kummen M, Eribe I, et al. Chronic Abdominal Pain and Symptoms 5 Years After Gastric Bypass for Morbid Obesity. Obes Surg. 2017;27(6):1438–1445. [DOI] [PubMed] [Google Scholar]
- 39.Sandvik J, Hole T, Klöckner CA, Kulseng BE, Wibe A. High-Frequency of Computer Tomography and Surgery for Abdominal Pain After Roux-en-Y Gastric Bypass. Obesity Surgery. 2018(28):2609–2616. [DOI] [PubMed] [Google Scholar]
- 40.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neovius M, Narbro K, Keating C, et al. Health care use during 20 years following bariatric surgery. Jama. 2012;308(11):1132–1141. [DOI] [PubMed] [Google Scholar]
- 43.Pardue B, Thomas A, Buckley J, Suggs WJ. An Opioid-Sparing Protocol Improves Recovery Time and Reduces Opioid Use After Laparoscopic Sleeve Gastrectomy. Obes Surg. 2020;30(12):4919–4925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.