Abstract
Safe depigmenting agents are currently increasing in the cosmetic or pharmaceutical industry because various compounds have been found to have undesirable side effects. Therefore, the present study aimed to investigate the melanogenesis inhibitory effects of Prunus cerasoides Buch. -Ham. D. Don. flower extracts and their molecular mechanism in B16F10 mouse melanoma cells. Moreover, we also examined phenolic and flavonoid contents, antioxidant activity, chemical constituents of potential extracts, and molecular docking. The highest phenolic and flavonoid contents with the greatest scavenging activity were found in the butanol extract of the P. cerasoides flower compared to other extracts. From all extracts, only crude, diethyl ether, and butanol extracts showed an inhibition of mushroom tyrosinase activity, cellular tyrosinase activity, and melanin content as well as the downregulation of the gene expression of the microphthalmia-associated transcription factor (MITF), tyrosinase, tyrosinase-related protein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2) in α-MSH-stimulated B16F10 cells. Based on the molecular docking study, n-hexadecanoic acid, heptadecanoic acid, octadecanoic acid, 9,12-octadecadienoic acid, 9,12,15-octadecanoic acid, and eicosanoic acid might show an inhibitory effect against tyrosinase and MITF. In conclusion, this finding demonstrates that both the diethyl ether and butanol extracts of the P. cerasoides flower can effectively reduce tyrosinase activity and melanin synthesis through the downregulation of the melanogenic gene expression in B16F10 cells and through the molecular docking study. Taken together, the diethyl ether and butanol extracts of the P. cerasoides flower could be an anti-melanogenic ingredient for hyperpigmentary or melasma treatment.
Keywords: Prunus cerasoides, antioxidant activity, phenolic content, tyrosinase, melanin content, molecular docking
Introduction
Melanin is the major color pigment in the human skin which protects the skin from the adverse effects of ultraviolet radiation (UVR) and oxidative stress.1 Overproduction of melanin results in skin hyperpigmentary disorders such as melasma, freckles, solar lentigines, and postinflammatory hyperpigmentation (PIH).2 Thus, the control of the overproduction of melanin synthesis is one of the treatment approaches to reduce abnormal pigmentary disorders for cosmetical or pharmacological use.3
UVR is the major factor in inducing hyperpigmentary disorders. UV-induced DNA damage activates a p53 tumor supressor protein, which binds and promotes the transcription of the proopiomelanocortin (POMC) gene. The POMC is enzymatically cleaved to produce adrenocorticotropic hormone, -melanocyte stimulating hormone (-MSH), and -endorphin. The -MSH binds to the melanocortin 1 receptor (MC1R), a G protein-coupled receptor on a melanocyte, which results in the increased transcription of the microphthalmia-associated transcription factor (MITF).4 The MITF is a significant factor in regulating melanogenic genes including tyrosinase, tyrosinase-related protein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2) which play important roles in melanin synthesis.5
Tyrosinase is the crucial enzyme in the two subsequent steps of melanin synthesis, catalyzing the conversion of L-tyrosine to 3,4-dihydroxyphenylalanine (L-DOPA) and the oxidation of L-DOPA to dopaquinone. Therefore, the first target for melanin synthesis is tyrosinase inhibition, which is one of the effective ways to treat hyperpigmentary disorders.6 There are various known melanogenesis inhibitors such as hydroquinone, kojic acid, arbutin, ascorbic acid, deoxyarbutin, resorcinol, and aloin.7,8 As well known, hydroquinone is the best depigmentation agent which has many side effects such as skin irritation, allergic contact dermatitis, and exogenous ochronosis.9 Kojic and arbutin are commonly used in cosmetic products which have low efficacy, poor stability, and less skin penetration.6 Nowadays, novel compounds derived from plant extracts with anti-melanogenic activity are considered because safety is an important concern.
Prunus cerasoides Buch.-Ham. D. Don. (wild Himalayan cherry) is a dedicious cherry tree found mainly in East Asia, South Asia, and South East Asia. It has traditionally been used in various Ayurveda formulations for skin treatment.10 The stem bark of P. cerasoides has been reported to have an anti-microbial activity which contains diterpenes, cardiac glycosides, phytosterols, saponin, alkaloids, flavonoid, and isoflavones.11,12 The leaf extracts of P. cerasoides have shown the ability to reduce testosterone in the prostate gland and to exhibit anti-diuretic, antioxidant, anti-microbial, anti-elastase, anti-collagenase, anti-inflammatory, and anti-tyrosinase activities; and the leaves are a rich source of phenolics and flavonoids similar to those found in wine made from fruits.13–15 The flowers have been used to treat diuretic and laxative conditions.16 However, there are numerous studies in many fields where the effect of P. cerasoides on melanogenesis has not been elucidated.
In the present study, we aimed to investigate the effect of P. cerasoides flower extracts on tyrosinase activity and melanin synthesis in -MSH-induced B16F10 mouse melanoma cells as well as the molecular mechanism involved. Moreover, we also investigated phenolic and flavonoid contents, antioxidant activity, chemical constituents of potential extracts, and molecular docking (tyrosinase, MC1R, and MITF with potential compounds).
Materials and Methods
Chemicals and Reagents
Analytical grade reagents including acetic acid, butanol, chloroform, diethyl ether, and ethyl acetate were purchased from RCI Labscan, Ltd (Bangkok, Thailand). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 3,4-dihydroxyphenylalanine (L-DOPA), gallic acid, aluminium chloride, quercetin, ascorbic acid, potassium persulfate, kojic acid, tyrosinase, fetal bovine serum (FBS), sodium hydroxide, triton X-100, and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). 2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and Folin-Ciocalteu's phenol reagent were purchased from Sisco Research Laboratories (Mumbai, India). Dulbecco's Modified Eagle Medium/high glucose, penicillin/streptomycin, and trypsin/EDTA were purchased from Thermo Scientific Hyclone (Logan, Utah, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was purchased from Applichem (Darmstadt, Germany). All specific primers were synthesized by Macrogen (Seoul, Korea).
Plant Collection
Mature flowers of Prunus cerasoides Buch.-Ham. D. Don. were collected from highlands reforestation located in Nakhon Thai, Phitsanulok, Thailand (16°25´23.4´´N, 98°47´39.1´´E). A voucher specimen (No. 01549-01551) was authenticated and deposited at Botanic Garden.
Extraction and Fractionation of P. cerasoides Flower Extracts
The gathered flowers of P. cerasoides were air-dried at 30 °C, subsequently oven-dried at 50 °C for 24 h, and then pulverized and stored in an air-tight container. The powdered flowers (25 g) were extracted with 250 mL of acidified aqueous methanol solution containing 1% acetic acid and 50% methanol in deionized water at the ratio of 1:10. The extract was macerated at 4 °C for 7 days with frequent agitation. The extract was centrifuged to remove remaining plant debris and filtered through a 0.2 µM polyethersulfone filter. P. cerasoides crude extract was concentrated at 50 °C for 24 h using an air-circulating drying oven.
Crude extract was completely reconstituted with 250 mL of deionized water and fractioned by different polarities of solvents by liquid-liquid partitioning.17 Firstly, diethyl ether (diethyl ether extract) was used to fractionate non-polar fraction. The aqueous part was further acidified with acetic acid to achieve a pH of 3.5, and the non-phenolic part was fractioned with chloroform (chloroform extract). Then, the acidified aqueous part was alkalinized with NaHCO3 and the fractioned phenolic part with ethyl acetate (ethyl acetate extract). Residual phenolic content was separated by butanol (butanol extract), leaving residual salt solubilized as a residual fraction (aqueous residual extract). Each fraction was concentrated under reduced pressure and at a controlled temperature of 50 °C to give final extracts, which were stored at −20 °C until further use.
Total Phenolic Content
The Folin-Ciocalteu method was chosen to determine the total phenolic content of the individual extracts.18 Briefly, 50 µL of extract solution (400 µg/mL) or standard gallic acid was mixed with the Folin-Ciocalteu reagent in a 96-well plate. Then, 50 µL of Na2CO3 was added into the mixture and stood at room temperature. After 1 h, the absorbance of the mixture was measured using a microplate reader at 750 nm. All tests were performed in triplicate. The total phenolic content of each sample was expressed as mg gallic acid equivalents per g (mg GAE/g) of dry extract.
Total Flavonoid Content
Total flavonoid contents of the P. cerasoides extracts were measured using the aluminium chloride colorimetric method.19 Briefly, 50 µL of extract (400 µg/mL) or quercetin was mixed with 50 µL of 2% AlCl3 solution. The mixture was gently shaken and incubated at room temperature. After 30 min, the absorbance of the mixture was measured at 415 nm against a blank. All tests were run in triplicate. The outcome data were expressed as mg quercetin equivalents per g (mg QE/g) of dry extract.
Antioxidant Activity
DPPH Radical Scavenging Activity
Free radical scavenging activity of the P. cerasoides flower extracts was determined using the DPPH method.20 Briefly, 10 µL of extract solution or ascorbic acid in methanol was added to 90 µL of DPPH working solution (an absorbance of 0.7 ± 0.02 at 517 nm). The mixture was incubated in a dark area for 30 min. The absorbance of each sample was measured at 517 nm against a DPPH working reagent and methanol as a blank. Ascorbic acid served as the positive control. All tests were done in triplicate. The percentage of DPPH scavenging activity was calculated by using the formula: 100 (Acontrol – Asample) / Acontrol. Data were also expressed as the concentration for 50% scavenging activity of DPPH radical (IC50).
ABTS Radical Scavenging Activity
Antioxidant activity of the flower extracts was determined by ABTS assay.21 ABTS•+ radical was prepared by mixing between 7 mM of ABTS and 2.45 mM of potassium persulfate at a ratio of 1:1 and kept in a dark condition at room temperature. After 16 h, the mixture was diluted with methanol until it reached the absorbance values of 0.7 ± 0.02 at 734 nm. Each extract solution or ascorbic acid (10 µL) was mixed with 90 µL of ABTS working solution and kept at room temperature. After 45 min, the decreased absorbance was measured at 734 nm against a blank. All experiments were run in triplicate. The percentage of ABTS free radical scavenging activity was calculated using the formula: 100 × (Acontrol – Asample) / Acontrol. The data were also expressed in terms of the decreased DPPH scavenging activity by 50%.
Evaluation of Mushroom Tyrosinase Activity
Tyrosinase inhibition activity was performed using L-DOPA as a substrate according to a slightly modified method.22 Firstly, the assay was carried out in a 96-well plate with each well containing 20 µL of extract (10 mg/mL) or kojic acid (as the positive control), 20 µL of tyrosinase enzyme (100 units/L), and 160 µL of sodium phosphate buffer (0.1 M, pH 6.8). The reaction was pre-incubated at room temperature. After 10 min, 20 µL of L-DOPA was added into each well and then incubated at 37 °C for 1 h. The absorbance of dopachrome was measured at 475 nm against a blank. All experiments were run in triplicate. The percentage of mushroom tyrosinase activity was calculated using the formula: 100 × (Acontrol – Asample) / Acontrol.
B16F10 Mouse Melanoma Cell Culture
B16F10 mouse melanoma cells were obtained from The American Type Culture Collection (ATCC, Rockville, MD). The cells were cultivated in Dulbecco's Modified Eagle Medium and supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin and streptomycin) at 37 °C and 5% CO2 in a humidified incubator.
MTT Assay and Trypan Blue Dye Exclusion Method
Cytotoxicity assay was carried out using the MTT method.23 Briefly, the B16F10 mouse melanoma cells were seeded in a 96-well plate at a density of 5 × 103 cells per well for 24 h. The cells were treated with 1 µM of -MSH with ether extract or kojic acid for 48 h. After incubation, the MTT reagent (0.5 mg/mL) was added into each well and incubated for an additional 4 h. The insolubilized MTT formazan was solubilized with DMSO and gently shaken. The absorbance of each well was measured at 550 nm using a microplate reader. All tests were performed in triplicate. Surviving cells (%) were expressed and calculated as a percentage of the control (treated with only -MSH).
Cell viability was investigated using a trypan blue exclusion assay.24 Approximately 5 × 103 cells per well were plated in 96-well plates and allowed to attach for 24 h. The cells were different concentrations of each extract and 1 µM of -MSH for another 48 h. After incubation, the cells were washed twice with phosphate buffer solution (PBS) and trypsinized with 0.25% trypsin-EDTA solution. The cells were suspended with PBS and centrifuged to separate the cell pellets. The cells were re-suspended in 100 µL of PBS. Dye exclusion test was performed by mixing 20 µL of the extract-treated cell and 20 µL of trypan blue (0.4%). The mixture was incubated for 2 min at room temperature and loaded in a hemocytometer. Total number of viable and non-viable cell counts were recorded and calculated as total number of viable cells per mL by multiplying the dilution factor for trypan blue. Viable cells (%) were calculated as a percentage of live treated cells with respect to live control cells.
Measurement of Melanin Content
To measure the melanin content, the B16F10 melanoma cells were seeded in a 96-well plate at a density of 1 × 105 cells per well for 24 h.25 Then, the cells were treated with -MSH and each extract or positive control (kojic acid) for an additional 48 h. After treatment, the cells were washed twice with phosphate buffer saline (PBS) and harvested using trypsin and culture medium. The harvested cells were then centrifuged at 15,000 g for 10 min. The cell pellet with a known cell number was dissolved in 1 N NaOH at 70 °C for 1 h. All experiments were done in triplicate. The melanin content was measured at 475 nm using a microplate reader.
Cellular Tyrosinase Activity Assay
Cellular tyrosinase activity was determined using the L-DOPA oxidation method.26 The B16F10 cells (1 × 105 cells) were plated in a 96-well plate and incubated for 24 h. The cells were treated for 48 h with -MSH and each extract or positive control (kojic acid). After incubation, the cells were washed with cold PBS and then lysed with 1% Triton X-100 in PBS. Lysates were centrifuged at 12,000 g for 10 min to obtain the supernatant. The quantity of each lysate was adjusted in 0.1 M of sodium phosphate buffer to obtain equal protein concentration (40 µg) in each sample. The reaction mixture contained 50 µL of each lysate and 50 µL of 2.5 mM L-DOPA in a 96-well plate. The mixture was incubated at 37 °C for 1 h. Dopachrome formation was measured at 475 nm. All tests were run in triplicate. Cellular tyrosinase activity was expressed and calculated as a percentage of the control.
Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
Total RNA was isolated from 48-h -MSH-treated cells with each extract using a Trizol reagent, and 1 µg of total RNA was converted to cDNA using an Accupower® RT Premix & Master Mix (Bioneer, Oakland, CA, USA). Each reaction was performed using a 5 × Hot FIREPol Evagreen qPCR Mix plus (Solis Bio-Dyne Co, Estonia). Quantitative RT-PCR analysis was performed using an ABI StepOne Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Primer sequences were as follows: GAPDH forward 5′ CTTTGTCAAGCTCATTTCCTGG 3’, reverse 5′ TCTTGCTCAGTGTCCTTGC 3’; MITF forward 5′AGGACCTTGAAAACCGACAG 3’, reverse 5′ GGTGGATGGGATAAGGGAAAG 3’; tyrosinase forward 5′ CTAACTTACTCAGCCCAGCATC 3’, reverse 5′ GGGTTTTGGCTTTGTCATGG 3’; TRP-1 forward 5′AGCCCCAACTCTGTCTTTTC 3’, reverse 5′ GGTCTCCCTACATTTCCAGC 3’; TRP-2 forward 5′ TCCAGAAGTTTGACAGCCC 3’, reverse 5′ GGAAGGAGTGAGCCAAGTTATG 3’.27 The reactions were performed in a 96-well plate under the following conditions: initial denaturation at 95 °C for 5 min and followed by 40 cycles at 95 °C for 20 s, 56 °C for 1 min, and 72 °C for 1 min. All experiments were tested in triplicate. Data were analyzed using the method and presented as the mean ± SEM normalized to GAPDH and relative to the control sample.
Identification of Phytochemicals
Phytochemicals present in the chosen P. cerasoides flower extracts were identified using a Gas Chromatography-Agilent 7890B combination with an Agilent 5977A triple quadrupole mass spectrometer (Agilent Technologies Inc, USA). Gas chromatograph-mass spectrometer (GC-MS) analysis is a common confirmation test, which is used to make an effective chemical analysis. An inlet temperature of 250 °C with the split ratio of 7:1 was employed, and helium was used as a carrier gas at the constant flow rate of 7 ml/min. The oven temperature was initially maintained at 50 °C for 5 min and increased at a rate of 5 °C/min to 250 °C for 15 min. For MS detection, an electron ionization mode was used with an ionization energy of 70 eV, ion source temperature of 230 °C, and scan mass range of m/z 30–500. The components were identified based on a correlation of the recorded fragmentation patterns of mass spectra that were provided in the GC-MS system software version Wiley10 and NIST14. All procedures were performed at the Scientific Equipment Center, Prince of Songkla University, Songkhla, Thailand.28 To analyze the biological activity of chemical compounds from GC-MS, phytochemical screening and computational chemistry techniques were used to identify possible new tyrosinase inhibitor candidates. The PubChem database (https://pubmed.ncbi.nlm.gov/) and Molinspiration (http://www.molinspiration.com//cgi/properties) were employed to check the chemical structure and the biological property of these ligand molecules, including the inhibitor of the G-protein coupled receptor (GPCR), nuclear receptor, and enzyme.29
Molecular Docking Study
Based on the bioactivity predictions, the efficiency of the compounds towards the G protein-coupled receptor (GPCR), nuclear receptor (MITF), and tyrosinase enzyme inhibition were initially assessed through molecular docking. The Lamarckian Genetic Algorithm was used for performing molecular docking using AutoDock 4.2, considering the docking parameters from a previously described method.30 A total number of 50 independent dockings ran with a population size of 200 were performed for each ligand. The lowest free energy of binding (G) and inhibition constant (Ki) were considered for choosing the most favorable pose. The molecular interactions between the compounds and receptors were studied using BIOVIA Discovery Studio.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 9.0.0, and all data were presented as mean ± standard error of the mean (SEM). The data were subjected to one-way ANOVA with Dunnett's post-hoc test using a statistical difference of p < 0.05.
Results
Extraction Yield
The extraction yield of P. cerasoides from maceration is shown in Table 1. The percentage of extraction yield was calculated against the initial amount of dry flower material. Extraction with acidified aqueous methanol (crude extract) gave the highest yield compared to all other extracts. The yields of extraction by other solvents decreased in the following order: aqueous residual > butanol > ethyl acetate > diethyl ether > chloroform. The results indicated that the acidified aqueous methanol was the best solvent for extraction.
Table 1.
Extraction Yield, Total Phenolic and Flavonoid Contents of P. cerasoides Flower Extracts.
| Extract | Extraction yield (% w/w of dry weight) | Total phenolic content (mg GAE/g of dry extract) | Total flavonoid content (mg QE/g of dry extract) |
|---|---|---|---|
| Crude | 19.67 | 62.53 3.06 | 18.36 1.58 |
| Diethyl ether | 2.03 | 65.97 2.23 | 59.68 5.81 |
| Chloroform | 1.62 | 1.59 0.09 | 1.68 0.17 |
| Ethyl acetate | 2.09 | 61.96 5.81 | 26.42 1.74 |
| Butanol | 2.40 | 107.17 5.63 | 67.09 5.44 |
| Aqueous residual | 8.12 | 4.78 0.27 | 2.37 1.05 |
Data are expressed as mean ± SEM (n = 3). %w/w: percentage of extract weight per initial weight of dry flower; GAE: gallic acid equivalent; QE: quercetin equivalents.
Total Phenolic Content
Total phenolic content was determined using a Folin-Ciocalteu reagent. The amount of phenolic compounds was expressed as mg gallic equivalent per g (mg GAE/g) of dry extract using the standard curve of gallic acid (Table 1). The values ranged from 1.59 ± 0.09 to 107.17 ± 5.63 mg GAE/g in the following order: butanol > diethyl ether > crude > ethyl acetate > aqueous residual > chloroform. Based on the results of the total phenolic content, the butanol extract was the best source of phenolic compounds.
Total Flavonoid Content
Total flavonoid content of the P. cerasoides flower was expressed as mg quercetin per g (mg QE/g) of dry extract as shown in Table 1. The flavonoid values were recorded in the range of 1.68 ± 0.17 to 67.09 ± 5.44 mg QE/g of dry extract. The content of flavonoid compounds was in the following order: butanol > diethyl ether > ethyl acetate > crude > aqueous residual > chloroform. Therefore, the butanol extract was the richest flavonoid content compared with the other extracts.
Antioxidant Activity
DPPH Radical Scavenging Activity
The results obtained from the free radical scavenging activity of the P. cerasoides extracts are shown in Figure 1a. At a concentration of 100 µg/mL, the scavenging activities of crude, diethyl ether, chloroform, ethyl acetate, butanol, and aqueous residual extracts were 96.42 ± 0.60, 85.85 ± 1.45, 3.01 ± 0.06, 88.69 ± 1.99, 95.14 ± 0.41, and 71.93 ± 1.30%, respectively. By contrast, the scavenging activity of ascorbic acid (10 µg/mL) was 96.43 ± 0.19% (IC50 = 4.42 ± 0.10 µg/mL). As shown in Figure 1b, the greatest DPPH radical scavenging activity with a minimum IC50 value was found in the butanol extract (9.59 ± 0.34 µg/mL), followed by crude (23.53 ± 0.36 µg/mL), ethyl acetate (26.37 ± 0.32 µg/mL), diethyl ether (50.14 ± 0.17 µg/mL), and aqueous residual (907.00 ± 16.33 µg/mL) extracts. A concentration of 50% DPPH radical scavenging activity was not found in the chloroform extract. Most of the scavenging activity was found in the butanol extract compared to the other extracts.
Figure 1.
Antioxidant activity of P. cerasoides extracts and ascorbic acid. (a) DPPH and ABTS radical scavenging activities of P. cerasoides extracts and ascorbic acid; (b) IC50 values of radical scavenging activities of P. cerasoides extracts. The values are the mean ± SEM from three independent experiments. Ascorbic acid served as the positive control.
ABTS Radical Scavenging Activity
The ABTS scavenging potency of P. cerasoides flower extracts was evaluated and presented in Figure 1a. At a concentration of 100 µg/mL, the ABTS scavenging activities of crude, diethyl ether, and ethyl acetate were more than 90%. By contrast, a lower scavenging activity was found in chloroform (17.74 ± 0.47%) and aqueous residual (12.72 ± 0.27%) extracts. The radical scavenging activity of ascorbic acid (10 µg/mL) was 94.44 ± 0.08% (IC50 = 4.92 ± 0.03 µg/mL). As shown in Figure 1b, it was found that the butanol extract possessed the strongest ABTS scavenging activity with the lowest IC50 value of 15.03 ± 0.38 µg/mL, followed by crude (16.91 ± 0.22 µg/mL), diethyl ether (22.36 ± 0.18 µg/mL), ethyl acetate (34.56 ± 0.33 µg/mL), chloroform (568.72 ± 8.79 µg/mL), and aqueous residual (628.79 ± 3.79 µg/mL) extracts. Thus, the butanol extract had the strongest scavenging activity compared to the other extracts.
Anti-Mushroom Tyrosinase Activity
To determine whether P. cerasoides flower extracts showed an inhibitory effect against the key enzyme in melanin synthesis, in vitro cell-free mushroom tyrosinase activity was carried out. Kojic acid (0.05 mg/mL) served as the positive control, which significantly reduced the tyrosinase enzyme at p < 0.001 (IC50 = 0.032 ± 0.004 mg/mL). The effect of each extract is represented in Figure 2. At a concentration of 10 mg/mL, significant inhibitory activity was found in crude, diethyl ether, butanol, and aqueous residual extracts. By contrast, chloroform and ethyl acetate extracts did not decrease mushroom tyrosinase activity when compared to the control group. The minimum IC50 value was found in the diethyl ether extract (14.83 ± 0.08 mg/mL), followed by butanol (14.97 ± 0.26 µg/mL), crude (17.58 ± 0.56 µg/mL), and aqueous residual (35.00 ± 0.01 µg/mL) extracts. The diethyl ether extract showed the greatest inhibitory activity against tyrosinase enzyme with the lowest IC50 value.
Figure 2.
Anti-mushroom tyrosinase activity of P. cerasoides extracts and kojic acid. (a) Tyrosinase activities of P. cerasoides extracts compared with the control; (b) IC50 of tyrosinase inhibition of P. cerasoides extracts and kojic acid. Kojic acid (0.050 mg/mL) served as the positive control. Average data (n = 3) are presented with SEM. A value of **p < 0.01 and ***p < 0.001 was performed by one-way ANOVA and compared to the control group without any extract.
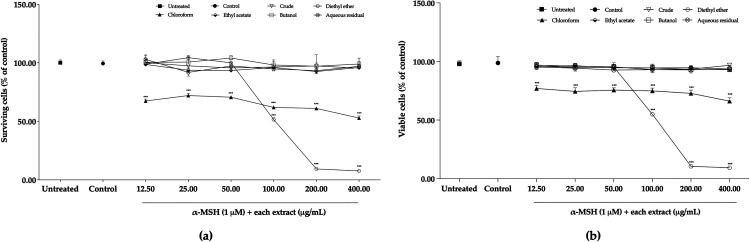
Effect of P. cerasoides Extracts on Cytotoxicity and Cell Viability of B16F10 Mouse Melanoma Cells
To examine the concentration of each extract without a cytotoxicity effect, the B16F10 cells were treated with 1 µM of -MSH and each extract at concentrations of 12.50–400.00 µg/mL. The results from the MTT assay (Figure 3a) showed that there were no differences between the untreated cells (no treatment) and control cells (treated with only -MSH). The chloroform extract had a cytotoxic effect on the B16F10 cells in every concentration, so it was not used for the next experiment. While the diethyl ether extract had some cytotoxic effect in a concentration-dependent manner, the concentration was not toxic in the cells at concentrations of 12.50–50.00 µg/mL. Under the concentrations of 12.50–400.00 µg/mL, crude, ethyl acetate, and aqueous residual extracts exhibited no cytotoxic effect on the cells. However, kojic acid at 125.00–500.00 µg/mL showed no apparent cytotoxicity to the cells.
Figure 3.
Effect of P. cerasoides extracts on cytotoxicity and cell viability of B16F10 mouse melanoma cells. The symbols indicate the percentage of surviving cells and viable cells of cells treated with 1 μM of α-MSH and each extract in indicated concentrations (12.50-400.00 μg/mL) for 48 h and subjected to (a) MTT assay and (b) trypan blue dye exclusion method. Data are represented from three independent experiments and shown as mean with standard errors of mean in triplicate; ***p < 0.001 (compared to the control group).
Trypan blue dye exclusion method was performed to measure changes in viable cell number by the extracts. As shown in Figure 3b, increasing the concentrations of chloroform extract from 12.50–400.00 µg/mL reduced cell viability, similar to that observed in the MTT test. Diethyl ether-treated cells at 100.00–400.00 µg/mL showed a significant reduction in viability. While crude, ethyl acetate, and aqueous residual extracts at 12.50–400.00 µg/mL showed no difference in viable cells when compared to control cells. The results obtained from the trypan blue dye exclusion method also matched with that obtained using MTT assay. To determine the anti-melanogenic activity of the P. cerasoides extract, we chose three non-toxic concentrations of crude (100.00-400.00 µg/mL), diethyl ether (12.50-50 µg/mL), ethyl acetate (100.00-400.00 µg/mL), butanol (100.00-400.00 µg/mL), and aqueous residual (100.00-400.00 µg/mL) extracts.
Effect of P. cerasoides Extracts on Cellular Melanin Content
To determine anti-melanogenic activity, the inhibitory activity of each extract was assessed for melanin content in the B16F10 cells. As shown in Figure 4, the melanin content in the control group (only -MSH) significantly increased when compared with that of the untreated group. Kojic acid (500 µg/mL) served as the positive control, which significantly reduced the amount of melanin. Crude (200 and 400 µg/mL), diethyl ether (12.50, 25, and 50 µg/mL), and butanol (200 and 400 µg/mL) extracts had an inhibitory effect on the melanin content. However, the ethyl acetate extract-treated group at concentrations of 200 and 400 µg/mL had significantly increased the melanin content compared with the control group. By contrast, the aqueous residual extract did not exhibit melanin inhibition. From all results, crude, diethyl ether, and butanol extracts effectively decreased the melanin content in a dose-dependent manner.
Figure 4.
Effect of P. cerasoides extracts on melanin content. The B16F10 mouse melanoma cells were treated with 1 μM of α-MSH in the presence of non-toxic concentrations of each extract for 48 h. Each percentage of bar for the treated cells is shown to compare with the control group. The results are shown as the mean with standard errors of mean and are representative of three independent experiments; ###p < 0.001 compared with the untreated group; **p < 0.01, ***p < 0.001 compared with the control group.
Effect of P. cerasoides Extracts on Cellular Tyrosinase Activity
The effect of P. cerasoides extract on intracellular tyrosinase activity was examined in the B16F10 mouse melanoma cells. The cells were treated with 1 µM of -MSH and each chosen extract or kojic acid (500 µg/mL) for 48 h. As a result, the control group (stimulated with only -MSH) significantly increased the intracellular tyrosinase activity when compared to the untreated group (without any treatment) as shown in Figure 5. Crude (400 µg/mL), diethyl ether (12.50, 25, and 50 µg/mL), and butanol (200 and 400 µg/mL) extracts significantly decreased tyrosinase activity compared with that of the control group. By contrast, the ethyl acetate extract (100 and 200 µg/mL) markedly increased tyrosinase activity compared to the control group. However, the aqueous residual extract did not exhibit tyrosinase inhibition activity. Kojic acid as the positive control (500 µg/mL) significantly decreased tyrosinase activity compared to the control group. The inhibition tyrosinase activity of diethyl ether (50 µg/mL) and butanol (400 µg/mL) extracts was higher than kojic acid. From all results, the concentrations of crude, diethyl ether, and butanol extracts decreased the intracellular tyrosinase activity in a concentration-dependent manner.
Figure 5.
Effect of P. cerasoides extracts on intracellular tyrosinase activity. The B16F10 cells were treated with α-MSH and each extract in non-toxic concentrations for 48 h. Results are presented as a percentage based on the control group. Data are expressed as mean with standard errors of mean (n = 3); ###p < 0.001 compared with the untreated group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
Effect of P. cerasoides Extracts on the Expression of Melanogenesis-Related Genes
We examined the mRNA expression of MITF, tyrosinase (TYR), tyrosinase-related protein-1 (TRP-1), and tyrosinase-related protein-2 (TRP-2), which are involved in melanogenesis. From all results, the mRNA levels of MITF TYR, TRP-1, and TRP-2 in the control group (stimulated with only -MSH) were significantly increased when compared with the untreated group (without any treatment). As shown in Figure 6a-d, the crude extract at 200 and 400 µg/mL downregulated the mRNA expression of MITF (by 0.49-fold, 0.63-fold), TYR (by 0.79-fold, 0.84-fold), TRP-1 (by 0.57-fold, 0.85-fold) and TRP-2 (by 0.51-fold, 0.61-fold), when compared the control group. Diethyl ether extract at 50 µg/mL caused the reduction of MITF, TYR, TRP-1, TRP-2 transcripts of 0.22-fold, 0.41-fold, 0.69-fold, and 0.34-fold, respectively, in comparison with control. While, butanol extract at 200, and 400 µg/mL decreased the expression of MITF (by 0.64-fold, 0.81-fold), TYR (by 0.63-fold, 0.75 fold), TRP-1 (by 0.12-fold, 0.15-fold) and TRP-2 (by 0.74-fold, 0.80-fold), relative to control group. However, kojic acid (500 µg/mL) as a positive control significantly reduced the level of MITF, TYR, and TRP-1 by 0.18-fold, 0.76-fold, and 0.85-fold, respectively, of the control group levels. But the positive control did not decrease the TRP-2 mRNA level. The results indicate that crude, diethyl ether, and butanol extracts decreased melanin synthesis through the downregulation of the expression of MITF, TYR, TRP-1, and TRP-2.
Figure 6.
Effect of P. cerasoides extracts on the expression of melanogenesis-related genes. The B16F10 cells were co-treated with α-MSH and each extract in non-toxic concentrations for 48 h. After treatment, the mRNA expression of (a) MITF, (b) TYR, (c) TRP-1, and (d) TRP-2 was determined, normalized to GAPDH expression, and compared to the control group (α-MSH-treated group). Values are represented as the mean with standard errors of mean (n = 3); #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the untreated group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group.
Phytochemical Constituents Identified in Selected P. cerasoides Extracts and Bioactivity Prediction
Phyto-compounds were described and characterized by comparing the mass spectra of the constituents of the Wiley10 and NIST14 libraries. We selected crude, diethyl ether, and butanol extracts, which decreased melanin content, tyrosinase activity, and gene expression of melanogenesis-related genes. GC-MS analysis of three selected P. cerasoides extracts with various phytochemicals that contributed to the plant's medicinal activities is shown in Table 2. The most prevailing compounds in the crude extracts from 37 compounds were acetic acid (52.57%), benzoic acid (7.67%), dihyrdroxyacetone (6.44%), 1,2-cyclopentanedione (6.28%), 2-oxopropanol (3.96%), 3-hydroxy-2,3-dihydromaltol (2.28%). From the 41 compounds in the diethyl ether extract, 5 compounds were reported to have high content including acetic acid (22.70%), benzoic acid (20.33%), n-hexadecanoic acid (12.20%), 9,12-octadecadienoic acid or alpha-linoleic acid (6.70%), and octadecanoic acid (6.20%). Quantitative analysis of the butanol extract showed that it contained predominantly the top five high chemical contents from 32 compounds such as benzoic acid (37.13%), acetic acid (15.43%), hydrocoumarin (6.18%), dihydroxyacetone (4.03%), and mandelamide (3.02%). To screen the bioactivity of each compound from the GC-MS profiles, a SMILE format from a total of 100 compounds was initially obtained from the Pubchem database and investigated by Molinspiration cheminformatics against the G-protein coupled receptor (GPCR), nuclear receptor, and enzyme. The Molinspiration bioactivity had a value > 0.00, which showed good bioactivity. As shown in Table 2, the results showed that only one compound (3,6-anhydroglucose)) had enzyme inhibitory activity, which might be represented as anti-tyrosinase activity. By contrast, the diethyl ether extract had 20 compounds that represented an inhibitory effect against the enzyme. Moreover, 14 and 15 molecules from the diethyl ether extract had inhibitory activity against the GPCR and nuclear receptor, respectively. Three potential molecules (hexadecanoic acid, octadecanoic acid, and levoglucosan) in the butanol extract were qualified in bioactivity screening. Thus, a list of 25 compounds from all fractions was further selected for the virtual study using molecular docking against the melanocortin 1 receptor, microphthalmia-associated transcription factor, and tyrosinase enzyme.
Table 2.
Chemical Constituents in Crude, Diethyl Ether, and Butanol Extracts of P. cerasoides Flower by GC-MS and Bioactivity Prediction.
| Retention Time | Identified Compound | Nature of compounds | Molecular weight (g/mol) | Formula | Area (%) | Molinspiration bioactivity | ||
|---|---|---|---|---|---|---|---|---|
| GPCR ligand | Nuclear receptor ligand | Enzyme inhibitor | ||||||
| Crude extract | ||||||||
| 8.0461 | (S)-Propane-1,2-diol | Propanone | 76.09 | C3H8O2 | 0.08 | No | No | No |
| 8.3076 | 2-Butenoic acid | Fatty acid | 86.09 | C4H6O2 | 0.21 | No | No | No |
| 8.6795 | 2-Oxopropanol | Propanone | 74.08 | C3H6O2 | 3.96 | No | No | No |
| 8.9575 | 2-Hydroxyethanol | Aldehyde | 60.05 | C2H4O2 | 2.09 | No | No | No |
| 11.7343 | Vinylidenglycol | Aldehyde | 86.09 | C4H6O2 | 1.20 | No | No | No |
| 12.0736 | Acetic acid | Carboxylic acid | 60.05 | C2H4O2 | 52.57 | No | No | No |
| 12.9801 | Methylpyruvate | Pyruvate ester | 102.09 | C4H6O3 | 2.26 | No | No | No |
| 13.8117 | Formic acid | Carboxylic acid | 46.025 | CH2O2 | 2.14 | No | No | No |
| 14.8044 | Propanoic acid | Fatty acid | 74.08 | C3H6O2 | 0.11 | No | No | No |
| 15.1245 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furanone | Furans | 144.12 | C6H8O4 | 0.23 | No | No | No |
| 15.9364 | 4-Cyclopentene-1,3-dione | Organooxygen | 96.08 | C5H4O2 | 0.57 | No | No | No |
| 17.3997 | 2-Furanmethanol | Furans | 98.1 | C5H6O2 | 1.04 | No | No | No |
| 19.3275 | 2(5H)-furanone | Furans | 84.07 | C4H4O2 | 0.14 | No | No | No |
| 19.6704 | 1,2-Cyclopentanedione | Organooxygen | 98.1 | C5H6O2 | 6.28 | No | No | No |
| 21.6283 | 3-Hydroxy-2,3-dihydromaltol | Pyrans | 144.12 | C6H8O3 | 0.09 | No | No | No |
| 24.1612 | 5,6-Dihydropyrran-2,5-dione | Pyrans | 112.08 | C5H4O3 | 0.59 | No | No | No |
| 24.3729 | Phenol | Phenol | 94.11 | C6H6O | 0.13 | No | No | No |
| 25.0416 | 2,5-Dimethylfuran-3,4(2H,5H)-dione | Furans | 128.13 | C6H8O3 | 0.40 | No | No | No |
| 25.7342 | Dihydroxyacetone | Ketotriose | 90.08 | C3H6O3 | 6.44 | No | No | No |
| 25.9985 | 3,6-Anhydroglucose | Aldehyde and an anhydrohexose | 162.14 | C5H8O4 | 0.81 | No | No | Yes |
| 27.4257 | 2-Hydroxy-gamma-butyrolactone | Furans | 130.14 | C4H6O3 | 1.37 | No | No | No |
| 27.6870 | 2-Acetyltetrahydrofuran | Furans | 128.13 | C6H10O2 | 0.20 | No | No | No |
| 29.1215 | 3-Hydroxy-2,3-dihydromaltol | Pyrans | 144.12 | C6H8O4 | 2.28 | No | No | No |
| 29.4828 | Hydrocoumarin | Coumarin | 162.14 | C9H8O2 | 1.18 | No | No | No |
| 29.9223 | 12,3-Propanetriol | Glycerol | 95.11 | C3H8O3 | 0.94 | No | No | No |
| 30.4405 | 2-Acetyl-2-hydroxy-gamma-butyrolactone | Gamma-lactone | 128.13 | C6H8O4 | 0.60 | No | No | No |
| 31.3470 | 4-Vinylphenol | Benzenoids | 120.15 | C8H8O | 1.64 | No | No | No |
| 31.9565 | Benzoic acid | Benzenoids | 122.12 | C7H6O2 | 7.67 | No | No | No |
| 32.5056 | Coumarin | Coumarin | 146.14 | C9H6O2 | 0.46 | No | No | No |
| 32.7612 | (S)-(+)-2’,3'-Dideoxyribonolactone | Lactones | 116.12 | C5H8O3 | 0.19 | No | No | No |
| 33.1293 | 5-Hydroxymethylfurfural | Furans | 126.11 | C6H6O3 | 0.54 | No | No | No |
| 34.0618 | Phenylacetic Acid | Benzenoids | 136.15 | C8H8O2 | 0.17 | No | No | No |
| 34.5939 | 3-Hydroxybutyrolactone | Furans | 102.09 | C4H6O3 | 0.39 | No | No | No |
| 40.6004 | Decanoic acid | Fatty acid | 172.26 | C10H20O2 | 0.10 | No | No | No |
| 42.3558 | 1,4-Benzenediol | Phenolic | 151.16 | C6H6O2 | 0.13 | No | No | No |
| 42.5682 | 2-Oxobutanoic acid | Keto acid | 106.07 | C4H6O3 | 0.27 | No | No | No |
| 45.3844 | Mandelamide | Benzenoids | 151.16 | C8H9NO2 | 0.52 | No | No | No |
| Diethyl ether extract | ||||||||
| 6.0189 | 1-Butanol | Primary alcohol | 74.12 | C4H10O | 0.05 | No | No | No |
| 8.8271 | Hydroxyacetone | Propanone | 74.08 | C3H6O2 | 0.08 | No | No | No |
| 9.1068 | 2-Hydroxyacetaldehyde | Aldehyde | 60.05 | C2H4O2 | 0.06 | No | No | No |
| 12.1713 | Acetic acid | Carboxylic acid | 60.05 | C2H4O2 | 22.70 | No | No | No |
| 19.9235 | Hydroxycyclopentenone | Organooxygen | 98.1 | C5H6O2 | 0.22 | No | No | No |
| 24.4439 | Phenol | Phenol | 94.11 | C6H6O | 0.54 | No | No | No |
| 25.0517 | Dodecyl acrylate | Fatty acid | 240.38 | C15H28O2 | 0.55 | No | No | No |
| 27.6641 | Nonanoic acid | Fatty acid | 158.24 | C9H18O2 | 0.20 | No | No | No |
| 28.0549 | 4-Vinylguaiacol | Phenolic | 150.17 | C9H10O2 | 1.30 | No | No | No |
| 28.2295 | (E)-1-(Benzylamino)-3-penten-2-ol | Alkaloids | 175.23 | C12H17NO | 0.16 | No | No | No |
| 28.6571 | 1,3-Diacetin | Fatty acid | 176.17 | C7H12O5 | 0.04 | No | No | No |
| 29.0309 | Methyl palmitate | Fatty acid | 370.6 | C17H34O2 | 1.65 | No | Yes | Yes |
| 29.3732 | Hydrocoumarin | Coumarin | 162.14 | C9H8O2 | 2.00 | No | No | No |
| 30.0704 | Phenol, 2,4-bis-(1,1-dimethylethyl) | Phenolic | 220.35 | C14H22O | 0.77 | No | Yes | No |
| 30.9181 | Methyl nonylate | Fatty acid | 186.29 | C10H20O2 | 0.07 | No | No | No |
| 31.2808 | 2-Hydroxycinnamic acid | Cinnamic acids | 164.16 | C9H8O3 | 8.80 | No | No | No |
| 31.8562 | Benzoic acid | Benzenoids | 122.12 | C7H6O2 | 20.33 | No | No | No |
| 32.4877 | Methyl stearate | Fatty acid | 298.5 | C19H38O2 | 0.90 | No | Yes | Yes |
| 32.7777 | Methyl elaidate | Fatty acid methyl ester | 296.5 | C19H36O2 | 0.13 | Yes | Yes | Yes |
| 33.1243 | [Dodecanoyl(methyl)amino]acetic acid | Acetate salt | 271.4 | C15H29NO3 | 0.29 | Yes | No | Yes |
| 33.4785 | Methyl linolelaidate | Fatty acyls | 294.5 | C19H34O2 | 2.66 | Yes | Yes | Yes |
| 34.4959 | alfa-Methyl linolenate | Fatty acyls | 292.5 | C19H32O2 | 0.60 | Yes | Yes | Yes |
| 34.9177 | 1-Hexadecanol | Fatty alcohol | 242.44 | C16H34O | 0.16 | No | No | Yes |
| 35.3693 | Phytol | Diterpenoid | 296.5 | C20H40O | 0.67 | Yes | Yes | Yes |
| 35.7587 | Methyl arachidate | Fatty acid | 326.6 | C21H42O2 | 0.15 | Yes | Yes | Yes |
| 36.4122 | Myristic acid | Fatty acid | 228.37 | C14H28O2 | 0.35 | No | No | Yes |
| 36.8447 | Mandenol | Fatty acid | 308.5 | C20H36O2 | 0.29 | Yes | Yes | Yes |
| 37.9240 | Methyl icosa-11,14,17-trienoate | Fatty acid | 320.5 | C21H36O2 | 0.18 | Yes | Yes | Yes |
| 40.4667 | n-Hexadecanoic acid | Fatty acid | 256.42 | C16H32O2 | 12.20 | Yes | Yes | Yes |
| 40.9472 | Methyl 4-hydroxybenzoate | Benzenoids | 175.14 | C8H8O3 | 0.22 | No | No | No |
| 41.1072 | 4-Hydroxybenzaldehyde | Benzaldehyde | 122.12 | C7H6O2 | 0.15 | No | No | No |
| 41.9797 | 1-Tetradecanol | Long-chain fatty alcohol | 214.36 | C14H30O | 0.26 | No | No | No |
| 42.3241 | Heptadecanoic acid | Long-chain fatty alcohol | 270.5 | C17H34O2 | 0.45 | Yes | Yes | Yes |
| 42.7127 | 2-Tridecenoic acid | Long-chain fatty acid | 212.33 | C13H24O2 | 0.91 | No | No | Yes |
| 43.7417 | Octadecanoic acid (or Stearic acid) | Long-chain fatty acid | 284.5 | C18H36O2 | 6.20 | Yes | Yes | Yes |
| 43.9641 | Methyl ferulate | Phenylpropanoids | 208.21 | C11H12O4 | 1.11 | No | No | No |
| 44.975 | 9,12-Octadecadienoic acid (or alpha-Linoleic acid) | Fatty acid | 280.4 | C18H32O2 | 6.70 | Yes | Yes | Yes |
| 46.186 | 9,12,15-Octadecatrienoic acid (or Linolenic acid) | Fatty acid | 278.4 | C18H30O2 | 3.98 | Yes | Yes | Yes |
| 47.3832 | Eicosanoic acid | Fatty acid | 312.5 | C20H40O2 | 0.77 | Yes | Yes | Yes |
| 48.9748 | Methyl 4-hydroxycinnamate | Phenylpropanoids | 178.18 | C10H10O3 | 0.74 | No | No | No |
| 49.4327 | 1-Octadecanol | Long-chain fatty alcohol | 270.5 | C18H38O | 0.44 | No | Yes | Yes |
| Butanol extract | ||||||||
| 9.0786 | 2-Oxopropanol | Propanone | 74.08 | C3H6O2 | 2.48 | No | No | No |
| 11.8739 | Vinylidenglycol | Aldehyde | 86.09 | C4H6O2 | 1.28 | No | No | No |
| 12.3799 | Acetic acid | Carboxylic acid | 60.05 | C2H4O2 | 15.43 | No | No | No |
| 13.1593 | Methyl pyruvate | Pyruvate ester | 102.09 | C4H6O3 | 1.11 | No | No | No |
| 14.0764 | Formic acid | Carboxylic acid | 46.025 | CH2O2 | 1.15 | No | No | No |
| 15.2171 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furanone | Furans | 144.12 | C6H8O4 | 1.08 | No | No | No |
| 16.0629 | 4-Cyclopentene-1,3-dione | Organooxygen | 96.08 | C5H4O2 | 0.24 | No | No | No |
| 17.4520 | 2-Furanmethanol | Furans | 98.1 | C5H6O2 | 0.86 | No | No | No |
| 19.8171 | 1,2-Cyclopentanedione | Organooxygen | 98.1 | C5H6O2 | 2.59 | No | No | No |
| 21.6775 | Dihydromaltol | Pyrans | 144.12 | C6H8O3 | 0.16 | No | No | No |
| 21.8304 | 4-Hydroxy-5,6-dihydro-(2H)-pyran-2-one | Pyrans | 114.1 | C5H6O3 | 0.42 | No | No | No |
| 24.2638 | 5,-Dihydro-2-pyranone | Pyrans | 98.1 | C5H4O3 | 0.26 | No | No | No |
| 24.4298 | Phenol | Phenol | 94.11 | C6H6O | 0.45 | No | No | No |
| 25.0922 | Furaneol | Furans | 128.13 | C6H8O3 | 0.45 | No | No | No |
| 25.7914 | Dihydroxyacetone | Ketotriose | 90.08 | C3H6O3 | 4.03 | No | No | No |
| 26.6149 | 2-Hydroxy-3-oxobutanal | Butanal | 102.09 | C4H6O3 | 0.50 | No | No | No |
| 27.4882 | Vinylcarbinol | Phenolic | 261.8 | C3H6O | 0.41 | No | No | No |
| 28.0895 | 4-Vinylguaiacol | Phenolic | 150.17 | C9H10O2 | 1.60 | No | No | No |
| 28.2467 | 4-Methylbenzaldehyde | Tolualdehyde | 120.15 | C8H8O | 0.39 | No | No | No |
| 29.1389 | 3-Hydroxy-2,3-dihydromaltol | Pyrans | 144.12 | C6H8O4 | 2.13 | No | No | No |
| 29.4294 | Hydrocoumarin | Coumarin | 162.14 | C9H8O2 | 6.18 | No | No | No |
| 30.4137 | 2-Acetyl-2-hydroxy-gamma-butyrolactone | Gamma-lactone | 128.13 | C6H8O4 | 0.67 | No | No | No |
| 31.3186 | 2-Hydroxycinnamic acid | Phenylpropanoids | 164.16 | C9H8O3 | 9.61 | No | No | No |
| 31.9108 | Benzoic acid | Benzenoids | 122.12 | C7H6O2 | 37.13 | No | No | No |
| 32.4883 | Coumarin | Coumarin | 146.14 | C9H6O2 | 1.72 | No | No | No |
| 33.1439 | 5-Hydroxymethylfurfural | Furans | 126.11 | C6H6O3 | 0.78 | No | No | No |
| 34.0617 | Phenylacetic acid | Carboxylic acid | 60.05 | C8H8O2 | 0.23 | No | No | No |
| 34.6087 | 3-Hydroxybutyrolactone | Furans | 102.09 | C4H6O3 | 0.26 | No | No | No |
| 40.5704 | Hexadecanoic acid | Fatty acid | 256.42 | C16H32O2 | 0.95 | Yes | Yes | Yes |
| 43.8071 | Octadecanoic acid | Long-chain fatty acid | 284.50 | C18H36O2 | 0.95 | Yes | Yes | Yes |
| 45.3815 | Mandelamide | Amide | 151.16 | C8H9NO2 | 3.02 | No | No | No |
| 47.1149 | Levoglucosan | Anhydrohexose | 162.14 | C6H10O5 | 1.46 | No | No | Yes |
Molecular Docking Study
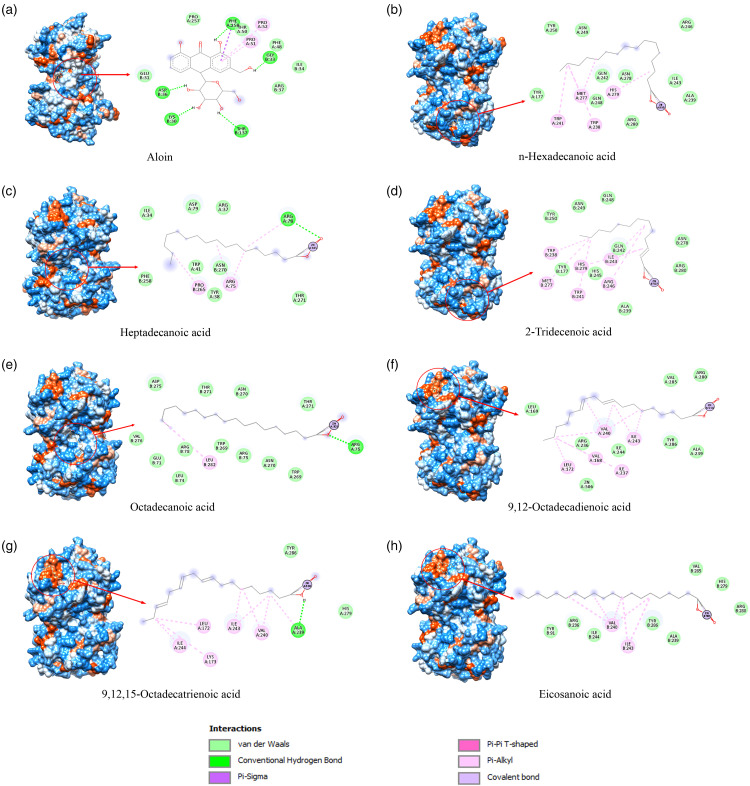
Tyrosinase enzyme is a multifunctional copper-containing enzyme that is responsible for skin pigmentation. This enzyme is controlled by binding between the melanocortin-1 receptor and -MSH molecule, which further stimulates the microphthalmia-associated transcription factor (MITF) for binding to a promoter of melanogenesis-related proteins. Thus, tyrosinase inhibition is a target for hyperpigmentation or melasma treatment. As shown in Table 3, n-hexadecanoic acid, heptadecanoic acid, 2-tridecenoic acid, octadecanoic acid (or stearic acid), 9,12-octadecadienoic acid (or alpha-linoleic acid), 9,12,15-octadecatrienoic acid (or linolenic acid) and eicosanoic acid were potential molecules that showed strong binding affinity against tyrosinase with better binding energy and inhibition constant when compared to all positive drugs or known inhibitors for tyrosinase. Among potent biomolecules, the 2-tridecenoic acid compound has shown the strongest binding affinity with a binding energy at −10.98 kcal/mol and an inhibition constant at 8.99 nM (Table 3). Moreover, the 2-tridecenoic acid reacted with the tyrosinase pocket site at the amino acid residues including TYR177, TRP238, ALA239, TRP241, GLN242, ILE243, HIS245, ARG246, GLN248, ASN249, TYR250, MET277, ASN278, HIS279, ARG280, and also zinc metal molecule (Figure 7D). The binding site of other potential compounds and aloin molecules (positive control) against tyrosinase is represented in Figure 7, and most of the compounds formed hydrogen bonding, van der Waals, and hydrophobic interaction to the target protein. In addition to the tyrosinase enzyme, the MITF was also inhibited with better binding affinity than the positive control by n-hexadecanoic acid, heptadecanoic acid, octadecanoic acid, 9,12-octadecadienoic acid, 9,12,15-octadecatrienoic acid, and eicosanoic acid (Table 3). The similar pocket binding pocket sites of these compounds are represented in Figure 8 with van der Waals and hydrophobic interaction to the MITF protein at amino acid molecules such as GLU28, ARG32, LEU35, ASN31, GLU31, HIS30, LYS27, and HIS34 of Chain A and B of the protein (Figure 8B-8F). Therefore, the docking results indicate that the potential compounds were found in the diethyl ether and butanol extracts of the P. cerasoides flower, which might inhibit the tyrosinase and MITF proteins.
Table 3.
The Binding Energies and Inhibition Constants of Selected Compounds Derived from Crude, Diethyl Ether, and Butanol Extracts Docked Against Molecular Targets.
| Compounds | Nature of compounds | Tyrosinase (PDB 3NQ1) | Melanocortin 1 receptor (Melanocyte-stimulating hormone receptor; Q01726) | Microphthalmia-associate transcription factor (PDB 4C7N) | |||
|---|---|---|---|---|---|---|---|
| ΔG (kcal mol−1) | Ki | ΔG (kcal mol−1) | Ki | ΔG (kcal mol−1) | Ki | ||
| Positive control | |||||||
| Hexylresorcinol | −6.23 | 27.35 µM | −4.79 | 310.39 µM | −3.30 | 3.83 mM | |
| Deoxyarbutin | −6.24 | 26.82 µM | −5.69 | 67.92 µM | −4.09 | 1.00 mM | |
| Kojic acid | −4.91 | 250.04 µM | −5.53 | 88.49 µM | −3.25 | 4.07 mM | |
| Aloin | −6.75 | 11.35 µM | −5.42 | 106.64 µM | −4.05 | 1.08 mM | |
| Compounds from crude extract | |||||||
| 3,6-Anhydroglucose | Aldehyde and an anhydrohexose | −4.68 | 370.39 µM | ND | ND | ND | ND |
| Compounds from diethyl ether extract | |||||||
| Methyl palmitate | Fatty acid | −4.10 | 990.94 µM | ND | ND | −1.89 | 41.03 mM |
| Phenol, 2,4-bis(1,1-dimethylethyl) | Phenolic | ND | ND | ND | ND | −4.25 | 769.09 µM |
| Methyl stearate | Fatty acid | −6.67 | 12.83 µM | ND | ND | ND | ND |
| Methyl elaidate | Fatty acid methyl ester | −5.89 | 48.06 µM | −4.62 | 412.86 µM | −1.41 | 93.28 mM |
| [Dodecanoyl(methyl)amino] acetate | Acetate salt | −5.72 | 63.76 µM | −4.47 | 528.58 µM | ND | ND |
| Methyl linolelaidate | Fatty acyls | −5.51 | 91.48 µM | −4.85 | 278.75 µM | −2.27 | 21.77 mM |
| alfa-Methyl linolenate | Fatty acyls | −5.49 | 94.48 µM | −4.98 | 223.89 µM | −1.95 | 37.20 mM |
| 1-Hexadecanol | Fatty alcohol | −5.90 | 47.01 µM | ND | ND | ND | ND |
| Phytol | Diterpenoid | −5.12 | 175.73 µM | −5.20 | 155.48 µM | −1.94 | 38.12 mM |
| Methyl arachidate | Fatty acid | −2.75 | 9.68 mM | −3.64 | 2.14 mM | −0.97 | 196.15 mM |
| Myristic acid | Fatty acid | −5.56 | 83.88 µM | ND | ND | ND | ND |
| Mandenol | Fatty acid | −4.69 | 362.64 µM | −4.55 | 459.29 µM | −1.36 | 101.50 mM |
| Methyl icosa-11,14,17-trienoate | Fatty acid | −4.69 | 362.64 µM | −4.33 | 667.11 µM | −2.36 | 18.64 mM |
| n-Hexadecanoic acid | Fatty acid | −9.47 | 114.89 nM | −3.96 | 1.26 mM | −8.27 | 874.24 nM |
| Heptadecanoic acid | Long-chain fatty alcohol | −8.83 | 339.11 nM | −3.89 | 1.40 mM | −7.56 | 2.88 µM |
| 2-Tridecenoic acid | Long-chain fatty acid | −10.98 | 8.99 nM | ND | ND | ND | ND |
| Octadecanoic acid (or Stearic acid) | Long-chain fatty acid | −8.50 | 591.97 nM | −3.81 | 1.61 mM | −7.79 | 1.94 µM |
| 9,12-Octadecadienoic acid (or alpha-Linoleic acid) | Fatty acid | −9.21 | 176.05 nM | −4.42 | 578.89 µM | −8.59 | 506.96 nM |
| 9,12,15-Octadecatrienoic acid (or Linolenic acid) | Fatty acid | −9.94 | 51.34 nM | −5.01 | 213.72 µM | −8.62 | 476.37 nM |
| Eicosanoic acid | Fatty acid | −8.36 | 748.79 nM | −3.19 | 4.60 mM | −7.46 | 3.38 µM |
| 1-Octadecanol | Long-chain primary fatty alcohol | −5.36 | 117.28 µM | ND | ND | ND | ND |
| Compounds from butanol extract | |||||||
| Hexadecanoic acid | Fatty acid | −8.67 | 440.98 nM | −4.41 | 589.19 µM | −7.89 | 1.66 µM |
| Octadecanoic acid | Long-chain fatty acid | −8.84 | 333.54 nM | −4.16 | 890.46 µM | −7.94 | 1.51 µM |
| Levoglucosan | Anhydrohexose | −4.85 | 278.45 µM | ND | ND | ND | ND |
ΔG (kcal mol−1) = Binding energy, Ki = Inhibition constant.
Figure 7.
Molecular docking study of the binding site of compounds and tyrosinase enzyme: (a) Aloin, (b) n-Hexadecanoic acid, (c) Heptadecanic acid, (d) 2-Tridecenoic acid, (e) Octadecanoic acid, (f) 9,12-Octadecadinoic acid, (g) 9,12,15-Octadecatrienoic acid, and (h) Eicosanic acid.
Figure 8.
Molecular docking study of the binding site of compounds and MITF: (a) Deoxyarbutin, (b) n-Hexadecanoic acid, (c) 9,12-Octadecadinoic acid, (d) 9,12,15-Octadecatrienoic acid, and (e) Octadecanoic acid.
Discussion
Natural, semi-synthetic, and fully synthetic sources of tyrosinase inhibitors have become increasingly important for medicinal and cosmetic products.31 Finding new extracts and active ingredients of natural products with anti-melanogenic and antioxidant activities has recently become of interest in the formulation of the products used for hyperpigmentation disorders or melasma.32 Many researchers have focused on the inhibition of melanin formation, scavenging free radicals, tyrosinase inhibition, molecular mechanism, identification, and molecular docking of new tyrosinase inhibitors, which can be developed as new, safe, and potent anti-tyrosinase agents for treating skin disorders.33 Thus, in this study, we evaluated the amount of phenolics and flavonoids and the antioxidant activities of different extracts of the P. cerasoides flower and their effects on melanin synthesis, tyrosinase activity, gene expression of melanogenesis-related genes, and molecular docking of selected compounds.
Plants are well known as the largest group of phenolic compounds which are responsible for bioactivity in plant extracts. Plants produce the main classes of polyphenols containing phenolic acids, flavonoids, stilbenes, and lignans.34 In a previous study, phenolic and flavonoid compounds were found in the methanolic extract of stem bark,35 leaf extracts (chloroform, ethyl acetate, acetone, and methanol),13 wood extracts,36 gum exudates,37 and methanolic extract of fruits.38 In this study, solvents of different polarities were used to extract the flower part of P. cerasoides. The plant material was extracted by homogenizing with solvent maceration and then separated by fractionation using the liquid-liquid partitioning. Different polarity of solvents was used to fractionate the extracts: diethyl ether for the fractionation of non-polar compounds; chloroform for the fractionation of non-phenolic polar compounds; diethyl ether for the fractionation of low-polar polyphenolic compounds; butanol for the fractionation of high polar polyphenolic compounds.17,39,40 Our result was implied that the butanol solvent was the most appropriate in extracting the phenolic and flavonoid compounds of the P. cerasoides flower, the same as the methanol solvent (a high polarity solvent) in extracting the phenolic and flavonoid compounds of stem barks and leaves. It was also stated that all extracts from P. cerasiodes contained flavonoid and phenolic contents. The high amount of phenolic and flavonoid compounds might relate to antioxidant and anti-tyrosinase activities.41 Moreover, polyphenol groups can also react with copper at the binding site of tyrosinase.42
Reactive oxygen species (ROS) are produced by ultraviolet radiation (UVR) from sunlight. ROS activates the G-protein coupled signaling pathway, which leads to an increase in melanin in human epidermal melanocytes.43 Many researchers have exploited some natural products to attenuate ROS induced by UVR, and antioxidants in natural products have the potential to stimulate the endogenous capacity in the skin and reduce ROS products.44 Antioxidant activities in P. cerasoides extracts were analyzed by DPPH and ABTS assay. Overall, the butanol extract of the P. cerasoides flower had the highest phenolic and flavonoid compounds and displayed the strongest antioxidant activities. In addition to our finding, strong antioxidant capacity was found in the methanolic extract of stem bark,35 leaf extracts (chloroform, ethyl acetate, acetone, and methanol),13 wood extracts,36 gum exudates,37 and methanolic extract of fruits.38 Antioxidant activity might depend on the phenolic and flavonoid compounds in these extracts of P. cerasoides which were responsible for good electron donors by their hydroxyl groups.45
Tyrosinase is an enzyme involved in melanin synthesis and the abnormal accumulation of melanin production leading to hyperpigmentation disorders or melasma.46 Nowadays, tyrosinase is one of the targets to treat pigment disorders. Tyrosinase inhibitors from natural products attract more attention than synthetic chemical compounds in the cosmetic industry.32 There are many structure types of tyrosinase inhibitors such as phenolics,47 anthraquinones,48 flavonoids,49 phenylpropanoids,50 olefinic unsaturated compounds,51 and unsaturated fatty acids.52 To investigate the melanin inhibition of P. cerasoides flower extracts, we determined the inhibitory activity of the P. cerasoides extracts on mushroom tyrosinase and cellular tyrosinase activities. Melanin content was also investigated after treatment with -MSH and P. cerasoides extracts with non-toxic concentrations in the B16F10 melanoma cells. Crude, diethyl ether, butanol, and aqueous residual extracts significantly decreased mushroom tyrosinase activity as a cell-free assay system. However, only three extracts including crude, diethyl ether, and butanol effectively reduced cellular tyrosinase activity and melanin production. In another study of P. cerasoides, fruit extracts with 80% acetone showed anti-mushroom tyrosinase activity.53 It seems that crude, diethyl ether, and butanol extracts contain phenolics and flavonoids, which have an influence on tyrosinase activity. Moreover, nonphenolic and flavonoid compounds may exert melanin inhibition through anti-tyrosinase activity. The tyrosinase inhibitory activity may also depend on the hydroxyl group of the phenolic and flavonoid compounds of P. cerasoides extracts which can form hydrogen bonds to the active site of tyrosinase enzymes.54 Moreover, the antioxidant activity of phenolics may be one of the reasons for tyrosinase inhibitory activity, caused by their redox properties (hydrogen donors and single oxygen quenchers).47 Thus, we also identified the chemical contents of crude, diethyl ether, and butanol extracts by GC-MS analysis.
The microphthalmia-transcription factor (MITF) is one of the most nuclear transcription factors which regulates melanogenic genes including TYR, TRP-1, and TRP-2.55 The upregulation of -MSH by UV binds to the melanocortin-1 receptor (MC1R), which leads to increased cyclic AMP (cAMP). cAMP further activates protein kinase A (PKA), which activates the phosphorylation of cAMP responsive-element-binding protein (CREB). The phosphorylation of CREB induced by -MSH leads to the activation of various downstream targets including MITF, which promotes the expression of TYR, TRP-1, and TRP-2 for melanin synthesis.56 In the present study, crude, diethyl ether, and butanol extracts of the P. cerasoides flower effectively downregulated the mRNA expression of MITF, TYR, TRP-1, and TRP-2 in -MSH-treated B16F10 melanoma cells. The previous study has shown that anti-melanogenic activity is controlled by turning off MITF signaling, hydroalcoholic extracts of Spartium juncerm L. flowers reduced mRNA levels of MITF, TYR, TRP-1, and TRP-2 by qPCR, and the amount of MITF protein by Western blot assay.57 From another study, Kalanchoe thyrsiflora Harv., Myrsine pillansii, and Vachellia karroo downregulated the tyrosinase gene expression as well as lipoic acid. Like a lipoic acid, these plant extracts inhibited tyrosinase directly and at the mRNA level.58 Natural compounds from plants contain flavonoids, terpenoids, coumarins, and polysaccharides which have shown anti-melanogenic functions. Other studies have reported the inhibition of MITF activity by phenolic compounds especially flavonoids. Among flavonoids, isoorientin, catechin, coumaric acid, kaempferol 7-O-glucuronide showed the downregulation of MITF expression.59 Prunus genus contains flavonoid compounds such as 7 kaempferol derivatives, 15 quercetin derivatives, and catechin. The inhibition of MITF activity could be related to the action of phytochemicals including kaempferol, quercetin and catechin which also inhibited tyrosinase activity and melanin content.60 The present study suggests that crude, diethyl ether, and butanol extracts of the P. cerasoides flower suppressed the expression of the transcription factor or MITF as well as its downstream melanogenesis-related genes (TYR, TRP1, and TRP-2) and reduced melanin production.
Major compounds of the selected extracts of the P. cerasoides flower were identified by GC-MS analysis. The chromatogram of crude extracts revealed high levels of acetic acid, benzoic acid, dihydroxyacetone, 1,2-cyclopentanedione, 2-oxypropanol, and 3-ydroxy-2,3-dihydromaltol. In addition, from another study, 40% of acetic acid-treated skin (used as chemical peeling) revealed more irregular and matted morphology compared to untreated skin.61 Crude extract (52.57% of acetic acid) is not suitable as an ingredient in the cosmetic industry. As shown in Table 2, P. cerasoides contained various types of compounds, including aldehyde, diterpenoid, furan, pyran, phenolic, coumarin, fatty acid, carboxylic acid, organooxygen, and amide groups. After the screening process by Molinspiration tools, some compounds in aldehyde, diterpenoid, furan, pyran, phenolic, coumarin, fatty acid, carboxylic acid, organooxygen, and amide groups might miss in this step due to the limitation of the prediction program. The good binding energies and inhibition constants of the compounds in the extracts against tyrosinase were found in n-hexadecanoic acid, heptadecanoic acid, 2-tridecenoic acid, octadecanoic acid, 9,12-octadecadienoic acid, 9,12,15-octadecanoic acid, and eicosanoic acid. The 2-tridecenoic acid from the diethyl ether extract was the best tyrosinase inhibitor, which reacted at the amino acid residues of the tyrosinase pocket site and zinc molecule. Both hexadecenoic acid and eicosanoic acid were found in both the diethyl ether and butanol extracts, but other compounds were found in only the diethyl ether extract. These compounds were fatty acids that could inhibit tyrosinase and melanogenic effect by synergistic activity. Tyrosinase degradation was previously reported to be regulated by fatty acids. Tyrosinase breakdown was enhanced by linoleic acid (an unsaturated fatty acid).62 Moreover, the n-hexadecanoic acid, 9,12-octadecadienoic acid, 9,12,5-octadecanoic acid, and octadecanoic acid also showed better binding affinity against MITF. These compounds had the van der Waals and hydrophobic interaction with MITF protein. The hydrophobic interactions help to stabilize the ligands at the site of action while it also alters binding affinity and therapeutic efficacy.63
From all results, n-hexadecanoic acid, heptadecanoic acid, 2-tridecenoic acid, octadecanoic acid, 9,12-octadecadienoic acid, 9,12,15-octadecanoic acid, and eicosanoic acid might be a tyrosinase inhibitor; while n-hexadecanoic acid, 9,12-octadecadienoic acid, 9,12,5-octadecanoic acid, and octadecanoic acid might be a MITF inhibitor. As the same with our results, 9,12-octadecadienoic acid (alpha-linoleic acid) had an inhibitory effect on melanin synthesis through tyrosinase inhibition.64,65 Moreover, both alpha-linoleic acid and 9,12,15-octadecanoic acid (linoleic acid) inhibited melanin production through the activation of the desquamation of melanin pigment in the epidermis layer.66 From all results, the diethyl ether, and butanol extracts had the potential compounds which showed inhibitory activity against tyrosinase and MITF as targets of hyperpigmentation treatment.
Conclusion
Our study demonstrated that the diethyl ether and butanol extracts of the P. cerasoides flower with high levels of phenolic and flavonoid contents had strong antioxidant activity, tyrosinase inhibition, and anti-melanogenic effects through the downregulation of the gene expression of MITF, tyrosinase, TRP-1, and TRP-2. In addition, the fatty acid compounds containing n-hexadecanoic acid, heptadecanoic acid, octadecanoic acid, 9,12-octadecadienoic acid, 9,12,15-octadecanoic acid, and eicosanoic acid had an inhibitory effect against tyrosinase and MITF based on the molecular docking study. The results of this study demonstrate that both the diethyl ether and butanol extracts of the P. cerasoides flower can be used as a useful component for an anti-hyperpigmentation agent. However, further investigation is needed to clarify the isolation and identification of bioactive compounds using high-performance liquid chromatography or liquid chromatography-mass spectrometry. This will be the aim of further studies.
Acknowledgements
The authors would like to thank the Research Institute for Health Sciences, the Research Excellence Center for Innovation and Health Products, and Research Article Publication Support Unit, Walailak University, for their support. We would like to thank Assistant Professor Anchalee Chiabchalard, PhD (Faculty of Allied Health Sciences, Chulalongkorn University) for the cell line. Lastly, we are pleased to thank Professor Duncan R. Smith, PhD (Institute of Molecular Biosciences, Mahidol University) for manuscript editing.
Footnotes
Authors’ Contributions: NK and AT: conceptualization, methodology, validation, data analysis, writing—original draft preparation, and writing—review and editing. KY: Data analysis, writing—review and editing. MC: conceptualization, methodology, validation, data analysis, writing—original draft preparation, writing—review and editing, supervision of this study, and funding acquisition. All authors have read and agreed to the publish version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: No ethical approval was obtained because this study did not involve laboratory animals and humans.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Individual Research Grant, Research Institute for Health Sciences, Walailak University, Grant Number WU-IRG-63-072.
ORCID iDs: Nateelak Kooltheat https://orcid.org/0000-0001-7623-854X
Moragot Chatatikun https://orcid.org/0000-0003-3264-1155
References
- 1.Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol. 2014;134(6):1512-1518. doi: 10.1038/jid.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho SG, Chan HH. The Asian dermatologic patient: Review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009;10(3):153-168. doi: 10.2165/00128071-200910030-00002 [DOI] [PubMed] [Google Scholar]
- 3.Zasada M, Debowska R, Pasikowska M, Budzisz E. The assessment of the effect of a cosmetic product brightening the skin of people with discolorations of different etiology. J Cosmet Dermatol. 2016;15(4):493-502. doi: 10.1111/jocd.12249 [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Weng QY, Fisher DE. UV Signaling pathways within the skin. J Invest Dermatoogy. 2014;134(8):2080-2085. doi: 10.1038/jid.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Mello SAN, Finlay GJ, Baguley BC, Askarian-Amiri ME. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17(7):1144. doi: 10.3390/ijms17071144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masum MN, Yamauchi K, Mitsunaga T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev Agric Sci. 2019;7:41-58. doi: 10.7831/ras.7.41 [DOI] [Google Scholar]
- 7.Tan C, Zhu W, Lu Y. Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosinase. Chin Med J (Engl). 2002;115(12):1859-1862. [PubMed] [Google Scholar]
- 8.Ortonne J-P, Bissett DL. Latest insights into skin hyperpigmentation. J Investig Dermatol Symp Proc. 2008;13(1):10-14. doi: 10.1038/jidsymp.2008.7 [DOI] [PubMed] [Google Scholar]
- 9.Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J Investig Dermatol Symp Proc. 2008;13(1):20-24. doi: 10.1038/jidsymp.2008.8 [DOI] [PubMed] [Google Scholar]
- 10.Joseph N, Anjum N, Tripathi Y. Prunus cerasoides D. Don: A review on its ethnomedicinal uses, phytochemistry and pharmacology. Int J Pharm Sci Rev Res. 2018;48(1):62-69. [Google Scholar]
- 11.Arora DS, Mahajan H. Major phytoconstituents of Prunus cerasoides responsible for antimicrobial and antibiofilm potential against some reference strains of pathogenic bacteria and clinical isolates of MRSA. Appl Biochem Biotechnol. 2019;188(4):1185-1204. doi: 10.1007/s12010-019-02985-4 [DOI] [PubMed] [Google Scholar]
- 12.Arora DS, Mahajan H. In vitro evaluation and statistical optimization of antimicrobial activity of Prunus cerasoides stem bark. Appl Biochem Biotechnol. 2018;184(3):821-837. doi: 10.1007/s12010-017-2571-8 [DOI] [PubMed] [Google Scholar]
- 13.Joseph N, Anjum N, Tripathi Y. Phytochemical screening and evaluation of polyphenols, flavonoids and antioxidant activity of Prunus cerasoides D. Don Leaves. J Pharm Res. 2016;10(7):502-508. [Google Scholar]
- 14.Dhrubajyoti S. Efficacy of different leaf extract fractions of Prunus cerasoides D. Don on benign prostate hyperplasia. Int J Pharm Pharm Sci. 2016;1(2):22-27. [Google Scholar]
- 15.Rana A, Singh HP. Bio-utilization of wild berries preparation of high valued herbal wines. Indian J Nat Prod Resour. 2012;4(2):165-169. [Google Scholar]
- 16.Tiwari C, Chubey S, Kurele R, Nautiyal R. A review on padmaka (Prunus cerasiodes D. Don): Different species and and their medicial uses. Ayushdhara. 2017;4(1):1051-1055. [Google Scholar]
- 17.Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47(9):2196-2201. doi: 10.1016/j.fct.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Goulas V, Georgiou E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods. 2019;9(1):20. doi: 10.3390/foods9010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwansyah AC, Manh TD, Andriana Y, et al. Effects of various drying methods on selected physical and antioxidant properties of extracts from moringa oliefera leaf waste. Sustainability. 2020;12(20):8586. doi: 10.3390/su12208586. [DOI] [Google Scholar]
- 20.Moragot C, Pitaksit S, Patcharaporn P, et al. Antioxidant and tyrosinase inhibitory properties of an aqueous extract of Garcinia atroviridis Griff. ex. T. Anderson fruit pericarps. Pharmacogn J. 2020;12(1):71-78. doi: 10.5530/pj.2020.12.12 [DOI] [Google Scholar]
- 21.Chaves N, Santiago A, Alías JC. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants. 2020;9(1):76. doi: 10.3390/antiox9010076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deguchi T, Tamai A, Asahara K, et al. Anti-tyrosinase and anti-oxidative activities by asana: The heartwood of Pterocarpusmarsupium. Nat Prod Commun. 2019;14(10):1934578X19883727. doi: 10.1177/1934578X19883727 [DOI] [Google Scholar]
- 23.Chung YC, Kim S, Kim JH, et al. Pratol, an o-methylated flavone, induces melanogenesis in B16F10 melanoma cells via p-p38 and p-JNK upregulation. Molecules. 2017;22(10):1704. doi: 10.3390/molecules22101704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebeau PF, Chen J, Byun JH, Platko K, Austin RC. The trypan blue cellular debris assay: A novel low-cost method for the rapid quantification of cell death. MethodsX. 2019;6:1174-1180. doi: 10.1016/j.mex.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panichakul T, Rodboon T, Suwannalert P, et al. Additive effect of a combination of Artocarpus lakoocha and Glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals (Basel). 2020;13(10):310. doi: 10.3390/ph13100310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sim M-O, Choi I-Y, Cho J-H, Shin H-M, Cho H-W. Anti-melanogenesis and anti-oxidant of Salix pseudo-lasiogyne water extract in α-MSH-induced B16F10 melanoma cells. Food Agric Immunol. 2017;28(6):1003-1016. doi: 10.1080/09540105.2017.1325840 [DOI] [Google Scholar]
- 27.Chatatikun M, Yamauchi T, Yamasaki K, Chiabchalard A, Aiba S. Phyllanthus acidus (L.) skeels and Rhinacanthus nasutus (L.) Kurz leaf extracts suppress melanogenesis in normal human epidermal melanocytes and reconstitutive skin culture. Original article. Asian Pac J Trop Med. 2019;12(3):98-105. doi: 10.4103/1995-7645.254935 [DOI] [Google Scholar]
- 28.Tedasen A, Khoka A, Madla S, Sriwiriyajan S, Graidist P. Anticancer effects of piperine-free Piper nigrum extract on cholangiocarcinoma cell lines. Original article. Pharmacogn Mag. 2020;16(68):28-38. doi: 10.4103/pm.pm_288_19 [DOI] [Google Scholar]
- 29.Thangarasu P, Thamarai Selvi S, Manikandan A. Unveiling novel 2-cyclopropyl-3-ethynyl-4-(4-fluorophenyl)quinolines as GPCR ligands via PI3-kinase/PAR-1 antagonism and platelet aggregation valuations; development of a new class of anticancer drugs with thrombolytic effects. Bioorg Chem. 2018;81:468-480. doi: 10.1016/j.bioorg.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 30.Tedasen A, Choomwattana S, Graidist P, Tipmanee V. Structure-guided cancer blockade between bioactive bursehernin and proteins: Molecular docking and molecular dynamics study. J Mol Graph Model. 2017;74:215-224. doi: 10.1016/j.jmgm.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 31.Zolghadri S, Bahrami A, Hassan Khan MT, et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34(1):279-309. doi: 10.1080/14756366.2018.1545767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SY, Baek N, Nam T-g. Natural, semisynthetic and synthetic tyrosinase inhibitors. J Enzym Inhib Med Chem. 2016;31(1):1-13. doi: 10.3109/14756366.2015.1004058 [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Hao M-M, Sun Y, et al. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules. 2018;23(1):106. doi: 10.3390/molecules23010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S, Kaur I, Kariyat R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int J Mol Sci. 2021;22(3):1442. doi: 10.3390/ijms22031442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rai J, Sharma K, Pokharel Y. Antioxidant and alpha amylase inhibitory activity of Nepalese medicinal plants from Gorkha district. J Pharmacogn Phytotherapy. 2020;12(2):28-35. doi: 10.5897/JPP2020.0571 [DOI] [Google Scholar]
- 36.Smailagić A, Ristivojević P, Dimkić I, et al. Radical scavenging and antimicrobial properties of polyphenol rich waste wood extracts. Foods (Basel, Switzerland). 2020;9(3):319. doi: 10.3390/foods9030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malsawmtluangi C, Thanzami K, Lalhlenmawia H, et al. Physicochemical characteristics and antioxidant activity of Prunus cerasoides D. Don gum exudates. Int J Biol Macromol. 2014;69:192-199. doi: 10.1016/j.ijbiomac.2014.05.050 [DOI] [PubMed] [Google Scholar]
- 38.Jangwan JS, Painuly AM. A new constituent from Prunus cerasoides. Nat Prod Indian J. 2007;3(3):126-128. [Google Scholar]
- 39.Kooltheat N, Chujit K, Nuangnong K, et al. Artemisia lactiflora extracts prevent inflammatory responses of human macrophages stimulated with charcoal pyrolysis smoke. J Evid Based Integr Med. 2021;26:2515690X211068837. doi: 10.1177/2515690X211068837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kooltheat N, Sranujit RP, Chumark P, Potup P, Laytragoon-Lewin N, Usuwanthim K. An ethyl acetate fraction of Moringa oleifera Lam. Inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients. 2014;6(2):697-710. doi: 10.3390/nu6020697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muddathir AM, Yamauchi K, Batubara I, Mohieldin EAM, Mitsunaga T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S Afr J Bot. 2017;109:9-15. doi:0.1016/j.sajb.2016.12.013 [Google Scholar]
- 42.Ríha M, Karlícková J, Filipský T, Jahodár L, Hrdina R, Mladenka P. In vitro copper-chelating properties of flavonoids. Free Radic Biol Med. 2014;75(Suppl 1):S46. doi: 10.1016/j.freeradbiomed.2014.10.807 [DOI] [PubMed] [Google Scholar]
- 43.Dumbuya H, Hafez SY, Oancea E. Cross talk between calcium and ROS regulate the UVA-induced melanin response in human melanocytes. FASEB J. 2020;34(9):11605-11623. doi: 10.1096/fj.201903024R [DOI] [PubMed] [Google Scholar]
- 44.Dunaway S, Odin R, Zhou L, Ji L, Zhang Y, Kadekaro AL. Natural antioxidants: Multiple mechanisms to protect skin from solar radiation. Front Pharmacol. 2018;9:392-392. doi: 10.3389/fphar.2018.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendary E, Francis RR, Ali HMG, Sarwat MI, El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci. 2013;58(2):173-181. doi: 10.1016/j.aoas.2013.07.002 [DOI] [Google Scholar]
- 46.Chen W-C, Tseng T-S, Hsiao N-W, et al. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci Rep. 2015;5(1):7995. doi: 10.1038/srep07995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L, Guo Y, Zhang Y, Zhuang Y. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in B16 mouse melanoma cells. Front Pharmacol. 2017;8:104. doi: 10.3389/fphar.2017.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng HJ, Sun DQ, Chu SH, Zhang JJ, Hu GZ, Yang R. Inhibitory effects of four anthraquinones on tyrosinase activity: Insight from spectroscopic analysis and molecular docking. Int J Biol Macromol. 2020;160:153-163. doi: 10.1016/j.ijbiomac.2020.05.193 [DOI] [PubMed] [Google Scholar]
- 49.Kim D, Park J, Kim J, et al. Flavonoids as mushroom tyrosinase inhibitors: A fluorescence quenching study. J Agric Food Chem. 2006;54(3):935-941. doi: 10.1021/jf0521855 [DOI] [PubMed] [Google Scholar]
- 50.Boghrati Z, Naseri M, Rezaie M, et al. Tyrosinase inhibitory properties of phenylpropanoid glycosides and flavonoids from Teucrium polium L. var. gnaphalodes. Iran J Basic Med Sci. 2016;19(8):804-811. doi: 10.22038/ijbms.2016.7460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen YS, Lee SM, Lin CC, Liu CY, Wu MC, Shi WL. Kinetic study on the tyrosinase and melanin formation inhibitory activities of carthamus yellow isolated from Carthamus tinctorius L. J Biosci Bioeng. 2013;115(3):242-245. doi: 10.1016/j.jbiosc.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 52.Ando H, Wen ZM, Kim HY, et al. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem J. 2006;394(Pt 1):43-50. doi: 10.1042/BJ20051419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh H, Lily MK, Dangwal K. Evaluation and comparison of polyphenols and bioactivities of wild edible fruits of north-west Himalaya. India. Asian pac J trop dis. 2015;5(11):888-893. doi: 10.1016/S2222-1808(15)60951-3 [DOI] [Google Scholar]
- 54.Jakimiuk K, Sari S, Milewski R, Supuran CT, Şöhretoğlu D, Tomczyk M. Flavonoids as tyrosinase inhibitors in in silico and in vitro models: Basic framework of SAR using a statistical modelling approach. J Enzyme Inhib Med Chem. 2022;37(1):421-430. doi: 10.1080/14756366.2021.2014832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawakami A, Fisher DE. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Invest. 2017;97(6):649-656. doi: 10.1038/labinvest.2017.9 [DOI] [PubMed] [Google Scholar]
- 56.García-Borrón JC, Abdel-Malek Z, Jiménez-Cervantes C. MC1R, The cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014;27(5):699-720. doi: 10.1111/pcmr.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanni V, Canuti L, Gismondi A, Canini A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine. 2018;46:1-10. doi: 10.1016/j.phymed.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 58.Stapelberg J, Nqephe M, Lambrechts I, Crampton B, Lall N. Selected South African plants with tyrosinase enzyme inhibition and their effect on gene expression. S Afr J Bot. 2019;120:280-285. doi: 10.1016/j.sajb.2018.08.013 [DOI] [Google Scholar]
- 59.Qian W, Liu W, Zhu D, et al. Natural skin-whitening compounds for the treatment of melanogenesis (review). Exp Ther Med. 2020;20(1):173-185. doi: 10.3892/etm.2020.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang GH, Kim HW, Lee MK, et al. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J Biol Sci. 2018;25(8):1622-1631. doi: 10.1016/j.sjbs.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamamoto Y, Uede K, Yonei N, Kishioka A, Ohtani T, Furukawa F. Effects of alpha-hydroxy acids on the human skin of Japanese subjects: The rationale for chemical peeling. J Dermatol. 2006;33(1):16-22. doi: 10.1111/j.1346-8138.2006.00003.x [DOI] [PubMed] [Google Scholar]
- 62.Ando H, Watabe H, Valencia JC, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J Biol Chem. 2004;279(15):15427-15433. doi: 10.1074/jbc.M313701200 [DOI] [PubMed] [Google Scholar]
- 63.Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS One. 2010;5(8):e12029. doi: 10.1371/journal.pone.0012029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imanaka H, Ando H, Ryu A, et al. Liposomal linoleic acid is useful as a skin lightening agent. J Soc Cosmet Chem Japan. 1999;33(3):277-282. doi: 10.5107/sccj.33.3_277 [DOI] [Google Scholar]
- 65.Huang H-C, Wei C-M, Siao J-H, et al. Supercritical fluid extract of spent coffee grounds attenuates melanogenesis through downregulation of the PKA, PI3K/Akt, and MAPK signaling pathways. Evid Based Complement Alternat Med. 2016;2016:5860296. doi: 10.1155/2016/5860296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res. 1998;290(7):375-381. doi: 10.1007/s004030050320 [DOI] [PubMed] [Google Scholar]