Abstract
Background:
Symptom burden differences may contribute to racial disparities in breast cancer survival. We compared symptom changes from before to during chemotherapy among women with breast cancer.
Methods:
This observational study followed a cohort of Black and White women diagnosed with Stage I-III, hormone-receptor positive breast cancer from a large cancer center in 2007-2015, and reported symptoms before and during chemotherapy. We identified patients who experienced a one-standard deviation (SD) increase in symptom burden after starting chemotherapy using four validated composite scores (General Physical Symptoms, Treatment Side-Effects, Acute Distress, and Despair). Kitagawa-Blinder-Oaxaca decomposition was used to quantify race differences in symptom changes explained by baseline characteristics (sociodemographic, baseline scores, cancer stage) and first-line chemotherapy regimens.
Results:
Among 1,273 patients, Black women (n=405, 31.8%) were more likely to report one-SD increase in General Physical Symptoms (55.6% vs. 48.2%, p=0.015), Treatment Side-Effects (74.0% vs. 63.4%, p<0.001), and Acute Distress (27.4% vs. 20.0%, p=0.010) than White women. Baseline characteristics and first-line chemotherapy regimens explained a large and significant proportion of the difference in Acute Distress changes (93.7%, p=0.001), but not General Physical Symptoms (25.7%, p=0.25) or Treatment Side-Effects (16.4%, p=0.28).
Conclusions:
Black women with early-stage breast cancer were more likely to experience significant increases in physical and psychological symptom burden during chemotherapy. Most of the difference in physical symptom changes remained unexplained by baseline characteristics, which suggests inadequate symptom management among Black women.
Impact:
Future studies should identify strategies to improve symptom management among Black women and reduce differences in symptom burden.
Keywords: breast cancer, chemotherapy symptoms, race difference, patient-reported outcomes, Kitagawa-Blinder-Oaxaca decomposition
Introduction
Survival outcomes for women with breast cancer have improved in the past few decades.(1) However, the death rate declined more slowly among Black women than White women.(2) Persistent race-based differences in cancer outcomes have been driven by decades of structural racism(3), leading to unfavorable socioeconomic status, restricted access to care, high exposures to environmental risk factors, as well as explicit and implicit provider bias.(4-7) In turn, Black women are less likely to receive guideline-concordant treatment (8,9) and have their symptoms evaluated and managed during treatment (10), which contributes to lower treatment adherence and efficacy (11-15), and racial disparities in cancer outcomes.
Symptom burden is a contributing factor to chemotherapy adherence and efficacy. Most prior studies of symptom burden race differences during chemotherapy were cross-sectional (16,17) or conducted only after treatment initiation (18,19), thus were unable to control for treatment heterogeneity or differences in baseline symptom burden. Our prior study found that Black women started chemotherapy with higher baseline physical symptoms typically associated with chemotherapy and lower distress symptoms compared to White patients.(20) To date, the comparison of symptom burden changes during chemotherapy by race is limited.
The underlying cause of any observed differences in symptom burden during treatment is complex and may occur through various mechanisms, including social determinants of health, differential treatments due to underlying health conditions, and under-evaluated and inadequately treated symptoms.(4,21-25) Evidence shows that race differences in symptom burden were attenuated but remained significant after adjusting for demographics and disease-related characteristics.(6) However, previous analysis using multivariable regression to adjust for patient characteristics assumes that patients from different comparison groups share the same risks or benefits from covariates (i.e., coefficients) towards study outcomes. In other words, while the attenuated racial differences from the multivariable regression approach inform us about differences due to differences in baseline characteristics we adjust for, it does not formally estimate potential disparities due to differential risks or benefits from covariates.
To fill these gaps, our study used unique longitudinal patient-reported symptom data collected with a validated survey to examine racial differences in four composite measures of physical and psychological symptom burden changes from before to after initiating chemotherapy among women diagnosed with early-stage hormone receptor-positive breast cancer. We used the Kitagawa-Blinder-Oaxaca decomposition model to quantify the extent to which differential symptom burden experienced by Black and White women during chemotherapy is explained by factors such as clinical characteristics and social determinants of health. This model also allows us to quantify potential racial disparities (i.e. unexplained differences) in symptom burden due to differential risks or benefits from covariates. A deeper understanding of the mechanisms that explain differences in symptom burden by race can inform future efforts to improve physician training and clinical practice.
Materials and Methods
Data and Analytical Sample
Our cohort came from the West Cancer Center and Research Institute (WCCRI) – a large multidisciplinary cancer center consisting of 9 clinics and over 70 physicians serving 60% of all patients in the tri-state area of west Tennessee, north Mississippi, and east Arkansas. Patient-reported symptoms were routinely collected at WCCRI during patients’ visits using the Patient Care Monitor (PCM; ConcertAI).(26) The PCM is a tablet-based platform used to collect patient-reported outcomes at the point of care. Patient responses were integrated into their electronic health record (EHR), and summary reports highlighted severe symptoms (≥7 out of 0-10 scale). The University of Tennessee Health Science Center institutional review board approved the study protocol and waived the requirement for written informed consent for participants in this study. This study was conducted in accordance with the U.S. Common Rule and followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies.
Using EHR data, we identified patients diagnosed with a first primary, Stage I-III hormone-receptor positive breast cancer between January 1, 2007 and December 31, 2015 at WCCRI, who initiated chemotherapy within 180 days of diagnosis, identified as a White or Black race only given limited sample sizes of other race groups, with ≥1 PCM report up to 45 days before chemotherapy and ≥1 PCM report after chemotherapy initiation, and those with non-missing residence zip code (n=1,273, Supplemental Figure 1). Other types of breast cancer (e.g., late stage, hormone-receptor negative, etc.) were excluded to homogenize the sample and treatment trajectories. We obtained additional data from the Tennessee cancer registry, Medicare and Medicaid claims to retrieve patients’ cancer history, cancer treatments, and other healthcare services received outside the WCCRI system. We also extracted neighborhood-level sociodemographic information from the 2011-2015 American Community Survey 5-year estimates file.
We conducted sensitivity analyses among subsamples of: 1) Medicare patients with continuous Fee-For-Service coverage in the year before their diagnosis (n=126) to control for baseline comorbidities; 2) patients with surgery dates identified prior to chemotherapy and one PCM report collected between surgery and chemotherapy initiation (n=617) to control for symptom burden from surgeries among adjuvant chemotherapy recipients; and 3) patients receiving ≥4 cycles of chemotherapy (i.e. no early discontinuation) (n=1,231).
Patient Reported Symptoms
We focused on four validated composite scores derived from individual items: General Physical Symptoms (11 items), Treatment Side-Effects (8 items), Acute Distress (4 items) and Despair (7 items). [Supplemental Table 1] Patients rated the severity of each symptom they experienced in the past week from 0 (not a problem) to 10 (as bad as possible). Each composite score was calculated by summing the individual item scores and then transformed into a normalized T score with a mean of 50 and a standard deviation (SD) of 10. These composite scores were automatically calculated by the PCM platform using algorithms detailed in previous validation studies. (26,27)
Baseline symptoms were extracted using the closest report within 45 days up to the first date of chemotherapy claim. We also identified the most severe composite score among all symptom reports collected during the window between the first chemotherapy claim to up to 30 days after the last chemotherapy claim. We focused on symptom change from baseline to most severe score to capture the full range of toxicity changes while receiving chemotherapy.
The primary outcome was a one-SD increase for each composite score. Previous research finds that a 5%-10% increase in symptom burden reflects a clinically important change in a patient-reported outcome score.(28) We used a threshold of one-SD (10-point) increase to categorize changes that exceed a clinically meaningful threshold for symptom burden change.(28) Aligned with WCCRI’s monitoring practice, we also examined individual symptoms reported as severe (i.e., scored ≥7 points). Of note, General Physical Symptoms and Treatment Side-Effects scores each assesses a range of individual physical symptoms, but Treatment Side-Effects scores include symptoms that are more likely to be exacerbated by chemotherapy.
Covariates
We accounted for baseline sociodemographic and clinical characteristics that might affect patients’ symptom burden, as well as baseline symptom scores in our analysis. Sociodemographic factors consisted of age, neighborhood-level income and education, and state of residence. Neighborhood-level median household income and percent of adults with a high school degree were linked using patients’ residence zip codes or county codes where zip code-level data were not available. Clinical factors included cancer stage at diagnosis, days between cancer diagnosis and chemotherapy initiation. We included the number of days between cancer diagnosis and chemotherapy initiation because of the substantial evidence that Black women delay cancer treatment initiation, impacting outcomes and potentially symptom burden.(29-31) First-line chemotherapy regimens were identified using patients’ first chemotherapy claim and categorized into 4 groups: 1) Anthracycline-based; 2) Taxane-based; 3) Cyclophosphamide, Methotrexate, and Fluorouracil (CMF); and 4) Other. Chemotherapy was identified using Healthcare Common Procedure Coding System codes. (32) [Supplemental Table 2] Each chemotherapy cycle had to be ≥7 days apart, and a gap of ≥60 days were considered as discontinuation.
For our sensitivity analyses, we constructed the number of days between surgery and baseline symptom report among the subsample with a surgery date and the number of comorbidities among the Medicare subsample. Twelve common comorbid conditions were evaluated according to the National Cancer Institute Comorbidity Index.(33)
Statistical Analyses
Baseline characteristics were compared between Black and White women using the Student t-test for continuous variables, and χ2 or the Fisher exact test for categorical variables. We compared the prevalence of severe individual symptoms (≥7) from each of the four most severe composite scores between two race groups. Kitagawa-Blinder-Oaxaca decomposition was used to quantify the Net Differences by race in reported symptom changes “explained” by differences in observable covariates and “unexplained” differences.(34,35) Specifically, we first ran Ordinary Least Square (OLS) models among White and Black patients separately and obtained the coefficients for each covariate. The “explained” difference was then calculated as the sum of the difference in White and Black patient characteristics multiplied by coefficients from the White patients’ OLS model. A negative “explained” difference would mean that, if Black patients had shared the same baseline characteristics as White patients, their symptom burden would be lower. The “unexplained” difference quantifies the change in Black patients’ symptom scores when applying White patients’ coefficients to Black patients’ characteristics (i.e. differences in coefficients). “Unexplained” differences are interpreted as differences in reporting patterns that would remain if patients had the same baseline characteristics. We used Stata 13 for data analyses. Statistical significance was determined using two-sided tests with an alpha of 0.05.
Data availability
Data underlying this article are not publicly available. Access to the data requires an institutional review board approval, and signed data use agreements with WCCRI and Centers for Medicare & Medicaid Services.
Results
Table 1 shows patient characteristics by race. Overall, 1,273 women were included in our analysis, with a mean age of 54 years old (SD = 11.3). Compared to White patients (n=868, 68.2%), Black patients (n=405, 31.8%) were younger (52 vs. 55 years old, p<0.001), more likely to live in neighborhoods with lower median household income (p<0.001), with fewer adults attaining high school education (p<0.001), and were more likely to have initiated their chemotherapy later (p<0.001). A higher proportion of White women received CMF than Black women (11.8% vs. 4.2%, p<0.001, Table 1). Black and White women had similar numbers of chemotherapy cycles (9.7 vs. 9.4, p=0.49) and numbers of PCM reports during chemotherapy (6.5 vs. 6.4, p=0.60).
Table 1.
Patient Characteristics by Race
| Black (n=405), No. (%) |
White (n=868), No.(%) |
Total (N=1,273), No. (%) |
P-value1 | |
|---|---|---|---|---|
| Age, Mean (SD) | 51.9 (10.5) | 55.0 (11.5) | 54.1 (11.3) | <.001 |
| Neighborhood-level median household income in thousands, Mean (SD) | 42.3 (18.8) | 58.5 (24.6) | 53.3 (24.1) | <.001 |
| Neighborhood -level % adults with high school degree, Mean (SD) | 81.4 (8.6) | 86.3 (9.2) | 84.7 (9.3) | <.001 |
| Residence state | <.001 | |||
| Arkansas | 8 (2.0%) | 47 (5.4%) | 55 (4.3%) | |
| Mississippi | 85 (21.0%) | 263 (30.3%) | 348 (27.3%) | |
| Tennessee | 307 (75.8%) | 517 (59.6%) | 824 (64.7%) | |
| Other | 5 (1.2%) | 41 (4.7%) | 46 (3.6%) | |
| Stage at diagnosis | 0.10 | |||
| Stage I | 105 (25.9%) | 257 (29.6%) | 362 (28.4%) | |
| Stage II | 207 (51.1%) | 453 (52.2%) | 660 (51.9%) | |
| Stage III | 93 (23.0%) | 158 (18.2%) | 251 (19.7%) | |
| Days between diagnosis and chemotherapy initiation, Mean (SD) | 64.9 (34.4) | 56.7 (28.0) | 59.3 (30.4) | <.001 |
| Number of chemotherapy cycles, Mean (SD) | 9.7 (7.2) | 9.4 (7.0) | 9.5 (7.1) | 0.49 |
| Number of PCM reports during chemotherapy, Mean (SD) | 6.5 (4.0) | 6.4 (3.5) | 6.4 (3.7) | 0.60 |
| First-line Chemotherapy regimen | <.001 | |||
| Anthracycline-based | 215 (53.1%) | 408 (47.0%) | 623 (48.9%) | |
| Taxane-based | 149 (36.8%) | 327 (37.7%) | 476 (37.4%) | |
| Cyclophosphamide, Methotrexate, and Fluorouracil | 17 (4.2%) | 102 (11.8%) | 119 (9.4%) | |
| Other | 24 (5.9%) | 31 (3.6%) | 55 (4.3%) |
Notes: CMF= Cyclophosphamide, Methotrexate, and Fluorouracil.
Student t-test used for mean comparison; Chi-square test or Fisher exact test for categorical measures.
General Physical Symptoms Score
For 3 out of 11 symptom items in General Physical Symptoms Scores, Black patients reported severe burden (score of ≥7) more often than White patients during chemotherapy, including sweating (24.8% vs. 15.3%, p<0.001), itching skin (18.3% vs. 12.7%, p =0.008), and numbness (22.5 vs. 13.5%, p <0.001, Figure 1).
Figure 1. Prevalence of Reporting 7 Points or Higher in Individual Symptoms by Race.
Individual symptom items are scored on a 0-10 scale, where “0” indicates “not a problem” and “10” indicates “as bad as possible”. Symptoms identified based on the most severe composite score among all symptom reports collected after the first chemotherapy claim up to 30 days after the last chemotherapy claim.
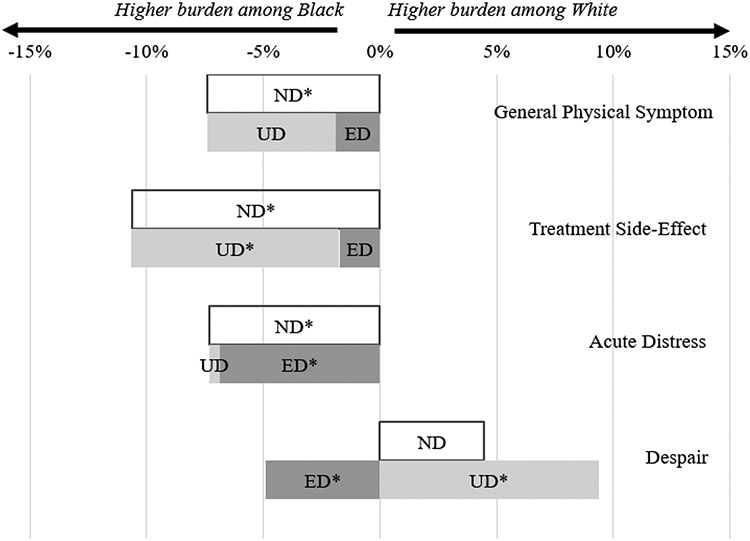
Black and White patients started with similar baseline General Physical Symptoms levels (44.4 vs. 44.6, p=0.67) and Black patients reported a marginally higher most severe General Physical Symptoms score during chemotherapy (55.6 vs. 54.6, p=0.06). When comparing symptom changes from baseline, 55.6% of Black women reported a one-SD increase compared to 48.2% of White women (p=0.015, Table 2). Decomposition of this net difference (−7.4 percentage points [ppts]) showed that baseline characteristics and first-line chemotherapy regimens only explained 25.7% of the net difference (−1.9 ppts out of −7.4 ppts, p=0.34), meaning that collectively Black patient characteristics were associated with on average 1.9 ppts higher chance of experiencing a one-SD increase in General Physical Symptom than White patients. Most of the difference remained unexplained by differences in the included covariates (74.3%, −5.5 ppts out of −7.4 ppts, p=0.10, Figure 2). In other words, assuming Black and White patients shared the same characteristics, Black patients would still be 5.5 ppts more likely than White patients to report a one-SD increase in General Physical Symptoms.
Table 2.
Average Composite Scores and Percent with one-SD Increases by Race Before and After Chemotherapy Initiation
| Black (Mean) | White (Mean) | P-value1 | |
|---|---|---|---|
| General Physical Symptom | |||
| Baseline Score | 44.4 | 44.6 | .67 |
| Most Severe Score During Chemotherapy | 55.6 | 54.6 | .06 |
| Percent with one-SD Increase2 (%) | 55.6 | 48.2 | .015 |
| Treatment Side Effects | |||
| Baseline Score | 44.3 | 43.7 | .029 |
| Most Severe Score During Chemotherapy | 58.1 | 55.9 | <.001 |
| Percent with one-SD Increase2 (%) | 74.0 | 63.4 | <.001 |
| Acute Distress | |||
| Baseline Score | 48.4 | 51.1 | <.001 |
| Most Severe Score During Chemotherapy | 53.4 | 53.9 | .51 |
| Percent with one-SD Increase2 (%) | 27.4 | 20.0 | .010 |
| Despair | |||
| Baseline Score | 47.6 | 47.8 | .77 |
| Most Severe Score During Chemotherapy | 52.0 | 52.0 | .97 |
| Percent with one-SD Increase2 (%) | 22.4 | 26.9 | .19 |
Student t-test for mean comparison; Chi-square for percentage comparison.
Percent of patients who experienced 10-point increase in the corresponding composite score from the baseline score to the most severe score reported during chemotherapy.
Figure 2. Kitagawa-Blinder-Oaxaca Decomposition of one-SD Increases in Symptom Index Scores by Race.
Abbreviation: ND = Net difference; ED = Explained difference; UD = Unexplained difference; SD = Standard deviation. One-SD increase in symptom scores determined using changes from baseline symptom reports collected within 45 days up to the first date of chemotherapy claim to the most severe composite score among all symptom reports collected after the first chemotherapy claim up to 30 days after the last chemotherapy claim.
* p<.05
Treatment Side-Effects Score
Black patients more often reported a severe burden in 3 out of the 8 individual items included in Treatment Side-Effect score during chemotherapy treatment: appetite change (16.1% vs. 12.0%, p=0.046), taste change (33.1% vs. 22.1%, p<0.001), and hair loss (72.8% vs. 53.8%, p<0.001, Figure 1).
Black women reported higher symptom burdens that may be exacerbated with chemotherapy treatment before initiating chemotherapy, compared with White women (44.3 vs. 43.7, p=0.029), and this gap increased during chemotherapy (58.1 vs. 55.9, p<0.001). Black women were more likely to have a one-SD increase in Treatment Side-Effects than White women (74.0% vs. 63.4%, p<0.001, Table 2). When this net difference of −10.6 ppts was decomposed, we found a non-significant explained difference (−1.7 ppts out of −10.6 ppts, 16.4%, p=0.38). In other words, if the average Black patient had the same baseline characteristics and first-line chemotherapy regimens as the average White patient, she would have 72.3% rather than 74% chance of having a one-SD increase in Treatment Side-Effects score. The remaining unexplained difference was large and statistically significant (−8.9 ppts out of −10.6 ppts, 84.0%, p=0.003, Figure 2), suggesting higher reporting of Treatment Side-Effects by Black patients even assuming the same characteristics between two race groups.
Acute Distress Score
Black patients started with a lower baseline Acute Distress score (48.4 vs. 51.1, p<0.001). During chemotherapy, the prevalence of the 4 individual items in Acute Distress score remained relatively low (Figure 1) and the composite score was similar between Black and White patients (53.4 vs. 53.9, p = 0.51, Table 2). Accordingly, a higher percentage of Black women experienced a one-SD increase in Acute Distress than White women (27.4% vs. 20.0%, p=0.010). Differences in Black-White characteristics explained majority of this net difference (−6.9 ppts out of −7.3 ppts, 93.7%, p=0.001, Figure 2). Thus, assuming the same characteristics between Black and White patients, they would have reported similar changes in Acute Distress.
Despair Index Score
Both groups reported a low prevalence of severe individual items in the Despair score before (47.6 vs. 47.8, p=0.77) and during chemotherapy (52.0 vs. 52.0, p=0.97). A higher percentage of White women (26.9%) experienced a one-SD increase in Despair score compared to Black patients (22.4%, p=0.19, Table 2). When we decomposed the net difference (4.5 ppts), we found a significant and negative explained difference of 4.9 ppts (p=0.024), resulting in an even larger and significant unexplained difference (9.4 ppts, p=0.018, Figure 2). This indicates that Black patients’ characteristics were associated with 4.9 ppts higher Despair score (i.e. higher burden). However, this was offset by an even higher reporting of Despair by White patients, resulting in a positive net difference in mean Despair scores.
Sensitivity Analyses
Kitagawa-Blinder-Oaxaca decomposition models with and without adjusting for comorbidities among Medicare beneficiaries (Supplemental Table 3), with and without adjusting for the time from surgery to chemotherapy initiation among surgery recipients (Supplemental Table 4), and among patients with at least 4 cycles of chemotherapy (Supplemental Table 5) showed comparable results to the primary analysis.
Discussion
To our knowledge, this is the largest cohort study to compare patient-reported physical and psychological symptom changes during chemotherapy among Black and White women with early-stage breast cancer. Using unique longitudinal data from a single cancer center, we found that Black women were more likely to experience large, clinically meaningful increases in General Physical Symptoms, Treatment Side-Effects, and Acute Distress, but less likely to experience an increase in Despair than White women after initiating chemotherapy.
Based on the most severe symptom report during chemotherapy, Black women reported significantly higher Treatment Side-Effects and marginally higher General Physical Symptoms, but similar levels of Acute Distress. However, because Black and White women started with different baseline levels, (20) when we examined symptom changes from baseline, race disparities became more prominent. A higher proportion of Black patients experienced a one-SD increase for General Physical Symptoms, Treatment Side-Effects, and Acute Distress. Compared to cross-sectional data or data collected only after chemotherapy initiation in prior studies,(6,16,17,36) our longitudinal data with the inclusion of baseline scores allowed us to compare within-patient symptom changes before and after starting chemotherapy. Thus, our analytical approach identified symptom burden changes correlated with the receipt of chemotherapy while eliminating differences due to time-invariant reporting preferences or baseline health status.
Decomposition results showed that Black patients’ baseline characteristics were consistently associated with a higher likelihood of symptom burden increase, indicated by the consistently negative explained difference found in our decomposition models. (Figure 2) Similarly, earlier studies found that patients’ sociodemographic and disease characteristics contributed to higher symptom burden and lower survival among Black women.(20,37) For Acute Distress, most of the observed differences were attributable to differences in baseline characteristics. Explained differences for two physical symptom scores were also negative, but small relative to net differences and not statistically significant.
After accounting for differences in baseline characteristics, large and statistically significant unexplained differences remained for physical symptom changes. This is concerning as it implies that Black patients may indeed experience higher symptom burdens with chemotherapy or poorer symptom management, which necessitates earlier and more active symptom monitoring and management approach. Among individual symptoms, Black women reported a higher prevalence of sweating, itching, numbness, change in taste and appetite, and hair loss. Symptom burden plays an important role in treatment intensity and adherence, (38-40) which contribute to survival outcomes.(41) More research is needed to understand how differences in symptom burden changes contribute to Black-White disparities in cancer outcomes.
Under-evaluation of symptoms and inadequate symptom management among Black women may be due to systemic racism, and explicit and implicit physician biases. Prior studies found that Black women were less likely to have pain levels evaluated and treated.(42-46) Black cancer survivors more commonly reported not having their symptoms validated by clinicians, having to be their own advocate to obtain information about treatment-related symptoms, and expressed dissatisfaction with symptoms management.(10,47) Differences in social support networks may also play a role. White cancer survivors were more likely to seek information from the healthcare system, such as attending educational classes at the institutions, while Black survivors were more likely to rely on information from their support networks.(48) Conceptual frameworks that capture the complexity of symptom management and include multilevel factors that influence cancer care will be instrumental in identifying new approaches to eliminate disparities in symptom management and cancer outcomes.(49)
Our finding also highlights the importance of rethinking symptom management systems. Symptom monitoring has been increasingly incorporated into routine cancer treatment. One randomized controlled trial found that symptom monitoring with e-mail alerts to providers significantly improved chemotherapy adherence and cancer survival compared to usual care,(41,50) suggesting the potential of routine symptom monitoring to improve cancer disparities. While patient symptoms were routinely collected and used for symptom monitoring at WCCRI, our finding indicates that new interventions are needed to ensure equitable symptom management among all patients.
Previous evidence suggests that psychological health is associated with treatment adherence (15,51) and survival outcomes for patients with cancer.(52) Thus, new approaches to improve psychological symptom assessment and management may be as important as that for physical symptoms for reducing racial disparities in cancer outcomes. Early identification of symptoms and evaluation of the symptom trajectory over the treatment course are critical and allow for timely intervention. Remote monitoring using mobile devices may be particularly useful for patients facing barriers to in-person care, such as lack of transportation, job flexibility, and time.(53,54) More research into tailored symptom monitoring and interventions for Black women may help to alleviate some of these symptom disparities.
Limitations
Our study has limitations. First, although our analyses adjusted for a wide range of patient and clinical covariates, this is an observational study and therefore we cannot rule out unmeasured confounding due to omitted variable bias. For example, early discontinuation may be associated with symptom severity but could not be clearly identified in our data. We tackled this issue by controlling for standard chemotherapy regimens in our analysis, and conducting sensitivity analyses among patients with ≥ 4 chemotherapy cycles. We also conducted sensitivity analyses among subgroups of Medicare beneficiaries to control for comorbidities and for whom we have confirmed surgery dates prior to chemotherapy to account for potential differences between neoadjuvant and adjuvant chemotherapy recipients and symptoms from prior surgery. Both subgroup analyses showed consistent patterns as our main analysis. In addition, by using longitudinal data and measuring within-patient symptom changes, our analytic approach adjusted for time-stable patient characteristics. However, measurement equivalence issues for different racial groups may still exist.(55,56) Lastly, our study is restricted to hormone-receptor positive, early-stage breast cancer women from a single cancer center and results are not generalizable to other disease conditions or study settings.
Conclusion
In this large, single-center cohort study of 1,273 women diagnosed with early-stage hormone-receptor positive breast cancer between 2007-2015, Black patients were more likely to experience a clinically meaningful increase in symptom burden after starting chemotherapy. Differences in baseline sociodemographic and clinical characteristics contributed to the increasing symptom burden among Black patients. However, most of the physical symptom changes remained unexplained by these characteristics, which suggests inadequate symptom management among Black women. Future studies should examine how differences in symptom burden affect racial disparities in treatment adherence and breast cancer outcomes.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Cancer Institute (R21CA208161, I. Graetz).
Footnotes
Conflicts of Interests
X. Hu reported receiving grants from PhRMA Foundation predoctoral fellowship outside the submitted work. C.M. Kaplan reported receiving grants from National Institute of Health, Agency for Healthcare Research and Quality, and American Cancer Society outside the submitted work. M.Y. Martin reported receiving grants from University of Tennessee Health Science Center Research Funding for the project during the conduct of the study. M.S. Walker reported receiving grants from AbbVie, Amgen, Astellas, Bayer, BMS, Daiichi Sankyo, Eisai, EMD Serono, Genentech, Gilead, GSK, Janssen, Eli Lilly, Merck, Merck KGaA, Novartis, and Pfizer outside the submitted work. G.A. Vidal reported receiving personal fees from Roche/Genetech, Eli Lilly, Pfizer, AstraZeneca, and Myriad; research funding from Roche/Genetech, Puma, Celcuity, Merck, BMS, Eli Lilly, Astrazeneca, Gilead, and GSK; and ownership of Oncodisc. L.S. Schwartzberg reported receiving grants from the National Institutes of Health during the conduct of the study; personal fees from Pfizer, Helsinn, Myriad, Bayer, Napo, BeyondSpring Pharmaceuticals, AbbVie, Coherus Biosciences, Illumina, Mirati Therapeutics, GlaxoSmithKline, Genentech, Amgen, Lilly, AstraZeneca, Seagen, Sanofi, and Merck outside the submitted work. I. Graetz reported receiving a research grant from Pfizer Oncology outside the submitted work. No other disclosures were reported.
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69(6):438–51 doi 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin 2019;69(3):211–33 doi 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 3.Goel N, Westrick AC, Bailey ZD, Hernandez A, Balise RR, Goldfinger E, et al. Structural Racism and Breast Cancer-specific Survival: Impact of Economic and Racial Residential Segregation. Annals of Surgery 2022;275(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker-Seeley RD. Social Determinants of Health and Disparities in Cancer Care for Black People in the United States. JCO Oncology Practice 2021;17(5):261–3 doi 10.1200/OP.21.00229. [DOI] [PubMed] [Google Scholar]
- 5.Sadigh G, Gray RJ, Sparano JA, Yanez B, Garcia SF, Timsina LR, et al. Assessment of Racial Disparity in Survival Outcomes for Early Hormone Receptor–Positive Breast Cancer After Adjusting for Insurance Status and Neighborhood Deprivation: A Post Hoc Analysis of a Randomized Clinical Trial. JAMA Oncology 2022;8(4):579–86 doi 10.1001/jamaoncol.2021.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Check DK, Chawla N, Kwan ML, Pinheiro L, Roh JM, Ergas IJ, et al. Understanding racial/ethnic differences in breast cancer-related physical well-being: the role of patient-provider interactions. Breast Cancer Res Treat 2018;170(3):593–603 doi 10.1007/s10549-018-4776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nong P, Raj M, Creary M, Kardia SLR, Platt JE. Patient-Reported Experiences of Discrimination in the US Health Care System. JAMA Network Open 2020;3(12):e2029650–e doi 10.1001/jamanetworkopen.2020.29650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Li CI. Racial Disparities in Breast Cancer Diagnosis and Treatment by Hormone Receptor and HER2 Status. Cancer Epidemiology Biomarkers & Prevention 2015;24(11):1666 doi 10.1158/1055-9965.EPI-15-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X-C, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, et al. Influence of Race, Insurance, Socioeconomic Status, and Hospital Type on Receipt of Guideline-Concordant Adjuvant Systemic Therapy for Locoregional Breast Cancers. Journal of Clinical Oncology 2011;30(2):142–50 doi 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 10.Samuel CA, Schaal J, Robertson L, Kollie J, Baker S, Black K, et al. Racial differences in symptom management experiences during breast cancer treatment. Support Care Cancer 2018;26(5):1425–35 doi 10.1007/s00520-017-3965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCall MK, Connolly M, Nugent B, Conley YP, Bender CM, Rosenzweig MQ. Symptom Experience, Management, and Outcomes According to Race and Social Determinants Including Genomics, Epigenomics, and Metabolomics (SEMOARS + GEM): an Explanatory Model for Breast Cancer Treatment Disparity. J Cancer Educ 2020;35(3):428–40 doi 10.1007/s13187-019-01571-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer 2017;123(11):2061–9 doi 10.1002/cncr.30575. [DOI] [PubMed] [Google Scholar]
- 13.Linden W, Girgis A. Psychological treatment outcomes for cancer patients: what do meta-analyses tell us about distress reduction? Psycho-Oncology 2012;21(4):343–50 doi 10.1002/pon.2035. [DOI] [PubMed] [Google Scholar]
- 14.Knisely AT, Michaels AD, Mehaffey JH, Hassinger TE, Krebs ED, Brenin DR, et al. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery 2018;164(2):195–200 doi 10.1016/j.surg.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Walker MS, Stepanski E, Kaplan CM, Martin MY, Vidal GA, et al. Racial Differences in Patient-Reported Symptoms and Adherence to Adjuvant Endocrine Therapy Among Women With Early-Stage, Hormone Receptor-Positive Breast Cancer. JAMA Netw Open 2022;5(8):e2225485 doi 10.1001/jamanetworkopen.2022.25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulls HW, Chang P-H, Brownstein NC, Zhou J-M, Hoogland AI, Gonzalez BD, et al. Patient-reported symptom burden in routine oncology care: Examining racial and ethnic disparities. Cancer Reports 2022;5(3):e1478 doi 10.1002/cnr2.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejeda S, Stolley MR, Vijayasiri G, Campbell RT, Estwing Ferrans C, Warnecke RB, et al. Negative psychological consequences of breast cancer among recently diagnosed ethnically diverse women. Psychooncology 2017;26(12):2245–52 doi 10.1002/pon.4456. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro LC, Samuel CA, Reeder-Hayes KE, Wheeler SB, Olshan AF, Reeve BB. Understanding racial differences in health-related quality of life in a population-based cohort of breast cancer survivors. Breast Cancer Res Treat 2016;159(3):535–43 doi 10.1007/s10549-016-3965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowen DJ, Alfano CM, McGregor BA, Kuniyuki A, Bernstein L, Meeske K, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat 2007;106(1):85–95 doi 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Chehal PK, Kaplan C, Krukowski RA, Lan RH, Stepanski E, et al. Characterization of Clinical Symptoms by Race Among Women With Early-Stage, Hormone Receptor–Positive Breast Cancer Before Starting Chemotherapy. JAMA Network Open 2021;4(6):e2112076–e doi 10.1001/jamanetworkopen.2021.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warsame R, D’Souza A. Patient Reported Outcomes Have Arrived: A Practical Overview for Clinicians in Using Patient Reported Outcomes in Oncology. Mayo Clinic Proceedings 2019;94(11):2291–301 doi 10.1016/j.mayocp.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi HY, Graetz I, Shaban-Nejad A, Schwartzberg L, Vidal G, Davis RL, et al. Social Disparities of Pain and Pain Intensity Among Women Diagnosed With Early Stage Breast Cancer. Frontiers in Oncology 2022;12 doi 10.3389/fonc.2022.759272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A 2016;113(16):4296–301 doi 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeder-Hayes KE, Wheeler SB, Mayer DK. Health disparities across the breast cancer continuum. Semin Oncol Nurs 2015;31(2):170–7 doi 10.1016/j.soncn.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne R, Medina E, Hampton JW. Quality of life concerns in patients with breast cancer: evidence for disparity of outcomes and experiences in pain management and palliative care among African-American women. Cancer 2003;97(1 Suppl):311–7 doi 10.1002/cncr.11017. [DOI] [PubMed] [Google Scholar]
- 26.Fortner B, Baldwin S, Schwartzberg L, Houts AC. Validation of the Cancer Care Monitor items for physical symptoms and treatment side effects using expert oncology nurse evaluation. J Pain Symptom Manage 2006;31(3):207–14 doi 10.1016/j.jpainsymman.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Abernethy AP, Zafar SY, Uronis H, Wheeler JL, Coan A, Rowe K, et al. Validation of the Patient Care Monitor (Version 2.0): a review of system assessment instrument for cancer patients. J Pain Symptom Manage 2010;40(4):545–58 doi 10.1016/j.jpainsymman.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer 2007;110(1):196–202 doi 10.1002/cncr.22799. [DOI] [PubMed] [Google Scholar]
- 29.Nurgalieva Z, Franzini L, Morgan R, Vernon S, Liu C, Du X. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Medical Oncology 2013;30(1):419. [DOI] [PubMed] [Google Scholar]
- 30.Green AK, Aviki EM, Matsoukas K, Patil S, Korenstein D, Blinder V. Racial disparities in chemotherapy administration for early-stage breast cancer: a systematic review and meta-analysis. Breast cancer research and treatment 2018;172(2):247–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004-2006. Journal of Clinical Oncology 2010;28(27):4135–41. [DOI] [PubMed] [Google Scholar]
- 32.Nusgart M HCPCS Coding: An Integral Part of Your Reimbursement Strategy. Adv Wound Care (New Rochelle) 2013;2(10):576–82 doi 10.1089/wound.2013.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53(12):1258–67 doi 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 34.Blinder AS. Wage discrimination: reduced form and structural estimates. Journal of Human resources 1973:436–55. [Google Scholar]
- 35.Jann B The Blinder–Oaxaca Decomposition for Linear Regression Models. The Stata Journal 2008;8(4):453–79 doi 10.1177/1536867X0800800401. [DOI] [Google Scholar]
- 36.McFarland DC, Shaffer KM, Tiersten A, Holland J. Physical Symptom Burden and Its Association With Distress, Anxiety, and Depression in Breast Cancer. Psychosomatics 2018;59(5):464–71 doi 10.1016/j.psym.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jemal A, Robbins AS, Lin CC, Flanders WD, DeSantis CE, Ward EM, et al. Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. J Clin Oncol 2018;36(1):14–24 doi 10.1200/jco.2017.73.7932. [DOI] [PubMed] [Google Scholar]
- 38.Griggs JJ, Sorbero MES, Stark AT, Heininger SE, Dick AW. Racial Disparity in the Dose and Dose Intensity of Breast Cancer Adjuvant Chemotherapy. Breast Cancer Research and Treatment 2003;81(1):21–31 doi 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 39.Griggs JJ, Culakova E, Sorbero MES, Poniewierski MS, Wolff DA, Crawford J, et al. Social and Racial Differences in Selection of Breast Cancer Adjuvant Chemotherapy Regimens. Journal of Clinical Oncology 2007;25(18):2522–7 doi 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 40.Wagner LI. Patient-Reported Outcomes Bridge an Important Gap in Identifying Risk for Early Endocrine Therapy Discontinuation. J Natl Cancer Inst 2021;113(8):945–7 doi 10.1093/jnci/djab024. [DOI] [PubMed] [Google Scholar]
- 41.Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016;34(6):557–65 doi 10.1200/jco.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in Opioid Prescriptions Among Part D Medicare Recipients From 2007 to 2012. Am J Med 2016;129(2):221.e21–30 doi 10.1016/j.amjmed.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J Clin Oncol 2014;32(16):1721–6 doi 10.1200/jco.2013.52.5196. [DOI] [PubMed] [Google Scholar]
- 44.Lee P, Le Saux M, Siegel R, Goyal M, Chen C, Ma Y, et al. Racial and ethnic disparities in the management of acute pain in US emergency departments: Meta-analysis and systematic review. Am J Emerg Med 2019;37(9):1770–7 doi 10.1016/j.ajem.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Stein KD, Alcaraz KI, Kamson C, Fallon EA, Smith TG. Sociodemographic inequalities in barriers to cancer pain management: a report from the American Cancer Society's Study of Cancer Survivors-II (SCS-II). Psycho-Oncology 2016;25(10):1212–21 doi 10.1002/pon.4218. [DOI] [PubMed] [Google Scholar]
- 46.Enzinger AC, Ghosh K, Keating NL, Cutler DM, Landrum MB, Wright AA. U.S. trends and racial/ethnic disparities in opioid access among patients with poor prognosis cancer at the end of life (EOL). Journal of Clinical Oncology 2020;38(15_suppl):7005- doi 10.1200/JCO.2020.38.15_suppl.7005. [DOI] [Google Scholar]
- 47.Anderson JN, Graff JC, Krukowski RA, Schwartzberg L, Vidal GA, Waters TM, et al. "Nobody Will Tell You. You've Got to Ask!": An Examination of Patient-provider Communication Needs and Preferences among Black and White Women with Early-stage Breast Cancer. Health Commun 2020:1–12 doi 10.1080/10410236.2020.1751383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis KR, Black KZ, Baker S, Cothern C, Davis K, Doost K, et al. Racial Differences in the Influence of Health Care System Factors on Informal Support for Cancer Care Among Black and White Breast and Lung Cancer Survivors. Fam Community Health 2020;43(3):200–12 doi 10.1097/fch.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taplin SH, Anhang Price R, Edwards HM, Foster MK, Breslau ES, Chollette V, et al. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr 2012;2012(44):2–10 doi 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017;318(2):197–8 doi 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theofilou P, Panagiotaki H. A literature review to investigate the link between psychosocial characteristics and treatment adherence in cancer patients. Oncol Rev 2012;6(1):e5 doi 10.4081/oncol.2012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berchuck JE, Meyer CS, Zhang N, Berchuck CM, Trivedi NN, Cohen B, et al. Association of Mental Health Treatment With Outcomes for US Veterans Diagnosed With Non–Small Cell Lung Cancer. JAMA Oncology 2020;6(7):1055–62 doi 10.1001/jamaoncol.2020.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards R, Kinnersley P, Brain K, McCutchan G, Staffurth J, Wood F. Use of Mobile Devices to Help Cancer Patients Meet Their Information Needs in Non-Inpatient Settings: Systematic Review. JMIR Mhealth Uhealth 2018;6(12):e10026 doi 10.2196/10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naughton MJ, Moon J, Loyan H, Peng J, DeGraffinreid C, Paskett ED. Provision of smartphones in a symptom monitoring program of gynecologic and breast cancer patients during active therapy. Journal of Clinical Oncology 2020;38(29_suppl):255- doi 10.1200/JCO.2020.38.29_suppl.255. [DOI] [Google Scholar]
- 55.Kim G, Wang SY, Sellbom M. Measurement Equivalence of the Subjective Well-Being Scale Among Racially/Ethnically Diverse Older Adults. The journals of gerontology Series B, Psychological sciences and social sciences 2020;75(5):1010–7 doi 10.1093/geronb/gby110. [DOI] [PubMed] [Google Scholar]
- 56.Lix LM, Acan Osman B, Adachi JD, Towheed T, Hopman W, Davison KS, et al. Measurement equivalence of the SF-36 in the Canadian Multicentre Osteoporosis Study. Health Qual Life Outcomes 2012;10:29 doi 10.1186/1477-7525-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying this article are not publicly available. Access to the data requires an institutional review board approval, and signed data use agreements with WCCRI and Centers for Medicare & Medicaid Services.