Abstract
Introduction
Obesity affects cardiac geometry, causing both eccentric (due to increased cardiac output) and concentric (due to insulin resistance) remodelling. Following bariatric surgery, reversal of both processes should occur. Furthermore, epicardial adipose tissue loss following bariatric surgery may reduce pericardial restraint, allowing further chamber expansion. We investigated these changes in a serial imaging study of adipose depots and cardiac geometry following bariatric surgery.
Methods
62 patients underwent cardiac magnetic resonance (CMR) before and after bariatric surgery, including 36 with short-term (median 212 days), 37 medium-term (median 428 days) and 32 long-term (median 1030 days) follow-up. CMR was used to assess cardiac geometry (left atrial volume (LAV) and left ventricular end-diastolic volume (LVEDV)), LV mass (LVM) and LV eccentricity index (LVei – a marker of pericardial restraint). Abdominal visceral (VAT) and epicardial (EAT) adipose tissue were also measured.
Results
Patients on average had lost 21kg (38.9% excess weight loss, EWL) at 212 days and 36kg (64.7% EWL) at 1030 days following bariatric surgery. Most VAT and EAT loss (43% and 14%, p<0.0001) occurred within the first 212 days, with non-significant reductions thereafter. In the short-term LVM (7.4%), LVEDV (8.6%) and LAV (13%) all decreased (all p<0.0001), with change in cardiac output correlated with LVEDV (r=0.35,p=0.03) and LAV change (r=0.37,p=0.03). Whereas LVM continued to decrease with time (12% decrease relative to baseline at 1030 days, p<0.0001), both LAV and LVEDV had returned to baseline by 1030 days. LV mass:volume ratio (a marker of concentric hypertrophy) reached its nadir at the longest timepoint (p<0.001). At baseline, LVei correlated with baseline EAT (r=0.37,p=0.0040), and decreased significantly from 1.09 at baseline to a low of 1.04 at 428 days (p<0.0001). Furthermore, change in EAT following bariatric surgery correlated with change in LVei (r=0.43,p=0.0007).
Conclusions
Cardiac volumes show a biphasic response to weight loss, initially becoming smaller and then returning to pre-operative sizes by 1030 days. We propose this is due to an initial reversal of eccentric remodelling followed by reversal of concentric remodelling. Furthermore, we provide evidence for a role of EAT contributing to pericardial restraint, with EAT loss improving markers of pericardial restraint.
Keywords: obesity, epicardiac adipose tissue, weight loss, bariatric surgery, cardiac remodelling, cardiac geometry
Introduction
Although obesity implies a global increase in total body fat, on an individual basis fat is deposited differentially into specific subcutaneous and visceral compartments of the body and also ectopically within organs (1). It is now becoming clear that the increased cardiovascular risk associated with obesity (2) is not solely the result of increased adiposity, but is also affected by the distribution of this excess fat, with evidence that visceral depots are more strongly linked to increased cardio-metabolic risk than subcutaneous depots (3–5). As a result, the pattern of fat distribution within an individual is likely to provide a better picture of cardiovascular risk than global measures of obesity (6, 7). Furthermore, there is emerging evidence that the greater adverse effect of visceral fat extends beyond cardiovascular risk factors and results in an increased likelihood of left ventricular (LV) hypertrophy (8, 9).
The combination of increased total adipose tissue and skeletal muscle volumes results in higher cardiac output and total blood volume. This leads to LV cavity dilatation and eccentric hypertrophic remodelling (10, 11) secondary to increased wall stress imposed by this cavity dilatation (12, 13). Although this accounts for the eccentric hypertrophic pattern, it is now becoming clear that concentric remodelling also occurs in obesity (14–16). The cause of this concentric remodelling has been shown to be related to insulin resistance (17), hyperleptinaemia (18) and myocardial steatosis (19) driven by visceral adipose tissue (20). In line with this, in diabetes, concentric remodelling with small LV cavity size is commonly reported (17).
Following bariatric surgery, many studies have reported reverse remodelling. LV mass has been universally shown to be reduced following weight loss and proportionally related to weight loss over time (21–24). With the reduction in both subcutaneous (driving volume load and eccentric hypertrophy) and visceral adiposity (driving insulin resistance and concentric remodelling) following bariatric surgery, this would be expected. The results for left ventricular cavity size changes are far less consistent, with some reporting a reduction in LV cavity size (22, 25) whilst others no change (26, 27), or an increase (28). In this case, the early reduction in volume load that accompanies total fat mass reduction would be expected to reduce LV cavity size, however, the reversal of insulin resistance that accompanies bariatric surgery (29), may be expected over time to reduce concentric remodelling and thus increase LV cavity size. Whether a reduction in epicardial adipose tissue allows additional expansion due to relief of external compression (30) or had a local paracrine effect on ventricular muscle given it’s anatomical location (31), is unknown.
We hypothesised that LV geometric remodelling would follow a biphasic time-course characterised initially by LV cavity reduction and reduced LV mass (as eccentric remodelling reverses), followed by relative LV cavity dilation with the return of insulin sensitivity (and reversal of concentric remodelling). We also hypothesised that a reduction in epicardial adipose tissue (EAT) following bariatric surgery would increase space within the pericardium, thus decreasing pericardial restraint. In order to assess these hypotheses, we performed a serial imaging study involving 62 participants who underwent CMR imaging to assess cardiac geometry alongside epicardial and visceral adipose tissue, before and after bariatric surgery. The study included 36 participants who underwent short-term (median 212 days), 37 medium-term (median 428 days) and 32 long-term (median 1030 days) imaging follow up.
Materials and methods
Cohort
62 participants (28 gastric bypass, 26 sleeve gastrectomy, 8 adjustable gastric band) were recruited from the bariatric surgery clinics at the Oxford University Hospitals Trust and Metabolic Diseases and Nutrition, Centre Hospitalier Universitaire Nord, Marseille, France and underwent a CMR scan prior to surgery as described below. Participants attended follow up CMR scans at varying timepoints after bariatric surgery, including at a median follow up of 212 days (n=36), 428 days (n=37) and 1030 days (n=32) (43 participants were imaged at two follow up points and 19 participants were imaged at one follow up point). The surgical procedure proportion was similar across timepoints.
The study was approved by the local research ethics committee (NHSREC Ref 15/SC/004, or local REC in Marseille (NCT01284816)), and informed written consent obtained from all volunteers Exclusion criteria included uncontrolled atrial fibrillation, established cardiac disease such as history or symptoms of flow-limiting coronary artery disease, infarction on CMR, severe valvular heart disease, recent change in medications, previous bariatric surgery, and standard contraindications to MR scanning (pregnancy, breastfeeding, implanted metallic devices, severe claustrophobia) All study visits were performed with the volunteer fasted for 8 hours.
Anthropomorphic and biochemical assessment
Height, weight and body composition were measured using digital scales (InBody 770, InBody Co Ltd, South Korea). Fasting venous blood was taken and biomarkers were analysed by the Oxford University Hospitals clinical biochemistry laboratory according to standardised protocols. Fasting insulin resistance was represented by HOMA-IR ((glucose x insulin)/22.5).
Magnetic resonance imaging
Visceral fat depots
Visceral adipose tissue (VAT) was measured with a 5mm transverse slice at the level of the 5th lumbar vertebral body, using a water-suppressed turbo spin echo (TSE) sequence and analysed with manual contouring as previously described (23).
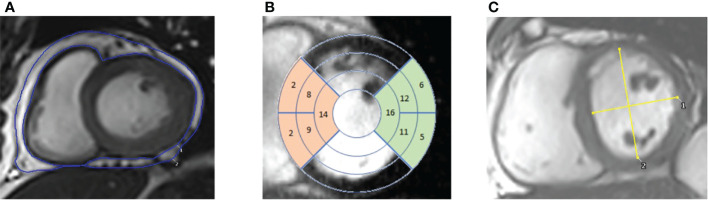
Epicardial adipose tissue (EAT) was manually delineated on the same short axis stack images from the atrioventricular valve annuli to the apex in end-ventricular systole ( Figure 1A ). Surfaces were then summed and multiplied by slice thickness to obtain epicardial adipose tissue volume.
Figure 1.
CMR images. (A) – contouring of epicardial adipose tissue in the short axis view. (B) – 17 segment view overlayed on short axis images. Highlighting the septal segments (in orange) and the lateral segments (in green). (C) – Short axis view with measurements of the maximal anterior-posterior (AP) diameter parallel to the septum and maximal septal-lateral (SL) diameter orthogonal to the AP diameter, with left ventricular eccentricity index being calculated by diving AP/SL diameters.
Cardiac imaging
Cardiac imaging to quantify ventricular volumes and function was acquired using an SSFP sequence (echo time 1.5ms, repetition time 3ms), which was performed with cardiac triggering and during end-expiratory breath-hold, on a 3T MR system (Tim Trio, Siemens, Munich, Germany/Verio, Siemens, Erlangen, Germany) as previously described (32). Typical SSFP sequence parameters were slice thickness 8 mm, gap 2 mm, retrospective gating, TE 1.5 ms, TR 46 ms, flip-angle 50°, FOV 400 mm, matrix size 256 in frequency encode direction. Endocardial and epicardial left ventricular contours were drawn and analysed using a semi-automated system to produce left ventricular mass (LVM), left ventricular end-diastolic volume (LVEDV) and left ventricular stroke volume (LVSV) (cmr42, Circle Cardiovascular Imaging Inc, Calgary, Canada) as previously described (33). Left atrial volumes (LAV) were acquired from contouring long axis cine images in ventricular end-systole. Average septal and lateral wall thickness was calculated by averaging wall thickness values from AHA segments 2, 3, 8, 9 and 14 (septal) and 5, 6, 11, 12 and 16 (lateral) in ventricular end-diastole ( Figure 1B ). In the short axis view at the level of the papillary muscles in ventricular end-diastole, maximal anterior-posterior (AP) LV diameter parallel to the septum and maximal septal-lateral (SL) distance perpendicular to the septum were measured ( Figure 1C ). Left ventricular eccentricity index (LVei) was calculated by dividing AP length by SL length.
Statistical analysis
All statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, California USA). Data are expressed as means ± standard deviation (SD) unless otherwise stated. For imaging parameters, percentage change relative to baseline was calculated to account for individual variation in parameters. All continuous variables were normally distributed. As not all participants were scanned at each time point, differences between timepoints were assessed using a mixed-effects model with the Geisser-Greenhouse correction. Bivariate correlations were performed to compute Pearson correlation coefficients. When assessing change in parameters (EAT, VAT and LVei) over the course of the study for the correlation analysis, the difference in maximal timepoints was used (e.g. if a participant was scanned at 3 timepoints, the difference in values between the first and final timepoints were used). A probability value of p < 0.05 was considered significant and two-tailed p values were used for all statistics.
Results
Baseline characteristics
Patients included in this cohort had a mean age of 44 (SD 9.9) years, with 74% of the participants being female. Average weight was 124.0kg (SD 19.1), average excess body weight was 54.5kg (SD 15.3) and average BMI was 44.6kg/m2 (SD 5.3). Participants had an average systolic and diastolic blood pressure of 129.6 mmHg (SD 18.6) and 74.3 mmHg (SD 10.1), respectively. Seven patients (11%) had diagnosed hypertension with four patients receiving medication for hypertension. Fasting venous blood samples revealed an average fasting glucose of 5.9 mmol/L (SD 2.0), total cholesterol of 4.9 mmol/L (SD 1.0), triglycerides of 1.3 mmol/L (SD 0.8) and a HOMA-IR of 4.4 (SD 3.5). Eight (13%) participants had dyslipidaemia and 9 (15%) had diabetes ( Table 1 ), with 6 and 5 receiving treatment for these conditions respectively.
Table 1.
Baseline characteristics of patients undergoing bariatric surgery.
| Participants included in analysis (n=62) | |
|---|---|
| Age (years) | 44.4 (9.9) |
| Sex (females; n (%)) | 46 (74%) |
| Weight (kg) | 124.0 (19.1) |
| BMI (kg/m2) | 44.6 (5.3) |
| Excess Body Weight (kg) | 54.5 (15.3) |
| Systolic Blood Pressure (mmHg) | 129.6 (18.6) |
| Diastolic Blood Pressure (mmHg) | 74.3 (10.1) |
| Hypertension (n (%)) | 7 (11%) |
| Fasting Glucose (mmol/L) | 5.9 (2.0) |
| Total Cholesterol (mmol/L) | 4.9 (1.0) |
| Fasting Triglycerides (mmol/L) | 1.3 (0.8) |
| Dyslipidaemia (n (%)) | 8 (13%) |
| HOMA-IR | 4.4 (3.5) |
| Diabetes (n (%)) | 9 (15%) |
Values are mean (standard deviation) unless otherwise stated. BMI, body mass index, HOMA-IR, homeostatic model assessment of insulin resistance.
Sequence of body changes following bariatric surgery
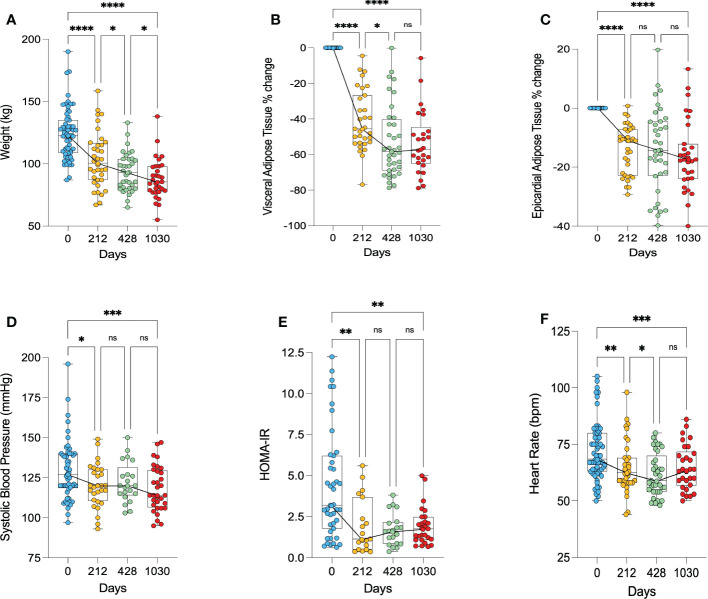
Following surgery, participants weight on average decreased from 124kg to 103kg at 212 days (38.9% excess weight loss), before decreasing further to 93kg at 428 days (56.5% excess weight loss) and 88kg at 1030 days (64.7% excess weight loss) ( Figure 2A ).
Figure 2.
Anthropomorphic changes following bariatric surgery. (A) – change in weight, (B) – percentage change in visceral adipose tissue, (C) – percentage change in epicardial adipose tissue, (D) – change in systolic blood pressure, (E) – change in HOMA-IR as a marker of insulin resistance, (F) – change in heart rate. HOMA-IR, homeostatic model assessment of insulin resistance. * < 0.05, ** < 0.01, ***<0.001, ****<0.0001, ns, Non significant.
The majority of VAT was lost in the first 212 days, with an average 43% decrease in VAT (p<0.0001). At the long-term follow up point of 1030 days, there was a 53% decrease in VAT relative to baseline (p<0.0001) ( Figure 2B ). Similarly, the majority of EAT loss occurred in the first 212 days, showing on average a 14% decrease from baseline (p<0.0001). At 1030 days, there was on average a 16% decrease in EAT from baseline (p<0.0001) ( Figure 2C ).
There was a decrease in systolic blood pressure from 130 mmHg pre-operatively to 120 mmHg at 212 days post-surgery (p=0.046) and 118 mmHg at 1030 days (p=0.0005) ( Figure 2D ). Insulin resistance, as measured by HOMA-IR, significantly decreased in the first 212 days (p=0.002), and remained lower at 1030 days (p=0.007) ( Figure 2E ). Heart rate significantly decreased following bariatric surgery, remaining significantly lower at 1030 relative to baseline (p=0.0006) ( Figure 2F ). Three of the 7 participants with hypertension, 2 of the 8 participants with dyslipidaemia, and 7 of the 9 patients with diabetes had resolution of their conditions.
Cardiac geometry changes following bariatric surgery
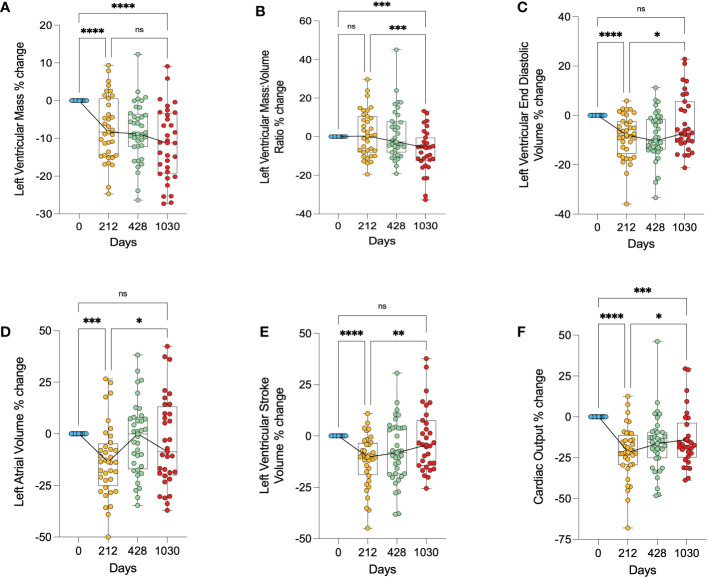
There was on average a 7.4% decrease in left ventricular mass (LVM) in the first 212 days (p<0.0001), with a further decrease of 12% relative to baseline at 1030 days (p<0.0001) ( Figure 3A ). Left ventricular mass:volume ratio (LVMVR - a marker of concentric remodelling) appeared to only decrease after 428 days, showing on average a 7.3% decrease at day 1030 relative to baseline (p=0.0010) ( Figure 3B ). LVEDV decreased significantly in the short term (day 212) by 8.6% (p<0.0001) but had increased to a volume not significantly different from pre-operative LVEDV by day 1030 ( Figure 3C ). LAV and LVSV also appeared to show this biphasic relationship. Both decreased significantly in the first 212 days (LAV by 13% (p<0.0001) and LVSV by 12% (p<0.0001)) but returned to values not significantly different from baseline at day 1030 ( Figures 3D, E ). Cardiac output decreased on average by 21% during the first 212 days (p<0.0001) and remained significantly lower at 1030 days (p=0.0001), although did show a small increase from 212 days to 1030 days (7.6% points, p=0.0230) ( Figure 3F ). The initial fall in cardiac output correlated with the decrease in both LVEDV (r=0.35, p=0.03) and LAV (r=0.37, p=0.03).
Figure 3.
Changes in cardiac geometry following bariatric surgery. (A) – percentage change in left ventricular mass, (B) – percentage change in left ventricular mass:volume ratio, (C) – percentage change in left ventricular end diastolic volume, (D) – percentage change in left atrial volume, (E) – percentage change in left ventricular stroke volume, (F) – percentage change in cardiac output. * < 0.05, ** < 0.01, ***<0.001, ****<0.0001, ns, Non significant.
Overall, this shows a biphasic response of the left ventricular and left atrial sizes, initially getting smaller, and then larger, returning to baseline values. It also suggests that whilst LV mass was reduced continually through follow up, initially this loss was proportional to the LV cavity size reduction, and at later timepoints showed a reduction in concentric remodelling.
Local effect of epicardial adipose tissue on cardiac geometry
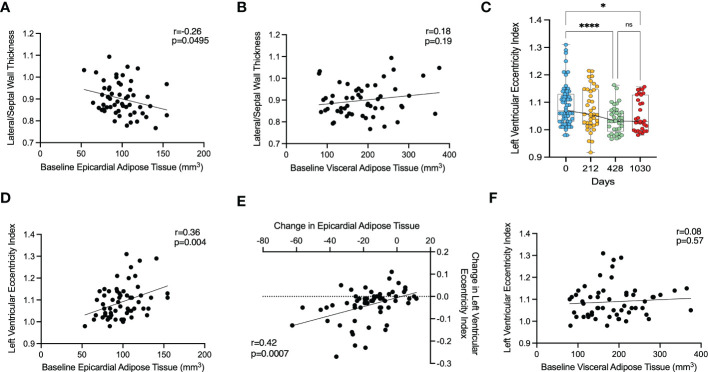
In order to assess whether epicardial adipose tissue has a local effect on ventricular wall thickness, we examined the relationship between EAT and regional LV thickness. For this we compared the relationship between EAT volume, and the lateral-to-septal wall thickness ratio, since the lateral wall is in close proximity to EAT and the septal wall is not, enabling assessment for regional effects. EAT was weakly, but inversely correlated with a lateral-to-septal wall thickness ratio (r=-0.26, p=0.0495) ( Figure 4A ), suggesting that increased EAT was not related to local LV hypertrophy. However, when looking at the correlation between EAT and lateral or septal wall thickness individually, no relationship was found (r=0.05, p=0.72 and r=0.22, p=0.10, respectively). Baseline VAT was not associated with lateral/septal wall thickness ratio (r=0.18, p=0.19) ( Figure 4B ). Increasing VAT was associated with both increased lateral (r=0.47, p=0.0003) and septal (r=0.34, p=0.0118) wall thickness.
Figure 4.
The effect of epicardial adipose tissue on cardiac geometry following bariatric surgery. (A) – correlation between baseline lateral/septal wall thickness ratio and baseline epicardial adipose tissue, (B) – correlation between baseline lateral/septal wall thickness ratio and baseline visceral adipose tissue, (C) – change in left ventricular eccentricity index, (D) - correlation between left ventricular eccentricity index and baseline epicardial adipose tissue, (E) - correlation between change in left ventricular eccentricity index and change in epicardial adipose tissue, (F) – correlation between left ventricular eccentricity index and baseline visceral adipose tissue. *<0.05, *** < 0.001, **** < 0.0001 ns, Non significant.
Overall, this would suggest that there was no local effect of EAT on lateral wall thickness, and that the observed relationship was driven more by VAT volume.
Effects of epicardial adipose tissue on pericardial restraint
Finally, we investigated the left ventricular eccentricity index (LVei) in end-diastole as a marker of ventricular interdependence and thus pericardial restraint. LVei decreased significantly from a mean of 1.09 (SD 0.08) at baseline to 1.04 (SD 0.05) at 428 days (p<0.0001) and was maintained with no further reduction at 1030 days (mean 1.06, SD 0.06, p=0.048) ( Figure 4C ). LVei appeared to be positively correlated with baseline EAT (r=0.37, p=0.0040) ( Figure 4D ) and moreover, change in EAT over maximal timepoints was correlated with change in LVei over maximal timepoints (r=0.43, p=0.0007) ( Figure 4E ). On the other hand, baseline VAT did not correlate with LVei (r=0.08, p=0.5713) ( Figure 4F ) and change in VAT over maximal timepoints was not correlated with change in LVei over maximal timepoints (r=0.10, p=0.4627).
This would suggest that epicardial adipose tissue causes some pericardial mass effect and pericardial restraint, which is improved with weight loss.
Discussion
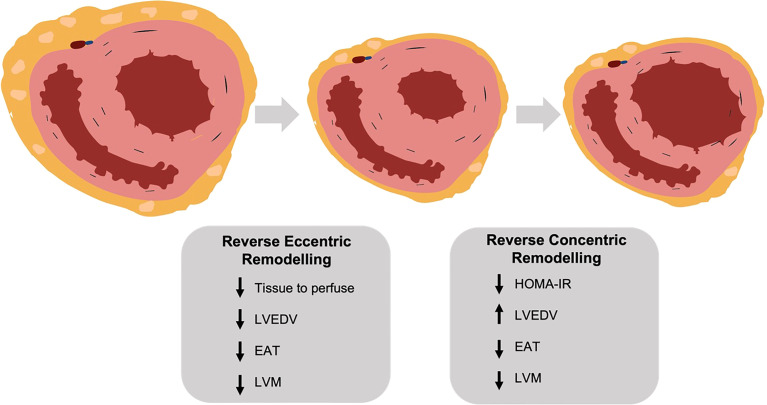
The cardiac remodelling in obesity is characterised by mixed eccentric and left ventricular hypertrophy and left atrial dilatation. In this study we show that following bariatric surgery there is an initial reduction in LV and LA cavity size mirroring the reduction in cardiac output, followed by an increase in chamber size with longer-term assessment. Associated with this is a reduction in epicardial adipose tissue and reduced LV eccentricity index, indicating reduced pericardial constraint. Additionally, we show that whilst LV mass was reduced continually throughout follow up, in early follow up this loss was proportional to the LV cavity size reduction, and at later timepoints a reduction in concentric remodelling is seen. Furthermore, we show that epicardial adipose tissue has no local effect on LV hypertrophy in obesity. A such we provide evidence for a biphasic cardiac response to weight loss (summarised in Figure 5 ).
Figure 5.
(central figure) – following bariatric surgery the left ventricle undergoes a biphasic remodelling response. Initially there is a reversal of eccentric remodelling as total body tissue to perfuse falls, with reductions in left ventricular mass, end diastolic volume and epicardial adipose tissue. In the longer term there is a reversal of concentric remodelling as insulin resistance (HOMA-IR) decreases, with a further fall in left ventricular mass but an increase in end diastolic volume. LVEDV, left ventricular end diastolic volume; EAT, epicardial adipose tissue; LVM, left ventricular mass; HOMA-IR, homeostatic model assessment of insulin resistance. Created with BioRender.com.
Changes in left ventricular geometry following weight loss
Eccentric LV remodelling
As expected, in our study significant decreases in total body weight and VAT were seen quickly following bariatric surgery. This rapid reduction in total body tissue to perfuse decreases the cardiac output requirement which was also observed. We see here that this reduction in cardiac output is a result of both decreased heart rate and LV end-diastolic volume, leading to reduced stroke volume. With a smaller stroke volume and LV cavity size it would be expected that LV mass would also fall, and we observe this to be the case in this study with the fall in LV mass being proportional to the reduction in LV cavity size. This initial reversal of eccentric remodelling occurs early post bariatric surgery with the majority of the change seen at the earliest timepoint of 212 days. These results agree with a prior study relating regression in LV mass to reductions in VAT (34), and are particularly important given the increasing recognition for VAT playing a pathophysiology role in obesity-related heart failure (35).
Concentric LV remodelling
However, the hormonal milieu in obesity also contributes to LV hypertrophy, with insulin and leptin both stimulating myocyte hypertrophy. Indeed, we have shown that diabetes and insulin resistance is associated with smaller LV cavity size and concentric remodelling (19). When put together with elevation in blood pressure, which is commonly associated with obesity, concentric remodelling is also observed with increased body mass index (14–16). As weight loss is known to reduce leptin, insulin and blood pressure (17, 18, 36), this aspect of LV remodelling should also reverse as shown in a previous study (37). In line with this we observed a significant decrease in insulin resistance (HOMA-IR) and systolic blood pressure and a reduction in LV mass-to-volume ratio at the longest term. The fall in insulin resistance in the short term is likely to take time to translate into reversal of concentric remodelling, explaining the delayed improvement in concentric remodelling. The reductions in LV remodelling, in tandem with decreases in volume overload, may be associated with salutary reductions in cardiac filling pressures, as previously reported following weight loss (38).
A biphasic ventricular response
Following bariatric surgery, studies have reported conflicting results regarding LV end-diastolic volume, with increased (28), decreased (22, 25) and no change being reported (26, 27). This study shows a biphasic response of the LV cavity following weight loss, with the initially observed decrease in LV cavity size being followed by a longer-term increase in cavity size, back to the baseline size. We propose that this cavity size increase is due to the improvement in insulin sensitivity, reduction in LV concentric remodelling, and the reduction in epicardial adipose tissue, that allows the LV cavity to expand. In line with this local volume effect of EAT, the significant decrease in EAT observed in this study was correlated with improvements in LV eccentricity index, a marker of ventricular interdependence and pericardial restraint (30, 39, 40). As such, the heterogeneity of reported changes in LV cavity size following bariatric surgery may simply be due to reporting at variable time intervals. In keeping with this, those that have reported up to one year after surgery (22, 25), in general report reduced LV cavity size, and those with no change or increase tend to report over two years from surgery (28, 37, 41).
Left atrial geometry and weight loss
Similar heterogeneity exists in reports of LA cavity size following bariatric surgery (42). In this study, LA cavity size followed a similar trajectory to LV cavity size, initially falling in size and then increasing from 212 days onwards. Previous studies have also shown a decrease in LA volume within 1 year of bariatric surgery (43, 44), with other studies reporting no change in LA volume after 1 year (41, 45, 46), and increased LA volume after 5 years (34). Whilst the initial reduction in size follows the reduction in cardiac output, as for the LV, the loss of EAT from the pericardial space may again allow further space for this LA cavity dilation to occur. It may also be that we are simply observing LA enlargement over time as a result of normal ageing (47) which is likely to be greater in a population with obesity (48). Despite observing no change in LA volume over the longer-term post bariatric surgery in this study, we feel it is likely that this still represents an improvement relative to those who don’t undergo weight loss who would be expected to have an increase in LA volume (46).
Local effect of epicardial adipose tissue
EAT is a visceral fat depot located entirely within the pericardium and thus is in direct contact with the myocardium. In keeping with its role as an ectopic fat depot, studies have shown that increased EAT is associated with increased myocardial steatosis (49, 50). However, beyond its role as a fat storage depot, EAT is being increasing recognised as a metabolically active tissue, with paracrine effects on the myocardium through adipokine release (51). Given that the lateral wall of the left ventricle is in direct contact with EAT, whilst the septal wall is not, we hypothesised that if a local paracrine hypertrophic effect exists, a difference in wall thickness at these two locations would be observed. Interestingly we found no evidence for such a difference between lateral and septal wall thickness, suggesting that a local hypertrophic effect does not exist.
Limitations
This study has a number of limitations. Firstly it is a relatively small sample size of 62, with variable follow up time points. Furthermore, participants in this cohort underwent different types of surgery and this may affect the amount of weight lost. Whilst this population was a relatively healthy cohort, some participants did have hypertension (11%) or diabetes (15%), and these conditions themselves or their treatment may have an impact on cardiac geometry. There was also significantly more females (74%) than males in this cohort, and this too may have an impact on cardiac geometry.
Conclusion
In summary, in this serial imaging study we provide evidence for a biphasic response in LV and LA remodelling following weight loss induced by bariatric surgery. This may be explained by an initial reversal of eccentric remodelling given the decreased demands of the body from global tissue loss, followed by a longer-term reversal of concentric remodelling as insulin sensitivity is restored over a longer duration of time. We also provide evidence for a role of EAT in contributing to pericardial restraint in the setting of obesity, and furthermore show that decreasing EAT correlates with improved markers of pericardial restraint. This study provides insights into how bariatric surgery effects the left ventricle and atrium, and provides an explanation for the inconsistent results previously reported in the field. Furthermore, it adds more evidence to support the beneficial role of decreasing EAT on cardiac geometry and function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the local research ethics committee (NHSREC Ref 15/SC/004, or local REC in Marseille (NCT01284816)). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JH, IA and OR conceptualised and designed the study. Data was collected by IA, AL, JR, MB, FK, AS, BS, JB, TB. Data analysis was conducted by JH, OD and OR, and BB, AD, BG, SN and OR contributed to data interpretation. The manuscript was written by JH and OR. All authors contributed to the article and approved the submitted version.
Funding Statement
The study was designed, conducted, analysed, and reported entirely by the authors. The work was supported by the Oxford Partnership Comprehensive Biomedical Research Centre, with funding from the Department of Health’s NIHR Biomedical Research Centres, a British Heart Foundation Intermediate Clinical Fellowship FS/16/70/32157, the National Institutes of Health and Assistance Publique des Hopitaux de Marseille. The views expressed are those of the author(s) and not necessarily those of the BHF, BRC, NHS, NIHR, NIH or Department of Health and Social Care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments. Circulation (2007) 116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 2. Clark K. Obesity and the risk of heart failure. N Engl J Med (2002) 347(1):305–13. doi: 10.1056/NEJMoa020245 [DOI] [PubMed] [Google Scholar]
- 3. Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol (2008) 28(6):1039–49. doi: 10.1161/atvbaha.107.159228 [DOI] [PubMed] [Google Scholar]
- 4. Vague J. A determinant factor of the forms of obesity. Obes Res (1996) 4(2):201–3. doi: 10.1002/j.1550-8528.1996.tb00535.x [DOI] [PubMed] [Google Scholar]
- 5. Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol (2004) 24(5):923–9. doi: 10.1161/01.atv.0000125702.26272.f6 [DOI] [PubMed] [Google Scholar]
- 6. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nat (2006) 444(7121):875–80. doi: 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- 7. Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther (2001) 298(2):461–8. [PubMed] [Google Scholar]
- 8. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nat (2006) 444(7121):881–7. doi: 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 9. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol (2013) 62(10):921. doi: 10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alpert MA. Obesity cardiomyopathy: Pathophysiology and evolution of the clinical syndrome. Am J Med Sci (2001) 321(4):225–36. doi: 10.1097/00000441-200104000-00003 [DOI] [PubMed] [Google Scholar]
- 11. Alexander JK. Obesity and the heart. Heart Dis Stroke (1993) 2(4):317–21. doi: 10.1161/01.cir.96.9.3248 [DOI] [PubMed] [Google Scholar]
- 12. Ku CS, Lin SL, Wang DJ, Chang SK, Lee WJ. Left ventricular filling in young normotensive obese adults. Am J Cardiol (1994) 73(8):613–5. doi: 10.1016/0002-9149(94)90347-6 [DOI] [PubMed] [Google Scholar]
- 13. Messerli FH. Cardiopathy of obesity — a not-So-Victorian disease. New England Journal of Medicine (2009) 314(6):378–80. doi: 10.1056/NEJM198602063140608 [DOI] [PubMed] [Google Scholar]
- 14. Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle: The multi-ethnic study of atherosclerosis (MESA). JACC Cardiovasc Imaging. (2010) 3(3):266–74. doi: 10.1016/j.jcmg.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S, et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson (2009) 11(1). doi: 10.1186/1532-429X-11-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rider OJ, Lewandowski A, Nethononda R, Petersen SE, Francis JM, Pitcher A, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Hear J (2013) 34(4):292–9. doi: 10.1093/eurheartj/ehs341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Athithan L, Gulsin GS, McCann GP, Levelt E. Diabetic cardiomyopathy: Pathophysiology, theories and evidence to date. World J Diabetes (2019) 10(10):490. doi: 10.4239/wjd.v10.i10.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rider OJ, Petersen SE, Francis JM, Ali MK, Hudsmith LE, Robinson MR, et al. Ventricular hypertrophy and cavity dilatation in relation to body mass index in women with uncomplicated obesity. Heart (2011) 97(3):203–8. doi: 10.1136/hrt.2009.185009 [DOI] [PubMed] [Google Scholar]
- 19. Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, et al. Relationship between left ventricular structural and metabolic remodelling in type 2 diabetes mellitus. Diabetes (2016) 65(1):44. doi: 10.2337/db15-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, et al. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol (2016) 68(1):53–63. doi: 10.1016/j.jacc.2016.03.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voglino C, Tirone A, Ciuoli C, Benenati N, Paolini B, Croce F, et al. Cardiovascular benefits and lipid profile changes 5 years after bariatric surgery: A comparative study between sleeve gastrectomy and roux-en-Y gastric bypass. J Gastrointest Surg (2019) 24(12):2722–9. doi: 10.1007/s11605-019-04482-9 [DOI] [PubMed] [Google Scholar]
- 22. Rider OJ, Francis JM, Ali MK, Petersen SE, Robinson M, Robson MD, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol (2009) 54(8):718–26. doi: 10.1016/j.jacc.2009.02.086 [DOI] [PubMed] [Google Scholar]
- 23. Rayner JJ, Banerjee R, Francis JM, Shah R, Murthy VL, Byrne J, et al. Normalization of visceral fat and complete reversal of cardiovascular remodeling accompany gastric bypass, not banding. J Am Coll Cardiol (2015) 66(22):2569–70. doi: 10.1016/j.jacc.2015.09.071 [DOI] [PubMed] [Google Scholar]
- 24. Karason K, Wallentin I, Larsson B, Sjöström L. Effects of obesity and weight loss on cardiac function and valvular performance. Obes Res (1998) 6(6):422–9. doi: 10.1002/j.1550-8528.1998.tb00374.x [DOI] [PubMed] [Google Scholar]
- 25. Kaier TE, Morgan D, Grapsa J, Demir OM, Paschou SA, Sundar S, et al. Ventricular remodelling post-bariatric surgery: is the type of surgery relevant? a prospective study with 3D speckle tracking. Eur Hear J - Cardiovasc Imaging (2014) 15(11):1256–62. doi: 10.1093/ehjci/jeu116 [DOI] [PubMed] [Google Scholar]
- 26. Van Schinkel LD, Sleddering MA, Lips MA, Jonker JT, De Roos A, Lamb HJ, et al. Effects of bariatric surgery on pericardial ectopic fat depositions and cardiovascular function. Clin Endocrinol (Oxf) (2014) 81(5):689–95. doi: 10.1111/cen.12402 [DOI] [PubMed] [Google Scholar]
- 27. Schneiter SM, Warrier R, Lefkovits L, Laurie C, O’Brien PE, Taylor AJ. Effects of weight loss on pericardial fat and left ventricular mass assessed with cardiac magnetic resonance imaging in morbid obesity. Int J Clin Med (2011) 2011(04):360–6. doi: 10.4236/ijcm.2011.24062 [DOI] [Google Scholar]
- 28. Jhaveri RR, Pond KK, Hauser TH, Kissinger KV, Goepfert L, Schneider B, et al. Cardiac remodeling after substantial weight loss: a prospective cardiac magnetic resonance study after bariatric surgery. Surg Obes Relat Dis (2009) 5(6):648–52. doi: 10.1016/j.soard.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 29. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA (2004) 292(14):1724–37. doi: 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 30. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: Implications for pathophysiology and treatment. JACC Hear Fail (2019) 7(7):574–85. doi: 10.1016/j.jchf.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab (2011) 22(11):450. doi: 10.1016/j.tem.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dass S, Holloway CJ, Cochlin LE, Rider OJ, Mahmod M, Robson M, et al. No evidence of myocardial oxygen deprivation in nonischemic heart failure. Circ Hear Fail (2015) 8(6):1088–93. doi: 10.1161/CIRCHEARTFAILURE.114.002169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rider OJ, Ntusi N, Bull SC, Nethononda R, Ferreira V, Holloway CJ, et al. Improvements in ECG accuracy for diagnosis of left ventricular hypertrophy in obesity. Heart (2016) 102(19):1566–72. doi: 10.1136/heartjnl-2015-309201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorimachi H, Obokata M, Omote K, Reddy YNV, Takahashi N, Koepp KE, et al. Long-term changes in cardiac structure and function following bariatric surgery. J Am Coll Cardiol (2022) 80(16):1501–12. doi: 10.1016/j.jacc.2022.08.738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J (2021) 42(16):1595–605. doi: 10.1093/eurheartj/ehaa823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the framingham study). Am J Cardiol (1990) 65(5):371–6. doi: 10.1016/0002-9149(90)90304-J [DOI] [PubMed] [Google Scholar]
- 37. Shah RV, Murthy VL, Abbasi SA, Eng J, Wu C, Ouyang P, et al. Weight loss and progressive left ventricular remodelling: The multi-ethnic study of atherosclerosis (MESA). Eur J Prev Cardiol (2015) 22(11):1408. doi: 10.1177/2047487314541731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reddy YNV, Anantha-Narayanan M, Obokata M, Koepp KE, Erwin P, Carter RE, et al. Hemodynamic effects of weight loss in obesity: A systematic review and meta-analysis. JACC Heart Fail (2019) 7(8):678–87. doi: 10.1016/j.jchf.2019.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation (2017) 136(1):6–19. doi: 10.1161/circulationaha.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. Heart Fail (2020) 8(8):657–66. doi: 10.1016/j.jchf.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ikonomidis I, Mazarakis A, Papadopoulos C, Patsouras N, Kalfarentzos F, Lekakis J, et al. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: A 3-year follow-up study. J Hypertens (2007) 25(2):439–47. doi: 10.1097/HJH.0b013e3280115bfb [DOI] [PubMed] [Google Scholar]
- 42. Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart (2012) 98(24):1763–77. doi: 10.1136/heartjnl-2012-301778 [DOI] [PubMed] [Google Scholar]
- 43. Strzelczyk J, Kalinowski P, Zieniewicz K, Szmigielski C, Byra M, Styczyński G. The influence of surgical weight reduction on left atrial strain. Obes Surg (2021) 31(12):5243–50. doi: 10.1007/s11695-021-05710-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, et al. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol (2012) 60(15):1381–9. doi: 10.1016/j.jacc.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 45. Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J Am Coll Cardiol (2011) 57(6):732–9. doi: 10.1016/j.jacc.2010.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Garza CA, Pellikka PA, Somers VK, Sarr MG, Seward JB, Collazo-Clavell ML, et al. Major weight loss prevents long-term left atrial enlargement in patients with morbid and extreme obesity. Eur J Echocardiogr (2008) 9(5):587. doi: 10.1093/ejechocard/jen117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, et al. Longitudinal changes in left ventricular stiffness: A community-based study: Borlaug et al: Age-related ventricular stiffening in humans. Circ Heart Fail (2013) 6(5):944–52. doi: 10.1161/CIRCHEARTFAILURE.113.000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Litwin SE, Adams TD, Davidson LE, McKinlay R, Simper SC, Ranson L, et al. Longitudinal changes in cardiac structure and function in severe obesity: 11-year follow-up in the Utah obesity study. J Am Hear Assoc Cardiovasc Cerebrovasc Dis (2020) 9(12). doi: 10.1161/JAHA.119.014542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol (2010) 105(12):1831–5. doi: 10.1016/j.amjcard.2010.01.368 [DOI] [PubMed] [Google Scholar]
- 50. Gaborit B, Kober F, Jacquier A, Moro PJ, Cuisset T, Boullu S, et al. Assessment of epicardial fat volume and myocardial triglyceride content in severely obese subjects: relationship to metabolic profile, cardiac function and visceral fat. Int J Obes (Lond) (2012) 36(3):422–30. doi: 10.1038/ijo.2011.117 [DOI] [PubMed] [Google Scholar]
- 51. Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DTL. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther (2014) 4(6):416. doi: 10.3978/j.issn.2223-3652.2014.11.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.