Immediate drug hypersensitivity reactions are characterized by a broad spectrum of symptoms varying from urticaria, angioedema, pruritus, upper and lower respiratory symptoms, gastrointestinal symptoms, hypotension up to anaphylaxis. Mechanistically, these reactions oftentimes involve mast cell (MC) degranulation as reflected by elevated serum tryptase levels.1,2
Some drugs, e.g. beta-lactam antibiotics or monoclonal antibodies, can induce FcεRI/IgE-dependent activation of MCs via specific IgE against the culprit drug.1 However, MC degranulation might also occur independently from FcεRI/IgE cross-linking. Indeed, some drugs have long been known to degranulate MCs directly, but the mechanism remained enigmatic until the discovery of Mas-related G-protein coupled receptor X2 (MRGPRX2). MRGPRX2 is abundantly expressed on skin MCs and its activation results in an IgE-independent degranulation (previously called pseudo-allergic or anaphylactoid). A group of cationic peptidergic drugs, e.g. icatibant, cetrorelix, leuprolide, is able to cause MC degranulation via MRGPRX2 and is associated with injection-site reactions, i.e. a local edema accompanied by itch and erythema3 (Figure 1). In addition, neuromuscular blocking agents (NMBA, e.g. mivacurium, atracurium, cisatracurium and rocuronium), opiates and antibiotics such as fluoroquinolons, and vancomycin may elicit both IgE-mediated and non-IgE-mediated hypersensitivity reactions.1,2 The drug binding sites for MRGPRX2 include a tetrahydroisoquinoline (THIQ) motif and/or positive charges.3 Traditionally, NMBA hypersensitivity, including life-threatening anaphylaxis during general anesthesia, was thought to be a result of IgE-mediated MC degranulation.1,2 Recently, it has been proposed that in some patients such reactions could be explained by an IgE-independent pathway involving MRGPRX2. In several cases rocuronium induced skin reactions in the absence of detectable serum IgE or positive basophil activation test (BAT) responses.1,2 The role of MRGPRX2 in immediate drug hypersensitivity has been proven by knock down models, inhibitors and siRNA silencing experiments.4 Conversely, in a study of 140 patients, rocuronium-induced hypersensitivity was associated with IgE-mediated rather than MRGPRX2-mediated activation as confirmed by an extensive diagnostic work-up including measurements of serum tryptase levels, quantification of specific IgE antibodies to rocuronium, intradermal skin testing, and BAT.5
Figure 1.
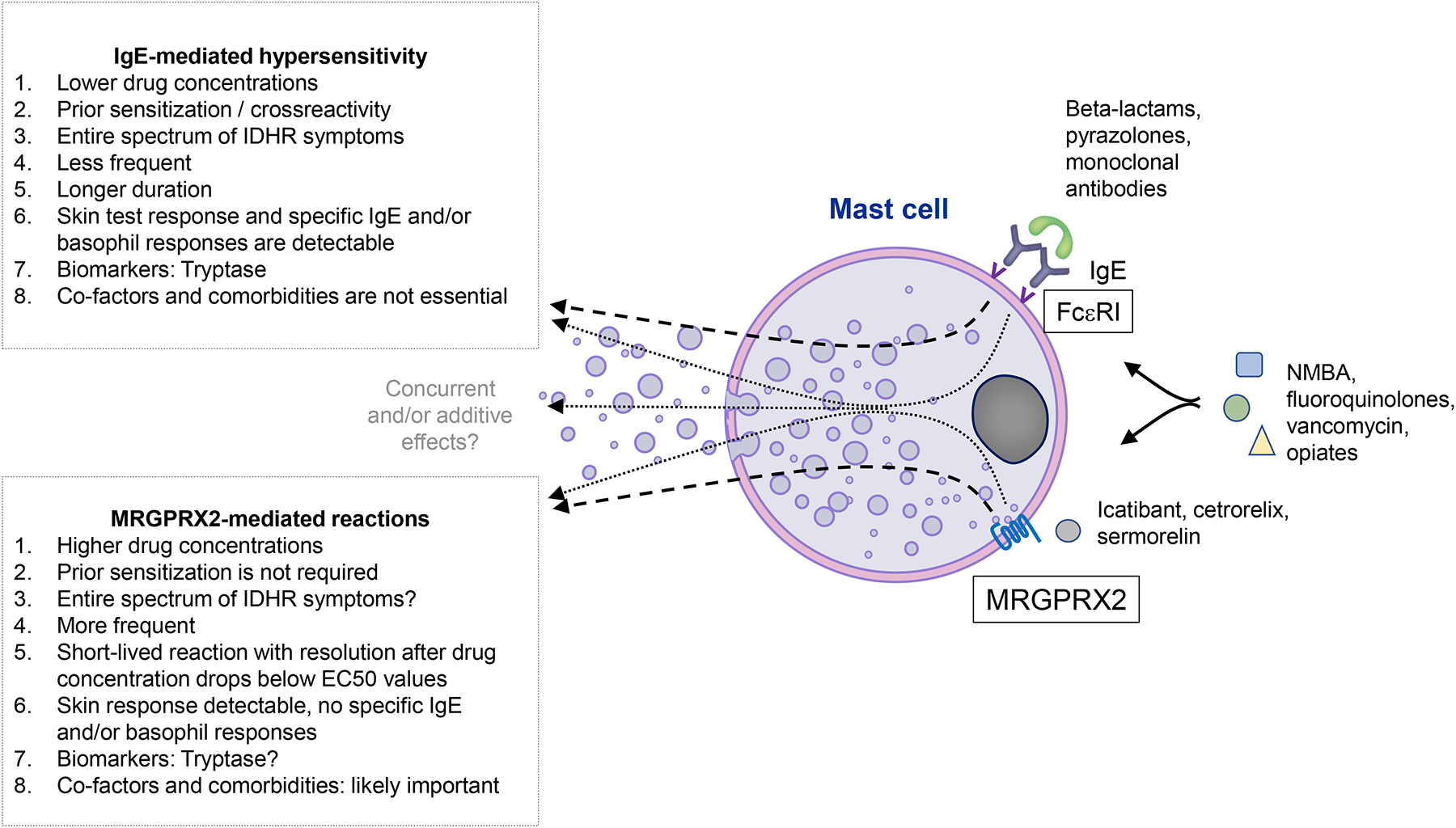
The similarities and differences of IgE-mediated and MRGPRX2-mediated drug reactions
The low-affinity nature of MRGPRX2, which might require high local concentration of the drug to trigger responses, is another issue in drug hypersensitivity reactions, making its real-life significance controversial.6 Also, MRGPRX2 is highly expressed on skin MCs, but found at low levels on lung and gut MCs.7 The dose of rocuronium used for skin tests likely activates skin MCs via MRGPRX2, while peak plasma concentrations of the drug (~15 μg/ml) are too low to activate the receptor expressed on the lung or gut MCs.5 McNeil recently associated EC50 values at MRGPRX2 with peak plasma drug concentrations achieved at a given injection speed to identify candidates potentially eliciting reactions via MRGPRX2.2 However, EC50 in plasma might differ from tissue EC50 and may also depend on MRGPRX2 isoforms and affinity for the ligand. These comparisons and results of other studies suggest that MRGPRX2 drives an entire spectrum of drug reactions from mild-to-moderate to severe.2,5,8 For example, presumably MRGPRX2-mediated reactions were noted in the 41% of patients with anaphylaxis, positive skin tests and negative specific IgE and BAT results.8
MRGPRX2-mediated anaphylaxis might develop in individuals with prolonged exposure to high plasma drug concentrations and/or harboring gain-of-function mutations in MRGPRX2 and/or mutations that would enhance the affinity of MRGPRX2 for a certain drug or induce a stronger intracellular response. Furthermore, MRGPRX2 is differentially expressed in MCs of different patients, and increased receptor expression on skin, lung and gut MCs and blood basophils may explain more pronounced drug reactions in certain patients and enhanced wheal reactions after the injection of MRGPRX2 agonists.2
Thus, whether anaphylaxis can be induced via the MRGPRX2 pathway of MC activation remains unclear. To investigate this, the differentiation between IgE and MRGPRX2-mediated reactions is needed. Here, MRGPRX2 tests, e.g. plasma drug concentration and MRGPRX2 gene polymorphisms (which may affect receptor function), and IgE tests, i.e. BAT and/or detection of allergen-specific IgE in serum, should be performed in tandem.2 Furthermore, the concentration of drugs to test IgE-mediated reactions, e.g. skin prick testing, should be standardized. The high expression of MRGPRX2 by cutaneous MCs should be taken into account when investigating the maximal non irritating drug concentration. Reliable biomarkers, e.g. mediators that are primarily or only released after the activation of one but not another receptor, would be also helpful in differential diagnosis.
There are factors that might predispose to MRGPRX2-mediated drug reactions, e.g. comorbidities.2 Numbers of MRGPRX2-positive cells and/or expression intensity are increased in patients with chronic urticaria and prurigo in comparison to control subjects.9 The combination of co-factors, e.g. temperature and NMBA administration during anesthesia, might have a cumulative effect for reaching the threshold for sufficient MC activation and degranulation.1
Finally, the use of MRGPRX2 antagonists and silencing will be of great help in dissecting the prevalence and relevance of both types of reactions and provide direct proof of MRGPRX2 involvement. There are several promising candidates in development, which can directly inhibit MRGPRX2 or inhibit MRGPRX2 downstream signaling. For example, a contribution of MRGPRX2-mediated MC activation in anaphylaxis due to NMBA can be investigated with the administration of an MRGPRX2 antagonist before surgical procedures.
Since IgE- and MRGPRX2-dependent routes act in an additive manner when stimulated concurrently, this also expands the spectrum of possible scenarios in which MRGPRX2 is involved.6 In this regard, reciprocal priming between both receptors should be also explored. In the same patients, some reactions might be mediated by drug-specific IgE and others by MRGPRX2.
In conclusion, we cannot exclude that MRGPRX2 reactions can be as severe as IgE-mediated reactions in some patients. However, compared to IgE-mediated drug allergy, MRGPRX2-mediated reactions can occur more frequently in drug naive patients, with resolution after decrease of drug concentration below EC50 values.2 Also, basophils and eosinophils might become more responsive to MRGPRX2-mediated activation in patients with some basophil/eosinophil-driven diseases where MRGPRX2 upregulation can be expected due to increased release of cytokines, e.g. IL-3 and IL-5.10 This might lead to decrease of threshold for MRGPRX2-mediated activation of these cells in circulation and more frequent systemic reactions with MRGPRX2-mediated drugs. Further studies should investigate the interaction between FcεRI and MRGPRX2 pathways of MC activation and delineate in detail the role of MRGPRX2 in immediate drug hypersensitivity reactions (current gaps and unmet needs are further discussed in Ref1).
COI Statements
Pavel Kolkhir received speakers fees, honoraria or travel support from Novartis and Roche.
Hydar Ali received seaker fees from Merck and Escient and received research funding from Numerate.
Magda Babina received consulting fees and honoraria from Health Advances and Escient
Marcus Maurer is or recently was a speaker and/or advisor for and/or has received research funding from Astria, Allakos, Alnylam, Amgen, Aralez, ArgenX, AstraZeneca, BioCryst, Blueprint, Celldex, Centogene, CSL Behring, Dyax, FAES, Genentech, GIInnovation, GSK, Innate Pharma, Kalvista, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Pfizer, Pharming, Pharvaris, Roche, Sanofi/Regeneron, Shire/Takeda, Third Harmonic Bio, UCB, and Uriach.
Didier Ebo, Vito Sabato, Jessy Elst, Stefan Frischbutter and Polina Pyatilova have no relevant conflict of interest in relation to this work
References
- 1.Porebski G, Kwiecien K, Pawica M & Kwitniewski M Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol 9, 3027., doi: 10.3389/fimmu.2018.03027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeil BD MRGPRX2 and Adverse Drug Reactions. Frontiers in Immunology 12, doi: 10.3389/fimmu.2021.676354 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeil BD et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241, doi: 10.1038/nature14022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elst J et al. Novel Insights on MRGPRX2-Mediated Hypersensitivity to Neuromuscular Blocking Agents And Fluoroquinolones. Front Immunol 12, 668962, doi: 10.3389/fimmu.2021.668962 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Gasse AL et al. Rocuronium Hypersensitivity: Does Off-Target Occupation of the MRGPRX2 Receptor Play a Role? J Allergy Clin Immunol Pract 7, 998–1003, doi: 10.1016/j.jaip.2018.09.034 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Babina M et al. FcεRI- and MRGPRX2-evoked acute degranulation responses are fully additive in human skin mast cells. Allergy, doi: 10.1111/all.15270 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Plum T et al. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 52, 404–416 e405, doi: 10.1016/j.immuni.2020.01.012 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Ebo DG et al. Immunoglobulin E cross-linking or MRGPRX2 activation: clinical insights from rocuronium hypersensitivity. Br. J. Anaesth 126, 27–29, doi: 10.1016/j.bja.2020.10.006 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Kolkhir P et al. Mast cells, cortistatin, and its receptor, MRGPRX2, are linked to the pathogenesis of chronic prurigo. J. Allergy Clin. Immunol S0091–6749, 00290–00291, doi: 10.1016/j.jaci.2022.02.021 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Wedi B, Gehring M & Kapp A The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy 75, 2229–2242, doi: 10.1111/all.14213 (2020). [DOI] [PubMed] [Google Scholar]


