Abstract
Clinical translation of mesenchymal stem/stromal cell (MSC) therapy has been impeded by the heterogenous nature and limited replicative potential of adult-derived MSCs. Human embryonic stem cell-derived MSCs (hESC-MSCs) that differentiate from immortal cell lines are phenotypically uniform and have shown promise in-vitro and in many disease models. Similarly, adipose tissue-derived MSCs (MSC(AT)) possess potent reparative properties. How these two cell types compare in efficacy, however, remains unknown. We randomly assigned mice to six groups (n=7-8 each) that underwent unilateral RAS or a sham procedure (3 groups each). Two weeks post-operation, each mouse was administered either vehicle, MSC(AT)s, or hESC-MSCs (5X105 cells) into the aorta. Mice were scanned with micro-MRI to determine renal hemodynamics two weeks later and kidneys then harvested. hESC-MSCs and MSC(AT)s were similarly effective at lowering systolic blood pressure. However, MSC(AT)s more robustly increased renal perfusion, oxygenation, and glomerular filtration rate in the post-stenotic kidney, and more effectively mitigated tubular injury, fibrosis, and vascular remodeling. These observations suggest that MSC(AT) are more effective than hESC-MSC in ameliorating kidney dysfunction and tissue injury distal to RAS. Our findings highlight the importance of tissue source in selection of MSCs for therapeutic purposes and underscore the utility of cell-based therapy for kidney disease.
Keywords: mesenchymal stem/stromal cells, renal artery stenosis, mouse
INTRODUCTION
Renal artery stenosis (RAS) is a prominent cause of secondary hypertension that may result in permanent kidney injury and functional decline. Patient management is centered around antihypertensive therapy, with revascularization used sparingly1. Although these treatments may control blood pressure (BP) and improve long-term outcomes, they often fail to prevent progressive renal dysfunction or tissue injury, especially in severe cases2–5. The search for alternative therapies that improve renal hemodynamics while preserving renal architecture has led to development of new treatment paradigms, including mesenchymal stem/stromal cells (MSCs) and their products.
MSCs are plastic-adherent cells that express characteristic markers and can differentiate into osteocytes, adipocytes, and chondrocytes in-vitro6. When administered in-vivo, these immuno-privileged cells exert their therapeutic effects largely though their secretome7. They can be isolated from bone marrow (MSC(M)), adipose tissue (MSC(AT)), umbilical cord blood (MSC(CB)), dental pulp, skin, liver, and other tissues8–12. Furthermore, MSCs can be derived from human embryonic stem cells (hESCs) or produced from somatic cells that had been induced to become pluripotent (iPSCs). In particular, MSC(AT) are readily accessible and endowed with myriad reparative properties, make them an attractive choice.
Despite potential benefits, clinical translation of MSC-based therapy has met noteworthy challenges, one of which is cellular heterogeneity. The properties of MSC(AT)s vary with extraction method13 and site14, as well as with donor age15, body mass index, and disease state. MSC(AT)s from obese donors have impaired differentiation and angiogenic potential16, calling into question the utility of autologous cells. In models of RAS, allogenic MSC(AT)s17 or their daughter extracellular vesicles (EVs)18 isolated from obese subjects failed to decrease inflammation or improve glomerular filtration rate (GFR), in contrast to MSC(AT)s or EVs from lean subjects. Moreover, scalability is a challenge because MSCs can only be passaged a limited number of times before cellular senescence degrades their replicative potential and therapeutic properties19,20.
Many such technical limitations may be circumvented using pluripotent stem cells such as hESCs, which are immortal and can differentiate into MSCs (hESC-MSCs), thus obviating the need to continuously identify new donors. Derivation of MSCs from a single hESC cell line may also potentially provide uniform and reproducible cell populations with a predictable phenotype. Furthermore, their immortality and clonality makes some hESC cell lines21 practical candidates for genetic modification to enhance the therapeutic efficacy or immuno-evasive properties of their daughter hESC-MSCs.
Several reliable protocols to induce differentiation of hESCs into MSCs have been developed. Interestingly, hESC-MSCs retain certain features of their parent pluripotent cells, including longer telomeres and faster doubling times than adult tissue-derived MSCs22. Moreover, their anti-inflammatory properties in-vitro and therapeutic properties in-vivo compare favorably to some adult MSCs22,23. However, how they functionally compare to MSC(AT)s in ischemic kidney repair remains unknown.
Therefore, we tested the hypothesis that hESC-MSCs would be at least as efficacious as healthy human MSC(AT)s in the treatment of injured kidneys in a well-established murine model of RAS.
METHODS
All animal protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee. Ten-week-old male 129-S1 mice (Jackson Lab, Bar Harbor, ME) were studied for 4 weeks following induction of RAS or sham surgery. They were randomly divided into sham+vehicle, sham+MSC(AT), sham+hESC-MSC, RAS+vehicle, RAS+MSC(AT), or RAS+hESC-MSC groups, consisting of 7-8 mice each.
An arterial cuff (0.15mm) was placed on the right renal artery to induce RAS, while control groups underwent a sham procedure without cuff placement24. After 2 weeks of RAS or sham, the carotid artery was cannulated via a vascular cut down, and 200μl of phosphate buffered saline (PBS), hESC-MSC, or human MSC(AT) (all cells 5×105 in 200μL PBS) were injected caudally into the aorta.
Two weeks after MSC delivery, the mice were scanned with 16.4T magnetic resonance imaging (MRI) to assess oxygenation and hemodynamics in the sham or stenotic kidneys (STK), and tail vein blood drawn for measurement of plasma creatinine (PCr) and plasma renin content. Mice were subsequently euthanized using carbon dioxide, and the kidney, heart, spleen, lung, and liver were collected.
Isolation and Expansion of Human MSC(AT)
Following Mayo Clinic Institutional Review Board approval, subcutaneous fats (~2g) were collected during kidney donation from healthy donors (n=7) with BMI<30kg/m2 who had given written informed consents. Exclusion criteria included pregnancy, chronic diseases (e.g. rheumatoid arthritis, active malignancy, cardiovascular diseases, solid organ transplant recipients, treatment with immunosuppression, or anticoagulation therapy). The tissue samples were aseptically homogenized, enzymatically digested using collagenase-H, and passed through a 100μm nylon filter. Plastic adherent cells were cultured in Advanced MEM (Gibco Cat. #12492021) supplemented with human platelet lysate (PLTMax; Mill Creek Life Sciences) at 37°C and 5% CO2, with the medium changed every other day. Upon reaching 70% confluency, cells were washed with PBS and passaged using TrypLE Express (ThermoFisher Scientific, Cat. #12604013). Cells underwent 3-4 passages before collection, characterization, and use.
Maintenance and Differentiation of hESC to MSC
The hESC-derived MSCs were provided by Cell Therapy R&D, Novo Nordisk A/S. The hESC line 121 (SA121 hESC) (Takara Bio, Cat. #Y00025)25 was cultured using a DEF-CS 500 Culture System (Takara Bio, Cat. #Y30010) at 37°C and 5% CO2. Tissue culture plastic was covered with DEF-CS 500 basal medium according to the manufacturer’s instructions. Medium was changed daily for 3-4 days, after which cells were passaged as a single cell suspension using TrypLE Express (ThermoFisher Scientific, #12605010). An inhibitor of Rho-associated protein kinase (ROCK) (5μM, Sigma-Aldrich, #Y0503) was added for the first 24h after passaging, and the cells frequently assessed for pluripotency markers.
SA121-hESC were then seeded into plastic flasks at 30,000 cells/cm2 and cultured for 48h, after which differentiation was initiated with a STEMdiff Mesenchymal Progenitor Kit (STEMCELL Technologies, #05240) by incubating the cells in STEMdiff-ACF mesenchymal induction medium for 4 days at 37°C and 5% CO2, with the medium changed every 24 hours. On the fifth day, cells were switched to MesenCult-ACF Plus Medium (STEMCELL Technologies, Cat. #05240), cultured for two days, and passaged at 10,000 cells/cm2 onto tissue culture plastic coated with Animal Component-Free Cell Attachment Substrate (STEMCELL Technologies, #05240), with 5μM ROCK inhibitor included for the first 24 hours. The medium was replenished daily, and cells passaged every 3-7 days for a total of five passages. Finally, hESC-MSCs were collected and cryopreserved in MesenCult-ACF freezing medium (STEMCELL Technologies, #05490) pending characterization.
Surface Marker Analysis and Trilineage Assay
All MSCs were characterized by imaging flow cytometry (FlowSight, Amnis, Seattle, WA, USA) to confirm expression of CD73 (Abcam, #ab106677), CD90 (Abcam, #ab124527), and CD105 (Abcam, #ab53321), and to rule out expression of CD45 (Abcam, #ab51482) and CD34 (BD Biosciences, Cat. #340441). Gating was performed in a manner previously described26 and data analyzed using Amnis® Image Data Exploration Analysis Software (IDEAS version 6.2).
A MSC functional identification kit (R&D Systems, #SC006) was used to verify trilineage differentiation capacity of hESC-MSCs and MSC(AT)s in accordance with the manufacturer’s instructions. Images (20x or 40x, at least 6 per marker) were quantified as the ratio of positive cells/total cells per field.
MSC Tracking
CellTrace™ Far Red Cell Proliferation (Invitrogen, Cat. #C34564) was used to label all MSCs before delivery as per manufacturer’s instructions. Frozen kidney sections (8μm) were subsequently also stained for CD3 (Abcam, #ab16669) to identify co-localization of T-lymphocytes and MSCs and thereby possible immune rejection. To assess their retention in the kidney, MSCs were counted at 20X magnification in 20-30 fields per group and averaged.
Systemic Measurements
The mice were weighed, and heart rate and BP were measured using a tail-cuff (Kent Scientific, Torrington, CT) at baseline, 2 weeks, and 4 weeks.
In-vivo Renal Perfusion and Oxygenation
Renal hemodynamics were measured using a vertical 16.4T MRI scanner (Bruker, Billerica, MA) as previously described24. Briefly, renal perfusion was measured using the arterial spin labeling technique employing the flow-sensitive alternating inversion-recovery sequence with rapid acquisition with relaxation enhancement. Renal oxygenation was measured using blood oxygen level-dependent (BOLD)-MRI. T2* was quantified by pixelwise mono-exponential fitting of the acquired MRI signal intensity over echo times, and R2* (1/T2*) used as an index of tissue hypoxia.
Histology
Tubulo-interstitial fibrosis was measured using mid-hilar renal cross-sections cut from paraffin-embedded tissue stained with Masson’s trichrome. Fibrosis was quantified semiautomatically in 10 fields (20x) using ZEN® 2012 (blue edition, Carl ZEISS), expressed as percent of cross-sectional surface area, and the results from all the fields were averaged. Glomerular score (number of ischemic glomeruli per 100) was also calculated. Sections stained with hematoxylin and eosin (H&E, 20x) were used to score tubular injury (atrophy, dilation, cell detachment, cast formation, etc.) on a scale of 0 to 5. An absence of injured tubules was scored 0, <10% of tubules injured = 1, 10-25% = 2, 26-50% = 3, 51-75% = 4, and >75% = 5. Peritubular capillary (PTC) density (in 40x images, the average number of capillaries associated with a tubule) was assessed using immunohistochemical staining for CD31 (Abcam, #ab77699) and quantified using ZEN®. Media-to-lumen ratio (M/L) was assessed in microvessels stained for α-smooth muscle actin (40x images) and quantified using ZEN®.
Statistical Analysis
Statistical analysis was performed using JMP software and GraphPad Prism-9 was used to generate graphs. Violin plots were used where feasible to reflect data distribution, and bar graphs or box plots where violin plots would have reduced legibility.
Kruskal-Wallis-H tests followed by Wilcoxon Rank Sum tests were used in most cases. When criteria for the use of parametric tests were met (e.g., blood pressure), one-way analysis of variance or unpaired t-tests were employed. A value of p≤.05 was considered significant.
RESULTS
MSC Characterization
Both MSC(AT) and hESC-MSC expressed CD90, CD105, and CD73 and were negative for CD45 and CD34 (Fig. 1A,B). Successful in-vitro differentiation of both cell types into adipocytes, osteocytes, and chondrocytes confirmed their multi-potency and identity as MSCs (Fig. 1C). Interestingly, hESC-MSC showed higher differentiation ratio into adipocytes and osteocytes than MSC(AT), possibly due to their embryonic source (Fig. 1D).
Figure 1.
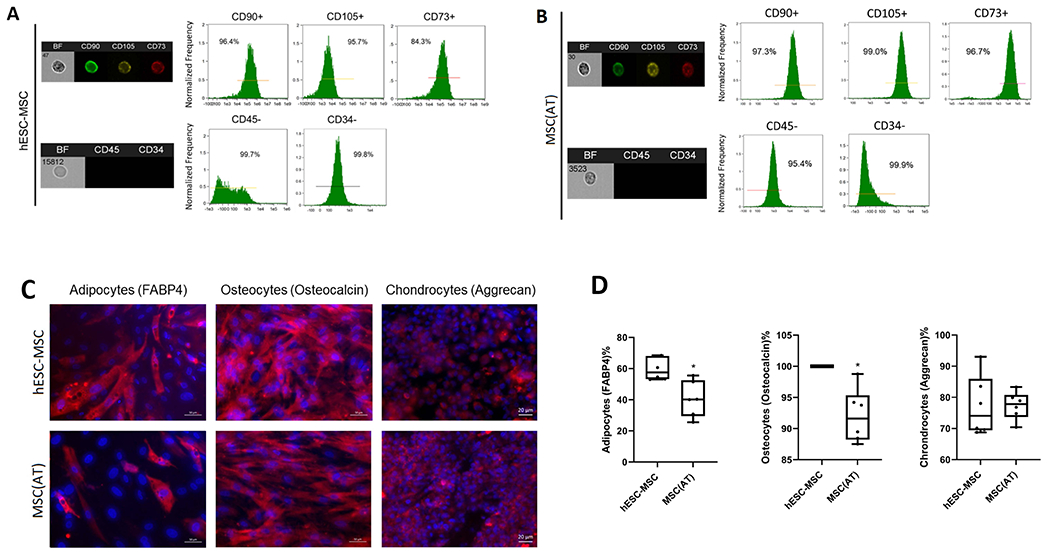
(A,B). Both hESC-MSCs and MSC(AT)s expressed the typical MSC markers CD90, CD105, and CD73, and were negative for CD45 and CD34. (C) Successful in-vitro differentiation into adipocytes (left), osteocytes (middle), and chondrocytes (right) confirmed the multi-potency of both cell types. (D) , hESC-MSC showed higher differentiation into adipocytes and osteocytes than MSC(AT).
MSCs Reduce Systolic BP (SBP)
Compared to sham, all mice that underwent RAS induction showed an overall increase in SBP of 22.5±7.5 mmHg (p≤.001). However, compared to baseline, only RAS+vehicle showed a significantly greater rise in SBP than sham+vehicle (p=.003), which was also greater than the change in SBP of RAS+MSC(AT) and RAS+hESC-MSC (p=.007 for both), suggesting that both types of MSCs attenuated the increase in SBP caused by RAS (Fig. 2A). Some data are missing from some mice due to technical reasons or motality.
Figure 2.
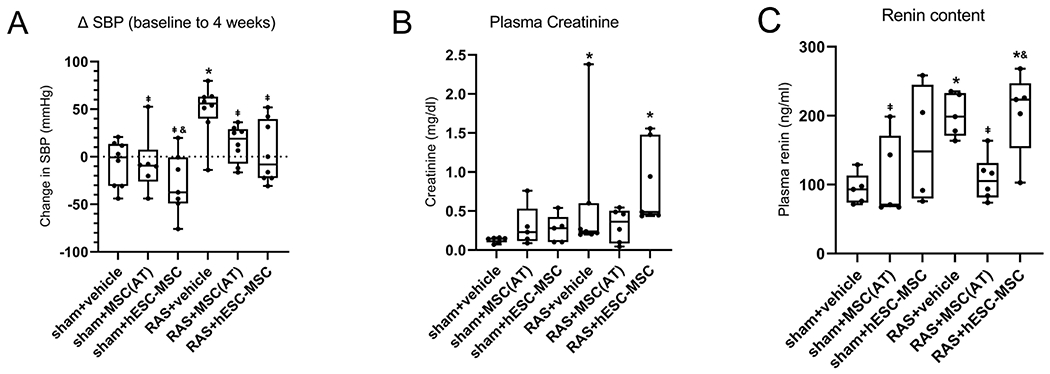
(A) The increase in systolic blood pressure (BP) in RAS+vehicle was greater compared to sham+vehicle, whereas changes in RAS+MSC(AT) and RAS+hESC-MSC were indistinguishable from sham+vehicle. (B) Plasma creatinine levels were similarly elevated compared to sham+vehicle in RAS+hESC-MSC and RAS+vehicle, but not in RAS+MSC(AT). (C) Plasma renin content remained elevated in RAS+hESC-MSC but returned to sham levels in RAS+MSC(AT). *p≤0.05 vs. sham+vehicle, ⱡp≤0.05 vs. RAS+vehicle, &p≤0.05 vs. RAS+MSC(AT). sham+vehicle n=5-8, sham+MSC(AT) n=5-6, sham+hESC-MSC n=4-7, RAS+vehicle n=5-8, RAS+MSC(AT) n=6-8, RAS+hESC-MSC n=5-8.
Plasma Creatinine and Renin Content Remain Elevated in RAS+hESC-MSC Mice
The RAS+vehicle group had significantly higher plasma creatinine (PCr) than sham+vehicle (p=.001). PCr was comparable to sham+vehicle in RAS+MSC(AT), but remained elevated in RAS+hESC-MSC (p=.001). Among sham groups, neither treatment affected PCr compared to vehicle (Fig. 2B).
Plasma renin content was elevated in RAS+vehicle compared to sham+vehicle (p=.008), but normalized in RAS+MSC(AT). Contrarily, in RAS+hESC-MSC it remained higher than sham+vehicle (p=.02) and similar to RAS+vehicle (Fig. 2C).
MSC(AT)s Restore Cortical Perfusion in the Stenotic Kidney
At 4 weeks (Fig. 3A, upper) STKs showed lower cortical and medullary perfusion in RAS+vehicle compared to sham+vehicle (p=.004 and p≤.001, respectively) (Fig. 3B,C), confirming hemodynamically significant RAS.
Figure 3.
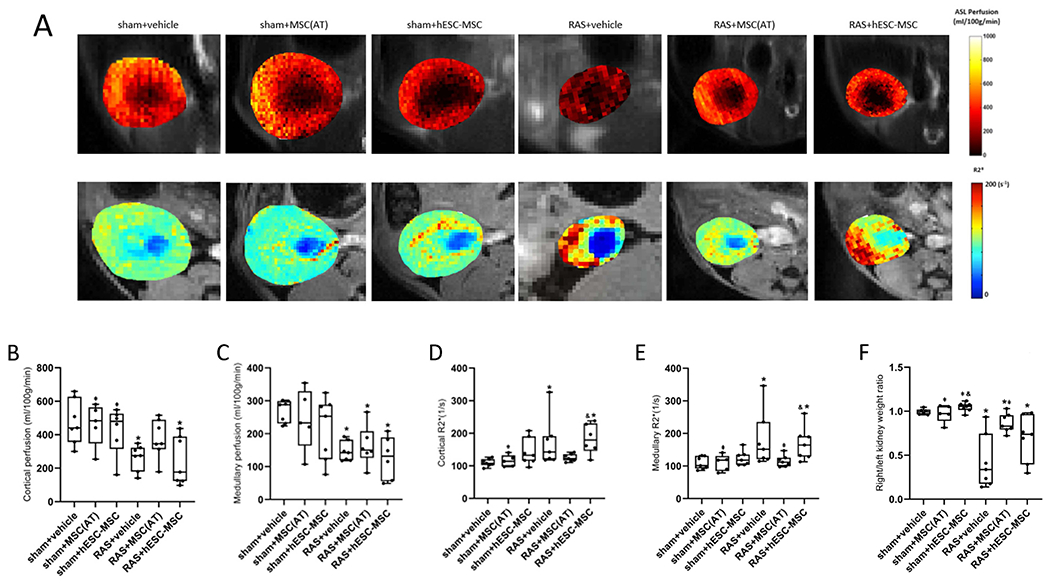
In vivo micro-magnetic resonance imaging at 4 weeks. (A) Representative renal perfusion and hypoxia maps obtained using arterial spin labeling and blood oxygenation level dependent magnetic resonance imaging, respectively. Increasing yellow color indicates higher perfusion, and increasing red means greater hypoxia. (B) Cortical perfusion in untreated post-stenotic kidneys (STK) was reduced compared to sham+vehicle. It normalized after administration of MSC(AT)s, but remained significantly lower than sham+vehicle after hESC-MSCs delivery. (C) Medullary perfusion was significantly below sham+vehicle in all RAS groups and remained unaltered after delivery of hESC-MSC and MSC(AT). (D,E) RAS+vehicle manifested cortical and medullary hypoxia. MSC(AT)-treated mice tended to show lower cortical and medullary hypoxia (both p=.01) than hESC-MSC treated mice and were comparable to sham+vehicle. Fluid-filled collection system structures (blue) were excluded from analysis. (F) Ratio of STK/contralateral kidney weight ex-vivo was reduced in RAS+vehicle and improved in RAS+MSC(AT). *p≤0.05 vs. sham+vehicle, ⱡp≤0.05 vs. RAS+vehicle, &p≤0.05 vs. RAS+MSC(AT). sham+vehicle n=7, sham+MSC(AT) n=5, sham+hESC-MSC n=7, RAS+vehicle n=7, RAS+MSC(AT) n=6-7, RAS+hESC-MSC n=6-7.
Cortical perfusion remained unchanged in RAS+hESC-MSCs and lower than sham+vehicle (p=.03). In RAS+MSC(AT), STK perfusion tended to be higher compared to RAS+hESC-MSC and RAS+vehicle (p=.13 and p=.07 respectively), and was similar to sham+vehicle. No significant differences were found among the sham groups (Fig. 3B).
Medullary perfusion in RAS was unchanged by either treatment (Fig. 3C).
MSC(AT)s were Superior to hESC-MSC at Reducing Renal Hypoxia
RAS+vehicle mice showed an increase in both cortical and medullary R2* (hypoxia) (Fig. 3A, lower) compared to sham+vehicle (p=.01 and p=.004, respectively). RAS+MSC(AT) displayed significantly lower cortical (p=.01) and medullary (p=.01) hypoxia than RAS+hESC-MSC and were similar to sham+vehicle, whereas RAS+hESC-MSC showed significantly more hypoxic cortex (p=.005) and medulla (p=.01) than sham+vehicle and were indistinguishable from RAS+vehicle. There were no differences in cortical or medullary hypoxia among the sham groups (Fig. 3D,E).
STK Atrophy Improved by MSC(AT)s
Ex-vivo STK-to-contralateral kidney (CLK) weight ratio was significantly lower in RAS+vehicle compared to sham+vehicle (p=.001), indicating renal atrophy. It increased in RAS+MSC(AT) compared to RAS+vehicle (p=.005) but remained significantly lower than sham+vehicle, whereas RAS+hESC-MSC remained indistinguishable from RAS+vehicle (Fig. 3F).
hESC-MSC Demonstrate High Retention in Stenotic Kidneys
The average number of MSC per field (20X magnification) revealed greater MSC retention in RAS+hESC-MSC versus RAS+MSC(AT) (p=.008), sham+MSC(AT) (p=.009), and sham+hESC-MSC (p=.02). Contrarily, hESC-MSC retention in sham+hESC-MSC, was indistinguishable from sham+MSC(AT) and RAS+MSC(AT) (Fig. 4A,B).
Figure 4.
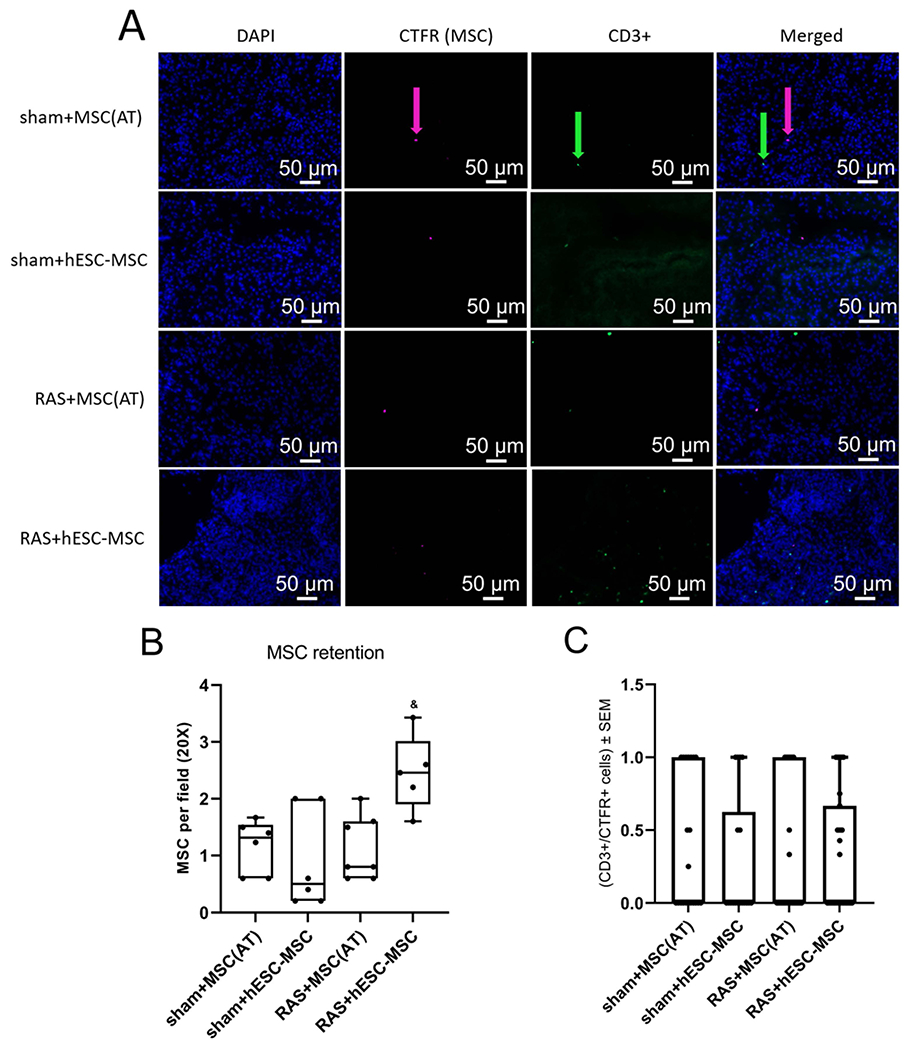
(A,B) RAS mice administered MSC(AT) retained a similar number of cells as sham mice, whereas RAS+hESC-MSC retained significantly more cells than any other group. (A,C) Co-localization of MSCs (pink arrows) and CD3+ cells (green arrows) was uncommon, with no significant differences among groups. &p≤0.05 vs. RAS+MSC(AT). sham+MSC(AT) n=5-6, sham+hESC-MSC n=6, RAS+MSC(AT) n=6-7, RAS+hESC-MSC n=5-6.
CD3-stained slides rarely showed CD3/CTFR co-localization (that might have been consistent with MSC rejection), with no significant differences among groups (Fig. 4A,C).
MSC(AT) Ameliorate Kidney Tissue Injury More Effectively Than hESC-MSC
Tubular injury score in RAS+vehicle was elevated compared to all other groups, including RAS+MSC(AT) (p=.002) and RAS-hESC-MSC (p=.03). MSC(AT)s tended to be more effective than hESC-MSCs at reducing tubular injury in RAS (p=.06 vs. RAS+hESC-MSC), although neither cell type normalized it. Sham+MSC(AT) and sham+hESC-MSC also showed some tubular injury (p=.04 and p=.002 versus sham+vehicle, respectively), which was greater in sham+hESC-MSC than sham+MSC(AT) (p=.02) (Fig. 5B).
Figure 5.
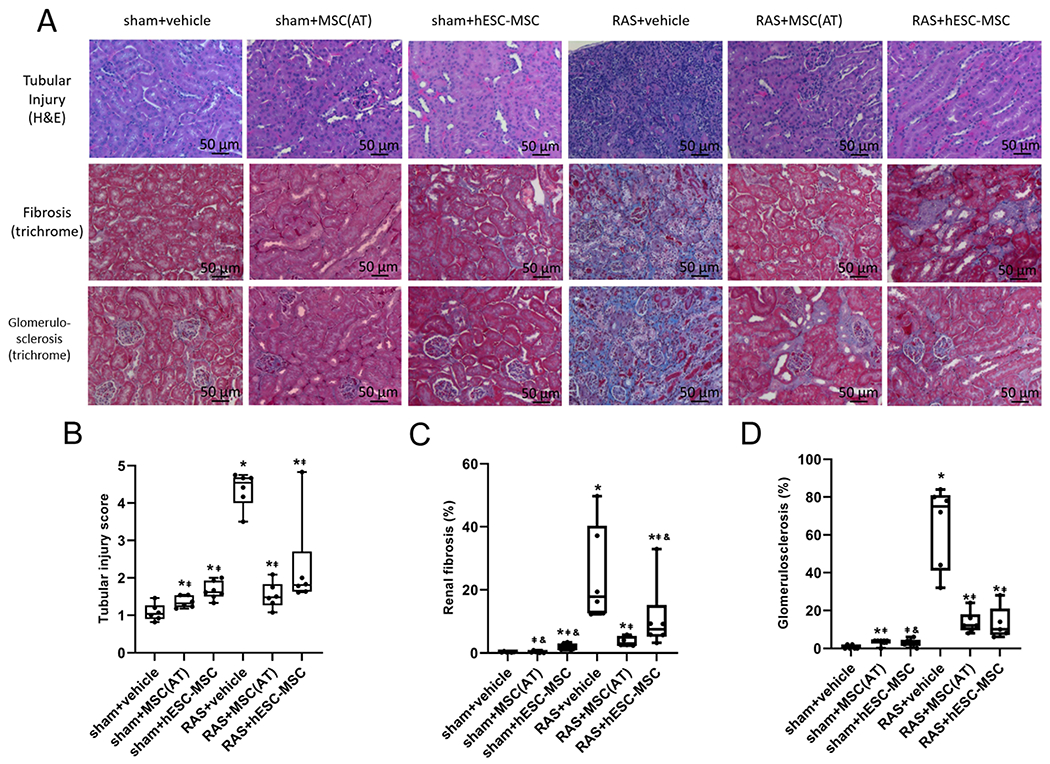
(A) Representative H&E and trichrome stained kidney sections. (B) MSC(AT)s were more effective than hESC-MSCs at reducing tubular injury in RAS mice kidneys, yet both cell types caused small but significant tubular injury in sham kidneys. (C) MSC(AT)s were more effective at reducing fibrosis in RAS than hESC-MSCs, although neither lowered it to sham+vehicle levels. In sham mice, hESC-MSCs provoked to greater fibrosis than vehicle (D) Both cell types dramatically lowered glomerulosclerosis in RAS kidneys but slightly increased it in sham. *p≤0.05 vs. sham+vehicle, ⱡp≤0.05 vs. RAS+vehicle, &p≤0.05 vs. RAS+MSC(AT). sham+vehicle n=6, sham+MSC(AT) n=6-7, sham+hESC-MSC n=6-7, RAS+vehicle n=6, RAS+MSC(AT) n=6-7, RAS+hESC-MSC n=5-6.
Renal fibrosis observed in RAS+vehicle was blunted but not normalized in RAS+MSC(AT) and RAS+hESC-MSC (p=.001 and p=.02 respectively), with RAS+MSC(AT) reducing fibrosis further than RAS+hESC-MSC (p=.01). Among the sham groups only sham+hESC-MSC developed significant fibrosis relative to sham+vehicle (p=.001) and sham+MSC(AT) (p≤.001) (Fig. 5C).
Glomerulosclerosis in RAS+vehicle was also prominent and higher than RAS+MSC(AT) and RAS+hESC-MSC (both p≤.001), although all RAS groups were higher than sham controls. Injecting sham mice with hESC-MSCs or MSC(AT)s tended to induce slight glomerulosclerosis compared to vehicle (p=.07 and p=.02, respectively) (Fig. 5D).
Peritubular capillary density was markedly decreased in RAS+vehicle and was significantly and similarly improved by both hESC-MSCs and MSC(AT)s (both p≤.001), yet remained lower than sham+vehicle. Among sham groups, peritubular capillary density was unchanged by either treatment (Fig. 6B).
Figure 6.
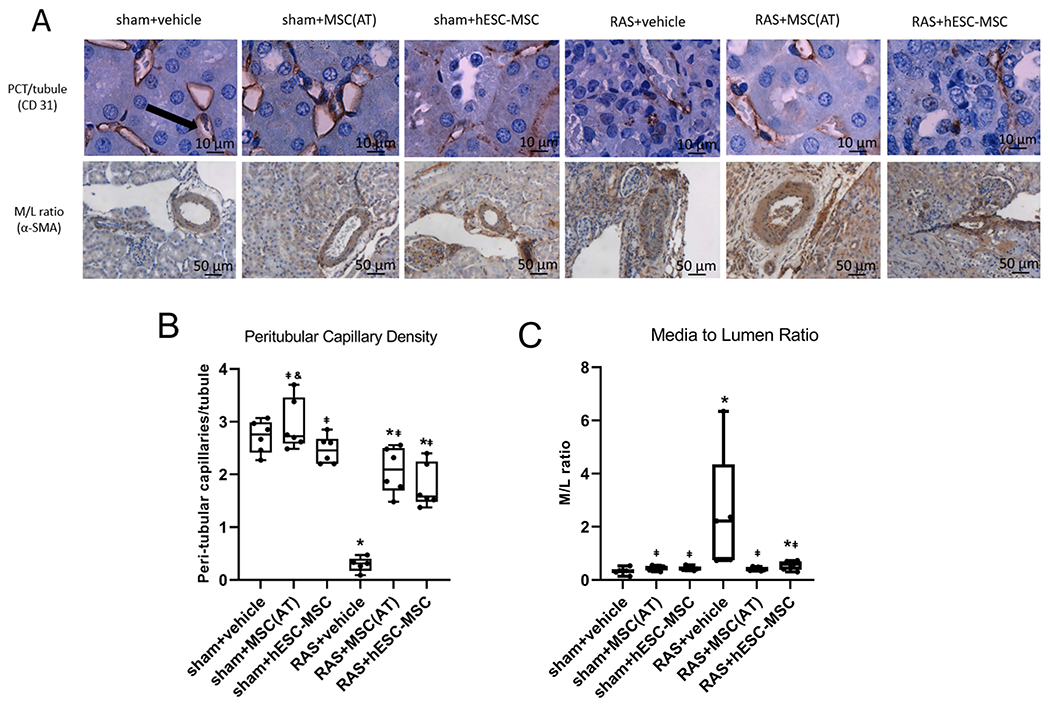
(A) Representative immunohistochemical staining of kidney sections for CD31 and α-SMA. (B) Both hESC-MSCs and MSC(AT)s improved peritubular capillary density in RAS mice. (C) MSC(AT)s and hESC-MSCs both reduced media-to-lumen ratio significantly in RAS, with MSC(AT)s restoring it to sham+vehicle levels. *p≤0.05 vs. sham+vehicle, ⱡp≤0.05 vs. RAS+vehicle, &p≤0.05 vs. RAS+MSC(AT). sham+vehicle n=6, sham+MSC(AT) n=6, sham+hESC-MSC n=6, RAS+vehicle n=5, RAS+MSC(AT) n=6, RAS+hESC-MSC n=6.
M/L ratio significantly increased in RAS+vehicle compared to sham+vehicle (p˂.01) and was reduced in both RAS+MSC(AT) and RAS+hESC-MSC (p˂.01 and p<.05 versus RAS+vehicle, respectively), whereas RAS+hESC-MSC remained elevated compared to sham+vehicle. No difference in M/L ratio was found among the sham groups (Fig. 6C).
DISCUSSION
This study shows that MSC(AT) are more effective than hESC-MSC in improving renal hemodynamics and function and blunting tissue injury in stenotic mouse kidneys. On the other hand, microvascular loss and remodeling are similarly improved by both therapies, consistent with uniform vasculo-protective effects of MSCs regardless of source. These findings underscore the need to match the type of MSCs used in cell-based therapy to the underlying disease etiology and ultimate goal of treatment.
We have previously shown that allogenic MSC(AT)s mitigate kidney injury distal to RAS and ameliorate microvascular remodeling in pig and mouse kidneys27,28. MSC phenotype and simplicity of extraction vary with the tissue of origin29. Of non-embryonic sources, adipose tissue is richer in MSCs than either bone marrow or cord blood and yields high rates of successful MSC isolation and colony formation. MSC(AT)s are less likely than MSC(CB)s to undergo early passage senescence30 and by some accounts may have greater proliferative, angiogenic, and immunomodulatory potential than MSC(M)s31–33. These properties, coupled with the minimally invasive accessibility to MSC(AT) harvesting, make adipose tissue an attractive MSC source.
Studies testing the therapeutic potential of MSC(AT)s delivered to post-stenotic kidneys have demonstrated their ability to improve GFR while reducing BP, fibrosis, microvascular remodeling, inflammation, apoptosis, and senescence in experimental RAS27,34–36. In a phase 1/2A trial, MSC(AT) infusion also increased cortical blood flow (possibly due to increased angiogenesis), reduced hypoxia, and stabilized GFR in patients with RAS, with no significant adverse effects or toxicity37.
The utility of hESC-MSC therapy in RAS, however, remained unknown. The impetus for this study was the versatility and scalability that characterizes pluripotent stem cell-based therapy, such as iPSCs and hESCs. The use of hESC-MSCs may eliminate the need for continuous sourcing from donors, produce phenotypically consistent cells, and is well suited to genetic modification. Furthermore, transcriptomic and proteomic studies have revealed hESC-MSCs to be broadly similar to adult-derived MSCs38, and in-vivo studies demonstrated their therapeutic efficacy in a range of experimental disease models, including some with pathophysiological similarities to renovascular disease. In murine models of colitis and arthritis, hESC-MSCs reduced neutrophilic infiltration and increased regulatory T-cell numbers, respectively39,40. In a mouse model of autoimmune encephalitis, hESC-MSCs reduced brain inflammation better than MSC(M)s41, and in murine lupus nephritis reduced PCr and proteinuria while improving survival and renal histopathology42. In rats with thioacetamide-induced liver cirrhosis, hESC-MSCs and their EVs reduced fibrosis, which is also the terminal histological manifestation of renovascular hypertension43,44. In a mouse model of monocrotaline-induced pulmonary arterial hypertension, hESC-MSCs had a superior capacity to decrease capillary and right ventricular remodeling compared to MSC(M)s45.
We compared the effects of MSC(AT)s and hESC-MSCs on measures of renal hemodynamics and function, including perfusion, oxygenation, BP, and PCr. RAS induces ischemia that persistently increases oxidative stress, hypoxia, and angiotensin-II and activates an inflammatory cascade that results in hypertension, tissue fibrosis, and loss of renal microcirculation. Rat46,47 and human17 MSC delivery has been previously shown to mitigate many of these processes in rodents with RAS .
Indeed, this study demonstrates the efficacy of both human MSC(AT)s as well as hESC-MSCs at blunting renovascular hypertension. However, unlike RAS+MSC(AT), renal cortical perfusion and oxygenation in RAS+hESC-MSC remained indistinguishable from RAS+vehicle, and renin levels were higher compared to RAS+MSC(AT). Interestingly, RAS+MSC(AT) showed cortical perfusion similar to sham+vehicle but medullary perfusion comparable to RAS+vehicle. These findings are underscored by previous studies in human patients37 where MSC(AT)s have been shown to increase cortical perfusion but exert more modest effects on medullary perfusion. Yet, in RAS+hESC-MSC both cortical and medullary perfusion were lower than sham+vehicle and comparable to RAS+vehicle. This pattern was even more pronounced in BOLD imaging where, unlike RAS+MSC(AT), RAS+hESC-MSC displayed greater cortical as well as medullary hypoxia compared to sham+vehicle.
Histological studies supported our in-vivo findings. Fibrotic tissue replaced significantly more of the STK parenchyma in RAS+hESC-MSC compared to RAS+MSC(AT). Vascular remodeling that developed in STKs was also observed in RAS+hESC-MSC, which unlike RAS+MSC(AT), showed a significantly higher M/L ratio than sham+vehicle. These findings, coupled with higher tubular injury scores, are likely also responsible for the higher PCr in RAS+hESC-MSC, which remained comparable to RAS+vehicle and higher than sham+vehicle. In contrast, PCr in RAS+MSC(AT) was similar to sham+vehicle.
Peritubular capillary density, however, was similarly improved in both groups relative to RAS+vehicle, suggesting comparable angiogenic potential. Pertinently, MSC(AT)s release more angiogenic growth factors (endostatin, aFGF, thrombospondin-2, angiogenin and PLGF) than MSC(M)33. Analysis of hESC-MSC supernatant has also revealed significantly greater angiogenic potential compared to MSC(M) supernatant, a benefit maintained in-vivo45. Hence, both cell types possess notable angiogenic potency.
Trichrome staining showed that both RAS+hESC-MSC and RAS+MSC(AT) similarly reduced glomerulosclerosis compared to RAS+vehicle, whereas RAS+hESC-MSC displayed significantly more fibrosis than RAS+MSC(AT). This might be a consequence of the failure of hESC-MSCs to improve STK perfusion and oxygenation. Nevertheless, the anti-fibrotic effects of both cell types might be better realized at a different dose. In a diabetic mouse model the effects of hESC-MSCs on detrusor muscle underactivity were dose-dependent, and although their effectiveness at improving bladder voiding functions was not different between 2.5×105 and 5.0×105 cells, their anti-fibrotic effects were greatest at the higher dose48. Similarly, we found dose-dependent effects of MSC(AT) on STK perfusion and function in patients with RAS49.
Although MSC(AT) were more efficacious, RAS+hESC-MSC in fact retained a greater number of cells than RAS+MSC(AT). Incidentally, this difference between hESC-MSC and MSC(AT) retention was not observed in sham kidneys, suggesting that hESC-MSCs might respond more vigorously than MSC(AT)s to chemo-attractants produced by the STK. This finding is consistent with the superior migratory capacity into inflamed CNS tissue of hESC-MSC compared to MSC(M) in experimental autoimmune encephalitis41. Nevertheless, evidently hESC-MSCs failed to improve renal function as effectively as MSC(AT)s, suggesting lower reparative potency overall. Furthermore, both hESC-MSCs and MSC(AT)s induced small but significant tubular injury in sham kidneys, possibly secondary to undetected rejection or release of cytokines that characterize MSCs.
The use of xenotransplantation is a limitation of our study, although we did not find evidence for rejection. Another limitation is the use of a single cell dose. Yet, the optimal dose for one type of cell therapy might be different from the other, complicating comparisons. In addition, commercially available hESC cell-lines such as Royan-H643, ES438, MA0923,42, H9, AND-1, SHEF-139, ES345 and others might yield different MSCs phenotypes and efficacies. A range of culture and induction methods utilized in past studies, including embryoid body differentiation and the use of different induction media42,43 may also impact MSC reparative potency. Therefore, further studies are needed to optimize cellular phenotype and identify new niches to fill. Additional studies are also needed to elucidate the molecular basis for the different efficacies of both cell types.
CONCLUSION
The present study found MSC(AT)s to be more effective than hESC-MSCs at mitigating renal perfusion and oxygenation defects characteristic of RAS, although both cell types successfully blunted the associated hypertension and microvascular loss. Treatment with MSC(AT)s also reduced PCr and renin more effectively than hESC-MSCs. These functional and hemodynamic benefits were evident histologically as well, wherein MSC(AT)s were superior at preventing STK tubular injury, fibrosis, and vascular remodeling. This might be due to subclinical rejection of hESC-MSC or to cellular mechanisms that remain to be determined.
These observations highlight the importance of characterizing, validating, and carefully selecting MSCs for cell-based therapy to effectively harness their potential in kidney repair. These studies also support the need to further explore MSC(AT) therapy in treating renovascular disease and hypertension, and to standardize adult derived-MSC manufacturing. Future studies are needed to establish, standardize, and optimize MSC lines and confirm their efficacy in human subjects with RAS and other forms of kidney disease.
Funding Statement
This work was supported by a Novo Nordisk grant and partly supported by NIH [grants DK120292 and HL158691].
Footnotes
Conflicts of Interest
Dr. Lerman is an advisor to AstraZeneca, CureSpec, and Butterfly Biosciences. The authors declare no conflict.
Ethics Approval
This study was approved by the Mayo Clinic Institutional Review Board.
Consent for Participation
All participants included in this study provided written informed consent.
Consent for Publication
All authors have read and approved this manuscript for submission.
Code Availability
Not Applicable
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- (1.).Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, … Wright JT Jr (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71(19), e127–e248. [DOI] [PubMed] [Google Scholar]
- (2.).Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, & Grande JP (2010). Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 25(11), 3615–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3.).Gloviczki ML, Glockner JF, Crane JA, McKusick MA, Misra S, Grande JP, Lerman LO, & Textor SC (2011). Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension (Dallas, Tex. : 1979), 58(6), 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4.).Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, & Textor SC (2013). Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circulation. Cardiovascular interventions, 6(4), 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5.).Kim SR, Eirin A, Zhang X, Lerman A, & Lerman LO (2019). Mitochondrial Protection Partly Mitigates Kidney Cellular Senescence in Swine Atherosclerotic Renal Artery Stenosis. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology, 52(3), 617–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6.).Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D. j., & Horwitz E (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. [DOI] [PubMed] [Google Scholar]
- (7.).Vizoso FJ, Eiro N, Cid S, Schneider J, & Perez-Fernandez R (2017). Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. International journal of molecular sciences, 18(9), 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8.).Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, & Chen TH (2004). Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood, 103(5), 1669–1675. [DOI] [PubMed] [Google Scholar]
- (9.).Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, & Hedrick MH (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering, 7(2), 211–228. [DOI] [PubMed] [Google Scholar]
- (10.).da Silva Meirelles L, Chagastelles PC, & Nardi NB (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science, 119(Pt 11), 2204–2213. [DOI] [PubMed] [Google Scholar]
- (11.).Melo FR, Bressan RB, Forner S, Martini AC, Rode M, Delben PB, Rae GA, Figueiredo CP, & Trentin AG (2017). Transplantation of Human Skin-Derived Mesenchymal Stromal Cells Improves Locomotor Recovery After Spinal Cord Injury in Rats. Cellular and molecular neurobiology, 37(5), 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12.).Yigitbilek F, Conley SM, Tang H, Saadiq IM, Jordan KL, Lerman LO, & Taner T (2021). Comparable in vitro Function of Human Liver-Derived and Adipose Tissue-Derived Mesenchymal Stromal Cells: Implications for Cell-Based Therapy. Frontiers in cell and developmental biology, 9, 641792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13.).Bajek A, Gurtowska N, Olkowska J, Maj M, Kaźmierski Ł, Bodnar M, Marszałek A, Dębski R, & Drewa T (2017). Does the Harvesting Technique Affect the Properties of Adipose-Derived Stem Cells?-The Comparative Biological Characterization. Journal of cellular biochemistry, 118(5), 1097–1107. [DOI] [PubMed] [Google Scholar]
- (14.).Di Taranto G, Cicione C, Visconti G, Isgrò MA, Barba M, Di Stasio E, Stigliano E, Bernardini C, Michetti F, Salgarello M, & Lattanzi W (2015). Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy, 17(8), 1076–1089. [DOI] [PubMed] [Google Scholar]
- (15.).Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, Cooney DS, Lee WP, & Brandacher G (2014). Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transplant immunology, 30(4), 122–127. [DOI] [PubMed] [Google Scholar]
- (16.).Oñate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Díez-Caballero A, Ballesta-López C, Moscatiello F, Herrero J, & Badimon L (2012). The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(10), 4327–4336. [DOI] [PubMed] [Google Scholar]
- (17.).Zhu XY, Klomjit N, Conley SM, Ostlie MM, Jordan KL, Lerman A, & Lerman LO (2021). Impaired immunomodulatory capacity in adipose tissue-derived mesenchymal stem/stromal cells isolated from obese patients. Journal of cellular and molecular medicine, 25(18), 9051–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18.).Eirin A, Ferguson CM, Zhu XY, Saadiq IM, Tang H, Lerman A, & Lerman LO (2020). Extracellular vesicles released by adipose tissue-derived mesenchymal stromal/stem cells from obese pigs fail to repair the injured kidney. Stem cell research, 47, 101877. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19.).Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, & Bellantuono I (2004). Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem cells (Dayton, Ohio), 22(5), 675–682. [DOI] [PubMed] [Google Scholar]
- (20.).Sethe S, Scutt A, & Stolzing A (2006). Aging of mesenchymal stem cells. Ageing research reviews, 5(1), 91–116. [DOI] [PubMed] [Google Scholar]
- (21.).Heins N, Lindahl A, Karlsson U, Rehnström M, Caisander G, Emanuelsson K, Hanson C, Semb H, Björquist P, Sartipy P, & Hyllner J (2006). Clonal derivation and characterization of human embryonic stem cell lines. Journal of biotechnology, 122(4), 511–520. [DOI] [PubMed] [Google Scholar]
- (22.).Luzzani CD, & Miriuka SG (2017). Pluripotent Stem Cells as a Robust Source of Mesenchymal Stem Cells. Stem cell reviews and reports, 13(1), 68–78. [DOI] [PubMed] [Google Scholar]
- (23.).Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, & Lanza R (2014). Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem cells and development, 23(14), 1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24.).Jiang K, Ferguson CM, Ebrahimi B, Tang H, Kline TL, Burningham TA, Mishra PK, Grande JP, Macura SI, & Lerman LO (2017). Noninvasive Assessment of Renal Fibrosis with Magnetization Transfer MR Imaging: Validation and Evaluation in Murine Renal Artery Stenosis. Radiology, 283(1), 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25.).Heins N, Englund MC, Sjöblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, & Semb H (2004). Derivation, characterization, and differentiation of human embryonic stem cells. Stem cells (Dayton, Ohio), 22(3), 367–376. [DOI] [PubMed] [Google Scholar]
- (26.).Isik B, Thaler R, Goksu BB, Conley SM, Al-Khafaji H, Mohan A, Afarideh M, Abumoawad AM, Zhu XY, Krier JD, Saadiq IM, Tang H, Eirin A, Hickson LJ, van Wijnen AJ, Textor SC, Lerman LO, & Herrmann SM (2021). Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis. Stem cell research & therapy, 12(1), 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27.).Kim SR, Zou X, Tang H, Puranik AS, Abumoawad AM, Zhu XY, Hickson LJ, Tchkonia T, Textor SC, Kirkland JL, & Lerman LO (2021). Increased cellular senescence in the murine and human stenotic kidney: Effect of mesenchymal stem cells. Journal of cellular physiology, 236(2), 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28.).Zhao Y, Zhu X, Zhang L, Ferguson CM, Song T, Jiang K, Conley SM, Krier JD, Tang H, Saadiq I, Jordan KL, Lerman A, & Lerman LO (2020). Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem cells and development, 29(18), 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29.).Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, & Aicher WK (2016). Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem cells international, 2016, 5646384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30.).Kern S, Eichler H, Stoeve J, Klüter H, & Bieback K (2006). Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem cells (Dayton, Ohio), 24(5), 1294–1301. [DOI] [PubMed] [Google Scholar]
- (31.).Gorodetsky R, & Aicher WK (2021). Allogenic Use of Human Placenta-Derived Stromal Cells as a Highly Active Subtype of Mesenchymal Stromal Cells for Cell-Based Therapies. International journal of molecular sciences, 22(10), 5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32.).Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, & Ma ZJ (2015). Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem cell research & therapy, 6(1), 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33.).Amable PR, Teixeira MV, Carias RB, Granjeiro JM, & Borojevic R (2014). Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem cell research & therapy, 5(2), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34.).Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal Stem Cells and Endothelial Progenitor Cells Decrease Renal Injury in Experimental Swine Renal Artery Stenosis Through Different Mechanisms. STEM CELLS. 2013;31(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35.).Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, & Lerman LO (2012). Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem cells (Dayton, Ohio), 30(5), 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36.).Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, & Saraiva Camara NO (2009). Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem cells (Dayton, Ohio), 27(12), 3063–3073. [DOI] [PubMed] [Google Scholar]
- (37.).Saad A, Dietz AB, Herrmann S, Hickson LJ, Glockner JF, McKusick MA, Misra S, Bjarnason H, Armstrong AS, Gastineau DA, Lerman LO, & Textor SC (2017). Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. Journal of the American Society of Nephrology : JASN, 28(9), 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38.).Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, & Graumann J (2016). Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Scientific reports, 6, 21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39.).Sánchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R, Delgado M, & Menendez P (2011). Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem cells (Dayton, Ohio), 29(2), 251–262. [DOI] [PubMed] [Google Scholar]
- (40.).Gonzalo-Gil E, Pérez-Lorenzo MJ, Galindo M, Díaz de la Guardia R, López-Millán B, Bueno C, Menéndez P, Pablos JL, & Criado G (2016). Human embryonic stem cell-derived mesenchymal stromal cells ameliorate collagen-induced arthritis by inducing host-derived indoleamine 2,3 dioxygenase. Arthritis research & therapy, 18, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41.).Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, & Xu RH (2014). Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem cell reports, 3(1), 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42.).Thiel A, Yavanian G, Nastke MD, Morales P, Kouris NA, Kimbrel EA, & Lanza R (2015). Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Scientific reports, 5, 17685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43.).Mardpour S, Hassani SN, Mardpour S, Sayahpour F, Vosough M, Ai J, Aghdami N, Hamidieh AA, & Baharvand H (2018). Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury. Journal of cellular physiology, 233(12), 9330–9344. [DOI] [PubMed] [Google Scholar]
- (44.).Textor SC, & Lerman LO (2015). Paradigm Shifts in Atherosclerotic Renovascular Disease: Where Are We Now?. Journal of the American Society of Nephrology : JASN, 26(9), 2074–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45.).Zhang Y, Liao S, Yang M, Liang X, Poon MW, Wong CY, Wang J, Zhou Z, Cheong SK, Lee CN, Tse HF, & Lian Q (2012). Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell transplantation, 21(10), 2225–2239. [DOI] [PubMed] [Google Scholar]
- (46.).Oliveira-Sales EB, & Boim MA (2016). Mesenchymal stem cells and chronic renal artery stenosis. American journal of physiology. Renal physiology, 310(1), F6–F9. [DOI] [PubMed] [Google Scholar]
- (47.).Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Câmara NO, Bergamaschi CT, Campos RR, & Boim MA (2013). Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PloS one, 8(11), e78464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48.).Shin JH, Ryu CM, Ju H, Yu HY, Song S, Hong KS, Chung HM, Park J, Shin DM, & Choo MS (2020). Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study. Journal of clinical medicine, 9(9), 2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49.).Abumoawad A, Saad A, Ferguson CM, Eirin A, Herrmann SM, Hickson LJ, Goksu BB, Bendel E, Misra S, Glockner J, Dietz AB, Lerman LO, & Textor SC (2020). In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney international, 97(4), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


