Abstract
Introduction:
People with cerebellar ataxia (CA) can develop impulsive and compulsive behaviors that significantly affect their and their family’s quality of life. To further assess the decision-making process associated with these behaviors, we used the Iowa Gambling Task (IGT) to study people with CA.
Methods:
Sixty individuals with CA and thirty age-matched controls were enrolled in the study to complete the IGT. No participants had a prior or comorbid neurologic or psychiatric disorder associated with impulsivity. IGT performance in each of the five 20-trial blocks was compared between groups and the progression of participants’ performance was assessed with simple linear regression models. Subgroup analyses were performed with genetic and non-genetic CA cases.
Results:
CA cases obtained significantly lower IGT total scores than controls (−5.30 ± 37.53 vs. 21.30 ± 37.37, p = 0.004). In addition, those with CA made riskier decisions throughout the task compared to controls. Although both CA and controls learned to make decisions with more favorable outcomes over the course of completing the IGT, CA participants never matched the controls’ performance. IGT performance did not correlate with ataxia severity or depressive symptoms.
Conclusion:
The IGT may capture a unique behavioral symptom of CA. Future studies may help elucidate the mechanisms underlying impaired decision-making in CA and further the understanding of a broader spectrum of cerebellar cognitive affective syndrome.
Keywords: Cerebellum, Cerebellar Ataxia, Impulsivity, Gambling, Risk Taking, Decision Making
Introduction
The cerebellum has long been known for its role in motor functions [1], but newer research have also revealed the cerebellum’s critical role in modulating cognition and emotions [2]. The cerebellum has extensive direct and indirect connections to a variety of cerebrocortical and other brain regions, including the frontal lobes, basal ganglia, limbic system, and ventral tegmental area (VTA). Dysfunction of these cerebello-cortical connections contribute to impaired social function, cognitive and emotional processing, psychiatric disorders, and learning, among others [3,4].
Recent studies in animal models have identified the crucial role of the cerebellum in modulating reward processing: granule cells encode reward anticipation and delivery [5], climbing fiber activity is related to the prediction of reward and violation of expectations [6,7], and Purkinje cell firing is important for reward-based reinforcement learning [8]. An upswing of recent scholarship has helped highlight the role of the cerebellum in impulsivity and obsessive-compulsive behavior [9]. New onset of impulsive and aggressive behavior has been reported after cerebellar strokes and similar psychological traits are observed in people with cerebellar ataxia (CA) [10,11]. Specifically, recent publications have demonstrated that people with CA display increased impulsive and compulsive behaviors, including gambling, hoarding, and compulsive medication use, and that this behavior was driven by non-planning impulsivity, exemplifying the cerebellum’s role in reward processing [10,12,13].
One of the most prominent impulse control symptoms in individuals with CA is gambling [10]. To further investigate this behavior and its significant impact on the patient’s and family’s quality of life, we aimed to study the decision-making process in those with CA using the Iowa Gambling Task (IGT). In this task, participants make selections from four card decks with the goal of maximizing their game money by choosing the advantageous card decks. IGT measures the reward-based decision-making process [14,15]. We thus hypothesized that people with CA will make riskier decisions, which is consistent with the current understanding of impulsivity in CA [10,12,13].
Methods
Participants
We conducted a cross-sectional study that included 60 individuals with CA and 30 age-matched controls in a 2:1 study design. The study protocol was approved by the Columbia University institutional review board and all participants consented to participation in this study. Participants with CA were recruited from the Ataxia Clinic at Columbia University Medical Center. Among the 60 CA participants, 33 had genetic ataxia whereas 27 had non-genetic ataxia. Specifically, the genetic ataxia group was comprised of 26 individuals with spinocerebellar ataxia (SCA), 1 with ataxia with oculomotor apraxia type 2 (AOA2), 1 with fragile X-associated tremor/ataxia syndrome (FXTAS), 1 with cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) with RFC1 repeat expansions, 1 with episodic ataxia type 2 (EA2), 1 with Niemann Pick disease type C (NPC), 1 with CA due to genetic glycosylation defect, and 1 with Friedreich’s ataxia. The non-genetic ataxia group included 8 participants with multiple system atrophy-cerebellar type (MSA-C), 15 with idiopathic late-onset CA (ILOCA), 1 with traumatic brain injury to the cerebellum, 1 with immune-mediated ataxia, 1 with ataxia secondary to a left posterior pontine cavernoma, and 1 with tacrolimus induced ataxia. The spouses and friends of the participants with CA were recruited as age- and sex-matched controls. No participant had a prior neurologic or psychiatric disorder known to be associated with impulsivity, including dementia, attention-deficit/hyperactivity disorder, autism spectrum disorder, or bipolar disorder.
Study measures
The severity of motor dysfunction in ataxia was measured with the Scale of Assessment and Rating of Ataxia (SARA) (n = 37) [16]. Symptoms of depression were determined using the Patient Health Questionnaire-9 (PHQ-9) (n = 51) [17].
A computerized version of the IGT was used [18,19]. Participants made 100 selections from the four decks of cards identical in appearance. After each draw, the amount of money won or lost and the cumulative sum of money was displayed on the screen. Two of the decks were considered advantageous because of their conservative nature (i.e., lower amount of money gained and lost after choosing the cards) and because these decks produced a net gain after many selections. On the contrary, the other two decks were disadvantageous based on their risky nature and resulted in a long-term net loss for the player. Thus, taken as a whole, stronger preference for the disadvantageous decks was indicative of riskier and more impulsive behavior.
Statistical analysis
Basic demographic and clinical characteristics were compared between CA cases and controls. For continuous variables, the normality of the variables was first tested using the Kolmogorov-Smirnov test. Comparisons between the two groups were performed with the Student’s t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. The Fisher exact test was used for categorical variables.
IGT scores were calculated as the number of selections from the advantageous decks (C and D) minus the number of selections from the disadvantageous decks (A and B). IGT total scores were compared between patients and controls using the Mann-Whitney U test because the variables were non-normally distributed. To examine the progression of patients’ and controls’ IGT performance over time, the 100 selections from the 4 card decks were divided into five 20-trial blocks. A two-group by five-block repeated measures analysis of variance was performed. Then, single block comparisons of patients’ and controls’ scores were performed using the Mann-Whitney U test because all variables were non-normally distributed. To investigate participants’ learning and reward processing, their learning rate was calculated using simple linear regressions with block as the independent variable and IGT score as the dependent variable. The slopes of patients’ and controls’ learning rates were compared using an analysis of covariance.
Patients were further divided into those with genetic ataxia and those with non-genetic ataxia. A one-way analysis of variance followed by Tukey post-hoc tests were performed to compare the IGT total scores in controls, participants with genetic ataxia, and participants with non-genetic ataxia. Pearson correlation coefficients were computed to analyze whether IGT total scores correlated with SARA scores or PHQ-9 scores. All statistical analyses were performed using GraphPad Prism version 9.
Results
Basic demographics and clinical characteristics of individuals with CA and controls are shown in Table 1. There was no significant difference in age and gender between groups. CA cases had significantly higher PHQ-9 scores than controls, consistent with prior findings that those with CA are more prone to depression [17].
Table 1.
Demographic and clinical features of controls and participants with cerebellar ataxia.
| Control | Cerebellar Ataxia | p value | |
|---|---|---|---|
| n | 30 | 60 | |
| Age (years) | 54.53 ± 16.36 | 58.37 ± 14.17 | 0.254a |
| Gender, M:W | 17:13 | 32:28 | 0.825c |
| PHQ-9 | 2.27 ± 2.83 | 5.00 ± 4.04 | 0.024 b |
| SARA | N/A | 12.62 ± 5.81 |
Values represent mean ± standard deviation.
PHQ-9, Patient Health Questionnaire-9; SARA, Scale of Assessment and Rating of Ataxia.
Two independent samples t test.
Two independent samples Mann-Whitney U test.
Fisher exact test.
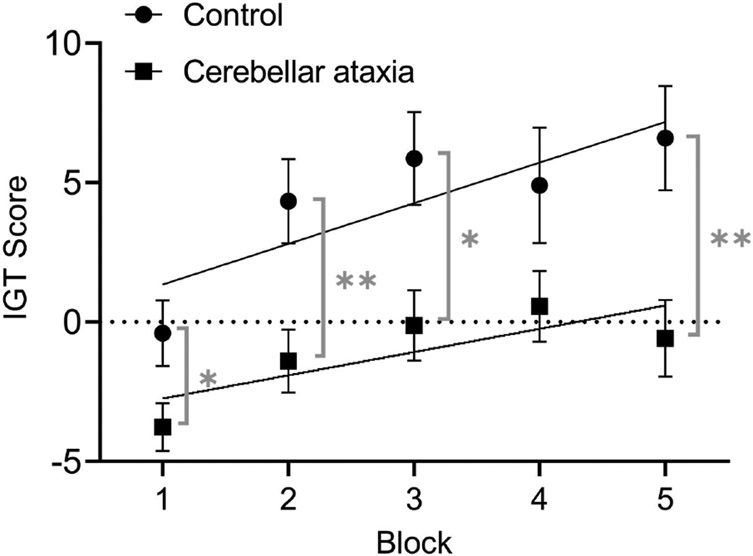
We next determined whether individuals with CA made riskier decisions on the IGT. CA cases had significantly lower IGT total scores than controls (−5.30 ± 37.53 vs. 21.30 ± 37.37, p = 0.004). A 2 (group) x 5 (block) ANOVA on the IGT scores indicated a significant main effect of group [F = 10.07, p = 0.002] and a significant main effect of block [F = 10.14, p < 0.001], but no interaction between group and block [F = 1.22, p = 0.303]. When we compared within each block, CA cases had lower IGT scores in all blocks (CA cases vs. controls: Block 1: −3.77 ± 6.68 vs. −0.40 ± 6.46, p = 0.022; Block 2: −1.40 ± 8.70 vs. 4.33 ± 8.24, p = 0.005; Block 3: −0.12 ± 9.79 vs. 5.87 ± 9.14, p = 0.011; Block 4: 0.57 ± 9.86 vs. 4.90 ± 11.35, p = 0.112; Block 5: −0.58 ± 10.63 vs. 6.60 ± 10.25, p = 0.006), although the difference in Block 4 did not reach statistical significance (Figure 1). These results demonstrate that participants with CA made riskier decisions consistently throughout the task.
Figure 1. Decision-making in cerebellar ataxia:
Mean ± SEM of the number of selections from the advantageous decks (C+D) minus the number of selections from the disadvantageous decks (A+B) over the course of five 20-trial blocks. Learning curves calculated through linear regression models. *p < 0.05, **p < 0.01
We next investigated whether participants learned over the course of completing the IGT to make favorable decisions (i.e., improving IGT scores) by performing simple linear regressions in both CA cases and controls. Linear regressions on the IGT scores across blocks showed a slope of 1.46 ± 0.53 (p = 0.007) for controls and a slope of 0.83 ± 0.38 (p = 0.028) for patients, indicating that both groups improved their IGT scores over time. There was no significant difference between the learning rates of patients and controls (p = 0.341). The results suggest that, similar to controls, CA cases can also learn to make favorable decisions over time, but overall, CA cases never catch up with controls in IGT performance.
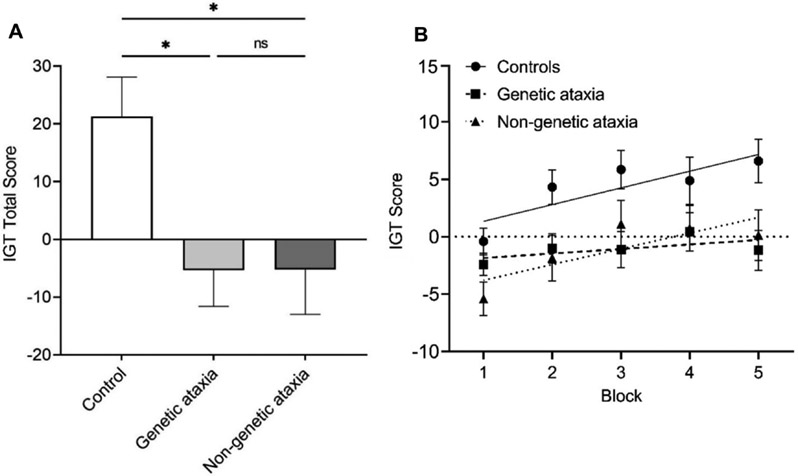
We further performed a subgroup analysis to investigate whether IGT total scores were different between genetic and non-genetic CA cases. A one-way ANOVA indicated a significant difference between the IGT total scores of controls, participants with genetic ataxia, and participants with non-genetic ataxia (F = 4.98, p = 0.009). Post-hoc analyses showed that controls (21.30 ± 37.37) had higher IGT scores than both genetic and non-genetic CA cases, whereas there was not a difference between genetic and non-genetic CA cases (−5.33 ± 35.90 vs. −5.26 ± 40.13, p > 0.999) (Figure 2A). Linear regression models also revealed no significant difference between the learning rates of those with genetic and non-genetic CA (p = 0.199) (Figure 2B).
Figure 2. Comparison between genetic and non-genetic ataxia:
(A) IGT total scores in controls, participants with genetic CA, and participants with non-genetic CA. (B) Mean ± SEM of IGT scores throughout the five 20-trial blocks in controls, participants with genetic CA, and participants with non-genetic CA. Learning curves calculated through linear regression models. *p < 0.05, ns: not significant.
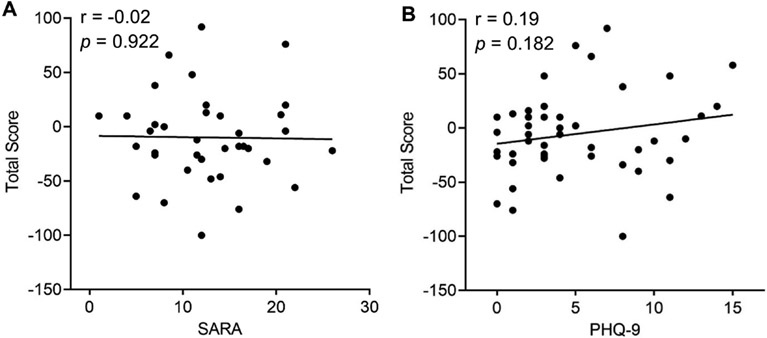
Finally, we examined whether IGT total scores in CA cases correlated with other clinical features, including ataxia severity and depressive symptoms. We found that IGT total scores in CA cases did not correlate with ataxia severity, measured by SARA scores (Pearson r = −0.02, p = 0.922; Figure 3A) or with depressive symptoms, measured by PHQ-9 scores (Pearson r = 0.19, p = 0.182; Figure 3B), demonstrating that IGT may capture a distinctive non-motor feature of CA patients.
Figure 3. Correlation with motor and non-motor symptoms of cerebellar ataxia:
(A) The correlation between patients’ IGT total scores and their SARA scores. (B) The correlation between patients’ IGT total scores and their PHQ-9 scores.
Discussion
In the present study, we found that people with CA showed significantly worse performance than controls in the IGT. Subjects with CA made riskier decisions from the very beginning of the IGT and this difference persisted throughout the task. Although CA cases, like controls, improved their IGT performance over time, they never matched the controls’ performance. Participants with genetic CA and those with non-genetic CA showed similar IGT performances. The absence of correlation between IGT performance and ataxia severity or depressive symptoms indicates that impaired decision making is a unique cognitive symptom in CA.
Previous studies have shown that individuals with CA are more impulsive and compulsive than controls and that these behaviors were specific to gambling, hobbyism-punding, and excessive medication use [10,12]. Consistent with these results, the present study found that those with CA made more impulsive decisions in the IGT.
Bechara et al. [14] hypothesized that poor performance in the IGT may result from three distinct deficits: hypersensitivity to reward, hyposensitivity to punishment, or myopia for the future. Chen et al. [13] showed that impulsivity in CA is driven by non-planning impulsivity. In this context, it is possible that the poor IGT performance observed in the present study resulted from the lack of consideration for the future penalties imposed by the disadvantageous decks. Further neuropsychological studies will be needed to fully explore the underlying mechanism of impaired decision making in IGT in CA cases.
The constellation of cognitive and behavioral symptoms in those with cerebellar dysfunction is called cerebellar cognitive affective syndrome (CCAS) [20]. This syndrome is characterized by deficits in executive function, visuospatial cognition, regulation of affect, and language [3]. Gambling behavior and impaired decision making could constitute a broader spectrum of CCAS.
Regarding the underlying pathophysiology of impulse control disorders in CA, further evidence is needed to better understand why individuals with cerebellar dysfunction exhibit such striking impulsive and compulsive behavior. In a previous publication, Miquel et al. [9] hypothesized that the cerebellum may have a modulatory function in behavior control by terminating or initiating actions through regulation of prefrontal cortices. In their model, cerebellar error prediction extends beyond motor control and encompasses other brain functions such as decision-making processes. Another potential explanation might be that the cerebellum influences gambling behavior and decision-making through its projections to the dopaminergic system via the VTA [21]. The IGT has been extensively studied in Parkinson’s disease [22] and affected individuals show a tendency to make riskier choices in the task similar to participants with CA in our study. It is therefore possible that the observed impulse control deficits in CA cases could be caused by impaired modulation of the VTA system leading to alterations in the reward system.
The IGT has been widely used to assess decision making in other neurologic and neurodegenerative disorders such as Parkinson’s disease [22], Huntington’s disease [23], and Alzheimer’s disease [24] and all three groups demonstrate impaired performance in the IGT. Specifically, Parkinson’s disease patients’ preference for risky selections in the IGT may be driven by deficits in anticipating unrewarding consequences or hyposensitivity to punishment [22]. Whereas impaired performance by those with Huntington’s disease may be associated with impaired working memory, increased recklessness, and reduced sensitivity to losses [25,26]. Similar to individuals with these other neurodegenerative disorders, the CA participants in the present study made riskier decisions in the IGT. Future studies focusing on the mechanisms underlying impaired performance in the IGT would help elucidate the pattern of impairments in different patient groups.
The present study has several limitations. First, the CA participants consisted of a heterogenous patient population, with many having extra-cerebellar involvement. Therefore, we cannot eliminate the possibility that other brain regions contributed to the risky decisions made by our participants. Future studies focusing on cases with “pure” cerebellar involvement, such as SCA6 and idiopathic late-onset CA, and those with pure cerebellar lesions, such as cerebellar strokes, would help further clarify the cerebellum’s distinct role in impulsivity. Second, 100 trials might not be sufficient to detect subtle performance differences in the IGT. Previous work found that when the IGT was extended beyond 100 trials, controls continued to improve their performance after the first 100 trials [27]. In our study, CA participants and controls had similar learning curves in the first 100 trials. Conducting a 200- or 300-trial IGT in participants with CA may thus be beneficial to determine whether the observed deficits of people with cerebellar dysfunction truly only manifest through disadvantageous and riskier choices or whether their learning curve may be distinct from healthy controls in more trials.
Given the significant and harmful impact that impulse control disorders can have on people with CA and their caregivers [12], it would be crucial to screen CA patients for impulsive and compulsive traits, particularly because such symptoms may not be voluntarily reported. Further work is needed to better understand the underlying pathophysiology of impulse control deficits in CA as well as to evaluate potential therapeutic interventions.
People with cerebellar ataxia (CA) made riskier decisions than controls
CA cases and controls both improved performance on the Iowa Gambling Task over time
CA cases never matched the controls’ performance
Risky decision making could be a unique cerebellar cognitive symptom
Funding
This work was supported by the National Institutes of Health [grant numbers NINDS #R01 NS104423, NINDS #R01 NS118179, NINDS #R01 NS124854]; and National Ataxia Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- [1].West JR, Gelderd JB, The Cerebellum, in: Conn PM (Ed.), Neuroscience in Medicine, Humana Press, Totowa, NJ, 2003: pp. 217–236. 10.1007/978-1-59259-371-2_10. [DOI] [Google Scholar]
- [2].Schmahmann JD, Ferdinando Rossi Lecture: the Cerebellar Cognitive Affective Syndrome—Implications and Future Directions, Cerebellum. (2022). 10.1007/s12311-022-01456-7. [DOI] [PubMed] [Google Scholar]
- [3].Schmahmann JD, Emotional disorders and the cerebellum: Neurobiological substrates, neuropsychiatry, and therapeutic implications, in: Handbook of Clinical Neurology, Elsevier, 2021: pp. 109–154. 10.1016/B978-0-12-822290-4.00016-5. [DOI] [PubMed] [Google Scholar]
- [4].Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JDE, Guell X, Heleven E, Lupo M, Ma Q, Michelutti M, Olivito G, Pu M, Rice LC, Schmahmann JD, Siciliano L, Sokolov AA, Stoodley CJ, van Dun K, Vandervert L, Leggio M, Consensus Paper: Cerebellum and Social Cognition, Cerebellum. 19 (2020) 833–868. 10.1007/s12311-020-01155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L, Cerebellar granule cells encode the expectation of reward, Nature. 544 (2017) 96–100. 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, Hull C, Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions, Nat Neurosci. 21 (2018) 1431–1441. 10.1038/S41593-018-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohmae S, Medina JF, Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice, Nat Neurosci. 18 (2015) 1798–1803. 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sendhilnathan N, Semework M, Goldberg ME, Ipata AE, Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum, Neuron. 106 (2020) 188–198.e5. 10.1016/j.neuron.2019.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miquel M, Nicola SM, Gil-Miravet I, Guarque-Chabrera J, Sanchez-Hernandez A, A Working Hypothesis for the Role of the Cerebellum in Impulsivity and Compulsivity, Front. Behav. Neurosci 13 (2019) 99. 10.3389/fnbeh.2019.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Amokrane N, Viswanathan A, Freedman S, Yang C, Desai NA, Pan M, Kuo S, Lin C, Impulsivity in Cerebellar Ataxias: Testing the Cerebellar Reward Hypothesis in Humans, Mov Disord. 35 (2020) 1491–1493. 10.1002/mds.28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tessier A, Cosin C, Mayo W, Pfeuty M, Misdrahi D, Sibon I, Impulsive aggressive obsessions following cerebellar strokes: a case study, J Neurol. 262 (2015) 1775–1776. 10.1007/s00415-015-7804-6. [DOI] [PubMed] [Google Scholar]
- [12].Amokrane N, Lin C-YR, Desai NA, Kuo S-H, The Impact of Compulsivity and Impulsivity in Cerebellar Ataxia: A Case Series, Tremor and Other Hyperkinetic Movements. 10(2020) 43. 10.5334/tohm.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen TX, Lin C-YR, Aumann MA, Yan Y, Amokrane N, Desai NA, Kang H, Claassen DO, Kuo S-H, Impulsivity Trait Profiles in Patients With Cerebellar Ataxia and Parkinson Disease, Neurology. 99 (2022) e176–e186. 10.1212/WNL.0000000000200349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bechara A, Tranel D, Damasio H, Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions, Brain. 123 (2000) 2189–2202. 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- [15].Bauer AS, Timpe JC, Edmonds EC, Bechara A, Tranel D, Denburg NL, Myopia for the future or hypersensitivity to reward? Age-related changes in decision making on the Iowa Gambling Task., Emotion. 13 (2013) 19–24. 10.1037/a0029970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang J-S, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schols L, Szymanski S, van de Warrenburg BP, Durr A, Klockgether T, Scale for the assessment and rating of ataxia: Development of a new clinical scale, Neurology. 66 (2006) 1717–1720. 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- [17].Lo RY, Figueroa KP, Pulst SM, Perlman S, Wilmot G, Gomez C, Schmahmann J, Paulson H, Shakkottai VG, Ying S, Zesiewicz T, Bushara K, Geschwind M, Xia G, Yu J-T, Lee L-E, Ashizawa T, Subramony SH, Kuo S-H, Depression and clinical progression in spinocerebellar ataxias, Parkinsonism & Related Disorders. 22 (2016) 87–92. 10.1016/j.parkreldis.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stoet G, PsyToolkit: A software package for programming psychological experiments using Linux, Behavior Research Methods. 42 (2010) 1096–1104. 10.3758/BRM.42.4.1096. [DOI] [PubMed] [Google Scholar]
- [19].Stoet G, PsyToolkit: A Novel Web-Based Method for Running Online Questionnaires and Reaction-Time Experiments, Teaching of Psychology. 44 (2017) 24–31. 10.1177/0098628316677643. [DOI] [Google Scholar]
- [20].Schmahmann JD, Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect, Trends in Cognitive Sciences. 2 (1998) 362–371. 10.1016/S1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- [21].Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K, Cerebellar modulation of the reward circuitry and social behavior, Science. 363 (2019) eaav0581. 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colautti L, Iannello P, Silveri MC, Antonietti A, Decision making in Parkinson’s disease: An analysis of the studies using the Iowa Gambling Task, Eur J of Neuroscience. 54 (2021) 7513–7549. 10.1111/ejn.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stout JC, Rodawalt WC, Siemers ER, Risky decision making in Huntington’s disease, J Int Neuropsychol Soc. 7 (2001) 92–101. 10.1017/S1355617701711095. [DOI] [PubMed] [Google Scholar]
- [24].Jacus J-P, Gély-Nargeot M-C, Bayard S, Ecological relevance of the Iowa gambling task in patients with Alzheimer’s disease and mild cognitive impairment, Revue Neurologique. 174 (2018) 327–336. 10.1016/j.neurol.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [25].Busemeyer JR, Stout JC, A contribution of cognitive decision models to clinical assessment: Decomposing performance on the Bechara gambling task., Psychological Assessment. 14 (2002) 253–262. 10.1037/1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- [26].Campbell MC, Stout JC, Finn PR, Reduced autonomic responsiveness to gambling task losses in Huntington’s disease, J Int Neuropsychol Soc. 10 (2004) 239–245. 10.1017/S1355617704102105. [DOI] [PubMed] [Google Scholar]
- [27].Bull PN, Tippett LJ, Addis DR, Decision making in healthy participants on the Iowa Gambling Task: new insights from an operant approach, Front. Psychol 6 (2015). 10.3389/fpsyg.2015.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]