Abstract
Gynostemma pentaphyllum saponin has a variety of biological properties. Classic separation methods of saponin, such as resin absorption and preparative chromatography are limited by environmental pollution and high cost. In the study, ultrasonic assisted membrane separation was firstly used to purify saponin from Gynostemma pentaphyllum. Total proteins, polysaccharides, saponin, gypenoside A and rutin were selected as indexes to optimize the pretreatment and purification parameters by response surface methodology. The fitted models were significant (p < 0.05) and the optimal conditions were: (1) removing protein and polysaccharides by MWCO 10,000 Da, ultrasonic power 400 W and pH 7.8; (2) separation flavonoids from saponin by MWCO 1000 Da, ultrasonic power 300 W and pH 7.9. The difficulty in separating saponin from flavonoids was solved by releasing flavonoids from micelles with ultrasonic assisted membrane method. The saponin content in Gynostemma pentaphyllum extracts reached 82.81%, which was more than four times of that obtained with resin adsorption method. The protective effect of saponins on SH-SY5Y cells injury induced by H2O2 was better than that of Gynostemma pentaphyllum extracts. The study suggested that ultrasonic assisted membrane method would be widely applied in the preparation of food materials.
Keywords: Gynostemma pentaphyllum, Ultrasonic assisted membrane, Saponin, Ultrafiltration
Introduction
Gynostemma pentaphyllum, a species of Cucurbitaceae, can be used as medicine and food (Yang et al., 2013; Xiao et al., 2012). Gynostemma pentaphyllum saponin is the main component, which has a variety of biological properties, such as antitumor, anti-atherosclerosis, liver protection and antioxidant (Liu et al., 2021; Huang et al., 2022; Shi et al., 2018; Zhai et al., 2021). Saponin is mainly separated and purified by resin absorption and preparative chromatography (Wan et al., 2008; Tsai et al., 2010; Wang et al., 2020). However, in resin adsorption and chromatograph separation method, the environmental pollution of organic solvents, high production costs, long separation period, and heat treatment-induced component conversion limit its industrial applications. Therefore, it is necessary to develop a green and efficient separation method.
Membrane separation methods includes microfiltration, ultrafiltration and nanofiltration, which had the technical advantages of normal temperature operation and high separation efficiency (Zhang et al., 2021; Zhu et al., 2022; Kaur et al., 2012). The separation principle of membrane separation methods involves molecular sieving, Donnan effect and steric hindrance effect. Microfiltration and ultrafiltration are mostly used to remove macromolecules, such as polysaccharides, polypeptides and proteins (Cao et al., 2018; Susanto et al., 2008). Nanofiltration was applied to treat water treatment and concentrate heat-sensitive ingredients (Luo et al., 2015; Arsuaga et al., 2006), but its separation efficiency relied on the pore size, charges, and trans-membrane pressure difference.
Saponins have surface-like activity, and can be associated as micelles in Gynostemma pentaphyllum aqueous solution. However, the association stability of micelles can be adjusted by ultrasound with cavitation effect (Bari et al., 2016; Hemar et al., 2020). The differences in physico-chemical properties between saponins micelles and other types of components are so obvious that it is theoretically feasible to separate saponins from Gynostemma pentaphyllum by regulating the properties with ultrasonic-assisted membrane methods.
The differences in molecular size and charge intensity among the ingredients of Gynostemma pentaphyllum can be enhanced to determine the separation mode of saponins. In this study, saponins, gypenoside A, rutin, polysaccharides and proteins were selected as indexes to optimize separation parameters of ultrasonic-assisted membrane methods with single factor analysis and response surface methodology (RSM). According to the component transfer rate and separation efficiency, the ultrasonic-assisted membrane separation method was verified with the complex solution of Gynostemma pentaphyllum.
Materials and methods
Reagents
Gynostemma pentaphyllum was purchased from Anhui Puren Pharmaceutical Co., Ltd (Bozhou, China). The reference substances of gypenoside A (purity ≥ 98.0%), D-glucose (purity ≥ 99.0%), and rutin (purity ≥ 91.9%) were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). BCA protein content detection kit was purchased from Jiangsu Kaiji Biotechnology Co., Ltd. Human neuroblastoma cancer SH-SY5Y cells were purchased from China Infrastructure of Cell Line Resources (Beijing, China). Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Acetonitrile and methanol were chromatographic grade and purchased from Merck (Darmstadt, Germany). Sulfuric acid, phenol and other chemicals were all analytical grades and obtained from Sinopharm Group Chemical Reagent Co. Ltd. (Shanghai, China). Ultrapure water and purified water was prepared by Millipore Direct-Q5 water purification system.
Pretreatment of Gynostemma pentaphyllum extracts
Gynostemma pentaphyllum was extract for 1 h with 15 times of the volume of purified water and the extraction was performed for two times. The extracts were centrifuged at 15,000 r/min in centrifuge tubes (Tianben, GQ125, Shanghai, China) to remove insoluble particles. Then, the polyethersulfone (PES) microfiltration membrane with a pore size of 0.1 μm and filtration area of 0.3 m2 was placed in TNZ-1 membrane separation equipment which purchased from Tuozhu Corporation (Nanjing, China). Gynostemma pentaphyllum extracts were placed in a storage tank and the trans-membrane pressure (TMP) was adjusted to 0.05 to 0.10 MPa so as to collect the filtrate.
Nanofiltration was carried out by using spiral membrane (NFG-1812) with a molecular weight cut-off (MWCO) of 800 Da and filtration area of 0.3 m2. The filtrate of microfiltration was placed in the stock tank of nanofiltration separation equipment and then the medium pressure plunger pump, digital pressure gage, flow velocity valve and membrane shell were assembled with pressure pipeline. When the solution volume in stock tank was reduced to 1/3 of its original volume, 2/3 of its original volume of purified water was supplemented in the tank for the nanofiltration separation. The operation was repeated 3 times to remove the small molecule components and regulate saponin concentration.
Ultrasonic-assisted ultrafiltration (UAU) separation
Through the microfiltration-nanofiltration pretreatment, the concentration of Gynostemma pentaphyllum saponin was regulated to enhance micelle state. As shown in Fig. 1, UAU separation system was used to solve the separation contradiction between saponins and flavones, which consisted of KQ-700DE ultrasonic equipment (Suzhou, China) (Li et al., 2020). During the experiment, the container with rejected solution of nanofiltration was placed in ultrasonic equipment and the peristaltic pump, digital pressure gage, flow velocity valve and membrane shell were assembled in the same way of nanofiltration separation equipment.
Fig. 1.

Instrumentation diagram of ultrasonic-assisted membrane system, (1) ultrasonic system, (2) stock solution tank, (3) peristaltic pump, (4) pressure gage, (5) membrane, (6) pressure gage, (7) speed regulator valve, (8) rejected solution, (9) inlet pipe and (10) filtrate solution
In order to enhance the separation difference between saponins and flavones, the MWCO of ultrafiltration membrane, ultrasonic power and pH were selected as single factor to adjust micelle existence and ionization degree of flavone (Abdellah et al., 2020). To clarify the effect of each single factor, the MWCO of ultrafiltration membrane was respectively 1000, 5000, 10,000, 30,000 and 50,000 Da. Ultrasonic power was set as 0, 100, 200, 300, 400, 500 and 600 W. The pH was set as 4.0, 5.0, 6.0, 7.0 and 8.0. During the experiment, the operating parameters were respectively fixed as follows: MWCO of 5000 Da, ultrasonic power of 100 W, pH 5.0, TMP of 0.1 MPa, and the solution temperature of 22 °C to 25 °C.
The micelle association state was regulated by ultrasound to further purify the total saponins from Gynostemma pentaphyllum (TSG). In order to further enhance the differences between saponins and flavones, the transmittance of gypenoside A, rutin and total protein (TP) were selected as the response value and MWCO (X1), ultrasonic power (X2) and pH (X3) were selected as independent variables based on the results of single factor analysis. The range and the levels of each independent variable were set as follows: X1 (1000, 5000 and 10,000 Da), X2 (200, 300 and 400 W) and X3 (6.0, 7.0 and 8.0).
Response surface methodology (RSM) has been widely used in pharmaceutical research due to its high computing efficiency and short computing time (Medina et al., 2021; Pinto et al., 2021). In this paper, Box–Behnken design was used to conduct the 3-factor 3-level experiment consisting of 17 experimental runs. The experimental results were analyzed in Design-Expert software version 8.06. The obtained experimental data were subjected to the regression analysis with a quadratic nonlinear polynomial model, indicating the relationship between regression coefficient and interaction of variables.
| 1 |
where Y is response variable. β0, βi, βii and βij are the constant, linear regression coefficient, quadratic regression coefficient and interaction regression coefficient, respectively. Xi and Xj are independent variables. The statistical significance was examined by the analysis of variance (ANOVA). F test and p value (p < 0.05) were used to evaluate the significance of each coefficient and the interaction between each independent variable.
Separation of gypenoside A and rutin
In order to analyze the adsorption and encapsulation characteristic between gypenoside A and rutin, 85.5 μg mL−1 gypenoside A, 16.2 μg mL−1 rutin, and the mixed solution of 85.5 μg mL−1 gypenoside A, 16.2 μg mL−1 rutin were respectively separated by ultrafiltraion. The separation parameters were set as follows: MWCO of 1000 Da, ultrasonic power of 300 W and pH 7.9.
Sample analysis
TP
TP was analyzed with bicinchoninic acid (BCA) protein assay kit purchased from Solarbio Technology Co., Ltd (Beijing, China). According to BCA method, TP in the samples was determined to analyze the effect of separation parameters on TP transmittance.
Polysaccharide
D-glucose reference was dried to constant weight and diluted to the concentration of 0.500 mg/mL with purified water in 10 mL volumetric flask. According to the anthracone sulfate method, 0.0, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5.0 mL D-glucose reference solution was accurately transferred into a 100 mL volumetric flask. The series of D-glucose solutions reacted with anthrone sulfate solution and then the absorbance (A) was measured by T6 ultraviolet–visible spectrophotometer at 620 nm. Linear regression analysis was conducted with D-glucose concentration (C) as the abscissa and A as the ordinate. The standard curve was plotted as A = 0.0144 C-0.0002 (r2 = 0.999 8). D-glucose concentration in the range of 10.00 to 25.00 mg mL−1 showed a good linear relationship with absorbance.
Rutin
Rutin reference was dissolved directly in methanol according to the concentration of 0.210 mg/mL, and then 0.10, 0.20, 0.50, 1.00 and 2.00 mL rutin reference solution was diluted to a volume of 5 mL with methanol and analyzed with a Waters e2695 high performance liquid chromatography (HPLC) system equipped with a 2998 PDA detector. The binary mobile phase was composed of methanol and 0.1% glacial acetic acid (32:68, v/v) and the analysis conditions were set as: detection wavelength of 257 nm, injection volume of 10 μL and flow rate of 1.0 mL mL−1. For the purpose of quantitative analysis, the standard curve was obtained by plotting the peak area (Y1) against rutin concentration (X1), Y1 = 15.72 X1 + 24.89, which was consistent with Beer’s Law (r2 = 0.999 3).
TSG
The reference substance of gypenoside A was dried to constant weight and dissolved in methanol according to the concentration of 2.0 mg mL−1. 20, 50, 100, 150 and 200 μL gypenoside A reference solution was accurately pipette into a 10 mL volumetric flask, evaporated to dryness in water bath at 65℃, and then reacted with 0.8 mL of perchloric acid and 0.2 mL of 5% vanillin—glacial acetic acid solution. The reaction was carried out under the conditions of water bath condition for 15 min and 5 mL glacial acetic acid was then added into a volumetric flask. After cooling down to room temperature, the absorbance of reaction solution was detected at 510 nm. With gypenoside A as a reference, the concentration of TSG was determined with T6 ultraviolet–visible spectrophotometer (Beijing General Instrument Co. Ltd, Beijing, China). The regression equation was calculated with the concentration of gypenoside A as X-axis and absorbance as Y-axis, Y = 4.308 6 X−0.088 3 (r2 = 0.999 6).
Gypenoside A
Gypenoside A was analyzed on Waters e2695 HPLC system equipped with a Thermo BDS C18 column (4.6 mm × 250 mm, 5 μm). The binary mobile phase consisting of acetonitrile (A) and 0.1% glacial acetic acid (B) was used for gradient elution (0–5 min, 20% A; 5–15 min, 20–40% A; 15–20 min, 40% A; 20–21 min, 40–20% A; 21–35 min, 20% A). In addition, the detection wavelength was 203 nm. The injection volume was 10 μL and flow rate was 1.0 mL/min. Taking the concentration as the X-axis, the peak area as the Y-axis, draw the standard curve, Y2 = 601 728 X2 − 35 681 (r2 = 0.998 6). The result showed that the concentration of gypenoside A in the range of 0.25 to 12.50 μg had a good linear relationship with the peak area. The precision, repeatability and recovery rate of the method met the analysis requirements and the stability of the tested solution was good within 24 h.
Protective effects of total saponins on H2O2 induced cell injury
SH-SY5Y cells were selected to evaluate the protective effects of total saponins with the positive control of Vitamin C according to MTT method. SH-SY5Y cells were seeded in 96-well culture plates and incubated for 24 h. H2O2 solution was added into the culture medium according to the concentration of 250 μM and incubated for 12 h at 37 °C. The supernatant medium was replaced with the fresh medium containing different concentrations of samples and incubated for 24 h at 37 °C. Then, the supernatant medium was replaced with MTT solution. The resultant formazan crystals in cells were solubilized with 100 μL of dimethyl sulfoxide and measured at 570 nm by SuPerMax 3000AL multifunctional ELISA instrument (Shanghai, China).
Data analysis
Transmittance
The percentage of transmittance of each component in microfiltration, ultrafiltration and nanofiltration is calculated as:
| 2 |
where T is the percentage of transmittance, Cf and CS are the concentration of each component in filtrate and stock solution.
Transfer rate
Transfer rate is one of the indexes reflecting the separation effect of components. The concentration of each component is calculated with standard curves based on the operating procedures of HPLC, spectrophotometry and test kit. With the volume before and after separation, the transfer rate (Tr) and total transfer rate (Ttr) are calculated as:
| 3 |
| 4 |
where Tr1 and Ttx are the component transfer rate of step 1 and step x. C1 and V1 are respectively the concentration and volume of each component before separation; C2 and V2 are respectively the concentration and volume of each component after separation.
Results and discussion
Influences of microfiltration and nanofiltration on components
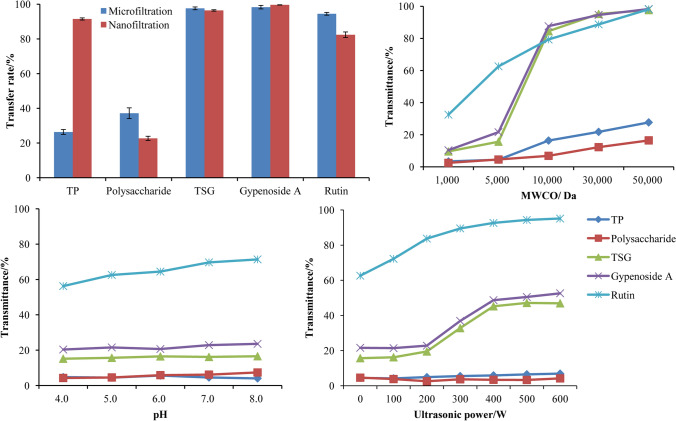
As a kind of commonly used membrane separating pretreatment technology, microfiltration, is widely applies in medicines, food and chemical fields (Oberholster et al., 2013; Silva et al., 2019; Manni et al., 2020). Microfiltration could efficiently remove proteins and polysaccharides from Gynostemma pentaphyllum extract while retaining the TSG, gypenoside A and rutin (Fig. 2A). Based on Donnan effect and membrane aperture sieving principle of nanofiltration (Mancini et al., 2022), the transfer rate of TP was significantly higher than polysaccharides because monosaccharides and disaccharides were easily to penetrate the nanofiltration membrane of 800 Da. The pretreatment of microfiltration and nanofiltration remove TP and polysaccharide while retaining TSG.
Fig. 2.
Transmittance or transfer rate of components. (A) Pretreatment of microfiltration—nanofiltration; (B) MWCO; (C) pH and (D) ultrasonic power
Single factor test
MWCO
MWCO of a membrane was related to the transmittance and separation efficiency of each component. In order to explore the transmittance difference between saponins and flavones, the membranes with the MWCO range of 1000 to 50,000 Da were selected for the single factor test. The transmittance of five indexes in Gynostemma pentaphyllum concentrate showed a significant transition (Fig. 2B). The transmittance of proteins and polysaccharides had no obvious response to membrane MWCO, because proteins and polysaccharides were macromolecules. The ultrafiltration transmittance of rutin showed the slow increasing trend with the increase in pH. However, TSG and rutin showed the difference in transmittance when the membrane MWCO was 1000 to 5000 Da, thus providing the theoretical feasibility for separating flavonoids and saponins from Gynostemma pentaphyllum.
pH
By adjusting pH, the existing state of flavonoids was changed to further enhance the separation difference between flavonoids and saponins. The pH value had no significant effect on the separation behavior of saponins, proteins and polysaccharides (Fig. 2C), but the transmittance of rutin increased gradually with the increase in pH. The pKa of rutin was 6.17. When pH rose from 6.0 to 8.0, rutin mainly existed in the form of ions and the transmittance increased from 60.5 to 71.4%. In addition, the separation difference between flavonoids and saponins increased accordingly with the increase in pH.
Ultrasonic power
Ultrasound was one of the most widely methods used to regulate micelles for the preparation of foods and drugs (Zhou et al., 2021; Krentz et al., 2022). In Fig. 2D, with the increase in ultrasonic power, the transmittance of TSG and gypenoside A firstly increased and then became stable and rutin showed the similar trend of transmittance. The existing state of TSG had been converted from the associative state into the molecular state after ultrasonic treatment, which did not directly affect the state of rutin. However, it was difficult to understand the phenomenon of increased transmittance of rutin increased with the increase in ultrasonic power. Based on the experimental results, it was assumed that rutin was adsorbed or embedded in TSG micelles and gradually released into the solution when the ultrasonic power increased in the range of 200 W to 400 W.
Statistical analysis and model fitting using RSM
A Box-Behnken experiment design of RSM was selected to establish a second order polynomial model of ultrafiltration separation of TSG from Gynostemma pentaphyllum with the ultrasonic assisted membrane separation technique. The effect of single factor on the separation behaviors of components indicated that the transmittance of polysaccharides and proteins were relatively stable when the membrane MWCO was between 1000 and 10,000 Da. The transmittance of gypenoside A was similar to TSG. The response variables of gypenoside A and rutin were explored.
The transmittances of gypenoside A ranged from 12.8 to 93.8% and the transmittance of rutin ranged from 72.6 to 99.8% (Table 1). The fitted model for describing the relationships between various factors and the response variables of gypenoside A and rutin is obtained as:
| 5 |
| 6 |
Table 1.
Response surface design and transmittance (%) results of ultrasonic assisted ultrafiltration
| Run | X1 | X2 | X3 | Gypenoside A | Rutin |
|---|---|---|---|---|---|
| 1 | 0 | 1 | 1 | 28.6 | 99.4 |
| 2 | − 1 | 1 | 0 | 22.4 | 87.3 |
| 3 | 1 | 1 | 0 | 93.8 | 97.1 |
| 4 | − 1 | 0 | − 1 | 16.0 | 72.6 |
| 5 | 0 | 1 | − 1 | 47.9 | 90.7 |
| 6 | 1 | 0 | − 1 | 90.6 | 94.3 |
| 7 | − 1 | − 1 | 0 | 12.8 | 77.2 |
| 8 | 0 | 0 | 0 | 34.2 | 93.8 |
| 9 | 0 | − 1 | 1 | 32.3 | 92.2 |
| 10 | 0 | 0 | 0 | 35.9 | 95.7 |
| 11 | 0 | − 1 | − 1 | 22.7 | 82.3 |
| 12 | 0 | 0 | 0 | 31.6 | 99.8 |
| 13 | 0 | 0 | 0 | 36.3 | 96.9 |
| 14 | − 1 | 0 | 1 | 13.9 | 86.7 |
| 15 | 1 | 0 | 1 | 89.8 | 94.5 |
| 16 | 0 | 0 | 0 | 33.6 | 93.7 |
| 17 | 1 | − 1 | 0 | 88.2 | 93.4 |
X1 MWCO, X2 Ultrasonic power, X3 pH
In the above equations, the values of the coefficients showed that the three factors had different effects on the response. MWCO, ultrasonic power and pH had a synergistic effect on the transmittance of rutin. However, unlike the equation for rutin, the regression equation for gypenoside A had the negative value of pH coefficient, indicating that the factors of MWCO and ultrasonic power had an antagonistic effect on the response.
The results of ANOVA for the two fitted models are shown in Table 2. The analysis results of gypenoside A are provided as follows. The F-value 395.04 with the p < 0.05 confirmed the validity of the model. The p-value of lack of fit was 0.4569, indicating that the fitting errors of Eq. (5) obtained with experimental data were smaller. The multivariate correlation coefficient R2, predicted R2 and adjusted R2 were respectively 0.998 0, 0.984 3, and 0.9955, indicating that the model could be used to predict and analyze the ultrafiltration separation behavior of gypenoside A. In addition, the p-values of X1, X2, X2X3 and X12 were less than 0.05, indicating that X1, X2, X2X3, and X12 had significant effect on the transmittance of gypenoside A.
Table 2.
Analysis of variance and significance of regression model
| Source | df | Gypenoside A | Rutin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of square | Mean square | F-value | p-value | Sum of square | Mean square | F-value | p-value | ||
| Model | 9 | 13,115.07 | 1457.23 | 359.04 | p < 0.05* | 896.43 | 99.60 | 24.82 | p < 0.05* |
| X1 | 1 | 11,048.41 | 11,048.41 | 2995.08 | p < 0.05* | 385.03 | 385.03 | 95.95 | p < 0.05* |
| X2 | 1 | 162.98 | 162.98 | 44.18 | p < 0.05* | 102.21 | 102.21 | 25.47 | p < 0.05* |
| X3 | 1 | 19.15 | 19.15 | 5.19 | 0.056 8 | 122.02 | 122.02 | 30.41 | p < 0.05* |
| X1X2 | 1 | 4.70 | 4.70 | 1.27 | 0.296 1 | 10.50 | 10.50 | 2.62 | 0.149 8 |
| X1X3 | 1 | 0.70 | 0.70 | 0.19 | 0.676 4 | 49.67 | 49.67 | 12.38 | p < 0.05* |
| X2X3 | 1 | 208.80 | 208.80 | 56.60 | p < 0.05* | 0.36 | 0.36 | 0.090 | 0.773 2 |
| X12 | 1 | 1032.58 | 1032.58 | 279.92 | p < 0.05* | 173.95 | 173.95 | 43.35 | p < 0.05* |
| X22 | 1 | 0.083 | 0.083 | 0.022 | 0.885 3 | 10.15 | 10.15 | 2.53 | 0.155 8 |
| X32 | 1 | 10.58 | 10.58 | 2.87 | 0.134 2 | 45.23 | 45.23 | 11.27 | p < 0.05* |
| Residual | 7 | 25.82 | 3.69 | 28.09 | 4.01 | ||||
| Lack of fit | 3 | 11.47 | 3.82 | 1.07 | 0.456 9 | 2.62 | 0.87 | 0.14 | 0.9327 |
| Pure Error | 4 | 14.53 | 3.59 | 25.47 | 6.37 | ||||
| Cor Total | 16 | 13,140.89 | 924.52 | ||||||
*Means significant
The analysis results of rutin are provided as follows. The model of rutin transmittance was significant (p < 0.05). The coefficients of X1, X2, X3, X1X3, X12 and X32 were significant, as indicated by p < 0.05 and the three factors had significant influence on the transmittance of rutin. The influences of three factors showed the following descending order: X1 > X3 > X2. The effects of X2 and X3 on the transmittance of gypenoside A were different from those on the transmittance of rutin, thus providing the basis for further parameter optimization. In addition, the predicted R2 of 0.969 6 was close to the adjusted R2 of 0.9306, indicating that the mathematical-model could be used to predict the transmittance of rutin and optimize the separation parameters for removing rutin from Gynostemma pentaphyllum.
Effects of UAU parameters on the transmittances
In order to analyze the independent variables and their interactive effects on the transmittances of gypenoside A, the contour maps were drawn against two variables while the other variable was kept constant (Fig. 3). The contour map in Fig. 3A displays the influences of MWCO and ultrasonic power on the transmittance of gypenoside A at pH 7.0. Increasing membrane MWCO and ultrasonic power could improve the transmittances of gypenoside A due to the synergistic effect of molecular sieving and cavitation (Arunkumar and Singh, 2021; Guo et al., 2021). Figure 3B shows the effect of MWCO and pH at a constant ultrasonic power of 300 W. The transmittance of gypenoside A was nearly unchanged when ultrasonic power was fixed, indicating that the interaction between MWCO and pH was insignificant. The influence of the interaction between pH and ultrasonic power on the transmittance of gypenoside A (Fig. 3C) showed that the higher ultrasonic power could achieve a higher transmittance of gypenoside A (94.3%) at pH 7.0. However, the negative coefficient of X1X3 showed that the transmittance of gypenoside A reduced with the increased in ultrasonic power and pH.
Fig. 3.
Interaction between variables on component transmittance. (A) X1X2 of gypenoside A; (B) X1X3 of gypenoside A; (C) X2X3 of gypenoside A; (D) X1X2 of rutin; (E) X1X3 of rutin and (F) X2X3 of rutin
The three contour maps of rutin transmittance had the similar tendency (Fig. 3D–F) and MWCO, ultrasonic power and pH were positively correlated with the transmittance. Under the fixed conditions of MWCO and pH, the transmittance of rutin increased with the increase in ultrasonic power, indicating that partial rutin were desorbed or released from micelles (Tai et al., 2020). Therefore, the removal efficiency of rutin from TSG can be improved by optimizing UAU parameters.
Optimization of UAU parameters
The TSG of Gynostemma pentaphyllum was separated by UAU in two steps. The first step was to remove proteins and polysaccharides from Gynostemma pentaphyllum. After the target transmittances of gypenoside A and rutin were respectively set as 90.0% and 95.0%, the UAU parameters were optimized in Design-Expert 8.06 as follows: MWCO (10,000 Da), ultrasonic power (400 W), and pH 7.8. Then, the second step was to remove flavonoids from TSG. After the target transmittances of gypenoside A and rutin were respectively set as 15.0% and 95.0%, the UAU parameters were optimized in Design-Expert 8.06 as follows: MWCO (1000 Da), ultrasonic power (300 W), and pH 7.9.
In order to verify the optimized parameters, the extracts of Gynostemma pentaphyllum were pretreated by microfiltration (0.1 μm) coupled with nanofiltration (800 Da). The results were shown in Table 3, the experimental transmittances of gypenoside A and rutin in first step were respectively 97.1% and 93.6%, which were close to the target transmittances. In second step, the experimental transmittances of gypenoside A and rutin in first step were respectively 6.6% and 92.1%, which were better than the target values.
Table 3.
Component transfer rate (%) by the coupling technology of membrane and ultrasound (‾x ± s, n = 3)
| Samples | TP | Polysaccharides | TSG | Gypenoside A | Rutin |
|---|---|---|---|---|---|
| Microfiltration | 27.3 ± 1.6 | 35.5 ± 2.3 | 98.2 ± 0.8 | 97.4 ± 1.1 | 95.7 ± 0.9 |
| Nanofiltration concentrate | 91.5 ± 0.4 | 22.7 ± 1.2 | 96.4 ± 0.4 | 99.6 ± 0.1 | 82.3 ± 1.6 |
| Ultrafiltrate of 10,000 Da | 5.2 ± 0.8 | 4.8 ± 0.5 | 97.1 ± 0.8 | 95.5 ± 0.7 | 93.6 ± 2.1 |
| Rejection of 1000 Da | 87.3 ± 1.7 | 32.6 ± 1.0 | 93.4 ± 1.3 | 97.2 ± 1.5 | 7.9 ± 0.3 |
| Total transfer rate | 1.1 | 0.1 | 85.9 | 90.1 | 5.8 |
The coupling technology of membrane and ultrasound was used to develop the purification mode of saponins from Gynostemma pentaphyllum based on the differences in physical and chemical properties of various components. The total transfer rate of TSG was 85.9%, and the transfer rates of other components were less than 6% (Table 3). In addition, after 5-h separation, the solid weight of Gynostemma pentaphyllum extracts decreased from 102.18 to 22.91 g and the content of TSG in Gynostemma pentaphyllum extracts increased from 17.40 to 82.81%, which was significantly higher than the result obtained with resin adsorption. With resin adsorption method, the TSG content reached 75.0% after 48-h separation. The higher TSG content in the study indicated that TSG could be efficiently separated from Gynostemma pentaphyllum with the ultrasonically assisted membrane method.
Adsorption or encapsulation characteristics of gypenoside A and rutin
The transmittance of rutin in single component solution was significantly higher than that in the mixed solution of gypenoside A and rutin with or without ultrasonic treatment. The transmittance of gypenoside A in single component solution and the mixed solution of gypenoside A and rutin was more stable. In the mixed solution, rutin was adsorbed or encapsulated in the micelles of gypenoside A and then was dissolved into solution without destroying micelles obviously (Li et al., 2021).
Protective effects on H2O2 induced cell injury
With vitamin C as the standard of protective compound, the protective effects of gypenoside A, Gynostemma pentaphyllum and TSG were analyzed by SH-SY5Y cells. The dosages of Gynostemma pentaphyllum and TSG were calculated with the content of gypenoside A. As shown in Fig. 4, the survival rate in H2O2 was 60.22%, and the recovered cell viability obtained with 50 μM gypenoside A and 50 μM TSG exceeded 73% (positive control of vitamin C, 68.77%), which was higher than that obtained with 50 μM Gynostemma pentaphyllum extracts. The protective effects of TSG against H2O2-induced SH-SY5Y cell hurt were dependent on dosage. The results indicated that the ultrasonic assisted membrane method could remove impurities while retaining active ingredients of saponin.
Fig. 4.
Protective effects of TSG on H2O2-induced damage of SH-SY5Y cells. Data are shown as mean ± SD (n = 3). #p < 0.05 and ##p < 0.01 as compared with blank; *p < 0.05 and **p < 0.01 as compared with H2O2
In this study, a feasible and efficient ultrasonic assisted membrane method was established to separate TSG from Gynostemma pentaphyllum. Saponins’ surface activity could decrease molecular surface energy and improve solute solubility under the critical micelle concentration. Due to the poor water solubility, flavones were easily distributed in Gynostemma pentaphyllum saponins micelles by adsorption and encapsulation. By optimizing the separation conditions with RSM, a three-step separation strategy was developed to purify total saponin from Gynostemma pentaphyllum. Firstly, the extracts of Gynostemma pentaphyllum were pretreated by 0.1 μm membrane coupled with 800 Da nanofiltration membrane. Secondly, proteins and polysaccharides were removed under the conditions of MWCO 10,000 Da, ultrasonic power 400 W and pH 7.8. Thirdly, separation flavonoids from saponin by MWCO 1000 Da, ultrasonic power 300 W and pH 7.9. The TSG content in Gynostemma pentaphyllum extract was increased by more than four times with the ultrasonic assisted membrane technology. The protective effect of TSG on SH-SY5Y cells injury induced by H2O2 was better than that of Gynostemma pentaphyllum extracts. Saponins were usually separated by macroporous resin and preparative chromatography, which was limited by long separation period, heat treatment and environmental pollution. Membrane separation technologies are mostly applied in pretreatment or sterilization filtration, but it is difficult to separate ingredients from a complex solution system with membrane separation technologies. According to the physical and chemical properties of ingredients, the separation behavior difference was enhanced by adjusting the existence states of components through changing external force field and pH.
The adsorption behavior of TSG was not apparent in the hydrophilic material membrane module and the membrane fouling and flux decline was still unavoidable in the separation process of microfiltration. The trans-membrane pressure difference was controlled within the range of 0.05 to 0.10 MPa so as to reduce concentration polarization and then washed with 0.1% sodium hydroxide solution under ultrasound treatment, which could promote membrane flux recovery of more than 95%. It was suggested that gel layer formed by polysaccharides, proteins and flavones on the membrane surface was the main factor of membrane pollution (Doan and Lai, 2021).
Acknowledgements
The authors wish to express their sincere gratitude to the National Natural Science Foundation of China (Grant Nos. 82274106 and 82074006), Natural Science Foundation of Jiangsu Province (Grant No. BK20211303) and 333 High-level Talents Training Project of Jiangsu Province (Grant No. 2022-3-16-449).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cunyu Li, Email: licunyuok@163.com.
Yun Ma, Email: myunok@163.com.
Xinglei Zhi, Email: 275682520@qq.com.
Guoping Peng, Email: 300632@njucm.edu.cn.
References
- Abdellah MH, Scholes CA, Liu L, Kentish SE. Efficient degumming of crude canola oil using ultrafiltration membranes and bio-derived solvents. Innovative Food Science & Emerging Technologies. 2020;59:102274. doi: 10.1016/j.ifset.2019.102274. [DOI] [Google Scholar]
- Arsuaga JM, López-Muñoz MJ, Sotto A, Rosario G. Retention of phenols and carboxylic acids by nanofiltration/reverse osmosis membranes: sieving and membrane-solute interaction effects. Desalination. 2006;200:731–733. doi: 10.1016/j.desal.2006.03.502. [DOI] [Google Scholar]
- Arunkumar A, Singh N. Ultrafiltration behavior of recombinant adeno associated viral vectors used in gene therapy. Journal of Membrane Science. 2021;620:118812. doi: 10.1016/j.memsci.2020.118812. [DOI] [Google Scholar]
- Bari S, Chatterjee A, Mishra S. Ultrasonication assisted and surfactant mediated synergistic approach for synthesis of calcium sulfate nano-dendrites. Ultrasonics Sonochemistry. 2016;31:39–50. doi: 10.1016/j.ultsonch.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Cao DQ, Song X, Hao XD, Yang WY, Iritani E, Katagiri N. Ca2+-aided separation of polysaccharides and proteins by microfiltration: Implications for sludge processing. Separation and Purification Technology. 2018;202:318–325. doi: 10.1016/j.seppur.2018.03.070. [DOI] [Google Scholar]
- Doan NTT, Lai QD. Ultrafiltration for recovery of rice protein: Fouling analysis and technical assessment. Innovative Food Science & Emerging Technologies. 2021;70:102692. doi: 10.1016/j.ifset.2021.102692. [DOI] [Google Scholar]
- Guo C, Liu J, Li XH, Yang SQ. Effect of cavitation bubble on the dispersion of magnetorheological polishing fluid under ultrasonic preparation. Ultrasonics Sonochemistry. 2021;79:105782. doi: 10.1016/j.ultsonch.2021.105782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemar Y, Xu C, Wu S, Ashokkumar M. Size reduction of “reformed casein micelles” by high-power ultrasound and high hydrostatic pressure. Ultrasonics Sonochemistry. 2020;63:104929. doi: 10.1016/j.ultsonch.2019.104929. [DOI] [PubMed] [Google Scholar]
- Huang YP, Wang YS, Liu BW, Song Z, Liang XS, Teng Y, Zhang J, Yin ZQ, Pan K. Dammarane-type saponins with proprotein convertase subtilisin/kexin type 9 inhibitory activity from Gynostemma pentaphyllum. Phytochemistry. 2022;194:113005. doi: 10.1016/j.phytochem.2021.113005. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sundarrajan S, Rana D, Matsuura T, Ramakrishna S. Influence of electrospun fiber size on the separation efficiency of thin film nanofiltration composite membrane. Journal of Membrane Science. 2012;392–393:101–111. doi: 10.1016/j.memsci.2011.12.005. [DOI] [Google Scholar]
- Krentz A, García-Cano I, Ortega-Anaya J, Jiménez-Flores R. Use of casein micelles to improve the solubility of hydrophobic pea proteins in aqueous solutions via low-temperature homogenization. Journal of Dairy Science. 2022;105:22–31. doi: 10.3168/jds.2021-20902. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhao CG, Ma Y, Chen W, Zheng YF, Zhi XL, Peng GP. Optimization of ultrasonic-assisted ultrafiltration process for removing bacterial endotoxin from diammonium glycyrrhizinate using response surface methodology. Ultrasonics Sonochemistry. 2020;68:105215. doi: 10.1016/j.ultsonch.2020.105215. [DOI] [PubMed] [Google Scholar]
- Li CY, Ma Y, Ma L, Zhi XL, Peng GP. Improving the clarity and sensitization of polysorbate 80 by ultrasonic-assisted ultrafiltration technology. European Journal of Pharmaceutical Sciences. 2021;159:105719. doi: 10.1016/j.ejps.2021.105719. [DOI] [PubMed] [Google Scholar]
- Liu H, Li XM, Duan Y, Xie JB, Piao XL. Mechanism of gypenosides of Gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. Journal of Ethnopharmacology. 2021;271:113907. doi: 10.1016/j.jep.2021.113907. [DOI] [PubMed] [Google Scholar]
- Luo JQ, Zeuner B, Morthensen ST, Meyer AS, Pinelo M. Separation of phenolic acids from monosaccharides by low-pressure nanofiltration integrated with laccase pre-treatments. Journal of Membrane Science. 2015;482:83–91. doi: 10.1016/j.memsci.2015.02.022. [DOI] [Google Scholar]
- Mancini E, Ramin P, Styrbæck P, Bjergholt C, Mansouri SS, Gernaey KV, Luo JQ, Pinelo M. Separation of succinic acid from fermentation broth: Dielectric exclusion, Donnan effect and diffusion as the most influential mass transfer mechanisms. Separation and Purification Technology. 2022;281:119904. doi: 10.1016/j.seppur.2021.119904. [DOI] [Google Scholar]
- Manni A, Achiou B, Karim A, Harrati A, Sadik C, Ouammou M, Alami YS, Bouari AE. New low-cost ceramic microfiltration membrane made from natural magnesite for industrial wastewater treatment. Journal of Environmental Chemical Engineering. 2020;8:103906. doi: 10.1016/j.jece.2020.103906. [DOI] [Google Scholar]
- Medina MB, Resnik SL, Munitz MS. Optimization of a rice cooking method using response surface methodology with desirability function approach to minimize pesticide concentration. Food Chemistry. 2021;352:129364. doi: 10.1016/j.foodchem.2021.129364. [DOI] [PubMed] [Google Scholar]
- Oberholster A, Carstens LM, Toit WJ. Investigation of the effect of gelatine, egg albumin and cross-flow microfiltration on the phenolic composition of Pinotage wine. Food Chemistry. 2013;138:1275–1281. doi: 10.1016/j.foodchem.2012.09.128. [DOI] [PubMed] [Google Scholar]
- Pinto D, Vieira EF, Peixoto AF, Freire C, Freitas V, Costa P, Delerue-Matos C, Rodrigues F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chemistry. 2021;334:127521. doi: 10.1016/j.foodchem.2020.127521. [DOI] [PubMed] [Google Scholar]
- Shi GH, Wang XD, Zhang H, Zhang XS, Zhao YQ. New dammarane-type triterpene saponins from Gynostemma pentaphyllum and their anti-hepatic fibrosis activities in vitro. Journal of Functional Foods. 2018;45:10–14. doi: 10.1016/j.jff.2018.03.016. [DOI] [Google Scholar]
- Silva Fabiana Luisa, Zin Guilherme, Rezzadori Katia, Longo Luana Cristina, Tiggemann Lídia, Soares Lenilton Santos, Cunha Petrus José Carlos, Vladimir de Oliveira José, Di Luccio Marco. Changes in the physico-chemical characteristics of a protein solution in the presence of magnetic field and the consequences on the ultrafiltration performance. Journal of Food Engineering. 2019;242:84–93. doi: 10.1016/j.jfoodeng.2018.08.016. [DOI] [Google Scholar]
- Susanto H, Arafat H, Janssen EML, Ulbricht M. Ultrafiltration of polysaccharide–protein mixtures: Elucidation of fouling mechanisms and fouling control by membrane surface modification. Separation and Purification Technology. 2008;63:558–565. doi: 10.1016/j.seppur.2008.06.017. [DOI] [Google Scholar]
- Tai ZG, Huang YP, Zhu QG, Wu W, Yi T, Chen ZJ, Lu Y. Utility of Pickering emulsions in improved oral drug delivery. Drug Discovery Today. 2020;25:2038–2045. doi: 10.1016/j.drudis.2020.09.012. [DOI] [PubMed] [Google Scholar]
- Tsai YC, Lin CL, Chen BH. Preparative chromatography of flavonoids and saponins in Gynostemma pentaphyllum and their antiproliferation effect on hepatoma cell. Phytomedicine. 2010;18:2–10. doi: 10.1016/j.phymed.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Wan JB, Zhang QW, Ye WC, Wang YT. Quantification and separation of protopanaxatriol and protopanaxadiol type saponins from Panax notoginseng with macroporous resins. Separation and Purification Technology. 2008;60:198–205. doi: 10.1016/j.seppur.2007.08.007. [DOI] [Google Scholar]
- Wang ZC, Wang Z, Huang WH, Suo JM, Chen XT, Ding KQ, Sun Q, Zhang HR. Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. International Journal of Biological Macromolecules. 2020;145:484–491. doi: 10.1016/j.ijbiomac.2019.12.213. [DOI] [PubMed] [Google Scholar]
- Xiao LL, Zheng HW, Yong XZ, Su JL, Ding WZ, Sheng XX, Zhen HP. Isolation and antitumor activities of acidic polysaccharide from Gynostemma pentaphyllum Makino. Carbohydrate Polymers. 2012;89:942–947. doi: 10.1016/j.carbpol.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Yang F, Shi HM, Zhang XW, Yang HH, Zhou Q, Yu LL. Two new saponins from tetraploid jiaogulan (Gynostemma pentaphyllum), and their anti-inflammatory and α-glucosidase inhibitory activities. Food Chemistry. 2013;141:3606–3613. doi: 10.1016/j.foodchem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Zhai XF, Zu ML, Wang YR, Cui WY, Duan Y, Yang C, Piao XL. Protective effects of four new saponins from Gynostemma pentaphyllum against hydrogen peroxide-induced neurotoxicity in SH-SY5Y cells. Bioorganic Chemistry. 2021;106:104470. doi: 10.1016/j.bioorg.2020.104470. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhu LN, Zhao SY, Wang DH, Guo ZG. A robust and repairable copper-based superhydrophobic microfiltration membrane for high-efficiency water-in-oil emulsion separation. Separation and Purification Technology. 2021;256:117751. doi: 10.1016/j.seppur.2020.117751. [DOI] [Google Scholar]
- Zhou L, Li AY, Wang HF, Sun WQ, Zuo SJ, Li CH. Preparation and characterization of luteolin-loaded MPEG-PCL-g-PEI micelles for oral Candida albicans infection. Journal of Drug Delivery Science and Technology. 2021;63:102454. doi: 10.1016/j.jddst.2021.102454. [DOI] [Google Scholar]
- Zhu TT, Zhou Z, Qu FS, Liu B, Bruggen BV. Separation performance of ultrafiltration during the treatment of algae-laden water in the presence of an anionic surfactant. Separation and Purification Technology. 2022;281:119894. doi: 10.1016/j.seppur.2021.119894. [DOI] [Google Scholar]