Abstract
Left ventricular assist device (LVAD) use has revolutionized the care of patients with advanced heart failure, allowing for more patients to survive until heart transplant and providing improved quality for patients unable to undergo transplantation. Despite these benefits, LVADs are associated with neurological complications despite an improvement in device technology and better clinical care and experience.
This review provides information on the incidence, risk factors, and management of neurological complications among LVAD patients. While scant guidelines exist for the evaluation and management of neurological complications in LVAD patients, a high index of suspicion can prompt early detection of neurological complications which may improve overall neurological outcomes. A better understanding of the implications of continuous circulatory flow on systemic and cerebral vasculature is necessary to reduce the common occurrence of neurological complications in this population.
Summary
This review provides information on the incidence, risk factors, and management of neurological complications among LVAD patients. We present different stroke risks between the contemporary continuous flow LVADs. A high index of suspicion can prompt early detection of neurological complications, which may improve overall neurological outcomes. A better understanding of the implications of continuous circulatory flow on systemic and cerebral vasculature is necessary to reduce the common occurrence of neurological complications in this population.
Introduction
Left ventricular assist device (LVAD) implantation is considered for patients with advanced heart failure refractory to medical therapy and may be implanted as a bridge to cardiac transplantation, a temporary bridge to recovery for cardiogenic shock, or a destination therapy for patients for whom cardiac transplantation is not feasible. As the number of patients with advanced heart failure has increased despite a finite number of available donor hearts for transplantation, LVAD implantations have similarly increased over the past decade and hence, more patients are supported with LVADs as a chronic therapy for a prolonged device support time.1 Furthermore, LVAD implantation is associated with improved quality of life and long-term neurocognitive outcomes with new LVADs having improved outcomes with less functional debility2, shifting the paradigm for care of patients with advanced heart failure.3,4
Prior to 2015, neurological complications including strokes were the leading cause of death for patients on LVAD support.1 While this has improved in the modern era, neurological complications still remain the second most common cause of death.1 Although the majority of neurological complications in this population are ischemic stroke (IS) and hemorrhagic stroke (HS), other complications involving the nervous system may arise due to unique LVAD-related alterations in cerebral blood flow, autoregulation, and perfusion with their non-physiological non-pulsatile continuous blood flow. Herein, we provide a review on the incidence, risk factors, and management of neurological complications in LVAD patients with a focus on the pathophysiology of LVAD-associated neurologic complications
LVAD Specific Stroke Pathophysiology:
Endothelial Dysfunction and Arterial Stiffness
In healthy individuals, the arterial vasculature experiences cyclical shear stress related to systole and diastole, which is believed to be a contributor to the expression of endothelial nitric oxide synthase and normal peripheral vascular functioning.5 Cardiac output among patients with CF-LVAD is not sufficient to provide this mechanical stress, resulting in impairments in endothelial function. Flow-mediated dilation (a measure of peripheral vascular reactivity) was significantly higher among patients with pulsatile LVAD compared with CF-LVAD.6 Additionally, implantation of CF-LVAD resulted in reduction of endothelial nitric oxide availability compared with healthy controls and pulsatile LVAD patients, underlying the importance of pulsatility for perfusion of the peripheral vasculature.7 Impaired endothelial function can result in increased arterial stiffness, which is independently associated with a higher risk of stroke8 Low pulsatility in CF-LVAD patients has been associated with increases in aortic wall thickening and stiffness with reduced aortic compliance compared with healthy patients and pulsatile LVAD patients9, and increased aortic stiffness was associated with higher rates of stroke among patients with CF-LVAD compared to patients without increased aortic stiffness.10
Impaired Cerebral Autoregulation
Cerebral autoregulation allows for the maintenance of an adequate supply of cerebral blood flow despite changes in cerebral perfusion pressure. Impaired cerebral autoregulation may result in either IS or HS via cerebral hypo- or hyperperfusion respectively. Impaired cerebral autoregulation has been previously demonstrated in non-pulsatile cardiopulmonary bypass and is associated with perioperative ischemic stroke.11,12 Whether CF-LVAD results in measurable impairment in cerebral autoregulation is unclear and data are limited. Patients undergoing CF-LVAD implantation had preserved cerebral autoregulation (as measured by transcranial Doppler and near-infrared spectroscopy) in the immediate postoperative period compared with matched patients who underwent coronary artery bypass grafting.13 In another cohort, patients with CF-LVAD (with median duration of LVAD support of 3 months) had preserved cerebral autoregulation in response to a sit-stand maneuver compared to healthy controls and patients with a pulsatile LVAD, and cerebral autoregulation was preserved across a range of LVAD pump speeds.14 However, both of these studies were limited due to investigating the perioperative and short-term periods, while changes in cerebral autoregulation may take longer to manifest. Both HMII and HM3 patients had impaired cerebrovascular metabolic reactivity as measured by increases in mean flow velocities in the cerebral vasculature in response to breath-holding challenge compared to healthy control patients or patients with heart failure.15 The median LVAD duration at time of testing in this study was 1762 days in HMII patients and 283 days in HM3 patients, suggesting that alterations in cerebral hemodynamics may be a later occurring phenomenon. Additionally, CF-LVAD patients demonstrate impaired cerebral blood flow and intracranial vasodilation during exercise compared with age-matched healthy controls and patients with heart failure16, although this effect was ameliorated by a cardiac rehabilitation exercise program.17 Impaired cerebral autoregulation may contribute the high incidence of neurological complications and functional debility among patients with CF-LVAD.
Dysautonomia
Autonomic dysfunction is common in patients with advanced heart failure and is characterized by increased activation of the sympathetic nervous system and decreased parasympathetic nervous system activity18. Blood pressure variability was more common among LVAD patients than matched advanced heart failure patients or controls19, and patients with CF-LVAD had increased muscle sympathetic nerve activity in response to tilt table testing compared to healthy controls and pulsatile LVAD patients.20 Fluctuations in heart rate and blood pressure may contribute to the increased risk of both IS and HS among LVAD patients especially in the setting of impaired cerebral autoregulation. Several mechanisms may underlie the autonomic dysfunction identified in some LVAD patients. It has been hypothesized that the lack of pulsatility and flow oscillations in CF-VAD result in offloading of carotid baroreceptors with resultant abnormal sympathetic tone and in one series, 12 patients who completed autonomic testing before and after implantation of a CF-LVAD were found to have impaired cardiac baroreceptor sensitivity.21
Ischemic Stroke
Epidemiology
For the past several decades, LVAD technology has significantly improved from the original first-generation pulsatile pumps to smaller (“miniaturization”) and more durable second-generation continuous-flow devices (such as HeartMate II [HMII])22, to the current third-generation centrifugal pumps (such as HeartWare [HVAD] and HeartMate 3[HM3]).23 The newer generation devices are not only associated with higher overall survival rates but also a lower incidence of stroke, particularly for HM3.24 However, stroke remains a major complication even in newer LVADs.1 An Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) registry study of 9,489 patients from 2014–2017 showed that 16% patients suffered one or more stroke, with a nearly equal frequency of IS and HS (with the caveat that HM3 was underrepresented in this sample).25
The incidence of IS varies based on device type. Incidence of stroke among first generation LVAD ranged from 20% to as high as 55%.26 Among the newer generation continuous flow (CF)-LVADs, the incidence of stroke was 6.3% or 0.294 event per patient year (EPPY) during the early postoperative period (within 90 days) and 4.1% or 0.094 EPPY after 90 days from LVAD implantation according to the INTERMACS report in 2020.1 Much of the comparative data between devices on the incidence of IS are derived from two large randomized controlled trials: ENDURANCE27 (comparing HMII and HVAD) and MOMENTUM 32 (comparing HMII and HM3). HMII had a lower incidence of IS than HVAD in ENDURANCE (8% [0.06 EPPY] vs 18% [0.17 EPPY], p=0.007) but a higher incidence of IS than HM3 in MOMENTUM 3 (13% [0.11 EPPY] vs 6% [0.05 EPPY]). In the absence of head-to-head clinical trial comparing the two contemporary 3rd generation CF-LVADs (HVAD and HM3), a propensity matching analysis by Cho et al. of 6205 LVAD patients in the INTERMACS registry demonstrated that HM3 carried a much lower risk of IS (3.4% vs. 7.7%, p<0.001, hazard ratio [HR] 5.37) as well as HS (2.0% vs 7.2%, p<0.001, HR 6.79) at 12-months (Figure 1).28 In June of 2021, the U.S. Food and Drug Administration and Medtronic simultaneously advised physicians to stop new HVAD implantations due to the increased risk of both IS and HS compared to HM3, and HVAD was removed from the market29. Despite these differences, prophylactic replacement of HVAD is not recommended due to risks associated with explantation and repeat implantation of alternate device; as such, many patients continue to live with an HVAD necessitating physicians being aware of the increased incidence of IS and HS associated with this device.
Figure 1.
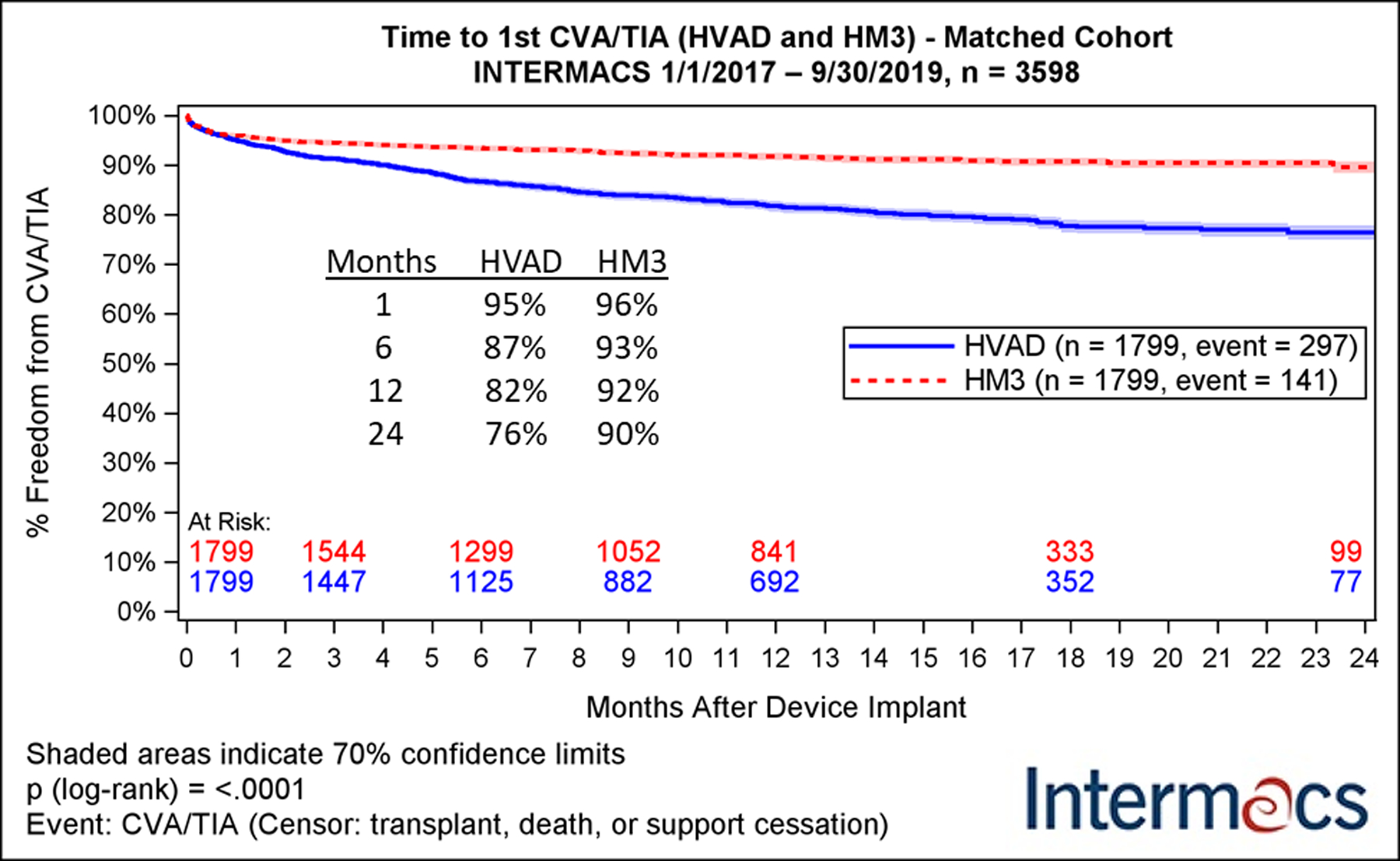
Kaplan-Meier analysis of freedom from major neurologic adverse events in a propensity-matched cohort of patients receiving HeartWare (HVAD, blue) or Heartmate III (HM3, red) devices. CVA: cerebrovascular accident; HM3: Abbott HeartMate3; HVAD: Medtronic HeartWare HVAD; INTERMACS: Interagency Registry for Mechanically Assisted Circulatory Support; TIA: transient ischemic attack.
Adapted with permission from “Cerebrovascular Events in Patients with Centrifugal-Flow Left Ventricular Assist Devices: Propensity Score–Matched Analysis From the Intermacs Registry.” Cho et al., Circulation, 2021.
The risk of IS follows a bimodal distribution with the greatest risk occurring within the first 3 months of implantation (with an incidence of 4%, 0.03 EPPY) and a subsequent peak at 9–12 months (incidence 6%, 0.04 EPPY).30 One-third of IS occurs perioperatively with median time to stroke of 5 days and may represent complications of cardiac surgery more so than device-associated stroke . Once a patient suffered an IS, there was a higher chance of a subsequent IS, and 13% suffered recurrent IS following their first stroke.25 This is in contrast with patients who suffered HS where no similar increased risk of subsequent HS was identified.25 The majority of IS in LVAD patients were cortical and multi-focal, and about one-third of strokes were due to a large vessel occlusion.31,32
Risk factors
Several risk factors for IS have been identified in LVAD patients including traditional IS risk factors such as age33 and hypertension32,34. LVAD-specific risk factors include pump thrombosis, LVAD infection, and inadequate anticoagulation therapy (Figure 2). Conflicting data exist on atrial fibrillation35–37 and diabetes38,39 as risk factors. History of stroke prior to LVAD implantation was not a risk factor for IS occurrence after LVAD implantation;40 however, there may be a selection bias as patients with a moderate to large stroke may be excluded from LVAD candidacy. Early IS (during the index admission for LVAD implantation) and late IS (after discharge from index admission) appear to have different risk factors. Subtherapeutic anticoagulation, hypertension, and pre-operative left ventricular thrombus were found to be risk factors associated with early IS.41 Optimal management to reduce risk of stroke in of patients with pre-operative cardiac thrombus is uncertain and an area for future study, although a case report of two patients who underwent concomitant cardiac thrombectomy during LVAD implantation reported uneventful postsurgical recovery without post-operative stroke.42 Late IS is attributed to traditional stroke risk factors (including hypertension, age, prior stroke, and diabetes), as well as ineffective anticoagulation coupled with enhanced thrombogenesis and inflammation related to LVAD.40,41
Figure 2.
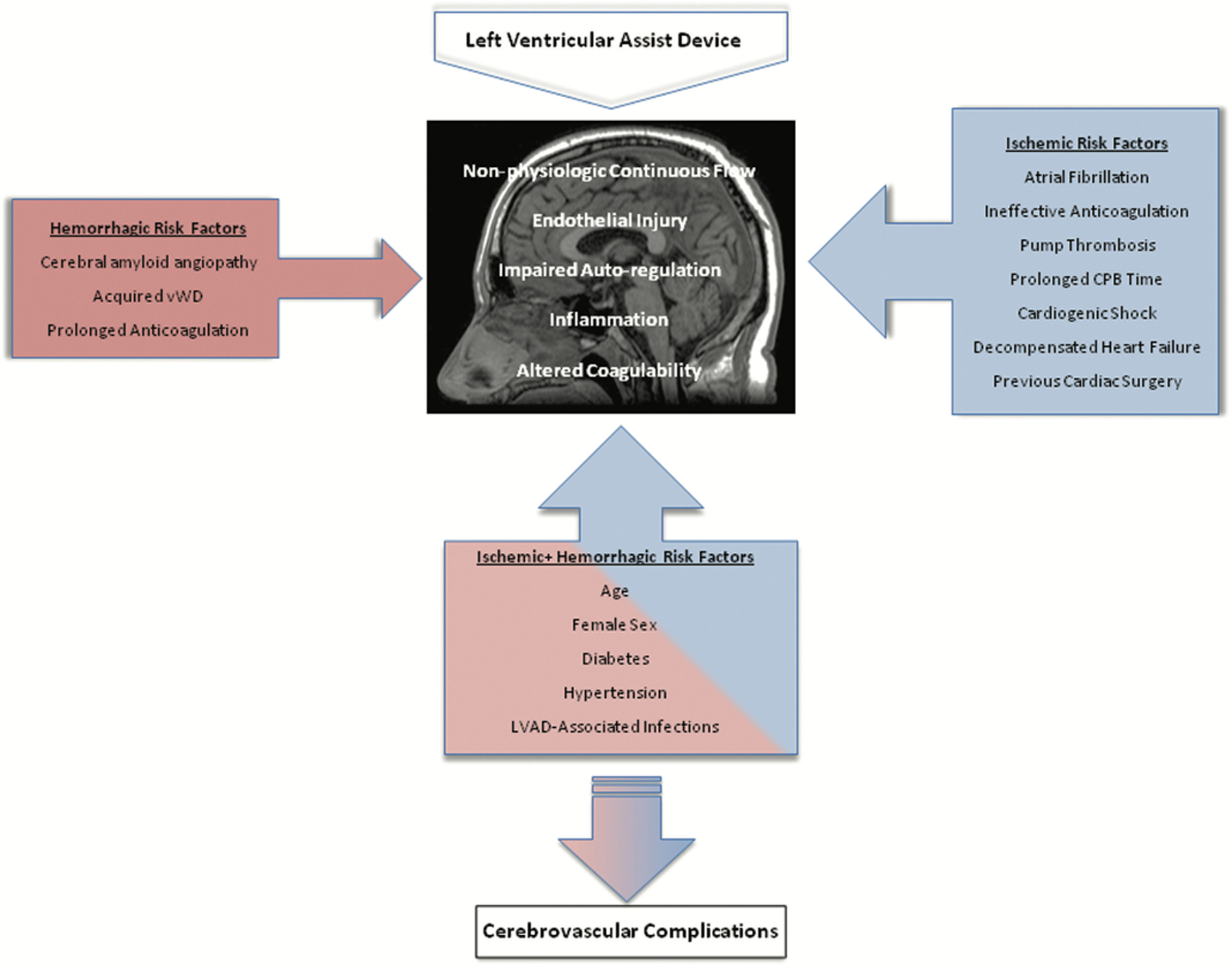
Risk factors for the development of LVAD-associated ischemic and hemorrhagic stroke. CPB: cardiopulmonary bypass, vWD: von Willebrand disease.
Adapted with permission from “A Comprehensive Review of Risk Factor, Mechanism, and Management of Left Ventricular Assist Device-Associated Stroke”. Cho et al., Seminars in Neurology, 2021.
Hypertension is the number one risk factor of IS in the general population, and this effect is exaggerated among patients with LVAD. LVAD patients not on antihypertensive medications are at a higher risk of stroke.43 Both mean arterial pressure (MAP) >85 mmHg and systolic blood pressure (SBP) >100 mmHg were found to be a risk factor for both IS and HS in LVAD patients, and every 5mmHg rise in SBP was found to increase the stroke risk by 19% at mean follow-up of 16 months.34,37,41 In the ENDURANCE trial, patients with HVAD had significant higher risk of stroke (29.7% vs 12.1%) compared to HMII group, which was thought to be due to poorly controlled hypertension in HVAD cohort. In the subsequent ENDURANCE supplemental trial, HVAD patients had a 24% reduction in IS with implementation of a MAP target of <85 mmHg.44
Infection affects 39% of the LVAD patients and is one of the most common complications and a leading cause of death after LVAD implantation.1 The highest risk of IS was found to be within the first 6 weeks after an LVAD-associated infection.45 In a single centre retrospective observational study with 402 patients, the most common types of infection after CF-LVAD were bacteremia (27%), driveline (17%), followed by surgical wound (8%), and pump pocket infection (6%).45 They reported that all types of LVAD-associated infection increased the risk of early IS, but only pump pocket infection increased the risk of late IS.45 Possible mechanisms for acute infection causing stroke in LVAD may include bloodstream infection with endocarditis, cerebral septic emboli, mycotic aneurysm, or cerebral vasculitis,46–49 and the non-pulsatile blood flow resulting in endothelial damage, which predisposes LVAD patients to fungal adhesion and fungemia.50
Another important risk factor for IS in LVAD is pump thrombosis which may occur due to embolus formation secondary to aberrant flow conditions, inadequate antithrombotic regimen, and systemic hypercoagulability. Although the rate of pump thrombosis has decreased significantly with the improved device technology,51 2–13% of the LVAD patients still suffered from pump thrombosis.1 Pump thrombosis is recognized as an important risk factor for LVAD-associated IS..46,52 In a retrospective single centre study investigating the etiology of 61 patients with LVAD-associated IS, about one third of all IS was secondary to pump thrombosis, and close to half of those IS patients had subtherapeutic International Normalized Ratio (INR) values at the time of acute IS.46,52 However, this risk has been significantly reduced with increased utilization of HM3 which is associated with a five year pump thrombosis risk of only 2% compared to 18% with HMII.53
Prevention
Antithrombotic therapy
Differing opinions exist about the optimal strategy of initiation of anticoagulation after LVAD implantation. While heparin bridging to warfarin reduced pump thrombosis in HMII patients, similar benefit was not demonstrated in HM3 trials which may be due in part to the significantly low pump thrombosis rate in HM3 patients. A retrospective study of 418 patients undergoing HMII implantation suggested initiation of oral warfarin and aspirin in the immediate post-operative period (compared with intravenous heparin bridging oral aspirin and warfarin) to be safe with a lower rate of bleeding complications (18% vs 32%, p=0.04) without a rise in perioperative IS (3% vs 5%) or pump thrombosis (2% vs 3%).54 Although some variability exists in the antithrombotic regimen for LVAD patients across different institutions and types of LVAD, single or dual antiplatelet agents plus vitamin K antagonist is the current mainstay therapy. The 2013 International Society for Heart and Lung Transplantation guidelines (ISHLT) recommends long-term treatment with aspirin 81–325 mg daily and warfarin with an INR goal based on device manufacturer instructions.55 Because of the lower prevalence of pump thrombosis and ischemic stroke in HM3, the feasibility of removal of aspirin from the antithrombotic regimen is currently being evaluated in the Antiplatelet Removal and Hemocompatibility Events with the HM3 pump (ARIES HM3) study, a randomized controlled study of warfarin and aspirin versus warfarin and placebo. The study has recently completed enrollment with 628 patients.56
A specific INR target has been a source of debate over the past decade given the need to balance the risk of both IS and HS. In a post-hoc analysis of the ENDURANCE trial, both supratherapeutic (>3.0) and subtherapeutic (<2.0) INR values were associated with increased risk of both HS and IS, which may reflect the difficulty of maintaining INR in therapeutic target range with day-to-day fluctuations.41 Given these findings and the paucity of high quality evidence, it appears reasonable to target an INR of 2.0–3.0 in order to prevent IS while reducing the risk of HS while keeping in mind the role that individual patients risk factors may play in balancing ischemic and hemorrhagic complications.39 With significant debate and problems with finding an adequate INR range to balance thrombotic and bleeding events, direct oral anticoagulants (DOACs) have been suggested as an alternative to warfarin. However, studies are currently lacking with direct comparison between warfarin and DOAC except dabigatran. A recent randomized control trial assessed dabigatran vs coumadin was terminated early due to an increased rate of thromboembolic events in patient treated with dabigatran. The main challenge, however, is that near half of LVADs patients were found to have inadequate or ineffective antithrombotic therapy at the time of their IS, and more than half of the patients were off anticoagulation therapy due to recent hemorrhagic complications.31,46
Phosphodiesterase Type 5 Inhibitors
Phosphodiesterase type 5 inhibitors (PDEis), frequently used in LVAD patients for treatment of pulmonary hypertension and right ventricular unloading, have been associated with reduced IS risk. This reduction in IS risk is likely due to platelet inhibition as well as improvement in hemodynamics and cardiac function resulting in decreased risk of thrombosis. Several single centre observational studies identified a reduction in ischemic stroke and mortality with PDE5i use in addition to standard antithrombotic regimen.57,58 Additionally, in an Intermacs analysis of LVAD patients, PDE5i use was associated with significant reduction in the IS and all-cause mortality without increased risk in HS, and this benefit was seen in both axial and CF-LVADs59. However, other studies have shown mixed results of PDE5i use. In a single centre cohort retrospective study of 318 LVAD patients, PDE5i use was not associated with improved clinical outcomes60 and in another retrospective study of 109 LVAD patients of whom 75 received long term PDE5i therapy, an increased bleeding risk was identified (23% vs 6%, p=0.03)61. A meta-analysis of 13 observational studies in 2020 showed that there was no association with PDE5i use and IS, HS, or other major bleeding; however, this meta-analysis was published prior to the aforementioned largest Intermacs analysis demonstrating benefit. As there are limitations of antithrombotic therapy in this population and the current literature remains uncertain, a randomized clinical trial of PDE5i use in LVAD is warranted.
Management of Ischemic Stroke
Sparse data exist on the management of IS in LVAD patients including the use of either tPA or endovascular thrombectomy (EVT). ISHLT guidelines do not routinely recommend the use of intravenous tissue plasminogen activator (tPA) in IS55. LVAD patients are typically not eligible for tPA due to therapeutic anticoagulation, prior history of HS, or recent major bleeding event or surgery and may be at increased risk of hemorrhagic transformation due to acquired von Willebrand Factor deficiency as well as cerebral microbleeds.31 Similar to patients without LVAD, when LVAD patients present with signs and symptoms of IS, an emergent non-contrast CT brain is recommended to rule out HS given differences in management for each condition (Figure 3). A CT angiography (CTA) of the head and neck with contrast and potentially CT perfusion to assess for large vessel occlusion and candidacy for EVT.62. The current data on the outcome in LVAD patients who underwent EVT are very limited. Although case series have shown that LVAD patients with LVO that underwent EVT have higher mortality and rate of hemorrhagic transformation compared to control patients without LVAD, this is largely due to the post-operative status of LVAD patients. The LVAD patients with stroke occurring outside of the post-operative period showed no significant difference in overall mortality, with odds of discharge home comparable to control patients.63,64 Additional research is needed to evaluate the long-term benefits of EVT after AIS in patients on LVAD support .
Figure 3.
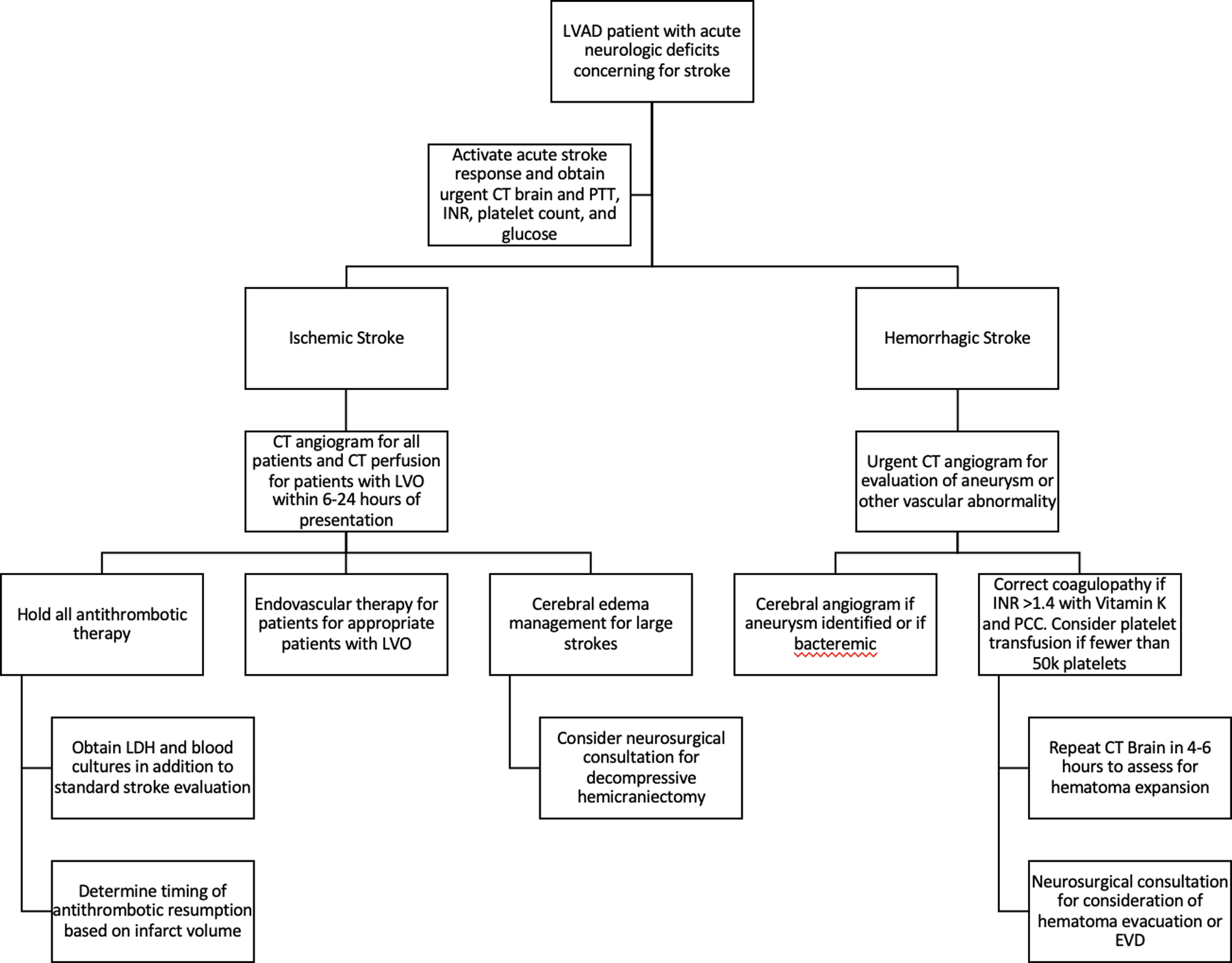
Proposed algorithm for the management of LVAD patients presenting with acute stroke. EVD: External Ventricular Drain, INR: International Normalized Ratio, LDH: Lactate Dehydrogenase, LVO: Large Vessel Occlusion, PCC: Prothrombin Complex Concentrate, PTT: Partial Thromboplastin Time
Optimal blood pressure management in LVAD patients after IS is unclear and it is difficult to extrapolate as LVAD patients have non-pulsatile blood flow. The American Stroke Association (ASA) recommends permissive hypertension (<220/120 mm Hg for patients without intravenous thrombolysis and <180/105 mm Hg for those treated with intravenous thrombolysis) after IS in non-LVAD patients.62 The safety and impact of permissive hypertension in patients with LVAD, who are afterload dependent and at high risk for hemorrhagic transformation, is unclear. However, a short-term permissive hypertension is reasonable in order to maintain adequate cerebral perfusion following IS. In patients with large hemispheric infarct who are at risk for cerebral edema, brain compression, and neurological deterioration, decompressive hemicraniectomy can be a life-saving measure.65 It may be offered after careful selection of patient’s candidacy including age, overall premorbid function, life expectancy and goals of care. A course of hyperosmolar therapy is also a reasonable bridge prior to hemicraniectomy.66
There is limited literature on timing of antithrombotic therapy resumption in LVAD patients. Antiplatelet agent is typically resumed immediately after IS regardless of infarct size in LVAD. The ASA recommends starting oral anticoagulation for atrial fibrillation within 4–14 days following IS.67,68 Based on the data in patients with atrial fibrillation, full anticoagulation can be resumed in 4–14 days, if not earlier as LVADs carry a higher risk of thromboembolic events vs. atrial fibrillation and resumption should be considered within the first week after IS.68
Hemorrhagic stroke
Epidemiology
The incidence of HS is similar to IS and varies between 0.01–0.11 EPPY or 3–11% across different studies for HVAD and HMII.69–73 When HVAD was directly compared to HMII in the ENDURANCE trial, the HS event rate was found much higher (0.11 vs. 0.03 EPPY). However, there was a 50% reduction in the rate of HS in the HVAD arm in the supplemental trial after the implementation of a blood pressure management protocol.24 Importantly, HS among HM3 patients had an incidence of 4% (0.03 EPPY) in the MOMENTUM3 trial, and 9% in HMII, although this difference did not reach clinical significance.23 As previously discussed, in a propensity matched Intermacs analysis HVAD was associated with higher rates of HS than HM3 (7.2% vs 2.0%, p<0.001), leading to the device’s withdrawal from the market. The characteristics and subtypes of HS were not well-described in the LVAD population. In a single centre cohort study of 73 LVAD patients with HS, intraparenchymal hemorrhage was the most common subtype followed by subarachnoid hemorrhage and subdural hemorrhage, and multiple concurrent hemorrhage subtypes were seen in 22% of patients with HS.74
The presence of HS significantly increases patient mortality. The 30-day and 2-year mortality rate after HS is between 33%−55% and 39%−100%, respectively.40,75–77 Not surprisingly, in-hospital (59% HS vs 28% IS) and overall mortality (HS 71% vs. IS 31%) for patients with HS are much higher compared to patients with IS.78,79 Notably, the highest risk of HS is in the immediate post-operative period and the risk increases again after 9–12 months, similar to IS, with 3% incidence of early HS (0.02 EPPY) and 7% incidence of late HS (0.05 EPPY).30
Risk factors
HS and IS in LVAD share many of the similar risk factors including infection, female sex, and hypertension (Figure 2). Anticoagulation use during LVAD support increases the risk of HS, however there is higher rate of HS in LVAD patients compared to patients only on chronic anticoagulation without LVAD,80–83 raising the question of other mechanisms related to LVAD use, especially when other systemic bleeding complications are also common in LVAD patients.
Acquired von Willebrand factor disease
The vWF is a protein expressed by vascular endothelial cells that binds to exposed collagen of damaged blood vessels and platelet receptors to induce activation, adhesion, and aggregation.84 Homeostasis of the vWF multimer size is important to prevention pathologic coagulopathy and excessive cleavage of the vWF multimeters will result in pathologic bleeding as seen in acquired vWF disease.84,85 Acquired vWF disease affects patients with severe aortic stenosis secondary to either high shear stress across the calcified aortic valve or increased luminal pressure in the vasculature and lowered pulse pressure leading to conformational changes of the vWF multimers and predisposing them to proteolytic cleavage.84 Non-pulsatile continuous blood flow by LVAD can induce high shear stress on the endothelium, and patients with LVAD have similar bleeding patterns to those described in severe aortic stenosis.86 Multiple studies have shown that nearly all axial-flow LVAD patients developed some degree of acquired vWF disease.87–89 HM3 patients had higher preservation of high molecular weight vWF multimers compared with HMII90 and HVAD.91 While mechanisms for the superior high molecular weight vWF preservation in HM3 are unclear, it may be related to differences in interior rotor design, wider consistent flow paths, and differences in pump speeds in HM3 resulting in less shear stress on the vWF molecule. In addition to loss of vWF multimers, impaired platelet aggregation in LVAD patients with minor or major bleeding has been identified; however, the relative contribution of impaired platelet aggregation and vWF disease has yet to be elucidated.92
Cerebral Microbleeds
Cerebral microbleeds (CMBs) are small (<5mm) areas of intracerebral hemorrhage visible as areas of hypointensity on susceptibility weighted imaging MRI sequences or at autopsy believed to be related to damage of small blood vessels. In one retrospective study of 37 patients with a brain MRI after CF-LVAD explantation, 97% of patients had at least one CMB and the number of CMBs was associated with increased risk of prior HS.93 In a follow-up study by the same investigators of an expanded cohort of 49 patients with CF-LVAD, CMBs were detected in significantly more patients with prior CF-LVAD (98%) than matched patients with chronic heart failure without history of LVAD (18%) or healthy patients (6%).94 One autopsy study of 21 patients identified CMBs in 19 (90%) LVAD patients, with 6 (29%) of these patients having had gross HS.95 Possible mechanisms for CMBs in patients with LVAD include infection93, chronic anticoagulant therapy96, and endotheliopathy related to vascular changes from CF-LVAD. CF-LVADs have been associated with altered angiogenesis and elevated levels of angiopoietin-2, which was associated with higher rates of non-surgical bleeding after LVAD implantation.97 CMBs are known to be associated with increased risk of HS in patients without LVAD who are on anticoagulant therapy98, and the increased frequency of CMBs may partly explain the increased risk of HS in the LVAD population, especially as LVAD patients are invariably on chronic anticoagulant therapy. Further research is needed to better understand CMB’s long-term implication and neurological outcome such as cognition.
Iatrogenic coagulopathy
Long-term antithrombotic therapy in LVAD patients to prevent thromboembolic events along with underlying acquired coagulopathy place LVAD patients at higher risk for bleeding, and all LVAD-associated HS occurred while on anticoagulation99. The current evidence suggests that IS and HS are minimized with dose-adjusted warfarin targeting INR 2.0–3.0 in addition to 81 mg of aspirin therapy, as both IS and HS risk increases with subtherapeutic and supratherapeutic antithrombotic regimens.41,100 Balancing bleeding and thrombosis risks is a critical component of LVAD patient management moving forward, and further research into the use of direct oral anticoagulant agents and PDE5is in addition to advancement in device technology may benefit this balance.
Management
Similar to IS, there is sparse research pertaining to acute management of LVAD patients with HS. After HS is identified on CT, a CTA study should be performed to evaluate for mycotic aneurysm and digital subtraction angiography should be considered due to its increased sensitivity for aneurysm, especially if there is a prior history of LVAD-associated infection. The ISHLT guideline recommends discontinuing or reversing anticoagulation after the occurrence of a HS.55 Warfarin, which is the typical anticoagulation agent for LVAD patients should be reversed with 10 mg of IV Vitamin K and prothrombin complex concentrate (PCC). 4-factor PCC is recommended over fresh frozen plasma (FFP), however, when 4-factor PCC is unavailable, 3-factor PCC with factor VIIa, or FFP can be used. 4-factor PCC is favored due to a faster administration/INR correction, smaller volume, lower risk of thrombosis, and less likely to have transfusion reaction compared to FFP.101 Although there are limited data, reversal of warfarin with either PCC or FFP seems to be safe in LVAD patients based on retrospective observation studies with very low rate of thromboembolic complications during hospitalization.102,103 Current evidence does not support routine platelet transfusion in patients with normal platelet count regardless of antiplatelet use at the time of HS.104
In addition to anticoagulation reversal, strict blood pressure control is another important intervention to prevent hematoma expansion which is associated poor outcome in HS.105,106 It remains unclear whether acute blood pressure lowering after HS impairs cerebral blood flow within the perihematomal region resulting in secondary ischemia; while some studies did not demonstrate higher rates of secondary IS with acute blood pressure lowering 105,106, a post-hoc analysis of patients presenting with SBP ≥220 mmHg reported higher rates of neurological deterioration and acute kidney injury.107 In patients without LVAD, American Heart Association (AHA) recommends targeting a SBP of 140 mmHg within the first hour of presentation with a goal of maintaining SBP between 130–150 mmHg.108 Although the SBP goal may not apply to LVAD patients, targeting a MAP of < 85–90 mmHg is considered reasonable and safe.
A large HS may cause mass effect from cerebral edema, brain compression, hydrocephalus and elevated ICP. AHA guidelines recommend placing an external ventricular drain in patients with intraventricular extension of HS causing hydrocephalus, and craniotomy for patients with cerebellar HS causing brainstem compression, hydrocephalus, or a hematoma volume ≥15 mL.108 In patients with supratentorial HS, there is limited evidence support hematoma evacuation and hemicraniectomy, but minimally invasive hematoma evacuation for HS ≥20mL may reduce mortality compared with medical management alone.109,110 Additionally, all patients should get repeat imaging to assess hematoma expansion or stability within 4 hours from initial CT brain and subsequent CT with any concerns of exam changes. Acute ICP elevation or life-threatening mass effect can be treated with standard neurocritical care interventions such as hypertonic saline or mannitol as well as elevation of the head of bed to 30 degrees and antipyretics to aggressively manage hyperthermia.111
The optimal timing for antithrombotic resumption following HS is unknown in both non-LVAD and LVAD patients. In the non-LVAD patients, the observational studies suggested waiting for 4–8 weeks following HS is safe while minimizing the risk of IS while off anticoagulation and HS after resumption.112,113 Early resumption around 1–2 weeks after HS is deemed feasible for high risk patients such as patients with mechanical valve or intra-cardiac thrombus.114 Patients with mechanical circulatory support device such as LVAD should be considered as a high risk population for thromboembolic events while off anticoagulation. One retrospective study of 36 LVAD-associated HS reported that withholding aspirin for 1 week and warfarin for 10 days was sufficient and safe to reduce the risk of hemorrhage expansion or recurrent HS in LVAD patients while minimizing the risk of IS and pump failure.76 Another observational study of 39 LVAD-associated HS reported that the resumption of aspirin alone was associated with higher thromboembolic events compared to warfarin alone and warfarin plus aspirin, although patients with warfarin resumption had a higher rate of non-fatal recurrent hemorrhage.102 Therefore, we advise to resume anticoagulation and aspirin within 7–10 days after HS with the exact timing being determined by evaluating unique patient characteristics and considering risks and benefits as part of a multidisciplinary team.
Stroke impact on Patient Outcome
LVAD-associated stroke significantly impacts patient’s quality of life, candidacy for heart transplantation, and their survival. Depending on the severity of the stroke and location of the stroke (involvement of eloquent neuroanatomical structures), the neurological deficits from stroke can often lead to a significant disability and poor quality of life. More importantly, stroke often compromises a patient’s candidacy for heart transplant for those with bridge to transplantation. Stroke leads to a lower probability of receiving a heart transplant compared to those without (15.6% vs. 33.3%) and negatively impact on survival in LVAD patients.40 Both IS and HS increase the in-hospital mortality by 4 and 18-fold, respectively, based on a US population study and is an independent predictor for subsequent in-hospital mortality (HR=6.1).115,116 Survival at one year is also much lower in patients with a stroke (43.7%) compared to those without a stroke (80%).40 IS that occurred after discharge was an independent predictor of mortality78, while periprocedural HS and HS after discharge were both independent predictors of mortality.78
Cognition and Cognitive Decline.
Advanced heart failure is associated with chronic decreased cerebral perfusion and has been associated with subsequent cognitive impairment.117 Pre-LVAD cognitive performance is predictive of LVAD-associated stroke as well as mortality after LVAD implantation.118 Importantly, LVAD implantation in patients with advancement heart failure was associated with an improvement in cognitive function. In one study, 56 patients underwent cognitive screening as part of LVAD candidacy evaluation and again after LVAD implantation.119 Patients undergoing cognitive screening before and after LVAD implantation demonstrated increases in Montreal Cognitive Assessment (MOCA) scores from 23.6 to 25.1 with significant improvements in visuospatial, executive, and delayed recall domains. Additionally, the Beck Depression Inventory improved significantly from 12.8 pre-LVAD (indicative of mild mood disturbance) to 6.64 at 8 months post-LVAD (within normal range). In an analysis of patients with HVAD and HMII, 80% of patients had stable or improved neurocognitive function at 6, 12, and 24 months after LVAD implantation.4 In the same study, when patients had cognitive decline, male sex, stroke, and HVAD (vs. HMII) were risk factors4, highlighting the importance of stroke prevention and device selection.
Conclusions
Despite significant improvements in LVAD design/technology and an increased recognition of device-associated complications over the past decade, neurological complications of LVAD remain a leading cause of morbidity and mortality and stroke is a major outcome in LVAD clinical trials. While neurological complications in LVAD patients are increasingly recognized, sparse evidence and clinical guidelines exist on optimal strategy for stroke prevention and management. Future research to further elucidate the underlying mechanisms of neurological complications is necessary in order to develop better prevention and management strategies.
References
- 1.EJ M, P S, MS K, et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg 2021;111(3):778–792. doi: 10.1016/J.ATHORACSUR.2020.12.038 [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Uriel N, Naka Y, et al. A Fully Magnetically Levitated Left Ventricular Assist Device — Final Report. N Engl J Med 2019;380(17):1618–1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 3.Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol 2015;66(16):1747–1761. doi: 10.1016/J.JACC.2015.07.075 [DOI] [PubMed] [Google Scholar]
- 4.Cho SM, Floden D, Wallace K, et al. Long-Term Neurocognitive Outcome in Patients With Continuous Flow Left Ventricular Assist Device. JACC Heart Fail 2021;9(11):839–851. doi: 10.1016/J.JCHF.2021.05.016 [DOI] [PubMed] [Google Scholar]
- 5.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 2009;10(1):53–62. doi: 10.1038/NRM2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amir O, Radovancevic B, Delgado RM, et al. Peripheral vascular reactivity in patients with pulsatile vs axial flow left ventricular assist device support. J Heart Lung Transplant 2006;25(4):391–394. doi: 10.1016/J.HEALUN.2005.11.439 [DOI] [PubMed] [Google Scholar]
- 7.Witman MAH, Garten RS, Gifford JR, et al. Further Peripheral Vascular Dysfunction in Heart Failure Patients With a Continuous-Flow Left Ventricular Assist Device: The Role of Pulsatility. JACC Heart Fail 2015;3(9):703–711. doi: 10.1016/J.JCHF.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014;63(7):636–646. doi: 10.1016/J.JACC.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AC, Dodson RB, Cornwell WK, et al. Dynamic Changes in Aortic Vascular Stiffness in Patients Bridged to Transplant With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail 2017;5(6):449–459. doi: 10.1016/J.JCHF.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 10.Rosenblum H, Pinsino A, Zuver A, et al. Increased Aortic Stiffness Is Associated With Higher Rates of Stroke, Gastrointestinal Bleeding and Pump Thrombosis in Patients With a Continuous Flow Left Ventricular Assist Device. J Card Fail 2021;27(6):696–699. doi: 10.1016/J.CARDFAIL.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 11.Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg 2010;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth 2012;109(3):391–398. doi: 10.1093/BJA/AES148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono M, Joshi B, Brady K, et al. Cerebral Blood Flow Autoregulation Is Preserved After Continuous-Flow Left Ventricular Assist Device Implantation. J Cardiothorac Vasc Anesth 2012;26(6):1022–1028. doi: 10.1053/J.JVCA.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornwell WK, Tarumi T, Aengevaeren VL, et al. Effect of pulsatile and nonpulsatile flow on cerebral perfusion in patients with left ventricular assist devices. J Hear Lung Transplant 2014;33(12):1295–1303. doi: 10.1016/j.healun.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 15.Stöhr EJ, Ji R, Akiyama K, et al. Cerebral vasoreactivity in HeartMate 3 patients. J Heart Lung Transplant 2021;40(8):786–793. doi: 10.1016/J.HEALUN.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Smith KJ, Suarez IM, Scheer A, et al. Cerebral Blood Flow during Exercise in Heart Failure: Effect of Ventricular Assist Devices. Med Sci Sports Exerc 2019;51(7):1372–1379. doi: 10.1249/MSS.0000000000001904 [DOI] [PubMed] [Google Scholar]
- 17.Smith KJ, Moreno-Suarez I, Scheer A, et al. Cerebral blood flow responses to exercise are enhanced in left ventricular assist device patients after an exercise rehabilitation program. J Appl Physiol 2020;128(1):108–116. doi: 10.1152/JAPPLPHYSIOL.00604.2019/ASSET/IMAGES/LARGE/ZDG0012032500006.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014;114(11):1815–1826. doi: 10.1161/CIRCRESAHA.114.302589 [DOI] [PubMed] [Google Scholar]
- 19.Castagna F, McDonnell BJ, Mondellini GM, et al. Twenty-four-hour blood pressure and heart rate variability are reduced in patients on left ventricular assist device support. J Hear Lung Transplant 2022;41(6):802–809. doi: 10.1016/J.HEALUN.2022.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markham DW, Fu Q, Palmer MD, et al. Sympathetic neural and hemodynamic responses to upright tilt in patients with pulsatile and nonpulsatile left ventricular assist devices. Circ Hear Fail 2013;6(2):293–299. doi: 10.1161/CIRCHEARTFAILURE.112.969873 [DOI] [PubMed] [Google Scholar]
- 21.Sailer C, Edelmann H, Buchanan C, et al. Impairments in Blood Pressure Regulation and Cardiac Baroreceptor Sensitivity Among Patients With Heart Failure Supported With Continuous-Flow Left Ventricular Assist Devices. Circ Hear Fail 2021;14(1):E007448. doi: 10.1161/CIRCHEARTFAILURE.120.007448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller LW, Pagani FD, Russell SD, et al. Use of a Continuous-Flow Device in Patients Awaiting Heart Transplantation. N Engl J Med 2007;357(9):885–896. doi: 10.1056/nejmoa067758 [DOI] [PubMed] [Google Scholar]
- 23.Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376(5):440–450. doi: 10.1056/nejmoa1610426 [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Goldstein DJ, Uriel N, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N Engl J Med 2018;378(15):1386–1395. doi: 10.1056/nejmoa1800866 [DOI] [PubMed] [Google Scholar]
- 25.Kirklin JK, Naftel DC, Myers SL, Pagani FD, Colombo PC. Quantifying the impact from stroke during support with continuous flow ventricular assist devices: An STS INTERMACS analysis. J Hear Lung Transplant 2020;39(8):782–794. doi: 10.1016/j.healun.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Schmid C, Wilhelm M, Rothenburger M, et al. Effect of high dose platelet inhibitor treatment on thromboembolism in Novacor patients. Eur J Cardio-thoracic Surg 2000;17(3):331–335. doi: 10.1016/S1010-7940(00)00334-1 [DOI] [PubMed] [Google Scholar]
- 27.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017;376(5):451–460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 28.Cho S-M, Mehaffey JH, Meyers SL, et al. Cerebrovascular Events in Patients With Centrifugal-Flow Left Ventricular Assist Devices: Propensity Score-Matched Analysis From the Intermacs Registry. Circulation 2021;144(10):763–772. doi: 10.1161/CIRCULATIONAHA.121.055716 [DOI] [PubMed] [Google Scholar]
- 29.FDA Alerts Health Care Providers to Stop New Implants of Certain Ventricular Assist Device System | FDA. Accessed September 23, 2022. https://www.fda.gov/news-events/press-announcements/fda-alerts-health-care-providers-stop-new-implants-certain-ventricular-assist-device-system [Google Scholar]
- 30.Frontera JA, Starling R, Cho SM, et al. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Hear Lung Transplant 2017;36(6):673–683. doi: 10.1016/j.healun.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 31.Rice CJ, Cho SM, Zhang LQ, Hassett C, Starling RC, Uchino K. The management of acute ischemic strokes and the prevalence of large vessel occlusion in left ventricular assist device. Cerebrovasc Dis 2019;46(5–6):213–217. doi: 10.1159/000495080 [DOI] [PubMed] [Google Scholar]
- 32.Tahsili-Fahadan P, Curfman DR, Davis AA, et al. Cerebrovascular Events After Continuous-Flow Left Ventricular Assist Devices. Neurocrit Care 2018;29(2):225–232. doi: 10.1007/s12028-018-0531-y [DOI] [PubMed] [Google Scholar]
- 33.Coffin ST, Haglund NA, Davis ME, et al. Adverse neurologic events in patients bridged with long-term mechanical circulatory support: A device-specific comparative analysis. J Hear Lung Transplant 2015;34(12):1578–1585. doi: 10.1016/j.healun.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 34.Nassif ME, Tibrewala A, Raymer DS, et al. Systolic blood pressure on discharge after left ventricular assist device insertion is associated with subsequent stroke. J Hear Lung Transplant 2015;34(4):503–508. doi: 10.1016/j.healun.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enriquez AD, Calenda B, Gandhi PU, Nair AP, Anyanwu AC, Pinney SP. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol 2014;64(18):1883–1890. doi: 10.1016/j.jacc.2014.07.989 [DOI] [PubMed] [Google Scholar]
- 36.Stulak JM, Deo S, Schirger J, et al. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg 2013;96(6):2161–2167. doi: 10.1016/j.athoracsur.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 37.Teuteberg JJ, Slaughter MS, Rogers JG, et al. The HVAD Left Ventricular Assist Device: Risk Factors for Neurological Events and Risk Mitigation Strategies. JACC Hear Fail 2015;3(10):818–828. doi: 10.1016/j.jchf.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 38.Morgan JA, Brewer RJ, Nemeh HW, et al. Stroke while on long-term left ventricular assist device support: Incidence, outcome, and predictors. ASAIO J 2014;60(3):284–289. doi: 10.1097/MAT.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 39.Boyle AJ, Jorde UP, Sun B, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: An analysis of more than 900 heartmate II outpatients. J Am Coll Cardiol 2014;63(9):880–888. doi: 10.1016/j.jacc.2013.08.1656 [DOI] [PubMed] [Google Scholar]
- 40.Acharya D, Loyaga-Rendon R, Morgan CJ, et al. INTERMACS Analysis of Stroke During Support With Continuous-Flow Left Ventricular Assist Devices: Risk Factors and Outcomes. JACC Hear Fail 2017;5(10):703–711. doi: 10.1016/j.jchf.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho SM, Starling RC, Teuteberg J, et al. Understanding risk factors and predictors for stroke subtypes in the ENDURANCE trials. J Hear Lung Transplant 2020;39(7):639–647. doi: 10.1016/j.healun.2020.01.1330 [DOI] [PubMed] [Google Scholar]
- 42.Guglin M, Cousin E, Scholfield M, Faber C, Caldeira C, Guglin M. Treatment options for patients with mobile left ventricular thrombus and ventricular dysfunction: a case series. Hear Lung Vessel 2014;6(2):88. Accessed October 20, 2022. /pmc/articles/PMC4095835/ [PMC free article] [PubMed] [Google Scholar]
- 43.Lampert BC, Eckert C, Weaver S, et al. Blood pressure control in continuous flow left ventricular assist devices: Efficacy and impact on adverse events. Ann Thorac Surg 2014;97(1):139–146. doi: 10.1016/j.athoracsur.2013.07.069 [DOI] [PubMed] [Google Scholar]
- 44.Milano CA, Rogers JG, Tatooles AJ, et al. HVAD: The ENDURANCE Supplemental Trial. JACC Hear Fail 2018;6(9):792–802. doi: 10.1016/j.jchf.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 45.Cho SM, Moazami N, Katz S, Bhimraj A, Shrestha NK, Frontera JA. Stroke Risk Following Infection in Patients with Continuous-Flow Left Ventricular Assist Device. Neurocrit Care 2019;31(1):72–80. doi: 10.1007/s12028-018-0662-1 [DOI] [PubMed] [Google Scholar]
- 46.Cho SM, Hassett C, Rice CJ, Starling R, Katzan I, Uchino K. What Causes LVAD-Associated Ischemic Stroke? Surgery, Pump Thrombosis, Antithrombotics, and Infection. ASAIO J 2019;65(8):775–780. doi: 10.1097/MAT.0000000000000901 [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal A, Gupta A, Kumar S, et al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J 2012;58(5):509–513. doi: 10.1097/MAT.0b013e318260c6a6 [DOI] [PubMed] [Google Scholar]
- 48.Lee T, Buletko AB, Matthew J, Cho SM. Bloodstream infection is associated with subarachnoid hemorrhage and infectious intracranial aneurysm in left ventricular assist device. Perfus (United Kingdom) 2020;35(2):117–120. doi: 10.1177/0267659119858853 [DOI] [PubMed] [Google Scholar]
- 49.Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail 2015;21(2):119–125. doi: 10.1016/j.cardfail.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 50.Hasin T, Matsuzawa Y, Guddeti RR, et al. Attenuation in peripheral endothelial function after continuous flow left ventricular assist device therapy is associated with cardiovascular adverse events. Circ J 2015;79(4):770–777. doi: 10.1253/circj.CJ-14-1079 [DOI] [PubMed] [Google Scholar]
- 51.Malone G, Abdelsayed G, Bligh F, et al. Advancements in left ventricular assist devices to prevent pump thrombosis and blood coagulopathy. J Anat Published online 2022. doi: 10.1111/joa.13675 [DOI] [PMC free article] [PubMed]
- 52.Inamullah O, Chiang YP, Bishawi M, et al. Characteristics of strokes associated with centrifugal flow left ventricular assist devices. Sci Rep 2021;11(1). doi: 10.1038/s41598-021-81445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehra MR, Goldstein DJ, Cleveland JC, et al. Five-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices in the MOMENTUM 3 Randomized Trial. JAMA 2022;328(12):1233–1242. doi: 10.1001/JAMA.2022.16197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slaughter MS, Naka Y, John R, et al. Post-operative heparin may not be required for transitioning patients with a HeartMate II left ventricular assist system to long-term warfarin therapy. J Hear Lung Transplant 2010;29(6):616–624. doi: 10.1016/j.healun.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 55.Feldman D, Pamboukian SV., Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: Executive summary. J Hear Lung Transplant 2013;32(2):157–187. doi: 10.1016/j.healun.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 56.Mehra MR, Crandall DL, Gustafsson F, et al. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo-controlled, non-inferiority ARIES HM3 trial. Eur J Heart Fail 2021;23(7):1226–1237. doi: 10.1002/EJHF.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zayat R, Ahmad U, Stoppe C, et al. Sildenafil Reduces the Risk of Thromboembolic Events in HeartMate II Patients with Low-Level Hemolysis and Significantly Improves the Pulmonary Circulation. Int Heart J 2018;59(6):18–001. doi: 10.1536/IHJ.18-001 [DOI] [PubMed] [Google Scholar]
- 58.Saeed O, Rangasamy S, Selevany I, et al. Sildenafil Is Associated With Reduced Device Thrombosis and Ischemic Stroke Despite Low-Level Hemolysis on Heart Mate II Support. Circ Heart Fail 2017;10(11). doi: 10.1161/CIRCHEARTFAILURE.117.004222 [DOI] [PubMed] [Google Scholar]
- 59.Xanthopoulos A, Tryposkiadis K, Triposkiadis F, et al. Postimplant phosphodiesterase type 5 inhibitors use is associated with lower rates of thrombotic events after left ventricular assist device implantation. J Am Heart Assoc 2020;9(14):15897. doi: 10.1161/JAHA.119.015897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts KL, Shuster JE, Britt NS, et al. Evaluation of clinical outcomes with phosphodiesterase-5 inhibitor therapy for right ventricular dysfunction after left ventricular assist device implantation. ASAIO J 2019;65(3):264–269. doi: 10.1097/MAT.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 61.Jakstaite AM, Luedike P, Schmack B, et al. Increased bleeding risk with phosphodiesterase-5 inhibitors after left ventricular assist device implantation. ESC Hear Fail 2021;8(4):2419–2427. doi: 10.1002/ehf2.13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 63.Kitano T, Sakaguchi M, Yamagami H, et al. Mechanical thrombectomy in acute ischemic stroke patients with left ventricular assist device. J Neurol Sci 2020;418. doi: 10.1016/J.JNS.2020.117142 [DOI] [PubMed] [Google Scholar]
- 64.Ibeh C, Mandigo GK, Sisti JA, Lavine SD, Willey JZ. Mechanical thrombectomy after acute ischemic stroke in patients with left ventricular assist devices: A nationwide analysis. Int J Stroke 2022;00(X). doi: 10.1177/17474930221097271 [DOI] [PubMed] [Google Scholar]
- 65.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009;8(4):326–333. doi: 10.1016/S1474-4422(09)70047-X [DOI] [PubMed] [Google Scholar]
- 66.Wijdicks EFM, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(4):1222–1238. doi: 10.1161/01.str.0000441965.15164.d6 [DOI] [PubMed] [Google Scholar]
- 67.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with Non-Vitamin-K oral anticoagulants (RAF-NOACs) Study. J Am Heart Assoc 2017;6(12). doi: 10.1161/JAHA.117.007034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seiffge DJ, Werring DJ, Paciaroni M, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol 2019;18(1):117–126. doi: 10.1016/S1474-4422(18)30356-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-U.S. food and drug administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: A prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circul. J Am Coll Cardiol 2011;57(19):1890–1898. doi: 10.1016/j.jacc.2010.10.062 [DOI] [PubMed] [Google Scholar]
- 70.John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011;92(4):1406–1413. doi: 10.1016/j.athoracsur.2011.05.080 [DOI] [PubMed] [Google Scholar]
- 71.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125(25):3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412 [DOI] [PubMed] [Google Scholar]
- 72.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J Hear Lung Transplant 2013;32(7):675–683. doi: 10.1016/j.healun.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 73.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017;376(5):451–460. doi: 10.1056/nejmoa1602954 [DOI] [PubMed] [Google Scholar]
- 74.Shoskes A, Hassett C, Gedansky A, et al. Implications of Causes of Intracranial Hemorrhage During Left Ventricular Assist Device Support. Neurocrit Care Published online April 12, 2022. doi: 10.1007/S12028-022-01494-3 [DOI] [PMC free article] [PubMed]
- 75.Yavar Z, Cowger JA, Moainie SL, Salerno CT, Ravichandran AK. Bleeding complication rates are higher in females after continuous-flow left ventricular assist device implantation. ASAIO J 2018;64(6):748–753. doi: 10.1097/MAT.0000000000000734 [DOI] [PubMed] [Google Scholar]
- 76.Wilson TJ, Stetler WR, Al-Holou WN, Sullivan SE, Fletcher JJ. Management of intracranial hemorrhage in patients with left ventricular assist devices: Clinical article. J Neurosurg 2013;118(5):1063–1068. doi: 10.3171/2013.1.JNS121849 [DOI] [PubMed] [Google Scholar]
- 77.Chou J, Bermudez C, Kormos R, Teuteberg J. Permanent Continuous Flow Left Ventricular Assist Devices Use After Acute Stabilization for Cardiogenic Shock in Acute Myocardial Infarction. ASAIO J 2017;63(2):e13–e17. doi: 10.1097/MAT.0000000000000398 [DOI] [PubMed] [Google Scholar]
- 78.Cho SM, Moazami N, Frontera JA. Stroke and Intracranial Hemorrhage in HeartMate II and HeartWare Left Ventricular Assist Devices: A Systematic Review. Neurocrit Care 2017;27(1):17–25. doi: 10.1007/s12028-017-0386-7 [DOI] [PubMed] [Google Scholar]
- 79.Willey JZ, Gavalas MV, Trinh PN, et al. Outcomes after stroke complicating left ventricular assist device. J Hear Lung Transplant 2016;35(8):1003–1009. doi: 10.1016/j.healun.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramey WL, Basken RL, Walter CM, Khalpey Z, Lemole GM, Dumont TM. Intracranial Hemorrhage in Patients with Durable Mechanical Circulatory Support Devices: Institutional Review and Proposed Treatment Algorithm. World Neurosurg 2017;108:826–835. doi: 10.1016/j.wneu.2017.09.083 [DOI] [PubMed] [Google Scholar]
- 81.López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: Systematic review, network meta-Analysis, and cost effectiveness analysis. BMJ 2017;359. doi: 10.1136/bmj.j5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: Meta-analysis with estimates of risk and benefit. Ann Intern Med 2005;143(4). doi: 10.7326/0003-4819-143-4-200508160-00005 [DOI] [PubMed] [Google Scholar]
- 83.Van Es RF, Jonker JJC, Verheugt FWA, Deckers JW, Grobbee DE. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): A randomised controlled trial. Lancet 2002;360(9327):109–113. doi: 10.1016/S0140-6736(02)09409-6 [DOI] [PubMed] [Google Scholar]
- 84.John R, Boyle A, Pagani F, Miller L. Physiologic and pathologic changes in patients with continuous-flow ventricular assist devices. J Cardiovasc Transl Res 2009;2(2):154–158. doi: 10.1007/s12265-009-9092-y [DOI] [PubMed] [Google Scholar]
- 85.Zhou Z, Nguyen TC, Guchhait P, Dong JF. Von willebrand factor, ADAMTS-13, and thrombotic thrombocytopenic purpura. Semin Thromb Hemost 2010;36(1):71–81. doi: 10.1055/s-0030-1248726 [DOI] [PubMed] [Google Scholar]
- 86.Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Hear Fail 2011;4(6):779–784. doi: 10.1161/CIRCHEARTFAILURE.111.962613 [DOI] [PubMed] [Google Scholar]
- 87.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016 [DOI] [PubMed] [Google Scholar]
- 88.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg 2010;90(4):1263–1269. doi: 10.1016/j.athoracsur.2010.04.099 [DOI] [PubMed] [Google Scholar]
- 89.Crow S, Milano C, Joyce L, et al. Comparative analysis of von willebrand factor profiles in pulsatile and continuous left ventricular assist device recipients. ASAIO J 2010;56(5):441–445. doi: 10.1097/MAT.0b013e3181e5de0a [DOI] [PubMed] [Google Scholar]
- 90.Netuka I, Kvasnička T, Kvasnička J, et al. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J Hear Lung Transplant 2016;35(7):860–867. doi: 10.1016/J.HEALUN.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 91.Klaeske K, Dieterlen MT, Scholz U, et al. Acquired von Willebrand factor deficiency is reduced in HeartMate 3 patients†. Eur J Cardiothorac Surg 2019;56(3):444–450. doi: 10.1093/EJCTS/EZZ045 [DOI] [PubMed] [Google Scholar]
- 92.Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely Impaired von Willebrand Factor-Dependent Platelet Aggregation in Patients With a Continuous-Flow Left Ventricular Assist Device (HeartMate II). J Am Coll Cardiol 2009;53(23):2162–2167. doi: 10.1016/j.jacc.2009.02.048 [DOI] [PubMed] [Google Scholar]
- 93.Yoshioka D, Okazaki S, Toda K, et al. Prevalence of cerebral microbleeds in patients with continuous-flow left ventricular assist devices. J Am Heart Assoc 2017;6(9). doi: 10.1161/JAHA.117.005955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murase S, Okazaki S, Yoshioka D, et al. Abnormalities of brain imaging in patients after left ventricular assist device support following explantation. J Hear Lung Transplant 2020;39(3):220–227. doi: 10.1016/j.healun.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 95.Fan TH, Cho SM, Prayson RA, Hassett CE, Starling RC, Uchino K. Cerebral Microvascular Injury in Patients with Left Ventricular Assist Device: a Neuropathological Study. Transl Stroke Res 2021 132 2021;13(2):257–264. doi: 10.1007/S12975-021-00935-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic Drug Use, Cerebral Microbleeds, and Intracerebral Hemorrhage. Stroke 2010;41(6):1222–1228. doi: 10.1161/STROKEAHA.109.572594 [DOI] [PubMed] [Google Scholar]
- 97.Tabit CE, Chen P, Kim GH, et al. Elevated Angiopoietin-2 Level in Patients with Continuous-Flow Left Ventricular Assist Devices Leads to Altered Angiogenesis and Is Associated with Higher Nonsurgical Bleeding. Circulation 2016;134(2):141–152. doi: 10.1161/CIRCULATIONAHA.115.019692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson D, Ambler G, Shakeshaft C, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018;17(6):539. doi: 10.1016/S1474-4422(18)30145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elder T, Raghavan A, Smith A, et al. Outcomes After Intracranial Hemorrhage in Patients with Left Ventricular Assist Devices: A Systematic Review of Literature. World Neurosurg 2019;132:265–272. doi: 10.1016/j.wneu.2019.08.211 [DOI] [PubMed] [Google Scholar]
- 100.Boyle AJ, Russell SD, Teuteberg JJ, et al. Low Thromboembolism and Pump Thrombosis With the HeartMate II Left Ventricular Assist Device: Analysis of Outpatient Anti-coagulation. J Hear Lung Transplant 2009;28(9):881–887. doi: 10.1016/j.healun.2009.05.018 [DOI] [PubMed] [Google Scholar]
- 101.Bower MM, Sweidan AJ, Shafie M, Atallah S, Groysman LI, Yu W. Contemporary Reversal of Oral Anticoagulation in Intracerebral Hemorrhage. Stroke 2019;50(2):529–536. doi: 10.1161/STROKEAHA.118.023840 [DOI] [PubMed] [Google Scholar]
- 102.Cho SM, Moazami N, Katz S, Starling R, Frontera JA. Reversal and Resumption of Antithrombotic Therapy in LVAD-Associated Intracranial Hemorrhage. Ann Thorac Surg 2019;108(1):52–58. doi: 10.1016/j.athoracsur.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 103.Wong JK, Chen PC, Falvey J, et al. Anticoagulation Reversal Strategies for Left Ventricular Assist Device Patients Presenting with Acute Intracranial Hemorrhage. In: ASAIO Journal Vol 62. Lippincott Williams and Wilkins; 2016:552–557. doi: 10.1097/MAT.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 104.Baharoglu MI, Cordonnier C, Salman RAS, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016;387(10038):2605–2613. doi: 10.1016/S0140-6736(16)30392-0 [DOI] [PubMed] [Google Scholar]
- 105.Divani AA, Liu X, Di Napoli M, et al. Blood Pressure Variability Predicts Poor In-Hospital Outcome in Spontaneous Intracerebral Hemorrhage. Stroke 2019;50(8):2023–2029. doi: 10.1161/STROKEAHA.119.025514 [DOI] [PubMed] [Google Scholar]
- 106.Manning L, Hirakawa Y, Arima H, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: A post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol 2014;13(4):364–373. doi: 10.1016/S1474-4422(14)70018-3 [DOI] [PubMed] [Google Scholar]
- 107.Lobanova I, Qureshi AI, Huang W, et al. Outcomes of Intensive Systolic Blood Pressure Reduction in Patients With Intracerebral Hemorrhage and Excessively High Initial Systolic Blood Pressure: Post Hoc Analysis of a Randomized Clinical Trial. JAMA Neurol 2020;77(11):1355–1365. doi: 10.1001/JAMANEUROL.2020.3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022;53:282–361. doi: 10.1161/STR.0000000000000407 [DOI] [PubMed] [Google Scholar]
- 109.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 2005;365(9457):387–397. doi: 10.1016/S0140-6736(05)70233-6 [DOI] [PubMed] [Google Scholar]
- 110.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013;382(9890):397–408. doi: 10.1016/S0140-6736(13)60986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stevens RD, Shoykhet M, Cadena R. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocrit Care 2015;23:76–82. doi: 10.1007/s12028-015-0168-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuramatsu JB, Huttner HB. Management of oral anticoagulation after intracerebral hemorrhage. Int J Stroke 2019;14(3):238–246. doi: 10.1177/1747493019828555 [DOI] [PubMed] [Google Scholar]
- 113.Sembill JA, Kuramatsu JB, Schwab S, Huttner HB. Resumption of oral anticoagulation after spontaneous intracerebral hemorrhage. Neurol Res Pract 2019;1(1). doi: 10.1186/s42466-019-0018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuramatsu JB, Sembill JA, Gerner ST, et al. Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. Eur Heart J 2018;39(19):1709–1723. doi: 10.1093/eurheartj/ehy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 116.Parikh NS, Cool J, Karas MG, Boehme AK, Kamel H. Stroke risk and mortality in patients with ventricular assist devices. Stroke 2016;47(11):2702–2706. doi: 10.1161/STROKEAHA.116.014049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ovsenik A, Podbregar M, Fabjan A. Cerebral blood flow impairment and cognitive decline in heart failure. Brain Behav 2021;11(6). doi: 10.1002/brb3.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pavol MA, Boehme AK, Willey JZ, et al. Predicting post-LVAD outcome: Is there a role for cognition? Int J Artif Organs 2021;44(4):237–242. doi: 10.1177/0391398820956661 [DOI] [PubMed] [Google Scholar]
- 119.Bhat G, Yost G, Mahoney E. Cognitive function and left ventricular assist device implantation. J Hear Lung Transplant 2015;34(11):1398–1405. doi: 10.1016/J.HEALUN.2015.05.015 [DOI] [PubMed] [Google Scholar]


