Abstract
Apricot (Prunus armeniaca L.) kernels, one of the economical stone fruit kernels, are utilized worldwide for edible, cosmetic, and medicinal purposes. Oil from the apricot kernel is valued by the richness of unsaturated fatty acids, the high proportion of oleic acids, phenols, and tocopherol content. Oil yield with quality from apricot kernel varies with region, variety, and adopted method of oil extraction. This review discusses apricot kernel characterization, different conventional and novel methods of oil extraction, their merits and demerits as reported in the literature. Novel technologies such as microwave-assisted oil extraction, ultrasound-assisted oil extraction, enzyme-assisted oil extraction, and supercritical fluid oil extraction have emerged as the most promising extraction methods that allow efficient oil recovery in very environment-friendly ways. Knowledge of the extraction technique aids in giving higher oil recovery with minimal nutritional losses while retaining the original organoleptic properties.
Graphical abstract
Keywords: Apricot kernel, Quality, Oil extraction method, Oil recovery, Oil application
Introduction
Apricot, (Prunus armeniaca L.), in the family Rosaceae, is the most significant economic stone fruit, grown in temperate regions of the world (İncedayi et al., 2016; Sharma et al., 2014). Apricots are native to Armenia and are now grown in many temperate regions of other nations (Jo et al., 2015). During the last decade, apricot production has increased tremendously throughout the world. In the year 2020, global apricot harvested area and production were 562,475 ha and 3.72 million MT, respectively (FAO, 2022). Turkey is the highest apricot-producing country, followed by Uzbekistan, Iran, Algeria, Italy, Afghanistan, Spain, Greece, Pakistan, and Morocco.
Apricot fruits are a rich source of beneficial nutrients like sugars, carbohydrates, vitamins (A, C, thiamine, riboflavin, niacin), minerals, fibers, and many bioactive phytochemicals (phenolics, carotenoids, and antioxidants) (Ali et al., 2015; Wani et al., 2018). Mostly, apricot fruits are consumed fresh as table fruit and utilized for different value-added products viz dried apricot, juices, jams, chutney, alcoholic beverages, leather, toffee, and fruit bars (Saini et al., 2021; Sharma et al., 2014). Due to the high number of antioxidants, it has pharmacological importance as well. It is used as an emetic, antipyretic, antiseptic, ophthalmic and mild laxative (Ali et al., 2015). Apricot fruit is also reported to serve as hepatoprotective, antimicrobial, anti-inflammatory, antimutagenic, and cardioprotective (Tabasum et al., 2018).
Apricot kernel
Apricot kernel is the innermost part of the apricot fruit. Most of the time, apricot fruit seeds are discarded as waste or byproduct in the food processing units (Gezer et al., 2011), but it has good potential source of oil, protein, vitamins, fibre, essential minerals, and many bioactive components (Akhone et al., 2022). Stone/pit are obtained after the separation of fruit pulp. Stones are then dried and decorticated to get apricot kernel (Gupta and Sharma, 2009a; Kate et al., 2014b). Apricot fruit has an average weight in the range of 8.0–15.1 g, which gives about 12.7–22.2% stone recovery after the removal of the pulp. Apricot stones contain about 30.7–33.7% of the kernel (Gupta et al., 2012). Apricot kernels are rich sources of essential nutrients that are beneficial for the human body. Generally, sweet apricot kernels are consumed as dry fruit, whereas bitter kernels are utilized for the extraction of oil (Targais et al., 2011). Majorly it contains high amount of oil, mainly unsaturated fatty acids (approximately 50%), protein content (23–27%), as well as different essential amino acids, which account for 32–34% of total amino acids (Liu and Zhang, 2022; Zhang et al., 2020). It also has the richness of total tocopherol (Kostadinović Veličkovska et al., 2018). It possesses a high amount of minerals, especially potassium and magnesium (Shariatifar et al., 2017). Kernels have several pharmacological components which act as anti-inflammatory, anti-cancer, anti-oxidant, anti-asthmatic, and anti-microbial activities (Saini et al., 2021).
Apricot kernels, based on taste, are divided into three categories, viz. sweet apricot, semi-bitter apricot, and bitter apricot (Cherif et al., 2021; Kate et al., 2014a). The physical properties and proximate composition analysis of apricot kernels from previous studies of different regions are compiled and presented in Tables 1 and 2. These comparative tables show variability in physical properties and proximate composition analysis depending on the region and cultivar.
Table 1.
Physical properties of apricot kernel
| Cultivar location | Malatya, Turkey | Iran | West Azarbayjan, Iran | Egypt |
|---|---|---|---|---|
| Geometrical mean diameter (mm) | 9.75–10.21 | 9.79 | 9.13–9.78 | 8.4 |
| Sphericity (%) | 55–69 | 62.21 | 59.79–62.21 | 59.26 |
| Surface area, (mm2) | – | 302.17 | 264–302 | 222 |
| Volume, (cm3) | 0.45–0.58 | 0.46 | 0.442–0.463 | 0.312 |
| True density (kg/m3) | 930.89–1117.02 | 983.30 | 882.58–983.4 | 896.26 |
| Bulk density (kg/m3) | 555.45–574.62 | 406.79 | 406.8–471.6 | 424.71 |
| Porosity (%) | 39.98–49.95 | 51.32 | 51.33–52.68 | 52.61 |
| 1000 kernel mass (kg) | 0.46–0.58 | 0.45 | 0.382–0.448 | 0.723 |
| Coeff. static friction for wood | 0.474–0.516 | 0.38 | 0.368–0.3838 | 0.45 |
| Rupture force (N) | 40.17–155.13 | 16.20–91.22 | 16.6–23.44 | 67.9 |
| References | Gezer et al. (2003) | Ahmadi et al. (2009) | Fathollahzadeh et al. (2008) | Fayed et al. (2020) |
Table 2.
Proximate composition of apricot kernel
| Apricot varieties and location | Oil, % | Protein, % | Ash, % | Crude fiber, % | Carbohydrate, % | mg HCN/ 100 g kernel |
|---|---|---|---|---|---|---|
|
Çataloglu, Hachaliloglu and Hasanbey (sweet) Çöloglu (bitter), Malatya province in Turkey Özcan (2000) |
46.3–51.4 | 23.58–27.70 | 2.10–2.67 | 13.49–17.98 | – | – |
|
Wild apricot kernel, Himachal Pradesh, India Sharma et al. (2010) |
45–50 | 23.6–26.2 | 4.2 | 5.42 | 8.2 | 90 |
|
Bitter apricot kernels, Himachal Pradesh, India Gupta et al. (2012) |
45.6–46.3 | 24.4 | 2.2–2.4 | 5.4 | 8.2 | 148–173 |
|
Halmas, Nari, Travet and Charmagzi: Swat, Khyber and Pukhtonkha, Pakistan Manzoor et al. (2012) |
32.23–42.51 | 13.21–20.90 | 2.11–3.89 | 5.13–9.81 | – | – |
|
Early Orange, Goldrich Sun giant, Harcot, Hargrand and Somo, South-eastern Poland Stryjecka et al. (2019) |
32.2–44.2 | 14.9–19.2 | 3.07–4.82 | 5.17–9.17 | – | – |
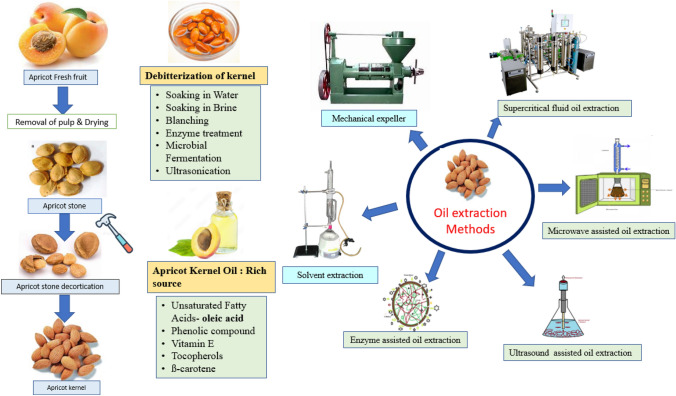
Debittering/detoxification of apricot kernel
Bitter apricot kernels have a strong bitter flavor & are toxicity due to the presence of amygdalin (C20H27NO11) (Akhone et al., 2022), which, after enzymatic breakdown, releases hydrocyanic acid (HCN) (Rampáčková et al., 2021; Silem et al., 2006). The HCN content in apricot seeds varies with cultivar/variety, region altitude, and maturity. Based on the amount of amygdalin content in apricot it has distinguished in sweet and bitter apricot (Huang et al., 2022). The bitter apricot kernel contains a high amount of HCN (220.55 to 317.7 mg/100 g) as compared to the sweet apricot kernel (30.20–79.20 mg/100 g). Amygdalin oral acute toxic dose is 0.1–0.2 g for humans and animals, which is equal to 20–30 apricot kernels (Song et al., 2017). Many researchers reported that detoxification of apricot kernel is necessary before consumption because of the possible toxicity of cyanide (Saini et al., 2021). The common principle for detoxification of apricot kernels is the removal of amygdalin content by soaking kernels in water or brine solution for a specific time and temperature. Apricot kernel grinding & soaking operation causes significant degradation of amygdalin due to endogenous ß- glycosidases (Silem et al., 2006). However, detoxification by water-soaking of kernels also adversely affects quality in terms of the leaching of soluble nutritional components, oxidation, and rancidity of products (Song et al., 2017). Therefore, nowadays, accelerated debittering/detoxification methods have been practiced for the removal of HCN from the apricot kernels, including soaking in water, fermentation, enzyme treatment, ultrasound treatment, microwave treatment, and vacuum methods (Song et al., 2017; Zhang et al., 2019). Amygdalin content upto 5 mg/kg in bitter apricot oil is considered as safe (Jin et al., 2018).
Apricot kernel oil (AKO) extraction
Apricot kernels are obtained from the decorticated/crushed stone, followed by separation (specific gravity separator) and kernel drying (Gupta and Sharma 2009a). Drying by sun exposure or mechanical dryer leads to reduce moisture content, which helps to minimize microbial activities and deteriorating chemical composition (Ouzir et al., 2021). Generally, kernels used for oil extraction should have moisture content below 10 percent (Kate et al., 2014a). Oil recovery depends on the cultivar, moisture content, pretreatments, and method of oil extraction. Many researchers have worked on oil yields from different apricot verities by different methods and have reported oil yields from 28.26 to 42.48% (Gezer et al., 2011), 38 to 55.3% (Shariatifar et al., 2017), 27.2–61.4% (w/w) dry weight basis (Górnaś et al., 2017) and 36.65 to 43.77% (Al-Juhaimi et al., 2021).
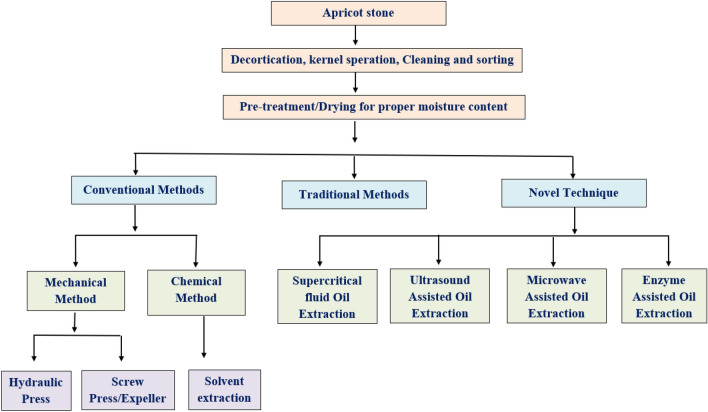
The Apricot kernel oil (AKO) can be extracted by different techniques such as traditional methods, conventional methods (mechanical methods, chemical methods), or combinations of these methods. Nowadays, many studies on novel oil extraction methods, viz. ultrasound-assisted extraction (Gayas et al., 2017), supercritical fluid extraction (Özkal et al., 2005), microwave-assisted extraction, and enzyme-assisted extraction (Bisht et al., 2015b) have been undertaken with the objectives of higher oil yield and retention of nutritional quality (Fig. 1).
Fig. 1.
Apricot kernel oil extraction methods
Traditional method
Traditional methods vary from region to region and are still practiced in some remote villages in the Ladakh region of India (Targais et al., 2011). Apricot stones are collected and stored in gunny bags during the fruiting season. At the time of utilization, stones are decorticated manually with the help of rounded stones. Kernels are sundried for 1–2 days for better oil extraction (Dwivedi and Dwivedi, 2007). Kernels are ground to obtain dough with the help of stone. The stone is shaped in such a way that a cup-shaped depression is given at one end to collect the oil. During oil extraction, a small amount of water is sprinkled on the dough and the stone is warmed with the help of fire under the stone. Oil from this method possesses a typical aroma which is lacking in the other oil extracted by modern techniques (Targais et al., 2011). Recovery from one kg apricot kernel is about 35–37% in this method (Dwivedi and Dwivedi, 2007; Kate et al., 2014a).
Conventional methods
Mechanical methods
In these methods, mechanical force is applied to kernels so that the oil-bearing cell wall is broken and oil is released easily. These methods are highly commercialized due to lower initial investment costs and simple operation. Oil quality obtained by this method has often been analyzed in terms of its iodine value, acid value, water content, and phosphorus content (Nde and Anuanwen, 2020). Mechanical oil extraction is usually done by power Ghani and oil expellers (screw type and hydraulic press). These expellers can be manually or animal driven or motor operated. Screw types are considered more efficient than the hydraulic press in terms of oil recovery (Gayas and Kaur, 2017). Oils obtained with the hydraulic press are considered as cold press oil, while during screw press oil temperatures may reach above 40–50 °C due to the friction between kernel and barrel/screw that causes a slight rise in the acid value, peroxide value, and oxidative stability (Catalán et al., 2016). The benefits of the mechanical press are that it does not require the use of solvents or additional heat. Thus, it helps in the retention of phenolics, flavonoids, essential fatty acids, and tocopherols in the oil (Pavlović et al., 2018). Gupta and Sharma (2009a) worked on apricot kernels oil extraction by using table oil expeller, baby oil expeller, and oil press (power Ghani) and reported oil yield as 37.5–38.5%, 31.5–32.0%, and 28.5–29.0%, respectively. It was also reported that the apricot oil yield from table oil expeller (43.5%) was higher than the traditional kolhu method (32.5%) (Kate et al., 2014a).
Solvent oil extraction method
In the solvent oil extraction method, oil from seeds is dissolved in solvents, and then by distillation, it is recovered from the solvent (Qin and Zhong, 2016). It is one of the widely applied methods for the extraction of oil from organic materials. Solvents usually used are hexane, diethyl ether, petroleum ether, and ethanol. Solvent selection is based on solubility, solvent–solute ratio, oil viscosity and solvent polarity, cost, and market availability (Nde and Anuanwen, 2020). Ethanol is generally regarded as safe for food oil extraction, but its oil extraction efficiency is lesser than conventional n-hexane solvent, which is toxic in nature (Gayas and Kaur, 2017). Oil extracted by solvent extraction is free from HCN. Gupta and Sharma (2009a) reported higher oil recovery (45.6–47.0%) through solvent extraction than mechanical oil expeller.
Novel techniques
Many drawbacks, like low oil recovery, degradation during processing, and low storage life of the oil, have been observed in conventional methods (Rajendran and Gurunathan, 2021). These can be overcome by adopting novel techniques to obtain high oil recovery, good oil quality, and reduced oil wastage in oil cake (Nde and Anuanwen, 2020). During the last few decades, there have been many researchers working on novel techniques for the extraction of apricot kernel oil. The novel techniques such as Supercritical fluid oil extraction (SC-FOE), Ultrasound-assisted oil extraction (UAOE), Microwave-assisted oil extraction (MAOE), Enzyme-assisted oil extractions (EAOE), etc., are discussed in the following subsections.
Supercritical fluid oil extraction method (SC-FOE)
Supercritical fluid extraction is a separation process in which the solvent is in supercritical condition. Under the controlled condition, the substance at pressure and temperature above its critical point acts as a supercritical fluid (Catalán et al., 2016). Its properties get changed such that it diffuses easily through solids matrices like gas and dissolves material like liquid (Ahangari et al., 2021). Carbon dioxide, ethanol, propane, ethane, butane, and water are generally used as supercritical solvents. Carbon dioxide (critical temperature-31 °C, critical pressure-73.8 bar) is mostly preferred due to its environment-friendly properties like non-flammable and non-polluting nature (Qi et al., 2019). Supercritical CO2 extraction is an excellent technique for nonpolar analytes. The benefits of CO2 as a solvent are easy availability and low toxicity (Chemat et al., 2019).
Özkal et al. (2005) worked on the application of supercritical carbon dioxide for apricot kernel oil extraction for higher oil recovery. They reported parameters viz. solvent flow rate, temperature, pressure, and co-solvent concentration produce a significant effect on oil recovery. The mass transfer coefficient of the fluid is increased with decreased particle size and pressure. SC-CO2 extraction has major advantages over conventional oil extraction, such as a higher number of tocopherols and lesser amygdalin content (Pavlović et al., 2018). The value of fatty acid composition in oil by SC-CO2 extraction method was found similar to that of the cold press method and (hexane) solvent extraction method (Pavlović et al., 2018; Ouzir et al., 2021).
Ultrasound assisted oil extraction (UAOE)
UAOE is non-thermal technology in the field of oil extraction (Tiwari, 2015). Extraction temperature is one of the major contributing factor for oil recovery. Ultrasound waves (20–100 kHz) generate form of energy that has power to alter the physical and chemical properties of the exposed subject (Gayas et al., 2017; Singla and Sit, 2021). The mechanism of ultrasound assisted extraction (UAE) is often attributable to combine effect of the cavitation's, as well as the ultrasound's mechanical and thermal impacts, which can damage cell walls, lower particle sizes, and improve mass transfer, resulting in improved extraction efficiency (Fan et al., 2019; Zhang et al., 2020). Ultrasound penetrates deeply through solvent into solid and increases reaction area; as a result, it can easily diffuse the compounds into solvent (Mushtaq et al., 2020). The use of ultrasonic pre-irradiation may minimize the time it takes to extract oil from edible oils derived from plants, improving throughput in the commercial oil manufacturing operation (Sharma and Gupta, 2006).
Gayas et al. (2017) worked on apricot oil extraction by using ultrasound assisted method, and reported that the rheological characteristics were significantly affected by ultrasound. Shear values increased as exposure duration and increased temperature. Ultrasonic pre-treatment before aqueous enzymatic oil extraction improved apricot oil recovery in range 19–22% w/w, it also reduced the overall process time from 18 to 6 h (Sharma and Gupta, 2006). Ultrasound irradiation on apricot kernels helps to improve ß- glucosidase activity by the 34.67%, with optimized ultrasound parameters such as the temperature, ultrasound power, exposure time and frequency (Fan et al., 2019). Ultrasound irradiation has been shown speed up the debitterization of apricot kernels by increasing mass transfer and D-amygdalin breakdown (Zhang et al., 2019).
Microwave assisted oil extraction (MAOE)
Microwave assisted extraction has gained attention due to its faster heating which results in low extraction time (Nde et al., 2015). Microwave heats the particle with a dual component of ionic conduction and dipole rotation (Mandal et al., 2007). Microwaves are electromagnetic waves with frequencies ranging from 300 MHz to 300 GHz (Bagade and Patil, 2021). In food, microwave application are executed at frequencies of 915 MHz at industrial scale and 2450 MHz in domestic ovens (Gayas and Kaur, 2017). The principle of effective extraction is microwave penetrates in cell membrane and cause destruction of cell wall, denaturation of protein and also moves mass and heat transfer inside to outside (Kate et al., 2020). Tiger nut treated with microwave, scanning electron microscope micrographs showed rupture of cell membrane results in rapid and efficient oil extraction (Hu et al., 2018). Also, there was no negative effect found for the quality of extracted oil and fatty acid composition (Lozano-Grande et al., 2019; Rezvankhah et al., 2019).
Roasting of apricot kernel effects on oil yield and adds roasting aroma, and flavor. Tocopherols content, oxidative stability, antioxidant capacity, antiradical activity of AKO is improved due to roasting as compared to non-roasted AKO (Durmaz et al., 2010). Al-Juhaimi et al. (2021) worked on microwave roasting followed by oil extraction. They found increase in oil recovery from apricot (variety-Alyanak) kernel up to 43.77%, that is higher than oven roasted apricot kernel. The total phenolic contents, antioxidant activity, gallic acid contents, 3,4-dihydroxybenzoic acid contents, palmitic acid contents, oleic acid contents, linoleic acid contents in microwave roasted apricot kernel oil, are significantly higher in comparison with oven roasted apricot kernel. Another study, Jin et al. (2018) worked on volatile compounds and quality changes of bitter apricot kernel oil (AKO) at different roasting temperature 120, 130, 140 and 150 °C for 15 min. They reported AKO having a pleasant aroma with typical flavor and useful as edible oil. Sensory evaluation showed that roasted, nutty, sweet and oily aromas increased with increased roasting temperature. Hojjati et al. (2016) applied microwaves (480 W for 3 or 4 min) for roasting of wild almonds and they found roasting causes increase in oil recovery with characteristics aroma compounds. Microwaves for roasting wild almond are a quick, safe, and economical method.
Enzyme assisted oil extraction (EAOE)
Major component of cell wall of plant cell are made up from polysaccharides such as cellulose, hemicelluloses, starch, pectin, which are responsible for oil extraction efficiency (Nadar et al., 2018). The principle of EAOE is to hydrolyze the cell wall with the help of enzyme as a catalyst to liberate the oil (Garofalo et al., 2021). Many commercial enzymes such as protease, hemicellulose, cellulase, pectinase, protease, amylase, glucanase and many more are used as pretreatment before the extraction of oil. Most of the studies suggested that the mixture of enzymes in proper proportion gives better results (Liu et al., 2016). Selection of enzyme should be based on its specific functionalities at optimum process condition like temperature, pH, concentration, treatment duration, particle size, structure of cell wall (Puri et al., 2012).
Bisht et al. (2015b) worked on EAOE from apricot kernel by using enzymes viz. Pectazyme (Pectinase), Mashzyme (Cellulase) and mixture of Pectazyme + Mashzyme. They reported increase in oil recovery without any adverse effect of enzyme on oil quality, hence recommended for industrial commercial application. In comparison of solvent extracted oil with enzyme assisted extracted oil, both showed similar values for fatty acid profile, refractive index and saponification value for wild almond (Balvardi et al., 2015). Mahatre et al. (2012) studied optimization of enzyme assisted apricot kernel oil extraction with combination of enzyme Pectolytic and Cellulolytic in the ratio ranges from 30:70 to 70:30. They reported that important factors responsible for oil yield in decreasing order such as incubation period, enzyme ratio, moisture content and enzyme concentration (Tables 3 and 4).
Table 3.
Merits and demerits of different apricot kernel oil extraction methods
| Extraction method | Type of Equipment used | Merits | Demerits | References |
|---|---|---|---|---|
| Traditional method |
Pestle and mortar (Locally known as rThun and Ton-tsig) |
∙ Produced oil having distinct smell ∙ Less degradation of bioactive components ∙ Highley preferred by the consumers ∙ Aids in efficient use of the by-products such as the oilseed cake and shell |
∙ Unhygienic, Tedious, Time consuming ∙ Low oil yield ∙ Poor oil quality (HCN content) |
Dwivedi and Dwivedi (2007); Targais et al. (2011); Kate et al. (2014a) |
| Mechanical method | Oil Press (Ghani), Screw press, Hydraulic Press |
∙ Better oil yield ∙ Low cost of operation ∙ Light-colored oil with low free fatty acids |
∙ Oil contained HCN ∙ Loss of bioactive compound (hot press) |
Gupta and Sharma (2009a); Kate et al. (2014a); Bisht et al. (2015b) |
| Solvent oil extraction method | Solvent extractor |
∙ Oil is completely free of HCN ∙ High oil recovery as compared to other methods |
∙ Loss of bioactive compound ∙ Long processing times ∙ high solvent requirement ∙ high investment, ∙ Potential hazards to environment |
Gupta et al. (2012); Mahatre et al. (2012) |
| Supercritical fluid oil extraction | Supercritical fluid extraction system |
∙ Yields high oil recovery ∙ Environment friendly technique ∙ Separation of many compounds having different polarity and molecular compound ∙ Used for the extraction of valuable compounds such as vitamins, aromas, natural pigments or essential oils |
∙ Poor solubility for polar composites ∙ High power required ∙ High initial investment |
Pavlović et al. (2018); Ouzir et al. (2021) |
| Ultrasound assisted oil extraction | Ultrasonic cleaning bath + solvent |
∙ High productivity, yield and selectivity ∙ Environment friendly ∙ More favourable for thermally unstable compound ∙ Reduces extraction time for oil |
∙ Quality impairment of some products by the appearance or off flavours ∙ Modification in physical parameters ∙ Degradation of major and minor compounds ∙ Capable of producing radicals in molecules such as OH and H radicals |
Gayas et al. (2017); Nde and Anuanwen (2020) |
| Microwave assisted oil extraction method | Microwave oven + Solvent/Expeller |
∙ Low processing time ∙ Requires lesser solvent ∙ Produces high oil yield ∙ Lower energy requirement ∙ Environmentally friendly |
∙ High cost of operation ∙ Chances of degrading oil ∙ High susceptibility to oxidation and degradation |
Hu et al. (2018) and Kate et al. (2020) |
| Enzyme assisted oil extraction | Enzyme treatment + incubation + Solvent/Expeller |
∙ Minimizes usage of solvents, ∙ Increases oil yield ∙ Retains quality of product ∙ Environment friendly ∙ No adverse effect of enzyme on oil quality |
∙ High enzyme cost leads to increased operational cost ∙ Proper concentration of enzyme should be used, else require de-emulsification |
Bisht et al. (2015b) and Mahatre et al. (2012) |
Table 4.
Novel method application for apricot kernel oil extraction
| Sample | Location | Type of oil extraction method | Parameter Studied | Solvent/ Enzyme | Optimum Condition | Outcome/ Yield | References |
|---|---|---|---|---|---|---|---|
| Apricot kernel | Turkey | SC-CO2 extraction | Solvent mass rate: (2, 3 and 4 g/min), Temperature: (40, 50 and 60 °C), Co-solvent concentration (0, 1.5 and 3% ethanol), Pressure (30, 37.5 and 45 MPa) | SC-CO2 + ethanol | Particle diameter 0.850 mm, 4 g/min solvent flow rate, 3% ethanol at 45 MPa and 60 °C, 15 min extraction | 0.26 g/g kernel | Özkal et al. (2005) |
| Hungarian best—sweet apricot kernel | Kneževivinogradi, Croatia | SC-CO2 system | Extraction pressure:300 bar, Temperature: 40 °C, Mass flow: 2 kg/h | SC-CO2 | – | Yields Oil with high concentration of fatty acids and tocopherols | Pavlović et al. (2018) |
| Apricot kernel | Jammu & Kashmir, India | Ultrasonic bath (40 kHz) + Solvent extraction method | Temperature: (30, 45, 60 °C), Time: (30, 40, 50 min), Solvent solute ratio: (15:1, 20:1, 25:1) | n-hexane | Extraction time: 43.95 min; Extraction temperature: 51.72 °C; Solvent to sample ratio: 19.8:01, ultrasound frequency: 40 kHz | 40.86 to 46.01% | Gayas et al. (2017) |
| Apricot seeds | India | Ultrasonic pre-irradiation + Aqueous enzymatic oil extraction (AEOE) | – | Protizyme™ (mixture of three proteases) | pH 4.0, 40 °C. Ultrasonic pre-irradiation at 70 W for 2 min, Reduced the extraction time to 6 h | 77% w/w (Increased yield range 19–22%) | Sharma and Gupta (2006) |
| Apricot kernel |
Konya, Turkey |
Microwave-roasted + Solvent + Ultrasonic water bath + Centrifugation |
Microwave power capacity: 720 W/25 min, working frequency: 2450 MHz | Methanol, n-hexane | – | 36.65% (unroasted) to 43.77% (microwave-roasted) | Al-Juhaimi et al. (2021) |
| Bitter apricot | Hebei Province, China | Oven roast + Oil Press (Laboratory scale) | Roasting temperatures: 120, 130, 140 and 150℃ for 15 min | Not Applicable | 140℃ most suitable roasting temperature | Pyrazines (aroma) increased | Jin et al. (2018) |
| Apricot kernel | Uttarakhand, India | Enzyme treatment + Oil expeller | Pectinase, cellulase and combination pectinase + cellulase, Concentration: 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7%, 2 h at 50 (± 2) ºC | Pectinase, cellulase and combination (pectinase + cellulase) |
0.3% (Pectinase + cellulase) combination, 2 h at 50 (± 2) ºC |
47.3% oil recovery | Bisht et al. (2015b) |
| Wild apricot kernel | Uttarakhand, India | Enzyme treatment + Solvent extraction |
Moisture content: 20 –32% (wet basis), Enzyme concentration: 12–16%, Enzyme ratio: 3:7–7:3 (pectolytic: cellulolytic), Incubation period: 12–16 h |
Mixture of enzyme (Pectolytic and Cellulolytic): (30:70–70:30) | 60:40 (pectolytic: cellulolytic), 13 h | 0.14 to 2.53% | Mahatre et al. (2012) |
Apricot kernel oil (AKO)
AKO is gaining importance amongst the consumers due to its immense health benefitting properties. Bitter apricot kernel analysis shows that the kernel is a rich in oil (up to 54.21%) having 94.4% unsaturated fatty acids and low amount of saturated fatty acids (Kate et al., 2018; Zhou et al., 2016). The major fatty acids present in AKO are oleic acid (57.9–68.43%), linoleic acid (22.82–30.4%), linolenic acid (0.25–0.30%), palmitic acid (3.9- 8.1%), stearic acid (1.37–1.7%) and palmitoleic acid (0.98–1.3%) (Azcan and Demİrel, 2012; Jin et al., 2018). Additionally, AKO have many bioactive components such as ß-carotene (61.05 mg/g), tocopherols (50.76 mg/100 g), phenolic compounds, campesterol (11.8 mg/100 g), stigmasterol (9.8 mg/100 g), sitosterol (177.0 mg/100 g) and pro-vitamin A (Zhou et al., 2016). AKO is composed from 98% triacylglycerols, 1.1% phospholipids, 0.2% free fatty acids, and 0.7% unsaponifiable matters of the oil weight. In AKO triglyceride composition differences are due to incompletely separation of some triglycerides (Kiralan et al., 2019). Uluata (2016) reported total phenolic content of oil was 24.9 and 26.9 μg gallic acid/g oil and antioxidant activity were 137.5–238.9 μg Trolox/g oil, 151.2–168.8 μg Trolox/g oil and 110.6–162.6 μmol/100 g oil using DPPH, ABTS and ORAC assays respectively. Owing to vitamin E rich contents, these oils are suitable for use in preparation of cosmetic and moisturizing creams for dry skins, massaging oils and for industrial use (Gupta et al., 2012). High amount of fatty acid composition and biologically active compounds in AKO leads to suitability of oil for edible purpose as well as pharmaceutical applications. Characteristic aroma and pale light-yellow color of the oil leads to high overall acceptability by consumers. The dominant volatile compounds in AKO are ethyl acetate, hexyl acetate, limonene, 6-methyl-5-hepten-2-one, menthone, (2E)-2-hexenal, linalool, ß-ion-one, and ɣ-decalactone (Kiralan et al., 2018; Zhou et al., 2016). A study on the apricot has revealed that the composition of fatty acid, triacylglycerol and bioactive component is varied depending on the maturity, variety, altitude, location, and other environmental condition (Cherif et al., 2021; Naryal et al., 2019). The physico-chemical properties of AKO from different regions are presented in Table 5.
Table 5.
Physico-chemical properties of AKO from different location
| Characteristic | Values (Min to Max) | ||||
|---|---|---|---|---|---|
| Location | Uttarakhand, India | Himachal Pradesh, India | Malatya province of Turkey | Swat, Khyber and Pukhtonkha, Pakistan | South Eastern Poland |
| Visual appearance/color | Pale Yellow | Yellowish, Light to deep | – | – | – |
| Refractive index (40 °C) | 1.4638 | 1.465–1.475 | 1.527–1.549 | 1.465–1.479 | 1.4449–1.4785 |
| Specific gravity (25 °C) | 0.9172 | 0.91–0.917 | – | 0.87–0.93 | – |
| Iodine value (g I2/100 g oil) | 102 | 87.2–109.9 | – | 96.4–106.3 | 95.7–101.8 |
| Saponification value (mg KOH/g oil) | 190 | 185–194.4 | – | 189.1–199.4 | 185–195 |
| Acid value (mg KOH/g oil) | 4.05 | 3.6–5.27 | – | – | – |
| Peroxide value (meq/kg oil) | – | 5.12–5.39 | 0.833–8.294 | – | – |
| References | Bachheti et al. (2012) | Gupta et al. (2012) | Özcan et al. (2010) | Manzoor et al. (2012) | Stryjecka et al. (2019) |
Storage stability of AKO
AKO storage stability is highly susceptible for quality deterioration during storage due to high concentration of unsaturated fatty acids. During storage, lipid oxidation is major issue which leads to production of rancid aroma and adverse effect on nutritional quality of oil (Kiralan et al., 2019). Bisht et al. (2015a) worked on storage stability of wild apricot kernel oils (13.17 to 18.47 °C temperature and 60 to 70% RH) by using different packaging materials viz. transparent plastic bottles, amber colored plastic bottles, transparent polyethylene pouches and aluminum laminated pouches. They found that wild AKO in amber colored plastic bottles are highly stable and did not show any adverse effect in its physico-chemical qualities up to six months. Gupta and Sharma (2009b) studied storage stability of AKO for 6 months by using packaging viz. transparent glass bottles, amber colored glass bottles and polyethylene pouches at 20 °C. They found during storage a significant decrease in the iodine value, while significant increase in saponification value, peroxide value and acid value. AKO in amber color glass bottles and polyethylene pouches exhibited lower changes in quality characteristics as compared to oil stored in transparent glass bottles. Kiralan et al. (2018) worked and reported AKO thermal and photo-oxidation stability. They found that during storage of AKO, the peroxide value and diene values (K232) increased slowly in thermal and photo oxidation condition. Peroxide value increases above 20 meq O2/kg after 6 days storage and K232 value 3.5 after 10 days storage at 60 °C.
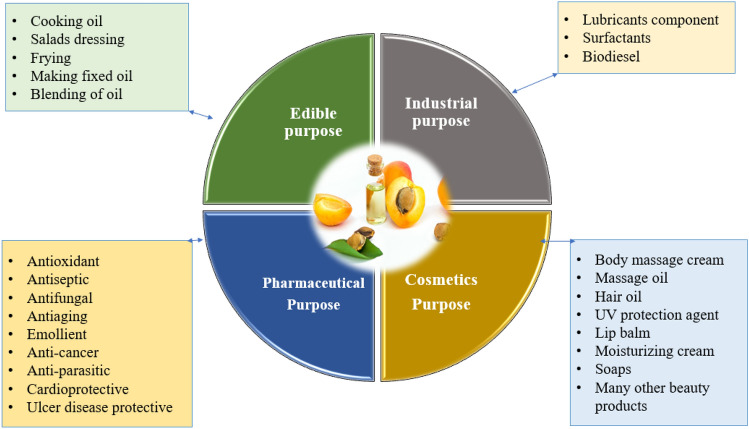
Utilization of AKO (Fig. 2)
Fig. 2.
Application of apricot kernel oil
As edible/cooking oil purpose
AKO having unique phytochemical composition and rich in tocopherol content makes it health benefitting oil. Also, AKO is rich in monosaturated fatty acids (oleic acid) hence the best potential to be used as edible oils, especially for heart attack patients. Additionally, carotenoids, benzaldehyde, and polyphenols in AKO have shown potential antioxidant and antibacterial activity, making their application as food additives or preservatives more attractive (Farag et al., 2022). Huge amount of bioactive components, natural antioxidants, phenolic compounds in AKO are utilized in the development of functional food development (Al-Juhaimi et al., 2021). AKO has a typical nutty flavour thus making it an excellent ingredient for desserts and other recipes. It is utilized for cooking and edible purpose such as salads dressing, frying and making fixed oil macaroon paste. Many bakery products like cakes, sweet biscuits and salted biscuits were prepared by using AKO without any additional oil treatment (Kiralan et al., 2019; Saini et al. 2021). Because of similar fatty acid compositions with almond oil, AKO can be utilized in the alteration of almond oil (Azcan and Demİrel, 2012). Moreover, AKO can be blended with other vegetable oils to increase their oxidative stability (Kiralan et al., 2019).
Application in pharmaceuticals
The AKO rich in essential fatty acids (linoleic and linolenic acids) aids for skin tissue care, hair nourishment, control on cholesterol metabolism and maintenance of cell membrane integrity. Thus, it possesses special pharmaceutical importance (Gupta and Sharma, 2009a). AKO is high valued for pharmaceutical components such as phenolics, carotenoids, volatiles, sterols, tocochromanols, which play important roles as antioxidant, antiseptic, antifungal, antiaging, and emollient (Pavlović et al., 2018; Yigit et al., 2009). AKO is also reported to have antimicrobial activity against some of the pathogenic bacteria like Salmonella typhimurium, Serratia marcescens, Staphylococcus aureus, E. coli and Streptococcus pyogenes (Saini et al., 2021). Many studies reported that AKO’s pharmacological effects are due to high contents of specific antioxidants, and mono and polyunsaturated fatty acids, which act as anti-cancer, anti-parasitic, anti-atheroscleration, cardioprotective, anti-anginal, anti-aging, hepatoprotective, renoprotective, ulcer diseases protective (Jaafar, 2021). In some remote villages AKO are still used to relieve backache and joint aches (Prakash et al., 2020). Squalene present in AKO acts as precursors of vitamin D, steroids hormones and cholesterols, also helps in protection against oxidative DNA damage in human mammary epithelial cells (Kiralan et al., 2019).
Application in cosmetics
AKO being rich in Vitamin E and fatty acid, are used in making cosmetic products like body massage cream, massage oil, hair oil, UV protection agent, lip balm, moisturizing cream, soaps, scrubbing cleaning cosmetic creams and many beauty products (Gupta et al., 2012; Stryjecka et al., 2019). Moreover, nowadays application of AKO as base oil for aromatherapy, sunbathing is widely used (Kiralan et al., 2019). A study reported, AKO can be utilized with palm stearin at ratio of 50% in fatty acid blend for manufacturing of toilet soap (Saini et al., 2021). Also, the cream prepared from AKO had good spread ability, absorbability, thermal stability, medium emollient, and it does not leave any oily feel on skin. AKO applied externally, protects the skin against the adverse effects of free radicals, exhibits anti-inflammatory activity, wound healing and also promotes skin barrier homeostasis due to components such as triglycerides, phospholipids, FFAs, phenolic compounds, and antioxidants, present in it (Stryjecka et al., 2019).
Industrial use
Apricot kernels have huge potential in many commercial areas like leather manufacturing, extraction of benzaldehyde (aroma industry), manufacturing of active carbon, furfural, extraction of amygdalin and hydrocyanic acid (Kaya et al., 2008). AKO has wide industrial application such as lubricants component, cosmetics, and surfactants (Jin et al., 2018). Nowadays biodiesel named Prunus armeniaca has been produced with wild AKO by trans esterification process, which can be used in automobile as substitute of regular petrol diesel fuel without making any major modification in engine (Saini et al., 2021; Yadav et al., 2018).
In conclusions, apricot fruit nutritional composition changes with origin, location, altitude, maturity, climate etc. Apricot kernels are byproduct in pulp industry and has huge potential for further oil extraction. Apricot kernels contain high amount of oil, protein, carbohydrates and many mineral contents. Amygdalin component in kernel is responsible for bittering and toxicity which are removed by grinding, soaking and other treatments. Conventional oil extraction methods are commercially used, but causes thermal degradation, oxidation and oil losses during processing. Novel oil extraction methods have several advantages with respect to oil yield, retention of oil quality and environment friendly operations. AKO is characterized by adequate proportion of free fatty acids with oleic acid as major free fatty acid, phenols and tocopherols, and hence it is traditionally used as edible oil for cooking, utilized in cosmetic products formulation, medicinal supplements and pharmaceutical products. However, very few studies have attempted to confirm the molecular mechanism of AKO for health benefits. In future, more research should concentrate on the retention of nutritional composition in oil, reduce oil losses during oil extraction, commercialization of novel oil extraction methods and molecular behavior of AKO with respect to health beneficial effects.
Acknowledgements
The authors are grateful to National Institute of Food Technology Entrepreneurship and Management (NIFTEM) (Institute of National Importance, Under MoFPI, Govt. of India), Kundli, Sonepat, Haryana, India for the institutional facility.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Krantidip R. Pawar, Email: kranti61@gmail.com
Prabhat K. Nema, Email: pknema@yahoo.co.in
References
- Ahmadi H, Fathollahz H, Mobli H. Post harvest physical and mechanical properties of apricot fruits, pits and kernels cultivated in Iran. Pakistan Journal of Nutrition. 2009;8:264–268. [Google Scholar]
- Ahangari H, King JW, Ehsani A, Yousefi M. Supercritical fluid extraction of seed oils - A short review of current trends. Trends in Food Science and Technology. 2021;111:249–260. [Google Scholar]
- Al-Juhaimi FY, Ghafoor K, Özcan MM, Uslu N, Babiker EE, Ahmed IA, Alsawmahi ON. Phenolic compounds, antioxidant activity and fatty acid composition of roasted alyanak apricot kernel. Journal of Oleo Science. 2021;70:607–613. doi: 10.5650/jos.ess20294. [DOI] [PubMed] [Google Scholar]
- Ali S, Masud T, Abbasi KS, Mahmood T, Hussai A. Apricot: nutritional potentials and health benefits-a review. Annals Food Science and Technology. 2015;16:175–189. [Google Scholar]
- Akhone MA, Bains A, Tosif MM, Chawla P, Fogarasi M, Fogarasi S. Apricot kernel: bioactivity, characterization, applications, and health attributes. Foods. 2022;11:2184. doi: 10.3390/foods11152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcan N, Demİrel E. Extraction parameters and analysis of apricot kernel oil. Asian Journal of Chemistry. 2012;24:1499–1502. [Google Scholar]
- Bachheti RK, Rai I, Joshi A, Rana V. Physico-chemical study of seed oil of Prunus armeniaca L. grown in Garhwal region (India) and its comparison with some conventional food oils. International Food Research Journal. 19: 577-581 (2012)
- Bagade S, Patil M. Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: a review. Critical Reviews in Analytical Chemistry. 2021;51:138–149. doi: 10.1080/10408347.2019.1686966. [DOI] [PubMed] [Google Scholar]
- Balvardi M, Rezaei K, Mendiola JA, Ibáñez E. Optimization of the aqueous enzymatic extraction of oil from Iranian wild almond. Journal of the American Oil Chemists' Society. 2015;92:985–992. [Google Scholar]
- Bisht TS, Sharma SK, Chakraborty B. Long term effect of different packaging materials on biochemical properties of wild apricot kernel oil. International Journal of Food and Fermentation Technology. 2015;5:69–74. [Google Scholar]
- Bisht TS, Sharma SK, Sati RC, Rao VK, Yadav VK, Dixit AK, Sharma AK, Chopra CS. Improvement of efficiency of oil extraction from wild apricot kernels by using enzymes. Journal of Food Science and Technology. 2015;52:1543–1551. doi: 10.1007/s13197-013-1155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán L, Alvarez-Ortí M, Pardo-Giménez A, Gomez R, Rabadan A, Pardo JE. Pistachio oil: A review on its chemical composition, extraction systems, and uses. European Journal of Lipid Science and Technology. 2016;119:1600126. [Google Scholar]
- Chemat F, Vian MA, Ravi HK, Khadhraoui B, Hilali S, Perino S, Fabiano Tixier AS. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules. 2019;24:3007. doi: 10.3390/molecules24163007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif AO, Messaouda MB, Abderrabba M, Moussa F. The content of carotenoids and tocochromanols in bitter, semi-sweet and sweet apricots depending on different harvest times and geographical regions. European Food Research and Technology. 2021;247:1609–1615. [Google Scholar]
- Durmaz G, Karabulut I, Topçu A, Asiltürk M, Kutlu T. Roasting-related changes in oxidative stability and antioxidant capacity of apricot kernel oil. Journal of the American Oil Chemists' Society. 2010;87:401–409. [Google Scholar]
- Dwivedi DH, Dwivedi SK. Traditional method of chuli oil extraction in Ladakh. Indian Journal of Traditional Knowledge. 2007;6:403–405. [Google Scholar]
- Fathollahzadeh H, Mobli H, Jafari A, Rafiee S, Mohammadi A. Some physical properties of tabarzeh apricot kernel. Pakistan Journal of Nutrition. 2008;7:645–651. [Google Scholar]
- Fan XH, Zhang XY, Zhang QA, Zhao WQ, Shi FF. Optimization of ultrasound parameters and its effect on the properties of the activity of beta-glucosidase in apricot kernels. Ultrasonics Sonochemistry. 2019;52:468–476. doi: 10.1016/j.ultsonch.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Farag MA, Eldin AB, Khalifa I. Valorization and extraction optimization of Prunus seeds for food and functional food applications: A review with further perspectives. Food Chemistry. 2022;388:132955. doi: 10.1016/j.foodchem.2022.132955. [DOI] [PubMed] [Google Scholar]
- Fayed MIA, El-Shal MS, Omar OA. Determination of some apricot seed and kernel physical and mechanical properties. Agricultural Engineering International: CIGR Journal. 2020;22:229–237. [Google Scholar]
- Food and Agriculture Organization. Fruit production in the World. Available from: https://www.fao.org/faostat. Accessed Jun. 21, 2022.
- Garofalo SF, Tommasi T, Fino D. A short review of green extraction technologies for rice bran oil. Biomass Conversion and Biorefinery. 2021;11:569–587. [Google Scholar]
- Gayas B, Kaur G. Novel oil extraction methods in food industry: A review. Journal of Oilseed Brassica. 2017;8:1–11. [Google Scholar]
- Gayas B, Kaur G, Gul K. Ultrasound-assisted extraction of apricot kernel oil: effects on functional and rheological properties. Journal of Food Process Engineering. 2017;40:e12439. [Google Scholar]
- Gezer İB, Haciseferoğulları H, Demir F. Some physical properties of Hacıhaliloğlu apricot pit and its kernel. Journal of Food Engineering. 2003;56:49–57. [Google Scholar]
- Gezer I, Hacıseferoğulları H, Özcan MM, Arslan D, Asma BM, Ünver A. Physico-chemical properties of apricot (Prunus armeniaca L.) kernels. South-Western Journal of Horticulture Biology and Environment. 2: 1-13 (2011)
- Górnaś P, Radziejewska-Kubzdela E, Mišina I, Biegańska-Marecik R, Grygier A, Rudzińska M. Tocopherols, tocotrienols and carotenoids in kernel oils recovered from 15 apricot (Prunus armeniaca L.) genotypes. Journal of the American Oil Chemists' Society. 94: 693-699 (2017)
- Gupta A, Sharma PC. Standardization of technology for extraction of wild apricot kernel oil at semi-pilot scale. Biological Forum-an International Journal. 2009;1:51–64. [Google Scholar]
- Gupta A, Sharma PC. Standardization of methods for apricot kernel oil Extraction, packaging and storage. Journal of Food Science and Technology. 2009;46:121–126. [Google Scholar]
- Gupta A, Sharma PC, Tilakratne BMKS, Verma A. Studies on physico-chemical characteristics and fatty acid composition of wild apricot (Prunus armeniaca L.) kernel oil. Indian Journal of Natural Products and Resources. 3: 366-370 (2012)
- Huang X, Xu J, Gao F, Zhang H, Guo L. Rapid quantitative typing spectra model for distinguishing sweet and bitter apricot kernels. Food Science and Biotechnology. 2022;31:1123–1131. doi: 10.1007/s10068-022-01095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjati M, Lipan L, Carbonell-Barrachina ÁA. Effect of roasting on physicochemical properties of wild almonds (Amygdalus scoparia) Journal of the American Oil Chemists’ Society. 2016;93:1211–1220. [Google Scholar]
- Hu B, Zhou K, Liu Y, Liu A, Zhang Q, Han G, Liu S, Yang Y, Zhu Y, Zhu D. Optimization of microwave-assisted extraction of oil from tiger nut (Cyperus esculentus L.) and its quality evaluation. Industrial Crops and Products. 115: 290-297 (2018)
- İncedayi B, Tamer CE, Sinir GÖ, Suna S, Çopur ÖU. Impact of different drying parameters on color, β-carotene, antioxidant activity and minerals of apricot (Prunus armeniaca L.). Food Science and Technology. 36: 171-178 (2016)
- Jaafar HJ. Effects of apricot and apricot kernels on human health and nutrition: a review of recent human research. Technium BioChemMed. 2021;2:139–162. [Google Scholar]
- Jin F, Wang J, Regenstein JM, Wang F. Effect of roasting temperatures on the properties of bitter apricot (Armeniaca sibirica L.) kernel oil. Journal of Oleo Science. 67: 813-822 (2018) [DOI] [PubMed]
- Jo Y, Lian S, Cho JK, Choi H, Chu H, Cho WK. De novo transcriptome assembly of two different apricot cultivars. Genomics Data. 2015;6:275–276. doi: 10.1016/j.gdata.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kate AE, Lohani UC, Pandey JP, Shahi NC, Sarkar A. Traditional and mechanical method of the oil extraction from wild apricot kernel: a comparative study. Research Journal of Chemical and Environmental Sciences. 2014;2:54–60. [Google Scholar]
- Kate AE, Lohani UC, Shahi NC. Development and testing of apricot (Prunus armeniaca L.) pit decorticator. Journal of Food Process Engineering. 41: e12690 (2018)
- Kate A, Shahi N, Sarkar A, Lohani UC, Jadhav PP. Moisture dependent engineering properties of wild apricot (Prunus armeniaca L.) pits. International Journal of Agriculture, Environment and Biotechnology. 7: 179-185 (2014b)
- Kate A, Singh A, Shahi N, Pandey JP, Praksah O. Modeling and kinetics of microwave assisted leaching based oil extraction from Bhat. Journal of Food Processing Engineering. 2020;43:e13503. [Google Scholar]
- Kaya C, Kola O, Ozer MS, Altan A. Some characteristics and fatty acids composition of wild apricot (Prunus pseudoarmeniaca L.) kernel oil. Asian Journal of Chemistry. 20: 2597-2602 (2008)
- Kiralan M, Kayahan M, Kiralan SS, Ramadan MF. Effect of thermal and photo oxidation on the stability of cold-pressed plum and apricot kernel oils. European Food Research and Technology. 2018;244:31–42. [Google Scholar]
- Kiralan M, Özkan G, Kucukoner E, Ozcelik MM. Apricot (Prunus armeniaca L.) Oil. pp. 505-519. In: Fruit Oils: Chemistry and Functionality. Ramadan M (ed). Springer, Cham, Switzerland AG (2019)
- Kostadinović Veličkovska S, Catalin Moţ A, Mitrev S, Gulaboski R, Brühl L, Mirhosseini H, Silaghi-Dumitrescu R, Matthäus B. Bioactive compounds and “in vitro” antioxidant activity of some traditional and non-traditional cold-pressed edible oils from Macedonia. Journal of Food Science and Technology. 2018;55:1614–1623. doi: 10.1007/s13197-018-3050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Gasmalla MAA, Li P, Yang R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innovative Food Science and Emerging Technologies. 2016;35:184–193. [Google Scholar]
- Liu MJ, Zhang QA. Valorization of the under-utilized apricot kernels protein based on the rheology and texture properties of dough. LWT-Food Science & Technology. 2022;169:114019. [Google Scholar]
- Lozano-Grande MA, Dávila-ortiz G, García-dávila J, íos-Cortés G, Espitia-Rangel E, Martínez-Ayala AL. Optimisation of microwave-assisted extraction of squalene from a maranthus spp . seeds. Journal of Microwave Power and Electromagnetic Energy. 53: 243-258 (2019)
- Mahatre RR, Kumbhar BK, Singh A, Lohani UC, Shahi NC. Optimization of parameters for enhanced oil recovery from enzyme treated wild apricot kernels. Journal of Food Science and Technology. 2012;49:482–488. doi: 10.1007/s13197-011-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal V, Mohan Y, Hemalatha S. Microwave assisted extraction-an innovative and promising extraction tool for medicinal plant research. Pharmacognosy Reviews. 2007;1:7–18. [Google Scholar]
- Manzoor M, Anwar F, Ashraf M, Alkharfy KM. Physico-chemical characteristics of seed oils extracted from different apricot (Prunus armeniaca L.) varieties from Pakistan. Grasas y Aceites. 63: 193-201 (2012)
- Mushtaq A, Roobab U, Denoya GI, Inam-Ur-Raheem M, Gullón B, Lorenzo JM, Barba FJ, Zeng XA, Wali A, Aadil RM. Advances in green processing of seed oils using ultrasound-assisted extraction: A review. Journal of Food Processing and Preservation. 2020;44:e14740. [Google Scholar]
- Nadar SS, Rao P, Rathod VK. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Research International. 2018;108:309–330. doi: 10.1016/j.foodres.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Naryal A, Acharya S, Bhardwaj AK, Kant A, Chaurasia OP, Stobdan T. Altitudinal effect on sugar contents and sugar profiles in dried apricot (Prunus armeniaca L.) fruit. Journal of Food Composition and Analysis. 76: 27-32 (2019)
- Nde DB, Anuanwen CF. Optimization methods for the extraction of vegetable oils: A review. Processes. 2020;8:209. [Google Scholar]
- Nde DB, Boldor D, Astete C. Optimization of microwave assisted extraction parameters of neem (Azadirachta indica A. Juss ) oil using the Doehlert’s experimental design. Industrial Crops and Products. 65: 233-240 (2015)
- Ouzir M, El Bernoussi S, Tabyaoui M, Taghzouti K. Almond oil: A comprehensive review of chemical composition, extraction methods, preservation conditions, potential health benefits, and safety. Comprehensive Reviews in Food Science and Food Safety. 2021;20:3344–3387. doi: 10.1111/1541-4337.12752. [DOI] [PubMed] [Google Scholar]
- Özcan M. Composition of some apricot (Prunus Armeniaca L.) kernels grown in Turkey. Acta Alimentaria. 29: 289-294 (2000)
- Özcan MM, Cundullah O, Unver A, Arslan D, Dursun N. Properties of apricot kernel and oils as fruit juice processing waste. Food and Nutrition Sciences. 2010;1:31–37. [Google Scholar]
- Özkal SG, Yener ME, Bayindirli L. Response surfaces of apricot kernel oil yield in supercritical carbon dioxide. LWT- Food Science and Technology. 2005;38:611–616. [Google Scholar]
- Pavlović N, Vidović S, Vladić J, Popović L, Moslavac T, Jakobović S, Jokić S. Recovery of tocopherols, amygdalin and fatty acids from apricot kernel oil: cold pressing vs. supercritical carbon dioxide. European Journal of Lipid science & Technology. 120: 1800043 (2018)
- Prakash O, Jain D, Nikumbhe P, Srivastava S, Raghuvanshi MS. Significance, status and scope of apricot in India : A review. International Journal of Chemical Studies. 2020;8:5–11. [Google Scholar]
- Puri M, Sharma D, Barrow CJ. Enzyme-assisted extraction of bioactives from plants. Trends in Biotechnology. 2012;30:37–44. doi: 10.1016/j.tibtech.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Qi Z, Xiao J, Ye L, Chuyun W, Chang Z, Shugang L, Fenghong H. The effect of the subcritical fluid extraction on the quality of almond oils: Compared to conventional mechanical pressing method. Food Science and Nutrition. 2019;7:2231–2241. doi: 10.1002/fsn3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zhong J. A review of extraction techniques for avocado oil. Journal of Oleo Science. 2016;65:e16063. doi: 10.5650/jos.ess16063. [DOI] [PubMed] [Google Scholar]
- Rajendran N, Gurunathan B. Optimization and technoeconomic analysis of biooil extraction from Calophyllum inophyllum L. seeds by ultrasonic assisted solvent oil extraction. Industrial Crops and Products. 162: 113273 (2021)
- Rampáčková E, Göttingerová M, Gála P, Kiss T, Ercişli S, Nečas T. Evaluation of protein and antioxidant content in apricot kernels as a sustainable additional source of nutrition. Sustainability. 2021;13:4742. [Google Scholar]
- Rezvankhah A, Emam-Djomeh Z, Safari M, Askari G, Salami M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with soxhlet extraction. Journal of Food Science and Technology. 2019;56:4198–4210. doi: 10.1007/s13197-019-03890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini D, Rawat N, Negi T, Barthwal R, Sharma S. c. Journal of Pharmacognosy and Phytochemistry. 10: 119-126 (2021)
- Shariatifar N, Pourfard IM, Khaniki GJ, Nabizadeh R, Akbarzadeh A, Nejad ASM. Mineral composition, physico-chemical properties and fatty acids profile of Prunus armeniaca apricot seed oil. Asian Journal of Chemistry. 2017;29:2011–2015. [Google Scholar]
- Sharma A, Gupta MN. Ultrasonic pre-irradiation effect upon aqueous enzymatic oil extraction from almond and apricot seeds. Ultrasonics Sonochemistry. 2006;13:529–534. doi: 10.1016/j.ultsonch.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sharma R, Gupta A, Abrol G, Joshi V. Value addition of wild apricot fruits grown in North-West Himalayan regions-a review. Journal of Food Science and Technology. 2014;51:2917–2924. doi: 10.1007/s13197-012-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma PC, Tilakratne BM, Gupta A. Utilization of wild apricot kernel press cake for extraction of protein isolate. Journal of Food Science and Technology. 2010;47:682–625. doi: 10.1007/s13197-010-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silem A, Günter HO, Einfeldt J, Boualia A. The occurrence of mass transport processes during the leaching of amygdalin from bitter apricot kernels: Detoxification and flavour improvement. International Journal of Food Science and Technology. 2006;41:201–213. [Google Scholar]
- Singla M, Sit N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrasonics Sonochemistry. 2021;73:105506. doi: 10.1016/j.ultsonch.2021.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhang QA, Fan XH, Zhang XY. Effect of debitterizing treatment on the quality of the apricot kernels in the industrial processing. Journal of Food Processing and Preservation. 2017;42:e13556. [Google Scholar]
- Stryjecka M, Kiełtyka-Dadasiewicz A, Michalak M, Rachoń L, Głowacka A. Chemical composition and antioxidant properties of oils from the seeds of five apricot (Prunus armeniaca L.) cultivars. Journal of Oleo Science. 68: 729-738 (2019) [DOI] [PubMed]
- Tabasum F, Omar B, Gousia G, Tashooq B, Nusrat J. Nutritional and health benefits of apricots. International Journal of Unani & Integrative Medicine. 2018;2:5–9. [Google Scholar]
- Targais K, Stobdan T, Yadav A, Singh SB. Extraction of apricot kernel oil in cold desert Ladakh. India. Indian Journal of Traditional Knowledge. 2011;10:304–306. [Google Scholar]
- Tiwari BK. Ultrasound: A clean, green extraction technology. Trends in Analytical Chemistry. 2015;71:100–109. [Google Scholar]
- Uluata S. Effect of Extraction method on biochemical properties and oxidative stability of apricot seed oil. Akademik Gıda. 2016;14:333–340. [Google Scholar]
- Wani SM, Masoodi FA, Ahmad M, Mir SA. Processing and storage of apricots: effect on physicochemical and antioxidant properties. Journal of Food Science and Technology. 2018;55:4505–4514. doi: 10.1007/s13197-018-3381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav AK, Pal A, Dubey AM. Experimental studies on utilization of Prunus armeniaca L. (wild apricot) biodiesel as an alternative fuel for CI engine. Waste and Biomass Valorization. 9: 1961-1969 (2018)
- Yigit D, Yigit N, Mavi A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Brazilian Journal of Medical and Biological Research. 42: 346-352 (2009) [DOI] [PubMed]
- Zhang N, Zhang QA, Yao JL, Zhang XY. Changes of amygdalin and volatile components of apricot kernels during the ultrasonically-accelerated debitterizing.Ultrasonics Sonochemistry. 58: 104614 (2019) [DOI] [PubMed]
- Zhang QA, Shi FF, Yao JL, Zhang N. Effects of ultrasound irradiation on the properties of apricot kernels during accelerated debitterizing. RSC Advances. 2020;10:10624–10633. doi: 10.1039/c9ra10965j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wang Y, Kang J, Zhong H, Prenzler PD. The quality and volatile-profile changes of Longwangmo apricot (Prunus armeniaca L.) kernel oil prepared by different oil-producing processes. European Journal of Lipid Science and Technology. 118: 236-243 (2016)