Abstract
Hydrolyzed vegetable proteins (HVPs) are widely used food flavorings. This study investigated the volatiles formed in thermally reacted model systems containing HVPs (made from defatted soy, corn gluten, and wheat gluten) and reducing sugars (glucose and fructose). Three types of HVPs, which had different free amino acid compositions, generated qualitatively and quantitatively different volatile compounds. In the results of principal component analysis, each thermally reacted system could be distributed according to type of HVPs and sugars. Aldehydes and pyrazines highly correlated with glucose- and fructose-containing model systems, respectively. In particular, model systems containing soy HVPs showed higher contents of sugar-degraded compounds, such as maltol, furfuryl alcohol, and cyclotene. However, some Strecker aldehydes and nitrogen-containing heterocyclic compounds, whose formation required amino acids, were more abundant in model systems containing corn and wheat HVP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01194-w.
Keywords: Hydrolyzed vegetable proteins, Reducing sugars, Maillard reaction, Volatile compounds
Introduction
Hydrolyzed vegetable proteins (HVPs) have been widely used as savory flavoring agents in various processed foods (Jung et al., 2020). Using acid hydrolysis, conventional HVPs are normally made from various plant sources, such as soy, wheat gluten, and maize (Jung et al., 2020). Through hydrolysis of HVP, proteins or peptides are decomposed into amino acids, which are precursors in the Maillard reaction.
The Maillard reaction, a non-enzymatic chemical reaction, generates various volatile compounds that can significantly affect the flavors of foods (Wang et al., 2021). Numerous volatiles, including heterocyclic compounds, can be made from the Maillard reaction between an amino group of amino acids and a carbonyl group of reducing sugars (Alpert et al., 2016). Also, Strecker degradation occurs through the degradation of amino acids initiated by dicarbonyls, causing the deamination and decarboxylation of amino acids. In addition, sugar degradation is involved in specific flavor components during the Maillard reaction. Nursten (2005) has classified the volatile compounds produced by the Maillard reaction into three groups: 1. simple sugar dehydration/fragmentation products, including furans, pyrans, cyclopentenes, carbonyls, and acids, 2. simple amino acid degradation products, including aldehydes and sulfur compounds, and 3. volatiles produced by further interactions, including pyrroles, pyridines, imidazoles, pyrazines, oxazoles, and thiazoles.
Several researchers have investigated the volatile compounds produced by the thermal reaction between HVPs and reducing sugars. Yoon et al. (1994) has analyzed the meat-like volatiles thermally formed from a mixture of defatted soy HVP and fructose syrup and identified volatile flavor compounds with meat-like odor, including 3-methylbutanal, 2-methyltetrahydrothiophene-3-one, and 3,4-dimethylthiophene. A previous study of the Maillard reaction of soybean protein hydrolysates with xylose and cysteine identified nitrogenous and sulfurated heterocyclic compounds, which can cause a meat-like flavor (Song et al., 2013). When cereal-based protein hydrolysates were thermally reacted with glucose, most of the flavor compounds derived from the cereal-based hydrolysates were similar to those from the animal-based products, suggesting the possibility of replacing animal-based products with cereal-based ones (Chiang et al., 2022). Karangwa et al. (2016) has found that the Maillard reaction of corn protein hydrolysates with xylose and cysteine generated kokumi-like flavoring.
HVPs are made from various plant protein sources, which have different amino acid compositions. Therefore, different amino acid compositions can impact the volatile compounds formed during thermal reactions, eventually affecting the flavor characteristics. In the Maillard reaction, the type of amino acid plays a key role not only in the rate of reaction but also in the properties of the flavor compounds produced. For example, sulfur-containing amino acids induce meat- or coffee-like flavor notes, whereas valine, leucine, and isoleucine cause chocolate-like flavor notes (Nursten, 2005). A previous study on the Maillard reaction demonstrated that different types of amino acids influenced volatile formation both quantitatively and qualitatively (Cho et al., 2010).
This study investigated the effects of types of HVP and reducing sugar on the formation of volatile compounds in model system. Different types of HVPs (soy, corn, and wheat) were reacted with the reducing sugars (fructose and glucose). Also, the amino acid composition and the degree of hydrolysis of the HVPs were identified in order to study the effects of the precursors of the volatile compounds.
Materials and methods
Chemicals
HVPs made from soy, corn, and wheat were provided by Sempio Food Company (Seoul, South Korea). Defatted soy, wheat gluten, and corn gluten were hydrolyzed with hydrochloric acid before the spray-drying process. Sodium chloride (NaCl), d-fructose, sodium sulfate, and internal standards, including (1S,2R,4S)-1,7,7-trimethylbicyclo [2.2.1] heptan-2-ol [(-)-borneol] for solid-phase microextraction (SPME) and tridecan-2-one for solvent extraction were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). HPLC-grade water and methylene chloride were obtained from J.T. Baker (Phillipsburg, NJ, USA).
Measurement of the degree of hydrolysis
The degree of hydrolysis (DH) of each HVP was measured. The ratio of α-amino nitrogen to total nitrogen was determined using the method of Zhou et al. (2021). The α-amino nitrogen was measured by formaldehyde titration, performed according to Lee et al. (1997). The Kjeldahl method was used to determine the total nitrogen and performed at the Korea Health Supplement Institute (Seongnam-si, Gyeonggi-do, South Korea).
Analysis of free amino acids
Free amino acid analysis was conducted by the Korea Health Supplement Institute using high-performance liquid chromatography (HPLC), following the Food Code of the Ministry of Food and Drug Safety (Cheongju-si, Chungcheongbuk-do, Korea). The amino acid standard mixture was purchased from Agilent Technologies (Palo Alto, CA, USA). A 1 g homogeneous sample was accurately weighed, and distilled water (J.T. Baker, Phillipsburg, NJ, USA) was added to extract the free amino acids. After centrifugation, the water layer was collected. Then, the residue obtained by concentrating and drying the water layer under reduced pressure was dissolved with a 0.2 M sodium citrate buffer (pH 2.2). Before injection into the HPLC, a pre-column derivatization of the free amino acids using the O-phthaldialdehyde reagent (OPA) was performed. The injection volume was 10 μL, and an Agilent liquid chromatograph system (Agilent Technology, Palo Alto, CA, USA) with a PDA (338 nm and 262 nm) detector, and a Capcellpak UG120 C18 (250 mm × 4.6 mm 5 μm, OSAKA SODA, Osaka, Japan) column was used for the analysis. The samples were eluted in a gradient mode with 40 mM NaCl (pH 3.5) for eluent A, and acetonitrile/methanol/water (45/45/10, v/v/v) for eluent B. The flow rate was 1.5 mL/min at 40 °C.
Preparation of Maillard reaction samples
The NaCl content of the HVPs varied, which could affect the Maillard reaction (Yamaguchi et al., 2009). Thus, the NaCl concentration was measured using a salt meter (model TM-30D, Takemura electric works. LTD., Tokyo, Japan). Then, the NaCl content was adjusted to that of the soy HVP, which had the highest original NaCl content. The amounts of NaCl added to the corn and wheat HVPs are given in Table 1. Before adding the NaCl, 6.25 g of each HVP was weighed. Then, 3.125 g of the reducing sugar (fructose and glucose) was added. These prepared samples were mixed with HPLC-grade water to a final volume of 50 mL before being transferred to a 250 mL stainless steel cylinder (Ilshinautoclave, Daejeon-si, Korea). The samples were named after the combination of initials for the HVPs and reducing sugars: CF, CG, SF, SG, WF, and WG, as shown in Table 2. Each sample was adjusted to pH 7.5 with 2 M NaOH, before the thermal reaction was conducted at 120 °C for 90 min in a drying oven (Eyela NDO-600SD, Rikakikai Co. Ltd., Tokyo, Japan).
Table 1.
Measured NaCl content and calculated amount of added NaCl
| Soy | Wheat | Corn | |
|---|---|---|---|
| NaCl (%) | 46.6 | 10.0 | 9.8 |
| NaCl in 6.25 g of Each HVP | 2.913 g | 0.625 g | 0.613 g |
| Added NaCl | 2.288 g | 2.300 g |
Table 2.
Model systems named as a combination of hydrolyzed vegetable proteins (HVPs) and reducing sugars
| HVPs | |||
|---|---|---|---|
| Corn | Soy | Wheat | |
| Reducing sugars | |||
| Fructose | CF | SF | WF |
| Glucose | CG | SG | WG |
Analysis of volatile compounds
Two types of extraction methods were used to analyze the volatile compounds. For SPME, which collects the volatile compounds in a headspace, 5 g of thermally reacted sample was transferred into a sealed vial, where it was allowed to equilibration time for 30 min at 40 °C. The adsorption time was 30 min with a fiber coated with polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm, Supelco, Bellefonte, PA, USA). After the volatile extraction, volatile compounds were analyzed using a 7890A gas chromatograph (GC, Agilent Technologies, Santa Clara, CA, USA) coupled with a 5977B mass selective detector (Agilent Technologies, Santa Clara, CA, USA). A DB-5MS capillary column (30 m length × 0.25 mm i.d. × 0.25 μm film thickness, J&W Scientific, Folsom, CA, USA) was used to separate the volatile compounds. Helium (99.999%) was used as the carrier gas at a flow rate of 0.8 mL/min. The GC oven temperature started at 40 °C, held for 5 min, rose to 185 °C at 3 °C/min, and reached 200 °C at a rate of 5 °C/min, where it was held for 5 min. The injector and the transfer line temperatures were 230 °C and 280 °C, respectively. These volatile compounds were monitored by the electron impact (EI) ion source at 70 eV and scanned in the range 35–350 atomic mass units (a.m.u.).
For the solvent extraction (SE), 50 mL of methylene chloride was added to a separating funnel containing the sample. After vigorously shaking the funnel at 250 rpm for 40 min using a separatory funnel shaker (JISICO, Seoul, South Korea), the lower layer of the sample was taken. The same was washed with HPLC-grade water three times, then dried to 0.3 mL using a gentle flow of nitrogen gas (99.999%). Using the same GC–MS instrument as above, 1 μL of the final sample was studied. The GC oven temperature was initially held at 40 °C for 5 min, then increased to 200 °C at 5 °C/min, and held for 5 min. Other conditions were identical to those above.
The compound was positively verified by comparing the retention time and mass spectral data of each volatile with those of standard compounds. If standards were unavailable, each volatile compound was tentatively identified using the NIST.08 and Wiley.9 mass spectral databases and relative retention index (RI) values. The RI values of volatile compounds were estimated using n-alkanes from C7 to C30 as external standards. The relative contents of volatile compounds were obtained by comparing the peak areas of the volatile compounds to that of an internal standard compound. All experiments were performed in triplicate.
Statistical analysis
The quantified results are presented as means ± standard deviation. All identified compounds were statistically analyzed using analysis of variance (ANOVA) using SPSS (IBM Corp., Armonk, NY, USA) to confirm the significant differences between samples. Duncan’s multiple range test was implemented at a significance level of α = 0.05. Principal component analysis (PCA) was also applied to the GC–MS data to verify the discrimination between model systems using the SIMCA-P software (version 16.0, Umetrics, Umea, Sweden).
Result and discussions
In this study, two different extraction methods for volatiles were performed to investigate the differences according to the types of vegetable proteins. Volatile compounds obtained from SPME and solvent extraction are listed in Tables 3 and 4, respectively. In the result of SPME analysis, volatile aldehydes, pyrazines, and sulfur containing compounds were more abundant than solvent extraction methods. On the other hand, more volatiles with relatively low volatility, such as lactones and nitrogen-containing heterocyclic compounds (pyrazines, pyrroles, pyridines, piperidines, pyrrolidines, and pyrimidine), were detected using solvent extraction.
Table 3.
Solid-phase microextraction (SPME) analysis of volatile compounds for each Maillard-reacted sample
| NOa | RIb | Compound | CAS | Relative peak areac | IDe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFd | SF | WF | CG | SG | WG | |||||
| Alcohol | ||||||||||
| A1 | 974 | heptan-1-ol | 111-70-6 | NDf | ND | ND | NDag | 0.003 ± 0.003a | NDa | A |
| A2 | 1031 | 2-ethylhexan-1-ol | 104-76-7 | NDa | NDa | 0.019 ± 0.004b | NDa | NDa | 0.021 ± 0.003b | A |
| A3 | 1177 | 5-methyl-2-propan-2-ylcyclohexan-1-ol | 1490-04-6 | ND | ND | ND | 0.006 ± 0.003a | 0.004 ± 0.004a | 0.023 ± 0.030a | A |
| A4 | 1373 | tetradecan-1-ol | 112-72-1 | ND | ND | ND | 0.004 ± 0.001b | NDa | NDa | A |
| A5 | 1374 | undecan-1-ol | 112-42-5 | 0.009 ± 0.008a | NDa | NDa | ND | ND | ND | A |
| A6 | 1474 | dodecan-1-ol | 112-53-8 | ND | ND | ND | NDa | 0.305 ± 0.037b | NDa | A |
| A7 | 1474 | dec-1-ene | 872-05-9 | 0.207 ± 0.020b | 0.284 ± 0.011c | 0.147 ± 0.012a | ND | ND | ND | A |
| A8 | 1592 | octadecan-1-ol | 112-92-5 | ND | ND | ND | NDa | 0.006 ± 0.001b | NDa | A |
| A9 | 1793 | hexacosan-1-ol | 506-52-5 | ND | ND | ND | NDa | 0.007 ± 0.001b | NDa | C |
| Aldehyde | ||||||||||
| D1 | 669 | 3-methylbutanal | 590-86-3 | NDa | 0.680 ± 0.006b | NDa | NDa | 0.907 ± 0.071b | NDa | A |
| D2 | 671 | pentanal | 110-62-3 | 0.525 ± 0.161b | NDa | 0.612 ± 0.064b | 1.038 ± 0.037b | NDa | 1.340 ± 0.035c | A |
| D3 | 805 | hexanal | 66-25-1 | ND | ND | ND | NDa | 0.041 ± 0.010b | NDa | A |
| D4 | 904 | heptanal | 111-71-7 | NDa | 0.012 ± 0.001b | NDa | NDa | 0.076 ± 0.010b | 0.045 ± 0.035b | A |
| D5 | 959 | benzaldehyde | 100-52-7 | 0.336 ± 0.014b | 0.232 ± 0.016a | 0.523 ± 0.028c | 0.216 ± 0.016a | 0.211 ± 0.016a | 0.500 ± 0.100b | A |
| D6 | 1003 | octanal | 124-13-0 | 0.034 ± 0.011b | 0.069 ± 0.004c | 0.016 ± 0.004a | 0.021 ± 0.002a | 0.246 ± 0.018c | 0.046 ± 0.004b | A |
| D7 | 1042 | 2-phenylacetaldehyde | 122-78-1 | NDa | 0.063 ± 0.007b | NDa | 0.075 ± 0.009c | NDa | 0.043 ± 0.001b | A |
| D8 | 1101 | (E)-5-methyl-2-propan-2-ylhex-2-enal | 35158-25-9 | NDa | 0.019 ± 0.001b | NDa | NDa | 0.014 ± 0.001b | 0.037 ± 0.003c | A |
| D9 | 1104 | nonanal | 124-19-6 | 0.151 ± 0.030b | 0.171 ± 0.016b | 0.092 ± 0.017a | 0.045 ± 0.022a | 0.499 ± 0.054b | 0.098 ± 0.012a | A |
| D10 | 1153 | 2-phenylprop-2-enal | 4432–63-7 | 0.025 ± 0.033a | NDa | NDa | ND | ND | ND | B |
| D11 | 1205 | decanal | 112-31-2 | 0.016 ± 0.009a | 0.039 ± 0.012b | 0.018 ± 0.007a | 0.026 ± 0.013a | 0.051 ± 0.015b | 0.030 ± 0.007ab | A |
| D12 | 1261 | (E)-dec-2-enal | 3913-81-3 | ND | ND | ND | NDa | 0.021 ± 0.005b | NDa | A |
| D13 | 1265 | (E)-2-phenylbut-2-enal | 4411-89-6 | 0.004 ± 0.003a | 0.004 ± 0.001a | 0.003 ± 0.001a | 0.011 ± 0.000b | 0.006 ± 0.001a | 0.007 ± 0.002a | A |
| D14 | 1307 | undecanal | 112-44-7 | 0.006 ± 0.006a | 0.024 ± 0.007b | NDa | 0.009 ± 0.004b | 0.022 ± 0.005c | NDa | A |
| D15 | 1363 | 4-methyl-2-phenylpent-2-enal | 26643-91-4 | NDa | 0.003 ± 0.001b | NDa | NDa | NDa | 0.020 ± 0.014b | A |
| D16 | 1363 | (E)-dodec-3-enal | 68083-57-8 | ND | ND | ND | NDa | 0.020 ± 0.006b | NDa | B |
| D17 | 1408 | dodecanal | 112-54-9 | 0.069 ± 0.036a | 0.106 ± 0.101a | 0.094 ± 0.030a | 0.073 ± 0.029a | 0.176 ± 0.057b | NDa | A |
| D18 | 1480 | (E)-5-methyl-2-phenylhex-2-enal | 21834-92-4 | 0.015 ± 0.003a | 0.033 ± 0.001b | 0.068 ± 0.001c | 0.006 ± 0.001a | 0.032 ± 0.004b | 0.064 ± 0.019c | A |
| D19 | 1510 | tetradecanal | 124–25-4 | ND | ND | ND | NDa | NDa | 0.152 ± 0.086b | A |
| D20 | 1750 | 3,5-ditert-butyl-4-hydroxybenzaldehyde | 1620-98-0 | ND | ND | ND | NDa | NDa | 0.006 ± 0.001b | B |
| Esters | ||||||||||
| E1 | 1112 | heptyl acetate | 112-06-1 | 0.017 ± 0.015ab | 0.025 ± 0.007b | NDa | 0.020 ± 0.008a | 0.021 ± 0.011a | 0.019 ± 0.006a | A |
| E2 | 1148 | 2-ethylhexyl acetate | 103-09-3 | NDa | NDa | 0.008 ± 0.002b | ND | ND | ND | A |
| E3 | 1369 | (3-hydroxy-2,4,4-trimethylpentyl) 2-methylpropanoate | 74367-34-3 | 0.031 ± 0.003a | 0.035 ± 0.002b | 0.048 ± 0.001c | 0.020 ± 0.006b | 0.051 ± 0.000c | NDa | C |
| E4 | 1369 | hexyl 2-methylpropanoate | 2349-07-7 | ND | ND | ND | NDa | NDa | 0.031 ± 0.017b | C |
| E5 | 1681 | bis(2-methylpropyl) hexanedioate | 141-04-8 | 0.003 ± 0.003a | NDa | NDa | ND | ND | ND | B |
| E6 | 1824 | propan-2-yl tetradecanoate | 110-27-0 | ND | ND | ND | NDa | 0.022 ± 0.004c | 0.009 ± 0.004b | A |
| Hydrocarbons | ||||||||||
| H1 | 1300 | tridecane | 629-50-5 | 0.002 ± 0.001a | 0.005 ± 0.000b | NDa | NDa | 0.003 ± 0.003a | NDa | A |
| H2 | 1321 | 2,6,10,14-tetramethylhexadecane | 638-36-8 | ND | ND | ND | 0.001 ± 0.002a | NDa | NDa | C |
| H3 | 1374 | octylcyclopropane | 1472-09-9 | NDa | NDa | 0.014 ± 0.006b | NDa | NDa | 0.014 ± 0.004b | C |
| H4 | 1400 | tetradecane | 629-59-4 | 0.011 ± 0.002a | 0.014 ± 0.003a | 0.007 ± 0.005a | NDa | 0.016 ± 0.001a | 0.065 ± 0.096a | A |
| H5 | 1410 | N,1,2,3,4,5-hexamethylcyclopenta-2,4-dien-1-amine | 138902-00-8 | NDa | NDa | 0.068 ± 0.004b | NDa | NDa | 0.035 ± 0.027b | C |
| H6 | 1474 | nonylcyclopropane | 74663-85-7 | NDa | 0.006 ± 0.002b | NDa | 0.041 ± 0.004b | NDa | NDa | C |
| H7 | 1530 | cyclodecane | 293-96-9 | 0.005 ± 0.005a | NDa | NDa | ND | ND | ND | A |
| H8 | 1530 | cyclododecane | 294-62-2 | 0.019 ± 0.001b | 0.017 ± 0.004b | 0.008 ± 0.003a | 0.006 ± 0.001a | 0.033 ± 0.009a | 0.895 ± 0.712b | C |
| H9 | 1532 | (5S,9S)-5,9-dimethylpentadecane | NDa | 0.015 ± 0.009b | NDa | ND | ND | ND | C | |
| H10 | 1532 | 2-methyltetradecane | 1560-95-8 | ND | ND | ND | NDa | NDa | 0.006 ± 0.004b | C |
| H11 | 1600 | hexadecane | 544-76-3 | 0.006 ± 0.002a | 0.009 ± 0.001b | 0.004 ± 0.002a | NDa | 0.009 ± 0.005b | 0.005 ± 0.001ab | A |
| H12 | 1676 | cyclotetradecane | 295–17-0 | NDa | NDa | 0.014 ± 0.001b | ND | ND | ND | B |
| H13 | 1677 | tetradec-1-ene | 1120–36-1 | NDa | 0.025 ± 0.002b | NDa | NDa | NDa | 0.052 ± 0.034b | C |
| H14 | 1714 | heptadecane | 629-62-9 | NDa | 0.017 ± 0.005b | NDa | NDa | 0.019 ± 0.006b | NDa | A |
| H15 | 1741 | 8-heptylpentadecane | 71005-15-7 | NDa | NDa | 0.007 ± 0.002b | ND | ND | ND | C |
| Ketones | ||||||||||
| K1 | 943 | 2-methyldecan-5-one | 54410-89-8 | ND | ND | ND | NDa | NDa | 0.006 ± 0.001b | C |
| K2 | 1022 | 3-methylcyclopentane-1,2-dione | 765-70-8 | ND | ND | ND | NDa | 0.003 ± 0.003a | NDa | B |
| K3 | 1062 | 1-phenylethanone | 98-86-2 | 0.070 ± 0.002a | 0.067 ± 0.001a | 0.170 ± 0.005b | 0.059 ± 0.002a | 0.068 ± 0.002b | 0.172 ± 0.003c | A |
| K4 | 1090 | nonan-2-one | 821-55-6 | 0.004 ± 0.004a | NDa | NDa | 0.005 ± 0.001a | 0.016 ± 0.003b | 0.008 ± 0.000a | A |
| K5 | 1120 | 1-phenylpropan-2-one | 103-79-7 | NDa | NDa | 0.004 ± 0.001b | ND | ND | ND | A |
| K6 | 1144 | (3aR,7aS)-7a-methyl-2,3,3a,4-tetrahydro-1H-inden-5-one | 17429-25-3 | 0.006 ± 0.003a | NDb | NDb | ND | ND | ND | C |
| K7 | 1191 | decan-2-one | 693-54-9 | ND | ND | ND | NDa | 0.023 ± 0.003b | NDa | A |
| K8 | 1291 | undecan-2-one | 112-12-9 | ND | ND | ND | NDa | 0.007 ± 0.001a | 0.019 ± 0.017a | A |
| K9 | 1346 | (Z)-2-methyl-1-phenylbut-2-en-1-one | 19847-64-4 | 0.002 ± 0.002a | NDa | NDa | ND | ND | ND | C |
| K10 | 1348 | (E)-4-phenylbut-3-en-2-one | 122–57-6 | NDa | NDa | 0.001 ± 0.001a | ND | ND | ND | A |
| K11 | 1415 | 1-(4-methoxyphenyl)ethanone | 100-06-1 | 0.037 ± 0.007b | NDa | NDa | ND | ND | ND | A |
| K12 | 1457 | 2,6-ditert-butylcyclohexa-2,5-diene-1,4-dione | 719–22-2 | 0.056 ± 0.018a | 0.066 ± 0.009a | 0.064 ± 0.011a | 0.014 ± 0.004a | 0.177 ± 0.017c | 0.092 ± 0.047b | B |
| K13 | 1466 | 2,5-ditert-butylcyclohexa-2,5-diene-1,4-dione | 2460-77-7 | NDa | 0.001 ± 0.002a | NDa | ND | ND | ND | B |
| K14 | 1670 | pentadecan-8-one | 818–23-5 | ND | ND | ND | NDa | 0.044 ± 0.011b | 0.048 ± 0.029b | C |
| Lactones | ||||||||||
| L1 | 948 | 5-methyloxolan-2-one | 108-29-2 | 0.024 ± 0.002c | 0.011 ± 0.001b | NDa | 0.023 ± 0.002b | NDa | NDa | A |
| N-containing compounds | ||||||||||
| N1 | 1135 | 2-phenylacetonitrile | 140-29-4 | ND | ND | ND | NDa | NDa | 0.032 ± 0.006b | A |
| N2 | 1136 | 2-methylbenzonitrile | 529-19-1 | NDa | NDa | 0.003 ± 0.000b | ND | ND | ND | C |
| Pyrazines | ||||||||||
| P1 | 824 | 2-methylpyrazine | 109-08-0 | 0.036 ± 0.010b | 0.041 ± 0.006b | NDa | ND | ND | ND | A |
| P2 | 911 | 2,6-dimethylpyrazine | 108-50-9 | 0.564 ± 0.050c | 0.239 ± 0.016b | NDa | ND | ND | ND | A |
| P3 | 911 | 2,5-dimethylpyrazine | 123-32-0 | NDa | NDa | 0.532 ± 0.011b | 0.075 ± 0.029b | NDa | 0.049 ± 0.001b | A |
| P4 | 930 | 2-ethenylpyrazine | 4177-16-6 | ND | ND | ND | 0.004 ± 0.006a | NDa | NDa | A |
| P5 | 995 | 2-ethyl-6-methylpyrazine | 13925-03-6 | NDa | 0.043 ± 0.004b | NDa | NDa | 0.034 ± 0.003c | 0.015 ± 0.000b | B |
| P6 | 999 | 2,3,5-trimethylpyrazine | 14667-55-1 | 0.084 ± 0.009b | NDa | 0.101 ± 0.017b | NDa | NDa | 0.048 ± 0.006b | A |
| P7 | 1014 | 2-ethenyl-6-methylpyrazine | 13925-09-2 | 0.011 ± 0.001b | NDa | NDa | 0.005 ± 0.008a | NDa | NDa | B |
| P8 | 1052 | 2-methyl-5-propan-2-ylpyrazine | 13925-05-8 | 0.071 ± 0.005c | NDa | 0.063 ± 0.003b | 0.030 ± 0.000b | NDa | 0.035 ± 0.004b | B |
| P9 | 1074 | 3-ethyl-2,5-dimethylpyrazine | 13360-65-1 | 0.174 ± 0.024b | 0.038 ± 0.004a | 0.211 ± 0.006c | 0.033 ± 0.004a | 0.051 ± 0.006b | 0.101 ± 0.002c | B |
| P10 | 1128 | 2,3-dimethyl-5-propan-2-ylpyrazine | 40790-21-4 | 0.013 ± 0.000c | NDa | 0.011 ± 0.000b | NDa | NDa | 0.009 ± 0.001b | C |
| P11 | 1156 | 2,5-dimethyl-3-propylpyrazine | 18433-97-1 | NDa | NDa | 0.024 ± 0.000b | ND | ND | ND | B |
| P12 | 1187 | 2,5-bis(1-methylethyl)pyrazine | 0.004 ± 0.000b | NDa | NDa | ND | ND | ND | C | |
| P13 | 1191 | 2,5-diethyl-3,6-dimethylpyrazine | 18903-30-5 | NDa | NDa | 0.037 ± 0.003b | ND | ND | ND | C |
| P14 | 1196 | 2,5-dimethyl-3-(2-methylpropyl)pyrazine | 32736-94-0 | 0.018 ± 0.001b | NDa | 0.045 ± 0.001c | 0.006 ± 0.000b | NDa | 0.038 ± 0.001c | B |
| P15 | 1233 | 2-methyl-3-(2-methylpropyl)pyrazine | 13925-06-9 | ND | ND | ND | NDa | NDa | 0.008 ± 0.009a | C |
| P16 | 1245 | 2-methyl-3-propylpyrazine | 15986-80-8 | NDa | NDa | 0.004 ± 0.001b | ND | ND | ND | C |
| P17 | 1245 | 2-butyl-3-methylpyrazine | 15987-00-5 | 0.002 ± 0.002a | NDa | NDa | ND | ND | ND | C |
| P18 | 1247 | 2-methyl-6-(3-methylbutyl)pyrazine | 91010-41-2 | 0.017 ± 0.001a | 0.008 ± 0.001a | 0.019 ± 0.010a | NDa | 0.007 ± 0.006a | 0.040 ± 0.039a | B |
| P19 | 1297 | 3,5-dimethyl-2-(2-methylbutyl)pyrazine | 56617-70-0 | 0.011 ± 0.000b | NDa | NDa | ND | ND | ND | B |
| P20 | 1297 | 2,5-dimethyl-3-(2-methylbutyl)pyrazine | 72668-36-1 | NDa | NDa | 0.040 ± 0.001b | NDa | NDa | 0.055 ± 0.030b | B |
| P21 | 1309 | 2,3-dimethyl-5-(3-methylbutyl)pyrazine | 18450-01-6 | ND | ND | ND | NDa | 0.007 ± 0.000b | NDa | B |
| P22 | 1309 | 2,5-dimethyl-3-(3-methylbutyl)pyrazine | 18433-98-2 | 0.164 ± 0.010b | NDa | 0.388 ± 0.014c | NDa | NDa | 0.144 ± 0.122a | B |
| P23 | 1309 | 2-butyl-3,5-dimethylpyrazine | 50,888–63-6 | NDa | 0.010 ± 0.000b | NDa | 0.023 ± 0.001b | NDa | NDa | C |
| P24 | 1349 | 2-(furan-2-yl)-5-methylpyrazine | 27610-38-4 | NDa | 0.031 ± 0.001b | NDa | ND | ND | ND | C |
| P25 | 1358 | 2,5-dimethyl-3-pentylpyrazine | 56617-69-7 | ND | ND | ND | NDa | 0.017 ± 0.023a | NDa | B |
| Pyridines | ||||||||||
| PD1 | 1541 | 4-methyl-6-phenylpyrimidine | 17759-27-2 | NDa | NDa | 0.007 ± 0.002b | ND | ND | ND | C |
| Furan and Furanones | ||||||||||
| OF1 | 857 | furan-2-ylmethanol | 98-00-0 | NDa | 0.023 ± 0.005b | NDa | NDa | 0.029 ± 0.006b | NDa | A |
| OF2 | 1125 | 1-(5-methylfuran-2-yl)propan-1-one | 10599-69-6 | NDa | 0.008 ± 0.001b | NDa | ND | ND | ND | B |
| OF3 | 1219 | 3-phenylfuran | 13679-41-9 | 0.065 ± 0.015a | 0.049 ± 0.003a | 0.049 ± 0.004a | 0.112 ± 0.008a | 0.143 ± 0.008b | 0.281 ± 0.008c | B |
| OF4 | 1335 | 5-Pentyl-2(5H)-furanone | ND | ND | ND | 0.006 ± 0.002b | NDa | NDa | B | |
| Oxides | ||||||||||
| ORX1 | 1307 | 2-hexadecyloxirane | 7390-81-0 | ND | ND | ND | NDa | NDa | 0.087 ± 0.113a | C |
| Phenols | ||||||||||
| PH1 | 1083 | 2-methoxyphenol | 90-05-1 | 0.052 ± 0.006a | NDb | NDb | 0.050 ± 0.007b | NDa | NDa | A |
| Sulfur-containing compounds | ||||||||||
| S1 | 741 | (methyldisulfanyl)methane | 624–92-0 | 0.495 ± 0.241b | 0.101 ± 0.014a | 0.306 ± 0.022ab | 0.172 ± 0.028b | 0.087 ± 0.021a | 0.238 ± 0.047b | A |
| S2 | 892 | 2-(methyldisulfanyl)propane | 40136-65-0 | NDa | NDa | 0.015 ± 0.004b | ND | ND | ND | B |
| S3 | 907 | 3-methylsulfanylpropanal | 3268-49-3 | 0.025 ± 0.003c | NDa | 0.017 ± 0.003b | 0.046 ± 0.007b | NDa | 0.040 ± 0.003b | A |
| S4 | 964 | (methyltrisulfanyl)methane | 3658-80-8 | 0.339 ± 0.080ab | 0.274 ± 0.008a | 0.387 ± 0.025b | 0.157 ± 0.017a | 0.153 ± 0.017a | 1.274 ± 1.746a | A |
| S5 | 968 | 2-methyl-1-(methyldisulfanyl)propane | 67421-83-4 | NDa | NDa | 0.007 ± 0.001b | ND | ND | ND | B |
| S6 | 1080 | 3-methylthiophene-2-carbaldehyde | 5834-16-2 | 0.011 ± 0.001a | NDa | 0.030 ± 0.002b | NDa | NDa | 0.018 ± 0.023a | A |
| S7 | 1209 | (methyltetrasulfanyl)methane | 5756-24-1 | NDa | NDa | 0.019 ± 0.002b | ND | ND | ND | A |
aAll volatile compounds are listed in their chemical class in the order of their RI values
bRetention indices were calculated using C6–C30 n-alkanes as external standards
cMean values of relative peak area to the area of internal standard ± standard deviation (n = 3)
dCF corn with fructose reacted model system, SF soy with fructose reacted model system, WF wheat with fructose reacted model system, CG corn with glucose reacted model system, SG soy with glucose reacted model system, WG wheat with glucose reacted model system
eIdentification of the compounds was based on the following: A mass spectrum and retention index agreed with those of standard compounds under the same conditions (positive identification); B mass spectrum and retention index were consistent with those from the NIST library (tentative identification); C mass spectrum was consistent with that of the W9N08 library (Wiley and NIST) and manual interpretation (tentative identification)
fND: Not detected
gDuncan’s multiple comparison test was used for both fructose models (CF, SF, and WF) and glucose models (CG, SG, and WG) to analyze significant difference (p < 0.05) between samples
Table 4.
Solvent extraction analysis of volatile compounds for each Maillard-reacted sample
| NOa | RIb | Compound | CAS | Relative peak areac | IDd | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CF | SF | WF | CG | SG | WG | |||||
| Alcohols | ||||||||||
| A1 | 1037 | phenylmethanol | 100-51-6 | 0.229 ± 0.214af | NDae | 0.104 ± 0.133a | NDa | NDa | 0.200 ± 0.305a | A |
| A2 | 1066 | (1R,2S)-cyclohexane-1,2-diol | 1792-81-0 | ND | ND | ND | NDa | NDa | 0.045 ± 0.078a | C |
| A3 | 1186 | 1-(2-butoxyethoxy)ethanol | 54446-78-5 | 0.115 ± 0.073b | NDa | NDa | ND | ND | ND | B |
| A4 | 1881 | heptacosan-1-ol | 2004-39-9 | 0.332 ± 0.575a | 0.829 ± 1.435a | ND | ND | ND | ND | C |
| Benzene derivatives | ||||||||||
| B1 | 1321 | cyclohexylbenzene | 827-52-1 | 0.139 ± 0.049a | 0.104 ± 0.033a | 0.173 ± 0.061a | 0.124 ± 0.060a | 0.063 ± 0.061a | 0.115 ± 0.040a | A |
| B2 | 1480 | N-(2-phenylethyl)formamide | 23069-99-0 | NDa | 0.015 ± 0.027a | NDa | 0.077 ± 0.097a | NDa | NDa | B |
| Acids | ||||||||||
| C1 | 1176 | octanoic acid | 124-07-2 | NDa | NDa | 0.049 ± 0.023b | 0.023 ± 0.026a | NDa | NDa | A |
| C2 | 1272 | nonanoic acid | 112-05-0 | NDa | NDa | 0.111 ± 0.096a | 0.084 ± 0.121a | 0.023 ± 0.040a | NDa | A |
| C3 | 1565 | dodecanoic acid | 143-07-7 | 0.069 ± 0.072a | NDa | NDa | 0.006 ± 0.010a | NDa | NDa | A |
| C4 | 1957 | hexadecanoic acid | 57-10-3 | 1.057 ± 0.838a | 0.408 ± 0.149a | 1.254 ± 0.027a | 0.739 ± 1.063a | 0.368 ± 0.441a | 1.337 ± 1.732a | A |
| Aldehydes | ||||||||||
| D1 | 792 | (E)-2-ethenylbut-2-enal | 20521-42-0 | ND | ND | ND | NDa | NDa | 0.169 ± 0.133b | B |
| D2 | 963 | benzaldehyde | 100-52-7 | 0.710 ± 0.317a | 0.523 ± 0.254a | 0.572 ± 0.081a | 1.364 ± 1.415a | 0.877 ± 0.853a | 0.818 ± 0.529a | A |
| D3 | 1044 | 2-phenylacetaldehyde | 122-78-1 | 1.498 ± 1.054a | 2.192 ± 0.956a | 0.587 ± 0.129a | 2.415 ± 1.984a | 7.011 ± 5.351a | 1.159 ± 0.404a | A |
| D4 | 1105 | nonanal | 124-19-6 | 0.101 ± 0.125a | NDa | 0.071 ± 0.061a | NDa | NDa | 0.007 ± 0.012a | A |
| D5 | 1263 | (E)-dec-2-enal | 3913-81-3 | NDa | NDa | 0.043 ± 0.009b | ND | ND | ND | A |
| D6 | 1268 | (E)-2-phenylbut-2-enal | 4411-89-6 | NDa | NDa | 0.024 ± 0.009b | 0.087 ± 0.039b | NDa | 0.037 ± 0.016a | A |
| D7 | 1372 | 4-hydroxybenzaldehyde | 123-08-0 | NDa | NDa | 0.052 ± 0.020b | NDa | NDa | 0.049 ± 0.058a | A |
| D8 | 1434 | 2-(dimethylhydrazono)-3,3-dimethoxypropanal | ND | ND | ND | 0.150 ± 0.107b | NDa | NDa | C | |
| D9 | 1437 | (E)-3-phenylprop-2-enal | 104-55-2 | NDa | NDa | 0.082 ± 0.042b | ND | ND | ND | A |
| D10 | 1484 | (E)-5-methyl-2-phenylhex-2-enal | 21834-92-4 | 0.009 ± 0.016a | NDa | 0.036 ± 0.001b | NDa | NDa | 0.040 ± 0.023b | A |
| Esters | ||||||||||
| E1 | 873 | 2-oxopropyl acetate | 592-20-1 | ND | ND | ND | 0.167 ± 0.246a | NDa | NDa | B |
| E2 | 1348 | (1-hydroxy-2,4,4-trimethylpentan-3-yl) 2-methylpropanoate | 74367-33-2 | 0.026 ± 0.026a | NDa | 0.025 ± 0.002a | NDa | NDa | 0.028 ± 0.009b | C |
| Hydrocarbons | ||||||||||
| H1 | 1073 | cyclooctane | 292-64-8 | NDa | 0.171 ± 0.176a | NDa | ND | ND | ND | C |
| H2 | 1400 | tetradecane | 629-59-4 | 0.041 ± 0.013b | NDa | 0.052 ± 0.025b | ND | ND | ND | A |
| H3 | 1475 | cyclodecane | 293-96-9 | NDa | 0.427 ± 0.351b | NDa | 0.133 ± 0.056b | NDa | NDa | C |
| H4 | 1475 | cyclododecane | 294-62-2 | 0.126 ± 0.065b | NDa | 0.123 ± 0.050b | NDa | 0.533 ± 0.204b | 0.161 ± 0.067a | C |
| H5 | 1500 | pentadecane | 629-62-9 | NDa | NDa | 0.077 ± 0.015b | 0.007 ± 0.011a | NDa | NDa | A |
| H6 | 1600 | hexadecane | 544-76-3 | 0.030 ± 0.026ab | NDa | 0.116 ± 0.071b | 0.115 ± 0.159a | 0.014 ± 0.025a | NDa | A |
| H7 | 1677 | tetradec-1-ene | 1120-36-1 | NDa | 0.095 ± 0.105a | NDa | ND | ND | ND | A |
| H8 | 1677 | tridec-1-ene | 2437-56-1 | ND | ND | ND | NDa | 0.113 ± 0.102a | NDa | C |
| H9 | 1677 | cyclotetradecane | 295-17-0 | 0.025 ± 0.043a | NDa | 0.088 ± 0.005b | NDa | NDa | 0.070 ± 0.030b | B |
| H10 | 1799 | octadecane | 593-45-3 | 0.026 ± 0.023ab | NDa | 0.102 ± 0.062b | NDa | 0.014 ± 0.024a | NDa | A |
| H11 | 1879 | octadec-1-ene | 112-88-9 | ND | ND | ND | NDa | 1.166 ± 2.019a | NDa | A |
| H12 | 2000 | icosane | 112-95-8 | 0.003 ± 0.006a | NDa | 0.046 ± 0.040a | ND | ND | ND | A |
| Ketones | ||||||||||
| K1 | 932 | cyclohex-2-en-1-one | 930-68-7 | 0.788 ± 0.402a | 1.499 ± 1.259a | 0.456 ± 0.031a | 1.763 ± 1.779a | 3.293 ± 3.493a | 0.775 ± 0.368a | A |
| K2 | 1024 | 2-hydroxy-3-methylcyclopent-2-en-1-one | 80-71-7 | 0.051 ± 0.032a | 0.280 ± 0.247a | NDa | 0.025 ± 0.043a | 0.590 ± 0.442b | 0.009 ± 0.016a | A |
| K3 | 1027 | 3-methylcyclopentane-1,2-dione | 765-70-8 | NDa | NDa | 0.017 ± 0.002b | ND | ND | ND | B |
| K4 | 1065 | 1-phenylethanone (acetophenone) | 98-86-2 | NDa | NDa | 0.214 ± 0.025b | ND | ND | ND | A |
| K5 | 1241 | 2-hydroxy-1-phenylethanone | 582-24-1 | 0.025 ± 0.027a | NDa | NDa | ND | ND | ND | A |
| K6 | 1301 | 2,3-dimethylcyclopent-2-en-1-one | 1121-05-7 | 0.124 ± 0.097b | NDa | NDa | 0.171 ± 0.032b | 0.090 ± 0.106ab | NDa | C |
| K7 | 1355 | 3,4,4-trimethylcyclopent-2-en-1-one | 30434-65-2 | 0.069 ± 0.047ab | 0.224 ± 0.134b | NDa | 0.088 ± 0.021b | NDa | NDa | C |
| K8 | 1361 | (E)-4-phenylbut-3-en-2-one | 1896-62-4 | 0.191 ± 0.168a | NDa | 0.017 ± 0.000a | ND | ND | ND | B |
| K9 | 1447 | 1-(4-hydroxyphenyl)ethanone | 99-93-4 | NDa | NDa | 0.046 ± 0.006b | ND | ND | ND | A |
| K10 | 1449 | 1-(4-hydroxy-2-methylphenyl)ethanone | 875-59-2 | NDa | 0.206 ± 0.112b | NDa | ND | ND | ND | A |
| Lactones | ||||||||||
| L1 | 952 | 5-methyloxolan-2-one | 108-29-2 | 2.186 ± 1.467a | 1.939 ± 0.920a | 0.660 ± 0.052a | 4.823 ± 4.618a | 3.102 ± 2.964a | 1.026 ± 0.460a | A |
| L2 | 959 | 4-methyloxolan-2-one | 1679-49-8 | 0.266 ± 0.390a | NDa | NDa | ND | ND | ND | C |
| L3 | 981 | 4,4-dimethyloxolan-2-one | 13861-97-7 | NDa | NDa | 0.045 ± 0.006b | ND | ND | ND | C |
| L4 | 1051 | 5-ethyloxolan-2-one | 695-06-7 | 0.044 ± 0.028b | NDa | 0.023 ± 0.001ab | 0.068 ± 0.038a | NDa | 0.188 ± 0.291a | A |
| L5 | 1160 | 4-hydroxyoxolan-2-one | 5469-16-9 | NDa | 0.064 ± 0.072a | NDa | NDa | NDa | 0.073 ± 0.058b | B |
| N-containing compounds | ||||||||||
| N1 | 1389 | 2-phenylacetamide | 103-81-1 | NDa | NDa | 0.161 ± 0.028b | 0.083 ± 0.081ab | NDa | 0.111 ± 0.038b | B |
| Pyrazines | ||||||||||
| P1 | 829 | 2-methylpyrazine | 109-08-0 | 1.248 ± 0.923a | 1.722 ± 1.442a | 0.312 ± 0.087a | 0.429 ± 0.362a | 2.020 ± 3.499a | 0.238 ± 0.201a | A |
| P2 | 913 | 2,6-dimethylpyrazine | 108-50-9 | 5.635 ± 3.218b | 3.536 ± 1.831ab | NDa | ND | ND | ND | A |
| P3 | 913 | 2,5-dimethylpyrazine | 123-32-0 | NDa | NDa | 2.371 ± 0.666b | 1.498 ± 1.576a | NDa | 0.823 ± 0.527a | A |
| P4 | 997 | 2-ethyl-6-methylpyrazine | 13925-03-6 | 0.055 ± 0.018a | 0.660 ± 0.754a | 0.052 ± 0.019a | NDa | 0.441 ± 0.470a | 0.060 ± 0.026a | A |
| P5 | 997 | 2-ethyl-5-methylpyrazine | 13360-64-0 | ND | ND | ND | 0.161 ± 0.184a | NDa | NDa | B |
| P6 | 1001 | 2,3,5-trimethylpyrazine | 14,667–55-1 | 0.418 ± 0.153a | 0.796 ± 0.787a | 0.321 ± 0.093a | 0.201 ± 0.129a | 0.090 ± 0.081a | 0.269 ± 0.113a | A |
| P7 | 1022 | 1-pyrazin-2-ylethanone | 22-047-25-2 | ND | ND | ND | 0.385 ± 0.484a | NDa | 0.146 ± 0.084a | A |
| P8 | 1055 | 2-methyl-3-propan-2-ylpyrazine | 15986-81-9 | ND | ND | ND | NDa | NDa | 0.047 ± 0.005b | B |
| P9 | 1055 | 2-methyl-5-propan-2-ylpyrazine | 13925-05-8 | 0.125 ± 0.073a | 0.036 ± 0.063a | 0.053 ± 0.007a | ND | ND | ND | B |
| P10 | 1076 | 3-ethyl-2,5-dimethylpyrazine | 13360-65-1 | 0.196 ± 0.057a | 0.125 ± 0.045a | 0.175 ± 0.023a | 0.179 ± 0.167a | 0.054 ± 0.047a | 0.205 ± 0.081a | A |
| P11 | 1113 | 1-(5-methylpyrazin-2-yl)ethanone | 22047-27-4 | NDa | NDa | 0.037 ± 0.017b | NDa | NDa | 0.017 ± 0.015a | C |
| P12 | 1119 | 1-(6-methylpyrazin-2-yl)ethanone | 22047-26-3 | 0.080 ± 0.031b | 0.014 ± 0.024a | 0.026 ± 0.007a | 0.190 ± 0.118b | NDa | 0.044 ± 0.007a | B |
| P13 | 1130 | 2,3-dimethyl-5-propan-2-ylpyrazine | 40790-21-4 | ND | ND | ND | 0.004 ± 0.007a | NDa | NDa | C |
| P14 | 1197 | 2-(2-methylpropyl)-3,6-dimethylpyrazine | 32736-94-0 | ND | ND | ND | 0.003 ± 0.005a | NDa | NDa | B |
| P15 | 1198 | 3,5-dimethyl-2-(2-methylpropyl)pyrazine | 70303-42-3 | 0.008 ± 0.014a | NDa | NDa | ND | ND | ND | B |
| P16 | 1202 | 2,5-dimethyl-3-(2-methylpropyl)pyrazine | 32736-94-0 | 0.214 ± 0.157b | NDa | 0.029 ± 0.001a | NDa | NDa | 0.026 ± 0.009b | B |
| P17 | 1250 | 2-methyl-6-(3-methylbutyl)pyrazine | 91010-41-2 | NDa | NDa | 0.010 ± 0.003b | ND | ND | ND | B |
| P18 | 1311 | 3,5-dimethyl-2-propylpyrazine | 32350-16-6 | ND | ND | ND | NDa | NDa | 0.014 ± 0.013a | B |
| P19 | 1311 | 2-butyl-3,5-dimethylpyrazine | 50888-63-6 | NDa | 0.163 ± 0.147a | NDa | ND | ND | ND | B |
| P20 | 1311 | 2,5-dimethyl-3-(3-methylbutyl)pyrazine | 0.064 ± 0.021b | NDa | NDa | ND | ND | ND | C | |
| P21 | 1311 | 3-butyl-2,5-dimethylpyrazine | NDa | NDa | 0.097 ± 0.027b | NDa | NDa | 0.067 ± 0.044b | C | |
| P22 | 1355 | 2-(furan-2-yl)-5-methylpyrazine | 27610-38-4 | ND | ND | ND | NDa | 0.291 ± 0.092b | NDa | C |
| Pyrroles | ||||||||||
| PR1 | 1065 | 1-(1H-pyrrol-2-yl)ethanone | 1072-83-9 | ND | ND | ND | 0.602 ± 0.443b | NDa | 0.474 ± 0.056ab | A |
| PR2 | 1127 | 1-methylpyrrole-2-carbaldehyde | 1192-58-1 | ND | ND | ND | NDa | 0.009 ± 0.015a | NDa | A |
| PR3 | 1128 | 5-methyl-1H-pyrrole-2-carbaldehyde | 1192-79-6 | ND | ND | ND | NDa | 0.017 ± 0.030a | NDa | C |
| PR4 | 1234 | 3-ethyl-4-methylpyrrole-2,5-dione | 20189-42-8 | NDa | NDa | 0.024 ± 0.001b | NDa | NDa | 0.024 ± 0.011b | B |
| PR5 | 1294 | 1-(2,4-dimethyl-1H-pyrrol-3-yl)ethanone | 2386-25-6 | NDa | NDa | 0.060 ± 0.061a | ND | ND | ND | C |
| PR6 | 1403 | 2,5-dimethyl-1-propylpyrrole | 20,282-39-7 | 0.071 ± 0.037b | NDa | NDa | ND | ND | ND | C |
| Pyridines | ||||||||||
| PD1 | 1155 | 1-nitroso-3,6-dihydro-2H-pyridine | 55556-92-8 | 0.070 ± 0.043b | NDa | NDa | ND | ND | ND | C |
| PD2 | 1463 | 2-phenylpyridine | 1008-89-5 | NDa | 0.008 ± 0.014a | NDa | ND | ND | ND | A |
| PD3 | 1463 | 3-phenylpyridine | 1008-88-4 | 0.026 ± 0.023a | NDa | NDa | 0.316 ± 0.470a | NDa | NDa | A |
| Piperidines | ||||||||||
| PP1 | 1179 | piperidin-2-one | 675-20-7 | ND | ND | ND | 0.006 ± 0.010a | NDa | NDa | A |
| Pyrrolidines | ||||||||||
| PL1 | 1079 | pyrrolidine-1-carbaldehyde | 3760-54-1 | 0.039 ± 0.035a | ND | ND | ND | ND | ND | A |
| PL2 | 1380 | 1-(6,7-dihydro-5H-pyrrolizin-3-yl)ethanone | 55041-85-5 | 0.066 ± 0.032b | NDa | NDa | NDa | NDa | 0.142 ± 0.155a | C |
| Quinolines | ||||||||||
| Q1 | 1211 | quinazoline | 253-82-7 | ND | ND | ND | 0.022 ± 0.032a | NDa | NDa | A |
| Q2 | 1547 | 1-methylisoquinoline | 1721-93-3 | 0.210 ± 0.027b | NDa | NDa | ND | ND | ND | A |
| Pyrimidine | ||||||||||
| PM1 | 1276 | 6-amino-5-methyl-1H-pyrimidin-2-one | 554-01-8 | NDa | 0.125 ± 0.047b | NDa | ND | ND | ND | C |
| PM2 | 1325 | 5-amino-2H-1,2,4-triazin-3-one | 931-85-1 | 0.014 ± 0.017a | NDa | NDa | ND | ND | ND | C |
| Imidazoles | ||||||||||
| IZ1 | 1480 | 1-methyl-3-(2-phenylethyl)imidazolidine-2,4,5-trione | 67867-43-0 | 0.049 ± 0.043a | NDa | NDa | ND | ND | ND | C |
| IZ2 | 1542 | 1-(1H-imidazo[4,5-c]pyridin-4-yl)ethanone | 146874-38-6 | 0.127 ± 0.049a | 0.264 ± 0.366a | 0.068 ± 0.009a | 0.040 ± 0.042a | NDa | NDa | C |
| Imines | ||||||||||
| I1 | 1597 | 1H-indol-5-ol | 1953-54-4 | ND | ND | ND | NDa | 0.022 ± 0.028a | NDa | B |
| Furans and Furanones | ||||||||||
| OF1 | 837 | furan-2-carbaldehyde | 98-01-1 | ND | ND | ND | 0.209 ± 0.309a | NDa | NDa | A |
| OF2 | 860 | furan-2-ylmethanol | 98-00-0 | 0.792 ± 0.983a | 0.783 ± 0.300a | 0.023 ± 0.005a | 0.007 ± 0.012a | 1.342 ± 1.544a | NDa | A |
| OF3 | 1063 | 4-hydroxy-2,5-dimethylfuran-3-one | 3658-77-3 | ND | ND | ND | 0.231 ± 0.200a | NDa | 0.019 ± 0.033a | A |
| OF4 | 1187 | 5-(hydroxymethyl)oxolan-2-one (5-(hydroxymethyl)dihydrofuran-2(3H)-one) | 10374-51-3 | 0.039 ± 0.068a | ND | ND | ND | ND | ND | C |
| OF5 | 1223 | 3-phenylfuran | 13679-41-9 | 0.105 ± 0.085a | NDa | 0.040 ± 0.024a | 0.070 ± 0.053b | NDa | 0.040 ± 0.004ab | A |
| OF6 | 1338 | 5-pentyl-2(5H)-furanone | 21963-26-8 | NDa | NDa | 0.025 ± 0.005b | ND | ND | ND | B |
| OF7 | 1358 | 2,5-dimethyl-3-ethylfuran | 10599-62-9 | ND | ND | ND | NDa | NDa | 0.053 ± 0.078a | C |
| OF8 | 1607 | 2-(phenylethenyl)tetrafuran | 0.073 ± 0.011b | NDa | NDa | ND | ND | ND | C | |
| Pyrans | ||||||||||
| OPN1 | 1107 | 3-hydroxy-2-methylpyran-4-one (maltol) | 118-71-8 | 0.166 ± 0.172a | 2.781 ± 2.525a | 0.120 ± 0.034a | 0.019 ± 0.034a | 4.335 ± 0.785b | 0.218 ± 0.140a | A |
| OPN2 | 1127 | 6-ethyl-4-methyl-3,6-dihydro-2H-pyran | 63500-69-6 | 0.010 ± 0.010a | NDa | 0.210 ± 0.021b | ND | ND | ND | C |
| OPN3 | 1134 | 6-methyl-3,4-dihydro-2H-pyran-2-carbaldehyde | 64881-26-1 | 0.143 ± 0.056b | NDa | NDa | ND | ND | ND | C |
| OPN4 | 1144 | 3,5-dihydroxy-6-methyl-2,3-dihydropyran-4-one | 28564-83-2 | ND | ND | ND | 0.084 ± 0.145a | NDa | 0.138 ± 0.107a | B |
| OPN5 | 1931 | 2-ethoxy-6-methyl-3,4-dihydro-2H-pyran | 52438-71-8 | 0.611 ± 1.058a | NDa | NDa | ND | ND | ND | C |
| Phenols | ||||||||||
| PH1 | 989 | phenol | 108-95-2 | 0.205 ± 0.185a | 1.372 ± 1.415a | NDa | 0.311 ± 0.189b | NDa | 0.026 ± 0.023a | A |
| PH2 | 1082 | 4-methylphenol | 106-44-5 | 0.104 ± 0.071a | 0.480 ± 0.279b | NDa | 0.181 ± 0.083b | NDa | NDa | A |
| PH3 | 1085 | 2-methoxyphenol | 90-05-1 | 0.251 ± 0.188b | 0.192 ± 0.057ab | NDa | 0.425 ± 0.334a | 0.247 ± 0.229a | 0.008 ± 0.013a | A |
| PH4 | 1346 | 2,6-dimethoxyphenol | 91-10-1 | 0.328 ± 0.223ab | 0.402 ± 0.052b | 0.077 ± 0.017a | 0.418 ± 0.091b | 0.374 ± 0.076b | 0.067 ± 0.035a | A |
| Sulfur-containing compounds | ||||||||||
| S1 | 909 | 3-methylsulfanylpropanal | 3268-49-3 | 1.126 ± 0.639a | 0.006 ± 0.359a | 0.485 ± 0.067a | 2.933 ± 2.833a | 0.021 ± 3.385a | 1.156 ± 0.667a | A |
| S2 | 969 | (methyltrisulfanyl)methane | 3658-80-8 | ND | ND | ND | 0.939 ± 1.598a | NDa | NDa | A |
| S3 | 993 | thiophene-2-carbaldehyde | 98-03-3 | NDa | NDa | 0.021 ± 0.002b | NDa | NDa | 0.140 ± 0.202a | A |
| S4 | 994 | 2,4-dimethylthiophene | 638-00-6 | 0.767 ± 1.317a | NDa | NDa | ND | ND | ND | B |
| S5 | 1243 | 2-methyl-3-methylsulfanylfuran | 63012-97-5 | NDa | NDa | 0.020 ± 0.009b | ND | ND | ND | C |
aAll volatile compounds are listed in their chemical class in the order of their RI values
bRetention indices were calculated using C6-C30 n-alkanes as external standards
cMean values of relative peak area to the area of internal standard ± standard deviation (n = 3)
dCF; corn with fructose reacted model system, SF; soy with fructose reacted model system, WF; wheat with fructose reacted model system, CG; corn with glucose reacted model system, SG; soy with glucose reacted model system, WG; wheat with glucose reacted model system
eIdentification of the compounds was based on the following: A, mass spectrum and retention index agreed with those of standard compounds under the same conditions (positive identification); B, mass spectrum and retention index were consistent with those from the NIST library (tentative identification); C, mass spectrum was consistent with that of the W9N08 library (Wiley and NIST) and manual interpretation (tentative identification)
fND: Not detected
gDuncan’s multiple comparison test was used for both fructose models (CF, SF, and WF) and glucose models (CG, SG, and WG) to analyze significant difference (p < 0.05) between samples
To compare the explanatory power of the model system obtained from SPME and SE analysis, PCA plots were applied (Fig. S1). The total variance of the PCA plot of SE was 45.9%, while that of the PCA plot for SPME was 62.5%. The SPME data showed higher model explanatory power than the SE data. It might be explained that model system obtained from SPME analysis could suggest more reliable variables which distinguish among samples. Therefore, the PCA model of SPME was applied to study the effects of the type of HVP and reducing sugar on the formation of volatile profiles.
Effect of sugar types on volatile formation
In Fig. S2, two types of PCA model (component 1 + 2 and component 1 + 3) were used to explain the differences of volatile profiles corresponding factors. The total explanatory powers were 45.7% (component 1 + 2) and 43.1% (component 1 + 3). In the results, CF and CG were discriminated by component 1, while SF and SG were separated by component 2. On the other hand, WF and WG were clearly separated by component 3.
In Fig. S2(A), some pyrazines, including 2-butyl-3-methylpyrazine, 3,5-dimethyl-2-(2-methylbutyl)pyrazine, and 2-ethenyl-6-methylpyrazine, were located near the CF model system. Based on this result, these pyrazines contributed to distinguishing CF from CG. In general, pyrazines are generated by the thermal reaction of amino acids and reducing sugars (Nursten, 2005). Fructose is more efficiently involved in the production of pyrazines compared to glucose, because of its higher reaction activity (Göğüş et al., 1998). Therefore, the increased production of pyrazines in the corn HVP system might contribute to discriminating fructose from glucose in this study.
The SF region was located on the positive side of component 2, while the SG region was located on the negative side of component 2 (Fig. S2(A)). Some aldehydes, including 3-methylbutanal, hexanal, nonanal, octanal, (E)-dec-2-enal, and (E)-dodec-3-enal, showed to correlate with SG. Glucose, an aldose form of sugar, were induced more abundant aldehydes in soy HVP model system. On the other hand, long-chain aldehydes, including hexanal, octanal, nonanal, (E)-dec-2-enal, and (E)-dodec-3-enal, are known to be mainly formed by lipid oxidation (Nursten, 2005). In general, there are trace amounts of lipids in the HVPs (Kang et al., 2017). However, the cause of the differing amounts of those long-chain aldehydes between fructose- and glucose- containing soy HVP model remains unclear.
WF and WG were distinguished mainly by component 3 in Fig. S2(B). In the result, pyrazines were located around the region associated with WF, while aldehydes were located near the WG region. Based on the result of CF and CG, it might assume that the Maillard reaction proceeded more actively the in the fructose-containing systems than the glucose-containing ones, leading to the production of many pyrazines (Göğüş et al., 1998).
Effect of HVP types on volatile formation
In the results of PCA models, component 1 contribute to distinguish between wheat and soy models, while component 2 separated the corn and wheat model systems [Fig. S2(A)]. Also, component 3 could discriminate the corn model from soy model [Fig. S2(B)].
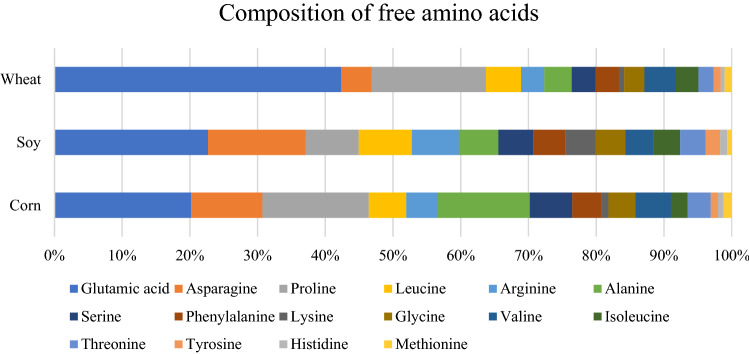
Tables 3 and 4 show all volatile compounds generated from different HVP and reducing sugar model systems obtained using SPME- and SE-GC-MS analysis. In order to correlate with the precursors (amino acids and reducing sugars) and volatile compounds, the contents and composition of free amino acids and degree of hydrolysis in each HVPs were showed in Table 5 & S1 and Fig. 1, respectively.
Table 5.
Free amino acid contents
| Amino acid | Amount (mg/g) | ||
|---|---|---|---|
| Soy | Wheat | Corn | |
| Tyrosine | 2.43 ± 0.28bA | 7.45 ± 0.47a | 5.88 ± 0.01a |
| Glycine | 4.59 ± 0.09a | 21.83 ± 0.13a | 21.87 ± 0.18a |
| Serine | 5.32 ± 0.11a | 26.28 ± 0.23a | 34.87 ± 0.52a |
| Alanine | 6.11 ± 0.06a | 29.75 ± 0.40a | 75.66 ± 0.11a |
| Glutamic acid | 23.89 ± 0.18c | 311.34 ± 0.23a | 112.18 ± 0.04b |
| Lysine | 4.64 ± 0.07b | 5.81 ± 0.16a | 5.6 ± 0.33a |
| Leucine | 8.57 ± 0.48b | 38.23 ± 0.25a | 30.71 ± 0.02a |
| Methionine | 0.69 ± 0.50b | 7.42 ± 0.37a | 6.71 ± 0.01ab |
| Valine | 4.31 ± 0.09b | 33.48 ± 0.06a | 29.62 ± 0.46a |
| Arginine | 7.51 ± 0.36a | 25.25 ± 0.09a | 25.34 ± 0.33a |
| Asparagine | 14.91 ± 0.28a | 33.36 ± 0.35a | 58.52 ± 0.23a |
| Isoleucine | 4.02 ± 0.11b | 25.45 ± 0.46a | 13.33 ± 0.32b |
| Threonine | 3.54 ± 0.60b | 16.66 ± 0.12ab | 19.33 ± 0.35a |
| Phenylalanine | 4.93 ± 0.10b | 25.01 ± 0.13a | 24.28 ± 0.33a |
| Proline | 8.33 ± 0.12b | 124.13 ± 0.45a | 87.17 ± 0.18a |
| Histidine | 1.35 ± 0.36b | 4.17 ± 0.15ab | 4.67 ± 0.16a |
| Cysteine | NDB | ND | ND |
| Tryptophane | ND | ND | ND |
ADuncan’s multiple comparison test was used for three types of free amino acids contents to analyze significant difference (p < 0.05) between three types of vegetable proteins (soy, wheat, and corn)
BND not detected
Fig. 1.
Composition of free amino acids expressed as percentiles
Volatiles from amino acids degradation
Figure 1 shows each HVP (corn, wheat, and soy) has different composition of free amino acids. In this study, Strecker aldehydes, which are generated via Strecker degradation of the corresponding α-amino acid, were found. When α-amino acids are oxidized to the corresponding aldehydes, ammonia is transported to other components of the reaction system and carbon dioxide is released (Nursten, 2005). Strecker aldehydes mainly have characteristic odors and low threshold values, which affect the organoleptic properties of foods (Cremer et al., 2000). In particular, some Strecker aldehydes, such as 3-methylbutanal, phenylacetaldehyde, and methional, can contribute to the flavor of meats (Mottram, 2007). 3-Methylbutanal was found only in SG and SF in this study. 3-Methylbutanal is derived from leucine, which is one of the dominant amino acids in soy HVP (Fig. 1). It has malty, green, fruity, and chocolate-like odor notes with a low odor threshold value (1.1 μg/kg in water) (Chen et al., 2020).
Several phenylalanine-induced aldehydes, including benzaldehyde, 2-phenylacetaldehyde, 2-phenylbut-2-enal, and 5-methyl-2-phenylhex-2-enal, were detected in this study. The content of phenylalanine was in the order as follows: wheat HVP (25.01 mg/g), corn HVP (24.28 mg/g), and soy HVP (4.93 mg/g). Wheat HVP, which had the largest amount of phenylalanine, produced the highest contents of benzaldehyde and (E)-5-methyl-2-phenylhex-2-enal. Benzaldehyde possesses sweet and floral odor notes (Karangwa et al., 2016). Additionally, phenyl methanol (benzyl alcohol), a benzaldehyde derivative (Nie et al., 2019), was found in the CF, WF, and WG models. 2-Phenylacetaldehyde, which has a strong floral green odor note (Zhang et al., 2014), was also detected in all model systems. 2-Phenylbut-2-enal was generated from the aldol condensation of phenylacetaldehyde, and possessed musty, floral, and green odor notes (Fadel et al., 2006). (E)-5-Methyl-2-phenylhex-2-enal, also derived from phenylacetaldehyde, is known to have a strong chocolate-flavor note (Barišić et al., 2019).
Methional, a Strecker aldehyde generated from methionine, was more abundant in corn and wheat model systems. Methionine, which is a precursor of methional, was detected in all HVPs, in the order as follows: wheat (7.42 mg/g), corn (6.71 mg/g), and soy (0.69 mg/g). The amount of methional appears to be strongly related to the level of methionine in HVP samples. Methional, which possessed cooked meat, potato, and onion-like odor notes, has been reported to be a key compound in beef (Hoa et al., 2012) and soy sauce (Steinhaus and Schieberle, 2007), however, it has also been associated with off-flavor in fresh lager beer and wine (Guedes de pinho and Silva Ferreira, 2006). Methionine was the only sulfur-containing amino acid detected in this study, and induced the formation of various sulfur-containing compounds, including methional, disulfides, and thiophenes.
Cysteine was not detected in this study. It has been reported that cysteine is destroyed completely during acidic hydrolysis (Aaslyng et al., 1998). When cysteine undergoes Strecker degradation, hydrogen sulfides are generated and involved in forming thiazoles (Nursten, 2005). In this study, thiazoles were not found, presumably due to the lack of cysteine.
Some sulfur-containing compounds with low threshold values and characteristic odor notes (Cerny, 2015) could contribute considerably to meat-like aroma. The content of methional was higher in wheat and corn HVPs compared to soy HVP. Several disulfides, which are derived from methionine, were also found in this study. Dimethyl disulfide and dimethyl trisulfide were identified in the wheat and corn model systems, while dimethyl tetrasulfide was found only in the WF model system. Wheat HVP contained more methionine than the corn and soy HVPs, yielding various sulfur-containing compounds.
Volatiles from the interaction between amino acids and reducing sugars
Some nitrogen-containing heterocyclic compounds have roasted and burnt odor notes, mainly providing desirable flavors to many foods. These compounds are generated by the interaction between amino acids and reducing sugars. At first, the condensation between sugars and amino acids produces Amadori and Heyns products, followed by sugar degradation, which releases amino compounds and aromatic compounds such as ketones, aldehydes, alcohols, and furans (Nursten, 2005). Through further reactions, diverse heterocyclic compounds, such as pyrroles, pyridines, pyrazines, and thiophenes, are generated. In this study, various pyrazines were formed in all model systems. A total of 22, 12, and 21 pyrazines were isolated from the corn, soy, and wheat model systems, respectively. The number and amounts of pyrazines were fewer in Soy HVP than the other HVPs, it might be explained that soy HVP had the lowest amount of free amino acids.
2,5-Dimethylpyrazine was the most dominant pyrazine in the wheat model system. It has been reported to be formed by the Maillard reaction of asparagine, valine, and leucine with reducing sugars (Cho et al., 2010). These amino acids were more abundant in wheat HVP than the other HVPs. On the other hand, the content of 2,6-dimethylpyrazine, which is derived from arginine, alanine, lysine, and glycine in the Maillard reaction, was higher in corn model systems, where those amino acids were more abundant compared to other HVPs. 2-Methylpyrazine was found abundantly in all the model systems. It is derived from the Maillard reaction of glycine, valine, leucine, and asparagine (Cho et al., 2010). 3-Ethyl-2,5-dimethylpyrazine, which has been reported as a product of the Maillard reaction of alanine (Van Lancker et al., 2012), lysine, arginine, and histidine (Wang et al., 2021), was more abundant in only the model systems containing corn HVP. This could be related to the higher contents of alanine, lysine, arginine, and histidine in corn HVP. On the other hand, 2,5-dimethyl-3-(3-methylbutyl)pyrazine, which could be formed from the Maillard reaction of leucine, lysine, threonine, and serine (Cho et al., 2010), was abundant in the model systems containing wheat HVP. It has been shown to contribute to meat aroma with a roasted odor (Tamura et al., 2022). In this study, 2,3,5-trimethylpyrazine was found in wheat and corn model systems. A previous study reported that alanine and glycine induce 2,3,5-trimethylpyrazine by the Maillard reaction (Kim et al., 1988). The contents of those amino acids were higher in wheat HVP than in wheat and corn HVP. Consequently, the composition of certain free amino acids significantly influenced the formation of some pyrazines in this study.
Nitrogen-containing heterocyclic compounds, including pyrroles and pyridines, are formed from proline through the Maillard reaction (Nursten, 2005). In particular, 2-acetylpyrrole, which has nutty, walnut, and bread-like odor notes (Tao et al., 2014), was more abundant in the corn and wheat model systems compared to the soy model systems. Wang and Kays (2000) has reported that 2-acetylpyrrole is main volatile compound in cooked beef, chicken, pork, coffee, and baked potato. Heterocyclic sulfur compounds, including thiophene-3-carbaldehyde, thiophene-2-carbaldehyde, and 2,4-dimethylthiophene, were found in the WF, WG, and CG model systems, respectively. Thiophenes, which are the products of Maillard reaction between sulfur-containing amino acids and the deoxyosones from sugar degradation, give a desirable meaty aroma in cooked meats (Shahidi, 2014).
Volatiles from sugar degradation
Furans, furanones, pyrans, and ketones, which were generated from sugar degradation (Nursten, 2005), were found. These compounds were characterized in the soy model system. The soy HVP had a smaller amount of amino acid precursors than the wheat and corn HVP, resulting the formation of volatile compounds from sugar degradation. Reducing sugars could be degraded themselves instead of interacting with the amino acids during thermal reactions. Many compounds derived from sugar degradation are reported as having sweet, fruity, berry, and caramel-like odors (Nursten, 2005). Furfuryl alcohol, which is a final product of dehydration and cyclization of reducing sugars (Albouchi and Murkovic, 2018), was one of the major components in the soy model systems. Higher content of furfuryl alcohol in the soy model systems might be correlated with lower contents of free amino acid. Maltol was found in all model systems; it was particularly abundant in the soy model systems. It is generated from hexose by losing three molecules of water (Nursten, 2005). Although the aroma strength of maltol is not strong, it is mainly used as a sweet flavor enhancer in foods (Lee et al., 2006). Cyclotene, which has a caramel-like odor description, is a volatile compound generated via the Maillard reaction (Nursten, 2005). It has been reported that the glucose skeleton leads to the formation of cyclotene during Maillard reaction. Cyclotene is recognized as a flavor enhancer at a minimum concentration in foods and also has been reported in smoked meat products (Issenberg and Lustre, 1970). Soy model systems contained higher content of cyclotene, compared to the wheat and corn model system. It might also be explained that lower total contents of free amino acids in soy HVP affected the formation of cyclotene. Therefore, the reducing sugars in soy model systems did not react with the amino acids completely, but it had a greater chance to undergo degradations themselves. Furfual and hydroxymethylfurfural (HMF) are generated through sugar degradation and are considered as main intermediates of the Maillard reaction (Nursten, 2005). HMF and furfural are generally generated from hexose and pentose, respectively (Nursten, 2005). In this study, HMF was not detected, and it might be explained that the generation of HMF is accelerated under acid conditions (Nursten, 2005), however, our reaction conditions was under alkaline pH.
This study investigated the profile of the volatile compounds generated by a thermal reaction between various HVPs, such as corn, soy, and wheat (majorly used HVPs), and the reducing sugars (glucose and fructose). In the results, the types of HVP, which had different amino acid compositions, strongly affected the formation of volatile compounds in each model system. The wheat and corn HVPs contained higher amounts of free amino acids than soy HVP. Therefore, the wheat and corn model systems produced various pyrazines derived from amino acids, such as alanine, asparagine, valine, and leucine. In particular, sulfur-containing compounds, such as dimethyl sulfides and thiophenes, were detected only in the corn and wheat model systems. On the other hand, furfuryl alcohol, maltol, and cyclotene, which are generated via sugar degradation, were found at higher levels in the soy model systems. In addition, the formation of volatiles was also different depending on the type of reducing sugar. In the result of PCA, glucose and fructose model systems were significantly correlated with abundant content of aldehydes, and pyrazines, respectively. In conclusion, these results demonstrated that the type of HVPs which has different contents and composition of free amino acids and that of reducing sugars significantly contribute to generated flavor profiles in the thermal model system.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant (NRF-2020R1A2C2004724) and project BK21 FOUR (Fostering Outstanding Universities for Research) (No. 4299990914600) funded by the Korea government (MSIT).
Declarations
Conflict of interest
None of the authors of this study has any financial interest or conflict with industries or parties.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aaslyng MD, Martens M, Poll L, Nielsen PM, Flyge H, Larsen LM. Chemical and sensory characterization of hydrolyzed vegetable protein, a savory flavoring. Journal of Agricultural and Food Chemistry. 1998;46:481–489. doi: 10.1021/jf970556e. [DOI] [PubMed] [Google Scholar]
- Albouchi A, Murkovic M. Formation kinetics of furfuryl alcohol in a coffee model system. Food Chemistry. 2018;243:91–95. doi: 10.1016/j.foodchem.2017.09.112. [DOI] [PubMed] [Google Scholar]
- Alpert HR, Agaku IT, Connolly GN. A study of pyrazines in cigarettes and how additives might be used to enhance tobacco addiction. Tobacco Control. 2016;25(4):444–450. doi: 10.1136/tobaccocontrol-2014-051943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barišić V, Kopjar M, Jozinović A, Flanjak I, Ačkar Đ, Miličević B, Babić J. The chemistry behind chocolate production. Molecules. 2019;24(17):3163. doi: 10.3390/molecules24173163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny C. The role of sulfur chemistry in thermal generation of aroma. pp. 187–210. In: Analysis and Perception in Food and Beverages. Parker JK, Elmore JS, Metheven L (ed). Woodhead Publishing. Sawston, UK (2015)
- Chen C, Zhou W, Yu H, Yuan J, Tian H. Evaluation of the perceptual interactions among aldehydes in a cheddar cheese matrix according to odor threshold and aroma intensity. Molecules. 2020;25(18):4308. doi: 10.3390/molecules25184308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IH, Lee SR, Jun HR, Roh HJ, Kim YS. Comparison of volatile Maillard reaction products from tagatose and other reducing sugars with amino acids. Food Science and Biotechnology. 2010;19:431–438. doi: 10.1007/s10068-010-0061-7. [DOI] [Google Scholar]
- Cremer DR, Vollenbroeker M, Eichner K. Investigation of the formation of Strecker aldehydes from the reaction of Amadori rearrangement products with α-amino acids in low moisture model systems. European Food Research and Technology. 2000;211:400–403. doi: 10.1007/s002170000196. [DOI] [Google Scholar]
- Chiang JH, Yeo MTY, Ong DSM, Henry CJ. Comparison of the molecular properties and volatile compounds of Maillard reaction products derived from animal- and cereal-based protein hydrolysates. Food Chemistry. 2022;383:132609. doi: 10.1016/j.foodchem.2022.132609. [DOI] [PubMed] [Google Scholar]
- Fadel HHM, Abdel Mageed MA, Abdel S, Abdel Kader ME, Lotfy SN. Cocoa substitute: Evaluation of sensory qualities and flavour stability. European Food Research and Technology. 2006;223:125–131. doi: 10.1007/s00217-005-0162-3. [DOI] [Google Scholar]
- Göğüş F, Wedzicha BL, Lamb J. Modelling of Maillard reaction during the drying of a model matrix. Journal of Food Engineering. 1998;35:445–458. doi: 10.1016/S0260-8774(98)00028-4. [DOI] [Google Scholar]
- de Pinho PG, Ferreira ACS. Role of Strecker aldehydes on beer flavour stability. Developments in Food Science. 2006;43:529–532. doi: 10.1016/S0167-4501(06)80125-1. [DOI] [Google Scholar]
- Hoa VB, Hwang IH, Jeong DW, Touseef A. Principle of meat aroma flavors and future prospect. pp. 145–176 In Latest Research into Quality Control. Akyar I (ed). IntechOpen, London, UK (2012)
- Issenberg P, Lustre AO. Phenolic components of smoked meat products. Journal of Agricultural and Food Chemistry. 1970;18:1056–1060. doi: 10.1021/jf60172a009. [DOI] [Google Scholar]
- Jung SY, Lee YM, Kim SS, Kim KO. Effect of added hydrolyzed vegetable proteins on consumers’ response for doenjang (Korean traditional fermented soybean paste) soup. Food Science and Biotechnology. 2020;29:45–53. doi: 10.1007/s10068-019-00646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Rahman MS, Kim AN, Lee KY, Park CY, Kerr WL, Choi SG. Comparative study of the quality characteristics of defatted soy flour treated by supercritical carbon dioxide and organic solvent. Journal of Food Science and Technology. 2017;54(8):2485–2493. doi: 10.1007/s13197-017-2691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karangwa E, Murekatete N, Habimana JD, Masamba K, Duhoranimana E, Muhoza B, Zhang X. Contribution of crosslinking products in the flavour enhancer processing: The new concept of Maillard peptide in sensory characteristics of Maillard reaction systems. Journal of Food Science and Technology. 2016;53:2863–2875. doi: 10.1007/s13197-016-2268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Kim OC, Lee JI, Yang KK. Formation of volatile compounds from Maillard reaction of D-glucose with DL-alanine in propylene glycol solution. Korean Journal of Food Science and Technology. 1988;20:157–163. [Google Scholar]
- Lee KY, Kim HS, Lee HG, Han O, Chang UJ. Studies on the prediction of the shelf-life of Kochujang through the physicochemical and sensory analyses during storage. Journal of the Korean Society of Food Science and Nutrition. 26(4): 558-594 (1997)
- Lee SM, Seo BC, Kim YS. Volatile compounds in fermented and acid-hydrolyzed soy sauces. Journal of Food Science. 2006;71:146–156. doi: 10.1111/j.1365-2621.2006.tb15610.x. [DOI] [Google Scholar]
- Mottram DS. The Maillard reaction: Source of flavour in thermally processed foods. pp. 269–283. In: Flavors and fragrances: Chemistry, bioprocessing and sustainability. R. G. Berger (ed). Springer, Berlin (2007)
- Nie C, Zhong X, He L, Gao Y, Zhang X, Wang C, Du X. Comparison of different aroma-active compounds of Sichuan dark brick tea (camellia sinensis) and Sichuan Fuzhuan brick tea using gas chromatography-mass spectrometry (GC-MS) and aroma descriptive profile tests. European Food Research and Technology. 2019;245:1963–1979. doi: 10.1007/s00217-019-03304-1. [DOI] [Google Scholar]
- Nursten HE, Royal Society of Chemistry (Great Britain). The Maillard reaction: chemistry, biochemistry and implications. Cambridge, England. pp.18–83 (2005)
- Shahidi FD. Cooking of meat: Maillard reaction and browning. pp. 391–403. In: Encyclopedia of Meat Sciences. Dikeman M, Devine C (ed). Academic Press, Oxford, UK (2014)
- Song N, Tan C, Huang M, Liu P, Eric K, Zhang X, Jia C. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chemistry. 2013;136:144–151. doi: 10.1016/j.foodchem.2012.07.100. [DOI] [PubMed] [Google Scholar]
- Steinhaus P, Schiberle P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. Journal of Agricultural and Food Chemistry. 2007;55(15):6262–6269. doi: 10.1021/jf0709092. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Iwatoh S, Miyaura K, Asikin Y, Kusano M. Metabolomic profiling reveals the relationship between taste-related metabolites and roasted aroma in aged pork. LWT-Food Science Technology. 2022;155:112928. doi: 10.1016/j.lwt.2021.112928. [DOI] [Google Scholar]
- Tao N, Wu R, Zhou P, Gu S, Wu W. Characterization of odor-active compounds in cooked meat of farmed obscure puffer (takifugu obscurus) using gas chromatography–mass spectrometry–olfactometry. Journal of Food and Drug Analysis. 2014;22:431–438. doi: 10.1016/j.jfda.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Shen H, Yang X, Liu T, Yang Y, Zhou X, Guo Y. Effect of free amino acids and peptide hydrolysates from sunflower seed protein on the formation of pyrazines under different heating conditions. RSC Advances. 2021;11:27772–27781. doi: 10.1039/D1RA05140G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kays SJ. Contribution of volatile compounds to the characteristic aroma of baked ‘jewel’ sweet potatoes. Journal of American Society of Horticultural Science. 2000;125:638–643. doi: 10.21273/JASHS.125.5.638. [DOI] [Google Scholar]
- Yamaguchi K, Noumi Y, Nakajima K, Nagatsuka C, Aizawa H, Nakawaki R, Chuyen NV. Effects of salt concentration on the reaction rate of Glc with amino acids, peptides, and proteins. Bioscience, Biotechnology, and Biochemistry. 73: 2379-2383 (2009) [DOI] [PubMed]
- Yoon SH, Lee JK, Nam HS, Lee HJ. Formation of meatlike flavors by Maillard reaction using hydrolyzed vegetable protein (HVP) Korean Journal of Food Science and Technology. 1994;26(6):781–786. [Google Scholar]
- Zhang Y, Huang M, Tian H, Sun B, Wang J, Li Q. Preparation and aroma analysis of Chinese traditional fermented flour paste. Food Science and Biotechnology. 2014;23(1):49–58. doi: 10.1007/s10068-014-0007-6. [DOI] [Google Scholar]
- Zhou X, Cui H, Zhang Q, Hayat K, Yu J, Hussain S, Tahir MU, Zhang X, Ho CT. Taste improvement of Maillard reaction intermediates derived from enzymatic hydrolysates of pea protein. Food Research International. 140: 109985 (2021) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.