Abstract
Objective
To compare glycaemic control and insulin dosage in people with type 1 diabetes treated by continuous subcutaneous insulin infusion (insulin infusion pump therapy) or optimised insulin injections.
Design
Meta-analysis of 12 randomised controlled trials.
Participants
301 people with type 1 diabetes allocated to insulin infusion and 299 allocated to insulin injections for between 2.5 and 24 months.
Main outcome measures
Glycaemic control measured by mean blood glucose concentration and percentage of glycated haemoglobin. Total daily insulin dose.
Results
Mean blood glucose concentration was lower in people receiving continuous subcutaneous insulin infusion compared with those receiving insulin injections (standardised mean difference 0.56, 95% confidence interval 0.35 to 0.77), equivalent to a difference of 1.0 mmol/l. The percentage of glycated haemoglobin was also lower in people receiving insulin infusion (0.44, 0.20 to 0.69), equivalent to a difference of 0.51%. Blood glucose concentrations were less variable during insulin infusion. This improved control during insulin infusion was achieved with an average reduction of 14% in insulin dose (difference in total daily insulin dose 0.58, 0.34 to 0.83), equivalent to 7.58 units/day.
Conclusions
Glycaemic control is better during continuous subcutaneous insulin infusion compared with optimised injection therapy, and less insulin is needed to achieve this level of strict control. The difference in control between the two methods is small but should reduce the risk of microvascular complications.
What is already known on this topic
Continuous subcutaneous insulin infusion (insulin pump therapy) produces good long term control of blood glucose concentrations in people with type 1 diabetes
Control of blood glucose concentration is substantially better on pump therapy than conventional (non-optimised) injection therapy
It is unclear how glycaemic control on pump therapy compares with modern optimised insulin injection regimens
What this study adds
Though glycaemic control was better during continuous subcutaneous insulin infusion than optimised insulin injection therapy, the difference was relatively small
Continuous subcutaneous insulin infusion is an effective form of intensive insulin therapy that should lower the risk of microvascular complications
Insulin pump therapy is unnecessary for most people with type 1 diabetes and should be reserved for those with special problems with optimised insulin injections
Introduction
Continuous subcutaneous insulin infusion, often called insulin pump therapy, was introduced in the 1970s as a way of achieving and maintaining strict control of blood glucose concentrations in people with type 1 (insulin dependent) diabetes.1 Short acting insulin is infused subcutaneously from a portable pump at one or more basal rates, with boosts in the dose activated by the patient at mealtimes. Overall control, as measured by mean blood glucose concentrations and percentage of glycated haemoglobin, is considerably improved during treatment with insulin infusion pumps compared with the non-optimised insulin injection therapy that was prevalent in management of diabetes until relatively recently.2,3 However, with the emergence of new treatment strategies such as insulin “pens,” which encourage multiple injection regimens, and the publication of the diabetes control and complications trial4 the importance and utility of intensive insulin injection regimens in achieving near normoglycaemia and slowing the development of microvascular complications has become increasingly apparent.
Though there have been several randomised controlled trials of insulin pumps compared with optimised insulin injection regimens, many had relatively small numbers of participants.5–8 Some of these studies showed better control with pumps,5,6 and others showed broadly similar control.7,8
We reviewed the literature on pump therapy and carried out a meta-analysis of glycaemic control and insulin dosage in randomised controlled trials that compared continuous subcutaneous insulin infusion and optimised insulin injection therapy.
Methods
Identification and selection of trials
To identify published trials that met the inclusion criteria we searched Medline (1975 to 2000) and Embase (1980-2000) for literature on insulin infusion systems/insulin infusion and the Cochrane database of randomised controlled trials. We also searched a personal (JP) collection of peer reviewed articles and reviews about infusion systems and lists of papers on pump therapy supplied by two manufacturers of insulin infusion pumps (MiniMed and Disetronic). We reviewed cited literature in retrieved articles and information and references supplied by INPUT, a support group for pump patients.
We selected only those studies that were randomised controlled trials of pump therapy compared with optimised insulin injection therapy. We considered optimised injection therapy as part of the trial design if multiple insulin injections were used, there was adjustment of insulin dosages or timing, or both, according to hospital and home monitored blood glucose concentrations, or the authors described the regimen as “intensive” or “optimised.” We did not included trials of alternative infusion and injection systems, such as the “pen infuser” and jet injectors, which are not based on electromechanical pumps or regular subcutaneous needle injection. We also excluded short term studies (two weeks' duration on either therapy), those in people with newly diagnosed type 1 diabetes, those in pregnant women with diabetes, controlled trials that were not randomised, those that used non-optimised (“conventional”) insulin injection therapy, and those when it was unclear whether injection therapy was optimised. When several publications reported different aspects of the same study—for example, effect on glycaemic control in one paper and subsequently effects on various microvascular complications in another paper—we chose only one paper to represent the trial data on glycaemic control.
We extracted data from text, tables, and graphs. Data were examined independently by two reviewers (JP and MM). Differences over inclusion of studies and interpretation of data were resolved by consensus reached after discussion.
Outcome measures
We assessed glycaemic control with each method as mean (SD) blood glucose concentration (to include whole blood, plasma, and serum glucose concentration) and percentage of glycated haemoglobin (to include HbA1c, HbA1 and glycated haemoglobin measurements made by different methods). We also noted total daily insulin dose on the two regimens. We recorded the type of pump, the type of insulin, and the insulin injection regimen.
Statistical analysis
We used a random effects model (StataCorp, College Station, TX, USA) for the meta-analysis. We calculated the weighted mean difference of the standardised blood glucose concentration, percentage of glycated haemoglobin, and insulin dosage on pump and injection therapy (that is, the number of SDs of the value) to compensate for different scales (for example, because of different methods of measuring glycated haemoglobin). We calculated the estimated treatment effects in absolute units by multiplying the combined treatment effects by the average pooled SDs in all studies.
We assessed potential publication bias by a funnel plot and Egger's test.9 Sensitivity to the estimate of publication bias was assessed by the trim and fill method.10 We assessed heterogeneity between trials by the χ2 test. Sources of heterogeneity were assessed with a random effects regression analysis with age, duration of diabetes and treatment, and year of study as independent variables. We tested the robustness of the analyses in sensitivity analyses by comparing the summary results of random effects meta-analysis with meta-analysis using a fixed effect model and analysis with data in absolute rather than standardised units.
We tested the hypothesis that variability in blood glucose concentration was less during continuous insulin infusion than during injection therapy by calculating the ratio of the minimum variance weighted geometric means of the SDs of blood glucose concentrations on the two regimens.
Results
We identified 13 randomised controlled trials that compared glycaemic control on continuous subcutaneous insulin infusion compared with optimised insulin injections.5–8,11–19 In one report the error terms were ambiguous. As we could not reach consensus about reliable extraction of data we omitted this trial from the analysis.15 The table shows the characteristics of the analysed trials. Eleven trials were of crossover design.5,6,8,11–14,16–19 Nine different infusion pumps were used. In total 301 participants were randomised to infusion pumps and 299 to optimised injections for between 2.5 and 24 months. This represented 2522 patient months of pump treatment.
Blood glucose control
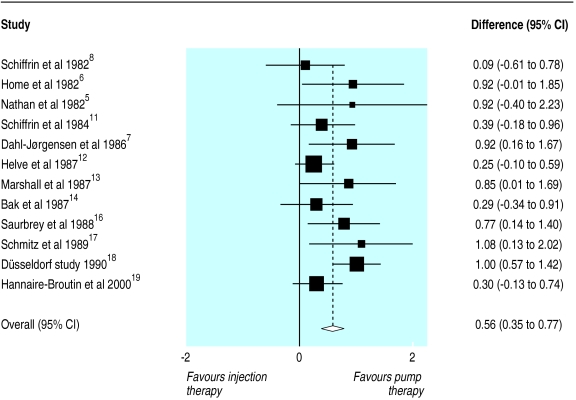
Figure 1 shows that glycaemic control was better during pump treatment. The standardised mean difference in blood glucose concentrations between insulin pump and optimised insulin injection therapy was 0.56 (95% confidence interval 0.35 to 0.77). The estimate from the fixed effects model was similar (0.53, 0.36 to 0.70). The treatment effect in terms of absolute units was 1.06 mmol/l (0.88, 0.52 to 1.24 mmol/l with unstandardised data).
Figure 1.
Standardised mean differences (95% confidence interval) in blood glucose concentration achieved during insulin pump compared with optimised insulin injection therapy
The results of the χ2 tests showed no significant heterogeneity among trials (P=0.17). There was no clear publication bias in a funnel plot, and the result of Egger's test was not significant (P=0.168). The trim and fill method gave an estimated corrected effect size of 0.39 (0.15 to 0.63). Only duration of treatment was related to effect size in a regression analysis (regression coefficient=0.32 (0.06 to 0.58)). This model estimated the effect size as 0.46 (0.14 to 0.77) at six months of treatment and 0.93 (0.30 to 1.57) at two years.
Glycated haemoglobin
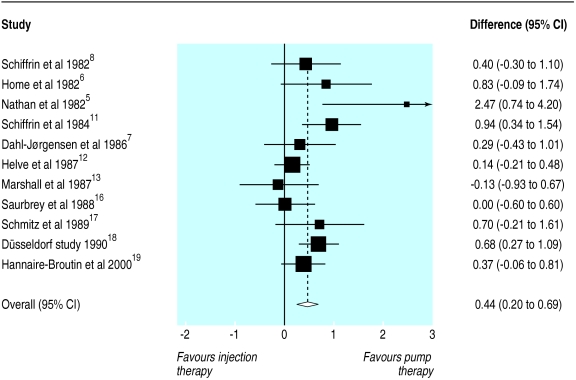
Figure 2 shows that the percentage of glycated haemoglobin was lower during pump therapy, the standardised mean difference being 0.44 (0.20 to 0.63). This is equivalent to an effect size of 0.51% in original units, consistent with that seen in a meta-analysis with unstandardised data (0.45%, 0.20% to 0.71%). The fixed effect model gave a similar standardised mean difference to the random model (0.41, 0.23 to 0.58). There was some evidence of heterogeneity (χ2 P=0.07), and a funnel plot and Egger's test (P=0.02) revealed some possible publication bias. The trim and fill method gave an estimated effect size corrected for bias of 0.31 (0.15 to 0.48). None of the measured variables was significantly related to effect size in regression analysis.
Figure 2.
Standardised mean differences (95% confidence interval) in percentage of glycated haemoglobin during insulin pump compared with optimised insulin injection therapy
Insulin dose
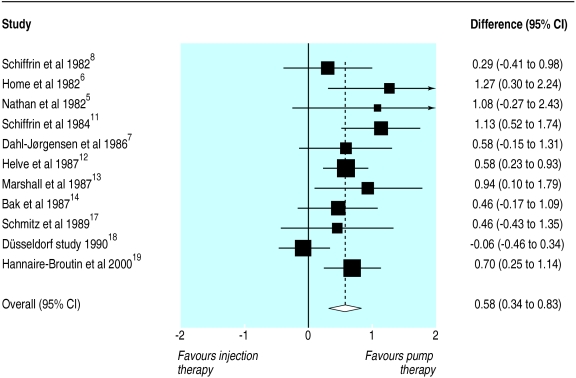
Figure 3 shows that the improved control during insulin pump therapy was achieved at a reduced total daily insulin dosage. The standardised mean difference in insulin dose was 0.58 (0.34 to 0.83). This represents a mean dosage reduction of 14% during pump therapy. The effect size was 7.58 units/day in original units, which was similar to that seen in a meta-analysis with unstandardised data (7.33, 4.07 to 10.59 units/day). The estimate from the fixed effect model was similar to that of the random effects model (0.53, 0.36 to 0.71).
Figure 3.
Standardised mean differences (95% confidence interval) in total daily insulin dose during insulin pump compared with optimised insulin injection therapy
Analysis of insulin dose showed some evidence of heterogeneity (P=0.07). The funnel plot showed some bias, though the result of Egger's test was not significant (P=0.17). The effect size corrected for bias was 0.42 (0.25 to 0.58). In regression analysis the duration of treatment was negatively related to effect size (regression coefficient=−0.41(−0.66 to −0.15)). The model estimated the effect size to be 0.66 (0.33 to 0.10) at six months and 0.05 (−0.59 to 0.70) at two years of treatment.
Variability in blood glucose concentration
Using SD of blood glucose concentration as a measure of glycaemic variability, we found the variability was significantly higher with insulin injections than with pump therapy (weighted geometric mean of the SD ratios 1.27, 1.11 to 1.47).
Discussion
Meta-analysis of 12 randomised controlled trials shows that use of insulin pumps results in better glycaemic control than optimised insulin injection therapy but that the difference is relatively small—about 1 mmol/l for blood glucose concentration and 0.5% for percentage of glycated haemoglobin. The main inclusion criterion in the studies was that patients should agree to and be capable of using the pump and its associated procedures. As this is the prerequisite of pump therapy in clinical practice20 the results of our meta-analysis are applicable to the general population of people with type 1 diabetes, though few of the participants in these studies had severe complications such as clinical nephropathy (with persistent proteinuria shown by positive result on dipstick testing).
Potential influences on glycaemic control
Our results of meta-analysis were not modified by the publication date of the trials, though there are several potential reasons why insulin pump therapy in the early 1980s might have been less effective than modern practice. Early pumps had few or no alarm features for events such as low battery or occlusion of delivery and no facility for automatic change in basal rate of infusion. The type of insulin used in the pump might also be important as unbuffered short acting insulin, used particularly in North America in the first years after the introduction of insulin pumps, was more likely than buffered insulins to occlude the delivery cannula and disrupt control.21,22 The most recent trial in this survey used the monomeric insulin analogue, lispro, in the pump.19 This is now considered to be the pump insulin of choice,23–26 but the results of the one lispro pump study in this meta-analysis were broadly consistent with the overall result of our analysis (standardised mean differences with lispro were 0.30 (−0.13 to 0.74) for blood glucose concentration and 0.37 (−0.06 to 0.81) for glycated haemoglobin, favouring pump treatment19).
Glycaemic control during optimised injection therapy may be affected by the regimen used and the intensity of its application. There were many different injection regimens used in the trials reported here, and we cannot make judgments about their appropriateness. Though this introduces some uncertainty into the conclusions, the results were surprisingly consistent across trials. The only identified source of heterogeneity was a tendency for trials with a longer duration to be associated with a larger difference in control of glycaemia between pump and injection therapy and a smaller difference in insulin dosage. This finding is consistent with the known effect of pump therapy in improving insulin sensitivity and reducing insulin resistance in people with type 1 diabetes.27,28
We excluded from our analysis the few trials in patients with newly diagnosed type 1 diabetes29 because the likely remaining endogenous β cell function would favour good control in any type of insulin therapy30 and obscure differences between pump and injection treatment. We also did not analyse trials in pregnant women because the number of studies is small31–33 and because we considered them to be a special group of patients, with changing control throughout pregnancy and a high level of motivation generally unrepresentative of most people with type 1 diabetes.
Clinical significance of improved control
What is the clinical significance of the small difference between the strict glycaemic control of pump and optimised injection therapy? Analysis of the results of the diabetes control and complications trial34 has shown that the risk of development and progression of microvascular complications extends over the entire range of glycated haemoglobin values and there is no threshold (short of normoglycaemia) below which there is no risk. The standardised mean difference for glycated haemoglobin of 0.44 in this meta-analysis corresponds to a reduction in HbA1c of about 0.5% in the diabetes control and complications trial (where the SD for HbA1c in the intensively managed group was 1.1-1.3%). This degree of improvement in control was associated with a reduction in risk of retinopathy of about 25%. However, the relation between the absolute risk (hazard rate per 100 patient years of treatment) and HbA1c was curvilinear, with a smaller rate at a lower than at a higher HbA1c. In people with intensively controlled glycaemia the absolute risk reduction for sustained progression in retinopathy (three steps on the early treatment of diabetic retinopathy scale) associated with a difference in HbA1c of 0.5% was about 0.5 cases per 100 patient years. Thus, maintaining this difference in control between insulin pump and injection therapy for 10 years would reduce the number of patients developing retinopathy of this degree by about 5%. The cost effectiveness of insulin pump versus insulin injections for this degree of benefit will need to be assessed.
Hypoglycaemia and variability of glycaemic control
A weakness of our study is that because of poor reporting and short duration of studies we could not assess the relative frequencies of potential side effects, particularly severe hypoglycaemia, ketoacidosis, and weight gain. For hypoglycaemia, for example, many studies were too short in duration to have more than one episode of severe hypoglycaemic reported on either treatment.5,6,8,11,16,17 However, as well as the lower mean blood glucose concentration, we found that oscillations in blood glucose concentration, as measured by SD, were also significantly less during pump treatment. This may contribute to the lower frequency of hypoglycaemia reported in other studies35–38 and is probably related to the lower variability in subcutaneous insulin absorption during pump infusion compared with injection treatment.39
Conclusions and recommendations
We conclude that continuous subcutaneous insulin infusion is an effective form of intensive insulin therapy for people with type 1 diabetes as glycaemic control is slightly but significantly better than during optimised insulin injections. However we consider that in general insulin pump should be reserved for those with special problems such as unpredictable hypoglycaemia or a marked increase in blood glucose concentration at dawn, despite best attempts to improve control with optimised injection regimens.20,40
Table.
Characteristics of trials included in meta-analysis of continuous subcutaneous insulin infusion versus intensive insulin injections
| Author | No of participants | Mean (SD or range) age (years) | Mean (SD or range) duration of diabetes (years) | Duration of treatment (months) | Type of pump | Type of insulin | Injection regimen |
|---|---|---|---|---|---|---|---|
| Schiffrin, 19828 | 16 | 24.9 (8.8) | 10.4 (5.1) | 6 | Mill Hill | Connaught/Lilly regular | Regular thrice daily; isophane insulin at bedtime |
| Home, 19826 | 10 | 40.4 (7.3) | 23.5 (8.3) | 2.5 | Mill Hill, Auto-Syringe | Pork Actrapid | Regular thrice daily; ultralente pm |
| Nathan, 19825 | 5 | 31 (5.7) | 7.4 (1.8) | 2-3 | Auto-Syringe | NA | Regular thrice daily; regular isophane insulin twice daily; ultralente before breakfast |
| Schiffrin, 198411 | 24 | 13-20 | 9 | 4 | Mill Hill | Connaught/Lilly regular | Regular thrice daily; isophane insulin pm/bedtime |
| Dahl-Jørgensen, 19867 | 15 | 26 (19-42)† | 12.8† | 24 | Nordisk | Pork Velosulin | Regular twice daily; isophane insulin am/bedtime |
| 15 | 26 (18-32)‡ | 12.8‡ | Auto-Syringe | ||||
| Helve, 198712 | 65 | 31.1 (1) | 12 (1) | 6 | Nordisk Auto-Syringe | Velosulin Actrapid | Multiple |
| Marshall, 198713 | 12 | 36 (21-50) | 18 (10-29) | 6 | Nordisk | Velosulin | Regular twice daily; isophane insulin twice daily/bedtime |
| Bak, 198714 | 20 | 24 (2) | 5.8 (3.8) | 6 | Graseby | Actrapid | Regular thrice daily; isophane insulin at bedtime |
| Saurbrey, 198816 | 21 | 32 (2.1) | 14.5 (1.4) | 2.5 | Auto-Syringe, Medix | NA | Regular thrice or four times daily; lente bedtime |
| Schmitz, 198917 | 10 | 36.5 (7.9) | 23.7 (2.9) | 6 | Nordisk | Velosulin | Regular thrice daily; isophane insulin at bedtime |
| Düsseldorf, 199018 | 47† | 32 (18-54) | 18 (3-44) | 24 | Nordisk, Promedos | NA | Regular twice, thrice, or four times daily |
| 49‡ | Betatron, Auto-Syringe | NA | Twice daily isophane insulin (or before breakfast injection/bedtime) | ||||
| Hannaire-Broutin, 200019 | 41 | 43.5 (10.3) | 20.0 (11.3) | 4 | MiniMed, Disetronic | Lispro | Thrice daily monomeric; twice daily isophane insulin (or before breakfast injection/bedtime) |
NA=data not available.
Regular=regular soluble or short acting insulin.
Participants on injections.
Participants on pump therapy.
Footnotes
Funding: None.
Competing interests: King's College London has received financial support for some studies on continuous subcutaneous insulin infusion from MiniMed, a manufacturer of insulin pumps.
References
- 1.Pickup JC, Keen H, Parsons JA, Alberti KGMM. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. BMJ. 1978;i:204–207. doi: 10.1136/bmj.1.6107.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauritzen T, Frost-Larsen K, Larsen HW, Deckert T. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet. 1983;i:200–204. doi: 10.1016/s0140-6736(83)92585-0. [DOI] [PubMed] [Google Scholar]
- 3.Kroc Collaborative Study Group. Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. N Engl J Med. 1984;311:365–372. doi: 10.1056/NEJM198408093110604. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Lou P, Avruch J. Intensive conventional and insulin pump therapy in adult type 1 diabetes. A crossover study. Ann Intern Med. 1982;97:31–36. doi: 10.7326/0003-4819-97-1-31. [DOI] [PubMed] [Google Scholar]
- 6.Home PD, Capaldo B, Burrin JM, Worth R, Alberti KGMM. A crossover comparison of continuous subcutaneous insulin infusion (CSII) against multiple insulin injections in insulin-dependent diabetic subjects: improved control with CSII. Diabetes Care. 1982;5:466–471. doi: 10.2337/diacare.5.5.466. [DOI] [PubMed] [Google Scholar]
- 7.Dahl-Jørgensen K, Brinchman-Hansen O, Hanssen KF, Ganes T, Kierulf P, Smeland E, et al. Effect of near-normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. BMJ. 1986;293:1195–1199. doi: 10.1136/bmj.293.6556.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiffrin A, Belmonte MM. Comparison between continuous subcutaneous insulin infusion and multiple injections of insulin. A one-year prospective study. Diabetes. 1982;31:255–264. doi: 10.2337/diab.31.3.255. [DOI] [PubMed] [Google Scholar]
- 9.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton AJ, Abrams KR, Jones DR, Sheldon T, Song F. Methods for meta-analysis in medical research. Chichester: Wiley; 2000. [Google Scholar]
- 11.Schiffrin AD, Desrosiers M, Aleyassine H, Belmonte MM. Intensified insulin therapy in the type 1 diabetic adolescent: a controlled trial. Diabetes Care. 1984;7:107–113. doi: 10.2337/diacare.7.2.107. [DOI] [PubMed] [Google Scholar]
- 12.Helve E, Koivisto VA, Lehtonen A, Pelkonen R, Huttunen JK, Nikkilä EA. A crossover comparison of continuous insulin infusion and conventional injection treatment of type 1 diabetes. Acta Med Scand. 1987;221:385–393. doi: 10.1111/j.0954-6820.1987.tb03360.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall SM, Home PD, Taylor R, Alberti KGMM. Continuous subcutaneous insulin infusion versus injection therapy: a randomized cross-over trial under usual diabetic clinic conditions. Diabetic Med. 1987;4:521–525. doi: 10.1111/j.1464-5491.1987.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 14.Bak JF, Nielsen OH, Pedersen O, Beck-Nielsen H. Multiple insulin injections using a pen injector versus insulin pump treatment in young diabetic patients. Diabetes Res. 1987;6:155–158. [PubMed] [Google Scholar]
- 15.Nosadini R, Velussi M, Fioretto P, Doria A, Avogaro A, Trevisan R, et al. Frequency of hypoglycaemic and hyperglycaemic-ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM. Diabetes Nutr Metab. 1988;1:289–296. [Google Scholar]
- 16.Saurbrey N, Arnold-Larsen S, Møller-Jensen B, Kühl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabetic Med. 1988;5:150–153. doi: 10.1111/j.1464-5491.1988.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz A, Sandahl-Christiansen J, Kjeldahl-Christiansen C, Hermansen K, Mogensen CE. Effect of pump versus pen treatment on glycaemic control and kidney function in long-term uncomplicated insulin-dependent diabetes mellitus (IDDM) Danish Med Bull. 1989;36:176–178. [PubMed] [Google Scholar]
- 18.Düsseldorf Study Group. Comparison of continuous subcutaneous insulin infusion and intensified conventional therapy in the treatment of type 1 diabetes: a two-year randomised study. Diabetes Nutr Metab. 1990;3:203–213. [Google Scholar]
- 19.Hannaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber J. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. Diabetes Care. 2000;23:1232–1235. doi: 10.2337/diacare.23.9.1232. [DOI] [PubMed] [Google Scholar]
- 20.Pickup JC, Keen H. Continuous subcutaneous insulin infusion in type 1 diabetes. BMJ. 2001;322:1262–1263. doi: 10.1136/bmj.322.7297.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mecklenburg RS, Guinn TS. Complications of insulin pump therapy: the effect of insulin preparation. Diabetes Care. 1985;8:367–370. doi: 10.2337/diacare.8.4.367. [DOI] [PubMed] [Google Scholar]
- 22.Eichner HL, Selam J-L, Woertz LL, Cornblath M, Charles MA. Improved metabolic control of diabetes with reduction of occlusions during continuous subcutaneous insulin infusion. Diabetes Nutr Metab. 1988;1:283–287. [Google Scholar]
- 23.Melki V, Renard E, Lassman-Vague V, Boivin S, Guerci B, Hanaire-Broutin H, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analogue in external pumps. Diabetes Care. 1998;21:977–981. doi: 10.2337/diacare.21.6.977. [DOI] [PubMed] [Google Scholar]
- 24.Renner R, Pfützner A, Trautman M, Harzer O, Sauter K, Landgraf R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Diabetes Care. 1999;22:784–788. doi: 10.2337/diacare.22.5.784. [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, Tildesley H, Chiasson J-L, Tsui E, Strack T. Insulin lispro in CSII: results of a double-blind crossover study. Diabetes. 1997;46:440–443. doi: 10.2337/diab.46.3.440. [DOI] [PubMed] [Google Scholar]
- 26.Schmauss S, König A, Landgraf R. Human insulin analogue [LYS(B28),PRO(B29)]: the ideal pump insulin? Diabetic Med. 1998;15:247–249. doi: 10.1002/(SICI)1096-9136(199803)15:3<247::AID-DIA547>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Beck-Nielsen H, Richelsen B, Hasling C, Nielsen OH, Hedding L, Sørensen NS. Improved in vivo insulin effect during continuous subcutaneous insulin infusion in patients with IDDM. Diabetes. 1984;33:832–837. doi: 10.2337/diab.33.9.832. [DOI] [PubMed] [Google Scholar]
- 28.Simonson DC, Tamborlane WV, Sherwin RS, Smith JD, DeFronzo RA.for the Kroc Collaborative Study Group. Improved insulin sensitivity in patients with type 1 diabetes mellitus after CSII Diabetes 198534(suppl 3)80–86. [DOI] [PubMed] [Google Scholar]
- 29.De Beaufort CE, Houtzagers CMGJ, Bruining GJ, Aarsen RSR, den Boer NC, Grose WFA, et al. Continuous subcutaneous insulin infusion (CSII) versus conventional injection therapy in newly diagnosed diabetic children: two-year follow-up of a randomized, prospective trial. Diabetic Med. 1989;6:766–771. doi: 10.1111/j.1464-5491.1989.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 30.Gonen B, Goldman J, Baldwin D, Goldberg RB, Ryan WG, Blix PM, et al. Metabolic control in diabetic patients. Effects of insulin-secretory reserve (measured by plasma C-peptide levels) and circulating insulin antibodies. Diabetes. 1979;28:749–753. doi: 10.2337/diab.28.8.749. [DOI] [PubMed] [Google Scholar]
- 31.Coustan DR, Reece EA, Sherwin RS, Rudolf MCJ, Bates JE, Sockin SM, et al. A randomized clinical trial of the insulin pump vs intensive conventional therapy in diabetic pregnancies. JAMA. 1986;255:631–636. [PubMed] [Google Scholar]
- 32.Carta Q, Meriggi E, Trossarelli GF, Catella G, Dal Molin V, Menato G, et al. Continuous subcutaneous insulin infusion versus intensive conventional insulin therapy in type I and type II diabetic pregnancy. Diabete Metabolisme. 1986;12:121–129. [PubMed] [Google Scholar]
- 33.Nosari I, Maglio ML, Lepore G, Cortinovis F, Pagani G. Is continuous subcutaneous insulin infusion more effective than intensive conventional insulin therapy in the treatment of pregnant diabetic women? Diabetes Nutr Metab. 1993;6:33–37. [Google Scholar]
- 34.Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the diabetes control and complications trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 35.Ng Tang Fui S, Pickup JC, Bending JJ, Collins ACG, Keen H, Dalton N. Hypoglycemia and counterregulation in insulin-dependent diabetic patients: a comparison of continuous subcutaneous insulin infusion and conventional insulin therapy. Diabetes Care. 1986;9:221–227. doi: 10.2337/diacare.9.3.221. [DOI] [PubMed] [Google Scholar]
- 36.Bending JJ, Pickup JC, Keen H. Frequency of diabetic ketoacidosis and hypoglycemic coma during treatment with continuous subcutaneous insulin infusion. Am J Med. 1985;79:685–691. doi: 10.1016/0002-9343(85)90518-2. [DOI] [PubMed] [Google Scholar]
- 37.Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type 1 diabetes. Diabetes Care. 1996;19:324–327. doi: 10.2337/diacare.19.4.324. [DOI] [PubMed] [Google Scholar]
- 38.Boland EA, Grey M, Oesterle A, Fredrickson L, Tamborlane WV. Continuous subcutaneous insulin infusion. A new way to lower risk of severe hypoglycemia, improve metabolic control and enhance coping in adolescents with type 1 diabetes. Diabetes Care. 1999;22:1799–1784. doi: 10.2337/diacare.22.11.1779. [DOI] [PubMed] [Google Scholar]
- 39.Lauritzen T, Pramming S, Deckert T, Binder C. Pharmacokinetics of continuous subcutaneous insulin infusion. Diabetologia. 1983;24:326–329. doi: 10.1007/BF00251817. [DOI] [PubMed] [Google Scholar]
- 40.Pickup JC. Is insulin pump treatment justifiable? In: Gill GV, Pickup JC, Williams G, editors. Difficult diabetes. Oxford: Blackwell Science; 2001. pp. 205–223. [Google Scholar]