Abstract
This study aims to prepare fish gelatin nanofibers extracted from fish waste by using electrospinning method and its encapsulation with fucoxanthin extracted from macroalgae Sargassum angustifolium. Four concentrations of gelatin and two concentrations of fucoxanthin were used under different voltage for preparing the nanofibers. The optimal conditions for producing the nanofibers were considered as 30%, 10 cm, 12 kV, and 5% for fish gelatin concentration, distance, voltage, and fucoxanthin, respectively. The average thickness of nanofibers was estimated 198 ± 0.073 nm. The FTIR results confirmed the presence of functional groups between fucoxanthin and gelatin. The loading efficiency of fucoxanthin in nanofibers and the free radical scavenging of DPPH were calculated 91% and 62%, respectively. Further, these nanofibers showed the antibacterial properties against bacteria. Based on the results, the fish gelatin nanofibers containing fucoxanthin can be proposed as a suitable coating for using in the food packaging industry.
Keywords: Electrospinning, Fish gelatin, Fucoxanthin, Seaweed, Food packaging
Introduction
Carotenoids have been used as the natural food coloring and antioxidants in a wide range of foods such as dairy products, beverages, breakfast cereals, candies, soups, and salad dressings (Horuz and Belibağlı, 2018). Fucoxanthin, which has the molecular structure of C42H58O6 is a carotenoid pigment with the xanthophyllic structure, which is obtained from the marine algae, especially brown algae (Wang et al., 2017). However, fucoxanthins have been extracted from algae species such as Cystoseira indica, Sragassum angostifolium, Sargassum horneri, and laminaria japonica (Mok et al., 2016). This pigment shows much stronger antioxidant properties compared to the other carotenoids related to its allenic groups. Moreover, it plays an important role in protecting against inflammation and oxidation, increasing the immunity and metabolism, improving the liver, heart, and eye function, and preventing the cancer, obesity, and diabetes (Šudomová et al., 2019). Although fucoxanthin has many health benefits, it faces challenges due to its low water solubility, and chemical and light instability (Huang et al., 2017). Encapsulation is an appropriate method for maintaining the compounds, which were sensitive to the environmental conditions such as pH, light, temperature, oxygen and relative humidity. This method leads to long-term stability of biological or medicinal compounds, the function of which shows no change. Recently, various methods were used for encapsulating fucoxanthin due to improving the stability, increasing the solubility of fucoxanthin, and the long-term stability, and bioavailability. Compounds such as chitosan and chitosan-casein using the spray drying method (Koo et al., 2016), as well as palm stearin (Wang et al., 2017), polyethylene glycol, and sunflower oil as a biodegradable coating (Vo et al., 2018) were used for its encapsulation. Based on the results, fucoxanthin was microencapsulated successfully and adding fucoxanthin along with a crosslinking agent such as improving the biostability by chitosan and reducing the dispersion of water in food (Koo et al., 2016). Moreover, fucoxanthin existed into microcapsules was more resistant to environmental conditions such as light, humidity, and temperature compare to the free fucoxanthin, and the efficiency of fucoxanthin encapsulation with palm stearin was reported to be 92–97% (Wang et al., 2017).
Electrospinning is one of the most widely used multifunctional encapsulation methods (Horuz and Belibağlı, 2018), which produces nanoscale fibers by stretching the synthetic or natural polymers under the electric field (Mahmood et al., 2019; Kwak et al., 2017). The electrospinning process requires a strong electric field applied to the polymer solution. The generated electric force reduces the surface tension of the polymer and converts the polymer droplets to nanofibers at the tip of the needle (spinneret) (Okutan et al., 2014). Nanofibers produced by using electrospinning method have the high surface-to-volume ratio, high porosity, high encapsulation ability without using temperature and increasing stability of the encapsulated compounds (Horuz and Belibağlı, 2018).
Nowadays, various polymers have been considered for producing the nanostructures by using electrospinning method (Huang et al., 2004; Rongthong et al., 2021), among which, gelatin extracted from fish waste is one of the most popular biopolymers which can be widely used (Gomes et al., 2013). Evaluating the electrospinning results of bovine, porcine, and fish gelatin indicated that the fish gelatin can be a good alternative to mammalian gelatin (Kwak et al., 2017). Chiu et al. (2019) evaluated the electrospinning process of different concentrations of the fish gelatin with Curcuma comosa turmeric. Based on the results, the nanofibers produced at a concentration of 30% gelatin with 5% turmeric under 15 kV voltage, current rate of 3 ml/h and a distance of 15 cm had the highest antioxidant and antimicrobial properties (Chiu et al., 2019).
This study aims to prepare the fish gelatin nanofibers obtained from Huso huso fish waste by using electrospinning method, the structural, mechanical, antioxidant, and biological properties of which were evaluated for using in the active food packaging industry by enriching with fucoxanthin extracted from Sargassum angustifolium.
Materials and methods
Materials
Ascorbic acid, butylated hydroxytoluene (BHT), 1,1-diphenyl-2-picrylhydrazine (DPPH), acetic acid (0.1 M), ethanol (96%), NaOH, acetone, n-hexane, methanole, and HCl. All Chemicals, reagents, and standards with premium quality were obtained from Sigma–Aldrich (St. Louis, MO, USA), and Nanochemia Company (Tehran. Iran).
Samples
The brown macroalgae Sargassum angustifolium was obtained from the coast of Persian Gulf was obtained from Alage Resource Development Technology Company (Shiraz, Iran). Macroalgae were first cleaned and then washed several times using fresh water. They were dried in an oven (Parsian Teb, Iran) at 37–38 °C and powdered. Finally, they were stored in plastic bags at—20 °C until needed for experiments (Ebrahimi et al. 2021). Fish gelatin (type A) extracted from skin of Beluga (Huso huso) (190 g bloom) (Mousavi et al., 2021).
Extraction and purification of fucoxanthin
Extraction of fucoxanthin was carried out by Oliyaei et al. (2020). The 10 g of the dried and powdered macroalgae (S. angustifolium) was mixed with %90 ethanol (1:20; W: V) and stored in shaker incubator at 37 °C for 24 h and then filtered via Whatman filter paper. The solution was concentrated at 39 °C in a rotary evaporator (Fara Azma, Iran) and then loaded to a column chromatography containing activated silica gel (100-200 mesh). Column chromatography is a method of separating and purification the pigments based on their density. Elution was performed with hexane:acetone (3:7; V:V) as mobile phase and the fucoxanthin was eluted by acetone. The extract was concentrated again in the rotary evaporator. The purity of fucoxanthin was about 54% on HPLC.
Electrospinning of fish gelatin
To prepare the electrospinning solution, different concentrations of fish gelatin (20, 25, 30 and 35%; W: V) were dissolved in water and acetic acid (50: 50; V: V) at 37 °C on a stirrer heater at 125 rpm for 24 h. Then, in order to obtain a desirable electrospinning solution, the electrospinning process was performed at flow rate of 0.2, 0.3, 0.5, and 1 ml/h, high voltages of 12, 15, 18 and 20 kV (Fnm High Voltage Power Supply-OC Series, Iran) was applied, positive electrode of high voltage was connected to the capillary needle and negative electrode was connected to rotating collector which was wrapped with aluminum foil. The distance of needle tip away from the collector was 15 and 10 cm. The solutions were injected into the electrospinning device (Fanavaran nano scale, Iran) with a 5 ml syringe equipped with stainless steel needle (22 gauges). The gelatin nanofibers were collected on the rotary spinning collector at 300 rpm (Kwak et al., 2017; Chiu et al., 2019; Mahmood et al., 2019). Gelatin solution with a concentration of 30%, a distance of 10 cm, voltages of 12 kV and a flow rate of 0.3 ml/h was selected as the optimal solution.
Development of fish gelatin nanofiber by fucoxanthin
The extracted fucoxanthin from S. angustifolium in different concentrations of (1, 5, 10, 15%; W: V) was added to the fish gelatin (30%) to provide a suitable substrate for the production of gelatin nanofibers enriched by fucoxanthin (Chiu et al., 2019). To prepare the nanofiber, the above solution was loaded into an electrospinning device with the optimal conditions obtained in the previous step (at the flow rate of 0.3 ml/h, voltages of 12 kV and a 10 cm distance of needle tip from the collector) at room temperature. After 4 h, the nanofibers were collected on the rotary spinning collector and used for further analysis (Kwak et al., 2017).
Characterization of produced nanofiber
Scanning electron microscopy (SEM)
The microstructure characterization of nanofibers was obtained from a scanning electron microscope (TESCAN vega3, Czech Republic) with accelerating voltage at 20 kV. A drop of nanofibers suspension in PBS was spread on an aluminum stub and left drying at room temperature. Dry suspension was covered with gold using a sputter coater (Desk Sputter Coater, DSR1, Nanostructural Coating Co., Iran). The microstructure photos of the surface of the samples were taken. In order to calculate the mean value of nanofibers diameters, 60 different nanofibers from SEM images were selected randomly, and their diameters were measured by using Digimizer software (5.3.5) (Ardekani et al., 2019).
Fourier transform infrared (FT-IR) spectroscopy
The interaction of functional groups in different nanofiber were evaluated by FT-IR spectrometer (TENSOR II, Bruker, Ettlingen, Germany) and FTIR spectra measured in absorbance from 4000 to 400 cm−1 wavenumber at room temperature (25 ± 1 °C). The number of scans and spectral resolution were measured 32 and 4 cm−1, respectively.
Encapsulation efficiency
Encapsulation efficiency (EE) of samples loaded with fucoxanthin was determined according to the indirect supernatant method.
10–20 mg of fish gelatin nanofiber loaded with fucoxanthin, fish gelatin nanofiber and fucoxanthin were dissolved into ethanol 70% and vortexed for 2 min and then shacked for 1 h at room temperature. The samples were centrifuged (SW14R, Feroilabo, France) at 6682 rpm for 2 min at 4 °C. The supernatant was removed, and their absorbance was measured at the wavelength of fucoxanthin (450 nm). The encapsulation efficiency is calculated using the following formula as described by (Oliyaei et al., 2020; Mousavipour et al., 2021):
| 1 |
Qe defines the amount of encapsulated material; Qt shows the amount of substance present in 100 ml of nanofibers.
Zeta potential
Zeta potential measurement was performed by (Dynamic Light Scattering (DLS) SZ-100 Horiba, Japan) to determined zeta potential and colloidal dispersion stability of samples. 10 mg of each nanofiber dispersed into 10 ml deionized water. All the measurements were performed in a cell placed in a temperature controlled holder (25 °C) (Akman et al., 2019).
DPPH free radical scavenging activity
In order to determine the antioxidant potential of fish gelatin nanofibers loaded with fucoxanthin, the DPPH free radical scavenging activities were measured according to Chiu et al. (2019) method. First, Different concentration (3.12, 6.25, 12.5, and 25 mg/l) of fish gelatin nanofibers loaded with fucoxanthin were prepared in 70% methanol. Then 100 μl of nanofiber solution and DPPH solution (1:1; V:V) were added to 96-well plates. The resulting solution was kept under darkness for 30 min at room temperature. Finally, the absorbance was read at 517 nm wavelength using a spectrophotometer (T7, PG Instruments Limited, Spain). The inhibitory percentage was obtained according to the following equation (Vardizadeh et al., 2021). All parameters were tested in triplicates.
Antibacterial activity (Disc diffusion method)
The Gram-negative bacteria, Escherichia coli (ATCC 25,922) and Gram-positive bacteria Staphylococcus aureus (ATCC 25,923) were supplied from the Pathobiology Department of the School of Veterinary Sciences and incubated in the nutrient broth medium for 24 h at 35 °C. A concentration of 0.5 McFarland bacteria was prepared and placed on a nutrient agar plate medium.
Paper discs (6 mm in diameter) were soaked with in fucoxanthin in concentration of 2.45 mg/ml and 1 mg/ml nanofibers load fucoxanthin with 2% DMSO soaked in NB broth, after that, they were placed on nutrient agar (NA) plates, which have been inoculated with test bacteria at 37 °C for 24 h. The gram positive and negative were used as positive controls and DMSO were used as negative control (Chiu et al., 2019). The diameter of the inhibition zone was measured using a digital caliper (Ebrahimi et al., 2021).
Statistical analysis
The data were analyzed using IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp, Armonk, NY, USA). Data analysis of different treatments on the criteria of interest were carried out using one-way ANOVA, after investigating the normal distribution (Kolmogorov–Smirnov test) and homogeneity of variance by Shapiro–wilk's and Levene’s tests, respectively. Statistical significance level was set at P ≤ 0.05 and the results were reported as mean ± SD.
Results and discussion
Extraction of fucoxanthin
The final fucoxanthin was produced with a purity of 54%, which is consistent with the results of the study of Oliyaei et al. (2020) (56%) and Sudhakar et al. (2013) conducted on Sargassum thunbergii algae. The difference in the purity of the extracted compounds may be related to differences in the type of extraction process. However, the purity above 50% is generally suitable for the food usage (Noviendri et al., 2011). Moreover, according to Oliyaei et al., (2020), 1H-NMR results exhibited the 5 methyl singlet forms (16-H, 17-H, 16’-H, 17’-H, 18’-H), 3 methyl in double bond (CH3–C=) (20-H, 19’-H, 20’-H), hydroxyl methyl (3’-H), 10 protons of a double bond (H-C =) and methylene (2’-H, 4’-H).
Effect of the gelatin concentration, voltage, and distance of needle tip away from the collector on electrospinning process of gelatin nanofibers
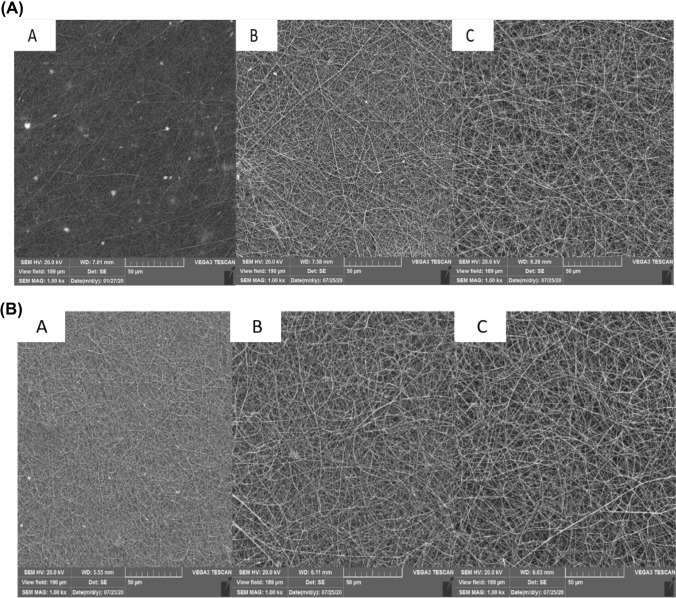
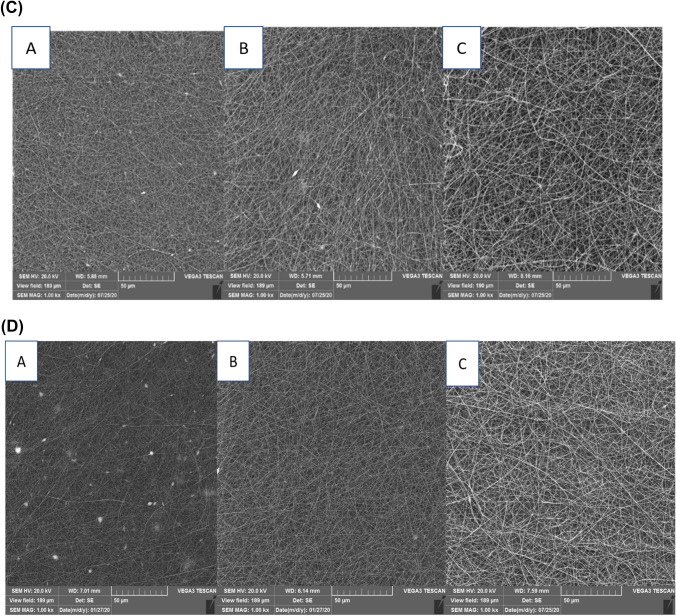
Various variables such as current rate, distance of needle tip away from the collector, and instrument voltage are considered as the importance factors for producing the nanofibers based on the electrospinning method (Kwak et al., 2017). SEM imaging showed that the gelatin solution 20% at different distances and voltages due to the low viscosity could not resist against electric force and did not form the nanofibers (Table 1A). However, as the Fig. 1A–D, the nanofibers were successfully formed at the concentrations of 25, 30, and 35%, under the voltages of 12 and 15 kV in the both distances of 10 and 15 cm (Table 1A). Concentration is one of the important factors for the nanofibers production, which is directly related to the viscosity. Increasing the fish gelatin concentration increased the diameter of the nanofibers, which resulted in producing the smooth and uniform nanofibers (Drosou et al., 2017). Although increasing the nanofiber diameter was observed at the concentration of 35% (Fig. 1A–D), viscous droplets formed on the syringe and collecting aluminum plate, which could be related to the electrical limitations during the electrospinning process. Therefore, the best fish gelatin nanofibers were obtained at the gelatin concentration 30% based on the SEM imaging, which are consistent with those of Mahmood et al. (2019), Chiu et al. (2019), Kwak et al. (2017). Moreover, in the study of Aliakbarshirazi et al. (2017), the results of SEM showed that a no grains were observed in the nanofibers at the concentration of 27.5% gelatin, and the average diameter of which was determined 95.19 nm.
Table 1.
A: The nanofibers characterization was prepared by different concentration of fish gelatin, voltage, and distance from spinneret to collector; B: Zeta potential of fish gelatin and different nanofibers; C: Mean diameter (mm) inhibition zone of samples against Escherichia coli and Staphylococcus aureus growth. Mean ± SD
| (A) | |||||
|---|---|---|---|---|---|
| Treatments | Concentration (W/V%) | Distance | Voltage | Present of beads | Nanofibers diameter (nm) |
| 1 | 20 | 10 | 12 | * | - |
| 2 | 20 | 10 | 15 | * | - |
| 3 | 20 | 15 | 12 | * | - |
| 4 | 20 | 15 | 15 | * | - |
| 5 | 25 | 10 | 12 | + | 181 ± 0.067 |
| 6 | 25 | 10 | 15 | + | 171 ± 0.065 |
| 7 | 25 | 15 | 12 | + | 167 ± 0.050 |
| 8 | 25 | 15 | 15 | + | 200 ± 0.075 |
| 9 | 30 | 10 | 12 | - | 206 ± 0.056 |
| 10 | 30 | 10 | 15 | + | 0.276±196 |
| 11 | 30 | 15 | 12 | + | 244 ± 0.042 |
| 12 | 30 | 15 | 15 | + | 246 ± 0.097 |
| 13 | 35 | 10 | 12 | - | 267 ± 0.057 |
| 14 | 35 | 10 | 15 | - | 241 ± 0.172 |
| 15 | 35 | 10 | 12 | - | 253 ± 0.094 |
| 16 | 35 | 15 | 15 | - | 304 ± 0.135 |
| (B) | |
|---|---|
| Sample | (Zeta potential (mv |
| Fish gelatin | 11.10 ± 0.75c |
| Fish gelatin nanofiber (FX) | 25.30 ± 0.82b |
| Fish gelatin nanofiber + 5% FX | 32.10 ± 1.93a |
| Fish gelatin nanofiber + 10% FX | 29.70 ± 1.44a |
| (C) | ||
|---|---|---|
| Sample | S. aureus | E. coli |
| Fucoxanthin (FX) | 22.7 ± 1.87 | 14.1 ± 1.14 |
| Fish gelatin nanofiber | 0 | 0 |
| Fish gelatin nanofiber + 5% FX | 19.6 ± 1.37 | 12.5 ± 0.40 |
(+) represent, there were beads in nanofibers. (-) represent, there were not beads in nanofibers. (*) represent, nanofibers were not formed. The lowercase letters indicate a significant difference in column. (P < 0.05). Mean ± SD
Fig. 1.
(A) SEM images of nanofibers produced by A 25%, B 30%, and C 35% of fish gelatin concentration at 15 kV and 15 cm distance of needle tip away from the electrospinning device collector; (B) SEM images of nanofibers produced by A 25%, B 30%, and C 35% of fish gelatin concentration at 12 kV and 15 cm distance of needle tip away from the electrospinning device collector; (C) SEM images of nanofibers produced by A 25%, B 30%, and C 35% of fish gelatin concentration at 15 kV and 10 cm distance of needle tip away from the electrospinning device collector; (D) SEM images of nanofibers produced by A 25%, B 30%, and C 35% of fish gelatin concentration at 12 kV and 10 cm distance of needle tip away from the electrospinning device collector
Evaluating the effect of the voltage on producing the gelatin nanofibers indicated that the electrostatic forces in the electrospinning process become stronger and the density of the electric charge increased by increasing the applied voltage (Fig. 1A, C). Therefore, the droplet at the tip of the needle was thrown faster, which reduced the diameter of the nanofiber (Drosou et al., 2017). As seen, the nanofibers did not form or had a granular structure at voltages of 18 and 20 kV (Table 1A). The ability for formation of the nanofibers as well as the diameter of the nanofibers at 12 kV voltage was higher compared to 15 kV voltage (Table 1A, B, D). However, the grains formed on the nanofibers decreased. Kwak et al. (2017) indicated that the lower the voltage used during the electrospinning process led to increasing the diameter of the nanofibers. The different voltage observed between the above-mention studies could be related to the type of gelatin, structural properties and viscosity of Huso huso fish gelatin.
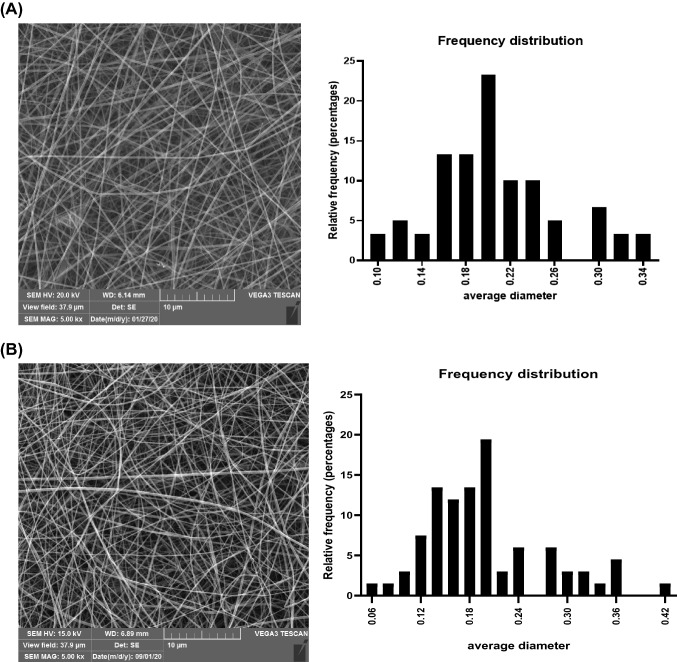
The greater distance from the collector to the nozzle caused the longer time for evaporating the used solvent (Fig. 1A, B). Therefore, the diameter of the fibers decreased, and the spherical grains between the fibers formed. The ability for producing the nanofibers increased and the nanofibers with the larger diameters were produced by reducing this distance (Drosou et al., 2017). The nanofibers with an average diameter of about 200 cm formed at a distance of 10 cm (Table 1A). No smooth and uniform nanofibers formed at a distance of 15 cm, and a number of spherical grains were observed simultaneously (Fig. 1A, B). Nanofibers were not observed at the distances higher than 15 cm due to the reduction of solvent evaporation rate (Table 1A). Okutan et al. (2014) and Akman et al. (2019) used a distance of 10 cm. The greater distance between the collector and the nozzle in the other studies can be attributed to the concentration of the acid used in the electrospinning process and the electrical force overcome on the surface of the polymer droplet (Gomes et al., 2013). The flow rate from the tip of the nozzle to the collector is another important factor of the electrospinning process, since it affected the rate of transferring the materials and solvent evaporation (Drosou et al., 2017). The best flow rate was obtained 0.3 ml/h, which showed the lower the flow rate led to producing more nanofibers and increasing the average diameter of nanofibers (Mahmood et al., 2019). Acetic acid with a concentration of 50% was used for reducing the surface tension of gelatin. Moreover, it was used as a suitable solvent for preparing the nanofibers during the electrospinning process. In the study of Kwak et al. (2017), the nanofibers produced by acetic acid (50:50, V:V) had the highest average diameter of the nanofibers compared to the control sample and distilled water. The results are consistent with those of Li et al. (2016) and Gomes et al. (2013) studies. The best nanofibers, the average diameter of which was estimated 206 ± 0.056 nm (Table 1A, Fig. 2A), were produced when the concentration of gelatin, voltage, flow rate, and the distance from nozzle to the collector were considered 30%, 12 kV, 0.3 ml/h, and 10 cm, respectively, based on the SEM image and the histogram of the nanofiber diameter distribution (Fig. 2A).
Fig. 2.
(A) SEM image and frequency distribution chart of fish gelatin electrospun nanofiber with 30% gelatin concentration, 12 kV, 10 Cm, rate 0.3 ml/h; (B) SEM image and frequency distribution chart of fish gelatin electrospun nanofiber with 30% gelatin concentration, 12 kV, 10 cm, rate 0.3 ml/h loaded by 5% fucoxanthin
Nanofibers loaded with fucoxanthin
Different percentages of fucoxanthin were added to the nanofibers solution after achieving the optimal conditions for the preparation of nanofibers. Evaluating the SEM images and the particle dispersion distribution diagram (Fig. 2B) indicated that adding more fucoxanthin to the solution caused a decrease in the viscosity of the solution and diameter of the produced nanofibers compared to the pure gelatin nanofibers (Akman et al., 2019). Further, Li et al. (2016) reported that adding the vitamin to the electrospinning solution made the diameter of the nanofibers smaller than the diameter of the pure gelatin. No nanofibers were obtained at a concentration of 15% fucoxanthin in the electrospinning solution. However, decreasing in the nanofiber diameter and producing in the spherical grain were observed by adding 10% of fucoxanthin. Finally, nanofibers loaded with 5% fucoxanthin with an average diameter of 198 ± 0.073 nm were selected as the best sample (Figs. 2B). As reported in the previous study, the gelatin nanofibers prepared with 5% turmeric, 15 cm distance, 15 kV voltage were considered as the optimal nanofibers (Chiu et al., 2019).
Encapsulation efficiency
Based on the results, the loading of fucoxanthin in the electrospun matrix of fish gelatin was determined 91 ± 0.03%, which is in line with that of the study of Oliyaei et al. (2020). Nanofibers produced by using electrospinning method showed an appropriate encapsulation performance due to the porosity and high surface to volume ratio, which could trap the particles of the bioactive materials well (Bhardwaj et al., 2010). In this regard, Yao et al. (2016) succeeded in loading 90% of rosehip seed oil into the zein matrix based on the electrospinning method (Yao et al., 2016). Moreover, the gelatin nanofibers prepared by using electrospinning method showed excellent performance (90–97%) in encapsulating the carotenoids of the tomato skin (Alehosseini et al., 2019). The researchers reported improving the solubility of carotenoids following their encapsulation in the gelatin nanofibers (Horuz et al., 2018). The results of loading curcumin into the gelatin matrix (Akman et al., 2019) and fucosantine into polyethylene glycol solution (Vo et al., 2018) indicated that the type of the encapsulation method, carrier, and fiber, as well as concentration of the materials used for loading was very effective in the final loading efficiency.
Zeta potential of the produced nanofibers
The zeta potential of gelatin, pure gelatin nanofibers, and loaded nanofibers is reported in Tables 1B. Whether the surface charge is positive or negative depended on the pH range of the gelatin. The isoelectric pH of the gelatin was + 4.8. The more positive zeta potential was obtained at the lower pH, and the higher pH caused the lower zeta potential (Anvari and Joyner, 2017). The gelatin had a surface charge of + 11.1 at pH 4. However, the zeta potential of electrospun nanofibers was significantly higher, which could be ascribed to the surface properties of electrospun nanofibers. Okutan et al. (2014) reported that the electrospun nanofibers had more electrostatic interactions with the environment at high voltage, which caused the more surface charge and positive charge (Okutan et al., 2014). The viscosity of the matrix and the diameter of the nanofibers decreased by adding fucoxanthin. Previous studies showed that the dispersion coefficient is inversely related to the diameter of the nanofibers. Therefore, decreasing the diameter led to an increase in the particle dispersion (Okutan et al., 2014).
Evaluating the structure of gelatin nanofibers using infrared spectroscopy
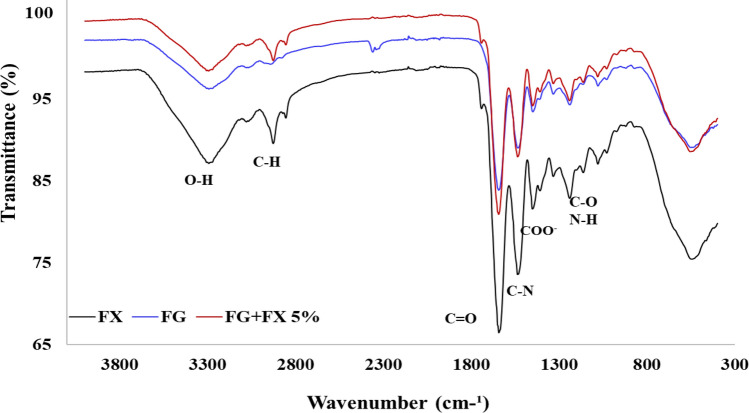
The FT-IR spectra of fucoxanthin, pure gelatin nanofibers, and nanofibers loaded with 5% fucoxanthin are shown in Fig. 3. In all the three samples, the peak appearing in the range of 552 cm−1 is attributed to hydrogen bond vibrations. In fucoxanthin FTIR spectrum, the peak at 1940 cm−1 was assigned to allenic bond (C=C=C) which is considered a functional group of fucoxanthin.
Fig. 3.
FTIR spectrum of fish gelatin (FG), Fucoxanthin (FX) and fish gelatin nanofiber loaded by 5% fucoxanthin (FG + FX 5%)
The peak observed at 1241 cm−1 is assigned to C=O and N–H stretching vibrations and C–H and COOH deformation vibrations. Moreover, the vibration band at 1451 cm−1 can be corresponded to COO− symmetric stretching vibration (Mahmood et al., 2019). The FTIR spectra of gelatin is usually determined as strong broad band 1534 cm−1 as the presence of amide II (Akman et al., 2019). The peak at 1644 cm−1 indicates the presence of amide I group in the pure gelatin nanofibers and the stretching and bending (stretching couple) of C=O and C –N groups (Mahmood et al., 2019). These are the most characteristic bands for gelatin. The peaks located at 2943 cm−1 can be ascribed to vinylic and aromatic bonds, asymmetric C–H groups, and C–H stretching bond in the polymers (Akman et al., 2019). Overall, the peaks in the range of 3100–3400 cm-1 are related to OH hydrogen bonds (Akman et al., 2019), which reduced in the fucoxanthin sample indicating the fucoxanthin hydrogen bond in the loaded nanofibers (Nootem et al., 2018). The pure sample of fucoxanthin shows the peak at 1647 cm−1 attributed to C=C double bonds (Yip et al., 2014) and the peaks appearing at 1044, 1086, 2895 and 2974 cm−1 assigned to the alkane group (CH) (Oliyaei et al., 2020).
The entire region of FTIR spectra exhibited similar bonds for fucoxanthin loaded-nanofibers and gelatin fibers, with some higher intensity in encapsulated form. Based on the FT-IR spectra, the intensity of the peaks increased in some regions by adding fucoxanthin to the electrospun gelatin solution, which can be attributed to the interaction of the groups of fucoxanthin with the functional groups of the gelatin. Some bands did not change after adding fucoxanthin to gelatin fibers, which indicated that both materials had no significant structural changes in the subsequent processing of the electrospinning process (Padrão et al., 2015).
Percentage of free radical scavenging of DPPH by using produced nanofibers
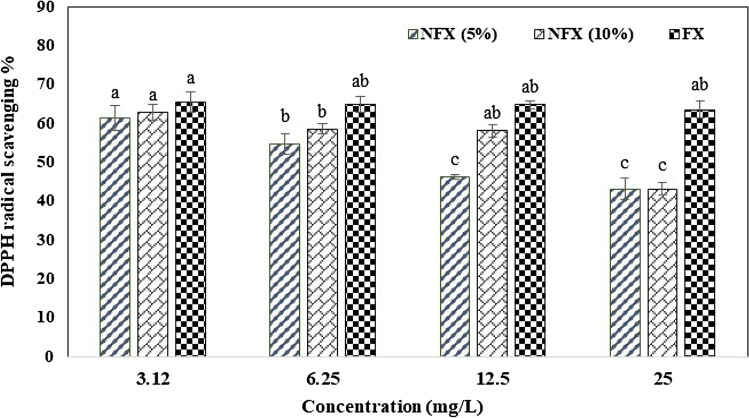
Fucoxanthin is a natural antioxidant due to its carotenoid structure, which showed free radical scavenging rate of 65% at the concentration of 2.4 mg/l, in nanofiber load 5% and 10% almost 61% in the present study (Fig. 4). This compound is unstable to light, heat, and some environmental conditions. Preventing these changes and maintaining the stability of it against oxidation can be provided by using the electrospinning process. Nootem et al. (2018) reported the similar results in the study of on astaxanthin. Nanofibers loaded with 5% turmeric showed high antioxidant properties compared to various other percentages of turmeric, which was reported 94% (Chiu et al., 2019). However, nanofibers loaded with 2.5% turmeric indicated much higher antioxidant properties compared to different percentages of curcumin, which was estimated 90% (Akman et al., 2019).
Fig. 4.
DPPH radical scavenging of fish gelatin nanofiber loaded by 5% and 10% fucoxanthin (FX 5% and FX 10%) and fucoxanthin (FX). Means with different capital letter superscripts are significantly different (p < 0.05)
Antibacterial properties
The antibacterial activities of nanofiber are shown in Table 1C. It was shown that the inhibition zone of Fucoxanthin was higher than that of fish gelatin nanofiber loaded by 5% Fucoxanthin. The inhibition zone of the fucoxanthin against S. aureus and E. coli were 22.69 and 14.08, respectively. Fish gelatin nanofiber + 5% fucoxanthin showed inhibition zone against S. aureus and E. coli were 19.63 and 12.54, respectively. In contrast, fish gelatin nanofiber had no antibacterial activity. The result exhibited a better antibacterial activity against Gram-positive bacteria than Gram-negative bacteria as well as others studies (Ardekani et al., 2019), which indicates that fucoxanthin biological activity depends on the differences in the cell wall structure and composition of both types of bacteria. The Gram-negative bacteria contain lipopolysaccharide in membrane component (Karpiński and Adamczak, 2019). The results revealed that antibacterial activity of fucoxanthin considerably decreased when encapsulated into fish gelatin nanofiber in contrast with (Akman et al., 2019) study. Unfortunately, the direct antibacterial mechanism of fucoxanthin action is not known, but, there is a relationship between the antioxidant and antibacterial properties of natural chemical compounds (Karpiński and Adamczak, 2019).
However, this study indicated that the gelatin obtained from the waste of the fish processing plants had an appropriate potential for producing the nanofibers based on the electrospinning method. The use of fucoxanthin extracted from Sargassum marine macroalgae as a natural pigment with the antioxidant and antibacterial properties played an effective role in improving the functional properties of the gelatin nanofibers. Therefore, the use of it for the packaging and storing the perishable food is suggested in future studies.
Acknowledgements
This Project funded by Shiraz University. Also, the authors thank the staff of the Seafood Processing Research Center and Laboratories of Shiraz University.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aida Azarshah, Email: ayda.azarshah@gmail.com.
Marzieh Moosavi-Nasab, Email: Marzieh.Moosavi-Nasab@Mail.McGill.ca.
Mohammad Khorram, Email: mkhorram@shirazu.ac.ir.
Sedigheh Babaei, Email: Babaei.Sedigheh@gmail.com.
Najmeh Oliyaei, Email: oliyaei_f@yahoo.com.
References
- Akman PK, Bozkurt F, Balubaid M, Yilmaz MT. Fabrication of curcumin-loaded gliadin electrospun nanofibrous structures and bioactive properties. Fibers and Polymers. 2019;20:1187–1199. doi: 10.1007/s12221-019-8950-8. [DOI] [Google Scholar]
- Alehosseini A, Gómez-Mascaraque LG, Martínez-Sanz M, López-Rubio A. Electrospun curcumin-loaded protein nanofiber mats as active/bioactive coatings for food packaging applications. Food Hydrocolloids. 2019;87:758–771. doi: 10.1016/j.foodhyd.2018.08.056. [DOI] [Google Scholar]
- Aliakbarshirazi S, Talebian A. Electrospun gelatin nanofibrous scaffolds for cartilage tissue engineering. Materials Today: Proceedings. 2017;4:7059–7064. [Google Scholar]
- Anvari M, Joyner HS. Effect of fish gelatin-gum arabic interactions on structural and functional properties of concentrated emulsions. Food Research International. 2017;102:1–7. doi: 10.1016/j.foodres.2017.09.085. [DOI] [PubMed] [Google Scholar]
- Ardekani NT, Khorram M, Zomorodian K, Yazdanpanah S, Veisi H, Veisi H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. International Journal of Biological Macromolecules. 2019;125:743–750. doi: 10.1016/j.ijbiomac.2018.12.085. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kundu SC. Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Chiu CM, Nootem J, Santiwat T, Srisuwannaket C, Pratumyot K, Lin WC, Mingvanish W, Niamnont N. Enhanced stability and bioactivity of Curcuma comosa Roxb. Extract in Electrospun Gelatin Nanofibers. Fibers. 2019;7:76. [Google Scholar]
- Drosou CG, Krokida MK, Biliaderis CG. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Drying Technology. 2017;35:139–162. doi: 10.1080/07373937.2016.1162797. [DOI] [Google Scholar]
- Ebrahimi S, Babaei S, Naseri M, Golmakani MT. Polyphenols content, antioxidant and antibacterial activities of seaweeds from the Persian Gulf. Environmental Engineering & Management Journal. 2021;20:1203–1211. doi: 10.30638/eemj.2021.112. [DOI] [Google Scholar]
- Gomes SR, Rodrigues G, Martins GG, Henriques CMR, Silva JC. In vitro evaluation of crosslinked electrospun fish gelatin scaffolds. Materials Science and Engineering: c. 2013;33:1219–1227. doi: 10.1016/j.msec.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Horuz T, Belibağlı KB. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chemistry. 2018;268:86–93. doi: 10.1016/j.foodchem.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Huang Z, Xu L, Zhu X, Hu J, Peng H, Zeng Z, Xiong H. Stability and bioaccessibility of fucoxanthin in nanoemulsions prepared from pinolenic acid-contained structured lipid. International Journal of Food Engineering. 2017;13:1. doi: 10.1515/ijfe-2016-0273. [DOI] [Google Scholar]
- Karpiński TM, Adamczak A. Fucoxanthin—An antibacterial carotenoid. Antioxidants. 2019;8:239. doi: 10.3390/antiox8080239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SY, Mok IK, Pan CH, Kim SM. Preparation of fucoxanthin-loaded nanoparticles composed of casein and chitosan with improved fucoxanthin bioavailability. Journal of Agricultural and Food Chemistry. 2016;64:9428–9435. doi: 10.1021/acs.jafc.6b04376. [DOI] [PubMed] [Google Scholar]
- Kwak HW, Shin M, Lee JY, Yun H, Song DW, Yang Y, Shin BS, Park YH, Lee KH. Fabrication of an ultrafine fish gelatin nanofibrous web from an aqueous solution by electrospinning. International Journal of Biological Macromolecules. 2017;102:1092–1103. doi: 10.1016/j.ijbiomac.2017.04.087. [DOI] [PubMed] [Google Scholar]
- Li H, Wang M, Williams GR, Wu J, Sun X, Lv Y, Zhu LM. Electrospun gelatin nanofibers loaded with vitamins A and E as antibacterial wound dressing materials. RSC Advances. 2016;6:50267–50277. doi: 10.1039/C6RA05092A. [DOI] [Google Scholar]
- Mahmood K, Kamilah H, Sudesh K, Karim AA, Ariffin F. Study of electrospun fish gelatin nanofilms from benign organic acids as solvents. Food Packaging and Shelf Life. 2019;19:66–75. doi: 10.1016/j.fpsl.2018.11.018. [DOI] [Google Scholar]
- Mok IK, Yoon JR, Pan CH, Kim SM. Development, quantification, method validation, and stability study of a novel fucoxanthin-fortified milk. Journal of Agricultural and Food Chemistry. 2016;64:6196–6202. doi: 10.1021/acs.jafc.6b02206. [DOI] [PubMed] [Google Scholar]
- Mousavi Z, Naseri M, Babaei S, Hosseini SMH, Shekarforoush SS. The effect of cross-linker type on structural, antimicrobial and controlled release properties of fish gelatin-chitosan composite films incorporated with ε-poly-L-lysine. International Journal of Biological Macromolecules. 2021;183:1743–1752. doi: 10.1016/j.ijbiomac.2021.05.159. [DOI] [PubMed] [Google Scholar]
- Mousavipour N, Babaei S, Moghimipour E, Moosavi-Nasab M, Ceylan Z. A novel perspective with characterized nanoliposomes: Limitation of lipid oxidation in fish oil. LWT. 2021;152:112387. doi: 10.1016/j.lwt.2021.112387. [DOI] [Google Scholar]
- Nootem J, Chalorak P, Meemon K, Mingvanish W, Pratumyot K, Ruckthong L, Srisuwannaket C, Niamnont N. Electrospun cellulose acetate doped with astaxanthin derivatives from Haematococcus pluvialis for in vivo anti-aging activity. RSC Advances. 2018;8:37151–37158. doi: 10.1039/C8RA08156E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noviendri D, Salleh HM, Taher M, Miyashita K, Ramli N. Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. Duplicatum. Journal of Medicinal Plants Research. 2011;5:2405–2412. [Google Scholar]
- Okutan N, Terzi P, Altay F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids. 2014;39:19–26. doi: 10.1016/j.foodhyd.2013.12.022. [DOI] [Google Scholar]
- Oliyaei N, Moosavi-Nasab M, Tamaddon AM, Fazaeli M. Double encapsulation of fucoxanthin using porous starch through sequential coating modification with maltodextrin and gum Arabic. Food Science & Nutrition. 2020;8:1226–1236. doi: 10.1002/fsn3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrão J, Machado R, Casal M, Lanceros-Méndez S, Rodrigues LR, Dourado F, Sencadas V. Antibacterial performance of bovine lactoferrin-fish gelatine electrospun membranes. International Journal of Biological Macromolecules. 2015;81:608–614. doi: 10.1016/j.ijbiomac.2015.08.047. [DOI] [PubMed] [Google Scholar]
- Rongthong W, Niamnont N, Srisuwannaket C, Paradee N, Mingvanish W. Electrospun gelatin fiber mats mixed with C. carandas extract and its enhanced stability and bioactivity. Journal of Pharmaceutical Sciences. 2021;110:2405–2415. doi: 10.1016/j.xphs.2020.12.031. [DOI] [PubMed] [Google Scholar]
- Sudhakar MP, Ananthalakshmi JS, Nair BB. Extraction, purification and study on antioxidant properties of fucoxanthin from brown seaweeds. Journal of Chemical and Pharmaceutical Research. 2013;5:169–175. [Google Scholar]
- Šudomová M, Shariati MA, Echeverría J, Berindan-Neagoe I, Nabavi SM, Hassan ST. A microbiological, toxicological, and biochemical study of the effects of fucoxanthin, a marine carotenoid, on Mycobacterium tuberculosis and the enzymes implicated in its cell wall: A link between mycobacterial infection and autoimmune diseases. Marine Drugs. 2019;17:641. doi: 10.3390/md17110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardizadeh F, Babaei S, Naseri M, Golmakani MT. Effect of marine sulfated polysaccharides derived from Persian Gulf seaweeds on Oncorhynchus mykiss oil stability under accelerated storage conditions. Algal Research. 2021;60:102553. doi: 10.1016/j.algal.2021.102553. [DOI] [Google Scholar]
- Vo DT, Saravana PS, Woo HC, Chun BS. Fucoxanthin-rich oil encapsulation using biodegradable polyethylene glycol and particles from gas-saturated solutions technique. Journal of CO Utilization. 2018;26:359–369. doi: 10.1016/j.jcou.2018.05.019. [DOI] [Google Scholar]
- Wang X, Li H, Wang F, Xia G, Liu H, Cheng X, Kong M, Liu Y, Feng C, Chen X, Wang Y. Isolation of fucoxanthin from Sargassum thunbergii and preparation of microcapsules based on palm stearin solid lipid core. Frontiers of Materials Science. 2017;11:66–74. doi: 10.1007/s11706-017-0372-1. [DOI] [Google Scholar]
- Wang T, Jonsdottir R, Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chemistry. 2009;116:240–248. doi: 10.1016/j.foodchem.2009.02.041. [DOI] [Google Scholar]
- Yao ZC, Chang MW, Ahmad Z, Li JS. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. Journal of Food Engineering. 2016;191:115–123. doi: 10.1016/j.jfoodeng.2016.07.012. [DOI] [Google Scholar]
- Yip WH, Joe LS, Mustapha WA, Maskat MY, Said M. Characterisation and stability of pigments extracted from Sargassum binderi obtained from Semporna, Sabah. Sains Malaysiana. 2014;43:1345–1354. [Google Scholar]