Abstract
Introduction
Central lymph node metastasis (CLNM) is common in papillary thyroid carcinoma (PTC). Prophylactic central lymph node dissection (PCLND) in clinically negative central compartment lymph node (cN0) PTC patients is still controversial. How to predict CLNM before the operation is very important for surgical decision making.
Methods
In this article, we retrospectively enrolled 243 cN0 PTC patients and gathered data including clinical characteristics, ultrasound (US) characteristics, pathological results of fine-needle aspiration (FNA), thyroid function, eight gene mutations, and immunoenzymatic results. Least absolute shrinkage and selection operator (LASSO) analysis was used for data dimensionality reduction and feature analysis.
Results
According to the results, the important predictors of CLNM were identified. Multivariable logistic regression analysis was used to establish a new nomogram prediction model. The receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA) curve were used to evaluate the performance of the new prediction model.
Discussion
The new nomogram prediction model was a reasonable and reliable model for predicting CLNM in cN0 PTC patients, but further validation is warranted.
Keywords: papillary thyroid carcinoma (PTC), central lymph node metastasis (CLNM), least absolute shrinkage and selection operator (LASSO), nomogram prediction model, clinically negative central compartment lymph nodes (cN0), prophylactic central lymph node dissection (PCLND)
Introduction
Papillary thyroid carcinoma (PTC) is the most common pathological type of thyroid cancer, accounting for more than 80% of thyroid cancers (1, 2). PTC easily metastasizes to cervical lymph nodes. The rate of central lymph node metastasis (CLNM) in PTC ranges from approximately 20% to 80% (3–5). At present, the diagnosis of CLNM mainly depends on preoperative ultrasound (US), but its diagnostic sensitivity is only approximately 20–40% before surgery (6, 7). In PTC patients, positive lymph node metastasis (LNM) is associated with a worse prognosis, more advanced TNM stage, and higher rate of tumor recurrence (8, 9).
Some studies have shown that 30–40% of clinically negative central compartment lymph node (cN0) PTC patients have pathologically confirmed CLNM after prophylactic central lymph node dissection (PCLND) (10–12). However, the use of PCLND in all cN0 PTC patients is still controversial (13, 14). PCLND can improve disease-free survival and decrease the local recurrence rate (15, 16), but it also increases the rate of surgical complications, such as recurrent laryngeal nerve injury and permanent hypothyroidism (17, 18). Thus, it is necessary to predict CLNM in cN0 PTC patients to determine whether PCLND is needed.
Materials and methods
Patients
A total of 243 cN0 PTC patients were retrospectively enrolled in our hospital from January 2019 to July 2021. cN0 PTC patients were referred to as PTC patients without LNM under imaging examination before surgery. After surgery, all patients were divided into pathologically CLNM positive (group A) and pathologically CLNM negative (group B) groups. The clinical characteristics, ultrasound (US) characteristics, pathological fine-needle aspiration (FNA) results, thyroid function, and immunoenzymatic results were retrospectively reviewed and analysed. Paraffin-embedded PTC tissue stored in the Department of Pathology was removed for eight-gene mutation testing. The exclusion criteria were as follows: ① patients had other types of thyroid malignancy; ② patients were confirmed to have CLNM or lateral LNM before the operation; ③ patient had a history of thyroid operations; and ④ patients refused PCLND. The requirement to obtain informed consent from the patients was waived for this retrospective study.
Operation methods
Standard operational procedures have been previously reported according to the “Chinese guidelines for diagnosis and treatment of thyroid diseases”. All patients underwent thyroidectomy with PCLND for unilateral PTC and total thyroidectomy with bilateral PCLND for bilateral PTC. All operations were performed by two experienced surgeons of the same surgery quality who had more than 10 years of working experience in our Department of General Surgery.
Clinical and US characteristics, thyroid function, and pathological results
The clinical characteristics collected for analysis included sex (male/female), age (<55 years or ≥55 years), history of thyroid drugs (levothyroxine/thiamazole), history of Hashimoto’s thyroiditis (HT), history of nodular goiter, and family history of thyroid cancer. The US characteristics included the location of the tumor (left/right/isthmus), tumor number, tumor size, aspect ratio (≤1/>1), margin, microcalcification, capsule involvement, blood flow signal, HT, hyperthyroidism and TI-RADS classification. Thyroid function included TSH, FT3, FT4, T3, T4, TPOAb, TRAb, and TgAb. Immunohistochemistry factors included MC, Gal-3, TTF1, TPO, Ki67, and CKHi. Detailed information is provided in Table 1 .
Table 1.
Clinical, US, thyroid function and pathological characteristics of patients undergoing PCLND in different groups.
| Variable | Group A (N=92) N (%) | Group B (N=151) N (%) | P value |
|---|---|---|---|
| Sex N (%) | 0.048 a | ||
| Female | 41 (44.6) | 47 (31.1) | |
| Male | 51 (55.4) | 104 (68.9) | |
| Age (mean ± SD) | 43.47 ± 12.37 | 45.45 ± 12.19 | 0.225 |
| ≥55 | 13 (14.3) | 43 (28.5) | 0.017 a |
| <55 | 78 (85.7) | 108 (71.5) | |
| Thyroid-related drugs | 0.714 | ||
| No | 91 (98.9) | 147 (97.4) | |
| Yes | 1 (1.1) | 4 (2.6) | |
| Hashimoto’s thyroiditis | 1 | ||
| Positive | 8 (8.7) | 12 (7.9) | |
| Negative | 84 (91.3) | 139 (92.1) | |
| Nodular goiter | 0.118 | ||
| Positive | 14 (15.2) | 12 (7.9) | |
| Negative | 78 (84.8) | 139 (92.1) | |
| Family history | 0.988 | ||
| Positive | 1 (1.1) | 3 (2.0) | |
| Negative | 91 (98.9) | 148 (98.0) | |
| Thyroid function b | |||
| TSH | 0.024 a | ||
| Positive | 33 (35.9) | 78 (51.7) | |
| Negative | 59 (64.1) | 73 (48.3) | |
| FT3 | 0.016 a | ||
| Positive | 28 (30.4) | 71 (47.0) | |
| Negative | 64 (69.6) | 80 (53.0) | |
| FT4 | 0.095 | ||
| Positive | 80 (87.0) | 142 (94.0) | |
| Negative | 12 (13.0) | 9 (6.0) | |
| T3 | 0.417 | ||
| Positive | 43 (46.7) | 80 (53.0) | |
| Negative | 49 (53.3) | 71 (47.0) | |
| T4 | 0.017 a | ||
| Positive | 82 (89.1) | 147 (97.4) | |
| Negative | 10 (10.9) | 4 (2.6) | |
| TgAb | 0.232 | ||
| Positive | 27 (29.3) | 57 (37.7) | |
| Negative | 65 (70.7) | 94 (62.3) | |
| TPOAb | 0.302 | ||
| Positive | 23 (25.0) | 63 (41.7) | |
| Negative | 69 (75.0) | 88 (58.3) | |
| Characteristics of US | |||
| Bilateral | 0.238 | ||
| No | 30 (32.6) | 62 (41.1) | |
| Yes | 62 (67.4) | 89 (58.9) | |
| Side N (%) | 0.317 | ||
| Right | 39 (42.4) | 79 (52.3) | |
| Left | 47 (51.1) | 63 (41.7) | |
| Isthmus | 6 (6.5) | 9 (6.0) | |
| Tumor Size N (%) | 0.074 | ||
| ≤10 mm | 54 (58.7) | 108 (72.0) | |
| >10 mm, ≤20 mm | 29 (31.5) | 35 (23.3) | |
| >20 mm | 9 (9.8) | 8 (4.7) | |
| Aspect Ratio N (%) | 0.331 | ||
| >1 | 68 (73.9) | 121 (80.1) | |
| <1 | 24 (26.1) | 30 (19.9)) | |
| Margin | 1 | ||
| Circumscribed | 14 (15.2) | 23 (15.2) | |
| Indistinct | 78 (84.8) | 128 (84.8) | |
| Microcalcification | 0.073 | ||
| Negative | 25 (27.2) | 59 (39.1) | |
| Positive | 67 (72.8) | 92 (60.9) | |
| Capsule Involvement | 0.186 | ||
| Negative | 73 (79.3) | 130 (86.1) | |
| Positive | 19 (20.7) | 21 (13.9) | |
| Internal Vascularity | 0.388 | ||
| Negative | 13 (14.1) | 29 (19.3) | |
| Positive | 79 (85.9) | 121 (80.7) | |
| TI-RADS | 0.042 a | ||
| 4A/4B | 18 (19.6) | 49 (32.5) | |
| 4C/5 | 74 (80.4) | 102 (67.5) | |
| Immunohistochemistry | |||
| MC | 0.451 | ||
| Negative | 6 (13.0) | 14 (20.3) | |
| Positive | 40 (87.0) | 55 (79.7) | |
| Gal3 | 0.393 | ||
| Negative | 8 (9.8) | 8 (5.7) | |
| Positive | 74 (90.2) | 132 (94.3) | |
| TTF1 | 0.061 | ||
| Negative | 17 (23.9) | 15 (12.4) | |
| Positive | 54 (76.1) | 106 (87.6) | |
| TPO | 0.245 | ||
| Negative | 75 (96.2) | 119 (90.8) | |
| Positive | 3 (3.8) | 12 (9.2) | |
| Ki67 ≥0.02, <0.02 | 0.101 | ||
| Negative | 24 (33.3) | 58 (46.4) | |
| Positive | 48 (66.7) | 67 (53.6) | |
| CKHi | 0.965 | ||
| Negative | 11 (19.6) | 15 (17.9) | |
| Positive | 45 (80.4) | 69 (82.1) | |
| Gene mutation | |||
| BRAF V600E | <0.001 a | ||
| Wild-type | 8 (8.7) | 85 (56.3) | |
| Mutant | 84 (91.3) | 66 (43.7) | |
| TERT C228T/250T | 0.663 | ||
| Wild-type | 90 (97.8) | 150 (99.3) | |
| Mutant | 2 (2.2) | 1 (0.7) | |
| KRAS G12C/G12V/Q61R | 1 | ||
| Wild-type | 91 (98.9) | 149 (99.3) | |
| Mutant | 1 (1.1) | 2 (0.7) | |
| HRAS Q61R | 1 | ||
| Wild-type | 92 (100) | 151 (100) | |
| Mutant | 0 | 0 | |
| NRAS Q61R | 1 | ||
| Wild-type | 92 (100%) | 151 (100%) | |
| Mutant | 0 | 0 | |
| CCDC6-RET | 1 | ||
| Wild-type | 92 (100%) | 151 (100%) | |
| Mutant | 0 | 0 | |
| PAX8-PPARG | 1 | ||
| Wild-type | 92 (100%) | 151 (100%) | |
| Mutant | 0 | 0 | |
| EVT6-NTRK3 | 1 | ||
| Wild-type | 92 (100%) | 151 (100%) | |
| Mutant | 0 | 0 | |
(a) Bold values indicate statistical significance (P<0.05). (b) The values of thyroid function were divided into positive and negative. Positive means outside the normal reference value range, and negative means within the normal reference value range.
Gene mutation testing
A thyroid cancer eight-gene detection kit (Rigen Bio, China) and SLAN-96S Real-Time PCR instrument (Hong Shi, China) were used for thyroid gene mutation testing. The kit included oncogene mutations (BRAF V600E, HRAS Q61R, KRAS G12C/G12V/Q61R NRAS Q61R, and TERT C228T/250T) and chromosome rearrangements (CCDC6-RET, PAX8-PPARG, and EVT6-NTRK3). Real-time PCR technology was used to detect point mutations and fusion mutations in eight thyroid cancer-related genes.
Statistical analysis
R (v4.1.2) and SPSS 25 were used for statistical analysis. Continuous variables with a normal distribution, such as age, tumor size and thyroid function, are presented as the mean ± standard deviation and were compared by the t test. Categorical variables, including clinical characteristics, US characteristics, pathological FNA results, thyroid function, immunoenzymatic results, and eight gene mutations, are presented as frequencies or percentages (%) and were compared by the chi-square test or Fisher’s test. We converted some continuous variables to categorical variables; for example, patient age was divided into <55 years and ≥55 years (AJCC/UICC TNM staging system version 8), tumor size was divided into ≤10 mm, 10 mm<T ≤ 20 mm, and >20 mm, and thyroid function was divided as well. All variables were analysed by least absolute shrinkage and selection operator (LASSO) analysis. The minimum error of the lambda (λ) value by the cross-validation method was calculated as the standard. The influencing factors of CLNM in cN0 PTC patients were screened out and used to establish a multifactor logistic regression model and then construct a nomogram prediction model. The RS of each patient was calculated by the mathematical formula. P<0.05 indicated a significant difference. Receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA) curves were used to evaluate the performance of the model.
Result
Clinical characteristics
There were 41 females and 51 males in group A and 47 females and 104 males in group B. The male/female ratios were 1:1.24 and 1:2.21, respectively (P<0.05). The average ages in groups A and B were 43.37 ± 12.37 and 45.45 ± 12.19 years, respectively (P<0.05). The numbers of patients <55 years old in groups A and B were 78 and 108, respectively, and those ≥55 years old were 13 and 43, respectively (P<0.05). A history of taking thyroid drugs, a history of HT, a history of nodular goiter, and a family history of thyroid cancer were not significantly different between groups A and B. Regarding US characteristics, only TI-RADS classification was significantly different (P<0.05) between groups A and B (74 cases and 102 cases, respectively). Other US features were not significantly different between groups A and B (P>0.05). TSH, FT3, and T4 were significantly different between groups A and B (P<0.05). FT4, T3, TPOAb, TRAb, and TgAb levels were not significantly different between groups A and B (P>0.05). Immunohistochemistry factors (MC, Gal-3, TTF1, TPO, Ki67, and CKHi) were not significantly different between groups A and B (P>0.05). BRAF V600E, KRAS G12C/G12V/Q61R, and TERTC228T/250T were selected for further analysis because the number of patients with other gene mutations in groups A and B was not sufficient. The number of patients with BRAF V600E was significantly different between groups A and B (84 and 66 cases, 91.3% and 43.7%, P<0.05). KRAS G12C/G12V/Q61R and TERT C228T/250T mutations were not significantly different between the two groups (P>0.05) ( Table 1 ).
Predictive model construction
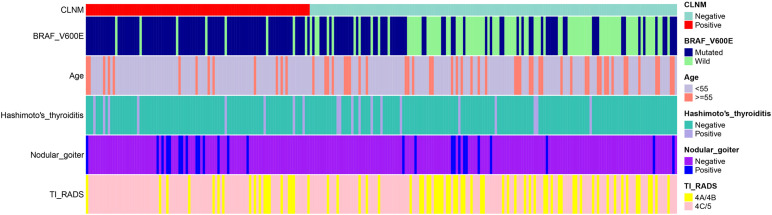
Five out of forty-nine factors, including age, history of nodular goiter, BRAF V600E mutation, history of HT, and TI-RADS classification, were identified as significant via LASSO regression ( Figure 1 and Table 2 ). Among them, BRAF V600E mutation was identified as the top risk factor. The statistical distribution of the five most powerful factors identified by LASSO regression analysis is visualized in Figure 2 .
Figure 1.
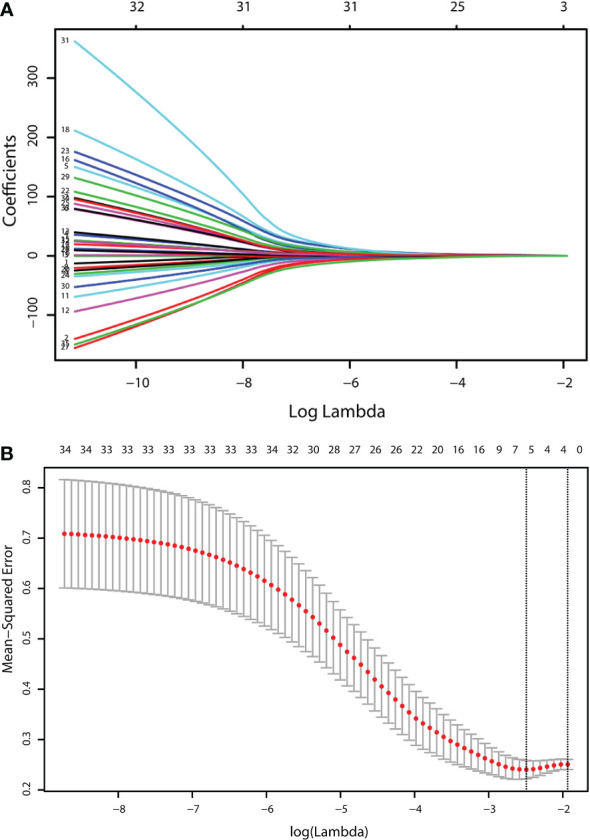
Identification of the influencing factors by LASSO regression. LASSO regression identified the following 5 most powerful predictors. (A) LASSO coefficient profiles of the 49 characteristics. A coefficient profile plot was produced against the log lambda (λ) sequence. (B) The relationship curve between the partial likelihood deviation (binomial deviation) and log(λ) was plotted.
Table 2.
Regression coefficients of the 5 most powerful factors identified by LASSO regression analysis.
| Variable | OR | Std. Error | P value |
|---|---|---|---|
| Age (≥55) | 0.317791 | 0.6785 | 0.0911 |
| Nodular goiter | 3.935329 | 1.2365 | 0.2679 |
| BRAF V600E | 4.04959 | 0.6449 | 0.0301a |
| Hashimoto’s thyroiditis | 1.124409 | 0.7038 | 0.8677 |
| TI-RADS (4C/5) | 1.752304 | 0.8284 | 0.4983 |
(a) Bold values indicate statistical significance (P<0.05).
Figure 2.
Heatmap of the top five significant variables identified based on LASSO regression analysis. Heatmap of the top five significant variables identified via LASSO regression, including BRAF V600E mutation, age, history of nodular goiter, history of Hashimoto’s thyroiditis and TI-RADS classification.
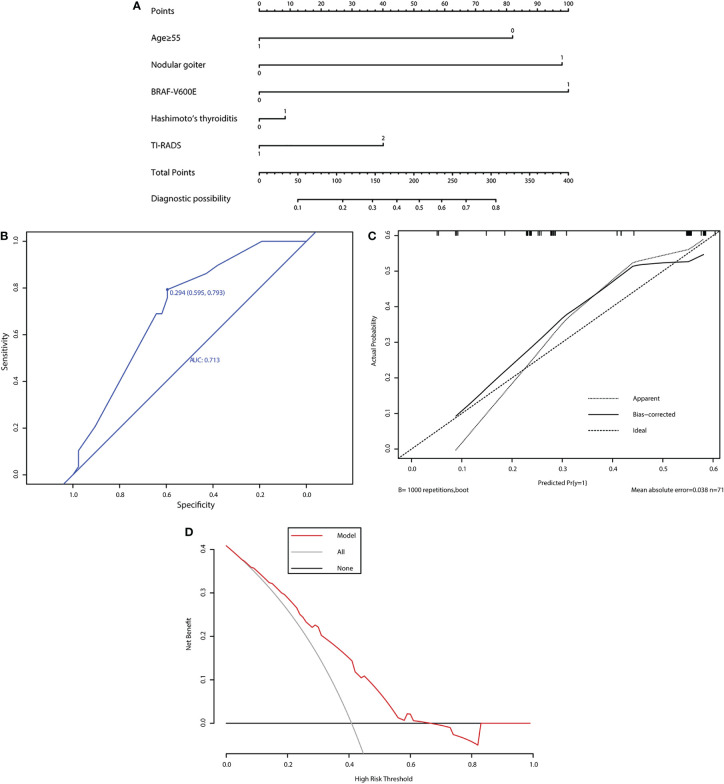
The nomogram prediction model was constructed based on these most powerful factors ( Figure 3A ). In the nomogram prediction model, each predictive factor was assigned a corresponding score. Among the predictive factors, age ≥55 years had a score of 1, and age <55 years had a score of 0. HT, nodular goiter, and BRAF V600E mutation each had a score of 1 for presence and 0 for absence. The TI-RADS classification T4A~T4B was assigned a score of 1, and T4C~T5 was assigned a score of 2 (AJCC/UICC TNM staging system version 8). According to the corresponding scores from each predictive factor, the total score can be obtained. The diagnostic possibility ranges from 0.1 to 0.8. A diagnostic possibility close to 0.1 was classified as low risk, and a diagnostic possibility close to 0.8 was classified as high risk. The probability of CLNM in cN0 patients could be predicted according to the diagnostic possibility.
Figure 3.
Evaluation of the performance of the new prediction model. (A) Nomogram for predicting CLNM in PTC patients based on five risk factors. (B) The ROC curve and AUC of the nomogram. ROC, receiver operating characteristic. (C) Calibration plots of the nomogram for predicting CLNM. (D) The DCA method evaluated the performance of the model.
Predictive model performance
The predictive model performance was evaluated by ROC curves, calibration curves, and DCA curves. The area under the ROC curve (AUC) of the model was 0.713 (95% CI 0.595–0.793) ( Figure 3B ). The approximate line and the bias-corrected line represent the performance of our model. After resampling for internal validation, the average absolute error was 3.8%. The threshold probability of CLNM metastasis was between 0.06 and 0.66. The net benefit level of the application of the nomogram prediction model was significantly higher than that of the “non-intervention” and “full intervention” schemes. Meanwhile, the calibration curve displayed a satisfactory consistency ( Figure 3C ). The DCA curve also suggested good predictive power ( Figure 3D ).
Discussion
National Comprehensive Cancer Network (NCCN) clinical practice guidelines for thyroid carcinoma and the American Thyroid Association (ATA) guidelines do not recommend PCLND in all cN0 PTC patients (19, 20). The efficacy of PCLND for cN0 PTC is uncertain (15, 21), and the incidence of complications, such as recurrent laryngeal nerve injury, permanent hypoparathyroidism (17, 21), and chyle leakage (18), is high. In contrast, the Chinese Thyroid Association guidelines still recommend PCLND because the rate of CLNM in cN0 PTC is up to 72% (22), which increases the recurrence rate. Reoperation leads to a higher rate of operative complications (23, 24). The 2015 version of the ATA management guideline marks a high rate of CLNM as a pivotal risk factor in the risk stratification evaluation of PTC patients. Therefore, it is necessary to determine the risk of CLNM in cN0 PTC patients and screen out high-risk patients for PCLND.
US, CT, and MRI are usually used for judging the condition of central lymph nodes, but their sensitivity and specificity are not sufficient (25, 26). Zhong et al (27) showed that the incidence of CLNM in cN0 PTC patients was 53.6%. Yasuhiro et al (28) found that the sensitivity of US diagnosis of lateral metastasis was only 27.2%. For this reason, it is unreliable to perform PCLND purely depending on preoperative US. Liu et al (26) showed that there was no significant difference in the diagnosis of lateral neck node metastases between MRI and US. A meta-analysis that included 17 studies showed that the sensitivity and specificity of CT in the detection of CLNM ranged from 23% to 83% and from 64% to 94%, respectively. The pooled sensitivity was 55%, and the pooled specificity was 87% (29). Zhan et al. reported that approximately 40% of cN0 PTC patients actually had CLNM (30). In our study, the rate of CLNM in cN0 patients was 21.40%.
Some previous studies have established diagnostic models for predicting LNM in PTC. Huang et al. (31) used the LASSO method to analyze all US features and some clinical features to establish a CLNM prediction model and web-based calculator, which presented good performance. Xue et al (32) analysed the relationship between US and contrast-enhanced US characteristics and then used univariate and multivariable logistic regression methods to establish a nomogram model. Park et al (33) aimed to develop a radiomics signature using US images of the primary tumor to preoperatively predict LNM in patients with conventional PTC. Zhao et al (34) used independent predictive factors, followed by multivariate logistic regression, to evaluate risk factors by using ROC curve analysis. Most of these articles included the characteristics of US-related factors and some clinical features. Our research included all information we could obtain before surgery, such as family history of thyroid cancer, history of HT, thyroid function and gene mutations.
CLNM is a complex problem, and the use of a single variable to predict CLNM is not reliable. Multivariable regression models are commonly used to identify significant independent risk factors in medical statistical analysis. The threshold of P<0.05 is artificially set, and it is easy to lose some important related factors. The LASSO method does not exclude any variables that might impact the outcome, but it did not play an independent role in univariate analysis. This method was properly used to reduce the number of variables. When the weight of low correlation variables is compressed to 0, they were finally eliminated. The LASSO method is a calculation method that is more suitable for datasets that include many variables. The use of the LASSO method before logistic regression follows the “second strike theory” from the combination of generalized genetic factors and environmental factors. We believe that the combination of many factors together brought about the final clinical event of CLNM. The use of LASSO before logistic regression in the calculation process of this study took into consideration the effects of multiple factors and did not arbitrarily rule out any of the possible factors.
In our article, the rate of positive CLNM in younger patients was higher than that in elderly patients, suggesting that age was negatively correlated with CLNM, which was similar to the findings of previous studies (35, 36). Importantly, the rate of positive CLNM in patients with other adverse prognostic factors, including a history of HT, a history of nodular goiter, worse TI-RADS classification and BRAF V600E mutation, was higher, which indicated that these factors were positively related to CLNM. Some studies demonstrated that the BRAF V600E mutation was correlated with CLNM (37, 38), but others obtained the opposite conclusion (39). The relationship between the BRAF V600E mutation and specific clinical pathological features of PTC remains controversial. However, in our research, only the BRAF V600E mutation was confirmed as a significant independent risk factor for CLNM.
All the related variables were subjected to selection by the LASSO method. Using this method, we did not exclude any variables that might definitely impact the outcome, and although these factors did not have not an independent role in univariate analysis, doing this improved the pertinence and accuracy of subsequent logistic regression.
The limitations of our article are as follows. First, this was a single-centre study, which may lead to data bias. Thus, it is necessary to conduct further prospective research and multicenter studies. Finally, the evaluation of some US features is subjective, and interobserver variability may occur. Our retrospective study preliminarily explored the possibility of using certain factors to predict CLNM in cN0 PTC patients.
In conclusion, the nomogram prediction model was able to predict the risk of preoperative CLNM in cN0 PTC patients and has a good predictive performance. Further prospective, multicenter, and larger sample size studies are needed to confirm our findings.
Data availability statement
The processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Requests to access the datasets should be directed to FZ, phillip_zhao@126.com.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2020-T346-1). The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding Statement
This study was supported by funding from the Clinical Research Program of 9th People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ202016). This research study was registered in the China clinical trial registry (ChiCTR2200057167).
Abbreviations
CLNM, central lymph node metastasis; PTC, papillary thyroid carcinoma; PCLND, prophylactic central lymph node dissection; FNA, fine-needle aspiration; LASSO, least absolute shrinkage and selection operator; ROC, receiver operating characteristic; DCA, decision curve analysis; cN0, clinically negative central compartment lymph node; LNM, lymph node metastasis; US, ultrasound; HT, Hashimoto’s thyroiditis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA: Cancer J Clin (2013) 63(6):374–94. doi: 10.3322/caac.21195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery (2003) 134(6):946–54. doi: 10.1016/s0039-6060(03)00424-0 [DOI] [PubMed] [Google Scholar]
- 4. Teixeira G, Teixeira T, Gubert F, Chikota H, Tufano R. The incidence of central neck micrometastatic disease in patients with papillary thyroid cancer staged preoperatively and intraoperatively as N0. Surgery (2011) 150(6):1161–7. doi: 10.1016/j.surg.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 5. Kim SY, Kim BW, Pyo JY, Hong SW, Chang HS, Park CS. Macrometastasis in papillary thyroid cancer patients is associated with higher recurrence in lateral neck nodes. World J Surg (2018) 42(1):123–9. doi: 10.1007/s00268-017-4158-5 [DOI] [PubMed] [Google Scholar]
- 6. Cho SY, Lee TH, Ku YH, Kim HI, Lee GH, Kim MJ. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery (2015) 157(1):111–8. doi: 10.1016/j.surg.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 7. Jianming L, Jibin L, Linxue Q. Suspicious ultrasound characteristics correlate with multiple factors that predict central lymph node metastasis of papillary thyroid carcinoma: Significant role of hbme-1. Eur J Radiol (2020) 123:108801. doi: 10.1016/j.ejrad.2019.108801 [DOI] [PubMed] [Google Scholar]
- 8. Wang TS, Sosa JA. Thyroid surgery for differentiated thyroid cancer - recent advances and future directions. Nat Rev Endocrinol (2018) 14(11):670–83. doi: 10.1038/s41574-018-0080-7 [DOI] [PubMed] [Google Scholar]
- 9. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncology: Off J Am Soc Clin Oncol (2015) 33(21):2370–5. doi: 10.1200/JCO.2014.59.8391 [DOI] [PubMed] [Google Scholar]
- 10. Enyioha C, Roman SA, Sosa JA. Central lymph node dissection in patients with papillary thyroid cancer: A population level analysis of 14,257 cases. Am J Surg (2013) 205(6):655–61. doi: 10.1016/j.amjsurg.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 11. Sadowski BM, Snyder SK, Lairmore TC. Routine bilateral central lymph node clearance for papillary thyroid cancer. Surgery (2009) 146(4):696–703. doi: 10.1016/j.surg.2009.06.046 [DOI] [PubMed] [Google Scholar]
- 12. Alvarado R, Sywak MS, Delbridge L, Sidhu SB. Central lymph node dissection as a secondary procedure for papillary thyroid cancer: Is there added morbidity? Surgery (2009) 145(5):514–8. doi: 10.1016/j.surg.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 13. Calo PG, Pisano G, Medas F, Marcialis J, Gordini L, Erdas E, et al. Total thyroidectomy without prophylactic central neck dissection in clinically node-negative papillary thyroid cancer: Is it an adequate treatment? World J Surg Oncol (2014) 12:152. doi: 10.1186/1477-7819-12-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conzo G, Mauriello C, Docimo G, Gambardella C, Thomas G, Cavallo F, et al. Clinicopathological pattern of lymph node recurrence of papillary thyroid cancer. Implications Surgery Int J Surg (2014) 12 Suppl 1:S194–7. doi: 10.1016/j.ijsu.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 15. Nixon IJ, Wang LY, Ganly I, Patel SG, Morris LG, Migliacci JC, et al. Outcomes for patients with papillary thyroid cancer who do not undergo prophylactic central neck dissection. Br J Surg (2016) 103(3):218–25. doi: 10.1002/bjs.10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yazıcı D, Çolakoğlu B, Sağlam B, Sezer H, Kapran Y, Aydın Ö, et al. Effect of prophylactic central neck dissection on the surgical outcomes in papillary thyroid cancer: Experience in a single center. Eur Arch Oto-Rhino-Laryngology (2020) 277(5):1491–7. doi: 10.1007/s00405-020-05830-1 [DOI] [PubMed] [Google Scholar]
- 17. Ataş H, Akkurt G, Saylam B, Tez M. Central neck dissection is an independent risk factor for incidental parathyroidectomy. Acta Chirurgica Belgica (2021) 121(1):36–41. doi: 10.1080/00015458.2020.1828677 [DOI] [PubMed] [Google Scholar]
- 18. Park I, Her N, Choe J-H, Kim JS, Kim J-H. Management of chyle leakage after thyroidectomy, cervical lymph node dissection, in patients with thyroid cancer. Head Neck (2018) 40(1):7–15. doi: 10.1002/hed.24852 [DOI] [PubMed] [Google Scholar]
- 19. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(8):925–51. doi: 10.6004/jnccn.2022.0040 [DOI] [PubMed] [Google Scholar]
- 20. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid: Off J Am Thyroid Assoc (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medas F, Canu GL, Cappellacci F, Anedda G, Conzo G, Erdas E, et al. Prophylactic central lymph node dissection improves disease-free survival in patients with intermediate and high risk differentiated thyroid carcinoma: A retrospective analysis on 399 patients. Cancers (2020) 12(6):E1658. doi: 10.3390/cancers12061658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He J, Li J, Cheng Y, Fan J, Jun G, Jiang Z, et al. [Guidelines of Chinese society of clinical oncology (CSCO) differentiated thyroid cancer]. J Cancer Control Treat (2021) 34(12):1164–201. [Google Scholar]
- 23. Ruggiero FP, Fedok FG. Outcomes in reoperative thyroid cancer. Otolaryngologic Clinics North America (2008) 41(6):1261–8. doi: 10.1016/j.otc.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 24. Pai SI, Tufano RP. Reoperation for Recurrent/Persistent well-differentiated thyroid cancer. Otolaryngologic Clinics North America (2010) 43(2):353–63. doi: 10.1016/j.otc.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 25. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of ct and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg (2008) 32(7):1552–8. doi: 10.1007/s00268-008-9588-7 [DOI] [PubMed] [Google Scholar]
- 26. Liu Z, Xun X, Wang Y, Mei L, He L, Zeng W, et al. Mri and ultrasonography detection of cervical lymph node metastases in differentiated thyroid carcinoma before reoperation. Am J Trans Res (2014) 6(2):147–54. [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong X, Lu Y, Yin X, Wang Q, Wang F, He Z. Prophylactic central lymph node dissection performed selectively with Cn0 papillary thyroid carcinoma according to a risk-scoring model. Gland Surg (2022) 11(2):378–88. doi: 10.21037/gs-21-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg (2005) 29(7):917–20. doi: 10.1007/s00268-005-7789-x [DOI] [PubMed] [Google Scholar]
- 29. Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Diagnostic performance of ct in detection of metastatic cervical lymph nodes in patients with thyroid cancer: A systematic review and meta-analysis. Eur Radiol (2019) 29(9):4635–47. doi: 10.1007/s00330-019-06036-8 [DOI] [PubMed] [Google Scholar]
- 30. Zhan J, Zhang L-H, Yu Q, Li C-L, Chen Y, Wang W-P, et al. Prediction of cervical lymph node metastasis with contrast-enhanced ultrasound and association between presence of Brafv600e and extrathyroidal extension in papillary thyroid carcinoma. Ther Adv Med Oncol (2020) 12:1758835920942367. doi: 10.1177/1758835920942367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C, Cong S, Shang S, Wang M, Zheng H, Wu S, et al. Web-based ultrasonic nomogram predicts preoperative central lymph node metastasis of Cn0 papillary thyroid microcarcinoma. Front Endocrinol (Lausanne) (2021) 12:734900. doi: 10.3389/fendo.2021.734900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xue T, Liu C, Liu J-J, Hao Y-H, Shi Y-P, Zhang X-X, et al. Analysis of the relevance of the ultrasonographic features of papillary thyroid carcinoma and cervical lymph node metastasis on conventional and contrast-enhanced ultrasonography. Front Oncol (2021) 11:794399. doi: 10.3389/fonc.2021.794399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park VY, Han K, Kim HJ, Lee E, Youk JH, Kim EK, et al. Radiomics signature for prediction of lateral lymph node metastasis in conventional papillary thyroid carcinoma. PloS One (2020) 15(1):e0227315. doi: 10.1371/journal.pone.0227315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao S, Yue W, Wang H, Yao J, Peng C, Liu X, et al. Combined conventional ultrasound and contrast-enhanced computed tomography for cervical lymph node metastasis prediction in papillary thyroid carcinoma. J Ultrasound Medicine: Off J Am Institute Ultrasound Med (2022). 9999(1-14):0278–4297 doi: 10.1002/jum.16024 [DOI] [PubMed] [Google Scholar]
- 35. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and Male sex are predictors of Large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid (2017) 27(10):1285–90. doi: 10.1089/thy.2017.0250 [DOI] [PubMed] [Google Scholar]
- 36. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid (2016) 26(6):807–15. doi: 10.1089/thy.2015.0429 [DOI] [PubMed] [Google Scholar]
- 37. So YK, Son Y-I, Park JY, Baek C-H, Jeong H-S, Chung MK. Preoperative braf mutation has different predictive values for lymph node metastasis according to tumor size. Otolaryngology–Head Neck Surgery: Off J Am Acad Otolaryngology-Head Neck Surg (2011) 145(3):422–7. doi: 10.1177/0194599811404649 [DOI] [PubMed] [Google Scholar]
- 38. Kim SK, Lee JH, Woo J-W, Park I, Choe J-H, Kim J-H, et al. Braf V600e mutation: Differential impact on central lymph node metastasis by tumor size in papillary thyroid carcinoma. Head Neck (2016) 38 Suppl 1:E1203–9. doi: 10.1002/hed.24192 [DOI] [PubMed] [Google Scholar]
- 39. Guo L, Ma Y-Q, Yao Y, Wu M, Deng Z-H, Zhu F-W, et al. Role of ultrasonographic features and quantified Brafv600e mutation in lymph node metastasis in Chinese patients with papillary thyroid carcinoma. Sci Rep (2019) 9(1):75. doi: 10.1038/s41598-018-36171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Requests to access the datasets should be directed to FZ, phillip_zhao@126.com.